Abstract
Introduction
The tyrosine kinase inhibitors regorafenib and cabozantinib remain the mainstay in second-line treatment of advanced hepatocellular carcinoma (HCC). There is currently no clear evidence of superiority in efficacy or safety to guide choice between the two treatments.
Methods
We conducted an anchored matching-adjusted indirect comparison using individual patient data from the RESORCE trial of regorafenib and published aggregate data from the CELESTIAL trial of cabozantinib. Second-line HCC patients with prior sorafenib exposure of ≥3 months were included in the analyses. Hazard ratios (HRs) and restricted mean survival time (RMST) were estimated to quantify differences in overall survival (OS) and progression-free survival (PFS). Safety outcomes compared were rates of grade 3 or 4 adverse events (AEs), occurring in >10% of patients, and discontinuation or dose reduction due to treatment-related AEs.
Results
After matching adjustment for differences in baseline patient characteristics, regorafenib showed a favorable OS (HR, 0.80; 95% CI: 0.54, 1.20) and ∼3-month-longer RMST over cabozantinib (RMST difference, 2.76 months; 95% CI: −1.03, 6.54), although not statistically significant. For PFS, there was no numerical difference in HR (HR, 1.00; 95% CI: 0.68, 1.49) and no clinically meaningful difference based on RMST analyses (RMST difference, −0.59 months; 95% CI: −1.83, 0.65). Regorafenib showed a significantly lower incidence of discontinuation (risk difference, −9.2%; 95% CI: −17.7%, −0.6%) and dose reductions (−15.2%; 95% CI: −29.0%, −1.5%) due to treatment-related AEs (any grade). Regorafenib was also associated with a lower incidence (not statistically significant) of grade 3 or 4 diarrhea (risk difference, −7.1%; 95% CI: −14.7%, 0.4%) and fatigue (−6.3%; 95% CI: −14.6%, 2.0%).
Conclusion
This indirect treatment comparison suggests, relative to cabozantinib, that regorafenib could be associated with favorable OS (not statistically significant), lower rates of dose reductions and discontinuation due to treatment-related AEs, and lower rates of severe diarrhea and fatigue.
Keywords: Hepatocellular carcinoma, Regorafenib, Cabozantinib, Indirect treatment comparison, Tyrosine kinase inhibitor, Decision-making
Introduction
The tyrosine kinase inhibitors (TKIs) regorafenib and cabozantinib remain the mainstay in second-line treatment of advanced hepatocellular carcinoma (HCC) after failure of immuno-oncology-based combinations and first-line sorafenib or lenvatinib TKI monotherapy [1, 2, 3]. Regorafenib and cabozantinib are approved for treating advanced HCC previously treated with sorafenib, having each demonstrated overall survival (OS) benefit compared to placebo in phase 3 trials [4, 5]. Ramucirumab, an antibody against VEGF receptor 2, is also approved in this setting but as second-line treatment only for patients with serum alpha-fetoprotein (AFP) levels ≥400 ng/mL [6]. Regorafenib and cabozantinib are both indicated and more commonly used in broader second-line populations, including those with AFP ≥400 ng/mL [4, 5]. Pembrolizumab monotherapy and nivolumab in combination with ipilimumab are also approved by the FDA under accelerated approvals for HCC previously treated with sorafenib, based on overall response rate and duration of response [7, 8]. Nivolumab monotherapy was previously approved for this indication but was voluntarily withdrawn after not meeting post-marketing requirements demonstrating confirmatory benefit [9].
Regorafenib was approved following the phase 3 RESORCE trial [5], which showed significantly longer survival than placebo (median OS, 10.6 months vs. 7.8 months; hazard ratio [HR], 0.63; 95% CI: 0.50, 0.79) for patients who were tolerant to sorafenib but had progressed on sorafenib treatment (N = 573). Cabozantinib was approved based on the phase 3 CELESTIAL trial [4], which showed a survival advantage over placebo (median OS, 10.2 months vs. 8.0 months; HR, 0.76; 95% CI: 0.63, 0.92) in patients who had progressed on or were intolerant to sorafenib, and who may have received up to two previous systemic regimens for advanced HCC, one of which had to be sorafenib (N = 707). The most common grade 3 or 4 treatment-emergent adverse events (TEAEs) for regorafenib and cabozantinib were diarrhea, fatigue, hypertension, and hand–foot skin reaction (HFSR) (assessed as palmar–plantar erythrodysesthesia [PPE] in CELESTIAL) [4, 5]. An increased aspartate aminotransferase (AST) level was also reported in CELESTIAL [4]. In the absence of head-to-head studies, an indirect comparison of these treatments' efficacy and safety is required to help guide treatment choices.
Standard methods for indirect treatment comparisons such as network meta-analysis or Bucher analysis use aggregate data from published randomized controlled trials that share common comparators to compare relative effects between treatments [10, 11]. Such methods assume similarity and homogeneity between trials. However, in the RESORCE and CELESTIAL trials, there is strong evidence of heterogeneity. For example, sorafenib-intolerant patients were excluded in RESORCE; these patients were likely earlier in their overall treatment journey and might have been less advanced. Furthermore, standard indirect treatment comparisons assume that relative treatment effects are the same across trials and that there is no difference between trials in the distribution of baseline characteristics that might impact treatment effects. Potential treatment effect-modifying characteristics are, however, imbalanced between the RESORCE and CELESTIAL trial populations, including the Eastern Cooperative Oncology Group (ECOG) score, the geographic region from which they were recruited, and the duration of prior sorafenib treatment.
When an imbalance in treatment effect modifiers is evident and individual patient data are available from at least one trial, population-adjusted indirect comparison (PAIC) methods such as matching-adjusted indirect comparison (MAIC) [12] and simulated treatment comparison (STC) [13] can be used to adjust for imbalances and estimate a less biased relative treatment effect than standard indirect treatment comparisons. These approaches can be applied with a common comparator (anchored) or without (unanchored). Anchored approaches are considered less biased whenever a common arm (e.g., a placebo arm in each trial) is available [14] since unanchored comparisons require much stronger assumptions in this situation, which is regarded as impossible [15].
Here, we conducted an anchored MAIC to compare the relative efficacy and safety of regorafenib versus cabozantinib in patients with HCC previously treated with sorafenib. An anchored STC, as well as standard indirect treatment comparison using unadjusted Bucher analysis, were also conducted to assess the sensitivity of the MAIC results versus alternative statistical methodologies.
Materials and Methods
Data Sources
The indirect treatment comparison was conducted with individual patient data from the RESORCE trial (cutoff date for progression-free survival [PFS], February 29, 2016; cutoff date for OS and safety, September 10, 2019) [5] and published aggregate patient characteristics and outcome data from the CELESTIAL trial [4, 16, 17].
Compatibility Assessment
A compatibility assessment was performed through a comparative review of the trial designs, inclusion and exclusion criteria, outcome definitions, and baseline characteristics of the trial populations to assess the similarities and differences between the trials. Differences that could potentially impact the results were adjusted in the analyses where possible.
There were key differences in inclusion and exclusion criteria between the studies: CELESTIAL allowed up to two prior lines of therapy, of which one had to be sorafenib, whereas RESORCE allowed only one prior therapy with sorafenib. This difference was adjusted for by including only patients in the second-line setting post-sorafenib from CELESTIAL. In addition, unlike the CELESTIAL trial, RESORCE required patients to be sorafenib tolerant (≥400 mg daily for at least 20 of the 28 days before discontinuation) and to have had documented radiological progression during sorafenib treatment. Due to lack of prior sorafenib-dosing information in the CELESTIAL trial, the differences were addressed by excluding patients with prior sorafenib exposure of <3 months from both trials since, as reasoned by Kelley et al. [17], this population in RESORCE represents fast progressors, whereas in CELESTIAL, it likely represents fast progressors but also some slow progressors intolerant to sorafenib. Thus, for PAIC, the population selected for analyses included only patients with ≥3 months of prior sorafenib treatment.
Important differences were also observed in baseline characteristics that are potential treatment effect modifiers between the studies among the patients with ≥3 months of prior sorafenib treatment (Table 1). Although the trials had similar designs, the geographic regions in which they were conducted differed; RESORCE had a higher proportion of patients from Asia (35% regorafenib, 38% placebo) than CELESTIAL (20% cabozantinib, 18% placebo). RESORCE had a smaller proportion of patients with the ECOG performance status score of 1 (versus 0) (33% regorafenib, 31% placebo) compared to CELESTIAL (44% cabozantinib, 43% placebo). Also, the proportion of patients with ≥6 months of prior sorafenib exposure was higher in RESORCE compared to CELESTIAL. Some small imbalances in distribution of gender, extrahepatic disease, macrovascular invasion, HBV, HCV, alcohol use, and AFP ≥400 ng/mL were also observed between trials. Differences in listed baseline characteristics were adjusted for in the PAIC.
Table 1.
Baseline characteristics before and after matching RESORCE population to CELESTIAL population with ≥3 months of prior sorafenib duration
| RESORCE |
CELESTIAL |
|||||
|---|---|---|---|---|---|---|
| regorafenib (N = 320) | regorafenib matched (ESS = 214) | placebo (N = 167) | placebo matched (ESS = 82) | cabozantinib (N = 241) | placebo (N = 117) | |
| ECOG status = 1, % | 33.4 | 44.4 | 30.5 | 42.7 | 44.4 | 42.7 |
| Geographic region = Asia, % | 34.7 | 20.3 | 37.7 | 17.9 | 20.3 | 17.9 |
| Prior sorafenib duration ≥6 months, % | 72.2 | 59.3 | 73.7 | 63.2 | 59.3 | 63.2 |
| Age, years | ||||||
| Median [range] | 64.0 [19–85] | 65.0 [19–85] | 62.0 [23–83] | 65.0 [23–83] | 65.0 [28–86]a | 67.5 [36–86]a |
| Male, % | 90.0 | 79.7 | 89.2 | 89.7 | 79.7 | 89.7 |
| Extrahepatic spread, % | 69.7 | 75.9 | 76.0 | 75.2 | 75.9 | 75.2 |
| Macrovascular invasion, % | 27.2 | 21.6 | 26.9 | 34.2 | 21.6 | 34.2 |
| Hepatitis B, % | 36.9 | 32.8 | 36.5 | 34.2 | 32.8 | 34.2 |
| Hepatitis C, % | 21.6 | 27.0 | 22.2 | 29.9 | 27.0 | 29.9 |
| Alcohol use, % | 25.3 | 24.5 | 29.9 | 13.7 | 24.5 | 13.7 |
| AFP ≥400 ng/mL, % | 41.6 | 38.2 | 43.7 | 38.5 | 38.2 | 38.5 |
| Child-Pugh class A, % | 98.1 | 97.5 | 97.0 | 95.8 | 98.8b | 98.8b |
Bold numbers indicate matched characteristics. AFP, alpha-fetoprotein; ECOG, Eastern Cooperative Oncology Group; ESS, effective sample size.
Reported in ref. [17] only for population who received sorafenib ≥6 months prior to screening.
Reported in ref. [16] only for the overall CELESTIAL population.
Both RESORCE and CELESTIAL used the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria to assess PFS, but PFS assessments were more frequent in RESORCE than in CELESTIAL (every 6 weeks vs. every 8 weeks). Moreover, in RESORCE, clinical progression (defined as worsening of the ECOG performance status to ≥3 or symptomatic deterioration including increase in liver function tests) as well as radiological progression were considered events, whereas in CELESTIAL, only radiological progression was considered an event. Although differences in assessment schedule and definition of clinical progression can substantially bias results in the unanchored PAIC setting, the impact of these differences is minimal in the anchored PAIC since it compares only relative treatment effects (TKI vs. placebo) of each trial.
Statistical Analysis
OS, PFS, proportion of patients with at least one dose reduction, rates of discontinuations due to treatment-related adverse events (AEs), and grade 3 or 4 TEAEs occurring in >10% of patients were compared. Efficacy and safety analyses were based on the intention-to-treat and safety population, respectively, of the subgroup of patients with prior sorafenib treatment duration of ≥3 months.
Baseline characteristics and safety outcomes for the CELESTIAL patients with ≥3 months of prior sorafenib exposure were derived by pooling published baseline characteristics and safety outcomes for the 3–6-month and ≥6-month subgroups [17]. Reconstructed individual patient data for OS and PFS from CELESTIAL were derived from Kaplan-Meier plots using the Guyot et al. [18] method. Statistical analysis was conducted using SAS Base 9.4 and R version 3.6.1.
Standard Indirect Treatment Comparison
A standard indirect treatment comparison was performed following the standard pairwise Bucher method [10] using unadjusted relative effects for regorafenib versus placebo from RESORCE and relative effects for cabozantinib versus placebo in the subgroup of the intention-to-treat population with prior sorafenib treatment duration of ≥3 months. An implicit assumption in standard indirect treatment comparison is that the populations of the two studies are comparable in regard to the distribution of baseline characteristics that are potential treatment effect modifiers, which is not the case for the RESORCE and CELESTIAL studies. Thus, PAICs were also conducted.
Population-Adjusted Indirect Comparisons
Baseline characteristics that are potential modifiers of efficacy outcomes were identified based on subgroup analyses, literature [4, 5], and evidence of imbalance between the studies. Ten such baseline characteristics were selected for adjustment in PAIC: ECOG status, geographic region, prior sorafenib treatment duration, gender, extrahepatic disease, macrovascular invasion, HBV, HCV, alcohol use, and AFP ≥400 ng/mL. The anchored MAIC analysis compared the relative effect of each treatment in their respective RCT, adjusting for these baseline characteristics following best practices [12, 14]. Briefly, the anchored MAIC balanced differences in baseline characteristics that are potential treatment effect modifiers through propensity score re-weighting of patients from RESORCE to produce a patient profile matching that of CELESTIAL. The relative treatment effect of regorafenib versus placebo for OS and PFS in the CELESTIAL-like population was quantified as an unstratified HR with a 95% CI derived from a weighted Cox regression analysis. Non-parametrical restricted mean survival time (RMST) difference and ratio with 95% CIs were also derived for OS and PFS to address violations of the proportional hazard assumption [19], indicated by crossing or lack of parallel log(−log(S[t]) versus log(t) lines where S is survival and t is time (see online suppl. Fig. S1–S4; for all online suppl. material, see www.karger.com/doi/10.1159/000527403). The RMST analyses were performed over the same follow-up period in both trials, defined as the minimum of the longest observed time among all four arms. The RMST analyses were also performed over the entire available follow-up period for each individual trial, defined as the minimum of the longest observed time between the two arms within each trial. For safety outcomes, the relative effects were expressed as absolute risk differences with a 95% CI. Due to the rarity of grade 3/4 TEAEs (with no grade 3/4 TEAEs in some treatment arms), the odds ratio was not considered an appropriate measure [20].
The anchored STC analysis was conducted following the method described by Ishak et al. [13]. For OS and PFS, HRs were estimated by fitting a Cox proportional hazards model to the RESORCE trial data, and RMST difference and ratio were estimated by fitting an ANCOVA-type model. These models included the same baseline characteristics as the MAIC analysis. Adjusting for baseline characteristics across trials was achieved by including interaction terms of treatment with the baseline characteristics in the models. STC analyses were not conducted for the safety comparisons due to small numbers (i.e., low incidence rates) of some events and, particularly grade 3/4 AEs in each arm, which would not allow for adjustment of all selected baseline characteristics using regression equations. After population adjustment with MAIC or STC, the relative treatment effect of regorafenib versus cabozantinib was calculated following the Bucher method [10] as for the standard indirect treatment comparison but using the adjusted relative effect instead of the unadjusted one from RESORCE.
Results
Matching Patient Baseline Characteristics
Prespecified target patient baseline characteristics for both studies are presented in Table 1. Before matching, there were considerable differences between populations in the proportions of patients with ECOG status of 1, patients from Asia, and patients with prior sorafenib treatment of ≥6 months. After re-weighting, all prespecified target patient characteristics were matched between the two study populations (Table 1; online suppl. Fig. S5). The original sample size of the regorafenib arm (N = 320) was reduced by 33% after matching (effective sample size = 214), and the placebo (N = 167) arm was reduced by 51% (effective sample size = 82).
Efficacy Outcomes
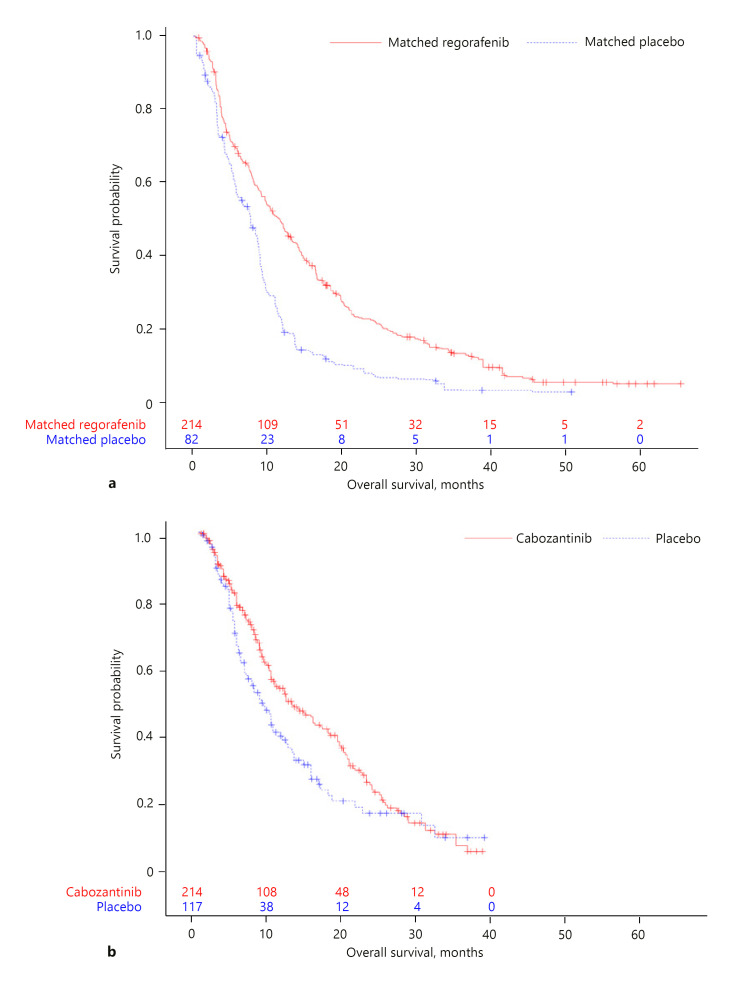
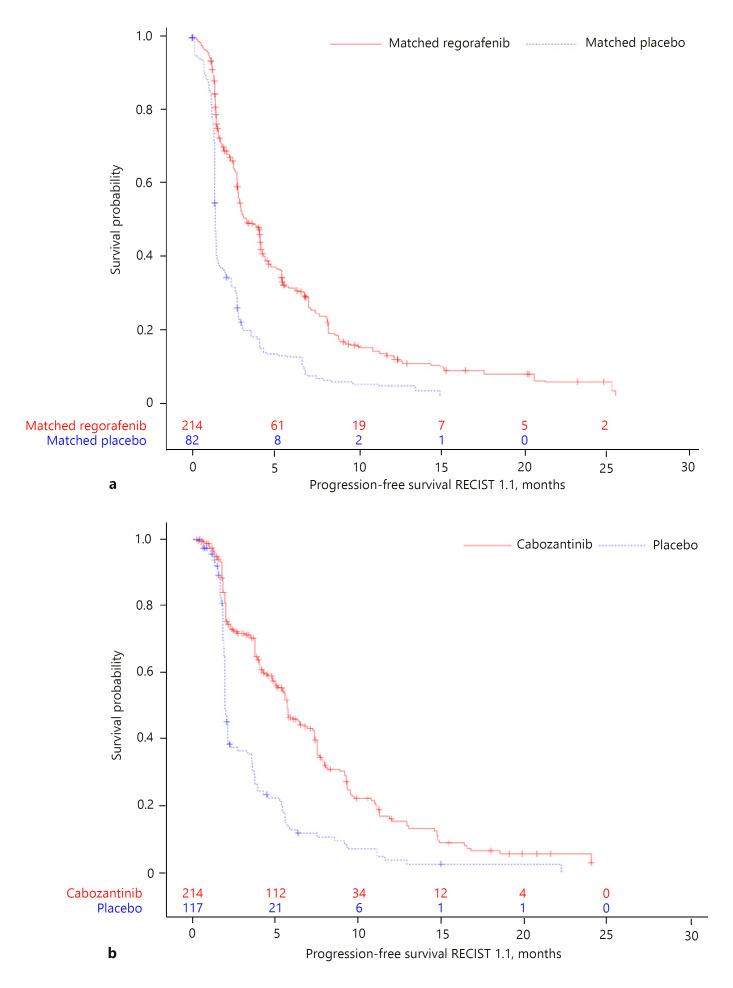
Kaplan-Meier curves for OS and PFS in the matching-adjusted RESORCE trial population and the CELESTIAL trial population are given in Figures 1 and 2. Results of the MAIC analyses suggested regorafenib was associated with a numerically lower risk of death than cabozantinib (HR, 0.80; 95% CI: 0.54, 1.20). There was no difference in PFS between the two drugs (HR, 1.00; 95% CI: 0.68, 1.49; Table 2). Because of potential violations of the proportional hazard assumption for OS and PFS in the trials (online suppl. Fig. S1–S4), non-parametrical RMST difference and ratio with 95% CIs were derived. For OS, at a 37.3-month cutoff (representing the minimum of the longest observed time among all four arms; Fig. 1), the difference in RMST suggested a survival gain of nearly 3 months with regorafenib over cabozantinib (RMST difference, 2.76 months; 95% CI: −1.03, 6.54; Table 2). The difference was not statistically significant. Results were similar when RMST analyses were performed over the entire available follow-up time for each individual trial, defined as the minimum of the longest observed time between arms within each trial (Table 2). No meaningful numerical differences in PFS were observed between regorafenib and cabozantinib (RMST difference, −0.59 months; 95% CI: −1.83, 0.65; at 15.2-month cutoff). Consistent results for OS and PFS were obtained from STC and standard ITC (online suppl. Table S1).
Fig. 1.
Kaplan-Meier curves for OS in the matching-adjusted RESORCE trial population (a) and the CELESTIAL trial population (b). Numbers within each plot are number of subjects at risk.
Fig. 2.
Kaplan-Meier curves for PFS in the matching-adjusted RESORCE trial population (a) and the CELESTIAL trial population (b). RECIST, Response Evaluation Criteria in Solid Tumors.
Table 2.
MAIC results for efficacy outcomes
| RESORCE Rego (ESS = 214) versus placebo (ESS = 82) | CELESTIAL Cabo (N = 241) versus placebo (N = 117)a | Rego versus cabo | |
|---|---|---|---|
| OS (months) | |||
| Overall HR (95% CI) | 0.59 (0.44, 0.79) | 0.74 (0.56, 0.97) | 0.80 (0.54, 1.20) |
| RMST difference (95% CI) at 37.3 months | 5.42 (2.91, 7.94) | 2.67 (−0.16, 5.50) | 2.76 (−1.03, 6.54) |
| RMST ratio (95% CI) at 37.3 months | 1.58 (1.25, 1.99) | 1.21 (0.98, 1.50) | 1.30 (0.95, 1.79) |
| RMST difference (95% CI)b | 6.01 (3.01, 9.01) | 2.67 (−0.16, 5.50) | 3.34 (−0.78, 7.46) |
| RMST ratio (95% CI)b | 1.61 (1.24, 2.10) | 1.21 (0.98, 1.50) | 1.33 (0.95, 1.87) |
| PFS (months) | |||
| Overall HR (95% CI) | 0.43 (0.32, 0.57) | 0.43 (0.33, 0.55) | 1.00 (0.68, 1.49) |
| RMST difference (95% CI) at 15.2 months | 2.51 (1.63, 3.39) | 3.10 (2.22, 3.97) | −0.59 (−1.83, 0.65) |
| RMST ratio (95% CI) at 15.2 months | 1.98 (1.51, 2.59) | 1.87 (1.54, 2.28) | 1.06 (0.76, 1.48) |
| RMST difference (95% CI)c | 2.51 (1.63, 3.39) | 3.38 (2.28, 4.47) | −0.87 (−2.27, 0.54) |
| RMST ratio (95% CI)c | 1.98 (1.51, 2.59) | 1.91 (1.51, 2.41) | 1.04 (0.72, 1.48) |
Cabo, cabozantinib; CI, confidence interval; ESS, effective sample size; HR, hazard ratio; MAIC, matching-adjusted indirect comparison; rego, regorafenib; RMST, restricted mean survival time.
Derived from recreated individual patient data from ref. [17].
Truncation time for RMST analyses was 50.6 months for RESORCE and 37.3 months for CELESTIAL, representing the minimum of the longest observed time between arms within each trial.
Truncation time for RMST analyses was 15.2 months for RESORCE and 22.2 months for CELESTIAL, representing the minimum of the longest observed time between arms within each trial.
Safety
The proportion of patients requiring dose reductions was significantly lower with regorafenib than with cabozantinib (MAIC risk difference, −15.2%; 95% CI: −29.0%, −1.5%). Similarly, regorafenib was associated with a statistically significant lower incidence of treatment-related AEs leading to permanent treatment discontinuation (MAIC risk difference, −9.2%; 95% CI: −17.7%, −0.6%; Table 3). Rates of grade 3 or 4 diarrhea and fatigue were also lower (not statistically significant) with regorafenib (MAIC risk difference for diarrhea, −7.1%; 95% CI: −14.7%, 0.4%; MAIC risk difference for fatigue, −6.3%; 95% CI: −14.6%, 2.0%). Differences in incidence of other TEAEs including AST increase, hypertension, and HFSR/PPE were small but all numerically in favor of regorafenib (Table 3). Standard ITC analysis yielded similar results (online suppl. Table S2).
Table 3.
MAIC results for safety outcomes
| Risk difference (regorafenib vs. cabozantinib) |
|||||
|---|---|---|---|---|---|
| % | 95% CI | ||||
| Dose reductions | −15.2 | −29.0, −1.5 | |||
| Treatment-related AE, leading to discontinuation | −9.2 | −17.7, −0.6 | |||
| Grade 3 or 4 TEAEs occurring in ≥10% of patients | |||||
| AST increased | −2.3 | −10.7, 6.1 | |||
| Diarrhea | −7.1 | −14.7, 0.4 | |||
| Fatigue | −6.3 | −14.6, 2.0 | |||
| HFSR/PPE | −0.9 | −7.8, 5.9 | |||
| Hypertension | −1.9 | −11.4, 7.7 | |||
Data are from the safety population of each trial. AE, adverse event; AST, aspartate aminotransferase; HFSR, hand–foot skin reaction; MAIC, matching-adjusted indirect comparison; CI, confidence interval; PPE, palmar–plantar erythrodysesthesia; TEAE, treatment-emergent adverse event.
Discussion
This anchored MAIC suggests regorafenib was associated with favorable OS over cabozantinib in the second-line post-sorafenib setting, although this difference did not reach statistical significance. The risk reduction in survival hazards was ∼20% and the increase in RMST was almost 3 months, or 30%. No difference in PFS was seen between the regorafenib- and cabozantinib-treated patients. Results from the MAIC also showed regorafenib to have a favorable safety and tolerability profile over cabozantinib, with a statistically significant lower incidence of treatment-related AEs (any grade) leading to permanent treatment discontinuation, a significantly lower proportion of patients requiring dose reductions, and a lower incidence of grade 3 or 4 diarrhea and fatigue.
The main strength of the study is its use of anchored indirect treatment comparison, thus maintaining the internal validity of the two randomized controlled trials and the relative treatment effects under comparison. The MAIC is further supported by alternative approaches and sensitivity analyses using standard Bucher indirect treatment comparison and STC. Its use of RMST as an alternative survival metric also corrects for the non-proportionality observed in both the RESORCE and CELESTIAL trials. This provides a more robust and valid measurement of the differences in survival benefit from the two treatments. Moreover, differences in rates of treatment discontinuation and dose reduction due to AEs provide a clearer and more general indication of the relative tolerability of the two treatments.
These strengths contrast with recent analyses by Kelley et al. [16] and Casadei-Gardini et al. [21], which used unanchored MAIC. Unanchored or single-arm comparisons assume that all prognostic factors and treatment effect modifiers were known, measured, and adjusted for, and that absolute treatment effects can be reliably predicted. These assumptions are impossible to meet [14, 15]. One example was the lack of matching and adjustment of definitions and assessment schedules of PFS, while a significant PFS difference was the main finding of both studies. PFS assessments were more frequent in RESORCE versus CELESTIAL (every 6 weeks vs. every 8 weeks), and the RESORCE trial included more qualifying events of progression (both clinical and radiological progression) than the CELESTIAL trial (radiological progression only). Since many patients progress within the first few cycles of treatment, the difference in PFS assessment schedules could potentially bias the results in favor of cabozantinib in an unanchored MAIC or STC comparison [22]. In the unanchored MAIC by Casadei-Gardini et al. [21], neither the definition nor the assessment schedule of progression was specified for the real-world regorafenib population used, which were likely to be heterogenous and poorly defined, making them even more difficult to compare with well-defined, uniformly assessed progression in a clinical trial. Additionally, neither study adjusted for the differences in proportions of patients with prior sorafenib duration of 3–6 months and ≥6 months to match the trial being compared.
A further limitation to the study by Kelley et al. [16] was that no attempt was made to adjust for the CELESTIAL trial's inclusion of patients intolerant to sorafenib, who were earlier in their disease course than patients having progressed after longer sorafenib treatment. This would also bias the comparison of OS and PFS in favor of cabozantinib. Additionally, contrary to claims by the authors, violation of proportional hazards is not a valid reason for conducting unanchored single-arm indirect comparisons since non-proportionality can be addressed as in the current study with the use of RMST. The procedure for estimating the difference in RMST (or t-mean survival) between two treatments is always valid without any proportional model assumptions, and thus can be used to quantify differences in PFS and OS between treatments when the proportional hazards assumption is not met [23].
The unanchored MAIC by Casadei-Gardini et al. [21] used real-world individual patient data from an unclear source for the regorafenib population to compare with aggregate data of the cabozantinib arm from the CELESTIAL trial. This may have introduced selection bias since clinical trial patients, per protocol, are highly selected, homogeneous, and generally healthier patients compared to real-world patients, who are more heterogenous (e.g., more comorbidities ineligible for trial enrollment), have worse performance status, and are expected to have poorer outcomes.
Both of the earlier MAIC studies found no difference in OS but a significant gain in PFS for cabozantinib compared to regorafenib. Such contradictions appeared to support the notion that PFS is not correlated with OS, thus further casting doubt on the clinical meaningfulness and patient relevance of PFS as an efficacy endpoint in advanced HCC [24].
In line with the current study, two previously conducted network meta-analyses [25, 26] ranked regorafenib the highest in OS benefit, comparing to cabozantinib, pembrolizumab, ramucirumab, brivanib, and placebo [26] or comparing to cabozantinib, pembrolizumab, and ramucirumab [25]. While these analyses support the findings of this study, these analyses were performed with aggregate data rather than individual patient data, which would limit their power to identify true differences, as they were not able to adjust for differences in inclusion criteria and treatment effect modifiers.
Also consistent with the current study, Kelley et al. [16] reported statistically significantly lower grade 3 or 4 diarrhea in the regorafenib population than in the cabozantinib population. Other grade 3 or 4 AEs including AST increase, hypertension, fatigue, and HFSR/PPE were to various degrees numerically in favor of regorafenib, although not statistically significant. A recent network meta-analysis also found regorafenib to be associated with significantly lower rates of grade 3 or 4 diarrhea (2 vs. 10%) and fatigue (6 vs. 10%) than cabozantinib [25]. Incidence differences between regorafenib and cabozantinib in other high-grade AEs were small but all numerically in favor of regorafenib. Overall, this supports better tolerability and safety of regorafenib, as further confirmed by the rates of discontinuation analyses in the current study.
Differences in strength of efficacy and tolerability between regorafenib and cabozantinib may be underpinned by the differences in their mechanisms of action. Both drugs are multi-kinase inhibitors with broad activities against VEGFR 1–3, RET, and KIT. Cabozantinib is unique in targeting MET and AXL, whereas regorafenib is unique in targeting FGFR1 and RAF [27, 28]. Regorafenib also inhibits the colony-stimulating factor 1 receptor, which may stimulate antitumor immunity by modulating tumor-associated macrophages [27, 29, 30].
While the current study has significant strengths over earlier analyses, some differences in trial design could not be fully adjusted for in the analyses. Specifically, RESORCE required patients to be sorafenib tolerant, whereas CELESTIAL did not. Full adjustment for this difference was not possible; however, partial alignment was achieved by excluding the population with <3 months of prior sorafenib exposure who, as argued by Kelley et al. [17], presumably represent sorafenib-intolerant or fast-progressing patients in CELESTIAL but only fast-progressing patients in RESORCE. The current results, therefore, are limited to sorafenib-tolerant patients who are not fast progressors (<3 months) on first-line sorafenib treatment. In addition, race (white vs. other) could not be adjusted for because race data were missing for 20% of the RESORCE patients due to reporting restrictions in some European countries. This was mitigated by adjusting for geographical region (Asia vs. rest of world). The difference in the definitions and assessment schedules of progression between RESORCE and CELESTIAL could not be fully adjusted for in the analyses; however, it is unlikely to have biased PFS comparison in an anchored setting. Finally, as with all anchored indirect treatment comparisons, the current study is limited by unobserved or unknown effect modifiers that could not be adjusted for. In addition, while of clinical interest to identify subgroup populations that might benefit particularly from one agent or the other, there are currently no published data for subgroups of the second-line CELESTIAL population (beyond subgroups of prior sorafenib duration) to compare with the second-line RESORCE population.
In conclusion, this anchored indirect treatment comparison supports regorafenib as the preferred treatment option in patients tolerant to and previously treated with sorafenib. This study addressed several methodological limitations of earlier MAICs to provide a less biased comparison of the efficacy and safety of second-line regorafenib versus cabozantinib treatment for advanced HCC. In the absence of a head-to-head comparative trial, these findings can help inform decisions for patients with HCC after previous treatment with sorafenib. While immunotherapy is becoming widely used in the frontline setting, sorafenib continues to be an appropriate option for patients not able to benefit from check-point inhibitors. And for patients who progress from sorafenib, regorafenib may offer longer survival and better tolerability and safety than cabozantinib. Furthermore, a recent analysis showed patients' quality of life is significantly better maintained with regorafenib than with placebo [31]. Further investigations are needed to determine the optimal treatment option for patients progressing from immunotherapy or lenvatinib frontline treatment.
Statement of Ethics
No institutional board review was required for this study since it was a post hoc analysis of previously published clinical data. The original clinical trials were approved by the local Institutional Review Boards for each study site and were conducted in line with the Declaration of Helsinki. All patients provided written informed consent before participating in the trials.
Conflict of Interest Statement
Philippe Merle has received consulting fees from Bayer, Ipsen, Eli Lilly, Eisai, Roche, AstraZeneca, Bristol-Myers Squibb, and Roche; travel expenses from Bayer, Ipsen, Roche, and Bristol-Myers Squibb; and research funding (to institution) from Ipsen. Masatoshi Kudo has received grants from Chugai, Otsuka, Takeda, Taiho, Sumitomo Dainippon, Daiichi Sankyo, MSD, Eisai, Bayer, EA Pharma, Ono Pharma, Gilead Sciences, and AbbVie; lecturing fees from Bayer, Eisai, MSD, BMS, EA Pharma, Eli Lilly, Chugai, and Ajinomoto; advisory and consultancy fees from Bayer, Eisai, Ono, Kowa, MSD, BMS, Roche, Chugai, and Taiho. Masatoshi Kudo is Editor-in-Chief of Liver Cancer. Stanimira Krotneva and Irina Proskorovsky are employed by Evidera. Kirhan Ozgurdal and Yun Su are employed by Bayer.
Funding Sources
This study was funded by Bayer. The authors employed by the sponsors were involved in the study's design, data interpretation, and preparation of the manuscript.
Author Contributions
Philippe Merle, Masatoshi Kudo, Stanimira Krotneva, Kirhan Ozgurdal, Yun Su, and Irina Proskorovsky contributed to the design of the study, were responsible for data interpretation, contributed to revising the manuscript, and reviewed and approved the final version. Stanimira Krotneva and Irina Proskorovsky were responsible for data analysis.
Data Availability Statement
The phase 3 CELESTIAL trial data that support the findings of this study are available in prior publications [4, 16, 17]. The phase 3 RESORCE trial data are also available in publications [5]. Availability of the data underlying this publication will be determined according to Bayer's commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, time point, and process of data access.
As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the USA and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014.
Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal.
Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an Independent Scientific Review Panel. Bayer is not involved in the decisions made by the Independent Review Panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Supplementary Material
Supplementary data
Acknowledgment
Medical writing was provided by Jonathan Pitt, PhD (Evidera, Paris, France), and funded by Bayer.
Funding Statement
This study was funded by Bayer. The authors employed by the sponsors were involved in the study's design, data interpretation, and preparation of the manuscript.
References
- 1.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76((3)):681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019 Apr 11;380((15)):1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 3.Vogel A, Martinelli E, ESMO Guidelines Committee; ESMO Guidelines Committee Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021 Jun;32((6)):801–805. doi: 10.1016/j.annonc.2021.02.014. Electronic address: clinicalguidelines@esmo.org. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018 Jul 5;379((1)):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017 Jan 7;389((10064)):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 6.Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019 Feb;20((2)):282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- 7.Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020 Nov 1;6((11)):e204564. doi: 10.1001/jamaoncol.2020.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018 Jul;19((7)):940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 9.Bristol Myers Squibb Bristol Myers Squibb Statement on Opdivo® (nivolumab) monotherapy post-sorafenib hepatocellular carcinoma U.S. Indication. 2021. Jul 23,
- 10.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50((6)):683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012 Jun;3((2)):98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Signorovitch JE, Sikirica V, Erder MH, Xie J, Lu M, Hodgkins PS, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15((6)):940–947. doi: 10.1016/j.jval.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Ishak KJ, Proskorovsky I, Benedict A. Simulation and matching-based approaches for indirect comparison of treatments. Pharmacoeconomics. 2015;33((6)):537–549. doi: 10.1007/s40273-015-0271-1. [DOI] [PubMed] [Google Scholar]
- 14.Phillippo D, Ades T, Dias S, Palmer S, Abrams KR, Welton N. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE. Sheffield: Decision Support Unit, ScHARR, University of Sheffield; 2016. [Google Scholar]
- 15.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Making. 2018 Feb;38((2)):200–211. doi: 10.1177/0272989X17725740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley RK, Mollon P, Blanc JF, Daniele B, Yau T, Cheng AL, et al. Comparative efficacy of cabozantinib and regorafenib for advanced hepatocellular carcinoma. Adv Ther. 2020 Jun;37((6)):2678–2695. doi: 10.1007/s12325-020-01378-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley RK, Ryoo BY, Merle P, Park JW, Bolondi L, Chan SL, et al. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: a subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open. 2020 Aug;5((4)):e000714. doi: 10.1136/esmoopen-2020-000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012 Feb 1;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013 Dec 7;13((1)):152. doi: 10.1186/1471-2288-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? BMJ. 1998;316((7136)):989–991. doi: 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casadei-Gardini A, Rimassa L, Rimini M, Yoo C, Ryoo BY, Lonardi S, et al. Regorafenib versus cabozantinb as second-line treatment after sorafenib for unresectable hepatocellular carcinoma: matching-adjusted indirect comparison analysis. J Cancer Res Clin Oncol. 2021 Mar 20;147((12)):3665–3671. doi: 10.1007/s00432-021-03602-w. [DOI] [PubMed] [Google Scholar]
- 22.Kapetanakis V, Prawitz T, Schlichting M, Ishak KJ, Phatak H, Kearney M, et al. Assessment-schedule matching in unanchored indirect treatment comparisons of progression-free survival in cancer studies. Pharmacoeconomics. 2019;37((12)):1537–1551. doi: 10.1007/s40273-019-00831-3. [DOI] [PubMed] [Google Scholar]
- 23.Pak K, Uno H, Kim DH, Tian L, Kane RC, Takeuchi M, et al. Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol. 2017 Dec 1;3((12)):1692–6. doi: 10.1001/jamaoncol.2017.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruix J. Endpoints in clinical trials for liver cancer and their value in evidence-based clinical decision making: an unresolved Gordian knot. J Hepatol. 2021 Jun;74((6)):1483–8. doi: 10.1016/j.jhep.2021.01.033. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Yang X, Lin J, Bai Y, Long J, Yang X, et al. Comparing the efficacy and safety of second-line therapies for advanced hepatocellular carcinoma: a network meta-analysis of phase III trials. Therap Adv Gastroenterol. 2020;13:175628482093248. doi: 10.1177/1756284820932483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonbol MB, Riaz IB, Naqvi SAA, Almquist DR, Mina S, Almasri J, et al. Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol. 2020 Dec 1;6((12)):e204930. doi: 10.1001/jamaoncol.2020.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granito A, Forgione A, Marinelli S, Renzulli M, Ielasi L, Sansone V, et al. Experience with regorafenib in the treatment of hepatocellular carcinoma. Therap Adv Gastroenterol. 2021;14:175628482110169. doi: 10.1177/17562848211016959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarcognato S, García-Lezana T, Villanueva A. Mechanisms of action of drugs effective in hepatocellular carcinoma. Clin Liver Dis. 2019 Aug;14((2)):62–65. doi: 10.1002/cld.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abou-Elkacem L, Arns S, Brix G, Gremse F, Zopf D, Kiessling F, et al. Regorafenib inhibits growth, angiogenesis, and metastasis in a highly aggressive, orthotopic colon cancer model. Mol Cancer Ther. 2013 Jul;12((7)):1322–1331. doi: 10.1158/1535-7163.MCT-12-1162. [DOI] [PubMed] [Google Scholar]
- 30.Arai H, Battaglin F, Wang J, Lo JH, Soni S, Zhang W, et al. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat Rev. 2019 Dec;81:101912. doi: 10.1016/j.ctrv.2019.101912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofheinz RD, Bruix J, Demetri GD, Grothey A, Marian M, Bartsch J, et al. Effect of regorafenib in delaying definitive deterioration in health-related quality of life in patients with advanced cancer of three different tumor types. Cancer Manag Res. 2021;13:5523–5533. doi: 10.2147/CMAR.S305939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Data Availability Statement
The phase 3 CELESTIAL trial data that support the findings of this study are available in prior publications [4, 16, 17]. The phase 3 RESORCE trial data are also available in publications [5]. Availability of the data underlying this publication will be determined according to Bayer's commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing.” This pertains to scope, time point, and process of data access.
As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the USA and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014.
Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal.
Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an Independent Scientific Review Panel. Bayer is not involved in the decisions made by the Independent Review Panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.