Abstract
The prefrontal cortex (PFC) has long been associated with arbitrating between approach and avoidance in the face of conflicting and uncertain motivational information, but recent work has also highlighted medial temporal lobe (MTL) involvement. It remains unclear, however, how the contributions of these regions differ in their resolution of conflict information and uncertainty. We designed an fMRI paradigm in which participants approached or avoided object pairs that differed by motivational conflict and outcome uncertainty (complete certainty vs. complete uncertainty). Behavioral data and decision-making parameters estimated using the hierarchical drift diffusion model revealed that participants’ responding was driven by conflict rather than uncertainty. Our neural data suggest that PFC areas contribute to cognitive control during approach-avoidance conflict by potentially adjusting response caution and the strength of evidence generated towards either choice, with differential involvement of anterior cingulate cortex and dorsolateral prefrontal cortex. The MTL, on the other hand, appears to contribute to evidence generation, with the hippocampus linked to evidence accumulation for stimuli. Although findings within perirhinal cortex were comparatively equivocal, some evidence suggests contributions to perceptual representations, particularly under conditions of threat. Our findings provide evidence that MTL and PFC regions may contribute uniquely to arbitrating approach-avoidance conflict.
Keywords: approach-avoidance conflict, memory, hippocampus, anterior cingulate cortex, dorsolateral prefrontal cortex
Introduction
Approach-avoidance (AA) conflict occurs when a stimulus with both positive and negative qualities simultaneously elicits incongruent goals, motivations, and/or responses (Ito and Lee 2016; Miller 1944). Deciding whether to approach or avoid in this scenario can have significant consequences for survival and research suggests that this form of decision-making may go awry in a number of disorders including anxiety, mood, and substance abuse (Ironside et al. 2020; Smith et al. 2021). It is important, therefore, to identify and understand fully the brain mechanisms that support AA conflict processing. Although frontal lobe regions like the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) have received the lion’s share of attention in supporting AA conflict processing (Talmi et al. 2009; Schlund et al. 2016; Aupperle et al. 2015; Zorowitz et al. 2019; Ironside et al. 2020; Rolle et al. 2022), converging evidence from both rodent and human research suggests that medial temporal lobe (MTL) structures—particularly the ventral (vHPC; rodent) or anterior (aHPC; human) portion, and the perirhinal cortex (PRC)—also play an important role (Bach et al. 2014; O’Neil et al. 2015; Schumacher et al. 2015; Loh et al. 2016; Chu et al. 2021). Yet, the distinct computational contributions of these regions remain unclear.
Within AA conflict, the ACC has been consistently speculated to be a primary hub of regulation when triggered by motivational and emotional conflict or uncertainty (Talmi et al. 2009; Mitchell 2011; Shenhav et al. 2013; Aupperle et al. 2015). Previous work has demonstrated greater ACC activation during high compared to low conflict conditions, and this has been thought to reflect a wide range of processes inherent to conflict regulation including encoding, monitoring, and resolving evidence towards one choice or another. Support for a conflict regulation hypothesis, however, is limited by a lack of findings defining the region’s precise contribution to conflict processing (Mitchell 2011; Aupperle et al. 2015). It is unknown whether ACC activity captures conflict monitoring, evidence generation, evidence evaluation, regulating and promoting a particular type of response (i.e. approach or avoid), or all the above.
Similarly, there are differing hypotheses about the involvement of DLPFC in AA conflict including the idea that it supports either cognitive control (Aupperle et al. 2015; Zorowitz et al. 2019), reward sensitivity (Rolle et al. 2022), or both. Concerning the former, evidence suggests that the DLPFC mediates a range of executive processes including goal selection and pursuit, and online manipulation of task relevant information and inhibitory control (Hoshi 2006; Knoch and Fehr 2007; Spielberg et al. 2015; Anderson et al. 2016; although see Aron et al. 2014). In the context of AA conflict, therefore, DLPFC may be sensitive to or track evidence quality and its current relationship to goals, outcomes, and responses. When evidence is weak, as would be the case for conflict or uncertainty, DLPFC may exert a stronger braking effect on the evidence generation process to ensure a more optimal choice consistent with current goals. Regarding reward sensitivity, DLPFC has been implicated in emotion regulation (Golkar et al. 2012; Etkin et al. 2015) and studies of motivational behavior have demonstrated greater DLPFC activity during conditions of high reward magnitude (Leon and Shadlen 1999; Ballard et al. 2011) and reduced evidence generated towards reward outcomes when DLPFC activity is disrupted (Rolle et al. 2022). According to this viewpoint, DLPFC activity would be selectively sensitive to reward information and might enhance evidence generation to maximize reward.
In contrast to the PFC, the roles of the HPC and PRC in conflict processing are somewhat less clear, as these regions have been linked with a number of functions including memory encoding and retrieval, stress and anxiety, and higher order perception (Gray and McNaughton 2003; Graham et al. 2010; Squire and Wixted 2011). Within AA conflict, the HPC has been posited to be involved in two potential computational mechanisms: behavioral inhibition and memory sampling. The behavioral inhibition theory suggests that the HPC is activated by the anxiety associated with conflict, which it resolves by inhibiting potentially risky behavior (i.e. promoting avoidance) or attenuating prepotent fear responses (i.e. promoting approach) (Gray and McNaughton 2003). This view aligns HPC function more closely with the ACC and DLPFC, suggesting that it might play a regulatory role. Support for this hypothesis would be reflected in the HPC being sensitive to conflict cues and consequently engaging in regulating the evidence generation process to promote an approach or avoid choice. An alternative perspective suggests that HPC’s role lies in finding and generating the evidence itself. This is in line with the region’s role in retrieving memories to support decision-making (e.g. by supplying stimulus representations incorporating featural, contextual, and valence information) and the simulation of possible future outcomes (Shadlen and Shohamy 2016) and may be reflected in an HPC neural signature during AA processing that differs from that in ACC and DLPFC.
While the literature proposes distinct predictions of HPC involvement in AA conflict, there is far less work to form specific hypotheses for the PRC. Distinct theories based on representational models of the MTL suggest that PRC may play a similar role to that of the HPC when AA conflict results from decontextualized objects rather than spatial and/or contextual stimuli (Graham et al. 2010; Saksida and Bussey 2010; Chu et al. 2021; Dhawan et al. 2022). Similar to the posited HPC theories, when objects are used, the PRC may be involved in memory sampling or behavioral inhibition. In support of the latter, trials placing demands on conflict resolution, but not memory for and/or perceptual and motor responses to conflict stimuli, were associated with left PRC activation (Chu et al. 2021) and optogenetic inhibition of PRC, but not ventral CA3, disrupted AA conflict behavior to object stimuli, a finding that was not present when context-dependent cues were used (Dhawan et al. 2022). An alternative theory is that the PRC, rather than assuming the same role as HPC for objects, may support HPC and PFC mediated conflict resolution by providing a fine-grained perceptual representation of the stimulus. Notably, this interpretation is also congruent with the representational model, which posits that the PRC sits atop the ventral visual stream and feeds information forward to the HPC to provide information scaffolding across modalities (Saksida and Bussey 2010). Evidence for this hypothesis would be reflected in PRC activity associated with a greater demand on perceptual processing for decisions that require more evidence or regulatory control from the HPC, ACC, and DLPFC.
The reviewed evidence suggests a number of outstanding questions about the specific roles played by PFC and MTL structures. Resolving these competing theories requires more sophisticated methods for distinguishing the predictions of different theoretical accounts beyond univariate neuroimaging analyses, which subtract averaged levels of activity across broad conditions of interest. Here, we argue that examining how trial-by-trial fluctuations in the activity of these regions contribute to choice, using computational modeling within an evidence accumulation framework, presents a fruitful approach to disentangling the predictions of different theories about these regions’ functions. Evidence accumulation models assume that choices are made by accumulating noisy or stochastic samples of evidence over time until the accumulated evidence passes a subjective criterion for making a choice. The precise type of evidence will depend on the task, but generally will depend on the information provided by the stimulus itself and/or retrieval of memory representations. A prominent example of this computational framework is the drift-diffusion model (DDM) (Ratcliff and Rouder 1998), which in its canonical form consists of three distinct parameters: a drift rate v capturing the average strength of the evidence on a given choice, a threshold a capturing response caution, and a non-decision time t capturing the length of time required to generate perceptual evidence and implement a motor response (Ratcliff and Rouder 1998; Ratcliff and Smith 2004). Importantly, such models appear to explain not only the choices people make, but also choice variability and response time (RT) with remarkable accuracy across a range of decision-making paradigms (Cavanagh et al. 2011; Mack and Preston 2016; Mandali et al. 2019; Ulrichsen et al. 2020; Pedersen et al. 2021). As the DDM captures the quality of information provided by a stimulus, varying trial-by-trial levels of information signal will be present and would be expected to differ when a stimulus contains conflicting or uncertain information. The latter is particularly relevant as previous behavioral tasks elicited conflict by manipulating the probability of a threat or reward occurring (Bach et al. 2014; O’Neil et al. 2015; Schlund et al. 2016), and both PFC and MTL regions have been implicated not only in AA conflict, but also in uncertainty, probabilistic decision-making, and sensitivity to unpredictable events (Vanni-Mercier et al. 2009; Schienle et al. 2010; Catena et al. 2012). An additional benefit of the DDM, therefore, is its ability to tease apart how conflict and uncertainty differentially impact profiles of evidence accumulation and their relationships with neural activity.
The previously discussed theories and findings make distinguishable predictions about the relationships between PFC and MTL in these regions and the computational parameters of the DDM. We hypothesized that regions most strongly involved in generating evidence would increase the drift rate, with a positive relationship for conditions of approach and a negative relationship in conditions of avoidance. In contrast, regions strongly involved in regulatory control, which may encompass evaluating evidence, response caution, and inhibition should be more involved in increasing threshold as a response to weaker evidence (as signaled by the drift rate). Given the close-knit functions of perception and memory inherent to the MTL, we hypothesized that MTL regions might instead or in addition support evidence generation by engaging in detailed perceptual representation, as reflected in a positive association with non-decision time.
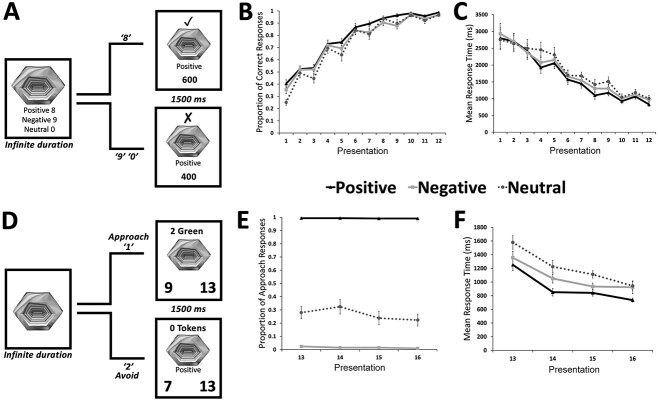
To shed light on the contributions of the HPC, PRC, ACC, and DLPFC to AA conflict processing, the present study examined the effects of conflict and outcome uncertainty on AA behavior and neural activity and used a combination of computational modeling and fMRI to elucidate the potentially distinct contributions of the MTL and PFC (Fig. 1). Participants first learned the valences of individual objects and then completed a decision task in which the previously learned objects were combined into No-Conflict (Positive & Neutral; Negative & Neutral; Neutral & Neutral) and Conflict (Positive & Negative) pairs, with each pair being associated with an outcome of either complete certainty (100% chance of receiving both outcomes) or complete uncertainty (50% chance of receiving one outcome or the other). By examining the relationship between DDM derived parameters and trial-by-trial activity in the MTL and PFC, we aimed to resolve the distinct computational roles of these brain regions to AA conflict processing.
Fig. 1.
A) Examples of positive, negative, and neutral object stimuli (actual stimuli were presented in color). B) Outline of study procedure, which included: (I) a calibration task in which participants placed a bid to purchase the right to avoid eating an aversive food mixture; (II) learn phase valence learning, in which participants learned the valences of individual objects. Participants were presented with the object and had to indicate whether the object was Positive (key press “8”), Negative (key press “9”), or Neutral (key press “0”). They were provided feedback on whether their response was correct or incorrect; (III) learn phase approach-avoidance responding, in which participants made approach-avoidance responses to the newly learned objects; (IV) decision phase, in which participants made approach-avoidance responses to pairs of objects; and (V) outcome, in which a decision phase trial was randomly drawn to determine each participant’s final outcome. G = green token; R = red token.
Methods
Participants
Twenty-four young adults (13 female, mean age = 24.67; SD = 3.73) from the University of Toronto student population completed the study, although data from three participants were excluded due to excessive motion during fMRI scanning. This resulted in a final sample of 21 participants (12 female, mean age = 24.67; SD = 3.99). All participants self-reported as being neurologically and psychiatrically healthy, with normal or corrected vision. Participants were recruited via email invitation and were screened for MRI contraindications as well as their willingness to eat an unpleasant food mixture (described below). Each participant gave written informed consent and was compensated $20/hour for their time, with the additional opportunity of winning up to $30 in bonus money (see section Behavioral task). This study was approved by the University of Toronto Research Ethics Board (Protocol #00036057).
Behavioral task
All experimental tasks were programmed using Eprime 2.0 (Psychology Software Tools, https://pstnet.com). Prior to scanning, participants completed a reward and punishment calibration, a learn phase, and a practice decision phase, with the latter two administered on a 12.5″ screen laptop computer. The final decision phase took place during fMRI data acquisition with stimuli being projected on a screen behind the MRI scanner bore and viewable via a mirror on the MRI head coil, and participants indicating their responses by using a button box in their right hand (see Fig. 1 for example stimuli and experiment timeline).
Participants were instructed that they would have the opportunity to obtain either a reward (i.e. cash prize of up to $30), punishment (i.e. 4 tablespoons of an unpleasant mixture consisting of fish sauce, mayonnaise, whole wheat flour, green food coloring, and a wet prune), or nothing (i.e. neither reward nor punishment) at the end of the study. Their outcome was determined by a token system employed in the learn and decision phases. There were three token types: green, red (both relevant to the learn and decision phases), and white (relevant to the decision phase only). Participants were instructed that tokens were collected independently of each other (i.e. different token types could be collected simultaneously) and could not be lost, only gained. At the end of the decision phase, a participant’s count of green, red, and white tokens was associated with their likelihood of obtaining the reward, punishment, or neither, respectively.
Reward and punishment calibration
For each subject, the magnitude of the reward was adjusted to the fixed punishment to ensure that each participant played the decision phase for a potential reward or punishment that they perceived to be of equal value. To achieve this, a variant of the Becker-DeGroot-Marschak (BDM) method was used, which determines a person’s true value for an item (Becker et al. 1964; Plassmann et al. 2010).
Participants were first informed that the calibration task was unrelated to the remainder of the tasks they would complete during the experimental session; rather, they were instructed that the purpose of this task was to collect food preference information for an entirely different study. Following the BDM procedure outlined in Plassmann et al. (2010), participants were told that they would be given $30, in increments of 25 cents, of “spending money.” Using this spending money, they could place a bid to purchase the right to avoid eating an aversive food at the end of the experiment, which was 4 spoons of the punishment mixture outlined above. Next, participants tasted the mixture and bid between $0 and 30, in increments of 25 cents, their willingness to pay (WTP), defined as the exact amount the participant would pay to avoid eating 4 spoons of the punishment mixture. Participants were incentivized to bid their true value by the rules of the BDM auction, which were made clear to them before the fixed punishment was revealed and their bid was placed. The rules were as follows: their bid would be compared to a selling price, which would be a randomly generated whole number (RGN) between 0 and 30. If their bid was greater than or equal to the RGN the subject was told they would pay a price equal to the RGN for the right to avoid eating the punishment. In contrast, if the bid was less than the RGN, the subject would have to eat all 4 spoons of the mixture, but would not have to pay anything (i.e. they keep all $30 of their spending money). Participants were discouraged from bidding less than their WTP because the price was determined by the RGN and thus, a lower bid would not affect the price they would pay. There was also no incentive to bid greater than their WTP since in doing so, the participant would end up paying more than they would have (i.e. keep less money), had they bid their WTP to avoid eating the mixture. In this way, the BDM auction elicited the participant’s true monetary value of the punishment. Once the bid for the 4 spoons was placed, participants were told that the RGN draw that determined their payout for the punishment would be completed at the end of the study when they were paid their base pay for participation. Unbeknownst to participants, however, this RGN draw and payout was never completed. Rather, their exact bid became their potential reward and 4 spoons of the mixture became their potential punishment during the decision phase. This information was revealed to them prior to explaining the decision phase rules.
Learn phase
The purpose of the learn phase was for participants to learn, through trial and error, the valence (positive, negative, or neutral) of 48 colored novel objects, each 565 × 565 pixels in size (stimuli from Yeung et al. 2017 and overlapping with those used in Chu et al. 2021). There were 16 possible objects of each type. The object valences were counterbalanced across participants so that the objects that were associated with each valence type were different for each third of the participants.
Participants saw all 48 objects 16 times across 4 learning runs. To facilitate learning, the 48 objects were divided into 8 subsets of 6 objects each (2 from each valence type), with each object being presented twice within each subset (i.e. 12 presentations per subset). Each of the 8 subsets was presented twice per learning run, leading to a total of 192 trials per learning run (4 repetitions per object). Individual object presentation order within each subset and subset order within run were pseudorandomized across participants.
There were two different types of learning runs: (i) valence learning (first 3 learning runs), in which participants were required to learn to identify the valence of each object; and (ii) AA responding (final learning run), which was designed to familiarize participants with the AA responses and the token system used in the decision phase. During the Valence Learning runs, an object was displayed in the center of the screen and the participant was required to indicate whether the object was positive (keyboard press of “8”), negative (keyboard press of “9”), or neutral (keyboard press of “0”) (Fig. 2A). Correct responses to any object, irrespective of valence type, resulted in a gain of 100 points. Incorrect responses to any object resulted in a loss of 100 points. Upon making their response, participants were presented with a feedback screen for 1.5 seconds indicating whether their response was correct as well as their total points. To facilitate learning, the object on the current trial and its associated valence were presented regardless of response accuracy. Participants were instructed to gain as many points as possible and were incentivized by a potential additional reward of $5 if they achieved 90% accuracy (i.e. a total of 15,200 points) on any of the three runs.
Fig. 2.
A) A sample trial of the valence learning task. Participants were presented with an object on the screen and indicated whether the object was positive, negative, or neutral. They received feedback indicating whether they had correctly or incorrectly identified the object’s valence and received point-based feedback to facilitate trial and error learning. B and C) Behavioral data from the valence learning task, with correct responses indicating correct identification of the object’s valence (± SE). D) A sample trial of the learn phase AA response task. Participants were presented with an object on the screen and made an approach or avoid response based on their knowledge of the object’s valence. Approaching positive and negative objects, respectively, resulted in green and red token gain. Approaching neutral objects or avoiding any object resulted in no change in cumulative tokens. E and F) Behavioral data from the learn phase AA response task (± SE).
For the final AA responding learning run, an object was displayed in the center of the screen until an approach (keyboard press of “1”) or avoidance (keyboard press of “2”) response was made (Fig. 2D). Approaching a positive or negative object resulted in green or red token gain, respectively. Approaching a neutral object as well as avoidance responses to all three object types resulted in zero tokens gained or lost. After making their response, participants were presented with a feedback screen for 1.5 seconds indicating the number and type of tokens acquired on that trial (e.g. 1 Green token) as well as their current count of green and red tokens (Fig. 2D). To facilitate learning, the object on the current trial was displayed during feedback and for all neutral objects (regardless of response) and avoidance responses, the object valence appeared beneath the object. Participants were instructed to maximize their green and minimize their red token count but receiving the cash reward or unpleasant food punishment at the end of the study was contingent only on decision phase outcomes.
Decision phase
During the decision phase, the 48 learned objects were presented in pairs on each trial (two images side by side in the middle of the screen). The objects within each pair were selected in a pseudorandomized manner to create three types of conflict: conflict (a positive object paired with a negative object); no-conflict positive (a positive object with a neutral object), no-conflict negative (a negative object with a neutral object), and no-conflict neutral (two neutral objects) (Fig. 3A). Between each object pair, the cue word “AND” or “OR” was presented to indicate whether the trial was associated with a certain or uncertain outcome. Considering all possible object pairing and outcome uncertainty combinations, there were, therefore, eight distinct conditions: (i) Conflict Certain, (ii) Conflict Uncertain, (iii) No-Conflict Positive Certain, (iv) No-Conflict Negative Certain, (v) No-Conflict Neutral Certain, (vi) No-Conflict Positive Uncertain, (vii) No-Conflict Negative Uncertain and (viii) No-Conflict Neutral Uncertain (Fig. 3A). Participants were instructed to make either an approach (response box button press “1”) or avoid (response box button press “2”) response to each presented object pair. Approaching an object pair with a certain outcome resulted in the participant receiving the token values of both objects on that trial, with positive, negative, and neutral objects associated with green, red, and white tokens, respectively. In contrast, approaching an object pair with a certain outcome led to a 50% chance of obtaining either the left- or right-side object’s token value. Thus, on each trial, participants could gain either a green token only, a red token only, a white token only, green and red, green and white, red and white, or two white tokens together upon making an approach response. Avoiding any pair (regardless of the condition) or failing to respond yielded the gain of one white token (see Fig. 3A for all possible trial outcomes). To prevent trial outcome from influencing participants’ responding and to minimize any new learning, participants did not receive feedback on the tokens they received after their response to each pair. The current paradigm, therefore, placed a primary emphasis on approach-avoidance conflict pertaining to outcome (i.e. probability of reward vs. punishment) (Bach et al. 2014; O’Neil et al. 2015; Korn et al. 2017; Chu et al. 2021). This contrasts to motivational conflict that can arise in relation to underlying decision-making variables, such as a conflict between heuristic variables (short-sighted variables that capture in-the-moment factors, i.e. current motivational state, immediate reward/punishment probabilities) and optimal policy (a longer term approach that fully considers multiple factors and all potential outcomes over an extended period of time) (Korn and Bach 2018, 2019).
Fig. 3.
A) Example decision phase stimuli and outcomes. Objects were combined into eight trial types based on four conflict conditions (No-Conflict Positive, No-Conflict Negative, No-Conflict Neutral, and Conflict) and two certainty conditions (Certain outcome, denoted by “AND,” and Uncertain outcome, denoted by “OR”). B) Decision phase trial structure; C and D) decision phase behavioral performance as measured by proportion of approach responses and RT (± SE); E–G) decision phase hDDM results. Posterior distributions of means for drift rate, threshold, and non-decision time. Blue represents No-Conflict Positive, red represents No-Conflict Negative, and green represents Conflict, with dark and light shades representing certainty and uncertainty, respectively.
It is important to note that neutral objects were included in the current paradigm so that outcome uncertainty did not result in differential outcomes for No-Conflict Positive and No-Conflict Negative pairs (e.g. in the absence of neutral objects, approaching a “positive AND positive” pair would lead to a gain of 2 green tokens whereas approaching a “positive OR positive” pair would only lead to the gain of a single green token). Furthermore, the No-Conflict Neutral Certain and Uncertain conditions were implemented to ensure that all objects were presented an equal number of times during the decision phase, thereby preventing any potential differences in incidental mnemonic processing. Given that these neutral pairs lacked a valence and were not associated with a motivationally driven, goal-directed outcome, they were not of interest and excluded from subsequent decision phase analyses. Notably, including these trials in the decision phase behavioral and univariate neuroimaging results led to a highly similar pattern of findings and were more significant likely due to the increase in statistical power when neutral trials were grouped with positive and negative ones. Excluding the neutral conditions improved model fit for the best fitting hDDM specification (Deviance Information Criterion [DIC] including neutral = 21692.89, DIC excluding neutral = 16844.21; see “Hierarchical drift diffusion model (hDDM) Fitting” section for details regarding model fit).
As part of the decision phase instructions, participants were told how their final reward or punishment (to be received after scanning) would be decided. To determine a participant’s final outcome, one trial was randomly selected out of all trials completed in the decision phase (Teoh et al. 2020). If, on that trial, the participant obtained only a green token or green and white tokens, they received their predetermined cash reward. In contrast, if the participant obtained only a red token or red and white tokens on that trial, they were required to consume the food punishment. If a trial was chosen on which the participant obtained both green and red tokens, they received both the monetary reward and the unpleasant food mixture. Finally, if the participant only received a white token on the selected trial, they received neither the reward nor punishment.
Participants completed 600 trials with 60 trials in each of the no-conflict conditions and 120 trials in each of the conflict conditions. Each trial began with presentation of the paired images (3,000 ms), followed by a jittered ITI of mean 3,000 ms (Fig. 3B). Participants completed 6 runs of the decision phase with 100 trials per run. Trial order was pseudorandomized within each run and run order was varied across subjects.
Practice phase
Prior to entering the fMRI scanner, participants completed a short practice version of the Decision task, which contained 20 trials covering all eight decision task trial types outlined above with 2 and 4 trials for No-Conflict and Conflict, respectively. Practice phase pairs were not repeated in the decision phase to prevent practice effects and differences in novelty across stimulus pairs. To emphasize to participants that responses on trials during the Decision task led to actual token outcomes, trial-specific feedback was implemented during the practice phase, with participants being presented with a 1.5 second feedback screen showing the type of token/s gained following each stimulus presentation. As well, following the practice phase, the random trial draw for the final outcome was simulated using the 20 practice trials for demonstrative purposes. Finally, although participants were not explicitly instructed what constituted a correct or desired response for each trial type, they were told that their ability to win additional cash was limited if they did not take risks on the conflict trials.
Neuroimaging data acquisition
All participants underwent magnetic resonance imaging at the Toronto Neuroimaging (ToNI) Facility at the University of Toronto St. George Campus. All data were acquired on a Siemens 3 T MAGNETOM Prisma scanner. Six runs of echo planar imaging (EPI) data were collected for each subject, using a multi-band echo pulse sequence. Each run comprised of 315 volumes acquired parallel to the HPC to prioritize temporal lobe signal (69 slices; voxel size = 2.0 × 2.0 × 2.0 mm; repetition time (TR) = 2,000 ms; echo time (TE) = 30 ms; flip angle (FA) = 90; field of view (FOV) = 220 mm; generalized autocalibrating partially parallel acquisitions (GRAPPA) factor = 2). Participants also received a standard high-resolution T1-weighted anatomical scan (160 slices; voxel size = 1.0 × 1.0 × 1.0 mm; TR = 2,000 ms; TE = 2.4 ms; FA = 9; FOV = 256 mm; GRAPPA factor = 2) and a high-resolution T2-weighted anatomical scan (42 slices; voxel size = 0.4 × 0.4 × 0.4 mm; TR = 4.13; TE = 66 ms; FA = 165; FOV = 220 mm; GRAPPA factor = 2). Fieldmaps using dual echo GRE sequences were acquired to address scanner magnetic field inhomogeneity artifacts (69 slices; voxel size = 1.5 × 1.5 × 1.5 mm; TR = 646 ms; TE1 = 4.91 ms; TE2 = 7.38 ms; FA = 45; FOV = 220 mm). Finally, one run of resting state data, comprised of 180 volumes, was collected for each subject (69 slices; voxel size = 1.5 × 1.5 × 1.5 mm; TR = 2,000 ms; TE = 30 ms; FA = 70; FOV = 20 mm). The T2 and resting state data were collected as part of a separate study and are not considered in the current manuscript.
Neuroimaging data preprocessing
Each participant’s task-based functional data were preprocessed prior to statistical analysis (FEAT version 6.0, FMRIB Software Library, https://fsl.fmrib.ox.ac.uk/fsl/) using the following steps: (i) removal of the first four volumes due to signal instability; (ii) brain extraction on anatomical and field map magnitude images (Brain Extraction Tool); (iii) motion correction using MCFLIRT; (iv) B0 unwarping to remove inhomogeneities in the scanner magnetic field; (v) application of a 50-s high-pass filter to remove low-frequency signal; (vi) spatial smoothing using a Gaussian kernel with a 4-mm full-width half-maxima; (vii) co-registration to the individual participant’s anatomical scan using boundary based registration (Greve and Fischl 2009); (viii) normalization to the MNI-152 2 mm standard space template using FSL’s linear registration tool (FLIRT) (Jenkinson et al. 2002); and (ix) MELODIC independent components analysis (ICA) to identify motion and artifact components.
Behavioral statistical analyses
Univariate hypothesis significance testing
Behavioral data were analyzed using RStudio (https://posit.co/products/open-source/rstudio/). In the learning phase, behavioral data were analyzed separately for the first three runs (Valence Learning) and the final run (AA Responding). For the first three runs, a repeated measures ANOVA was conducted on the proportion of correct responses and the mean RT with two within-subject factors of presentation (1–12) and valence (positive, negative, neutral). For the final run, a repeated measures ANOVA was conducted on the proportion of approach responses and the mean RT with two within-subject factors of presentation (1–4) and valence (positive, negative, neutral). These measures were behavioral indicators of approach and avoidance tendencies and have been used in previous human AA paradigms (Bach et al. 2014; O’Neil et al. 2015). In the decision phase, two repeated measures ANOVAs were conducted for the proportion of approach responses and mean RT with three within-subject factors of condition (No-Conflict Positive, No-Conflict Negative, Conflict), certainty (Uncertain, Certain) and run (1–6). For statistical robustness and to take into account the possibility that binomially distributed data may violate the assumptions necessary for certain statistical tests (Jaeger 2008), we determined the significance for all ANOVAs and post hoc tests using a permutation-based approach, in keeping with our previous work (Chu et al. 2021). Parametric statistical values (i.e. F/t values) were first calculated and then re-calculated for 10,000 different samples created by label shuffling within participant. The original statistical value was then compared to the permuted (null) distribution, and the probability of obtaining a permuted value greater than the original value was considered the significance of the original statistical test (all t-tests were two-tailed by using absolute t-values). 95% confidence intervals (CI) of all effect sizes (e.g. Cohen’s d, η2) were derived from the permuted data. All p-values for post hoc tests were corrected using the Holm-Bonferroni procedure.
hDDM fitting
Our behavioral modeling goal was to identify the underlying decision-making process by estimating the most parsimonious set of parameters that would account for patterns of choice and RT. To this end, we used the hDDM package (Wiecki et al. 2013) to estimate the influence of neural activity on different model parameters. The drift diffusion model (DDM) (Ratcliff and Rouder 1998) models decisions in two-choice tasks where each choice (e.g. approach or avoid) is represented as an upper and lower boundary. The model assumes that a drift process occurs whereby evidence is accumulated for one of the two choices until the choice boundary is crossed and an approach or avoid response is executed. In the present task, approach and avoid decisions during the decision phase relied on the reinstatement of memory representations (e.g. valence information) and the consideration of potential future outcomes (e.g. possibility of reward vs. punishment) in the context of motivational goal states. As such, we considered sampling from memory to be a key component of evidence accumulation. Importantly, given that there is noise in the drift process, the choice and time to respond will differ between trials within- and between-conditions. The following parameters output by the DDM characterize key components of the decision-making process: (i) drift rate, v, refers to the speed at which evidence is accumulated and is impacted by the amount of evidence provided by the stimulus; (ii) threshold, a, refers to the distance between the two boundaries and impacts the amount of evidence that must be accumulated prior to response. In trials where there is no correct response (e.g. conflict), larger thresholds are associated with more stable but slower choices as noise impacts the drift process less (Pedersen et al. 2021); (iii) non-decision time, t, accounts for the time required for processes that precede and follow evidence accumulation (i.e. perception, motor planning, and execution).
The hDDM package uses Markov Chain Monte Carlo (MCMC) simulations and hierarchical Bayesian parameter estimation to estimate the posterior distributions of the three hDDM parameters. Specifically, the hDDM assumes that participants within a group are similar, but not identical to each other; therefore, the hDDM estimates the above parameters in a hierarchical fashion whereby individual subject parameter estimates are constrained by the group-level distributions from which they belong (Shiffrin et al. 2008; Wiecki et al. 2013). For each hDDM specification, 10,000 samples were generated from the posterior distributions with a burn-in period of 2,000 samples, thinning set to 2, and use of informative priors (i.e. based on parameters from 23 studies that reported the best-fitting DDM parameters for multiple cognitive tasks) (Matzke and Wagenmakers 2009; Wiecki et al. 2013). For each model, the Gelman–Rubin statistic was used to assess model convergence (Gelman and Rubin 1992). This statistic compares the variance within- and between-chains in the same model. 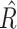 = 1 reflects perfect convergence and
= 1 reflects perfect convergence and  ≥ 1.02 indicative of poor chain convergence (Gelman and Rubin 1992). The
≥ 1.02 indicative of poor chain convergence (Gelman and Rubin 1992). The 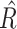 statistic was computed for all model parameters for the winning models, and the resulting statistics indicated good chain convergence.
statistic was computed for all model parameters for the winning models, and the resulting statistics indicated good chain convergence.
Several models with unique hDDM specifications were fit to the data, all using a software package available in Python (Wiecki et al. 2013). The three parameters of interest were estimated from DDM likelihood functions that translate choice (i.e. approach or avoid) and RT distributions that varied by the six conditions of interest (i.e. No-Conflict Positive Certain, No-Conflict Positive Uncertain, No-Conflict Negative Certain, No-Conflict Negative Uncertain, Conflict Certain, Conflict Uncertain). The first model (M1) allowed all parameters (v, a, t) to vary across subject but not within subjects, with no parameters varying by task condition. The second, third, and fourth models (M2–4) allowed a single parameter to vary by task condition, with the remaining two parameters permitted to vary across but not within subjects. The fifth, sixth, and seventh models (M5–7) permitted a combination of two parameters to vary by task condition, with the remaining parameter varying across but not within subject. The eighth model (M8) permitted all three parameters to vary by task condition; in other words, each condition could be weighted differently and individuals were presumed to use different drift rates, thresholds, and non-decision times according to condition. To compare the goodness of fit of each of the above DDM specifications, visual inspection and a Bayesian measure of model fit (Spiegelhalter et al. 2002), the DIC was used. The DIC is defined as a classical estimate of fit plus twice the effective number of parameters whereby a lower DIC value represents a better model fit (Spiegelhalter et al. 2002). Examination of the DIC indicated that the best fitting model was M8 (Supplementary Tables 1 and 2). Visual inspection of the posterior predicted likelihood plotted on top of each individual subject’s RT distribution indicated good fit of M8. With respect to statistical inference, Bayesian estimation allows for direct comparison of the posterior distributions generated from repeated samples. Specifically, the coefficients can be compared to the null hypothesis (B = 0) or one regression coefficient can be compared to another by determining the percentage of the 10,000 samples > 0 or the percentage of the difference between the 10,000 samples > 0, respectively. Within the hDDM results, the notation P refers to the probability of overlap between the posterior distributions for each condition (e.g. P = 0 here indicates no overlap between the distributions of two conditions as determined by 10,000 samples) and a value of P < 0.05 was similarly used to indicate significance.
Neuroimaging data analysis
Neuroimaging univariate analysis
Preprocessed functional runs were submitted to an event-related general linear model (GLM) that modeled condition and response type. The paradigm-related explanatory variables (EVs) in the model were: (i) No-Conflict Positive Certain Approach; (ii) No-Conflict Positive Uncertain Approach; (iii) No-Conflict Negative Certain Avoid; (iv) No-Conflict Negative Uncertain Avoid; (v) No-Conflict Neutral Certain; (vi) No-Conflict Neutral Uncertain; (vii): Conflict Certain Approach; (viii) Conflict Certain Avoid; (ix) Conflict Uncertain Approach; (x) Conflict Uncertain Avoid; and (xi) Errors, which included No-Conflict Positive and Negative trials with non-optimal responses and all trials for which a participant response was not recorded. Our goals for modeling response type in the GLM were twofold: first, this allowed us to remove the effects of incorrect responses to No-Conflict trials (e.g. when a participant accidentally avoided on a No-Conflict Positive trial). Second, this design best allowed us to identify potential differences in activity between approach and avoid responses. Additional confound EVs created by FSL motion outliers were included in the GLM to account for sudden movements during scanning. Each EV was convolved with a double-gamma hemodynamic response function and a 50-s high-pass temporal filter was applied. Parameter estimate (PE) images were created for all EVs and contrasts. Our contrasts were designed to examine the main effect of conflict (Conflict vs. No-Conflict), certainty ([Certain vs. Uncertain], and an interaction between the two (([Conflict Certain vs. Conflict Uncertain] vs. [No-Conflict Certain vs. No-Conflict Uncertain]). Within our main effect of conflict, we also examined whether response type influenced activation by examining approach and avoid to conflict and no-conflict separately (Conflict Approach vs. No-Conflict Positive; Conflict Avoid vs. No-Conflict Negative).
For each participant, all six decision phase runs were combined in a second-level fixed effects analysis. A subsequent group-level mixed effects analysis that incorporated within-session fixed effects and between-session/subject random effects variance was run (Woolrich et al. 2009). Due to our a priori hypotheses, we adopted a region of interest (ROI) approach focused on the MTL. A MTL mask was created using the Harvard-Oxford Subcortical and Cortical probabilistic atlases, binarized, and thresholded at 50% and included the bilateral hippocampus, and anterior and posterior parahippocampal gyrus. Significant activity was determined for each contrast of interest using a non-parametric approach as implemented by FSL’s randomize tool (10,000 permutations) (Winkler et al. 2014) with threshold-free cluster enhancement (TFCE) (Smith and Nichols 2009), and a significance threshold of p < 0.05, small volume correction (svc) applied. Whole brain activity was examined with p < 0.05 corrected.
hDDM specifications with fMRI data
We examined the relationship between trial-by-trial measures of brain activity (i.e. percent signal change in BOLD activity) to variations in drift rate, threshold, and non-decision time (Cavanagh et al. 2011; Wiecki et al. 2013). Given that No-Conflict Positive and No-Conflict Negative did not differ by uncertainty in the behavioral model, we examined whether per trial BOLD activity interacted with four conditions by collapsing the No-Conflict conditions across uncertainty (i.e. No-Conflict Positive, No-Conflict Negative, Conflict Certain, Conflict Uncertain). Our four a priori ROIs were the PRC, aHPC, ACC, and DLPFC. Within the MTL, we created a mask using the extent of activation thresholded at 0.95 in the left PRC (associated with the main effect contrast Conflict—No-Conflict) and left aHPC (associated with the main effect contrast No-Conflict—Conflict). The left, rather than right, aHPC was selected since preliminary analyses revealed poorer model fit (as indicated by the DIC) for the latter compared to the former. For the PFC, given the widespread activation across the PFC for the Conflict—No-Conflict contrast, we first identified the 50 most significant local maxima across both hemispheres. From these voxels, we then selected the two most significant voxels that corresponded to the ACC (−2, 22, 34) and DLPFC (−40, 28, 32) and extracted activity from an 8-mm sphere created around these voxels (Supplementary Fig. 1). The sphere size was chosen to ensure an appropriate number of voxels were sampled while at the same time ensuring that neighboring regions were not included. Trial parameter estimates were first created using an iterative least-squares single GLM approach in Feat (Mumford et al. 2012). On every trial, for all participants, a GLM was implemented in which one regressor modeled activity for that trial (i.e. 3,000 ms pair presentation) and a second regressor modeled activity for all other trials of stimulus pair presentation. Each predictor and its temporal derivative were convolved with a double-gamma hemodynamic response function and FILM prewhitening was applied, which included temporal autocorrelation correction. This resulted in 600 parameter estimate maps, for each run, for each subject. Percent signal change was then extracted from the aforementioned ROIs in these maps using the Featquery tool.
A strength of the hDDM is its ability to estimate the effects of activity in several regions on all three parameters simultaneously in one model, thereby not requiring multiple models and subsequent corrections for multiple comparisons. To this end, we ran a single model which assumed that both drift rate and threshold would vary trial-by-trial as a function of activity in each of our four ROIs, and non-decision time would vary according to the two MTL ROIs, with coefficients estimated separately for each condition. In other words, v, a, and t were described by a linear specification of ROI percent signal change, and this effect was assumed to interact with condition. Coefficients, or the weight of ROI activity on each parameter of interest, were estimated separately for the trials in each of the four conditions. To ensure the effect of ROI activity was estimated independently of the effects of v, a, and t on condition, each model permitted these three parameters to vary by condition to control for these effects. In each regression model, the condition Conflict Certain was set as the intercept and all other conditions were expressed relative to the intercept.
V t ~ Conditiont * PRC + Conditiont * aHPC + Conditiont * DLPFC + Conditiont * ACC.
A t ~ Conditiont * PRC + Conditiont * aHPC + Conditiont * DLPFC + Conditiont * ACC.
T t ~ Conditiont * PRC + Conditiont * aHPC + Conditiont.
All estimation and statistical inference followed the same procedures outlined in the previous section. Notably, this model significantly improved DIC fit compared to the baseline behavioral model and demonstrated the greatest DIC change relative to other models (ΔDIC = decrease of 361.56). To demonstrate that the selected ROIs did indeed improve explanatory power in the hDDM model, we ran the same model but using per trial activity from a voxel within the left superior parietal lobe (BA7; −10, −46, 56), a region that was not significantly activated in the Conflict > No-Conflict contrast. The DIC change for this model compared to the baseline behavioral model was an increase of 8.92 (i.e. a worse fit).
Results
Behavioral results
Calibration task
Participants placed a mean bid of $16.06 (SD = 8.5) with no significant relationships between bid amount and the proportion of approach responses made to Conflict Certain (r = 0.097, p = 0.68) and Conflict Uncertain (r = 0.26, p = 0.26) condition trials. Thus, subsequent Decision Task findings cannot be explained by perceived imbalances in reward and punishment.
Learn phase
Valence Learning (Fig. 2A–C, Table 1). Both object valence and the number of presentations had a significant impact on the proportion of correct responses (both p < 0.01) (Fig. 2C, Table 1). There was also a significant interaction between these two factors. Post hoc testing at every presentation to examine this interaction (i.e. 12 separate post hoc tests) indicated a significant effect of valence at the first, seventh, and ninth presentations of the stimuli (all p < 0.05), but not at any other presentations (all p > 0.27). Notably, participants demonstrated over 96.8% accuracy for all three object valences by the 12th presentation, which suggests that, through trial and error, they were able to successfully learn the object valences over time. RT data tell a similar story, with participants responding increasingly quickly to the objects across successive presentations (Fig. 2D, Table 1). We observed significant effects of valence and presentation (both p ≤ 0.03), as well as a significant interaction effect (p = 0.01) that was driven by a significant effect of valence at presentation 4 only (p < 0.01), with participants being slower to respond to neutral compared to positive objects (p = 0.02).
Table 1.
Learn phase valence learning behavioral results. All post hoc p-values are Holm-Bonferroni corrected and significant values have been highlighted in bold.
| Proportion of correct responses | ||||
| Omnibus 2 × 2 ANOVA | ||||
| Effect | DOF | F | p | ηG2 [95% CIs] |
| Valence (positive vs. negative vs. neutral) | 2, 40 | 7.29 | <0.01 | 0.03 [0.000069, 0.011] |
| Presentation (1–12) | 11, 220 | 158.80 | <0.001 | 0.71 [0.0055, 0.031] |
| Valence × presentation | 22, 440 | 2.13 | <0.01 | 0.02 [0.015, 0.051] |
| Post hoc one-way ANOVA at each presentation | ||||
| Effect | DOF | F | p | ηG2 [95% CIs] |
| Valence at presentations 1, 7, 9 | 2, 40 | all ≥6.50 | ≤0.046 | ≥0.06 [0.00031 to 0.038, 0.00086 to 0.125] |
| Valence at all other presentations | 2, 40 | all ≤ 4.51 | all ≥0.273 | ≤0.05 [0.00038 to 0.045, 0.00035 to 0.038] |
| Post hoc pairwise comparisons within presentations 1, 7, 9 | ||||
| Pair | DOF | t | p | d [95% CIs] |
| Positive vs. negative at presentations 1 and 7 | 20 | all ≤3.11 | all ≥0.50 | all ≤0.52 [−0.61 to 0.61, −0.63 to 0.63] |
| Positive vs. negative at presentation 9 | 20 | 3.75 | 0.016 | 0.82 [−0.61, 0.61] |
| Positive vs. neutral at presentations 1 and 7 | 20 | all ≥3.07 | all ≤ 0.012 | all ≥0.56 [−0.6 to 0.6, −063, 0.63] |
| Positive vs. neutral at presentations 9 | 20 | 2.07 | 0.71 | 0.55 [−0.61, 0.61] |
| Negative vs. neutral at presentations 1, 7, and 9 | 20 | all ≤ 2.69 | all ≥0.21 | all ≤0.84 [−0.61 to 0.61, −0.63 to 0.63] |
| RT | ||||
| Omnibus 2 × 2 ANOVA | ||||
| Effect | DOF | F | p | ηG2 [95% CIs] |
| Valence (positive vs. negative vs. neutral) | 2, 40 | 3.52 | 0.030 | 0.001 [0.000081, 0.01] |
| Presentations (1–12) | 11, 220 | 34.87 | <0.001 | 0.39 [0.0055, 0.03] |
| Valence × presentation | 22, 440 | 1.93 | <0.01 | 0.008 [0.016, 0.05] |
| Post hoc one-way ANOVA at each presentation | ||||
| Effect | DOF | F | p | ηG2 [95% CIs] |
| Valence at presentation 4 | 2, 40 | 10.24 | ≤0.001 | 0.06 [0.00022, 0.028] |
| Valence at all other presentations | 2, 40 | all ≤ 5.79 | all ≥0.07 | all ≤0.05 [0.00031 to 0.034, 0.00023 to 0.029] |
| Post hoc pairwise comparisons within presentation 4 | ||||
| Pair | DOF | t | p | d [95% CIs] |
| Positive vs. negative | 20 | 1.38 | 0.10 | 0.18 [−0.61, 0.63] |
| Positive vs. neutral | 20 | 3.85 | 0.02 | 0.57 [−0.61, 0.64] |
| Negative vs. neutral | 20 | 3.33 | 0.07 | 0.39 [−0.61, 0.64] |
AA Responding (Fig. 2D–F, Table 2). Both valence and presentation had a significant effect on the proportion of approach responses (both p < 0.02) and there was also a significant interaction effect between these two factors (p = 0.04), with significant differences between the three valences at all four presentations (all p < 0.01) (Fig. 2E, Table 2). Indicative of successful valence learning and transfer of this learning to an AA framework, participants approached positive objects (Proportion of Approach M = 0.99, SD = 0.014) and avoided negative objects (Proportion of Approach M = 0.016, SD = 0.023) the vast majority of the time. Responses to neutral objects were more mixed, although participants tended to avoid these stimuli (Proportion of Approach M = 0.27, SD = 0.20). Valence and presentation also had a significant impact on RTs (both p < 0.01), although there was a non-significant interaction effect (Fig. 2F, Table 2). Participants responded increasingly quickly across presentations and mean RT was fastest for positive (M = 920.19, SD = 213.64) followed by negative (M = 1066.28, SD = 306.82) and finally neutral objects (M = 1214.65, SD = 272.55).
Table 2.
Learn phase approach-avoidance responding behavioral results. Stimuli were presented 4 times each (presentations 13–16 following valence learning phase). All post hoc p-values are Holm-Bonferroni corrected and significant values have been highlighted in bold.
| Proportion of approach responses | ||||
|---|---|---|---|---|
| Omnibus 2 × 2 ANOVA | ||||
| Effect | DOF | F | p | ηG2 [95% CIs] |
| Valence (positive vs. negative vs. neutral) | 2, 40 | 402.50 | <0.001 | 0.91 [0.00023, 0.033] |
| Presentation (13–16) | 3, 60 | 3.52 | 0.010 | 0.01 [0.00092, 0.042] |
| Valence × presentation | 6, 120 | 2.24 | 0.040 | 0.02 [0.0054, 0.063] |
| Post hoc one-way ANOVA at each presentation | ||||
| Effect | DOF | F | p | ηG2 [95% CIs] |
| Valence at presentations 13–16 | 2, 40 | all ≥241.95 | all < 0.001 | all ≥0.89 [0.0012 to 0.17, 0.0012 to 0.17] |
| Post hoc pairwise comparisons within each presentation | ||||
| Pair | DOF | t | p | d [95% CIs] |
| Positive vs. negative | 20 | all ≥94.90 | all <0.001 | all ≥27.48 [−0.67 to 0.67, −0.68 to 0.68] |
| Positive vs. neutral | 20 | all ≥12.43 | all <0.001 | all ≥3.76 [−0.62 to 0.62, −0.63, 0.64] |
| Negative vs. neutral | 20 | all ≥4.29 | all ≤ 0.001 | all ≥1.34 [−0.64 to 0.64, −0.63, 0.6] |
| RT | ||||
| Omnibus 2 × 2 ANOVA | ||||
| Effect | DOF | F | p | ηG2 [95% CIs] |
| Valence (positive vs. negative vs. neutral) | 2, 40 | 26.02 | <0.001 | 0.12 [0.00026, 0.033] |
| Presentation (13–16) | 3, 60 | 45.10 | <0.001 | 0.28 [0.001, 0.041] |
| Valence × presentation | 6, 120 | 1.43 | 0.21 | 0.01 [0.0055, 0.063] |
| Post hoc pairwise comparisons between valences | ||||
| Pair | DOF | t | p | d [95% CIs] |
| Positive vs. negative | 20 | 4.56 | ≤ 0.001 | 0.55 [−0.61, 0.6] |
| Positive vs. neutral | 20 | 6.73 | <0.001 | 1.20 [−0.63, 0.62] |
| Negative vs. neutral | 20 | 3.27 | 0.010 | 0.51 [−0.63, 0.62] |
| Post hoc pairwise comparisons between presentations | ||||
| Pair | DOF | t | p | d [95% CIs] |
| 13 vs. 14/15/16 | 20 | all ≥6.18 | all <0.001 | all ≥1.06 [−0.62 to −0.62, 0.61 to 0.62] |
| 14 vs. 15 | 20 | 1.74 | 0.10 | 0.33 [−0.62, 0.62] |
| 14 vs. 16 | 20 | 4.75 | <0.001 | 0.71 [−0.63, 0.63] |
| 15 vs. 16 | 29 | 3.18 | 0.011 | 0.45 [−0.63, 0.61] |
Decision phase
Choice and RTs (Fig. 3C–D, Table 3). The proportion of approach data suggest that participants sought to maximize reward and minimize punishment during the decision phase and that their responses were driven primarily by condition, with a smaller effect of outcome uncertainty (Fig. 3C, Table 3). Both condition and certainty (both p < 0.03), but not run (P = 0.15) had a significant effect on the proportion of approach responses. There was also a significant interaction between condition and certainty (P < 0.01), with no other interactions reaching significance (all p ≥ 0.18). Although there was no significant effect of certainty for No-Conflict Positive and No-Conflict Negative trials (both p ≥ 0.08), there was a significant difference between Conflict Uncertain and Conflict Certain trials whereby participants approached more frequently on the former (M = 0.617, SD = 0.24) compared to the latter (M = 0.372, SD = 0.287).
Table 3.
Decision task behavioral results. All post hoc p-values are Holm-Bonferroni corrected and significant values have been highlighted in bold.
| Proportion of approach responses | ||||
|---|---|---|---|---|
| Omnibus 2 × 2 ANOVA | ||||
| Effect | DOF | F | p | ηG2 [95% CIs] |
| Condition (positive vs. negative vs. conflict) | 2, 36 | 279.90 | <0.001 | 0.8 [0.000072, 0.011] |
| Certainty (certain vs. uncertain) | 1, 18 | 6.07 | 0.020 | 0.04 [0.0000012, 0.0076] |
| Run | 5, 90 | 1.66 | 0.15 | 0.004 [0.00124, 0.019] |
| Condition × certainty | 2, 36 | 10.54 | 0.0003 | 0.097 [0.000072, 0.011] |
| Condition × run | 10, 180 | 1.41 | 0.18 | 0.0087 [0.0049, 0.031] |
| Certainty × run | 5, 90 | 0.43 | 0.82 | 0.0008 [0.0013, 0.019] |
| Condition × certainty × run | 10, 180 | 0.32 | 0.98 | 0.0014 [0.005, 0.012] |
| Post hoc pairwise comparisons at each condition | ||||
| Pair | DOF | t | p | d [95% CIs] |
| No-conflict positive | 18 | 0.85 | 0.39 | 0.15 [−0.66, 0.66] |
| No-conflict negative | 18 | 2.15 | 0.09 | 0.04 [−0.65, 0.65] |
| Conflict | 18 | 2.98 | 0.02 | 0.93 [−0.63, 0.65] |
| RT | ||||
| Omnibus 2 × 2 ANOVA | ||||
| Effect | DOF | F | p | ηG2 [95% CIs] |
| Condition (positive vs. negative vs. conflict approach vs. conflict avoid) | 3, 54 | 29.40 | <0.001 | 0.23 [0.00025, 0.011] |
| Certainty (certain vs. uncertain) | 1, 18 | 0.18 | 0.69 | 0.0001 [0.0000012, 0.0061] |
| Run | 5, 90 | 16.28 | <0.001 | 0.2 [0.001, 0.015] |
| Condition × certainty | 3, 54 | 0.14 | 0.94 | 0.0004 [0.00027, 0.011] |
| Condition × run | 15, 270 | 1.64 | 0.064 | 0.018 [0.00073, 0.032] |
| Certainty × run | 5, 90 | 0.97 | 0.45 | 0.0031 [0.001, 0.015] |
| Condition × certainty × run | 15, 270 | 0.66 | 0.82 | 0.0073 [0.007, 0.032] |
| Post hoc pairwise comparisons at each condition | ||||
| Effect | DOF | t | p | d [95% CIs] |
| Positive vs. negative | 227 | 0.17 | >0.90 | 0.016 [−0.19, 0.18] |
| Positive vs. conflict approach | 227 | 11.94 | <0.001 | 0.75 [−0.19, 0.18] |
| Positive vs. conflict avoid | 227 | 12.35 | <0.001 | 0.77 [−0.19, 0.18] |
| Negative vs. conflict approach | 227 | 11.64 | <0.001 | 0.70 [−0.18, 0.18] |
| Negative vs. conflict avoid | 227 | 13.00 | <0.001 | 0.71 [−0.18, 0.18] |
| Conflict approach vs. conflict avoid | 227 | 0.32 | >0.90 | 0.018 [−0.1, 0.19] |
| Post hoc pairwise comparisons at each run | ||||
| Pair | DOF | t | p | d [95% CIs] |
| 1 vs. 2/3/4/5/6 | 151 | all ≥5.67 | all < 0.001 | all ≥0.49 [−0.23, 0.23] |
| 2 vs. 3 | 151 | 1.69 | 0.28 | 0.12 [−0.23, 0.22] |
| 2 vs. 4/5/6 | 151 | all ≥3.67 | all ≤ 0.0028 | all ≥0.27 [−0.23, 0.22 to 0.23] |
| 3 vs. 4 | 151 | 2.05 | 0.16 | 0.16 [−0.23, 0.22] |
| 3 vs. 5/6 | 151 | both ≥4.58 | both < 0.001 | both ≥0.35 [−0.23 to −0.22, 0.23] |
| 4 vs. 5 | 151 | 2.44 | 0.085 | 0.17 [−0.22, 0.23] |
| 4 vs. 6 | 151 | 4.82 | <0.001 | 0.34 [−0.22, 0.23] |
| 5 vs. 6 | 151 | 3.21 | 0.010 | 0.18 [−0.22, 0.23] |
Consistent with previous work (O’Neil et al. 2015; Chu et al. 2021) and suggestive of greater cognitive resource allocation, participants were slower to respond to conflict compared to no-conflict object pairs, irrespective of outcome uncertainty, and RTs decreased over successive runs (Fig. 3D, Table 3). There were significant effects of condition and run (both p < 0.001), as well as a trending run by condition interaction (p = 0.064), with no other effects reaching significance (all p > 0.45). Conflict and no-conflict trials differed significantly (all p ≤ 0.001) but there was no difference between No-Conflict Positive or Negative, or Conflict Approach and Avoid (both p > 0.90). The main effect of run was driven by decreased RTs over time, irrespective of condition.
hDDM Modeling (Fig. 3E–G). No-Conflict Positive Certain and Uncertain trials had positive drift rates towards the approach boundary, which differed significantly from all other trials (all P = 0) but not each other (P = 0.46) (Fig. 3E). On the other hand, No-Conflict Negative Certain and Uncertain trials had a negative drift rate towards the avoidance boundary, which also differed significantly from all other trials (P = 0) but not each other (P = 0.82). There was, however, a significant difference between Conflict Certain and Conflict Uncertain trials (P = 0.0087), with the former associated with a negative drift rate and the latter a positive drift rate. Notably, in comparison to the No-Conflict conditions, participants were slow to accumulate evidence for both types of Conflict trials.
For decision threshold (a) (Fig. 3F), No-Conflict Negative trials had the highest threshold and differed significantly from all other conflict conditions (all P ≤ 0.02), although No-Conflict Negative Certain and Uncertain trials did not differ significantly (P = 0.22). While No-Conflict Positive trials were associated with a numerically greater threshold than Conflict trials, irrespective of uncertainty, this did not reach significance (0.77 ≤ P ≤ 0.88). Certain and Uncertain trials did not differ significantly within No-Conflict Positive (P = 0.44) and Conflict (P = 0.62) conditions. Together, the key finding indicates that No-Conflict Negative trials required more evidence for a decision to be made (i.e. a greater distance between the approach and avoid boundaries), with individuals providing more accurate and careful responses under clear threat. No-Conflict Positive and Conflict trials were associated with comparatively smaller thresholds, although they did not differ from each other.
Finally, non-decision time (t) also differed by conflict as opposed to uncertainty, with Conflict trials associated with significantly longer non-decision times compared to No-Conflict trials, irrespective of certainty (all P ≤ 0.008) (Fig. 3G). There were no significant differences between Conflict and No-Conflict Positive trials (0.14 ≤ P ≤ 0.17) and a trending difference between No-Conflict Positive and No-Conflict Negative (all P = 0.07). Non-decision time did not differ significantly between Certain and Uncertain trials within No-Conflict Positive, No-Conflict Negative, and Conflict trials (0.46 ≤ P ≤ 0.51). These results suggest that Conflict trials, followed by No-Conflict Positive and finally No-Conflict Negative trials, required retrieval of more distinct and detailed evidence, resulting in longer non-decision times.
Neuroimaging results
Univariate neuroimaging results
We first sought to identify greater activity during high AA conflict compared to no-conflict irrespective of outcome uncertainty (i.e. [Conflict Approach + Conflict Avoid]—[No-Conflict Positive + No-Conflict Negative]). Within the MTL ROI mask, significant activity was only observed in the left PRC (peak: −24, −8, −38; p = 0.043 svc, 3 voxels) (Fig. 4A) while at the whole brain level, significant activity was found in a large extensive cluster in the frontal cortex that encompassed multiple regions including the ACC (peak: −2, 22, 34; p(corrected) < 0.001) and DLPFC (peak: −40, 28, 32; p(corrected) = 0.008) (Fig. 4E, G). Other areas that demonstrated greater activity during high AA conflict included the orbitofrontal cortex, ventrolateral prefrontal cortex, posterior cingulate gyrus, thalamus, caudate, putamen, angular gyrus, and posterior supramarginal gyrus (Table 4). Notably, while activity in the left PRC was numerically greater for approach compared to avoid responses during high AA conflict, a contrast examining the interaction between conflict and response type regardless of certainty ([Conflict Approach—No-Conflict Positive Approach]—[Conflict Avoid—No-Conflict Negative Avoid]), revealed no significant regions of activity in the MTL. The same contrast at the whole brain level identified a significant cluster in the left frontal pole. In sum, predominant PRC, rather than aHPC, activity was observed in association with high AA conflict in the context of a wider network of regions including the ACC, DLPFC, orbitofrontal cortex, and ventrolateral prefrontal cortex.
Fig. 4.
A) Greater PRC activity during Conflict compared to No-Conflict conditions; B) relationship between PRC activity and threshold (a) and non-decision time (t); C) greater aHPC activity during No-Conflict compared to Conflict conditions; D) relationship between left aHPC activity and drift rate (v), a and t; E) greater ACC activity during Conflict compared to No-Conflict conditions; F) relationship between ACC activity and v and a; G) greater DLPFC activity during Conflict compared to No-Conflict conditions; H) relationship between DLPFC activity and v, and a. The relationships between ROIs and hDDM parameters are presented as regression coefficients indicating the strength and direction of the effect of per trial ROI regressors on parameter estimates. For v, the interpretation of the coefficient is dependent on whether the direction of the condition’s behavioral drift rate tended towards avoidance or approach. For No-Conflict Positive and Conflict Uncertain trials, which were associated with approach, a positive coefficient indicates that as ROI activity increased, drift rates were faster towards the approach boundary. In contrast, a negative coefficient suggests that increases in ROI activity were associated with slower evidence accumulation. For No-Conflict Negative and Conflict Certain trials, which were associated with avoidance, a negative coefficient indicates that as ROI activity increased, drift rates were faster (i.e. more negative) towards the avoidance boundary. A positive coefficient, however, suggests that as ROI activity increased, evidence accumulation was slower as captured by a less negative drift rate value. For a, a positive coefficient indicates that increases in ROI activity were associated with a greater amount of evidence required prior to choice or stable choices across trial for No-Conflict and Conflict trials, respectively. A negative coefficient indicates that increased ROI activity was associated with less evidence required or unstable, inconsistent choices across trial for No-Conflict and Conflict trials, respectively. For t, a positive coefficient indicates that increases in ROI activity were associated with a greater amount of time spent on processes outside of evidence accumulation. A negative coefficient, however, indicates that as ROI activity increases, there was less time spent on processes (e.g. perception, motor planning) supporting evidence accumulation. Activity was thresholded at P(corrected) < 0.05 and rendered on the MNI152 template. Asterisks indicate significant relationship between trial-by-trial activity and hDDM parameter.
Table 4.
Univariate analysis results beyond the MTL.
| Contrast | Region | BA | x | y | z | p-value | Voxels |
|---|---|---|---|---|---|---|---|
| Conflict–no conflict | Left DLPFC | 9 | −40 | 28 | 32 | <0.001 | 26,879 |
| Right frontopolar cortex | 10 | 22 | 68 | 12 | <0.001 | ||
| Left orbitofrontal cortex | 11 | −18 | 48 | −16 | <0.001 | ||
| Right ACC | 32 | −2 | 22 | 34 | <0.001 | ||
| Right DPLFC | 46 | 48 | 36 | 16 | <0.001 | ||
| Right ventrolateral PFC | 47 | 28 | 42 | −14 | <0.001 | ||
| (Conflict approach–no-conflict positive approach)–(conflict avoid–no-conflict negative avoid) | Left anterior PFC | 10 | −24 | 54 | 24 | 0.035 | 42 |
| Conflict–no conflict | Right pars orbitalis | 47 | 36 | 26 | −24 | <. 0.001 | 21,293 |
| (Approach only) | Right lateral occipital cortex | 18 | 30 | −88 | −2 | 0.001 | 742 |
| Right angular gyrus | 39 | 58 | −48 | 32 | <0.001 | 2,395 | |
| Left occipital fusiform gyrus | −28 | −74 | −2 | 0.001 | 791 | ||
| Left caudate | −12 | −6 | 16 | 0.002 | 479 | ||
| Right thalamus | 12 | −14 | 18 | 0.005 | 346 | ||
| Left posterior cingulate gyrus | 23 | −2 | −26 | 36 | 0.002 | 338 | |
| Right medial temporal gyrus | 21 | 54 | −33 | −16 | 0.006 | 235 | |
| Right inferior temporal gyrus | 20 | 54 | 4 | −40 | 0.007 | 46 | |
| Conflict–no conflict | Right frontopolar cortex | 10 | 28 | 50 | −12 | <0.001 | 20,884 |
| (Avoid only) | Right posterior supramarginal gyrus | 40 | 44 | −44 | 42 | <0.001 | 2,766 |
| Left angular gyrus | 39 | −50 | −50 | 52 | 0.003 | 1,242 | |
| Right middle temporal gyrus | 21 | 64 | −24 | −8 | 0.003 | 763 | |
| Bilateral posterior cingulate | 31 | 2 | −24 | 40 | 0.039 | 88 | |
| No conflict–conflict | Right precuneus | 18 | 16 | −50 | 6 | 0.045 | 354 |
| Right pre-motor supplemental gyrus | 6 | 22 | −12 | 72 | 0.046 | 41 |
Interestingly, a contrast to identify greater activity during no conflict compared to high AA conflict (i.e. [No-Conflict Positive + No-Conflict Negative]—[Conflict Approach + Conflict Avoid]), representing conditions in which outcome representation signals are strong and unambiguous, revealed significant bilateral aHPC activity within the MTL (right hemisphere peak: 30, −14, −16, p = 0.002 svc, 70 voxels; left hemisphere peak: −30, −16, −14, p = 0.015 svc, 106 voxels) (Fig. 4C), and right precuneus and right precentral gyrus activity at the whole brain level (Table 4). A contrast examining the interaction between No-Conflict and response type regardless of uncertainty [No-Conflict Positive Approach—Conflict Approach]—[No-Conflict Negative Avoid—Conflict Avoid] revealed no significant activity even when a liberal threshold was applied (P < 0.1).
Next, we investigated the impact of outcome uncertainty on neural activity regardless of conflict ([No-Conflict Uncertain + Conflict Uncertain] vs. [No-Conflict Certain + Conflict Certain]). Within the MTL, greater right parahippocampal cortex activity was found during uncertain compared to certain trials (22, −34, −14, p = 0.049 svc, 1 voxel). No significant clusters of activity were observed at the whole brain level, and the reverse contrast (Certain—Uncertain) did not identify any significant activity within or beyond the MTL. Examination of the interaction between uncertainty and conflict (i.e. [Conflict Uncertain vs. Conflict Certain] vs. [No-Conflict Uncertain vs. No-Conflict Certain]) did not yield any significant clusters of activity within the MTL or at the whole brain level.
Relationship between hDDM parameters and conflict-related activity
Activity in all ROIs, except for the PRC, was significantly associated with drift rate in one or more conditions, while the two PFC regions showed the strongest effects of modulating threshold (Fig. 4). Finally, left aHPC and PRC activity both modulated non-decision time in relation to conflict (Supplementary Table 3).
ACC activity was significantly related to drift rate positively for No-Conflict Negative and Conflict Certain (P ≤ 0.00075), and negatively for No-Conflict Positive (P = 0) (Fig. 4F). As No-Conflict Negative and Conflict Certain had negative drift rates behaviorally, a positive regression coefficient indicates that ACC activity was associated with a less negative, and therefore smaller drift rate magnitude. In contrast, since No-Conflict Positive trials had a behaviorally positive drift rate value, a negative regression coefficient with ACC activity indicates a less positive, and similarly, smaller drift rate magnitude. Thus, increased ACC activity occurred in the context of weaker evidence generation. With respect to threshold, ACC activity had a significant positive relationship with threshold for both Conflict conditions (P ≤ 0.011), and a significant negative relationship for No-Conflict Negative (P = 0.021) (Fig. 4F). Since, behaviorally, a larger threshold is indicative of greater response caution, positive activity coefficients indicate that increased ACC activity was associated with larger amounts of required evidence to make a decision for Conflict. The negative ACC coefficient for No-Conflict Negative indicates that greater ACC activity was associated with a lower threshold and amount of evidence required. Given the combination of greater ACC activation in the face of weaker evidence and higher thresholds, one possible interpretation could be that as Conflict trials lack information, they have a slower drift rate, and may trigger increases in ACC activity. We speculate that this modulation in activity then adjusts the threshold in a trial-specific manner, resulting in increased thresholds for Conflict but decreased ones for No-Conflict Negative. This would have the effect of increasing accuracy of responding in the face of conflict. A potential interpretation, therefore, is that the ACC is monitoring the quality of information and drift rate, and in turn adjusts the threshold to influence response caution.
In contrast to the ACC, which was associated with increased thresholds only for Conflict trials, DLPFC activity was most strikingly positively associated with threshold for all conditions (all P ≤ 0.043) indicating that greater activity was associated with larger amounts of required evidence for all conditions (Fig. 4H). With respect to drift rate, DPLFC demonstrated a similar pattern of a positive, significant relationship for No-Conflict Negative (P = 0.00038) and Conflict Certain (0.018), respectively, and a significant negative relationship for Conflict Uncertain (P = 0.026) (Fig. 4H). Like the coefficient patterns displayed by the ACC, DLPFC activity’s positive coefficient with No-Conflict Negative and Conflict Certain was associated with a smaller drift rate value. Given that Conflict Uncertain was behaviorally associated with a positive drift rate, and DLPFC activity had a negative coefficient with drift rate value in this condition, it similarly indicates a smaller drift rate magnitude. A potential interpretation of these results is that, just like ACC, DLPFC is sensitive to the trial-by-trial quality of the evidence provided by the stimulus as reflected in the drift rate. Unlike the ACC, however, DLPFC may respond with a global adjustment to threshold to allow for general cognitive control rather than a conflict-specific effect (see section Discussion).
In contrast to the pattern demonstrated by PFC activity, left aHPC activity was associated with a significant positive and negative relationship for No-Conflict Positive (P = 0) and No-Conflict Negative (P = 0), respectively (Fig. 4D) and was not associated with drift rate for Conflict trials. As No-Conflict Positive trials were associated with a positive drift rate, a positive coefficient with left aHPC activity indicated a larger drift rate magnitude. In contrast, since No-Conflict Negative and Conflict Certain trials had negative drift rates behaviorally, a negative coefficient similarly reflects a larger drift rate. This suggests that left aHPC activity increased the rate of evidence accumulation for No-Conflict conditions. With respect to threshold, left aHPC was negatively associated with threshold for Conflict Certain (P = 0.00013) (Fig. 4D), indicating that greater activity in the left aHPC was associated with smaller thresholds for Conflict. Left aHPC activity also demonstrated a significant positive relationship with non-decision time for Conflict Certain (P = 0.00025). A possible interpretation is that since No-Conflict conditions serve as relatively stronger memory reinstatement cues, these stimuli may trigger left aHPC activity to gather more detailed outcome representations that in turn take longer to process, resulting in a larger drift rate, while not necessarily triggering a greater amount of evidence, as indexed by the weak relationship with threshold in these conditions. Interestingly, the only condition in which the left aHPC was strongly related to threshold and non-decision time was Conflict Certain (Fig. 4D), albeit in opposite directions, with greater left aHPC activity reflecting the need for less evidence for a decision but greater engagement with non-decision-making processes.
Finally, there were no significant relationships between PRC activity and drift rate (0.13 ≤ P ≤ 0.45). There was, however, a significant negative relationship between PRC activity and threshold for No-Conflict Negative trials (P = 0.03) (Fig. 4B) revealing that activity was associated with smaller thresholds and less required evidence for No-Conflict Negative only. Finally, PRC activity and non-decision time were significantly negatively associated for Conflict Certain (P = 0.003) indicating that PRC activity was associated with less time engaging in decision-making supportive processes for Conflict Certain trials (Fig. 4B).
Discussion
Previous theories of AA conflict have attributed a variety of computational functions to PFC and MTL structures, with limited focus on the latter’s contributions. Using a novel paradigm with object stimuli and modeling trial-by-trial neural fluctuations to varying levels of conflict and uncertainty, we teased apart the distinct contributions of the PFC and MTL to AA conflict. At the behavioral and neural levels, we found that choice was driven primarily by conflict with only a minor contribution of uncertainty when selecting between conflict choices. Behaviorally, compared to no-conflict trials, conflict was associated with slower evidence generation, less cautious and unstable choices across trials, and placed greater demands on processes supporting evidence accumulation, although the effects of the latter two did not significantly differ from reward only trials. Our neural findings revealed a distinct pattern of results between the PFC and MTL’s contributions. We speculate that PFC regions were associated with inhibition and adjusting levels of control in response to the evidence generation profiles, while MTL regions were involved in the evidence generation process itself. Our findings lend important insights into the processing shaping AA conflict decision-making.
PFC regions
Our results suggest similar, though somewhat distinct, roles played by both the ACC and DLPFC, in resolving AA conflict, with the full pattern of results possibly representing a conflict-triggered regulatory role for both regions. First, consistent with the idea that these regions’ response is triggered by conflict, a univariate contrast of Conflict—No-Conflict revealed activity in a large network of PFC regions including ACC, DLPFC, orbitofrontal cortex and ventrolateral prefrontal cortex. Second, within conditions we also found consistent evidence that trial-by-trial weakening of the drift rate or evidence was generally associated with greater response in both regions. In other words, when the stimulus elicited greater behavioral conflict and produced weaker evidence generation, there were associated changes in activity in both the ACC and DLPFC at the trial level. Finally, we speculate that in response, both PFC regions adjust response caution and threshold. This is supported by prior work demonstrating that PFC regions such as the Supplementary Motor Area are related to conflict-triggered increases in response threshold (Wolpe et al. 2022), as well as findings that the relationship between medial prefrontal response and threshold can be reversed in the context of disrupted subthalamic nucleus stimulation (Cavanagh et al. 2011). It is critical to note, however, that we cannot infer directionality or causality with respect to whether drift rate or threshold changes occur first in each region. Indeed, it is conceivable that the relationship between the rate and amount or consistency of evidence accumulation is bidirectional across trials. As such, it is simultaneously possible that as the PFC activates in response to threshold fluctuations, it subsequently modulates drift rate through its interactions with regions involved in evidence generation. Regardless of the directionality, our findings provide evidence that ACC and DLPFC activity is associated with both weaker evidence generation and increased response caution.
A critical finding, however, is that the ACC and DLPFC engage in this response caution adjustment in different ways. The trial-wise response in the DLPFC was consistently associated with increases in threshold, irrespective of the original stimulus context, as evidenced by threshold increases across conditions. This lends support for the hypothesis that DLPFC plays a general inhibitory effect within AA conflict that is not specific to conditions of reward only (Rolle et al. 2022), with possible mechanisms including attentional control or reconfiguration, behavioral planning, working memory allocation, and cognitive allocation (Badre and Wagner 2007; Hart et al. 2013). In contrast, ACC activity had a more nuanced and contextually specific relationship with threshold, increasing it in cases of conflict, and decreasing it in the face of certain negative outcomes. In the context of conflict, raising the threshold (which results in greater requirements for evidence accumulation) results in choices more consistent with the individual’s goal state. In the context of certain negative choices, where the evidence is clear, lowering the threshold reduces effort without reducing accuracy. Broadly, the current results are also consistent with speculation that the ACC contributes to valuation of effort in a contextually appropriate manner (Shenhav et al. 2013, 2016).
MTL regions
Prior work on MTL involvement during AA conflict has pointed towards differing possibilities regarding its function, attributing its role to evidence generation in the form of memory sampling and/or behavioral inhibition and avoidance. Here, our univariate analysis revealed that, dissimilar to PFC regions and PRC, bilateral aHPC was more strongly activated for both positive and negative No-Conflict trials in comparison to Conflict trials. The absence of significant aHPC activity during conflict converges with our previous work on object-associated AA conflict that similarly found PRC but not aHPC activity during high AA conflict (Chu et al. 2021) and suggests that stimulus material (i.e. object vs. contextual information) may influence the involvement of differential MTL structures. The observation of greater activity during No-Conflict trials was, however, unexpected. We hypothesize this could be accounted for by differences in task structure, with HPC involvement increasing when the complexity in the relationships between the stimuli and their associated outcomes are greater. Previous paradigms relied on point gain/loss as the reward and punishment (O’Neil et al. 2015; Chu et al. 2021), whereas the present task requires two distinct reward (i.e. money) and punishment (i.e. food) representational spaces to be considered. The use of different modalities may activate the HPC differently given its role in match/mismatch detection computation (Duncan et al. 2012; Barnett et al. 2014), with HPC involvement in the low Conflict conditions potentially reflecting increased recruitment due to matching goal states (Duncan et al. 2012). An additional task factor that may influence the extent and nature of MTL structure involvement is the type of memory on which the task relies. In the current study, valence information was acquired in an episodic-like manner via stimulus-specific response feedback during the learning phase of the paradigm. An important research question, therefore, is to what extent MTL contributions during approach-avoidance conflict processing differ when other forms of valence information are required (i.e. generalized categorical valence information rather than stimulus specific valence information).
The relationship between aHPC activity and hDDM parameters was largely consistent with a proposed memory sampling role for this structure in decision-making (Mack and Preston 2016; Shadlen and Shohamy 2016). To make an approach or avoid decision on each trial of the decision phase of our task, participants were required to retrieve the valence information associated with each presented stimulus (i.e. memory retrieval) and to consider potential future outcomes (i.e. possibility of reward or punishment) in the context of overarching motivational goal states. Potentially reflective of these processes, we found that trial-by-trial activation was associated with stronger positive evidence generation in No-Conflict Positive trials, and stronger negative evidence generation in No-Conflict Negative trials. Given the correlative nature of fMRI data there is a limit to the extent to which we can conclude from the current study alone that the aHPC is contributing directly to memory sampling. It is important, however, to note that our findings converge with other studies that have suggested a role for the HPC in sampling during motivational decision-making, including fMRI and patient lesion work (Gluth et al. 2015; Bakkour et al. 2019). Our own recent work, for instance, has shown that focal damage to the HPC impacts drift rate during an approach-avoidance conflict paradigm similar to that used here (Le Duc et al. in preparation).
In contrast to drift rate, we found that the aHPC was only weakly related to threshold adjustments, with only a negative relationship observed for the Conflict Certain condition. While this may be interpreted as undermining a behavioral inhibition hypothesis (i.e. greater response caution and thresholds for conflict or negative information selectively), the aHPC’s contribution to inhibition cannot be ruled out definitively and further research will be necessary to investigate this further. Of particular interest will be the relationship between aHPC involvement and DDM parameters in a context-based AA conflict task, where we would predict greater aHPC recruitment during high compared to low Conflict. Finally, aHPC activity was also observed to be positively related to non-decision time for Conflict Certain trials. Notably, both the threshold and non-decision time effects were unique to these trials in which both positive and negative outcomes were certain to occur, although greater activity was associated with lower thresholds but larger non-decision times. The involvement of the aHPC, therefore, in evidence accumulation during conflict in the current task was in contrast to that of PFC regions and was found to support non-decision-making processes that were particularly emphasized when participants were faced with certain conflicting outcomes.
In addition to greater PRC activity during high compared to no conflict, per trial PRC activity was found to be associated with a smaller No-Conflict Negative threshold as well as reduced and lengthened non-decision times for Conflict Certain and No-Conflict Negative, respectively. This pattern of findings is somewhat challenging to interpret but one explanation may be that as No-Conflict Negative trials elicited the greatest response caution, richer PRC stimulus representations that can help confirm that the stimulus is indeed associated with punishment may reduce the overall amount of information required to make a choice, which is reflected in smaller thresholds but longer non-decision times. This stimulus representational information may then be fed forward to the HPC (Saksida and Bussey 2010) and the level of caution required to generate subsequent evidence may be reduced. The PRC’s relationship with non-decision time in Conflict Certain is notably in an opposing direction to that of the aHPC’s. While previous work has interpreted shorter non-decision times as an indicator of more efficient perceptual processing (Ulrichsen et al. 2020), it is unclear why the direction of the effect would occur in one, but not the other, MTL region. Future work will be needed to confirm and illuminate this effect.
A proposed model of MTL and PFC contributions to AA processing
Generally, MTL activity displayed an opposing directional pattern to that of the PFC in evidence accumulation, indicative of convergent top-down/bottom-up processes. Beginning with the MTL, our data suggests that while stimulus-specificity exerts some influence (i.e. PRC, but not aHPC, activity presents during Conflict > No-Conflict), it does not account for all the data, particularly within the context of the DDM. We suggest that in tasks with multimodal reward-punishment representational space, the HPC plays a role in reinstating representations from memory, detecting match/mismatch in the representation, and engaging in future outcome pre-play (e.g. imagining what eating the punishment food mixture may be like and weighing this to the cash prize) during evidence generation. We speculate that the PRC supports the HPC and PFC areas by providing a rich, detailed perceptual foundation, although it remains unclear how this process is affected by condition, task structure, or analysis type. At the top of the hierarchy, we hypothesize that DLPFC and ACC may be sensitive to trial level fluctuations in the quality of evidence generation and exert top-down cognitive control by adjusting the levels of response caution. We suspect that these regions, particularly the ACC, may provide feedback to MTL regions to modulate the actual comparisons during present-trial and future-trial evidence generation processes for conditions of conflict and uncertainty where more adjustments are required (Braem et al. 2017). In the PRC and HPC, respectively, these adjustments may take the form of more detailed stimulus perceptual representations or cleaner memory sampling.
Limitations and future directions
Although the present data revealed only a limited contribution of uncertainty on choice behavior and its associated neural correlates, it is important to point out that our task only manipulated two levels of uncertainty (i.e. complete uncertainty (50%) versus complete certainty (100%)). It is possible, therefore, that the incorporation of more widely varying and unpredictable levels of uncertainty in future work will provide greater insight into the potential impacts of outcome uncertainty on behavior and neural activity. Further, it is puzzling why the PRC was not linked with conflict evidence accumulation in the DDM, despite it being implicated in conflict in the current univariate GLM and our previous work (Chu et al. 2021). A possible explanation may be that signal to noise ratio (SNR) is lower in the PRC compared to HPC and PFC regions (Chu et al. 2021). In a smaller sample, the PRC may be less suited to the trial-by-trial approach implemented in the hDDM, particularly when it is competing for model variance with regions such as the HPC or PFC, resulting in discrepancies between the hDDM and analyses examining averaged signal. Indeed, the relatively small sample size (n = 21) used here needs to be acknowledged as a broader limitation and future work with a larger number of participants may be necessary to confirm and further clarify the current neural activity-related hDDM findings. Additionally, it is important to acknowledge the assumptions of the hDDM approach, in particular when contextualizing the link between the data and the associated cognitive processes. For example, while there is strong theoretical support for linking evidence generation to sampling mnemonic content, this cannot be causally verified using the current task. Further, present models assume independence between the parameters at all timepoints, but individuals may dynamically change thresholds across trials. This may explain how variance captured in the trial-by-trial DDM neural data may differ from averaged univariate neuroimaging findings. Finally, in light of our hypothesis that different reward and punishment modalities may elicit hippocampal activity through match/mismatch detection mechanisms, future work can aim to directly compare AA conflict created by similar (e.g. pleasant vs. unpleasant food) and different (e.g. money vs. unpleasant food) representational spaces in memory.
Conclusion
In summary, our data provide several novel contributions to our understanding of AA conflict. First, we highlight the importance of noting task structure complexity within the context of the DDM. For instance, when comparing our behavioral data (reward vs. punishment) to conflict created by reward vs. no reward (Mandali et al. 2019), we see divergences in drift rate and threshold despite having similar manipulations of conflict and uncertainty. Task structure may, therefore, be an important differentiating factor in addition to stimulus-type when determining MTL contributions to AA conflict. Second, we demonstrate that the PRC, HPC, ACC, and DLPFC make unique contributions to the AA conflict process using fMRI and the hDDM, although our findings in the PRC remain inconclusive. Our neural data suggest that the HPC supports evidence generation, while ACC and DLPFC may adjust response caution as a reaction to conflict and evidence strength.
Supplementary Material
Acknowledgments
We thank all participants for their time, Priya Abraham and Ali Golestani for their assistance with data collection, and Yang Teoh for his help with the hDDM analyses.
Contributor Information
Sonja Chu, Department of Psychological Clinical Science, University of Toronto, 1265 Military Trail, Toronto, ON M1C 1A4, Canada.
Cendri Hutcherson, Department of Psychological Clinical Science, University of Toronto, 1265 Military Trail, Toronto, ON M1C 1A4, Canada; Department of Psychology (Scarborough), University of Toronto, 1265 Military Trail, Toronto, ON M1C 1A4, Canada; Rotman School of Management, University of Toronto, 105 St. George Street, Toronto, ON M5S 3E6, Canada.
Rutsuko Ito, Department of Psychological Clinical Science, University of Toronto, 1265 Military Trail, Toronto, ON M1C 1A4, Canada; Department of Psychology (Scarborough), University of Toronto, 1265 Military Trail, Toronto, ON M1C 1A4, Canada; Department of Cell and Systems Biology, University of Toronto, 25 Harbord Street, Toronto, ON M5S 3G5, Canada.
Andy C H Lee, Department of Psychological Clinical Science, University of Toronto, 1265 Military Trail, Toronto, ON M1C 1A4, Canada; Department of Psychology (Scarborough), University of Toronto, 1265 Military Trail, Toronto, ON M1C 1A4, Canada; Rotman Research Institute, Baycrest Centre, 3560 Bathurst Street, Toronto, ON M6A 2E1, Canada.
Author contributions
Sonja Chu (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing), Cendri Hutcherson (Conceptualization, Formal analysis, Methodology, Writing—review & editing), Rutsuko Ito (Conceptualization, Funding acquisition, Methodology, Writing—review & editing), Andy Lee (Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Supervision, Visualization, Writing—review & editing).
Funding
This research was funded by a Canada Graduate Scholarship - Doctoral studentship (to S.C., 93007347) and project grant (to R.I., A.C.H.L., 156070) from the Canadian Institutes of Health Research, as well as a Canada Research Chair Award from the government of Canada (to C.H.).
Conflict of interest statement. All authors have no financial disclosures or conflicts of interest to declare.
Data availability statement
Experiment data and materials will be provided upon request by the corresponding authors.
References
- Anderson MC, Bunce JG, Barbas H. Prefrontal–hippocampal pathways underlying inhibitory control over memory. Neurobiol Learn Mem. 2016:134:145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci. 2014:18(4):177–185. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Francisco A, Paulus MP, Stein MB. Neural substrates of approach-avoidance conflict decision-making. Hum Brain Mapp. 2015:36(2):449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Guitart-Masip M, Packard PA, Miró J, Falip M, Fuentemilla L, Dolan RJ. Human hippocampus arbitrates approach-avoidance conflict. Curr Biol. 2014:24(5):541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007:45(13):2883–2901. [DOI] [PubMed] [Google Scholar]
- Bakkour A, Palombo DJ, Zylberberg A, Kang YH, Reid A, Verfaellie M, Shadlen MN, Shohamy D. The hippocampus supports deliberation during value-based decisions. elife. 2019:8:e46080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard IC, Murty VP, Carter RM, MacInnes JJ, Huettel SA, Adcock RA. Dorsolateral prefrontal cortex drives mesolimbic dopaminergic regions to initiate motivated behavior. J Neurosci. 2011:31(28):10340–10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AJ, O’Neil EB, Watson HC, Lee ACH. The human hippocampus is sensitive to the durations of events and intervals within a sequence. Neuropsychologia. 2014:64:1–12. [DOI] [PubMed] [Google Scholar]
- Becker GM, DeGroot MH, Marschak J. Measuring utility by a single-response sequential method. Behav Sci. 1964:9(3):226–232. [DOI] [PubMed] [Google Scholar]
- Braem S, King JA, Korb FM, Krebs RM, Notebaert W, Egner T. The role of anterior cingulate cortex in the affective evaluation of conflict. J Cogn Neurosci. 2017:29(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catena A, Perales JC, Megias A, Cándido A, Jara E, Maldonado A. The brain network of expectancy and uncertainty processing. PLoS One. 2012:7(7):e40252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci. 2011:14(11):1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S, Margerison M, Thavabalasingam S, O’Neil EB, Zhao YF, Ito R, Lee ACH. Perirhinal cortex is involved in the resolution of learned approach-avoidance conflict associated with discrete objects. Cereb Cortex. 2021:31(5):2701–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan S, Pinter C, Lee ACH, Ito R. Rodent perirhinal cortex, but not ventral hippocampus, inhibition induces approach bias under object-based approach-avoidance conflict. BioRxiv. 2022. 10.1101/2022.06.28.497792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: a high-resolution fMRI study of the human hippocampus. Hippocampus. 2012:22(3):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015:16(11):693–700. [DOI] [PubMed] [Google Scholar]
- Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Stat Sci. 1992:7(4):457–472. [Google Scholar]
- Gluth S, Sommer T, Rieskamp J, Büchel C. Effective connectivity between hippocampus and ventromedial prefrontal cortex controls preferential choices from memory. Neuron. 2015:86(4):1078–1090. [DOI] [PubMed] [Google Scholar]
- Golkar A, Lonsdorf TB, Olsson A, Lindstrom KM, Berrebi J, Fransson P, Schalling M, Ingvar M, Öhman A. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. PLoS One. 2012:7(11):e48107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010:48(4):831–853. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: an enquiry into the function of the septo-hippocampal system. Oxford: Oxford University Press; 2003. [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009:48(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013:70(2):185–198. [DOI] [PubMed] [Google Scholar]
- Hoshi E. Functional specialization within the dorsolateral prefrontal cortex: a review of anatomical and physiological studies of non-human primates. J Neurosci Res. 2006:54(2):73–84. [DOI] [PubMed] [Google Scholar]
- Ironside M, Amemori K, McGrath CL, Pedersen ML, Kang MS, Amemori S, Frank MJ, Graybiel AM, Pizzagalli DA. Approach-avoidance conflict in major depressive disorder: congruent neural findings in humans and nonhuman primates. Biol Psychiatry. 2020:87(5):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Lee ACH. The role of the hippocampus in approach-avoidance conflict decision-making: evidence from rodent and human studies. Behav Brain Res. 2016:313:345–357. [DOI] [PubMed] [Google Scholar]
- Jaeger TF. Categorical data analysis: away from ANOVAs (transformation or not) and towards logit mixed models. J Mem Lang. 2008:59(4):434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002:17(2):825–841. [DOI] [PubMed] [Google Scholar]
- Knoch D, Fehr E. Resisting the power of temptations: the right prefrontal cortex and self-control. Ann N Y Acad Sci. 2007:1104(1):123–134. [DOI] [PubMed] [Google Scholar]
- Korn CW, Bach DR. Heuristic and optimal policy computations in the human brain during sequential decision-making. Nat Commun. 2018:9(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn CW, Bach DR. Minimizing threat via heuristic and optimal policies recruits hippocampus and medial prefrontal cortex. Nat Hum Behav. 2019:3(7):733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn CW, Vunder J, Miró J, Fuentemilla L, Hurlemann R, Bach DR. Amygdala lesions reduce anxiety-like behavior in a human benzodiazepine-sensitive approach–avoidance conflict test. Biol Psychiatry. 2017:82(7):522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Duc W, Butler C, Argyropoulos G, Chu S, Ito R, Lee ACH. Insights into the role of the human hippocampus in approach-avoidance conflict processing from focal lesion hippocampal patients and drift diffusion modelling. In preparation.
- Leon MI, Shadlen MN. Effect of expected reward magnitude on the response of neurons in the dorsolateral prefrontal cortex of the macaque. Neuron. 1999:24(2):415–425. [DOI] [PubMed] [Google Scholar]
- Loh E, Kurth-Nelson Z, Berron D, Dayan P, Duzel E, Dolan R, Guitart-Masip M. Parsing the role of the hippocampus in approach–avoidance conflict. Cereb Cortex. 2016:27(1):201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack ML, Preston AR. Decisions about the past are guided by reinstatement of specific memories in the hippocampus and perirhinal cortex. NeuroImage. 2016:127:144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandali A, Weidacker K, Kim SG, Voon V. The ease and sureness of a decision: evidence accumulation of conflict and uncertainty. Brain. 2019:142(5):1471–1482. [DOI] [PubMed] [Google Scholar]
- Matzke D, Wagenmakers EJ. Psychological interpretation of the ex-Gaussian and shifted Wald parameters: a diffusion model analysis. Psychon Bull Rev. 2009:16(5):798–817. [DOI] [PubMed] [Google Scholar]
- Miller NE. Experimental studies of conflict. In: J. McV. Hunt, (ed). Personality and the behavior disorders, New York: Ronald Press, 1944. p 431–465. [Google Scholar]
- Mitchell DG. The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behav Brain Res. 2011:217(1):215–231. [DOI] [PubMed] [Google Scholar]
- Mumford JA, Turner BO, Ashby FG, Poldrack RA. Deconvolving BOLD activation in event-related designs for multivoxel pattern classification analyses. NeuroImage. 2012:59(3):2636–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil EB, Newsome RN, Li IH, Thavabalasingam S, Ito R, Lee AC. Examining the role of the human hippocampus in approach–avoidance decision making using a novel conflict paradigm and multivariate functional magnetic resonance imaging. J Neurosci. 2015:35(45):15039–15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen ML, Ironside M, Amemori K, McGrath CL, Kang MS, Graybiel AM, Pizzagalli DA, Frank MJ. Computational phenotyping of brain-behavior dynamics underlying approach-avoidance conflict in major depressive disorder. PLoS Comput Biol. 2021:17(5):e1008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010:30(32):10799–10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Rouder JN. Modeling response times for two-choice decisions. Psychol Sci. 1998:9(5):347–356. [Google Scholar]
- Ratcliff R, Smith PL. A comparison of sequential sampling models for two-choice reaction time. Psychol Rev. 2004:111(2):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolle CE, Pedersen ML, Johnson N, Amemori K, Ironside M, Graybiel AM, Pizzagalli DA, Etkin A. The role of the dorsal–lateral prefrontal cortex in reward sensitivity during approach–avoidance conflict. Cereb Cortex. 2022:32(6):1269–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksida LM, Bussey TJ. The representational–hierarchical view of amnesia: translation from animal to human. Neuropsychologia. 2010:48(8):2370–2384. [DOI] [PubMed] [Google Scholar]
- Schienle A, Köchel A, Ebner F, Reishofer G, Schäfer A. Neural correlates of intolerance of uncertainty. Neurosci Lett. 2010:479(3):272–276. [DOI] [PubMed] [Google Scholar]
- Schlund MW, Brewer AT, Magee SK, Richman DM, Solomon S, Ludlum M, Dymond S. The tipping point: Value differences and parallel dorsal-ventral frontal circuits gating human approach-avoidance behaviour. NeuroImage. 2016:136:94–105. [DOI] [PubMed] [Google Scholar]
- Schumacher A, Vlassov E, Ito R. The ventral hippocampus, but not the dorsal hippocampus is critical for learned approach-avoidance decision making. Hippocampus. 2015:26(4):530–542. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Shohamy D. Decision making and sequential sampling from memory. Neuron. 2016:90(5):927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013:79(2):217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. 2016:19(10):1286–1291. [DOI] [PubMed] [Google Scholar]
- Shiffrin RM, Lee MD, Kim W, Wagenmakers EJ. A survey of model evaluation approaches with a tutorial on hierarchical Bayesian methods. Cog Sci. 2008:32(8):1248–1284. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009:44(1):83–98. [DOI] [PubMed] [Google Scholar]
- Smith R, Kirlic N, Stewart JL, Touthang J, Kuplicki R, Khalsa SS, Paulus M, Aupperle R, T1000 Investigators. Greater decision uncertainty characterizes a transdiagnostic patient sample during approach-avoidance conflict: a computational modeling approach. J Psychiatry Neurosci. 2021:46(1):E74–E87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol. 2002:64(4):583–639. [Google Scholar]
- Spielberg JM, Miller GA, Heller W, Banich MT. Flexible brain network reconfiguration supporting inhibitory control. Proc Natl Acad Sci. 2015:112(32):10020–10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT. The cognitive neuroscience of human memory since HM. Annu Rev Neurosci. 2011:34:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmi D, Dayan P, Kiebel SJ, Frith CD, Dolan RJ. How humans integrate the prospects of pain and reward during choice. J Neurosci. 2009:29(46):14617–14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh YY, Yao Z, Cunningham WA, Hutcherson CA. Attentional priorities drive effects of time pressure on altruistic choice. Nat Commun. 2020:11(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrichsen KM, Alnæs D, Kolskår KK, Richard G, Sanders AM, Dørum ES, Ihle-Hansen H, Pedersen ML, Tornås S, Nordvik JE, Westlye LT. Dissecting the cognitive phenotype of post-stroke fatigue using drift diffusion modeling of sustained attention. Eur J Neurosci. 2020:52(7):3828–3845. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Mauguiere F, Isnard J, Dreher JC. The hippocampus codes the uncertainty of cue–outcome associations: an intracranial electrophysiological study in humans. J Neurosci. 2009:29(16):5287–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Sofer I, Frank MJ. HDDM: hierarchical bayesian estimation of the drift-diffusion model in python. Front Neuroinformatics. 2013:7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014:92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe N, Hezemans FH, Rae CL, Zhang J, Rowe JB. The pre-supplementary motor area achieves inhibitory control by modulating response thresholds. Cortex. 2022:152:98–108. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009:45(1):S173–S186. [DOI] [PubMed] [Google Scholar]
- Yeung L-K, Olsen RK, Bild-Enkin HE, D’Angelo MC, Kacollja A, McQuiggan DA, Keshabyan A, Ryan JD, Barense MD. Anterolateral entorhinal cortex volume predicted by altered intra-item configural processing. J Neurosci. 2017:37(22):5527–5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorowitz S, Rockhill AP, Ellard KK, Link KE, Herrington T, Pizzagalli DA, Widge AS, Deckersbach T, Dougherty DD. The neural basis of approach-avoidance conflict: a model based analysis. ENeuro. 2019:6(4):ENEURO.0115–ENEU19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experiment data and materials will be provided upon request by the corresponding authors.