Dear Editor,
Melanoma and basal cell carcinoma (BCC) are common cancerous skin lesions, and their incidence is steadily increasing. 1 , 2 Although both neoplasms require treatment, their associated mortality differs greatly causing a difference in urgency and rapidity of treatment. 2 , 3 Thus, adequate differentiation between melanoma and BCC is essential. However, differentiating melanoma from pigmented BCC may sometimes be challenging as clinical, and dermoscopic features of both entities may mimic one another. 4 For these equivocal lesions, more sophisticated non‐invasive imaging techniques such as reflectance confocal microscopy (RCM) may provide significant help, 4 , 5 whereas the role of conventional OCT seems to be more marginal in this particular differential diagnosis. 6
Line‐field confocal optical coherence tomography (LC‐OCT) is a newly introduced non‐invasive imaging tool combining the technical advantages of RCM (high isotropic resolution) and conventional OCT (high in‐tissue penetration). It provides images in both vertical and horizontal planes (depth 500 μm), which enables tridimensional (3D) reconstructions of the skin. It features an integrated dermoscopic camera, which enables dermoscopy‐aided LC‐OCT probe placement within the examined lesion. Technical details of the device are decribed elsewhere. 7
LC‐OCT criteria for melanoma and BCC have been suggested, 8 , 9 and the technique is able to increase the diagnostic performance of physicians in both cases. 10 , 11
Hereby we report a case of a 62‐year‐old woman, previously diagnosed with breast cancer treated by mastectomy with sentinel node procedure, chemotherapy and radiotherapy. The patient presented with a shiny nodular lesion on the lateral right upper arm of 6 mm in diameter (Figure 1A). Dermoscopic examination, revealed the following: polymorphous vascular structures including short fine arborizing vessels; brown and blue‐grey structureless areas asymmetrically distributed in the periphery; blue‐grey globules, blue‐white veil‐like structures; featureless, erythematous background in a portion of the lesion (Figure 1B). Based on the clinical and dermoscopic features, differential diagnoses included melanoma and pigmented nodular BCC.
FIGURE 1.
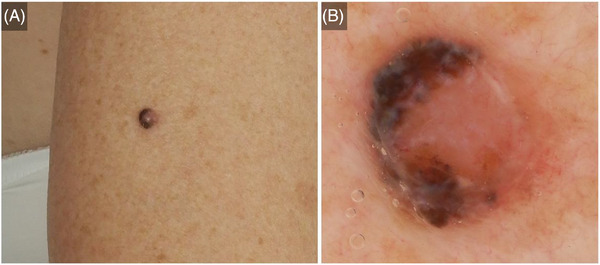
Nodular melanoma on the lateral right upper arm of a 62‐year‐old woman: clinical (A) and dermoscopic (B) presentation.
LC‐OCT examination rapidly prompted us to formulate the diagnosis of nodular melanoma, which was later confirmed on histopathology. Indeed, LC‐OCT examination of dermoscopically pigmented areas (Figure 2A–C) revealed dermal lobules containing tightly packed bright pleomorphic cells with dark nuclei and bright cytoplasm corresponding to atypical melanocytes on histopathology (Figure 2D) and on RCM (Figure 2E). Importantly, the vertical view of the LC‐OCT examination showed a good correlation with the histopathology whereas the horizontal view displayed a good correlation with RCM.
FIGURE 2.
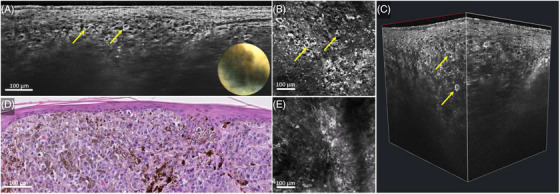
Nodular melanoma on the lateral right upper arm of a 62‐year‐old woman: line‐field confocal optical coherence tomography (LC‐OCT) (A–C), histopathology (D) and reflectance confocal microscopy (RCM) (E). Vertical LC‐OCT examination of the pigmented area (A) showed pleomorphic melanocytes (yellow arrows) arranged in dermal nests, corresponding to the nests on histopathology (D). Horizontal LC‐OCT examination (B) showed singularly arranged atypical melanocytes (yellow arrows) under the dermal epidermal junction, which correlated well to RCM (E).
Within the dermoscopically non‐pigmented areas, LC‐OCT displayed nests of hypo‐reflective cells, forming a more irregular and more hyper‐reflective wave‐like pattern than the one commonly found in benign melanocytic proliferations and recently decribed by Lenoir et al. (Figure 3). 12
FIGURE 3.
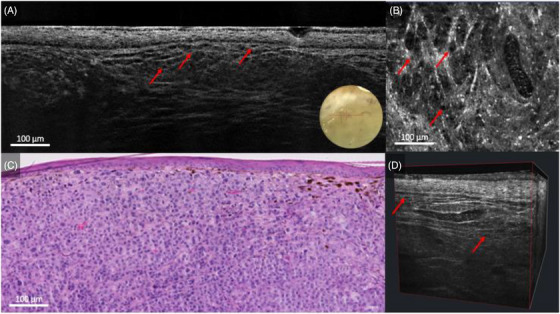
Nodular melanoma on the lateral right upper arm of a 62‐year‐old woman: line‐field confocal optical coherence tomography (LC‐OCT) (A, B, D) and histopathology (C). Vertical, horizontal, and 3D LC‐OCT examination of the non‐pigmented area (A, B, D), revealing a hyper‐reflective and irregular wave‐like pattern with hypo‐reflective melanocytes (red arrows), which correlated well with histopathology (C).
Criteria proper of nodular BCC (such as lobules featuring by the typical millefeuille pattern and surrounded by clefting and bright rim) 9 were not visualized.
This case illustrates not only the utility of LC‐OCT in distinguishing clinically/dermoscopically equivocal lesions non‐invasively but also the usefulness of the integrated dermoscopic camera, which enabled us to examine parts of the lesion with different dermoscopic characteristics. Future studies corroborating the available data on diagnostic performance of LC‐OCT and examining the association between dermoscopic criteria and LC‐OCT features are needed.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
The authors received no specific funding for this work.
ACKNOWLEDGEMENTS
The patient in this manuscript has given written informed consent to the publication of her case details. The authors are indebted to Clara Tavernier (DAMAE Medical, Paris, France) for her technical assistance with our LC‐OCT prototypes. The non‐invasive skin imaging research team of Hôpital Erasme, Université Libre de Bruxelles (Brussels, Belgium) would like to acknowledge the support of the Fonds Erasme (www.fondserasme.org).
Tom Wolswijk and Dilara Sanak contributed equally to this manuscript and share first authorship.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lomas A, Leonardi‐Bee J, Bath‐Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069‐1080. [DOI] [PubMed] [Google Scholar]
- 2. Dimitriou F, Krattinger R, Ramelyte E, et al. The world of melanoma: epidemiologic, genetic, and anatomic differences of melanoma across the globe. Curr Oncol Rep. 2018;20(11):1‐9. [DOI] [PubMed] [Google Scholar]
- 3. Yang D, Salciccioli J, Marshall D, Sheri A, Shalhoub J. Trends in malignant melanoma mortality in 31 countries from 1985 to 2015. Br J Dermatol. 2020;183(6):1056‐1064. [DOI] [PubMed] [Google Scholar]
- 4. Charles CA, Marghoob AA, Busam KJ, Clark‐Loeser L, Halpern AC. Melanoma or pigmented basal cell carcinoma: a clinical‐pathologic correlation with dermoscopy, in vivo confocal scanning laser microscopy, and routine histology. Skin Res Technol. 2002;8(4):282‐287. [DOI] [PubMed] [Google Scholar]
- 5. Yélamos O, Manubens E, Jain M, et al. Improvement of diagnostic confidence and management of equivocal skin lesions by integration of reflectance confocal microscopy in daily practice: prospective study in 2 referral skin cancer centers. J Am Acad Dermatol. 2020;83(4):1057‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12(12):CD013189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubois A, Levecq O, Azimani H, et al. Line‐field confocal optical coherence tomography for high‐resolution noninvasive imaging of skin tumors. J Biomed Opt. 2018;23(10):106007. [DOI] [PubMed] [Google Scholar]
- 8. Perez‐Anker J, Puig S, Alos L, et al. Morphological evaluation of melanocytic lesions with three‐dimensional line‐field confocal optical coherence tomography: correlation with histopathology and reflectance confocal microscopy. A pilot study. Clin Exp Dermatol. 2022;47(12):2222‐2233 [DOI] [PubMed] [Google Scholar]
- 9. Suppa M, Fontaine M, Dejonckheere G, et al. Line‐field confocal optical coherence tomography of basal cell carcinoma: a descriptive study. J Eur Acad Dermatol Venereol. 2021;35(5):1099‐1110. [DOI] [PubMed] [Google Scholar]
- 10. Schuh S, Ruini C, Perwein MKE, et al. Line‐field confocal optical coherence tomography: a new tool for the differentiation between nevi and melanomas? Cancers (Basel). 2022;14(5):1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gust C, Schuh S, Welzel J, et al. Line‐field confocal optical coherence tomography increases the diagnostic accuracy and confidence for basal cell carcinoma in equivocal lesions: a prospective study. Cancers (Basel). 2022;14(4):1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lenoir C, Perez‐Anker J, Diet G, et al. Line‐field confocal optical coherence tomography of benign dermal melanocytic proliferations: a case series. J Eur Acad Dermatol Venereol. 2021;35(6):e399401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


