Abstract
Basophil activation test (BAT) can tackle multiple mechanisms underlying acute and delayed hypersensitivity to drugs and vaccines and might complement conventional allergy diagnostics but its role in anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine-related hypersensitivity is ill-defined. Therefore, 89 patients with possible hypersensitivity (56 % with delayed mucocutaneous manifestations) to anti-SARS-CoV-2 vaccines were tested with BAT for Macrogol 3350, DMG-PEG 2000, PEG 20000, polysorbate-80 and trometamol and compared to 156 subjects undergoing pre-vaccine BAT. A positive BAT was associated with delayed reaction onset (p = 0.010) and resolution (p = 0.011). BAT was more frequently positive to DMG-PEG 2000 than to other excipients in both groups (p < 0.001). DMG-PEG 2000 reactivity was less frequent in vaccine-naïve (6 %) than vaccinated subjects (35 %, p < 0.001) and associated with mRNA-1273 vaccination. DMG-PEG 2000 BAT might therefore have a diagnostic role in subjects with delayed hypersensitivity reactions. Natural immunity might be a key player in basophil activation.
Keywords: Basophil activation test, SARS-CoV-2, COVID-19, Vaccine, Allergy, Hypersensitivity, Adverse reaction, Polyethylene glycol
1. Introduction
Vaccination is the mainstay of prevention for infectious diseases, including the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related disease (COVID-19). In this latter setting, a multitude of novel vaccine technologies such as polyethylene glycol (PEG)-bound liposomal preparations embedding mRNA particles, adenoviral vectored and saponin adjuvanted vaccines have been successfully tested and widely employed in clinical practice to stimulate host immunisation towards SARS-CoV-2. Similar to other vaccine preparations, these novel pharmacological tools might occasionally induce hypersensitivity reactions. Immediate hypersensitivity reactions to mRNA-based vaccines occur with an estimated frequency of 2.5–11.1 cases/million doses, which is higher than the reaction rate reported for conventional vaccines [1], [2], [3]. Delayed hypersensitivity has also been reported in association with anti-SARS-CoV-2 vaccination [4], [5], especially with the use of mRNA-1273 (in contrast to the association between BNT162b2 and acute urticaria) [6]. Exposure to dermal fillers has also been selectively associated with delayed hypersensitivity to mRNA-based vaccines [7]. Nonetheless, most patients with vaccine-related reactions are able to receive additional doses with mild or no symptoms, suggesting that non-conventional mechanisms, other than those involving deranged humoral and/or cellular adaptive immune responses might be implicated [8], [9], [10].
With the progression of mass vaccination campaigns and accumulating evidence on vaccine-related hypersensitivity, pathogenic hypotheses on potential molecular triggers rapidly accumulated. Polyethylene glycol (PEG)-2000 constitutes the main component of the molecular scaffold ensuring stability of mRNA-based liposomal vaccines [11]. Due to existing literature on PEG-related allergy and the widespread sources of potential sensitisation among commonly used drugs and cosmetics in the general population, PEG was first proposed as a potential culprit agent for anti-SARS-CoV-2 vaccine-related hypersensitivity reactions [12], [13], [14]. The PEG-2000 hypothesis is further corroborated by the detection of circulating anti-PEG IgG and IgE in humans [14], [15], [16] and by the potential association between anti-SARS-CoV-2 mRNA-based vaccine hypersensitivity and positive skin prick or intradermal tests with PEG [17], [18]. Nevertheless, anti-PEG antibodies are apparently not correlated with the occurrence of hypersensitivity reactions [19] and the rate of positive skin prick tests to PEG is low, especially without accurate pre-test stratification [10], [17], [20]. Furthermore, even patients with known history of PEG hypersensitivity (including anaphylaxis) have been shown to be able to safely receive mRNA-based vaccines, suggesting that PEG amounts in vaccine preparations are not able per se to sustain allergic responses to anti-SARS-CoV-2 vaccines [21]. Therefore, lipid components of mRNA-based vaccine formulations have eventually been proposed as potential candidate triggers or cofactors for vaccine related hypersensitivity reactions by means of mechanisms such as complement-mediated activation of the acute phase response [11] or clustering of existing anti-PEG antibodies on leukocytes [22].
Basophil activation test (BAT) is a safe and informative in vitro test able to assess systemic reactivity to exogenous stimuli and overcome potential limitations and confounding effects of tests such as skin tests and specific IgE measurement in blood [23]. Only scattered evidence is available on the potential role of BAT in complementing current diagnostic weaponry for anti-SARS-CoV-2 vaccine-related hypersensitivity [15], [24]. To address this issue and obtain potential additional clues on the pathophysiology of hypersensitivity in the setting of anti-SARS-CoV-2 vaccination, we set up a multicentre observational study focused on potential correlations among BAT reactogenicity of vaccine excipients (including PEG and lipid-bound PEG) and safety outcomes in patients having received or due to receive anti-SARS-CoV-2 vaccines.
2. Materials and methods
2.1. Patients
From April to June 2022, we collected clinical and laboratory data from consecutive patients attending seven allergy tertiary referral Centres within the Lombardy Region, Italy and reporting a history of hypersensitivity reactions following vaccination with anti-SARS-CoV-2 vaccines (test group) or undergoing precautionary allergy evaluation before being vaccinated (control group). Clinical and laboratory data were collected in an online database built through the surveymonkey.com online tool [25], devoid of individual identifying information and conforming to the indications of the European Commission for anonymisation [26] besides being in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. As the subjects were not identifiable, no specific approvals by the local Institutional Review Boards were required.
Inclusion criteria for the test group were: 1) age ≥ 18 years; 2) a history of hypersensitivity reaction after vaccination with any dose of any type of anti-SARS-CoV-2 vaccines. Inclusion criteria for the control group were: 1) age ≥ 18 years; 2) having not received any anti-SARS-CoV-2 vaccine dose; 3) having performed a precautionary BAT before vaccination due coexisting allergy or other supposed predisposing conditions (previous reactions to other vaccines). Hypersensitivity was defined according to the World Allergy Organisation nomenclature [27] and had to be validated by an experienced Allergist. Reactions were graded according to Common Terminology Criteria for Adverse Events (CTCAE) [9] and classified according to the presence of urticaria/angioedema, rash, itch, bronchospasm, hypotension, immediate diarrhoea. There were no exclusion criteria.
Demographic and general clinical information included age group (we defined 13 five-year age ranges with additional extremes at 18–25 and > 90 years of age, respectively), sex, history of allergy-spectrum disorders (anaphylaxis, chronic spontaneous urticaria/angioedema, atopic dermatitis, contact allergic dermatitis, respiratory allergy with or without allergic asthma, allergy to food, hymenopter venom and/or drugs) and chronic treatments for allergic symptoms.
Reaction-specific data included qualitative features of the reaction, ad hoc prophylactic medication or post-reaction treatments. Reactions were also dichotomised for timing, with immediate-onset reactions occurring within 4 h from vaccine exposure and delayed-onset reactions occurring at later times, respectively. Delayed resolution was defined as the complete disappearance of symptoms and signs after > 48 h from onset.
2.2. Basophil activation test
BAT was centralised at ICS Maugeri, Pavia, Italy following a standardised protocol [28]. Briefly, 50 μl whole blood samples underwent a 10-minute pre-incubation with 10 ng/ml of IL-3 at 37 °C before being incubated with an equal volume of wash buffer (phosphate buffered saline with 0.1 % human serum albumin, negative control), 200 ng/ml anti-IgE antibodies (clone G7-18, BD Biosciences, San Jose, CA; positive control) or diluted substances. Besides Dimyristoyl glycerol (DMG)-PEG 2000, we chose to test other available high-molecular weight PEGs (Macrogol 3350, PEG 20000) along with potential PEG cross-reactants (polysorbate 80, PS80) and other relevant mRNA vaccine excipients (trometamol). We preferred high-molecular weight PEGs over low-molecular weight PEGs in light of previous evidence of increasing diagnostic accuracy for PEG hypersensitivity with increasing PEG molecular weight [29]. For each test substance two concentrations were tested and those eliciting the highest CD63 expression considered for analysis in order to intercept the nearest point to the peak of substance bell-shaped dose–response curve [23]. Specifically, tested excipients (concentration) were: Macrogol 3350 (Moviprep, Norgine Amsterdam, the Netherlands, 5 mg/ml and 500 μg/ml in wash buffer); DMG-PEG 2000 (Avanti, Alabama, firstly diluted 10 mg/ml in Dimethyl sulfoxide and subsequently in wash buffer at 5 and 0.5 μg/ml); PEG 20000 (Merck, Darmstadt, Germany at 5 mg/ml and 500 μg/ml in wash buffer); PS80 (Merck, Darmstadt, Germany, firstly diluted in ETOH and subsequently in wash buffer at 0.5 and 0.05 μg/ml); Trometamol (Merck, Darmstadt, Germany, dilution tested 10 and 1 μg/ml in wash buffer). Concentrations were chosen after preliminary experiments in atopic and non-atopic subjects (Table S1) in order to select sub-toxic concentrations not inducing non-specific basophil activation. Each subject was also tested for a known tolerated substance as a negative control test (often paracetamol 20 and 5 μg/ml or another tolerated drug or food extract, Lofarma, Milan, diluted 1/100 in wash buffer). After twenty minutes of incubation, cells were stained with phycoerythrin (PE)-conjugated anti-CD123, peridinin-chlorophyll- protein (PerCP)-conjugated anti-Human Leukocyte Antigen (HLA)-DR and fluorescein isothiocyanate (FITC)-conjugated anti-CD63 monocolonal antibodies (BD Fastimmune, BD Biosciences, San Jose, CA) and incubated for further 20 min on ice, shielded from light. After erythrocyte lysis with ammonium chloride for seven minutes, samples were washed, centrifuged and resuspended in FACS Flow Sheath Fluid before being acquired and analysed through a BD FACS Canto II flow cytometer. Basophils were defined as side-scatter low, CD123 + HLA-DR- cells within the lymphocyte/monocyte gate. Data acquisition was limited to 1,000 basophil events. A stimulation index (SI) was calculated as the ratio of CD63 %-expression with the test substance and CD63 %-expression with negative control. Positive responses were defined as BAT showing CD63 expression > 5 % and SI > 2 [23], [30], [31].
2.3. Statistical analyses
Statistical analyses were performed with SPSS statistical software (version 20) and Statacorp STATA (version 15). Categorical data were compared among groups by using the chi square test with Fisher’s exact correction for small groups. Non-normally distributed continuous variable trends were compared among groups through the Mann-Whitney or the Kruskal-Wallis test. Data are expressed as median (interquartile range, IQR) unless otherwise specified.
3. Results
3.1. Characteristics of patients reporting vaccine-associated hypersensitivity
The test group consisted of 89 subjects reporting suspected hypersensitivity reactions after SARS-CoV-2 vaccination. Most patients were women and had a median age range of 41–45 years. One third of the tested subjects did not have any history of allergic reactions before anti-SARS-CoV-2 vaccination. Accordingly, only 3 % of patients in the test group were taking any anti-allergic medication at time of reaction. Nine subjects (10 %) reported a history of COVID-19 before performing BAT (Table 1 ). Most patients were vaccinated with either the BNT162b2 [Pfizer BioNTech] (54 %) or the mRNA-1273 [Moderna] (36 %) mRNA-based vaccines, while only seven subjects (8 %) received an adenoviral vectored vaccine [Astra Zeneca] at time of reaction (Table S2).
Table 1.
Demographic and clinical features of patients with post-vaccine hypersensitivity (N = 89).
| Clinical variable | N ( %) |
|---|---|
| Sex F ( %) | 71 (80) |
| Age < 45 years | 50 (56) |
| Allergy history | 59 (66) |
| Drug allergy | 29 (33) |
| Respiratory allergy without asthma | 18 (20) |
| Respiratory allergy with asthma | 12 (14) |
| DAC | 7 (8) |
| Atopic dermatitis | 4 (5) |
| Anaphylaxis | 4 (5) |
| Urticaria and angioedema | 3 (3) |
| Hymenoptera allergy | 0 (0) |
| No allergic history | 30 (34) |
| COVID-19 before BAT | 9 (10) |
| Chronic therapy for allergic diseases | 3 (3) |
Vaccine-associated hypersensitivity occurred with a slightly higher frequency after the first dose (39/89 subjects, 44 %), than after the second dose (30/89, 34 %) or the first booster dose (20/89 subjects, 22 %). Additional booster doses were not yet being administered in the general population at time of data collection. Most patients (56 %) reported delayed reactions, with 37 % of the reactions developing more than 48 h after vaccination. Delayed reactions were significantly more frequent than immediate reactions in patients with no previous allergy history (23/50, 46 % vs 7/39, 18 % p = 0.005). Conversely, immediate reactions were more frequent than delayed reactions in patients with history of respiratory allergy with asthma (9/39, 23 % vs 3/50, 6 % p = 0.019) and anaphylaxis (4/39, 10 % vs 0/50, 0 %, p = 0.034). Reaction resolution was delayed in 81 % of patients and lasted up to more than one month in more than one out of five patients (24 %). Hypersensitivity reactions were mainly mucocutaneous (80 %) and respiratory (24 %, Table 2 ). Most reactions were of moderate severity (median CTCAE score = 2.0 (1.0–2.0)). More than one out of six patients (17 %) reported having previously experienced similar reactions in their life. Hypersensitivity reactions were mainly mucocutaneous (80 %) and respiratory (24 %, Table 2). Most reactions were of moderate severity (median CTCAE score = 2.0 (1.0–2.0)). More than one out of six patients (17 %) reported having experienced similar reactions in their history.
Table 2.
Clinical features of vaccine-related hypersensitivity in the test group.
| Reaction features | N ( %) |
|---|---|
| Reaction timing | |
| Timing of onset | |
| Immediate (≤4h) | 39/89 (44) |
| Delayed (>4h) | 50/89 (56) |
| Timing of resolution | |
| Immediate (≤48 h) | 17/89 (19) |
| Delayed (>48 h) | 72/89 (81) |
| Very slow (>1 month) | 21/89 (4) |
| Clinical characteristics | |
| Mucocutaneous manifestations | 71/89 (80) |
| Respiratory manifestations | 21/89 (24) |
| Haemodynamic/cardiovascular instability | 4/89 (5) |
| Gastrointestinal manifestations | 4/89 (5) |
| Neurologic manifestations | 6/89 (7) |
3.2. BAT profile of patients in the test and control groups
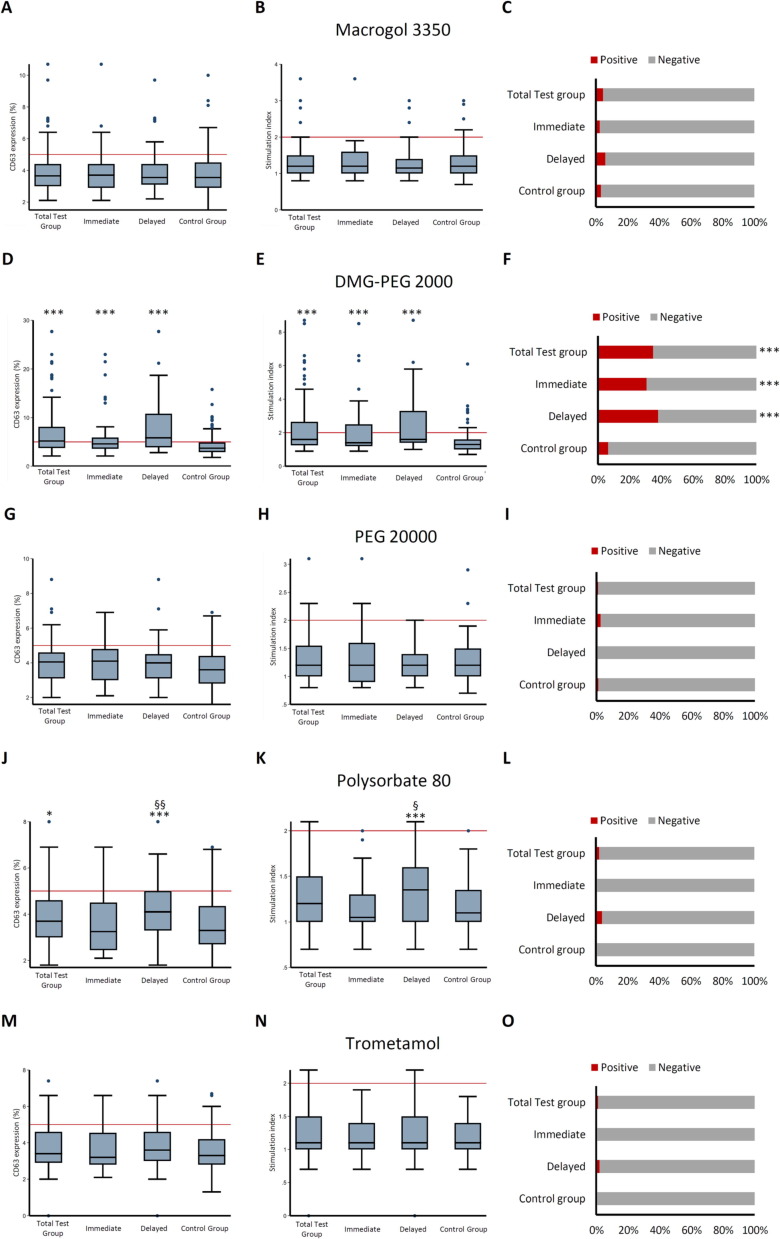
In the test group, we found 33/89 patients (37 %) with a positive BAT for at least one excipient. DMG-PEG 2000 was associated with the highest frequency of positive BAT (31/89, 35 % of all subjects, 94 % of all positive BAT. DMG-PEG 2000 BAT-positive patients had similar demographic and general clinical characteristics compared to the other subjects (Table S3).
Compared to patients with no BAT reactivity to DMG-PEG 2000, DMG-PEG 2000 BAT-positive patients had received the mRNA-1273 vaccine more frequently (p = 0.004) and their hypersensitivity reactions occurred more frequently after the first booster dose (p = 0.002). In addition, we found a higher prevalence of mucocutaneous reactions (p = 0.011) of delayed (p = 0.011) or very slow (p = 0.014) resolutions among patients with positive DMG-PEG 2000 BAT than in the remainder subjects (Table 3 ). There was no significant association with a history of previous COVID-19.
Table 3.
Comparison between patients with suspected hypersensitivity reactions and BAT positive or negative with DMG-PEG 2000.
| Positive DMG-PEG 2000 BAT N ( %) | Negative DMG-PEG 2000 BAT N ( %) | P | |
|---|---|---|---|
| Reaction-associated vaccines | 31/89 (35) | 58/89 (65) | |
| BNT162b2 (Pfizer BioNTech) | 12/31 (39) | 13/58 (22) | 0.004 |
| mRNA-1273 (Moderna) | 19/31 (61) | 7/58 (12) | |
| ChAdOx1 nCoV-19 (Astra Zeneca) | 0/31(0) | 1/58 (2) | |
| Ad26.COV2.S (Johnson&Johnson) | 0/31 (0) | 1/58 (2) | |
| NVX-CoV2373 (Novavax) | 0/31 (0) | 0/58 (0) | |
| Others | 0/31 (0) | 1/58 (2) | |
| First dose | 7/31 (23) | 32/58 (55) | 0.002 |
| Second dose | 11/31 (36) | 19/58 (33) | |
| First booster | 13/31 (42) | 7/58 (12) | |
| Reaction characteristics | |||
| Reaction timing: | |||
| Timing of onset | |||
| Immediate (≤4h) | 12/31 (39) | 31/58 (53) | 0.477 |
| Delayed (>4h) | 19/31 (61) | 27/58 (47) | |
| Delayed (>48 h) | 17/31 (55) | 16/58 (28) | 0.011 |
| Timing of resolution | |||
| Immediate (≤48 h) | 14/31 (45) | 42/58 (72) | 0.011 |
| Delayed (>48 h) | 17/31 (55) | 16/58 (28) | |
| Very slow (>1 month) | 12/31 (39) | 9/58 (16) | 0.014 |
| Reaction clinical features | |||
| Mucocutaneous manifestations | 29/31 (94) | 42/58 (72) | 0.018 |
| Respiratory manifestations | 8/31 (26) | 13/58 (22) | 0.719 |
| Haemodynamic/cardiovascular instability | 1/31 (3) | 3/58 (5) | 1.000 |
| Gastrointestinal manifestations | 1/31 (3) | 3/58 (5) | 1.000 |
| Neurologic manifestations | 1/31 (3) | 5/58 (9) | 0.661 |
| Previous similar reactions | 5/31 (16) | 10/58 (17) | 0.946 |
A control group of subjects who performed precautionary BAT for the same excipients before SARS-CoV-2 vaccination was included (n = 156). These subjects were older (p = 0.004), had a higher prevalence of allergy history (p = 0.001), especially to drugs (p < 0.001), and a higher prevalence of anaphylaxis (p = 0.001). A history of COVID-19 before BAT was less frequent in the control than in the test group (p = 0.017, Table S4). Among control subjects, DMG-PEG 2000 showed the highest BAT reactogenicity in comparison with other vaccine excipients (p < 0.001). However, DMG-PEG 2000 reactivity and the frequency of positive DMG-PEG 2000 BAT was significantly lower in control subjects (10/156, 6 %) than in test subjects (31/89, 35 % p < 0.001). The frequency of positive BAT for other excipients was low and comparable between groups (Fig. 1 ). CD63 expression and SI for tolerated substances (drugs or food) were comparable between the two groups: CD63 = 3.4 % (2.8–3.9) SI = 1.1 (0.9–1.3) and CD63 = 3.1 % (2.7–3.7) SI = 1.0 (1.0–1.2) respectively. When all subjects were considered together (test group + control group), the frequency of DMG-PEG 2000 positive BAT was higher in non-allergic subjects compared to allergic subjects (26 % vs 14 %, p = 0.033).
Fig. 1.
Differential BAT profiles among patients with immediate reactions, delayed reactions and controls.
In this figure, quantitative BAT responses to Macrogol 3350, DMG-PEG 2000, PEG 20000, Polysorbate 80 and trometamol among patients in the test group, patients with immediate and delayed reactions within the test group and in non-vaccinated control-group patients are depicted as boxplots. Specifically, left-side boxplots (panel A,D,G,J,M) show CD63 expression on basophils after stimulation with test substances, while central boxplots (panel B,E,H,K,N) depict the stimulation index. Red reference lines represent the cut-offs for positive CD63 expression (CD63 %=5 %) and stimulation index (SI = 2). Right-side panels show the percentage of positive BAT tests to each substance among groups. Symbols: *p < 0.050 compared to control group; ** p < 0.010 compared to control group; *** p < 0.001 compared to control group; § p < 0.05 compared to patients with immediate reactions, §§ p < 0.010 compared to patients with immediate reactions; §§§ p < 0.001 compared to patients with immediate reactions.
After further stratifying BAT results by timing of reaction in the test group, we found that patients with both delayed and immediate reactions had significantly higher reactivity to DMG-PEG 2000 than control group. Patients with immediate- and delayed reactions did not differ in terms of DMG-PEG 2000 reactivity. Patients with delayed reactions showed higher quantitative reactivity to PS80 than immediate reaction subjects and control group-subjects, although the frequency of tests crossing the significance thresholds were comparable among groups (Fig. 1).
3.3. Follow up data
Data on post-BAT vaccine administration were available from 15 subjects in the test group (11 with immediate and four with delayed reactions) and 22 subjects in the control group. Twelve of 15 patients in the test group received the same vaccine associated with the reaction without any adverse event. Two of them had positive BAT to DMG-PEG 2000. Three patients in the test group (none with DMG-PEG 2000-positive BAT) received a different vaccine, with one showing mild symptoms comparable to the index delayed reaction. Of the 22 control subjects with available follow-up data, one had a positive DMG-PEG 2000 BAT. All subjects tolerated vaccination.
4. Discussion
In this study, we tested BAT reactogenicity of an array of excipients embedded in current anti-SARS-CoV-2 vaccine formulation, in a cohort of patients reporting post-vaccine hypersensitivity and in a group of vaccine-naïve subjects receiving precautionary allergy work up. Clinical evidence showed that delayed and slowly resolving hypersensitivity reactions with prominent cutaneous involvement constituted the most common clinical presentation of immune-mediated adverse reactions to anti-SARS-CoV-2 vaccines, in line with previous reports from international registries and cohort studies [4], [6], [10], [32]. Experimental data indicated that DMG-PEG 2000 had high in vitro BAT reactogenicity both in patients exposed and non-exposed to anti-SARS-CoV-2 vaccines. We also found that DMG-PEG 2000 BAT responses were higher in exposed than in non-exposed subjects and peaked in those with slowly resolving reactions and skin manifestations. By selectively detecting an increased frequency of DMG-PEG 2000 BAT reactogenicity, our results suggest that lipid-bound high molecular weight PEG rather than unbound PEG might constitute the major trigger of inflammation for anti-SARS-CoV-2 vaccine-related inflammatory adverse events. Consistently, positive DMG-PEG 2000 BATs clustered with patients exposed to mRNA-1273 in contrast to patients exposed to BNT162b2, which contains N,N-ditetradecylacetamide-bound PEG 2000. In our cohort of patients with anti-SARS-CoV-2 vaccine hypersensitivity, we also did not find a correlation between PEG molecular weight and reactogenicity, in contrast to the expected allergenicity profile of PEG [33] but in line with other reports on mRNA-based vaccines [15]. Taken together, these results suggest that DMG-PEG 2000 BAT might tackle a specific key pathophysiological event in anti-SARS-CoV-2 vaccine-related hypersensitivity reactions.
Nonetheless, the reasons behind the detection of some degree of DMG-PEG reactivity in non-exposed subjects are not straightforward. Sensitisation to PEG with generation of antibodies has long been described as a relatively common event in the general population, due to the widespread use of PEG in drugs and cosmetics [7], [12], [34]. However, in the setting of mRNA vaccines, immune memory-independent, nanoparticle-triggered inflammatory responses might occur even in PEG-naïve subjects. In fact, mechanistically, BAT might be sensitive to both conventional antibody-induced and anaphylatoxin-mediated activation of basophils/mast-cells [23], [24], [35], [36]. Canonical IgE-mediated allergic responses to PEG were first claimed as potential causes of inflammatory adverse events in patients receiving anti-SARS-CoV-2 vaccines. In contrast to this hypothesis, limited evidence has so far been acquired on clinically relevant anti-PEG IgE in association with vaccine-related allergic events [14], [15], [16], [37]. Furthermore, vaccination occurs uneventfully in patients with established allergy to PEG [19], suggesting that either a) lipid-bound PEG rather than “naked” PEG might be the target antigen of canonical IgE-mediated allergic responses to anti-SARS-CoV-2 vaccines and/or that; b) non-IgE-mediated mechanisms might be involved in anti-SARS-CoV-2 vaccine hypersensitivity. Anti-PEG IgG have also been found in patients with allergic reactions to mRNA vaccines and positive BAT to PEG and might constitute an additional mechanism accounting for basophil activation and vaccine-related hypersensitivity [15]. Nonetheless, the majority of patients with previous reactions to anti-SARS-CoV-2 vaccines are able to receive additional vaccine doses without further reactions of any severity [4], [32] suggesting the existence of mechanisms independent on immunological memory. Consistently, we also did not find evidence of cross-reactivity among molecularly related substances such as PEG and PS80. Similar findings have been described in the setting of reactions to nanoparticle infusions, especially of anticancer drugs [38]. Therefore, complement activation–related pseudoallergy (CARPA) or non-specific engagement of leukocyte receptors by nanoparticles have been proposed as additional innate mechanisms of vaccine-induced hypersensitivity [11], [39]. In this setting, DMG-PEG 2000 might constitute a valuable proxy nanoparticle antigen for diagnostic purposes in the setting of anti-SARS-CoV-2 vaccine hypersensitivity.
Besides being involved in acute and hyper-acute reactions, basophils also sustain persisting local and systemic inflammation, especially in the skin [40], [41], [42], [43], [44]. Basophils also constitute the main granulocytic white cell subpopulation in delayed hypersensitivity reaction infiltrates [45] and may provide support to Th2 responses through IL4/IL13 release [46]. Furthermore, basophils are also crucial in IgE-mediated T-cell independent chronic inflammation [47]. Interestingly, basophil-enriched infiltrates, resembling those observed in Jones-Mote responses, constitute a hallmark of cutaneous delayed hypersensitivity reactions to mRNA vaccines [35]. Consistently, in vitro basophil responses in our cohort did not show significant differences between patients with immediate and delayed hypersensitivity reactions, with the latter being highly prevalent in the test group.
A number of limitations should be considered to interpret our results. First, DMG-PEG 2000 was the only lipid-bound excipient employed in our study, limiting further considerations on the allergenic profile of non-DMG-PEG 2000 containing vaccines, including BNT162b2 and ChAdOx1 nCoV-19. Second, vaccine formulations were also not available for testing. Third, our study did not encompass a group of bona fide healthy subjects as the majority of individuals in the control group had a history of allergy. Fourth, we had no data on concomitant PEG skin tests, which might have enabled further stratification of patients into subphenotypes. Notwithstanding these limitations, to the best of our knowledge, this is the first study evaluating basophil reactivity to both PEG with different molecular weights and to other vaccine excipients in a relatively large multicentre cohort of subjects who developed hypersensitivity reactions after SARS-CoV-2 vaccination in comparison to a group of non-vaccinated subjects. Moreover, these data have been obtained in a clinical real-world setting and in the majority of cases allowed a prompt decision-making activity for the single patient. Our data show also how BAT for excipients may be useful also in cases of delayed induced urticarial rash. Finally, our data support the potential clinical diagnostic utility of BAT for anti-SARS-CoV-2 vaccine hypersensitivity and provide novel clues for defining its molecular profile.
5. Conclusions
DMG-PEG 2000 has high BAT reactogenicity in subjects with anti-SARS-CoV-2 vaccine hypersensitivity and in vaccine-naïve individuals, correlates with exposure to DMG-PEG 2000 containing vaccines and with slowly resolving cutaneous reactions. DMG-PEG 2000 BAT might constitute a proxy for sensitivity to vaccine nanoparticles and add to current diagnostics in the setting of anti-SARS-CoV-2 vaccine hypersensitivity, independent on reaction timing of onset, while providing novel clues about its pathophysiology.
Funding
This research is independent and was partially supported by the Ricerca Corrente funding scheme of the Italian Ministry of Health to Istituti Clinici Scientifici Maugeri IRCCS Pavia.
Data statement
Data supporting this manuscript can be shared upon reasonable requests.
Contributorship
MRY, PP, GAR and LD designed the study. PP performed BAT. MR, PM, SN, CA, FDT, AT, BMG, AG, AM, GB, MBC, PP, GAR, PP and MRY collected clinical data. PP, GAR, CA and MR analysed the data. PP and GAR drafted the manuscript. MRY, SB, LD provided critical revision of data and manuscript. All authors approved the final version of the article and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work will appropriately be investigated and resolved.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the support of all medical and nurse staff involved in patient vaccination.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.06.039.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Luxi N., Giovanazzi A., Arcolaci A., Bonadonna P., Crivellaro M.A., Cutroneo P.M., et al. Allergic reactions to COVID-19 vaccines: risk factors, frequency, mechanisms and management. BioDrugs. 2022;36(4):443–458. doi: 10.1007/s40259-022-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Team CC-R, Food Drug A. Allergic reactions including anaphylaxis after receipt of the first dose of moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(4):125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shavit R., Maoz-Segal R., Offengenden I., Yahia S.H., Maayan D.M., Lifshitz Y., et al. Assessment of immediate allergic reactions after immunization with the Pfizer BNT162b2 vaccine using intradermal skin testing with the COVID-19 vaccines. J Allergy Clin Immunol Pract. 2022;10(10):2677–2684. doi: 10.1016/j.jaip.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon D.E., Kovarik C.L., Damsky W., Rosenbach M., Lipoff J.B., Tyagi A., et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: A registry-based study. J Am Acad Dermatol. 2022;86(1):113–121. doi: 10.1016/j.jaad.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal K.G., Freeman E.E., Saff R.R., Robinson L.B., Wolfson A.R., Foreman R.K., et al. Delayed large local reactions to mRNA-1273 vaccine against SARS-CoV-2. N Engl J Med. 2021;384(13):1273–1277. doi: 10.1056/NEJMc2102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitlick M.M., Joshi A.Y., Gonzalez-Estrada A., Chiarella S.E. Delayed systemic urticarial reactions following mRNA COVID-19 vaccination. Allergy Asthma Proc: Off J Regional State Allergy Societies. 2022;43(1):40–43. doi: 10.2500/aap.2022.43.210101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maoz-Segal R., Shavit R., Kidon M.I., Offengenden I., Machnes-Maayan D., Lifshitz-Tunitsky Y., et al. Late hypersensitivity reactions to the BNT162b2 SARS-CoV-2 vaccine are linked to delayed skin sensitization and prior exposure to hyaluronic acid. Life (Basel) 2022;12(12) doi: 10.3390/life12122021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu D.K., Abrams E.M., Golden D.B.K., Blumenthal K.G., Wolfson A.R., Stone C.A., et al. Risk of second allergic reaction to SARS-CoV-2 vaccines: a systematic review and meta-analysis. JAMA Intern Med. 2022;182(4):376–385. doi: 10.1001/jamainternmed.2021.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yacoub M.-R., Cucca V., Asperti C., Ramirez G.A., Della-Torre E., Moro M., et al. Efficacy of a rational algorithm to assess allergy risk in patients receiving the BNT162b2 vaccine. Vaccine. 2021;39(44):6464–6469. doi: 10.1016/j.vaccine.2021.09.048. [DOI] [PubMed] [Google Scholar]
- 10.Shavit R., Maoz-Segal R., Iancovici-Kidon M., Offengenden I., Haj Yahia S., Machnes Maayan D., et al. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4(8) doi: 10.1001/jamanetworkopen.2021.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moghimi S.M. Allergic reactions and anaphylaxis to LNP-based COVID-19 vaccines. Mol Ther: J Am Soc Gene Ther. 2021;29(3):898–900. doi: 10.1016/j.ymthe.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calogiuri G., Foti C., Nettis E., Di Leo E., Macchia L., Vacca A. Polyethylene glycols and polysorbates: Two still neglected ingredients causing true IgE-mediated reactions. J Allergy Clin Immunol Pract. 2019;7(7):2509–2510. doi: 10.1016/j.jaip.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese L.H., Kavanaugh A., Yeo A.E., Lipsky P.E. Frequency, distribution and immunologic nature of infusion reactions in subjects receiving pegloticase for chronic refractory gout. Arthritis Res Ther. 2017;19(1):191. doi: 10.1186/s13075-017-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Povsic T.J., Lawrence M.G., Lincoff A.M., Mehran R., Rusconi C.P., Zelenkofske S.L., et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J Allergy Clin Immunol. 2016;138(6):1712–1715. doi: 10.1016/j.jaci.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 15.Warren C.M., Snow T.T., Lee A.S., Shah M.M., Heider A., Blomkalns A., et al. Assessment of allergic and anaphylactic reactions to mRNA COVID-19 vaccines with confirmatory testing in a US regional health system. JAMA Netw Open. 2021;4(9) doi: 10.1001/jamanetworkopen.2021.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mouri M., Imamura M., Suzuki S., Kawasaki T., Ishizaki Y., Sakurai K., et al. Serum polyethylene glycol-specific IgE and IgG in patients with hypersensitivity to COVID-19 mRNA vaccines. Allergol Int: Off J Japanese Soc Allergol. 2022;71(4):512–519. doi: 10.1016/j.alit.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brockow K., Mathes S., Fischer J., Volc S., Darsow U., Eberlein B., et al. Experience with polyethylene glycol allergy-guided risk management for COVID-19 vaccine anaphylaxis. Allergy. 2022;77(7):2200–2210. doi: 10.1111/all.15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paoletti G., Racca F., Piona A., Melone G., Merigo M., Puggioni F., et al. Successful SARS-CoV-2 vaccine allergy risk-management: The experience of a large Italian University Hospital. World Allergy Organiz J. 2021;14(5) doi: 10.1016/j.waojou.2021.100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerrini G., Gioria S., Sauer A.V., Lucchesi S., Montagnani F., Pastore G., et al. Monitoring anti-PEG antibodies level upon repeated lipid nanoparticle-based COVID-19 vaccine administration. Int J Mol Sci. 2022;23(16) doi: 10.3390/ijms23168838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramirez G.A., Asperti C., Cucca V., Yacoub M.R. Challenges to vaccination against SARS-CoV-2 in patients with immune-mediated diseases. Vaccines (Basel) 2021;9(10) doi: 10.3390/vaccines9101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurst M., Jordan A., Capstick J., Brewerton M., Cockroft R., Ingram J. Patients with previous immediate hypersensitivity reactions to polyethylene glycol can safely receive the BNT162b2 mRNA COVID-19 vaccine. Intern Med J. 2022;52(10):1818–1820. doi: 10.1111/imj.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ju Y.i., Lee W.S., Pilkington E.H., Kelly H.G., Li S., Selva K.J., et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. ACS Nano. 2022;16(8):11769–11780. doi: 10.1021/acsnano.2c04543. [DOI] [PubMed] [Google Scholar]
- 23.Santos A.F., Alpan O., Hoffmann H.J. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy. 2021;76(8):2420–2432. doi: 10.1111/all.14747. [DOI] [PubMed] [Google Scholar]
- 24.Eberlein B., Mathes S., Fischer J., Darsow U., Biedermann T., Brockow K. Do basophil activation tests help elucidate allergic reactions to the ingredients in COVID-19 vaccines? Allergy. 2022;77(10):2924–2936. doi: 10.1111/all.15278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramirez G.A., Gerosa M., Beretta L., Bellocchi C., Argolini L.M., Moroni L., et al. COVID-19 in systemic lupus erythematosus: Data from a survey on 417 patients. Semin Arthritis Rheum. 2020;50(5):1150–1157. doi: 10.1016/j.semarthrit.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European-Commission. Article 29 data protection working party (0829/14/EN WP216). In: European-Commission, ed. 0829/14/EN WP216; 2014.
- 27.Pawankar R, Canonica G, Holgate S, Lockey R, Blaiss MJMWAO. WAO white book on allergy: update 2013; 2013.
- 28.Pignatti P., Yacoub M.-R., Testoni C., Pala G., Corsetti M., Colombo G., et al. Evaluation of basophil activation test in suspected food hypersensitivity. Cytometry B Clin Cytom. 2017;92(4):279–285. doi: 10.1002/cyto.b.21264. [DOI] [PubMed] [Google Scholar]
- 29.Bruusgaard-Mouritsen M.A., Jensen B.M., Poulsen L.K., Duus Johansen J., Garvey L.H. Optimizing investigation of suspected allergy to polyethylene glycols. J Allergy Clin Immunol. 2022;149(1) doi: 10.1016/j.jaci.2021.05.020. 168–175 e164. [DOI] [PubMed] [Google Scholar]
- 30.Dreborg S. Methodological cutoff of basophil activation test and basophil activation test diagnostic value. J Allergy Clin Immunol Pract. 2018;6(3):1089–1090. doi: 10.1016/j.jaip.2017.10.038. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann H.J., Knol E.F., Ferrer M., Mayorga L., Sabato V., Santos A.F., et al. Pros and cons of clinical basophil testing (BAT) Curr Allergy Asthma Rep. 2016;16(8):56. doi: 10.1007/s11882-016-0633-6. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson M.A., Zakaria A., Maung Z., Hart C., McCalmont T.H., Fassett M., et al. Incidence and characteristics of delayed injection site reaction to the mRNA-1273 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (Moderna) in a Cohort of hospital employees. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2022;74(4):591–596. doi: 10.1093/cid/ciab518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutkowski K., Mirakian R., Till S., Rutkowski R., Wagner A. Adverse reactions to COVID-19 vaccines: A practical approach. Clin Exp Allergy: J Br Soc Allergy Clin Immunol. 2021;51(6):770–777. doi: 10.1111/cea.13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garay R.P., El-Gewely R., Armstrong J.K., Garratty G., Richette P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin Drug Deliv. 2012;9(11):1319–1323. doi: 10.1517/17425247.2012.720969. [DOI] [PubMed] [Google Scholar]
- 35.Askenase P.W. Rare skin reactions after mRNA vaccination, similar to Jones-Mote Basophil responses. N Engl J Med. 2021;385(18):1720–1721. doi: 10.1056/NEJMc2111452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol Lett. 2010;128(1):36–45. doi: 10.1016/j.imlet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sellaturay P., Nasser S., Islam S., Gurugama P., Ewan P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin Exp Allergy: J Br Soc Allergy Clin Immunol. 2021;51(6):861–863. doi: 10.1111/cea.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szebeni J., Bedőcs P., Urbanics R., Bünger R., Rosivall L., Tóth M., et al. Prevention of infusion reactions to PEGylated liposomal doxorubicin via tachyphylaxis induction by placebo vesicles: a porcine model. J Control Release. 2012;160(2):382–387. doi: 10.1016/j.jconrel.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 39.Szebeni J., Storm G., Ljubimova J.Y., Castells M., Phillips E.J., Turjeman K., et al. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat Nanotechnol. 2022;17(4):337–346. doi: 10.1038/s41565-022-01071-x. [DOI] [PubMed] [Google Scholar]
- 40.Imamura S., Washio K., Mizuno M., Oda Y., Fukunaga A., Nishigori C. Activated steady status and distinctive FcepsilonRI-mediated responsiveness in basophils of atopic dermatitis. Allergol Int: Off J Japanese Soc Allergol. 2021;70(3):327–334. doi: 10.1016/j.alit.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Poddighe D., Dossybayeva K., Bexeitov Y., Mukusheva Z. Basophils in autoimmunity: Systemic lupus erythematosus and more? Autoimmun Rev. 2021;20(4) doi: 10.1016/j.autrev.2021.102790. [DOI] [PubMed] [Google Scholar]
- 42.Dema B., Lamri Y., Pellefigues C., Pacreau E., Saidoune F., Bidault C., et al. Basophils contribute to pristane-induced Lupus-like nephritis model. Sci Rep. 2017;7(1):7969. doi: 10.1038/s41598-017-08516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan Q., Feng Y., Peng Y., Zhou H., Deng Z., Li L.u., et al. Basophil recruitment to skin lesions of patients with systemic lupus erythematosus mediated by CCR1 and CCR2. Cell Physiol Biochem. 2017;43(2):832–839. doi: 10.1159/000481609. [DOI] [PubMed] [Google Scholar]
- 44.Miyake K., Ito J., Karasuyama H. Role of basophils in a broad spectrum of disorders. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.902494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dvorak H.F., Mihm M.C., Jr. Basophilic leukocytes in allergic contact dermatitis. J Exp Med. 1972;135(2):235–254. doi: 10.1084/jem.135.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandt E.B., Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2(3) doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukai K., Matsuoka K., Taya C., Suzuki H., Yokozeki H., Nishioka K., et al. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity. 2005;23(2):191–202. doi: 10.1016/j.immuni.2005.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.