Abstract
Despite growing evidence implicating thalamic functional connectivity atypicalities in autism spectrum disorder (ASD), it remains unclear how such alterations emerge early in human development. Because the thalamus plays a critical role in sensory processing and neocortical organization early in life, its connectivity with other cortical regions could be key for studying the early onset of core ASD symptoms. Here, we investigated emerging thalamocortical functional connectivity in infants at high (HL) and typical (TL) familial likelihood for ASD in early and late infancy. We report significant thalamo-limbic hyperconnectivity in 1.5-month-old HL infants, and thalamo-cortical hypoconnectivity in prefrontal and motor regions in 9-month-old HL infants. Importantly, early sensory over-responsivity (SOR) symptoms in HL infants predicted a direct trade-off in thalamic connectivity whereby stronger thalamic connectivity with primary sensory regions and basal ganglia was inversely related to connectivity with higher order cortices. This trade-off suggests that ASD may be characterized by early differences in thalamic gating. The patterns reported here could directly underlie atypical sensory processing and attention to social vs. nonsocial stimuli observed in ASD. These findings lend support to a theoretical framework of ASD whereby early disruptions in sensorimotor processing and attentional biases early in life may cascade into core ASD symptomatology.
Keywords: infancy, autism, fMRI, functional connectivity, thalamus
Introduction
Autism Spectrum Disorder (ASD) is a highly heritable developmental condition (Folstein and Rutter 1977; Thapar and Rutter 2021) that is characterized by social and communication deficits, restricted and repetitive behaviors and interests, as well as altered sensory processing (American Psychiatric Association 2013). Despite recent estimates showing that ASD affects 1 in 44 children by age 8 (CDC 2022), the early neurodevelopmental underpinnings of ASD remain poorly understood. Functional brain networks are consistently disrupted in ASD (Hernandez et al. 2015; Müller and Fishman 2018), and growing evidence suggests that these atypicalities may begin early in infancy (see Girault and Piven 2020 for review). However, the core behavioral symptoms of autism do not begin to emerge until the second year of life. Since ASD cannot be reliably diagnosed until around the second birthday based on behavioral criteria, currently a primary goal of the field is to understand how the earliest atypicalities in brain development relate to the early emergence of core behavioral symptoms.
Long-range functional connections in the brain strengthen during gestation (Thomason et al. 2015) and infancy (Wen et al. 2019). While functional networks undergo further refinement throughout adolescence and puberty (Goddings et al. 2019), the period before an infant’s first birthday is distinguished by especially dramatic and consequential maturations. Primary sensory networks are first to resemble adult networks, while supramodal and associative networks, such as default mode and dorsal attention networks, develop more gradually (Gao et al. 2015). In particular, thalamic projections to sensorimotor hubs and regions of the salience network—a network involved in orienting attention to relevant stimuli (Uddin 2015)—are already detectable in neonates. However, thalamic connections to medial visual and default mode hubs do not emerge until around the first birthday (Alcauter et al. 2014). Since early sensory and salience networks are the first to emerge in the young brain, they likely influence the downstream development of higher order associative networks, with implications for the larger functional organization of the human brain as well as the development of complex behaviors.
The thalamus is a subcortical brain structure primarily responsible for the integration, relay, and regulation of sensory information from primary sensory inputs to the rest of the brain. However, during development the thalamus also plays a crucial role in the specialization of the neocortex, whereby thalamic projections reciprocally interface with cortical areas in order to organize the neocortex according to environmental and sensory inputs (Nakagawa 2019). Such a foundational organizational role renders thalamocortical pathways prime candidates in the investigation of the early underpinnings of ASD. Indeed, past work has consistently demonstrated altered thalamocortical functional connectivity in ASD-diagnosed children and adolescents (see Hwang et al. 2022 for review). Specifically, these past studies have shown thalamic hyperconnectivity with sensorimotor, temporal (Nair et al. 2015; Woodward et al. 2017), and limbic regions, as well as hypoconnectivity with later-maturing prefrontal and association areas (Nair et al. 2015). Notably, recent work has replicated this pattern of prefrontal-thalamic hypoconnectivity and sensorimotor-thalamic hyperconnectivity in 6-week-old infants with a family history of ASD (Nair et al. 2021). This growing body of work suggests that functional thalamic atypicalities seen in ASD emerge early and remain relatively consistent across development. However, little is yet known about the relationship between atypical thalamocortical connectivity and core symptoms of ASD early in development.
These findings on atypical functional thalamic connectivity are compelling given that atypicalities in sensory processing—a main function of the thalamus—are prevalent in ASD. In particular, sensory over-responsivity (SOR) is a common and impairing symptom characterized by negative or aversive reactions to sensory stimuli such as loud noises, bright lights, scratchy clothing, or strong odors (Liss et al. 2006). Over half of ASD-diagnosed toddlers and children meet criteria for SOR (Baranek et al. 2006; Ben-Sasson et al. 2007), and sensory processing issues have been added as core ASD symptoms in the DSM-V (American Psychiatric Association 2013). SOR has been related to heightened sensory-limbic activation and reduced neural habituation to sensory stimuli (Green et al. 2015, 2019), as well as to aberrant thalamocortical connectivity during sensory processing in children and adolescents with ASD (Green et al. 2017). Moreover, SOR severity in ASD youth is related to abnormally low levels of thalamic GABA (inhibitory neurotransmitter) concentrations, with thalamic GABA levels directly related to altered thalamic functional connectivity with somatosensory cortices (Wood et al. 2021). Furthermore, ASD-diagnosed toddlers with sleep problems—which are common in ASD (Reynolds and Malow 2011) and are frequently related to sensory sensitivities (Tzischinsky et al. 2018)—also display thalamic overconnectivity with auditory cortex (Linke et al. 2021). Collectively, past work consistently implicates thalamic abnormalities in ASD, including from a very early age. Specifically, decreased thalamic inhibition and related overconnectivity with sensory/limbic regions could provide a causal mechanism by which sensory stimuli are attributed atypically strong emotional and attentional salience. Heightened attention to sensation could naturally detract from attention to socially relevant information (see Tsang et al. 2021, for example), initiating a developmental cascade in which early difficulties tuning out extraneous sensory stimuli may lead to atypical attentional biases that, in turn, may disrupt social development.
As infants who have an older sibling with ASD are at high likelihood (20%) for receiving a diagnosis (Ozonoff et al. 2011), prospectively studying these “infant sibs” offers a unique opportunity to study emerging brain atypicalities well before the onset of overt symptoms. With this paradigm, a growing body of work has shown that early functional networks are dysregulated in these infants (Emerson et al. 2017; Ciarrusta et al. 2020; Liu et al. 2020; Nair et al. 2021; Tsang et al. 2021). Atypicalities in functional connectivity at 6 months of age, for instance, may accurately predict later diagnosis (Emerson et al. 2017). Indeed, disruptions in short-range functional connections are even detectable soon after birth (Ciarrusta et al. 2020; Scheinost et al. 2022), and infant siblings also show significant structural and cellular brain abnormalities that, via experience-dependent neuronal pruning, could alter the development of systems-level brain networks (Piven et al. 2017). Taken together, past work indicates that very early functional network alterations may cascade into the complex and heterogeneous profile of core autism symptoms. However, more research is needed to understand how early atypicalities in thalamocortical connectivity relate to the emergence of specific symptoms of autism, such as SOR.
In this study, we investigated the early neural development of thalamocortical resting-state functional connectivity (rs-FC) during the first year of life and its relationship to early sensory symptoms in ASD. Here, we used a whole-thalamus seed-based approach, first examining differences between groups at typical likelihood (TL; no family history) and high likelihood of ASD (HL; older sibling with diagnosis) at 1.5 and 9 months of age. The interval between these two timepoints sees a number of developments and milestones, including—importantly for this study—a purported shift from largely subcortical to cortical control (Shultz et al. 2018). Furthermore, we also examined how functional connectivity during early and late infancy related to SOR symptoms assessed at 6 months of age. Based on prior work in young infants (Nair et al. 2021) and youth with ASD (Nair et al. 2015), we expected to find functional hypoconnectivity between the thalamus and higher order prefrontal and/or association cortices, as well as functional hyperconnectivity between the thalamus and primary sensory cortices in HL infants relative to TL infants. We also expected that more SOR symptoms would be associated with functional hyperconnectivity between the thalamus and sensory-limbic regions, which, if observed, could be considered a neural marker of a hyperactive sensory-limbic circuit.
Methods
Participants
Participants were enrolled in a longitudinal study of early brain-based markers of ASD as part of UCLA’s Autism Center for Excellence. Infants were assigned to groups based on family history of ASD: high likelihood (HL) infants had at least one older sibling with a clinical ASD diagnosis, whereas typical likelihood (TL) infants had no first- or second-degree relatives with ASD or any other neurodevelopmental disorder. Informed consent was obtained from all participants’ parents or legal guardians, and all study protocols were approved by the UCLA Institutional Review Board. Overall exclusionary criteria included: (i) genetic or neurological conditions associated with ASD risk (e.g. fragile X syndrome, tuberous sclerosis), (ii) chronic developmental condition or perinatal insult, (iii) severe visual, hearing, or motor impairment, and (iv) contraindication for MRI. Sibling pairs were not retained in the final sample in order to preserve independence of observations; thus, for sets of siblings who both yielded usable data, the sibling with higher-quality imaging data or more available behavioral data was included in the final sample (see MRI data acquisition).
At the 1.5-month timepoint, 105 infants underwent an MRI scan, including ten sibling pairs (5 HL, 5 TL); of these, we excluded from our analyses the sibling who displayed the most head motion during the MRI scan (5 HL, 5 TL excluded). An additional 10 HL (18.9%) and 3 TL (7.1%) infants were excluded due to excessive motion; and 5 (4 HL, 1 TL) were excluded due to failed registration or scanner artifacts. At the 9-month timepoint, 90 infants underwent MRI, including eight sibling pairs out of whom six infants were excluded (4 HL, 2 TL) due to greater head motion than their sibling, and two TL infants were excluded in favor of siblings who had more behavioral data available. An additional 4 HL (7.4%) and 2 TL (6.8%) infants were excluded due to excessive motion; and 4 were excluded due to scanner artifact or failed registration (2 HL, 2 TL). The rate of exclusion due to motion did not significantly differ between the two groups, at either age. Thus, the final imaging samples consisted of 77 participants (39 HL and 38 TL) at 1.5 months and 72 participants (48 HL and 24 TL) at 9 months. Seventeen HL and 18 TL participants provided usable data at both timepoints. These participants met all inclusion criteria described above, with rs-fMRI scans that met quality control thresholds for motion (mean relative motion <0.2 mm, maximum relative motion <3.0 mm). At both the 1.5- and 9-month imaging timepoints, groups did not significantly differ on sex, age, race, maternal education, or head motion during the MRI scan, but at both ages the HL and TL groups differed on ethnicity and birth order (Table 1). This study was significantly impacted by the COVID-19 pandemic, which hindered our recruitment of a larger HL sample as well as our ability to assess diagnostic outcome. Accordingly, our analyses had to be limited to comparisons between groups with different vulnerability to ASD based on family history.
Table 1.
Sample characteristics.
| HL | TL | HL vs. TL: t or χ2 (P) | ||||
|---|---|---|---|---|---|---|
| 1.5 Months | 9 Months | 1.5 Months | 9 Months | 1.5 Months | 9 Months | |
| N = 39 | N = 48 | N = 38 | N = 24 | |||
| Sex (female subjects) | 15 (38%) | 19 (40%) | 16 (42%) | 11 (46%) | 0.11 (P = 0.75) | 0.06 (P = 0.8) |
| Age (months) | 1.45 (0.29) | 9.20 (0.38) | 1.54 (0.25) | 9.13 (0.36) | 1.39 (P = 0.17) | 0.75 (P = 0.46) |
| Race | 0.72 (P = 0.40) | 0.63 (P = 0.43) | ||||
| White | 31 (79%) | 34 (71%) | 26 (68%) | 14 (58%) | ||
| Nonwhite | 8 | 14 | 12 | 10 | ||
| Ethnicity | 4.20 (P = 0.04*) | 4.33 (P = 0.04*) | ||||
| Hispanic or Latinx | 14 (36%) | 19 (40%) | 5 (13.2%) | 3 (13%) | ||
| Not Hispanic or Latinx | 25 | 29 | 33 | 21 | ||
| Maternal Ed. | <0.001 (P > 0.99) | 1.84 (P = 0.17) | ||||
| College and above | 37 (95%) | 42 (88%) | 37 (97.4%) | 24 (100%) | ||
| No college | 2 | 6 | 1 | 0 | ||
| Birth order | 18.25 (P < 0.001*) | 22.5 (P < 0.001*) | ||||
| First | 0 | 0 | 16 | 11 | ||
| Not first | 39 | 48 | 22 | 13 | ||
| Mean Abs. motion (mm) | 0.35 (0.31) | 0.24 (0.31) | 0.31 (0.27) | 0.26 (0.27) | 0.54 (P = 0.59) | 0.03 (P = 0.97) |
| Mean Rel. motion (mm) | 0.07 (0.04) | 0.07 (0.04) | 0.07 (0.04) | 0.07 (0.04) | 0.25 (P = 0.80) | 0.15 (P = 0.88) |
| Max Rel. motion (mm) | 1.26 (1.17) | 0.70 (1.18) | 0.89 (1.18) | 0.74 (0.88) | 1.38 (P = 0.17) | 0.16 (P = 0.87) |
| #Components removed | 24.72 (10.58) | 20.69 (7.90) | 25.50 (10.87) | 22.13 (8.67) | 0.32 (P = 0.75) | 0.68 (P = 0.50) |
Standard deviations given in parentheses. One participant at 1.5 months was missing maternal education data. Single asterisks mark comparisons that were significant at P < 0.05.
Behavioral measures and assessments
The Mullen Scales of Early Learning (Mullen 1995) were administered at 6, 12, and 36 months to evaluate five components of early language, motor, and cognitive development which, together, were combined into an Early Learning Composite (ELC). Early signs of ASD symptomatology were assessed at 12 months using the Autism Observation Scale for Infants (AOSI; Bryson et al. 2008) and at 36 months with the Autism Diagnostic Observation Schedule-2nd Edition (ADOS-2; Lord et al. 2012). At the 36-month outcome assessment, children were classified as falling into one of several Clinical Best Estimate groups: Typically Developing, Autism Spectrum Disorder, or Other Concerns. Children classified as “Other Concerns” showed speech/language delay, subclinical ASD symptoms, or another developmental delay as assessed by the licensed psychologist performing the diagnostic assessment. Diagnoses were not available for all children due to participant attrition or due to some participants not yet being old enough for the diagnostic follow-up. A full breakdown of diagnostic results by group is given in Supplementary Table 1. The Infant Sensory Profile (ISP; Dunn and Daniels 2002), a parent-reported measure of sensory processing across sensory seeking, avoiding, sensitivity, and sensory registration behaviors, was administered at 6 months of age to evaluate sensory over-responsivity (SOR). An SOR composite score was generated using the summed scores on four items measuring sensitivity to auditory and tactile stimuli (“Baby becomes upset by sudden everyday sounds,” “Baby looks away or becomes restless in noisy settings or with noisy toys,” “Baby becomes upset when having nails trimmed,” “Baby is startled by texture differences”). ISP scores were available for 18 HL infants with 1.5 month imaging data and for 28 infants with 9 month imaging data. Given the low rates of SOR in “typical” control samples (Green et al. 2016; Wood et al. 2021), our fMRI analysis involving SOR focused on HL infants only in order to evaluate ASD-related SOR.
Groups did not significantly differ on Mullen scores as assessed at 6 months. TL infants from the 1.5-month imaging sample had higher scores on the Mullen ELC at 12 months (P = 0.02), driven by the fine motor subscale, and TL infants from the 9 month sample had higher scores on the receptive language subscale (P = 0.037). HL infants from the 1.5-month sample had significantly lower scores on the Mullen at 36 months (P = 0.029), and HL infants from both imaging timepoints (1.5 and 9 months) had significantly lower receptive (P = 0.035 at 1.5 months, P = 0.02 at 9 months) and expressive (P = 0.019 at 1.5 months, P = 0.017 at 9 months) language scores at 36 months. As shown in Table 2, at 12 and 36 months the samples did not significantly differ on ASD symptomatology, likely reflecting not only the heterogeneity in the HL sample but also the fact that two TL infants from the 1.5-month imaging sample and three TL infants from the 9-month imaging sample later received an ASD diagnosis. Table 2 also shows that removing TL infants with a later ASD diagnosis revealed expected group differences in symptomatology on the AOSI; removing additional TL participants who displayed other developmental concerns (e.g. speech/language delay, subclinical ASD-like symptoms, or other developmental delays) revealed group differences on the ADOS as well. Likelihood groups did not significantly differ on SOR scores, including when TL participants with a later ASD diagnosis Other Concerns were excluded.
Table 2.
Behavioral characteristics.
| HL | TL | HL vs. TL: t or χ2 (P) | ||||
|---|---|---|---|---|---|---|
| 1.5 Months | 9 Months | 1.5 Months | 9 Months | 1.5 Months | 9 Months | |
| N = 39 | N = 48 | N = 38 | N = 24 | |||
| Mullen ELC 6moa | 96.6 (9.61) | 97.9 (9.62) | 100.0 (8.09) | 100.0 (7.63) | 1.54 (P = 0.13) | 1.00 (P = 0.32) |
| Gross motor | 46.5 (12.6) | 48.4 (9.81) | 49.5 (6.50) | 49.2 (6.77) | 1.25 (P = 0.22) | 0.40 (P = 0.69) |
| Fine motor | 50.0 (9.32) | 52.7 (7.76) | 51.0 (7.90) | 52.3 (6.83) | 0.45 (P = 0.66) | 0.20 (P = 0.85) |
| Visual reception | 48.3 (9.81) | 51.5 (8.96) | 52.0 (10.10) | 53.1 (10.8) | 1.55 (P = 0.13) | 0.63 (P = 0.54) |
| Receptive language | 48.1 (7.11) | 46.4 (7.29) | 49.8 (7.35) | 48.1 (8.11) | 0.92 (P = 0.36) | 0.87 (P = 0.39) |
| Expressive lang. | 46.7 (7.56) | 45.0 (7.07) | 47.0 (6.83) | 46.3 (6.79) | 0.13 (P = 0.90) | 0.76 (P = 0.45) |
| Mullen ELC 12mob | 102.5 (15.3) | 105.9 (13.9) | 110.6 (11.5) | 111.4 (11.2) | 2.36 (P = 0.02*) | 1.71 (P = 0.09) |
| Gross motor | 46.9 (18.3) | 50.8 (10.4) | 46.5 (17.8) | 49.8 (15.2) | 0.09 (P = 0.93) | 0.26 (P = 0.79) |
| Fine motor | 56.3 (10.9) | 58.5 (9.59) | 63.3 (7.97) | 65.0 (7.96) | 2.91 (P = 0.005**) | 2.92 (P = 0.005**) |
| Visual reception | 53.5 (10.1) | 55.9 (7.88) | 56.5 (7.80) | 56.6 (7.03) | 1.30 (P = 0.20) | 0.34 (P = 0.73) |
| Receptive language | 46.8 (8.32) | 46.5 (7.95) | 50.1 (6.77) | 50.2 (5.97) | 1.70 (P = 0.094) | 2.14 (P = 0.037*) |
| Expressive lang. | 47.8 (12.7) | 50.6 (11.0) | 51.1 (10.4) | 50.9 (10.6) | 1.09 (P = 0.28) | 0.12 (P = 0.90) |
| Mullen ELC 36moc | 104.8 (25.7) | 105.4 (24.8) | 119.5 (18.4) | 117.2 (21.8) | 2.27 (P = 0.029*) | 1.86 (P = 0.069) |
| Gross motor | N/A | N/A | N/A | N/A | ||
| Fine motor | 47.6 (15.8) | 51.1 (17.6) | 54.8 (14.1) | 55.1 (14.3) | 1.67 (P = 0.10) | 0.95 (P = 0.35) |
| Visual reception | 56.3 (18.2) | 57.4 (17.6) | 63.8 (12.2) | 61.5 (13.4) | 1.67 (P = 0.10) | 0.99 (P = 0.33) |
| Receptive language | 51.9 (15.0) | 49.7 (13.3) | 60.4 (11.6) | 58.8 (14.0) | 2.19 (P = 0.035*) | 2.40 (P = 0.02*) |
| Expressive lang. | 51.8 (12.7) | 50.9 (11.9) | 59.6 (9.04) | 58.6 (10.8) | 2.44 (P = 0.019*) | 2.48 (P = 0.017*) |
| AOSI 12mod | 4.93 (3.11) | 4.83 (2.70) | 3.84 (1.95) | 3.71 (2.08) | 1.54 (P = 0.13) | 1.82 (P = 0.08) |
| ♦ | “ | “ | 3.57 (1.67) | 3.33 (1.78) | 1.99 (P = 0.05*) | 2.53 (P = 0.01*) |
| ♦♦ | “ | “ | 3.6 (1.73) | 3.31 (1.82) | 1.89 (P = 0.07) | 2.47 (P = 0.02*) |
| ADOS-2 36moe | 6.3 (3.69) | 6.125 (4.11) | 5.5 (5.32) | 6.65 (5.96) | 0.60 (P = 0.55) | 0.33 (P = 0.74) |
| ■ | “ | “ | 4.38 (3.49) | 4.88 (4.00) | 1.77 (P = 0.08) | 0.97 (P = 0.34) |
| ■■ | “ | “ | 3.71 (2.15) | 3.87 (2.45) | 2.72 (P = 0.01**) | 2.15 (P = 0.04*) |
| SORf | 5.83 (2.96) | 5.54 (2.71) | 5.12 (2.42) | 5.25 (1.91) | 0.78 (P = 0.44) | 0.34 (P = 0.74) |
| ◊ | “ | “ | 5.19 (2.48) | 5.43 (1.99) | 0.69 (P = 0.49) | 0.12 (P = 0.91) |
| ◊◊ | “ | “ | 5.36 (2.62) | 5.67 (2.07) | 0.48 (P = 0.63) | 0.13 (P = 0.90) |
Standard deviations given in parentheses. Single asterisks marks P < 0.05. Double asterisks mark P < 0.01. ♦ = AOSI 12mo with ASD-diagnosed TL infants omitted (2 in 1.5 month sample, 3 in 9 month sample). ♦♦ = AOSI 12mo with additional TL infants removed who had other developmental concerns (speech/language delay, subclinical ASD symptoms, or other concerns; 3 in 1.5 month sample, 2 in 9 month sample). ■ = ADOS-2 36mo with ASD-diagnosed TL infants omitted (2 in 1.5 month sample, 3 in 9 month sample). ■■ = ADOS-2 36mo with additional TL infants removed who had other developmental concerns (speech/language delay, subclinical ASD symptoms, or other concerns; 3 in 1.5 month sample, 2 in 9 month sample). ◊ = SOR scores with ASD-diagnosed TL infants omitted (1 in 1.5-month sample, 1 in 9-month sample). ◊◊ = SOR scores with additional TL infants removed who had other developmental concerns (speech/language delay, subclinical ASD symptoms, or other concerns; 2 in 1.5 month sample, 1 in 9 month sample).
aMSEL Assessment (6mo): Missing from 4 in the HL cohort at 1.5 months; 3 from the HL cohort at 9 months; and 6 from the TL cohort at 1.5 months.
bMSEL Assessment (12mo): Missing from 7 in the HL cohort at 1.5 months; 5 in the HL cohort at 9 months; 9 in the TL cohort at 1.5 months; and 2 in the TL cohort at 9 months.
cMSEL Assessment (36mo): Missing from 16 in the HL cohort at 1.5 months; 13 from the HL cohort at 9 months; 13 from the TL cohort at 1.5 months; and 3 from the TL cohort at 9 months.
dAOSI (12mo): Missing from 9 in the HL cohort at 1.5 months; 9 in the HL cohort at 9 months; 13 in the TL cohort at 1.5 months; and 3 in the TL cohort at 9 months.
eADOS-2 (36mo): Missing from 24 in the HL cohort at 1.5 months; 30 in the HL cohort at 9 months; 12 in the TL cohort at 1.5 months; and 4 in the TL cohort at 9 months.
fSOR (6mo): Available for 18 in the HL cohort at 1.5 months; 17 in the TL cohort at 1.5 months; 28 in the HL cohort at 9 months; 8 in the TL cohort at 9 months.
MRI data acquisition
Data collection procedures were based on recommended guidelines for neuroimaging in young infants (Raschle et al. 2012). Resting-state fMRI (rs-fMRI) scans were collected in the evening during natural sleep on a Siemens Trio scanner (12-channel head coil) or, following an upgrade to scanning facilities, a Siemens Prisma scanner (32-channel head coil). Because of this change, scanner was included as a nuisance regressor in all group-level analyses. The 8-min rs-fMRI scan sequence was collected prior to other stimulus-evoked functional runs in order to avoid possible confounding effects. Scan parameters were as follows: TR = 2,000 ms, TE = 28 ms, matrix size 64 × 64, FOV = 192 mm, 34 slices, 3-mm in-plane resolution, with 4 mm-thick axial slices. After upgrading to the Siemens Prisma scanner, the sequence remained identical except with 33 slices. A high-resolution matched-bandwidth T2-weighted high-resolution echo planar scan was acquired co-planar to the functional scan for registration (Siemens Trio: TR = 5,000 ms, TE = 34 ms, matrix size 128 × 128, FOV = 192 mm, 34 slices, 1.5-mm in-plane resolution with 4-mm-thick axial slices; Siemens Prisma: identical parameters except with TE = 45 ms, 33 slices).
Parents were encouraged to emulate the infant’s normal bedtime routine and swaddle/rock the participant to sleep. Sleeping infants were then transferred to the scanner bed padded with linens and cushions. Malleable silicone earplugs and MiniMuffs Neonatal Noise Attenuators (Natus Medical, Inc., San Carlos, CA) were used for hearing protection; headphones, which were used to deliver auditory stimuli during a following scan, provided additional hearing protection. Infants were secured to the bed with a Velcro strap underneath a weighted blanket. A trained member of the study staff remained inside the scanner room with the participant to monitor for signs of wakefulness, discomfort, or distress.
fMRI data preprocessing
Functional imaging data were preprocessed and analyzed using FMRIB’s Software Library (FSL version 5.0.11; Smith et al. 2004). Functional scans underwent motion correction and structural images underwent skull stripping with manual quality checks using FSL’s Brain Extraction Tool. Functional scans were then registered to the infant’s own T2-weighted high-resolution structural scan with 6 degrees of freedom. This was followed by registration to a standard infant brain template (Shi et al. 2011) with 12 degrees of freedom. Scans collected at the 1.5-month timepoint were registered to the neonate template, while scans collected at 9 months were registered to the 1-year brain template (Shi et al. 2011). Registration quality was manually inspected for quality control, then data were spatially smoothed using a 6-mm Gaussian kernel and underwent 4D mean intensity normalization.
Independent Component Analysis—Automatic Removal of Motion Artifacts (ICA-AROMA; Pruim et al. 2015a, Pruim et al. 2015b) was used to automatically identify and remove signal components attributable to motion, in lieu of “scrubbing” individual volumes contaminated with motion artifacts. This approach has been shown to improve reproducibility in resting-state analyses (Carone et al. 2017) and is able to preserve temporal degrees of freedom throughout the scan sequence. Importantly, risk groups did not differ on several key metrics of motion (mean absolute motion, mean and maximum relative motion), nor the number of ICA-AROMA components removed (Table 1). Data were bandpass filtered (0.01 Hz < t < 0.1 Hz) to remove signal attributable to physiological noise such as heartbeat and respiration. Mean white matter time series, mean cerebrospinal fluid time series, and mean global time series were regressed out at the single-subject level. Global signal regression (GSR) was applied, as it has been shown to be an effective denoising strategy (Power et al. 2014), particularly when paired with ICA-AROMA (Parkes et al. 2018). Furthermore, previous work from our lab (Liu et al. 2020) has demonstrated that including GSR in the processing pipeline does not affect the reproducibility of rs-FC results in the same infant population.
fMRI data analysis
The bilateral thalamus, derived from a standard infant atlas (Shi et al. 2011) was used as the seed ROI for generating single-subject rs-FC maps across the whole brain. The time series averaged across the thalamus was extracted from processed residuals in standard space and correlated with that of every other voxel in the brain. The resulting correlation maps were then converted to z-statistic maps using Fisher’s r-to-z transformation. Group-level analyses were performed in FSL using FMRIB’s Local Analysis of Mixed Effects (FLAME 1 + 2), which assumes unequal variances between ASD likelihood groups, with scanner included as a demeaned nuisance regressor. Within-group analyses were thresholded at Z > 2.7, correcting for multiple comparisons at the cluster level (P < 0.05). The between-group contrasts were masked by regions that displayed significant connectivity in either group as per the results of the within-group analyses (Fig. 1). Given the role of the thalamus in the processing of sensory information, we additionally examined the relationship between thalamocortical rs-FC and sensory over-responsivity (SOR) as assessed at 6 months of age in the HL group. Whole-brain regression analyses were conducted within the HL group to examine how SOR at 6 months related to thalamocortical rs-FC connectivity at both imaging timepoints. All between-group contrasts and bottom-up behavioral regressions were thresholded at Z > 2.3, cluster corrected for multiple comparisons at P < 0.05; however, only clusters whose peaks exceeded a significance threshold of Z = 2.7 were reported.
Fig. 1.
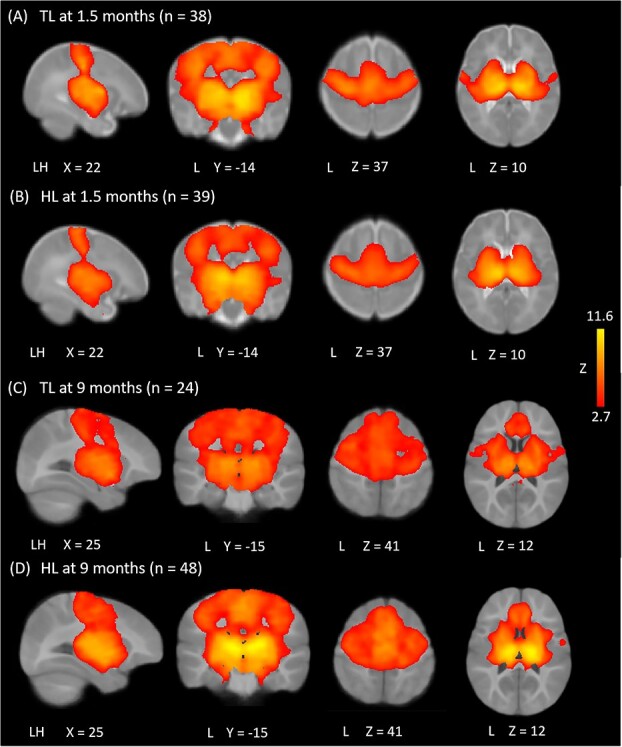
Thalamocortical functional connectivity within groups. At both timepoints, participants showed thalamic functional connectivity with primary sensory and motor cortices, supplementary motor area (SMA), middle cingulate, basal ganglia, insula, and amygdala, with the notable absence of connectivity with visual cortex (A–D). At 9 months, both groups showed additional connectivity with medial prefrontal cortices (C and D). Results thresholded at Z > 2.7, corrected for multiple comparisons at P < 0.05. Differences in coordinates between 1.5- and 9-month data reflect the use of two distinct age-appropriate atlases.
Post-hoc fMRI analyses
Finally, based on our findings whereby SOR in HL infants was positively associated with thalamic connectivity with sensorimotor cortices, inferior frontal gyrus, and basal ganglia, and negatively associated with thalamic connectivity with frontal, medial occipital, and parietal areas, we conducted post-hoc analyses to investigate the extent to which there was a direct trade-off in these SOR-related connectivity patterns. We first examined this relationship in the subset of HL participants who had SOR scores available (“HL Original Sample”, n = 28), and then tested whether this relationship would also generalize to two other subsets of participants: the HL group without SOR scores (“HL Validation Sample,” n = 20), and TL infants (excluding those who did not receive a later ASD diagnosis; “TL Sample,” n = 21). This provided a distinct HL sample in which to test the reproducibility of this finding. Finally, examining this relationship in the TL infants allowed us to test whether this inverse relationship may be uniquely associated with ASD likelihood.
Results
Seed-based analyses
As shown in Fig. 1, at both timepoints and in both the HL and TL groups, thalamic rs-FC maps showed robust connectivity with primary sensory, primary motor, supplementary motor, and middle cingulate cortices, as well as basal ganglia, insula, and amygdala. At 9 months, both groups also showed significant thalamo-cortical connectivity with medial prefrontal cortices. At 1.5 months, compared to TL infants, the HL group showed stronger functional connectivity between the bilateral thalamus and limbic regions, including the hippocampus, para-hippocampus, as well as left amygdala and left olfactory cortex (Fig. 2A; see Table 3 for coordinate peaks for group contrasts and regressions). At 9 months, compared to the TL group, HL infants displayed weaker thalamic connectivity with bilateral supplementary motor area (SMA) and superior frontal gyrus (dorsal), as well as left inferior frontal gyrus (pars triangularis), precentral gyrus, middle frontal gyrus, and operculum (Fig. 2B).
Fig. 2.
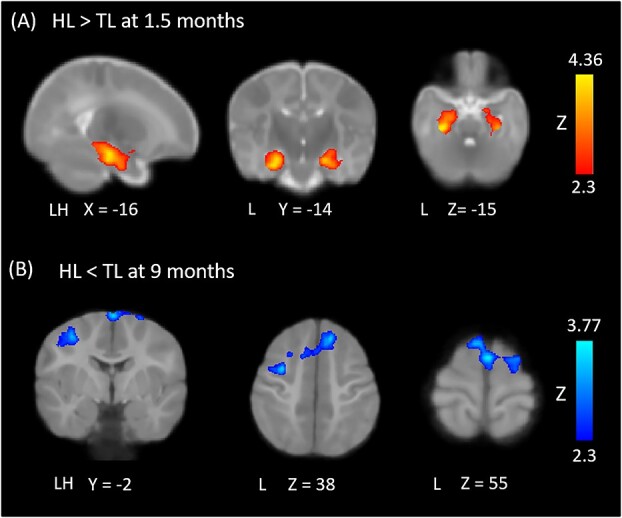
Between-group differences in thalamocortical functional connectivity. At 1.5 months, HL infants displayed functional overconnectivity between the thalamus and a limbic cluster encompassing hippocampus, parahippocampus, left amygdala, and left olfactory cortex (A). At 9 months, HL infants displayed functional underconnectivity between the thalamus and bilateral SMA and mPFC, as well as left precentral gyrus, operculum, and insula (B). All results were thresholded at Z = 2.3 and cluster-corrected for multiple comparisons.
Table 3.
Coordinate table for functional connectivity peaks in group comparisons and SOR regressions between-group contrasts and SOR regressions.
| Peak (voxel coordinates) | ||||||
|---|---|---|---|---|---|---|
| Cluster location | L/R | Max Z | x | y | z | |
| HL > TL at 1.5 months | Parahippocampus | L | 4.36 | 71 | 109 | 57 |
| R | 3.58 | 108 | 109 | 57 | ||
| Hippocampus | L | 4.2 | 73 | 110 | 60 | |
| R | 3.85 | 104 | 110 | 61 | ||
| Amygdala | L | 2.79 | 77 | 122 | 61 | |
| Olfactory | L | 2.74 | 81 | 127 | 61 | |
| SOR − at 1.5 months | SFG (dorsal) | L | 4.15 | −15 | 36 | 13 |
| OFC (middle) | L | 4.10 | −24 | 33 | −4 | |
| IFG (pars tri.) | L | 3.52 | −31 | 28 | 5 | |
| SFG (medial) | R | 3.14 | 2 | 35 | 10 | |
| OFC (superior) | L | 3.03 | −10 | 40 | −3 | |
| TL > HL at 9 months | IFG (pars tri.) | L | 3.75 | 59 | 135 | 93 |
| Precentral | L | 3.51 | 60 | 125 | 110 | |
| MFG | L | 2.99 | 60 | 129 | 116 | |
| Operculum | L | 2.89 | 54 | 132 | 87 | |
| SMA | L | 3.77 | 88 | 133 | 122 | |
| R | 3.66 | 90 | 122 | 126 | ||
| SFG (dorsal) | L | 3.66 | 101 | 150 | 108 | |
| R | 3.27 | 104 | 120 | 128 | ||
| SFG (medial) | R | 3.54 | 100 | 147 | 107 | |
| SOR+ at 9 months; HL only | Operculum | R | 4.29 | 131 | 115 | 79 |
| L | 2.99 | 46 | 123 | 80 | ||
| Putamen | R | 3.5 | 112 | 123 | 88 | |
| L | 2.95 | 74 | 143 | 70 | ||
| Insula | R | 3.43 | 115 | 122 | 87 | |
| L | 3 | 63 | 120 | 86 | ||
| Postcentral | R | 3.8 | 143 | 112 | 104 | |
| Precentral | R | 3.44 | 129 | 112 | 119 | |
| Heschl | L | 3.11 | 50 | 110 | 80 | |
| IFG (pars oper.) | L | 2.96 | 55 | 136 | 85 | |
| Caudate | L | 3.16 | 78 | 131 | 91 | |
| Pallidum | L | 2.84 | 78 | 129 | 74 | |
| SOR − at 9 months; HL only | MOG | L | 4.6 | 54 | 62 | 87 |
| R | 4.28 | 121 | 56 | 88 | ||
| Precuneus | L | 4.27 | 87 | 74 | 109 | |
| SFG (medial) | R | 4.65 | 94 | 179 | 74 | |
| L | 3.5 | 89 | 173 | 82 | ||
| OFC (medial) | R | 3.57 | 93 | 173 | 69 | |
| L | 3.51 | 85 | 158 | 64 | ||
| Rectus | R | 3.25 | 93 | 167 | 56 | |
HL: high likelihood for autism; TL: typical likelihood for autism; SOR+: coordinates where greater connectivity to thalamus was correlated with sensory over-responsivity; SOR−: coordinates where weaker connectivity to thalamus was correlated with sensory over-responsivity; IFG: inferior frontal gyrus; MFG: middle frontal gyrus; SMA: supplementary motor area; SFG: superior frontal gyrus; MOG: middle occipital gyrus; OFC: orbitofrontal cortex.
Regression analyses (HL only)
Greater SOR symptoms at 6 months of age were predicted by weaker thalamic connectivity, at 1.5 months of age, with frontal regions including the bilateral superior frontal gyrus, left orbitofrontal cortex, and left inferior frontal gyrus (pars triangularis; Fig. 3A). There were no positive associations between 6-month SOR and thalamic connectivity at 1.5 months.
Fig. 3.
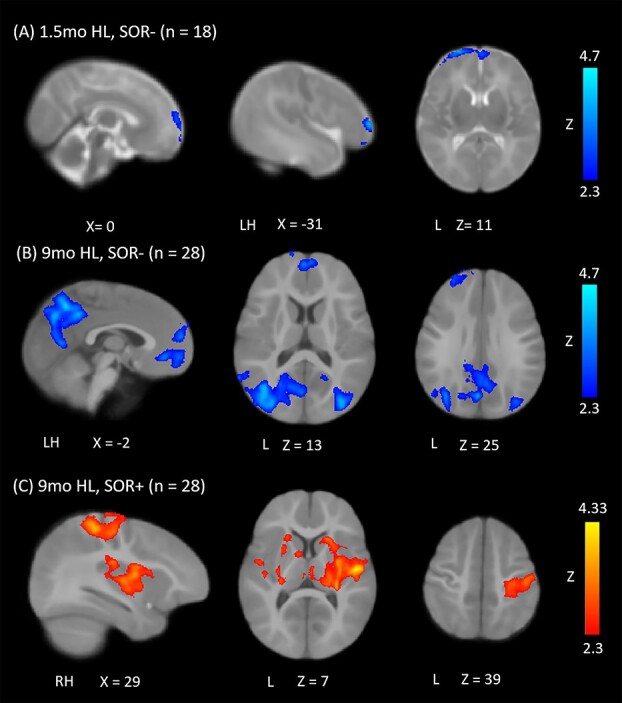
Regions whose thalamocortical connectivity is related to SOR at 6 months. Regions whose functional connectivity with the thalamus (A) at 1.5 months is negatively correlated with SOR; (B) at 9 months is positively correlated with SOR and (C) at 9 months is negatively correlated with SOR. All results were thresholded at Z = 2.3 and corrected for multiple comparisons.
Greater SOR symptomatology at 6 months of age predicted weaker thalamocortical connectivity later on, at 9 months of age, with medial prefrontal cortex, superior frontal gyrus, and bilateral middle occipital gyrus, as well as left precuneus (Fig. 3B). Greater SOR also predicted stronger thalamocortical connectivity with sensorimotor cortices (primary auditory, somatosensory, and motor cortex), as well as inferior frontal gyrus (pars opercularis), insula, operculum, and basal ganglia (caudate, pallidum, and putamen; Fig. 3C).
Post hoc analyses
Our SOR regressions with 9-month imaging data implicated two sets of regions (Fig. 3B and C) for which connectivity with the thalamus showed opposite relationships with SOR. This prompted us to ask whether, at the individual level, greater thalamic connectivity with sensory and subcortical regions, as a function of higher SOR, was inversely related to thalamic connectivity with higher order regions (mPFC and precuneus) that were associated with lower SOR. We extracted and correlated parameter estimates of functional connectivity from these two sets of regions (visible in Fig. 3B and C) in infants for the HL original sample (n = 27, one outlier removed). This analysis revealed a strong negative correlation (r = −0.62, P = 0.0006; Fig. 4A), indicating that increased thalamic connectivity with sensory and subcortical regions was consistently associated with decreased connectivity with higher order regions at the individual level.
Fig. 4.
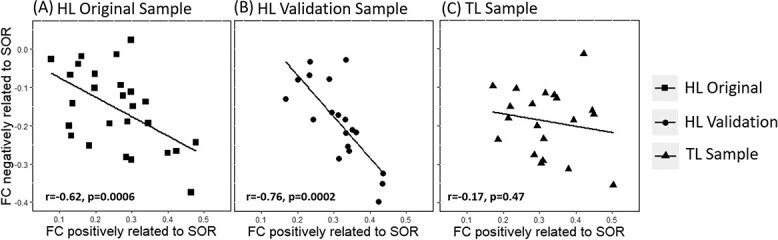
Functional trade-offs associated with SOR. Connectivity indices between the thalamus and regions positively associated with SOR (x-axis; derived from clusters in Fig. 3A) were plotted against connectivity indices between the thalamus and regions negatively related with SOR (y-axis; derived from clusters in Fig. 3B). This trade-off relationship was significant across the original sample of 9-month-old HL infants (n = 27, one outlier removed) included in the initial whole-brain SOR regression (A), as well as a “validation group” of HL infants (n = 19, one outlier removed) without SOR scores (B). This relationship was not observed in the subset of TL infants (C; n = 20, one outlier removed).
To further validate this finding, we then examined the extent to which this pattern held in the HL validation sample (n = 19, one outlier removed), who were not included in our original regression analysis given that SOR scores for these infants were not available. Again, we extracted parameter estimates of connectivity from the same clusters identified in the analysis with the HL original sample. Interestingly, this group also showed a significant negative correlation (r = −0.76, P = 0.0002) between regions showing stronger vs. weaker connectivity as a function of SOR such that infants who showed stronger connectivity with primary sensory-motor regions and basal ganglia also showed weaker connectivity with prefrontal regions and precuneus. Finally, in order to investigate whether this trade-off effect was specific to infants at high likelihood for ASD, we repeated this analysis with the TL Sample (n = 20; one outlier removed). This inverse functional relationship (Fig. 4C) was not observed in this group (r = −0.17, P = 0.47; Fig. 4C). Further analyses showed that the correlations observed in the HL original and HL validation groups (Fig. 4A and B) were both significantly stronger than that observed in the TL group (z = 1.74, P = 0.04 and z = 2.37, P = 0.009, respectively). Outliers did not significantly influence results; see Supplementary Figure 1.
Discussion
Here, we investigated resting-state functional connectivity of the thalamus as a function of family history for ASD in 1.5- and 9-month-old infants. Overall, we found that group differences in functional connectivity of the thalamus are detectable as early as 1.5 months of age with the HL group displaying thalamic hyperconnectivity with limbic regions including the hippocampus, parahippocampus, amygdala, and olfactory cortex. By contrast, at 9 months the HL group showed hypoconnectivity with several prefrontal regions including mPFC and motor areas. Given the role of the thalamus in sensory gating and the prevalence of sensory processing atypicalities in ASD, we additionally investigated whether patterns of thalamic connectivity within the HL infants might relate to individual differences in sensory responsivity. We found that fewer SOR symptoms at 6 months were associated with stronger thalamic connectivity with higher-order prefrontal cortices at both 1.5 and 9 months of age, as well as stronger thalamic connectivity with parietal and occipital association areas later in infancy. Stronger thalamic connectivity with subcortical and primary sensory regions in late infancy was associated with a higher prevalence of SOR symptoms.
In line with previous work in typically developing infants, at both ages and in both groups we found significant functional thalamic connections with sensorimotor and attentional regions, but not with visual or default mode network hubs (Alcauter et al. 2014), which purportedly develop later around the one-year mark. Relative to the TL group, HL infants displayed robust thalamic hyperconnectivity, at 1.5 months, with bilateral limbic regions (hippocampus, parahippocampus, left amygdala), as well as left olfactory cortex. This is consistent with prior work, as limbic overconnectivity with the thalamus is frequently reported in studies of ASD children and adolescents (e.g. Nair et al. 2015). Prior work has also shown that the amygdala shows overactive responses (Green et al. 2013, 2015, 2019) and a lack of habituation to sensory stimuli (Green et al. 2015, 2019); importantly, increased amygdala activity has also been linked to SOR severity (Green et al. 2015). Furthermore, in another relevant study (Green et al. 2017), youth with ASD showed stimulus-induced increases in connectivity between the pulvinar nucleus of the thalamus and the right amygdala as a function of SOR severity. Given this empirical link between pulvinar-amygdala connectivity, sensory stimulation, and SOR, the authors interpreted this heightened connectivity as a reflection of the aversiveness of the sensory stimuli and related increased arousal. Similarly, the heightened connectivity between thalamus and amygdala observed in our study, at a much earlier age, could be an early indication that HL infants may be more likely to perceive sensory stimuli as aversive and/or more arousing.
In contrast to prior work in a similar sample (Nair et al. 2021), in this study, we did not find hyperconnectivity in thalamic-motor and thalamic-occipital networks at 1.5 months of age. This may reflect differences in the data analytic approach used in the two studies: here, we examined thalamic connectivity using the whole thalamus as a seed, whereas Nair and colleagues examined cortico-thalamic connectivity starting with cortical seeds to identify functional connectivity with specific thalamic nuclei. While using the entire thalamus as the seed in our study may have resulted in a loss of sensitivity due to averaging signal across distinct thalamic nuclei, this approach allowed us to examine thalamic connectivity with both cortical and subcortical regions including the limbic lobe. As previously mentioned, subcortical limbic structures such as the amygdala consistently show atypicalities in ASD and have been implicated in core ASD symptomatology. Taken together, the two distinct yet complementary approaches employed in the present study and in Nair et al. (2021) provide converging evidence that atypicalities in thalamic functional connectivity associated with ASD risk can be detected exceedingly early in development.
At 9 months, HL infants showed significant thalamo-cortical hypoconnectivity compared to the TL group, with weaker connectivity between the thalamus and prefrontal cortices (including left inferior/middle frontal gyri, mPFC, and motor cortices). Given previous reports of thalamic-motor hyperconnectivity in the literature (Nair et al. 2015, 2021), the observed hypoconnectivity with left primary motor cortex was unexpected. However, the broader pattern of thalamic hypoconnectivity with other prefrontal regions corroborates prior work from our lab at 1.5 months (Nair et al. 2021), as well as studies in older youth with an ASD diagnosis (Nair et al. 2013, 2015) that reported weaker thalamic connectivity with the SMA and supramodal association cortices. Interestingly, prefrontal hypoconnectivity has also been observed with the salience network (Tsang et al. 2021) and the amygdala (Odriozola et al. 2019) in ASD. When taken together, these reports of prefrontal hyporconnectivity with sensory, limbic, and salience detection regions could represent an early-emerging disruption in top-down control that later cascades into broader, often complex, sensory, and social behavioral profiles. Thalamic hypoconnectivity with medial prefrontal areas in particular, as observed in this study, could reflect difficulties in top-down regulation of environmental sensory stimuli that have been postulated in the ASD literature (Cerliani et al. 2015; Green et al. 2019).
Given the known role of the thalamus in sensory gating and prior findings of atypical thalamic connectivity in ASD youth with SOR (Green et al. 2017), here we directly examined the relationship between SOR symptom severity and thalamocortical functional connectivity in HL infants. We observed that greater SOR symptoms measured at 6 months of age were significantly predicted by weaker connectivity between the thalamus and frontal cortices at 1.5 months. SOR severity at 6 months was also related to weaker thalamic-mPFC connectivity later on, at 9 months of age (with the addition of the precuneus and middle occipital gyri), suggesting that greater coactivation between the thalamus and multiple core social regions (mPFC and precuneus) commonly considered nodes of the default mode network (DMN) relates to milder sensory symptoms in the first year of life. In line with these results, past work has also shown atypical hypoconnectivity between subcortical/insular regions and the DMN in adolescents with ASD (Nomi and Uddin 2015; Guo et al. 2019). The DMN is generally thought to play a dynamic role in brain function, potentially modulating the interplay between external sensory information and internal representations (Yeshurun et al. 2021) and perhaps contributing to the development of conscious awareness in infancy (Hu et al. 2022). In this context, the heightened coactivation of these core social/attentional areas and the thalamus—the brain’s primary sensory gate—may make sense for infants with fewer SOR symptoms. For these infants, greater thalamic integration with higher order social/control and DMN regions could represent a more efficient balancing act between incoming external sensory inputs and emerging internal representations of the world.
At 9 months, a greater prevalence of 6 month SOR symptoms was additionally related to stronger thalamic connectivity with auditory and somatosensory cortices—primary sensory regions—as well as the inferior frontal gyrus, insula, and basal ganglia. Similarly, a recent study of toddlers with an ASD diagnosis found that thalamic hyperconnectivity with primary auditory cortex was also implicated in sleep problems (Linke et al. 2021), which are related to sensory sensitivity (Tzischinsky et al. 2018). These early patterns may even persist throughout development: a well-powered study of rs-FC (Cerliani et al. 2015) found that, independently of relationships with SOR, youth, and adults with ASD display atypically strong connectivity between subcortical structures (including thalamus and basal ganglia), both primary somatosensory and auditory cortices, and the left IFG. Our findings implicate virtually the same regions in early-emerging sensory symptoms of ASD, which suggests that sensory-associated atypicalities in functional connectivity previously observed in ASD youth are already present in infancy. Our results are also in line with more recent studies directly relating thalamic connectivity to sensory symptoms, which have shown that ASD youth display hyperconnectivity between primary somatosensory cortex and the thalamus during sensory stimulation (Green et al. 2017), and that SOR severity is associated with hyperconnectivity between the salience network and somatosensory cortices (Green et al. 2016). Additionally, in the present study we also found that 6-month SOR predicted greater thalamic connectivity with the basal ganglia at 9 months, which corroborates past work showing that ASD youth experience thalamic-putamen hyperconnectivity during sensory stimulation (Green et al. 2017). The current study thus suggests that the basal ganglia feedback loop—which is well known to gate sensory information between the thalamus and the cortex (Lanciego et al. 2012)—may begin contributing to SOR etiology in infancy. Altogether, our associations with early-emerging SOR are strikingly similar to past findings from toddlerhood to adolescence and, critically, demonstrate that network atypicalities observed in older populations may stem from hyperconnected sensory circuits very early in life.
The observation that, at 9 months, earlier SOR symptom severity was related to heightened thalamic connectivity with sensory cortices, basal ganglia, and insula, but weaker connectivity with higher-order regions (mPFC and precuneus) prompted us to examine whether these patterns reflect a possible functional trade-off whereby infants who showed thalamic hyperconnectivity with sensory cortices and basal ganglia would reliably also show hypoconnectivity with mPFC and precuneus. In the subset of HL participants for whom we had assessed SOR, connectivity patterns associated with sensory sensitivity confirmed a direct trade-off in thalamic connectivity with sensory cortices/basal ganglia, and higher-order associative regions. This indicates that greater connectivity between the thalamus and primary sensory regions may come at the expense of connectivity with higher-order regions involved in social cognition and cognitive control. This trade-off effect was replicated in our sample of HL infants for whom we did not have SOR measures, but not in our TL sample, suggesting that this effect may be specific to HL infants. In light of past work from our lab showing a similar trade-off in salience network connectivity with higher-order prefrontal vs sensory regions in HL infants (Tsang et al. 2021), converging evidence points to early atypicalities in the prefrontal modulation of thalamic gating and attentional systems in ASD. Moreover, past work has demonstrated that thalamus-DMN functional connectivity emerges during the first year of life while thalamic-sensorimotor connections undergo increases in specialization (Alcauter et al. 2014). In this context, our findings on the relationship between earlier sensory sensitivity and later atypicalities in functional connectivity could indicate that the early emergence of SOR is related to concurrent delays in both growth of thalamus-DMN connectivity and specialization between the thalamus and distinct sensorimotor areas. Overall, the strong thalamic connectivity with sensory cortices and basal ganglia observed in infants with higher SOR may reflect less filtering of extraneous sensory stimuli via thalamic gating, while the concomitant prefrontal hypoconnectivity may reflect reduced top-down modulation over incoming sensory information.
The COVID-19 pandemic affected recruitment and retention for this study, and this should be noted as a limitation. Although attrition rates from the 1.5-month to 9-month imaging visits were roughly similar across both groups, we experienced substantial challenges in recruiting additional TL participants to offset attrition in the 9-month imaging sample. In addition, attrition at the 36-month diagnostic visit, coupled with the inability to administer the ADOS due to mask mandates, resulted in the loss of outcome data for many participants (see Supplemental Table 1). Moreover, our sample size is far smaller than those deemed optimal to accurately estimate effect sizes for brain-behavior relationships (Marek et al. 2022). Indeed, samples this large do not yet exist for infants at high likelihood for autism, which is a limitation inherent to conducting research in this population. Nevertheless, while future studies are needed to establish reproducibility, this is the first study to examine the relationship between thalamocortical functional connectivity and SOR, a core feature of ASD, in infancy.
Conclusions and future directions
We identified significant alterations in functional thalamic networks across the first nine months of life, with thalamo-limbic hyperconnectivity in early infancy, thalamic hypoconnectivity with prefrontal cortices in late infancy, and a replicable connectivity profile associated with early SOR symptoms. Importantly, our findings also show that previously reported atypicalities in thalamic connectivity in older individuals with ASD can be observed very early in life, and thus hold promise as an index of ASD vulnerability well before symptoms can be behaviorally assessed. These findings deepen our understanding of the early development of sensory over-responsivity symptoms, indicating that early alterations in sensory, limbic, and associative circuits are already present in infancy. Notably, our results lend support to a current theoretical model (Piven et al. 2017) positing that complex ASD symptoms may unfold as a downstream effect of early disruptions in sensorimotor and attentional networks. Early atypicalities in prefrontal modulation of thalamic gating and attentional systems could directly underlie aberrant sensory processing and decreased salience attribution to social stimuli—core features of ASD. Future work should examine how early disruptions in thalamic connectivity might relate to salience, attentional, and default mode network development (see, e.g. Uddin 2015), as well as the onset of other core ASD symptoms, in order to yield a better understanding of the developing autistic brain and ultimately inform earlier and more targeted interventions.
Supplementary Material
Acknowledgments
The content contained herein is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We sincerely thank the families who have generously donated their time to participate in this research, without whom this work would not be possible.
Contributor Information
Lauren Wagner, Neuroscience Interdepartmental Program, University of California Los Angeles, Los Angeles, CA 90095, United States.
Megan Banchik, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA 90095, United States.
Nana J Okada, Department of Psychology, Harvard Medical School, Boston, MA 02138, United States.
Nicole McDonald, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA 90095, United States.
Shafali S Jeste, Division of Neurology, Children’s Hospital Los Angeles, Los Angeles, CA 90027, United States.
Susan Y Bookheimer, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA 90095, United States.
Shulamite A Green, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA 90095, United States.
Mirella Dapretto, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA 90095, United States.
Author contributions
Lauren Wagner (Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review and editing), Megan Banchik (Investigation, Writing—review and editing), Nana Okada (Investigation), Nicole McDonald (Funding acquisition, Writing—review and editing), Shafali S. Jeste (Funding acquisition, Writing—review and editing), Susan Y. Bookheimer (Funding acquisition), Shulamite Green (Conceptualization, Funding acquisition, Investigation, Supervision, Writing—review and editing), Mirella Dapretto (Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing—review and editing).
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health grant number P50 HD055784 to MD and SYB; the National Institute of Mental Health at the National Institutes of Health grant number R01 MH100028 to MD; and National Institute of Child Health and Human Development grant numbers T32 HD091059 and F31 HD108987 to LW. We are grateful for the generous support of the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, Capital Group Companies Charitable Foundation, William M. and Linda R. Dietel Philanthropic Fund, and Northstar Fund.
Conflict of interest statement: None declared.
Data availability
Upon direct request, authors will share code for statistical analyses and will advise on how to download this study’s data from the National Institutes of Mental Health (NIMH) Data Archive.
References
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, Gilmore JH, Gao W. Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci. 2014:34(27):9067–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.). 2013. [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory experiences questionnaire: discriminating sensory features in young children with autism, developmental delays, and typical development. J Child Psychol Psychiatry. 2006:47(6):591–601. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Carter AS, Kadlec MB, Dunn W. Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. Am J Occup Ther. 2007:61(5):584–592. [DOI] [PubMed] [Google Scholar]
- Bryson S, Zweigenbaum L, McDermott C, Rombough V, Brian J. The autism observation scale for infants: scale development and reliability data. Journal of autism and developmental disorders. 2008:731–738. [DOI] [PubMed] [Google Scholar]
- Carone D, Licenik R, Suri S, Griffanti L, Filippini N, Kennedy J. Impact of automated ICA-based denoising of fMRI data in acute stroke patients. NeuroImage: Clin. 2017:16:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . Autism and developmental disabilities monitoring (ADDM) network. Centers for Disease Control and Prevention. 2022. https://www.cdc.gov/ncbddd/autism/addm.html. [Google Scholar]
- Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C. Increased functional connectivity between subcortical and cortical resting-state networks in autism spectrum disorder. JAMA Psychiatry. 2015:72(8):767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarrusta J, Dimitrova R, Batalle D, O’Muircheartaigh J, Cordero-Grande L, Price A, Hughes E, Kangas J, Perry E, Javed A, et al. Emerging functional connectivity differences in newborn infants vulnerable to autism spectrum disorders. Transl Psychiatry. 2020:10(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W, Daniels DB. Initial development of the infant/toddler sensory profile. Journal of Early Intervention. 2002:25(1):27–41. [Google Scholar]
- Emerson RW, Adams C, Nishino T, Hazlett HC, Wolff JJ, Zwaigenbaum L, Constantino JN, Shen MD, Swanson MR, Elison JT, et al. Functional neuroimaging of high-risk 6-month-old infants predicts a diagnosis of autism at 24 months of age. Sci Transl Med. 2017:9(393):eaag2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry. 1977:18(4):297–321. [DOI] [PubMed] [Google Scholar]
- Gao W, Alcauter S, Elton A, Hernandez-Castillo CR, Smith JK, Ramirez J, Lin W. Functional network development during the first year: relative sequence and socioeconomic correlations. Cereb Cortex. 2015:25(9):2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JB, Piven J. The neurodevelopment of autism from infancy through toddlerhood. Neuroimaging Clin. 2020:30(1):97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddings A-L, Beltz A, Peper JS, Crone EA, Braams BR. Understanding the role of puberty in structural and functional development of the adolescent brain. J Res Adolesc. 2019:29(1):32–53. [DOI] [PubMed] [Google Scholar]
- Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, Tottenham N, Dapretto M, Bookheimer SY. Overreactive brain responses to sensory stimuli in youth with autism Spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013:52(11):1158–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, Dapretto M. Neurobiology of sensory overresponsivity in youth with autism spectrum disorders. JAMA Psychiatry. 2015:72(8):778–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Bookheimer SY, Dapretto M. Salience network connectivity in autism is related to brain and Behavioral markers of sensory overresponsivity. J Am Acad Child Adolesc Psychiatry. 2016:55(7):618–626.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Bookheimer SY, Dapretto M. Reduced modulation of thalamocortical connectivity during exposure to sensory stimuli in ASD. Autism Res. 2017:10(5):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Lawrence KE, Liu J, Tsang T, Yeargin J, Cummings K, Laugeson E, Dapretto M, Bookheimer SY. Distinct patterns of neural habituation and generalization in children and adolescents with autism with low and high sensory overresponsivity. AJP. 2019:176(12):1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Duan X, Suckling J, Heng C, Liao W, Cui Q, Huafu C. Partially impaired functional connectivity states between right anterior insula and default mode network in autism spectrum disorder. Hum Brain Mapp. 2019:40(4):1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez LM, Rudie JD, Green SA, Bookheimer S, Dapretto M. Neural signatures of autism spectrum disorders: insights into brain network dynamics. Neuropsychopharmacology. 2015:40(1):171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Cusack R, Naci L. Typical and disrupted brain circuitry for conscious awareness in full-term and preterm infants. Brain Commun. 2022:4(2):fcac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang WJ, Kwak YB, Cho KIK, Lee TY, Oh H, Ha M, Kim M, Kwon JS. Thalamic connectivity system across psychiatric disorders: current status and clinical implications. Biol Psychiatry Global Open Sci. 2022:2(4):332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2012:2(12):a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke AC, Chen B, Olson L, Ibarra C, Fong C, Reynolds S, Apostol M, Kinnear M, Müller R-A, Fishman I. Sleep problems in preschoolers with autism spectrum disorder are associated with sensory sensitivities and thalamocortical overconnectivity. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021:8(1):21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, Kinsbourne M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006:10(2):155–172. [DOI] [PubMed] [Google Scholar]
- Liu J, Okada NJ, Cummings KK, Jung J, Patterson G, Bookheimer SY, Jeste SS, Dapretto M. Emerging atypicalities in functional connectivity of language-related networks in young infants at high familial risk for ASD. Dev Cogn Neurosci. 2020:45:100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule. 2nd ed. Los Angeles (CA): Western Psychological Services. 2012. [Google Scholar]
- Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, Donohue MR, Foran W, Miller RL, Hendrickson TJ, et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022:603(7902):654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. Circle Pines, MN: AGS; 1995 [Google Scholar]
- Müller R-A, Fishman I. Brain connectivity and neuroimaging of social networks in autism. Trends Cogn Sci. 2018:22(12):1103–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Treiber JM, Shukla DK, Shih P, Müller R-A. Impaired thalamocortical connectivity in autism spectrum disorder: a study of functional and anatomical connectivity. Brain. 2013:136(Pt 6):1942–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Carper RA, Abbott AE, Chen CP, Solders S, Nakutin S, Datko MC, Fishman I, Müller R. Regional specificity of aberrant thalamocortical connectivity in autism. Hum Brain Mapp. 2015:36(11):4497–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Jalal R, Liu J, Tsang T, McDonald NM, Jackson L, Ponting C, Jeste SS, Bookheimer SY, Dapretto M. Altered thalamocortical connectivity in 6-week-old infants at high familial risk for autism spectrum disorder. Cereb Cortex. 2021:31(9):4191–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y. Development of the thalamus: from early patterning to regulation of cortical functions. WIREs Dev Biol. 2019:8(5):e345. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ. Developmental changes in large-scale network connectivity in autism. NeuroImage: Clin. 2015:7:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odriozola P, Dajani DR, Burrows CA, Gabard-Durnam LJ, Goodman E, Baez AC, Tottenham N, Uddin LQ, Gee DG. Atypical frontoamygdala functional connectivity in youth with autism. Dev Cogn Neurosci. 2019:37:100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson S, Carver LJ, Constantino JN, Dobkins K, et al. Recurrence risk for autism spectrum disorders: a baby siblings research consortium study. Pediatrics. 2011:128(3):e488–e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L, Fulcher B, Yücel M, Fornito A. An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI. NeuroImage. 2018:171:415–436. [DOI] [PubMed] [Google Scholar]
- Piven J, Elison JT, Zylka MJ. Toward a conceptual framework for early brain and behavior development in autism. Mol Psychiatry. 2017:22(10):1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014:84:320–341. 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, Buitelaar JK, Beckmann CF. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage. 2015a:112:278–287. [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. 2015b:112:267–277. [DOI] [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, Benasich AA, Gaab N. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann N Y Acad Sci. 2012:1252(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AM, Malow BA. Sleep and autism spectrum disorders. Pediatr Clin. 2011:58(3):685–698. [DOI] [PubMed] [Google Scholar]
- Scheinost D, Chang J, Lacadie C, Brennan-Wydra E, Foster R, Boxberger A, Macari S, Vernetti A, Constable RT, Ment LR, et al. Hypoconnectivity between anterior insula and amygdala associates with future vulnerabilities in social development in a neurodiverse sample of neonates. Sci Rep. 2022:12:16230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yap P-T, Wu G, Jia H, Gilmore JH, Lin W, Shen D. Infant brain atlases from neonates to 1- and 2-year-olds. PLoS One. 2011:6(4):e18746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz S, Klin A, Jones W. Neonatal transitions in social behavior and their implications for autism. Trends Cogn Sci. 2018:22(5):452–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL—ScienceDirect. NeuroImage. 2004:23(Supplement 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Thapar A, Rutter M. Genetic advances in autism. J Autism Dev Disord. 2021:51(12):4321–4332. 10.1007/s10803-020-04685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Grove LE, Lozon TA, Vila AM, Ye Y, Nye MJ, Manning JH, Pappas A, Hernandez-Andrade E, Yeo L, et al. Age-related increases in long-range connectivity in fetal functional neural connectivity networks in utero. Dev Cogn Neurosci. 2015:11:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang T, Green S, Liu J, Lawrence K, Jeste S, Bookheimer SY, Dapretto M. Altered salience network connectivity in 6-week-old infants at risk for autism. bioRxiv. 2021. 10.1101/2021.10.27.466195. [DOI] [PMC free article] [PubMed]
- Tzischinsky O, Meiri G, Manelis L, Bar-Sinai A, Flusser H, Michaelovski A, Zivan O, Ilan M, Faroy M, Menashe I, et al. Sleep disturbances are associated with specific sensory sensitivities in children with autism. Mol Autism. 2018:9(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015:16(1):55–61. [DOI] [PubMed] [Google Scholar]
- Wen X, Zhang H, Li G, Liu M, Yin W, Lin W, Zhang J, Shen D. First-year development of modules and hubs in infant brain functional networks. NeuroImage. 2019:185:222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ET, Cummings KK, Jung J, Patterson G, Okada N, Guo J, O’Neill J, Dapretto M, Bookheimer SY, Green SA. Sensory over-responsivity is related to GABAergic inhibition in thalamocortical circuits. Transl Psychiatry. 2021:11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Giraldo-Chica M, Rogers B, Cascio CJ. Thalamocortical dysconnectivity in autism spectrum disorder: an analysis of the autism brain imaging data exchange. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017:2(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshurun Y, Nguyen M, Hasson U. The default mode network: where the idiosyncratic self meets the shared social world. Nat Rev Neurosci. 2021:22(3):181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon direct request, authors will share code for statistical analyses and will advise on how to download this study’s data from the National Institutes of Mental Health (NIMH) Data Archive.


