Surveillance

Prof. M. Kudo Editor Liver Cancer

The incidence of hepatocellular carcinoma (HCC) increased rapidly in Japan after 1978 (Fig. 1), and most cases were caused by hepatitis C virus infection [1−3]. The mortality of HCC reached a peak in 2002 and has been declining since then. An HCC surveillance program was implemented in Japan after the rapid increase in incidence in the 1980s. At that time, ultrasound devices switched from contact compound scanning to higher-resolution electronic scanning, which enabled detection of small intrahepatic lesions measuring =2 cm, and the Japan Society of Hepatology (JSH) began to promote surveillance of HCC using ultrasonography and measurement of alpha-fetoprotein (AFP) levels. The JSH focused on improving the early diagnosis of HCC by actively recommending HCC surveillance for patients with chronic viral hepatitis and cirrhosis, who are at high risk for HCC, which led to efforts to lobby the Ministry of Health, Labour, and Welfare (MHLW) and hold public information sessions. The latest JSH Clinical Practice Guidelines for the Management of Liver Cancer [4] recommend surveillance by ultrasound examination and measurement of three tumor markers (AFP, Lens culinaris agglutinin-reactive AFP fraction (AFP-L3), and protein induced by vitamin K absence or antagonist II [PIVKA-II]) every 3–4 months for the super-high-risk HCC population (patients with hepatitis viral B- or C-related cirrhosis), as well as optional dynamic computed tomography (CT) or gadolinium ethoxybenzyl diethylene penta-acetic acid-enhanced-magnetic resonance imaging (Gd-EOB-MRI) once or twice a year. The guidelines for the high-risk group (patients with chronic hepatitis C, chronic hepatitis B, and non-viral cirrhosis) include surveillance by ultrasound examination and measurement of three tumor markers once every 6 months.
Fig. 1.
Changing number of deaths due to HCC in Japan.
Measurement of the tumor markers PIVKA-II and AFP-L3 became covered by the Japanese National Health Insurance in 1989 and 1996, respectively. This makes Japan the only country in the world where clinicians have been measuring the three tumor markers, AFP, PIVKA-II, and AFP-L3, in routine clinical practice since 1996. In addition, because ultrasound examinations are usually performed by hepatologists and gastroenterologists rather than by a radiologist, HCC can be detected at an early stage based on the levels of these three tumor markers together with ultrasound findings and risk stratification of the patients. According to the report of the 24th Nationwide Follow-up Survey of Primary Liver Cancer in Japan by the Japan Liver Cancer Association (JLCA) (formerly the Liver Cancer Study Group of Japan), 68% of HCC cases are detected as solitary HCC, and 53% of HCCs are detected when lesions are =3 cm, indicating that HCC is currently detectable at a very early stage [5]. Approximately 65% of HCC cases in Japan are detected at BCLC 0 or A stage. In 1999, the JSH launched a campaign to eradicate HCC. In this ongoing campaign, one or two JSH members appointed for each of the 47 prefectures in Japan are tasked with holding educational meetings at least once a year with healthcare professionals not specialized in hepatology (doctors, nurses, and allied health professionals such as public health workers) to address the early detection, diagnosis, and treatment of HCC. As a separate ongoing part of this campaign, the JSH holds public education meetings at least once a year to raise public awareness about the importance of early detection of HCC.
This campaign has increased knowledge and awareness of several topics, such as which patients are at high risk for HCC and the importance of surveillance for high-risk patients among non-hepatologist physicians and the general public in Japan. This in turn has promoted proactive surveillance efforts. The Japanese government and the MHLW, which is the ministry responsible for such public health efforts, have contributed to improving the early detection and treatment of HCC by actively following the advice of groups such as the JSH and Liver Disease Patient Associations and are also flexibly responding to needs in areas such as research funding and National Health Insurance coverage. As a result, a system is now in place to offer free testing for hepatitis B and C viruses in all medical institutions and public health centers in Japan. Annual corporate and community group health checkup programs also include testing for hepatitis B and C viruses, which provides another opportunity for the general public to learn whether they are personally at high risk for HCC. There is also a system in which patients who test positive for hepatitis B or C virus are strongly encouraged to visit a liver disease specialist. In university hospitals and many core hospitals, an alert function in the electronic medical record system recommends referral to a hepatologist when a patient receives a positive result on a preoperative hepatitis virus test ordered by a department other than gastroenterology or hepatology. These coordinated efforts of the national government, academic societies, and government agencies to eradicate HCC are the main reason why early detection of HCC is possible in Japan [6, 7].
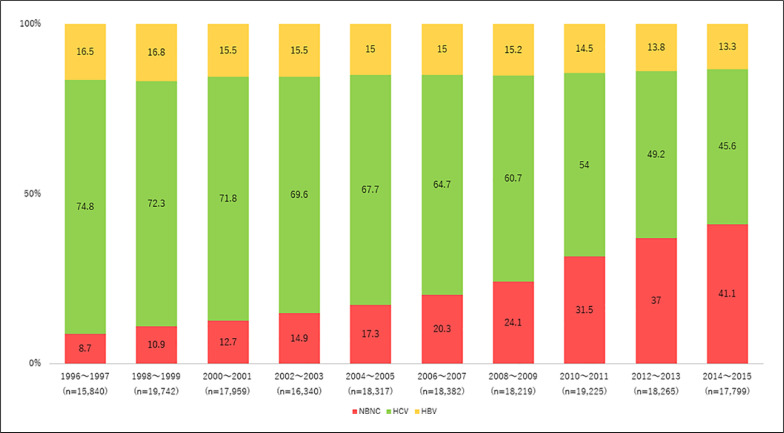
This surveillance system for the early detection of HCC is currently accessible to all community hospitals, general practitioners, and small clinics throughout Japan. Facilities that lack ultrasound equipment have systems in place for coordinating with larger hospitals to ensure that their patients undergo regular surveillance for HCC. This strict surveillance system should theoretically cover >80% of people in Japan who are at high risk for HCC. However, cases of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (NASH)-related HCC have been increasing in recent years, including in Japan, and are often missed by this surveillance system. This results in occasional delays in diagnosis in such cases, which is currently a major problem in Japan. In fact, the incidence of non-B non-C HCC is increasing in Japan [8]. The JLCA reported a prevalence of non-B non-C HCC of 8.7% in the report of its 14th Nationwide Follow-Up Survey of Primary Liver Cancer in Japan in HCC patients enrolled during 1996–1997 [9], and this figure has steadily increased with each follow-up survey, reaching 41.1% in the 24th report (Fig. 2) [5].
Fig. 2.
Changing trend in etiology of hepatocellular carcinoma (HCC) in Japan. HCC of non-viral etiology is gradually increasing.
Diagnosis
In Japan, helical CT became widely used around 1990 and multidetector row CT (MDCT) around 2000. The regulatory approval and widespread adoption of Gd-EOB-MRI in 2008 dramatically improved the accuracy of HCC diagnosis [10]. This resulted in a high success rate for the diagnosis of solitary HCC measuring =2 cm. In addition, Sonazoid® contrast-enhanced ultrasound, which was approved in 2007, has enabled the diagnosis of many nodules that are hypovascular in the arterial phase and do not exhibit a Kupffer defect in the Kupffer phase. This technique also facilitated the diagnosis of nodules that are hypovascular in the arterial phase on CT and Gd-EOB-MRI, do not show washout in the portal venous phase, and are isointense or have slightly low intensity in the hepatobiliary phase of Gd-EOB-MRI. These types of nodules are called “pathological early HCC” or HCC in the early stage of carcinogenesis, and they are diagnosed by resection or needle biopsy [11−13]. Japan was the first to establish the concept of “pathological” early-stage HCC [11−13], which is now accepted worldwide [14−16]. Carbon dioxide ultrasound angiography was also developed in Japan [17−19], as well as techniques such as CT hepatic arteriography and CT arterial portography, which are somewhat more invasive, but can isolate and detect arterial and portal venous blood flow in tiny nodules [20, 21]. As a result, Japan is the only country where specialized devices combining angiographic and CT technology are widely available. These specialized devices are called “CT angiography” or “interventional CT” devices. The concept of early HCC and its imaging findings were established in Japan by comparing CT angiography findings of intra-nodular arterial and portal venous blood flow with histopathological images [21−24]. Technologies such as Sonazoid® contrast-enhanced ultrasound [25, 26] and Gd-EOB-MRI have enabled detection of many extremely small nodules, which has considerably improved the diagnostic accuracy for early-stage HCC. A study from South Korea showed that diagnosis by MDCT plus Gd-EOB-MRI results in better treatment outcomes than diagnosis by MDCT alone [27].
Treatment Outcomes
Transarterial chemoembolization (TACE) was developed in Japan in the 1980s [28], became widely adopted across Japan around 1985, and was later adopted worldwide. Anatomical resection techniques, such as segmentectomy and subsegmentectomy, were also first developed in Japan and are now used worldwide [29]. In addition, percutaneous ethanol injection therapy was developed in Japan in the 1980s [30] and adopted worldwide later on. The widespread adoption of radiofrequency ablation across Japan in the 2000s has further improved the survival of patients with early-stage HCC in recent years [31]. Hepatic arterial infusion chemotherapy was also developed in Japan around 1995, has been widely adopted nationwide, and continues to be used for certain indications, such as vascular invasion, with favorable outcomes [32]. Hepatic arterial infusion chemotherapy is widely used in Asian countries, especially Taiwan and China, and is included in their HCC treatment guidelines [33, 34]. In addition, sorafenib was approved in 2009 [35, 36], regorafenib in 2017 [37], lenvatinib in 2018 [38], ramucirumab in 2019 [39], and atezolizumab/bevacizumab [40, 41] and cabozantinib [42] in 2020. Incidentally, durvalumab plus tremelimumab, as well as durvalumab monotherapy, were also approved in 2022 [43, 44]. Systemic therapy using these molecular-targeted agents and immunotherapy has been widely adopted for the treatment of advanced and intermediate-stage HCC in Japan. The effectiveness of such systemic therapy has also contributed to the recent improvements in treatment outcomes for HCC. In Japan, sorafenib-TACE sequential therapy, which was initially investigated as a treatment for intermediate-stage HCC in patients unsuitable for TACE [45−47], stimulated the development and widespread adoption of similar approaches such as lenvatinib-TACE sequential therapy [48−50] and ABC conversion therapy [51−53], which have also produced favorable treatment outcomes.
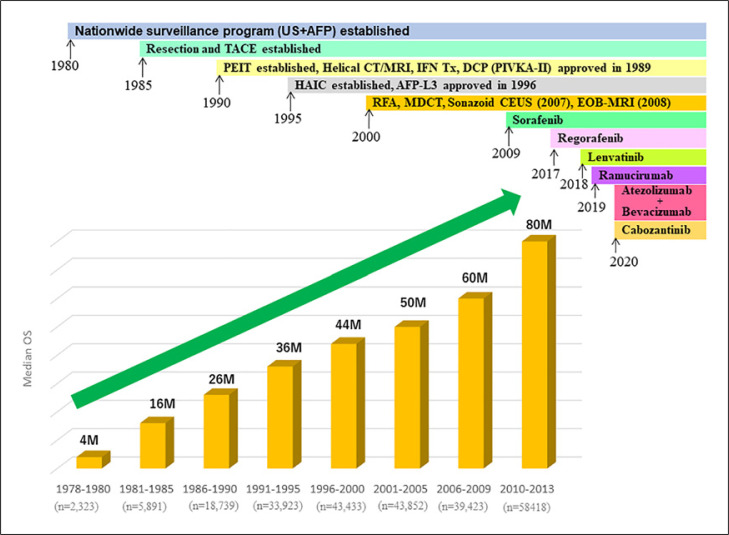
Advances in treatment methods have dramatically improved the treatment outcomes of patients with HCC in Japan over the past 40 years, and Japan has the best treatment outcomes in the world today. The report of the 24th Nationwide Follow-Up Survey of Primary Liver Cancer in Japan by the JLCA [5, 7] notes that the 5-year survival rate and median OS were 5% and 4 months, respectively, in 2,323 patients enrolled between 1978 and 1980, and they increased to 58% and 80 months, respectively, among 58,418 HCC patients of all BCLC stages enrolled between 2010 and 2013, showing a strong upward trend (Fig. 3, 4). These positive results could be attributed to the nationwide HCC surveillance system maintained in Japan since the 1980s, advances in diagnostic modalities, and recent dramatic advances in systemic therapy and other treatment modalities. In addition, the report of the latest 24th Nationwide Follow-up Survey of Primary Liver Cancer in Japan by the JLCA [5] shows that the treatment modality selected for patients registered between 2016 and 2017 was resection for 48%, ablation for 19%, and TACE for 27%. This indicates that resection or radiofrequency ablation was selected as the initial treatment modality in approximately 67% of patients who underwent curative therapy. Furthermore, resection is gradually becoming a more popular choice for curative therapy: before 2000, only 28–29% of patients underwent resection as initial treatment, and this percentage has gradually increased to 46–48% of patients in the most recent survey period from 2014 to 2017 (Fig. 5). This may be because the increase in non-B non-C HCC, especially NASH-HCC, has led to a relatively large number of patients diagnosed with huge HCC with mild fibrosis and good liver function, resulting in a higher proportion of resectable cases. As mentioned above, NASH-HCC is often missed by the HCC surveillance system, and the lesions are often large when detected because it is not easy to determine risk, in contrast to viral HCC. Patients with NASH-HCC are more likely to have mild fibrosis and good liver function than those with viral hepatitis-related HCC and thus may be more easily regarded as candidates for resection. More than 60% of recent surgical resections were performed in patients with non-B non-C HCC.
Fig. 3.
Improvement of 5-year survival rate in Japan in patients with all BCLC stage HCC.
Fig. 4.
Improvement median overall survival in Japan in patients with all BCLC stage HCC.
Fig. 5.
Changes of treatment modality for initially detected HCC in Japan. Rate of surgical resection is gradually increasing.
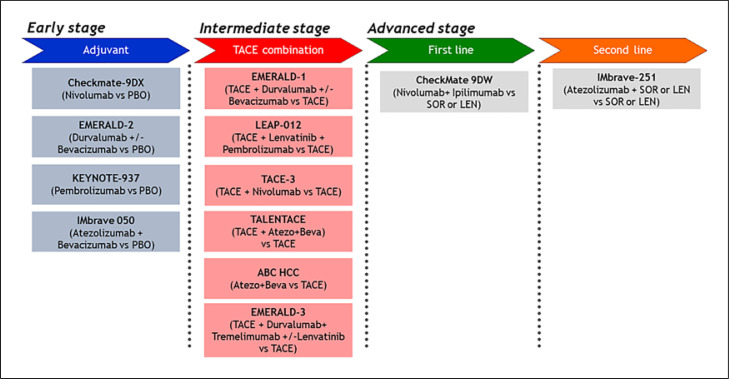
Trials of adjuvant therapies after curative resection or ablation for early HCC and TACE combination therapies for intermediate-stage HCC are currently ongoing (Fig. 6). On January 19, 2023, a press release was issued stating that the phase 3 IMbrave 050 trial of adjuvant therapy with atezolizumab plus bevacizumab met its primary endpoint of improving recurrence-free survival [54]. If other ongoing clinical trials are also successful, treatment outcomes for HCC should continue to improve.
Fig. 6.
Ongoing global phase III trials for HCC. IMbrave 050 trial met its primary endpoint of better recurrence-free survival over active surveillance according to the press release on January 2023 [54].
Conflict of Interest Statement
Lecture: Eli Lilly, Bayer, Eisai, Chugai, Takeda, and AstraZeneca. Grants: Gilead Sciences, Taiho, Otsuka, EA Pharma, AbbVie, Eisai, Chugai, and GE HealthCare. Advisory consulting: Chugai, Roche, AstraZeneca, and Eisai. Masatoshi Kudo is the Editor-in-Chief of Liver Cancer.
Funding Sources
There was no funding for this Editorial.
Author Contributions
Masatoshi Kudo conceived, wrote, and approved the final manuscript.
Funding Statement
There was no funding for this Editorial.
References
- 1.Kudo M. Recent advances in systemic therapy for hepatocellular carcinoma in an aging society 2020 update. Liver Cancer. 2020;9(6):640–662. doi: 10.1159/000511001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudo M. Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan. Liver Cancer. 2015;4(1):39–50. doi: 10.1159/000367727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Center Japan https//ganjoho.jp/reg_stat/statistics/stat/cancer/8_liver.html .
- 4.Hasegawa K, Takemura N, Yamashita T, Watadani T, Kaibori M, Kubo S, et al. Clinical practice guidelines for hepatocellular carcinoma the Japan society of hepatology 2021 version (5th JSH-HCC guidelines) Hepatol Res. 2023 doi: 10.1111/hepr.13892. [DOI] [PubMed] [Google Scholar]
- 5.Liver Cancer Study Group of Japan Report of the 24th Nationwide follow-up survey of primary liver cancer in Japan (2016-2017) Osaka. 2022.
- 6.Kudo M. Management of hepatocellular carcinoma in Japan as a world-leading model. Liver Cancer. 2018;7(2):134–147. doi: 10.1159/000484619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo M. Surveillance and treatment outcomes of hepatocellular carcinoma in Japan 2021 update. Liver Cancer. 2021;10(3):167–180. doi: 10.1159/000516491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tateishi R, Uchino K, Fujiwara N, Takehara T, Okanoue T, Seike M, et al. A nationwide survey on non-B non-C hepatocellular carcinoma in Japan 2011-2015 update. J Gastroenterol. 2019;54(4):367–376. doi: 10.1007/s00535-018-1532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liver Cancer Study Group of Japan . Report of the 14th follow-up survey of primary liver cancer (1996-1997) Kyoto: Liver Cancer Study Group of Japan; 2000. [Google Scholar]
- 10.Kudo M. Will Gd-EOB MRI change the diagnostic algorithm for hepatocellular carcinoma? Oncology. 2010;78(Suppl 1):S87–93. doi: 10.1159/000315235. [DOI] [PubMed] [Google Scholar]
- 11.Kojiro M, Sugihara S, Nakashima O. Pathomorphologic characteristics of early hepatocellular carcinoma. Gann Monogr Cancer Res. 1991;38:29–37. [Google Scholar]
- 12.Sakamoto M, Ojima H, Masugi Y. Pathology of early HCC. Liver Cancer. 2014;3:145. [Google Scholar]
- 13.Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1991;22(2):172–178. doi: 10.1016/0046-8177(91)90039-r. [DOI] [PubMed] [Google Scholar]
- 14.Kojiro M, Wanless IR, Alves V, Badve S, Balabaud C, Bedossa P, International Consensus Group for Hepatocellular Neoplasia Pathologic diagnosis of early hepatocellular carcinoma a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49(2):658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 15.Kudo M. Early hepatocellular carcinoma definition and diagnosis. Liver Cancer. 2013;2:69–72. doi: 10.1159/000343842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theise ND, Park YN, Curado MP, Sakamoto M, Franceschi S, Torbenson M, et al. Hepatocelular carcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. Lyon: IARC; 2010. pp. p. 205–216. [Google Scholar]
- 17.Kudo M, Tomita S, Tochio H, Mimura J, Okabe Y, Kashida H, et al. Small hepatocellular carcinoma diagnosis with US angiography with intraarterial CO2 microbubbles. Radiology. 1992;182(1):155–160. doi: 10.1148/radiology.182.1.1309216. [DOI] [PubMed] [Google Scholar]
- 18.Kudo M, Tomita S, Tochio H, Mimura J, Okabe Y, Kashida H, et al. Sonography with intraarterial infusion of carbon dioxide microbubbles (sonographic angiography) value in differential diagnosis of hepatic tumors. AJR Am J Roentgenol. 1992;158(1):65–74. doi: 10.2214/ajr.158.1.1309220. [DOI] [PubMed] [Google Scholar]
- 19.Kudo M, Tomita S, Tochio H, Kashida H, Hirasa M, Todo A. Hepatic focal nodular hyperplasia specific findings at dynamic contrast-enhanced US with carbon dioxide microbubbles. Radiology. 1991;179(2):377–382. doi: 10.1148/radiology.179.2.1849661. [DOI] [PubMed] [Google Scholar]
- 20.Matsui O, Takashima T, Kadoya M, Ida M, Suzuki M, Kitagawa K, et al. Dynamic computed tomography during arterial portography the most sensitive examination for small hepatocellular carcinomas. J Comput Assist Tomogr. 1985;9(1):19–24. doi: 10.1097/00004728-198501000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Matsui O, Kadoya M, Suzuki M, Inoue K, Itoh H, Ida M, et al. Work in progress dynamic sequential computed tomography during arterial portography in the detection of hepatic neoplasms. Radiology. 1983;146(3):721–727. doi: 10.1148/radiology.146.3.6298857. [DOI] [PubMed] [Google Scholar]
- 22.Kudo M. Atypical large well-differentiated hepatocellular carcinoma with benign nature a new clinical entity. Intervirology. 2004;47(3–5):227–237. doi: 10.1159/000078475. [DOI] [PubMed] [Google Scholar]
- 23.Kudo M. Multistep human hepatocarcinogenesis correlation of imaging with pathology. J Gastroenterol. 2009;44(Suppl 19):112–118. doi: 10.1007/s00535-008-2274-6. [DOI] [PubMed] [Google Scholar]
- 24.Kudo M. The 2008 Okuda lecture management of hepatocellular carcinoma: from surveillance to molecular targeted therapy. J Gastroenterol Hepatol. 2010;25(3):439–452. doi: 10.1111/j.1440-1746.2009.06207.x. [DOI] [PubMed] [Google Scholar]
- 25.Kudo M, Hatanaka K, Maekawa K. Sonazoid-enhanced ultrasound in the diagnosis and treatment of hepatic tumors. J Med Ultrasound. 2008;16(2):130–139. [Google Scholar]
- 26.Kudo M, Hatanaka K, Maekawa K. Newly developed novel ultrasound technique, defect reperfusion ultrasound imaging, using sonazoid in the management of hepatocellular carcinoma. Oncology. 2010;78(Suppl 1):40–45. doi: 10.1159/000315229. [DOI] [PubMed] [Google Scholar]
- 27.Kim HD, Lim YS, Han S an J, Kim GA, Kim SY, et al. Evaluation of early-stage hepatocellular carcinoma by magnetic resonance imaging with gadoxetic acid detects additional lesions and increases overall survival. Gastroenterology. 2015;148(7):1371–1382. doi: 10.1053/j.gastro.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 28.Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148(2):397–401. doi: 10.1148/radiology.148.2.6306721. [DOI] [PubMed] [Google Scholar]
- 29.Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161(4):346–350. [PubMed] [Google Scholar]
- 30.Sugiura N, Takara K, Ohto M, Okuda K, Hirooka N. Ultrasound image-guided percutaneous intratumor ethanol injection for small hepatocellular carcinoma. Acta Hepatologica Japonica. 1983;24:920. [Google Scholar]
- 31.Shiina S, Tagawa K, Unuma T, Takanashi R, Yoshiura K, Komatsu Y, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991;68(7):1524–1530. doi: 10.1002/1097-0142(19911001)68:7<1524::aid-cncr2820680711>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 32.Ueshima K, Ogasawara S, Ikeda M, Yasui Y, Terashima T, Yamashita T, et al. Hepatic arterial infusion chemotherapy versus sorafenib in patients with advanced hepatocellular carcinoma. Liver Cancer. 2020;9(5):583–595. doi: 10.1159/000508724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surveillance group. Diagnosis group. Staging group. Surgery group. Local ablation group. TACE/TARE/HAI group et al. Management consensus guideline for hepatocellular carcinoma 2016 updated by the taiwan liver cancer association and the gastroenterological society of taiwan. J Formos Med Assoc. 2018;117(5):381–403. doi: 10.1016/j.jfma.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Sun J, Guo R, Bi X, Wu M, Tang Z, Lau WY, et al. Guidelines for diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus in China (2021 edition) Liver Cancer. 2022;11(4):315–328. doi: 10.1159/000523997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 36.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 37.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE) a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 38.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 39.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2) a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 40.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 41.Cheng A-L, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Updated efficacy and safety data from IMbrave150 atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 42.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):2100070. doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 44.Kudo M. Durvalumab plus tremelimumab a novel combination immunotherapy for unresectable hepatocellular carcinoma. Liver Cancer. 2022;11(2):87–93. doi: 10.1159/000523702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma TACTICS trial. Gut. 2020;69(8):1492–1501. doi: 10.1136/gutjnl-2019-318934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Final results of tactics a randomized, prospective trial comparing transarterial chemoembolization plus sorafenib to transarterial chemoembolization alone in patients with unresectable hepatocellular carcinoma. Liver Cancer. 2022;11(4):354–367. doi: 10.1159/000522547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kudo M. Implications of the TACTICS trial establishing the new concept of combination/sequential systemic therapy and transarterial chemoembolization to achieve synergistic effects. Liver Cancer. 2022;11(6):487–496. doi: 10.1159/000527404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T, et al. Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child-pugh A liver function a proof-of-concept study. Cancers. 2019;11(8):1084. doi: 10.3390/cancers11081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma asia-pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9(3):245–260. doi: 10.1159/000507370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, et al. Management of hepatocellular carcinoma in Japan JSH consensus statements and recommendations 2021 update. Liver Cancer. 2021;10(3):181–223. doi: 10.1159/000514174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kudo M. A novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE upfront systemic therapy followed by curative conversion. Liver Cancer. 2021;10(6):539–544. doi: 10.1159/000519749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kudo M. Atezolizumab plus bevacizumab followed by curative conversion (ABC conversion) in patients with unresectable, TACE-unsuitable intermediate-stage hepatocellular carcinoma. Liver Cancer. 2022;11(5):399–406. doi: 10.1159/000526163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kudo M, Aoki T, Ueshima K, Tsuchiya K, Morita M, Chishina H, et al. Achievement of complete response and drug-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable intermediate-stage hepatocellular carcinoma a multicenter proof-of-concept study. Liver Cancer. 2023 doi: 10.1159/000529574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roche's Tecentriq plus Avastin is the first treatment combination to reduce the risk of cancer returning in people with certain types of early-stage liver cancer in a Phase III trial Roche Media Releases Ad Hoc Announcements. 2023. https://www.roche.com/media/releases/med-cor-2023-01-19 .