Goranci-Buzhala and Niessen highlight work from Moore and colleagues that uses high-resolution imaging and machine learning to examine tissue-scale patterns of Ca2+ signaling by basal stem cells of the skin epidermis.
Abstract
How adult stem cells signal in vivo over time to coordinate their fate and behavior across self-renewing tissues remains a challenging question. In this issue, Moore et al. (2023. J. Cell Biol. https://doi.org/10.1083/jcb.202302095) combine high-resolution live imaging in mice with machine learning tools to reveal temporally regulated tissue-scale patterns of Ca2+ signaling orchestrated by cycling basal stem cells of the skin epidermis.
In self-renewing tissues, the maintenance of functional tissue architecture, known as homeostasis, demands that stem cells communicate with their local neighbors and with cells positioned far away to coordinate the replacement of lost cells. Over the years, we have gained tremendous insight into how adult stem cells and their niches communicate locally to direct stem cell behavior. Yet, understanding how local signaling dynamics integrate into a tissue-wide information network to align large-scale cell behavior in regenerating organs has remained a formidable challenge in mammals. This challenge stems from the complexities in both monitoring and analyzing the signal dynamics from thousands of cells heterogenous in fate and behavior in an uninjured animal at high enough spatial and temporal resolution.
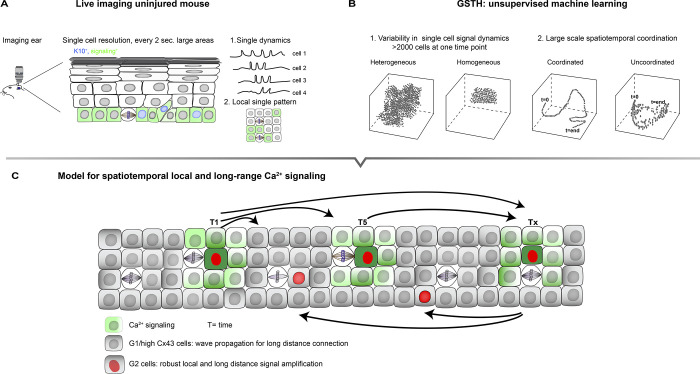
Moore et al. (1) took on both of these challenges (Fig. 1 A). Further advancing their intravital imaging setup previously used to show how the local neighborhood coordinates adult stem cell behavior in the epidermis (2), the authors now capture thousands of signals at single-cell resolution every 2 s, covering a large area of the stem cell compartment. At the same time, they developed a novel unsupervised machine learning tool called geometric scattering trajectory homology (GSTH) to analyze the generated complex intravital imaging datasets (3). GSTH employs PHATE, a method developed previously by the Krishnaswamy lab, to visualize both local and global structure and patterns within high-dimensional data sets (4). In brief, GSTH applications visualize complex imaging datasets to show how thousands of individual cells signal and, through PHATE time trajectories, whether individual cells are connected in space and time also over large distances (Fig. 1 B). GSTH thus allows the authors to assess whether different settings promote or disturb signal dynamics and signal coordination.
Figure 1.
Cell cycle–controlled short- and long-range Ca2+ signal dynamics coordinates stem cell cycle in the epidermis. (A and B) Moore et al. (1) combined high resolution intravital imaging of large areas of the basal epidermis (A) with new unsupervised machine learning called GSTH (B) to assess single cell signaling dynamics of thousands of cells and local and large distance spatiotemporal coordination of signals to monitor Ca2+ signaling. (C) Model for spatiotemporal coordination of Ca2+ signaling across the basal stem cell compartment of the epidermis. Cells in G2 are essential to amplify signals within local signaling neighborhoods to control and reinforce robust long-distance communication, whereas G1 cells, through high expression of Cx43, are essential to propagate signals between local neighborhoods.
Moore et al. (1) chose Ca2+ signaling because this pathway regulates diverse cellular processes that are central for (adult) stem cell function (5, 6). Moreover, sensitive genetic fluorescent sensors, such as GCaMP6s, allow for the imaging of cellular Ca2+ signaling dynamics that are well characterized in vivo, e.g., in mammalian neurons (7) and Drosophila epithelia (5). To ask how adult stem cells communicate in a regenerating setting, the authors focused on the stem cell compartment of the skin epidermis, the basal layer that also harbors progenitors already committed to differentiation (8).
Initial characterization of in vivo epidermis expressing the GCaMP6s sensor revealed that individual cells comprising both stem cells and committed progenitors display highly variable Ca2+ signal dynamics, and signaling was not spatially restricted to certain domains within the basal layer. This dynamic Ca2+ signaling is a unique emerging property of the basal layer as no signal events were observed in the suprabasal compartment within the 30-min time frame. Single cells spiked either in isolation or in clusters with connected Ca2+ signals as defined by spikes occurring within 10 s between two neighbors. The majority of these local signaling neighborhoods consisted of 2–10 cells with sporadical waves that spread across hundreds of cells. The larger neighborhoods also showed more persistent single signal events, suggesting that long distance propagation of waves require more robust signals.
This initial analysis indicated that Ca2+-signaling cells are connected locally with a few signal-connected neighborhoods spanning hundreds of cells. Do these signaling neighborhoods then function as independent units or are these integrated into larger networks over time across much larger tissue areas? To answer this question, the authors developed GSTH as described above. The resulting PHATE trajectories revealed that cellular Ca2+ signaling is consistently connected spatially and over time, as opposed to some other cell types, such as neurons, which are scattered using existing data, and which, under resting conditions, are known to spontaneously fire without any topographical or temporal organization. The epidermal basal stem cell compartment thus displays tissue-wide coordination of Ca2+ signaling.
Is the cellular state important for this coordination? Basal stem cells exhibit heterogeneity in the cell cycle stage (9), which can control cell fate (10). The authors found that although both G1/S or G2 propagate Ca2+ signals, GSTH revealed that cells in G2 are much more homogenous in signaling dynamics than G1/S cells. Is this difference in cell signal dynamics relevant for the spatiotemporal propagation of local and long-distance signals? Genetic or chemical depletion of G2 cells did not affect the size of the Ca2+ signal local neighborhood but strongly impaired the number of events and signal strength, resulting in temporally and spatially uncoordinated signals. Thus, cells in the G2 phase of the cell cycle are essential to initiate robust coordinated long-distance calcium signaling information flows within regenerating tissues. What was most surprising is that an increase in G2 cells did not seem to affect the Ca2+ circuit (e.g., by increasing the number of local neighborhoods that signal at the same time). This result suggests that G2 cells may not initiate Ca2+ signals but instead act as signal amplifiers essential to coordinate the propagation of signals.
What then are the molecular mediators that enable the spread of Ca2+ signals? Obvious candidates are connexins, proteins that form intercellular channels known as gap junctions (GJs), to exchange small molecules and second messengers (including Ca2+) between cells (11). Interestingly, Connexin 43 (Cx43)-containing GJs were most prominent in G1 cells and almost absent in G2 cells, suggesting that G2 cells communicate Ca2+ through other molecular players. Inactivating Cx43 in the epidermis revealed that Cx43 dampens Ca2+-signal intensity and duration, especially in local small signal neighborhoods. Moreover, Cx43 is essential to integrate these local signaling clusters into a tissue-wide temporally connected network. Interestingly, human Cx43 mutations linked to epidermal disease show increased GJ channel activity (11). Together, these results imply that amplification of the long distance Ca2+-signaling network may contribute to skin disease.
Finally, the authors explored the functional relevance of the temporally regulated Ca2+ signaling network. Manipulating Ca2+ signals either chemically or optogenetically changed the number of cycling cells, suggesting a positive feedback loop between cell cycle phase-directed long-distance communication of Ca2+ signals and large-scale coordinated stem cell proliferation.
Together, the impressive collection of data presented by Moore et al. (1) indicate that cells in different stages of the cell cycle cooperate to coordinate large-scale signaling waves in space and time to direct stem cell behavior. Their data suggest a model (Fig. 1 C) in which G2 cells function as amplifiers essential to create robust signals in local neighborhoods, which then, through Cx43-mediated GJ-containing G1 cells that are the most abundant basal population, spread locally and across long distances, perhaps through the very large signaling neighborhoods. G2 cells in the next neighborhood are then necessary to amplify and reinforce signals again to sustain long distance signaling over larger time periods. In the future, it will be important to examine how different signaling networks integrate over space and time and to explore the physiological relevance of such signaling networks in tissue-wide coordination of stem cell behavior during healthy and pathological regeneration.
Acknowledgments
We would like to thank Matthias Rübsam for help with the figure.
Work in the Niessen laboratory is supported by the German Krebshilfe, the Deutsche Forschungsgemeinschaft (DFG German Research Foundation) Germany’s Excellence Strategy – EXC 2030–390661388, Project-ID 73111208 - SFB 829, SPP 1782 NI1234/6-2, Project-ID 388932620 - FOR 2743 NI 1234/7-1, and Project-ID 411422114 - GRK 2550, ANR-DFG NI 1234/9-1, Center for Molecular Medicine Cologne and Köln Fortune.
References
- 1.Moore, J.L., et al. 2023. J. Cell Biol. 10.1083/jcb.202302095 [DOI] [Google Scholar]
- 2.Mesa, K.R., et al. 2018. Cell Stem Cell. 10.1016/j.stem.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhaskar, D., et al. 2023. bioRxiv. 10.1101/2023.03.22.533807 [DOI] [Google Scholar]
- 4.Moon, K.R., et al. 2019. Nat. Biotechnol. 10.1038/s41587-019-0336-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balaji, R., et al. 2017. Sci. Rep. 10.1038/srep42786 [DOI] [Google Scholar]
- 6.Snoeck, H.W., 2020. EMBO Rep. 10.15252/embr.202050028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, T.W., et al. 2013. Nature. 10.1038/nature12354 [DOI] [Google Scholar]
- 8.Cockburn, K., et al. 2022. Nat. Cell Biol. 10.1038/s41556-022-01021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiratsuka, T., et al. 2015. Elife. 10.7554/eLife.05178 [DOI] [Google Scholar]
- 10.Hu, X., et al. 2019. FEBS Lett. 10.1002/1873-3468.13625 [DOI] [Google Scholar]
- 11.Cocozzelli, A.G., and White T.W.. 2019. Int. J. Mol. Sci. 10.3390/ijms20246186 [DOI] [Google Scholar]