Abstract
The establishment of cortical representations critical for mounting language is supported by both ongoing neural maturation and experience-expectant plasticity as infants increasingly recognize the linguistic events that occur most often in their surrounding environment. Previous research has demonstrated that enhanced efficiency of syllabic representation and discrimination is facilitated by interactive attention-driven, nonspeech auditory experience. However, experience-dependent effects on syllable processing as a function of nonspeech, passive auditory exposure (PAE), remain unclear. As theta band-specific activity has been shown to support syllabic processing, we chose theta inter-trial phase synchrony to examine the experience-dependent effects of PAE on the processing of a syllable contrast. Results demonstrated that infants receiving PAE increased syllabic processing efficiency. Specifically, compared with controls, the group receiving PAE showed more mature, efficient processing, exhibiting less theta phase synchrony for the standard syllable at 9 months, and at 18 months, for the deviant syllable. Furthermore, the PAE modulatory effect on theta phase synchrony at 7 and 9 months was associated with language scores at 12 and 18 months. These findings confirm that supporting emerging perceptual abilities during early sensitive periods impacts syllabic processing efficiency and aligns with literature demonstrating associations between infant auditory perceptual abilities and later language outcomes.
Keywords: auditory plasticity, development, inter-trial phase locking, speech perception, training
Introduction
A distinctive characteristic of the infant’s brain is its developmentally regulated plasticity, which dynamically adapts in response to continuing environmental input, thus allowing representations of key sensory information to be established in the cortex (Hebb 1949). Ongoing neural plasticity inherent to normative development (maturational plasticity) occurs along with plasticity modulated by extrinsic experiences (experience-expectant plasticity), particularly during sensitive periods in which the brain is most responsive to important environmental cues and hence, more flexible to change (Greenough et al. 1987; Ismail et al. 2017; DeMaster et al. 2019). In that way, emerging neural connections are shaped not only by the anticipated maturational path common to all but also by the specific experiences unique to each one (Galvan 2010; Takesian and Hensch 2013; Kolb et al. 2017; Bennett et al. 2018; Reh et al. 2020). A clear example of this powerful combined plasticity is evident in the prelinguistic period when the foundations of language are being established (White et al. 2013; Gervain 2015; Werker and Hensch 2015).
Studies have demonstrated that before beginning to talk, young infants are able to decode and discriminate speech and nonspeech information in the tens of milliseconds (ms) range (Eilers et al. 1981; Aslin 1989; Benasich and Tallal 2002; Tallal 2004; Rivera-Gaxiola et al. 2005; Ortiz-Mantilla et al. 2013, 2016; Benasich et al. 2014). In addition, they learn to segment the speech stream into small syllabic units (Greenberg 1998), identify these acoustic segments as familiar linguistic tokens, and subsequently create accurate representations of these native sounds within the auditory cortex (Eilers et al. 1981; Werker and Tees 2005; Ortiz-Mantilla et al. 2016). During this time in which linguistic perceptual narrowing happens, infants use statistical and distributional learning, 2 domain-general mechanisms to facilitate the acquisition of the native language phonetic categories (Liu and Kager 2017; Reh et al. 2021). Syllabic segments are considered the basic units of speech perception that, once mapped within temporal cortical areas, facilitate access from the acoustic level to the mental lexicon (Bertoncini and Mehler 1981; Greenberg 1998; Eimas 1999; Jusczyk 1999; Jusczyk et al. 1999; Hickok and Poeppel 2007; Hagoort and Poeppel 2013; Räsänen et al. 2018).
Adults (Ding and Simon 2014; Greenberg and Kinsbury 1997; Jin et al. 2014; Obleser et al. 2012) and infants (Ortiz-Mantilla et al. 2016; Kalashnikova et al. 2018; Nacar et al. 2018) encode and discriminate syllabic information in the 4 to 7 Hz theta range which corresponds with the most frequent syllable duration of 200 ms (Greenberg 1998; Hickok and Poeppel 2000, 2007; Hickok 2001; Poeppel 2003; Giraud and Poeppel 2012; Ghitza 2013; Hyafil et al. 2015). Theta phase synchrony has a prominent role in shaping syllable perception (Kayser et al. 2012; Peelle and Davis 2012), relates to syllable identity (ten Oever and Sack 2015), and is critical for speech intelligibility (Ahissar et al. 2001; Luo and Poeppel 2007; Gross et al. 2013; Peelle et al. 2013). Phase modulation in the theta range during syllable processing has also been associated with later language abilities in typically developing infants (Ortiz-Mantilla et al. 2022) and in infants at familial risk for language and reading disorders (Cantiani et al. 2019; Mittag et al. 2022).
Most children master their prelinguistic tasks seamlessly with support from the brains’ rapid auditory processing (RAP) abilities; RAP enables fine-grained acoustic analysis within the tens of ms range, a fundamental skill required for decoding speech and for efficient establishment of the cortical representation of native language sounds (Aslin 1989). However, for some children, including those at familial risk for developmental language and reading disorders such as specific language impairment and dyslexia, language acquisition does not always proceed smoothly (Benasich et al. 2016). Genetically mediated deficits in RAP abilities that may appear during the first months of life (Riva et al. 2018) have been consistently linked to later language outcomes (Benasich et al. 2002, 2006; Benasich and Tallal 2002; Tallal 2004; Tsao et al. 2004; Guttorm et al. 2005; Leppänen et al. 2010; Choudhury and Benasich 2011; Benasich and Choudhury 2012; Maitre et al. 2013; Cantiani et al. 2016, 2019).
Infants at high risk for developmental language disorders (DLD) because of familial history might require additional assistance to help them master early prelinguistic abilities including RAP. Animal research has demonstrated a high degree of plasticity in primary auditory cortex with experience-dependent effects that tune and shape auditory cortical representations (Buonomano and Merzenich 1998; Zhang et al. 2001; de Villers-Sidani et al. 2007; Sanes and Bao 2009; Froemke and Jones 2011; Homma et al. 2020; Kudo et al. 2020), in particular during developmental critical or sensitive periods (Schreiner and Polley 2014). Therefore, a reasonable approach to early intervention would be to support the development of RAP efficiency by taking advantage of the experience-dependent plasticity that occurs when enhanced experiences are provided during these early sensitive periods (Minagawa-Kawai et al. 2011; Kolb et al. 2017). In humans, several studies have reported the impact of both musical and nonmusical experiences during development. For example, infants receiving active musical training improved their processing of auditory patterns, metrical perception, rhythm, and sensitivity to tonality (Gerry et al. 2010, 2012; Trainor et al. 2012; Cirelli et al. 2016); increased the development of prelinguistic communicative gestures (Gerry et al. 2012) and the temporal structure of both music and nonwords (Zhao and Kuhl 2016); and demonstrated enhanced language outcomes as compared with non-trained infants (Dondena et al. 2021). An active nonmusical auditory experience that consisted of spectrotemporally modulated nonspeech stimuli markedly improved processing of key prelinguistic cues of familiar and new nonspeech sounds (Benasich et al. 2014) as well as promoting fine-tuning of acoustic mapping (Musacchia et al. 2017). Further study revealed that this particular nonspeech intervention not only facilitated the formation of cortical phonemic representations (Ortiz-Mantilla et al. 2019) but also supported more accurate syllabic representation and discrimination, an effect that was associated with linguistic outcomes (Ortiz-Mantilla et al. 2022).
To the best of our knowledge, however, few studies have reported modulatory experience-dependent effects using passive auditory protocols. Infants passively exposed to musical recordings showed enhanced generalization responses to pitch tones (Trainor et al. 2012), increased differentiation of foreign rhythmic patterns (Hannon and Trehub 2005), and had stronger electrophysiological response to prenatally exposed melodies (Partanen et al. 2013). However, whereas no generalization effects on language were found with passive exposure to music (Gerry et al. 2012) early passive exposure to spectrotemporally modulated nonspeech sounds resulted in generalization to speech with spectral power modulation in the theta range at 9 months, promoting more efficient syllabic processing in exposed but not in control infants (Ortiz-Mantilla et al. 2019). It seems clear that both active experience and passive exposure to nonspeech stimuli containing spectrotemporal speech-like cues prompt experience-dependent effects during speech processing in typically developing infants. But, it is still not known whether the modulatory effect seen on theta spectral power during syllable processing in 9-month-olds who received passive auditory exposure at 4.5–6 months (Ortiz-Mantilla et al. 2019): (i) extends from the more general theta spectral power activation to the more precise intertrial theta phase-locked synchrony, (ii) persists beyond the first year of life, and more importantly, (ii) whether the impact of early passive exposure on syllable processing relates to later language abilities.
In this intervention study, that has the potential to help infants at familial risk for DLD, as well as typically developing infants, we aimed to support and optimize RAP prelinguistic abilities and thus, discrimination of the linguistic elements that are basic to language acquisition. We investigated whether using passive exposure to speech-like but nonlinguistic pairs of sounds, separated by interstimulus intervals (ISI) in the tens of ms range, influences later language abilities. Previous infant studies (Bosseler et al. 2013; Ortiz-Mantilla et al. 2013, 2016, 2019, 2022) have shown that activity in the theta frequency band supports syllable processing. Furthermore, variation of theta phase synchrony in the left auditory areas as a function of active auditory training is associated with later language (Ortiz-Mantilla et al. 2022). Thus, we examined theta phase synchrony modulation to syllables, in left (LAC) and right (RAC) auditory cortices, across ages, in infants that had received passive auditory exposure to nonspeech. Based on what is known about nonspeech active training paradigms (Ortiz-Mantilla et al. 2019, 2022; Dondena et al. 2021), we postulated that experience-dependent effects of early passive exposure to nonspeech might well modulate inter-trial phase synchrony in the theta range during syllabic processing, which, in turn, may optimize later language outcome.
Materials and methods
Methods for the auditory exposure paradigm, EEG–ERP recordings, source localization, and electrophysiological and statistical analyses used in this study closely follow those reported in previous publications from the Infancy Studies Laboratory as the same techniques, paradigms, and equipment are in use across studies (Hämäläinen et al. 2011, 2019; Ortiz-Mantilla et al. 2012, 2013, 2016, 2019, 2022; Musacchia et al. 2013, 2015, 2017; Benasich et al. 2014). Summarized versions of the methodology used are included in each subsection.
Participants
The initial sample included 117 typically developing infants, which were part of a larger, mixed-design, longitudinal study. Participants were assigned to the longitudinal group or to one of the 4 age-matched cross-sectional groups; a subset of these infants was also included in previous reports (Musacchia et al. 2013, 2017; Benasich et al. 2014; Ortiz-Mantilla et al. 2019, 2022). The 20 infants that comprised the longitudinal group (PEx group) were recruited at 4 months-of-age to participate in passive auditory exposure (PAE) to nonspeech sounds once a week for 6 weeks. The PEx group was followed longitudinally at 7, 9, 12, and 18 months. At 7 months, data for one subject were of poor quality; at both 9 and 12 months, data for 1 subject were missing, and 3 participants did not return for the 18-month visit. Fifteen PEx participants had good-quality data at each age point. For the control sample, 97 cross-sectional infants were initially recruited, 14 of which were later excluded because of data quality or technical issues leaving a final cross-sectional sample of 83 infants. Those 83 infants, 18 at 7 months, 23 at 9 months, 21 at 12 months, and 21 at 18 months of age, comprised the 4 cross-sectional naïve control (NC) groups (NC07, NC09, NC12, NC18). To tease apart maturational and experience-expectant from experience-dependent neural plasticity, we used combined cross-sectional and longitudinal design methodologies. The use of a longitudinal approach in which experimental trajectories are examined multiple times throughout a developmental period allows tracking of whether the brain activity has changed in association with training on a specific task or exposure to a particular sensory input. Because this approach is within-subject, it is advantageous in providing the optimal statistical power to identify, across time, neural changes associated with the experience of interest. On the other hand, cross-sectional samples comprised of subjects that have not undertaken the training or experience, although providing less statistical power than longitudinal samples, serve well to realistically assess normative developmental trajectories on the process investigated. All 103 participants in the final sample (52 females/51 males; PEx: 11/9; NC07: 9/9; NC09: 10/13; NC12: 12/9; NC18: 10/11) had uneventful prenatal and perinatal circumstances and were born healthy, full-term, normal birth weight into monolingual English families. Mothers of all infants completed high school (data for 6 mothers was not available) and 5 of the mothers did not have a college education (mean post-high school years of education: 4.99 years, SD: 2.30, range: 0–11 years). Participants were recruited from urban and suburban areas in New Jersey and had no family history of specific language impairment, autism, hearing loss, no repeated episodes of otitis media, or other relevant medical or neurological disorders, and had passed the newborn hearing screening. Parents received compensation for their time and infants were given a toy after the visit. The study was conducted following the Declaration of Helsinki. Written informed consent approved by our university’s Human Subjects Institutional Review Board was obtained from caregivers before the child’s inclusion in the study.
Procedure
Behavioral protocol for passive auditory exposure (PAE)
The PAE protocol administered was identical to that described in previous reports (Benasich et al. 2014; Musacchia et al. 2017; Ortiz-Mantilla et al. 2019). After their initial evaluation at 4 months of age, infants in the PEx group visited the lab once a week for 6 consecutive weeks between 4.5 and 6 months of age (mean: 4.7 [SD: 0.2]–5.98 [0.2] months). During each visit, the infant sat in an infant seat placed equidistant between left and right speakers in a sound-attenuated and electrically shielded sound booth (Industrial Acoustics Company). The stimuli were passively presented in the free field, whereas the infant was quietly entertained with puppets/toys to maintain alertness. Thus, no infant response or overt attention was required during sound exposure. Two blocks of stimuli were presented in random order at each session, for 10 min at 40 ms ISI and 10 min at 70 ms ISI, both ISIs are typical of transitions within the speech range. Three different pairs of nonspeech auditory stimuli were used in the PAE protocol as follows: weeks 1 and 2, complex tones (standard (STD): 800–800 Hz; deviant (DEV): 800–1,200 Hz); weeks 3 and 4, bandpass noise (STD: 400–1,900 Hz and 400–1,900 Hz; DEV: 400–1,900 Hz and 800–1,900 Hz); and weeks 5 and 6, simple sweeps (STD: 1,600–1,200 Hz and 1,600–1,200 Hz; DEV: 1,600–1,200 Hz and 1,200–1,600 Hz). The PAE protocol was designed to increase rapid auditory spectrotemporal processing efficiency through controlled background exposure (Benasich et al. 2014).
Event-related potentials
Event-related potentials stimuli
The stimuli used for the event-related potentials (ERP) were computer-generated consonant-vowel syllables that differed by 40 ms in voice onset time (VOT), which allowed us to investigate the generalization effects of the nonspeech PAE on speech. The STD stimulus was the syllable /da/ (VOT = 0 ms; 100 ms duration) and the DEV stimulus was /ta/ (VOT = 40 ms; 100 ms duration). Both syllables shared the same place of articulation and contained a stop consonant, voiced for the STD and voiceless for the DEV, followed by a 60 ms steady-state vowel. The fundamental frequency of each syllable was 120 Hz and the 3 formants F1, F2, and F3 were 750, 1,200, and 2,500 Hz, respectively. The stimulus onset-to-onset interval was 1,020 ms, and the offset-to-onset interval was 920 ms. The ERP stimuli were presented in a pseudo-randomized passive oddball paradigm, using a block design comprised of 85% STD and 15% DEV, with at least 3 and no more than 12 STD given before each DEV stimulus. Syllables were presented binaurally using E-Prime software (Psychology Software Tools) in a sound-attenuated free field environment at 75 dB SPL (Ortiz-Mantilla et al. 2019, 2022).
EEG recording and ERP data processing
Dense array EEG/ERP recordings were acquired at each follow-up visit (7, 9, 12, and 18 months), whereas participants were seated on their parent’s lap, watching a silent movie, or entertained with silent toys to keep them calm and engaged (Musacchia et al. 2015). The EEG was recorded from 125-channel EGI pediatric sensor nets (Electrical Geodesic, Inc. Eugene, Oregon) for most of the longitudinal (PEx07: 18; PEx09: 17; PEx12: 17; PEx18: 16) and cross-sectional (NC07: 14; NC09: 17; NC12: 17; NC18: 14) participants. For a subset, it was recorded with 125-channel EGI sensor Hydrocel nets (PEx07: 2; PEx09: 2; PEx12: 2; PEx18: 1; NC07: 4; NC09: 6; NC12: 4; NC18: 7). The vertex channel was used as an online reference, with a sampling rate of 250 Hz, and 0.1–100 Hz high pass–low pass filters. Artifact correction of eye movements was conducted on the raw data using an automatic correction algorithm based on the principal component analysis method (PCA), a standard routine included in the Brain Electrical Source Analysis (BESA GmbH) 7.1 software. To keep consistency, all EEG–ERP recordings previously processed using BESA 5.3 and 6.1 software, a subset of which has already been published (Ortiz-Mantilla et al. 2019, 2022, respectively), were re-processed using the updated BESA 7.1 software version. For ERP processing, EEGs were band-pass filtered off-line 1–15 Hz and re-referenced to a whole-head average reference. EEG/ERP data were then segmented into epochs according to stimulus type (STD, DEV) with 300 ms pre-stimulus, 1,020 ms poststimulus times, and 100 ms before stimulus onset used as the baseline. Epochs with signals exceeding ±300 μV from the baseline were excluded. After data cleaning, a total of 158 recordings were included in the analyses. However, it is important to note that the grand average ERP waveforms included only data from participants for which EEG data were recorded from 125-channel EGI sensor nets (PEx: 68, NC: 62). For a small subset (PEx: 7, NC: 21), it was recorded with 125-channel EGI sensor Hydrocel nets that have a different sensor layout precluding combining data acquired from both sensor nets in the grand average ERP file. The experience-dependent effects of early nonspeech auditory exposure on syllable representation were examined using the STD stimulus. The STD response denotes a short-term memory trace in the auditory cortex of the regularity of a repetitive sound (Näätänen and Alho 1997; Näätänen and Winkler 1999). Experience-dependent effects of PAE on syllable discrimination were examined using the DEV stimulus, which reflects the detection of a sporadic stimulus incongruent with the memory representation of the preceding sound (Näätänen et al. 2007). In oddball paradigms, the STD is the most repeated sound, which facilitates the formation of a strong memory trace, and a robust cortical phonemic representation (Haenschel et al. 2005; Azaiez et al. 2022). The STD syllable was presented 566 times assuring a good signal-to-noise ratio and thus, an increased probability of having a more reliable measure of syllable representation, whereas the DEV syllable was presented 100 times. A minimum of 70% STD (396) and DEV (70) artifact-free epochs per infant were required for inclusion in ERP averaging. The groups did not differ in the number of STD and DEV trials accepted for ERP analysis at any of the ages examined. The mean average number of trials across the 4 age points was: STD: 481 for PEx, 472 for NC; DEV: 86 for PEx, 83 for NC.
Source localization of ERP generators
Source localization is a technique used to identify the loci of the neural activation registered at the scalp surface. This approach enables the high temporal resolution provided by EEG/ERP to be combined with structural images thus allowing a closer spatial approximation as to where in the brain neural responses are being generated. The source localization technique has proven very helpful to examine lateralization of auditory responses to speech and nonspeech sounds during early development (Musacchia et al. 2013, 2015, 2017; Ortiz-Mantilla et al. 2013, 2016, 2019, 2022; Cantiani et al. 2019; Hämäläinen et al. 2019). To localize source generators of the perceptual response to the STD and DEV syllables, EEG/ERPs were mapped onto a 0–18 months head template provided by BESA Research 7.1 software. Based on PCA the first positive peak (P1) for the STD and DEV responses was identified in the grand average ERP of each NC group and the PEx group at each age. The P1 peak was chosen for source localization as it is an obligatory speech response that reflects not only the detection of the sound but also initial phoneme identification (Hämäläinen et al. 2015; Kuuluvainen et al. 2016; Azaiez et al. 2022). A discrete dipole source model (Scherg and Von Cramon 1985) using a 4-shell ellipsoidal head model was applied to the P1 within each STD and DEV condition for source localization. Following a protocol developed for infant source modeling (Hämäläinen et al. 2011; Ortiz-Mantilla et al. 2012) a time window of 40 ms (± 20 ms around the peak) was used for dipole fitting. A 2-dipole model explaining most of the P1 variance identified sources of activation in left (LAC) and right (RAC) auditory cortices. The source montage generated during discrete dipole fitting in the grand average of each condition (STD, DEV) and group (PEx, NC) at each age (7, 9, 12, and 18 months) was saved for further time-frequency analyses at the source level.
Time-frequency analyses
Spectrotemporal changes in event-related oscillations during STD and DEV syllable processing were then examined at the source level following an established protocol (Ortiz-Mantilla et al. 2013, 2016, 2019, 2022). The previously saved 2-source montage (that functions as a fixed spatial filter) from the grand average source model for each condition and age was applied to the raw 125-channel recording of everyone in the corresponding group to transform the continuous EEG into 2-channel source space (Hoechstetter et al. 2004). To control for low-frequency activity (slow drifts) while at the same time preserving as much of the frequency information as possible, only a low cutoff of 0.5–1 Hz was applied to the raw EEG data (Cohen 2014; Paul et al. 2021). A complex demodulation method with 1 Hz wide frequency bins and 50 ms time resolution, from −300 to 1020 ms in the range of 2–90 Hz was used next for decomposing the single-trial EEG data into time-frequency representations (Paul et al. 2021). As theta frequency-band is particularly suited to processing syllabic information, in this study, event-related changes in the magnitude of inter-trial phase synchrony (Tallon-Baudry et al. 1996; Hari and Salmelin 1997; Tallon-Baudry and Bertrand 1999) were investigated within the lower frequency bands (2–10 Hz), using inter-trial phase-locking (ITPL). ITPL is a measure of phase synchrony that determines the stability of the phase alignment across trials. ITPL is reported between 0 and 1, in which 0 represents random phase alignment and 1 total phase alignment. ITPL individual results, generated for the STD and DEV in left and right auditory sources for each group at each age, were exported to MATLAB R2021b (MathWorks) for plotting graphics across subjects.
Standardized behavioral measures
Cognitive assessment
The Bayley Scales of Infant and Toddler Development, Third Edition (Bayley 2006) evaluates developmental status between 1 and 42 months using 5 developmental domains: adaptative behavior, cognitive, language, motor, and social–emotional. The Cognitive Subscale, which examines sensorimotor development, exploration, and manipulation, object relatedness, memory, and habituation among others, was administered at each age to assess cognitive abilities. The Cognitive Composite standard score is reported for this subscale (mean: 100, SD:15).
Language assessment
The Preschool Language Scale-4th ed. (PLS-4; Zimmerman et al. 2002), which assesses receptive (Auditory Comprehension) and expressive (Expressive Communication) language skills in children from birth to 6 years 11 months of age, was also administered at each age. In this study, standard scores for the Auditory Comprehension and Expressive Communication subscales were used (mean: 100, SD:15).
The MacArthur Communicative Development Inventories (CDI; Fenson et al. 1993) is a parental questionnaire that provides a valid and efficient means of assessing communicative gestures and play, early imitation, language comprehension, language production, and the early stages of grammatical development. At 12 months, parents completed the infant version (Words and Gestures) that measures expressive language (words produced), language comprehension (words understood), and nonlinguistic communication (number of gestures). At 18 months, parents filled out the toddler version (Words and Sentences) that primarily measures language expression (words produced) and early syntactic language skills (irregular words and sentence complexity). The CDI provides age- and gender-normed percentile scores shown to be stable over time as well as highly correlated with other language and communication measures. However, in this study we followed the infant literature (Bettoni et al. 2020; Bosseler et al. 2021; Mittag et al. 2022) and compared raw scores for these measures, converting them to z-scores for statistical analyses where appropriate.
Statistical analyses
Descriptive statistics, demographic analyses, and behavioral comparisons were conducted using analysis of variance (ANOVAs) and chi-square in SPSS Statistics 28 (SPSS, Inc.) software. All time-frequency analyses were conducted in BESA Statistics 2.0 (BESA GmbH, Gräfelfing, Germany) software. High-density EEG experiments frequently result in high-dimensional outcomes, particularly in the time-frequency domain; cluster-based permutation tests take care of the multiple comparison problem, providing high statistical power while controlling the false positive error rate (Sassenhagen and Draschkow 2018). For comparisons between 2 groups, BESA Statistics uses parameter-free, cluster-based permutation testing based on Student’s t-test, and for within and between comparisons in which more than 2 comparisons are estimated, the comparison is based on permutation ANOVA (Maris and Oostenveld 2007). Correction for multiple comparisons in post hoc tests is addressed using a Bonferroni–Holm correction. Results are considered corrected for multiple comparisons as only those clusters will be identified that have higher cluster values than 95% of all clusters derived by random permutation of data. For a more detailed description of these methods refer to the BESA Statistics website and User-Manual (Bornfleth et al. 2020, https://www.besa.de/products/besa-statistics/besa-statistics-overview/; https://www.besa.de/wp-content/uploads/2014/05/BESA-Statistics-2.1-User-Manual.pdf). In this study, there were no predefined clusters as after 1,000 permutations, time-frequency regions (clusters) with significant changes in the magnitude of ITPL within/between ages/groups are automatically identified. To determine the experience-dependent effects of the PAE on syllable processing, ITPL in the theta range was examined. For the PEx longitudinal analysis, a 2 × 4 (source [LAC, RAC] × age [07, 09, 12, 18]) within-group permutation ANOVA was conducted followed by a post hoc permutation Scheffe’s test to determine which pairwise comparisons were responsible for the age main effect. Effects of normative maturation were examined cross-sectionally between the NC groups using a 2 × 4 (source [LAC, RAC] × group [NC07, NC09, NC12, NC18]) permutation ANOVA followed by post hoc permutation Scheffe’s test to determine which pairwise comparisons were responsible for the group main effect. Group differences in theta ITPL between the PEx and the age-matched NC group were examined at each age using permutation t-tests. To assess the association between inter-trial phase synchrony and linguistic outcome, cluster permutation testing based on Pearson correlation analysis (Freedman and Lane 1983; O’Gorman 2012) was conducted in BESA Statistics 2.0. For permutation 2-sided analyses, significance was determined at alpha < 0.025.
Data availability: the data used in the analyses reported here are not yet publicly available as it is part of a large longitudinal study still underway. At the study’s end, the data will be made available upon request by e-mail to the corresponding author (S.O.M.) or the senior author (A.A.B.), contingent on a formal data-sharing agreement.
Results
The preliminary analyses did not find differences in gestational age (F(4, 100) = 2.143, P = 0.081), birth weight (F(4, 97) = 1.01, P = 0.41), sex (X2 = 1.06, P = 0.90), or maternal education (F(4, 96) = 1.41, P = 0.235) among the groups (Table 1).
Table 1.
Characteristics of the final sample for the longitudinal PEx group and the 4 age-matched cross-sectional NC groups.
| Group (# infants) | Sex F/M | Gestational age weeks (SD) | GA range | Birth weight grams (SD) | BW range |
|---|---|---|---|---|---|
| PEx (20) | 11/9 | 39.2 (.83) | 38–40 | 3410.2 (510.4) | 2579.8–4592.5 |
| NC07 (18) | 9/9 | 39.8 (1.1) | 38–42 | 3504.1 (425.3) | 2778.2–4224.0 |
| NC09 (23) | 10/13 | 39.6 (1.0) | 38–41 | 3364.9 (468.0) | 2693.2–4507.5 |
| NC12 (21) | 12/9 | 39.9 (1.3) | 38–42 | 3554.6 (462.5) | 2721.5–4224.0 |
| NC18 (21) | 10/11 | 39.1 (1.0) | 37–41 | 3306.9 (290.9) | 2693.2–3968.9 |
| Total (103) | 52/51 | 39.5 (1.1) | 37–42 | 3421.3 (438.9) | 2579.8–4592.5 |
Notes: F/M, female/male; GA, gestational age; SD: standard deviation; BW, birth weight; PEx, passive exposure group; NC07, 7-months naïve control group; NC09, 9-months naïve control group; NC12, 12-months naïve control group; NC18, 18-months naïve control group.
Standardized behavioral assessments
The PEx and NC groups had cognitive and language scores within the normal range at 12 and 18 months. No significant differences in expressive and receptive language or cognitive abilities were found between the PEx and age-matched groups. It seems clear that the large standard deviations may have accounted for the lack of significance between groups, though it is interesting to note that the means for the PEx group are consistently higher than for the NC group. At the individual level, 2 participants in the PEx group at 12 months and one participant in the NC12 group scored below the standard deviation (<85) for PLS-4 receptive language. The accuracy of the scoring was confirmed by a careful review of the videos recorded during the test session, the scoring sheets, and the written comments from the examiner. Participants that scored below the norm in PLS-4 receptive language at 12 months had expressive language scores within the normal range at that age and their scores in both receptive and expressive language were within the normal range at 18 months, thus, we kept them in the analysis (means, standard deviations, and P-values are provided in Table 2).
Table 2.
Descriptive statistics (means, standard deviations, and P-values) for standardized Bayley cognitive scores, PLS-4, and CDI language assessments at 12 and 18 months of age.
| Measure | PEx12 | NC12 | P-value | PEx18 | NC18 | P-value |
|---|---|---|---|---|---|---|
| BAY-CCS | 106.1 (8.9) | 110.9 (9.8) | 0.108 | 107.1 (11.7) | 100.2 (9.7) | 0.057 |
| PLS-EXS | 106.7 (8.2) | 105.1 (7.4) | 0.521 | 103.4 (10.7) | 104.3 (7.6) | 0.759 |
| PLS-RCS | 96.9 (10.8) | 94.8 (10.6) | 0.541 | 103.9 (11.1) | 97.3 (12.7) | 0.099 |
| CDI-TGR | 25.6 (12) | 24.3 (11) | 0.997 | |||
| CDI-WUR | 105.2 (94) | 65.17 (51) | 0.999 | |||
| CDI-WPR | 8.05 (8.4) | 5.83 (7.4) | 1.000 | 128.7 (114) | 78.95 (95) | 0.995 |
Notes: PEx12, passive exposure group at 12 months; NC12, 12-months naïve control group; PEx18, passive exposure group at 18 months; NC18, 18-months naïve control group; BAY-CCS, Bayley Cognitive Composite Score; PLS, The Preschool Language Scale-4th ed; PLS-EXS, PLS-4 Expressive standard score; PLS-RCS, PLS-4 Receptive standard score; CDI, MacArthur-Bates Communicative Development Inventory; CDI-TGR, # of total gestures raw score at 12 months; CDI-WUR, # of words understood raw score at 12 months; CDI-WPR, # Words Produced raw score at 12 and 18 months. Note that raw scores were converted to z-scores for statistical analyses.
Source localization of ERP generators
The ERP response to the STD and DEV stimuli at each age for the PEx and each age-matched NC group was characterized by a fronto-central positivity followed by a negative deflection resembling those reported in other infancy studies using syllables differing in VOT (Rivera-Gaxiola et al. 2005; Ortiz-Mantilla et al. 2012, 2013, 2016). The grand average ERP waveforms included only data from participants for which EEG/ERP was recorded from 125-channel EGI sensor nets (PEx: 68, NC: 62). Generators of the P1 ERP response to STD and DEV were identified from the grand average ERP waveforms and a 2-dipole model freely fitted for each group, stimulus, and age. The free dipole fitting procedure placed dipoles in LAC and RAC (Fig. 1) explaining ~95% of the variance for STD and DEV (see Table 3 for the number of infants included in the grand average source localization for each group and specific residual variance for each group, age, and stimulus).
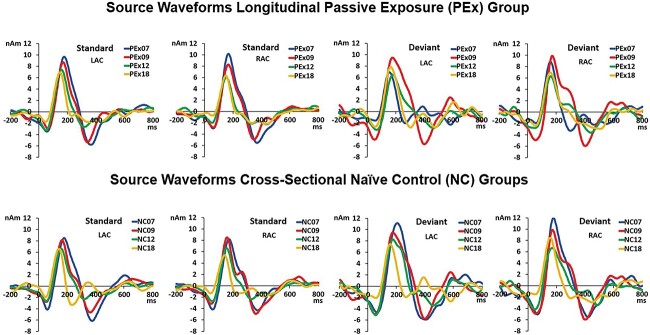
Fig. 1.
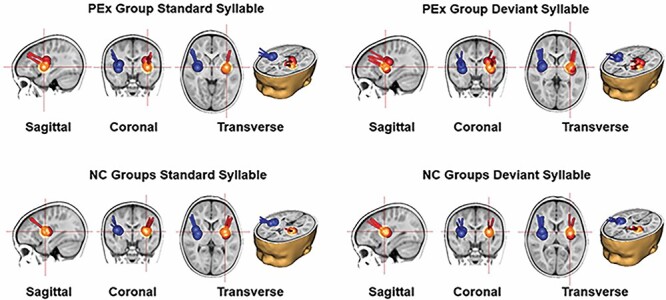
Source localization: source localization of the P1 peak generators based on the grand average ERP is shown in sagittal, coronal, and transverse views in a 0–18 months infant brain template. Dipoles in red represent sources localized in the LAC and in blue, in the RAC, whereas the red crosshairs highlight the left dipole location. In the first row to the left, the dipole models representing the response to the STD syllable /da/ for the longitudinal PEx group at 7, 9, 12, and 18 months of age are displayed superimposed in the infant brain template. In the first row to the right, the dipole models representing the response to the DEV syllable /ta/ at all 4 ages are displayed superimposed in the infant brain template. In the second row, source localization for the STD to the left and the DEV to the right for the 4 cross-sectional NC groups (NC07, NC09, NC12, NC18) are displayed superimposed in the infant brain template.
Table 3.
Amplitude and latency of the P1 response in the grand average source waveform for the PEx longitudinal group and the 4 NC cross-sectional groups.
| Group | N | RV (%) | Amplitude (nAm) | Latency (ms) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STD | DEV | STD LAC | STD RAC | DEV LAC | DEV RAC | STD LAC | STD RAC | DEV LAC | DEV RAC | ||
| Longitudinal PEx group | |||||||||||
| PEx07 | 17 | 4.28 | 3.22 | 9.87 | 10.78 | 10.89 | 10.38 | 172 | 164 | 184 | 176 |
| PEx09 | 18 | 3.63 | 2.81 | 8.73 | 8.27 | 9.78 | 9.63 | 164 | 160 | 180 | 168 |
| PEx12 | 17 | 3.22 | 5.08 | 7.39 | 6.00 | 6.50 | 6.28 | 152 | 148 | 172 | 156 |
| PEx18 | 16 | 3.77 | 4.75 | 6.96 | 6.32 | 7.81 | 6.94 | 144 | 144 | 156 | 152 |
| Cross-sectional NC groups | |||||||||||
| NC07 | 14 | 3.14 | 1.99 | 8.47 | 8.19 | 11.13 | 11.96 | 172 | 168 | 200 | 172 |
| NC09 | 17 | 2.45 | 2.88 | 8.11 | 8.50 | 9.46 | 9.84 | 160 | 156 | 168 | 168 |
| NC12 | 17 | 2.40 | 2.75 | 6.48 | 6.89 | 8.25 | 6.69 | 152 | 152 | 172 | 164 |
| NC18 | 14 | 2.97 | 2.62 | 6.58 | 5.37 | 7.42 | 8.49 | 136 | 136 | 152 | 148 |
Notes: PEx07, passive exposure group at 7 months; PEx09, passive exposure group at 9 months; PEx12, passive exposure group at 12 months; PEx18, passive exposure group at 18 months; NC07, 7-months naïve control group; NC09, 9-months naïve control group; NC12, 12-months naïve control group; NC18, 18-months naïve control group; N, number of participants included in the grand average source waveform; RV, residual variance unexplained by the 2-dipole model; %, percentage; STD, standard stimulus; DEV, deviant stimulus; LAC, left auditory cortex; RAC, right auditory cortex; nAm, nanoampere meters; ms, milliseconds; PEx, passive exposure; NC, naïve control.
The grand average source waveforms in each group and age followed the positive–negative pattern seen in the original ERP waveforms indicating a good model fit to the data (Fig. 2). The longitudinal group across age as well as the 4 cross-sectional groups showed a tendency to decrease both the amplitude and latency of the P1 as infants got older (Fig. 2). This can be seen in Table 3 in which the amplitude and latency of the P1 response to the STD and DEV syllables at LAC and RAC for each group and age are provided.
Fig. 2.
Source waveforms: first row: grand average source waveforms for the longitudinal PEx group followed across age. The first 2 source waveforms represent responses in the LAC and RAC to the STD syllable /da/. The next 2 source waveforms represent responses in LAC and RAC to the DEV syllable /ta/. The source waveforms are shown superimposed at 7 months in blue, at 9 months in red, at 12 months in green, and at 18 months in yellow. Second row: grand average source waveforms for the 4 cross-sectional NC groups. The first 2 source waveforms represent responses in the LAC and RAC to the STD syllable /da/. The next 2 source waveforms represent responses in LAC and RAC to the DEV syllable /ta/. The source waveforms are shown superimposed for the NC07 group in blue, for the NC09 group in red, for the NC12 group in green, and for the NC18 group in yellow. Positivity is plotted up, time is shown in ms on the x-axis, and amplitude is given in nanoampere meters (nAm) on the y-axis.
Time-frequency analysis
Theta ITPL analyses across age
Evaluation of ITPL during speech perception (first 500 ms after syllable onset) was obtained via data clustering in combination with permutation testing. The P-value reported in this series of analyses represents the P-value of the cluster already corrected for multiple comparisons. Significant differences in theta ITPL dynamics during syllable representation were seen in the response to the STD stimulus. Longitudinal analyses within the PEx group [n = 15] 2 × 4 (source by age) permutation ANOVA showed an age effect with an overall bilateral decrease in ITPL with age (cluster size: 50–500 ms, 2–8 Hz; LAC: P = 0.012; RAC: P = 0.002). Post hoc permutation Scheffe’s tests conducted to determine which pairwise comparisons were responsible for the age main effect showed that the significance was driven by a decrease in ITPL from 7 to 12 months in the RAC (P = 0.006), from 9 to 18 months in LAC (P = 0.008), and from 7 to 18 months in both LAC (P = 0.005) and RAC (P = 0.006) sources.
Similarly, cross-sectional analyses between the NC groups 2 × 4 (source by group [n = 83]) permutation ANOVA, showed a group effect (P = 0.007) with less amount of ITPL seen in older compared to younger groups only in the LAC source (cluster size: 150–500 ms, 2–7 Hz). Pairwise comparisons found that the NC07 group exhibited larger ITPL in the LAC than the NC18 group (P = 0.002). No significant difference in the amount of ITPL was seen for the DEV (a proxy for syllable discrimination) across age for either the longitudinal PEx or the cross-sectional NC groups.
Theta ITPL group comparison analyses
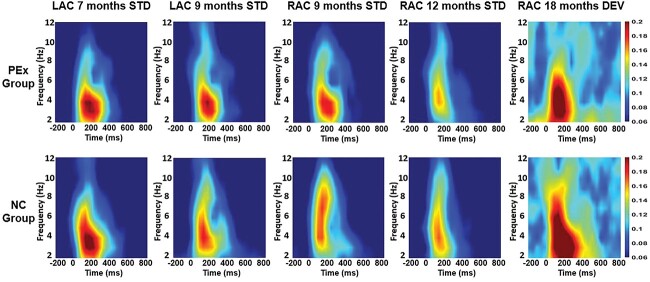
Group comparison conducted at each age between the PEx and corresponding age-matched NC group found differences in theta ITPL for the STD processing. The NC group showed larger ITPL than the PEx group in the LAC (P = 0.002) and RAC sources (P = 0.005) at 9 months; at 7 and 12 months, the difference between the groups was not significant (P > 0.025) (see Table 4 for temporal-spectral cluster size definition and ITPL means). No group differences in theta ITPL were found for STD at 18 months. However, the NC18 group showed larger ITPL than PEx18 during DEV processing in the RAC source (P = 0.000). Thus, the modulatory effect of ITPL supported more efficient processing of syllabic representation in the LAC and RAC sources at an earlier age (9 months) and more efficient syllable discrimination in the RAC source at 18 months (Fig. 3).
Table 4.
Group differences in theta ITPL between PEx group and age-matched NC groups.
| Group G1 G2 |
N G1 G2 |
Stim. | Cluster time | Cluster Freq. | Source | Mean ITPL G1 | Mean ITPL G2 | P |
|---|---|---|---|---|---|---|---|---|
| NC07 > PEx07 | 18 19 | STD | 0–100 | 4–7 | LAC | 0.1124 | 0.0792 | 0.026 |
| NC09 > PEx09 | 23 19 | STD | 350–500 | 2–5 | LAC RAC | 0.1094 0.1025 | 0.0788 0.0673 | 0.002* 0.005* |
| NC12 > PEx12 | 21 19 | STD | 0–200 | 2–3 | RAC | 0.1085 | 0.0809 | 0.031 |
| NC18 > PEx18 | 21 17 | DEV | 350–500 | 3–7 | RAC | 0.1380 | 0.0953 | 0.000* |
Notes: ITPL, inter-trial phase-locking; PEx, passive exposure group; NC, naïve control groups; G1, group 1 (NC); G2, group 2 (PEx); >, larger than; N, number of participants in each group; Stim, stimulus; Time, given in milliseconds; Freq, frequency (given in Hz); P, P-value of the cluster-based permutation; NC07, 7-months naïve control group; PEx07, passive exposure group at 7 months of age; NC09, 9-months naïve control group; PEx09, passive exposure group at 9 months of age; NC12, 12-months naïve control group; PEx12, passive exposure group at 12 months of age; NC18, 18-months naïve control group; PEx18, passive exposure group at 18 months of age; LAC, left auditory cortex source; RAC, right auditory cortex source; STD, standard stimulus syllable /da/; DEV, deviant stimulus syllable /ta/. *: P-value significant at <0.025 level for 2-sided analyses.
Fig. 3.
ITPL plots: time-frequency plots illustrating group differences in inter-trial phase synchrony (ITPL) as a response to the STD syllable /da/ and DEV syllable /ta/ in the theta frequency band. In the first row, ITPL plots are shown for the PEx group, and, in the second row, for the age-matched NC group. In the first column, ITPL for the PEx response to the STD at 7 months in the LAC source is compared with ITPL from the NC07 group; in the second and third columns, ITPL for the PEx response to the STD at 9 months in LAC and right (RAC) sources is compared with corresponding ITPL from the NC09 group; in the fourth column, ITPL for PEx response to the STD at 12 months in RAC is compared with ITPL from the NC12 group; in the fifth column, ITPL from PEx response to the DEV at 18 months in RAC is compared with ITPL from NC18 group. ITPL was significantly smaller for the PEx than the matched NC group as reported in Table 4 for the STD at 9 months and for the DEV at 18 months. The result at 7 and 12 months was considered not significant (P > 0.025). Time is shown in ms on the x-axis and frequency is shown in Hz on the y-axis.
Correlations between theta ITPL and language
To examine the relationship between the plasticity-dependent effect of PAE on theta ITPL at 7 and 9 months and language abilities at 12 and 18 months, cluster-based permutation correlational analyses were conducted in the PEx longitudinal group.
A pattern of negative associations was found between the magnitude of theta ITPL in infancy and later language abilities. Specifically, less ITPL in LAC for the STD processing at 7 months was related to higher expressive language scores at 12 months (CDI-total number of gestures: P = 0.012). Likewise, less ITPL for the DEV processing at 9 months was associated with higher expressive language scores in both LAC and RAC sources at 18 months (PLS4-Expressive: LAC: P = 0.016, RAC: P = 0.008). To note, contrary to the consistent pattern of negative associations described above, a single positive correlation was found in the RAC between the amount of ITPL at 7 months during DEV processing and the receptive language score (PLS-Receptive: P = 0.018) at 12 months (time frame and frequency range definition of the clusters in which significant associations were found are provided in Table 5).
Table 5.
Experience-dependent plasticity effects of PAE: Associations between early (7 and 9 months) ITPL and later (12 and 18 months) expressive and receptive language abilities for the PEx group.
| Language | Stim | TW (ms) | Freq (Hz) | Source | Direction | P |
|---|---|---|---|---|---|---|
| Theta ITPL at 7 months | ||||||
| PLS4-EXS 12 m | STD | 350–500 | 6–10 | LAC | Negative | 0.044 |
| CDI-TGR 12 m | STD | 100–250 | 7–9 | LAC | Negative | 0.017* |
| PLS4-EXS 18 m | STD | 350–500 | 3–6 | LAC | Negative | 0.031 |
| PLS4-RCS 12 m | DEV | 350–550 | 2–5 | RAC | Positive | 0.018* |
| Theta ITPL at 9 months | ||||||
| PLS-EXS 12 m | STD | 350–500 | 3–6 | LAC | Negative | 0.035 |
| CDI-TGR 12 m | STD | 150–300 | 7–9 | LAC | Negative | 0.035 |
| PLS4-EXS 18 m | STD | 400–500 | 3–5 | LAC | Negative | 0.050 |
| PLS4-EXS 12 m | DEV | 300–450 | 3–5 | LAC | Negative | 0.045 |
| CDI-WPR 12 m | DEV | 250–400 | 2–5 | LAC | Negative | 0.040 |
| CDI-WUR 12 m | DEV | 200–300 | 3–6 | RAC | Negative | 0.036 |
| CDI-TGR 12 m | DEV | 300–500 | 2–4 | RAC | Negative | 0.026 |
| PLS4-EXS 18 m | DEV | 150–400 200–500 |
2–6 2–5 |
LAC RAC |
Negative Negative |
0.016* 0.008* |
| CDI-WPR 18 m | DEV | 300–450 | 2–4 | RAC | Negative | 0.039 |
Notes: PAE, passive auditory exposure; ITPL, inter-trial phase-locking; PEx, passive exposure group; m, months; Stim, stimulus; TW, time window for the cluster; ms, milliseconds; Freq, frequency range for the cluster; Hz, hertz; P, P-value of the cluster-based permutation for the correlation; PLS, The Preschool Language Scale-4th ed; PLS-EXS, PLS-4 Expressive standard score; PLS-RCS, PLS-4 Receptive standard score; CDI, MacArthur-Bates Communicative Development Inventory; CDI-TGR, number of total gestures raw score; CDI-WPR, number of words produced raw score; CDI-WUR, number of words understood raw score; LAC, left auditory cortex; RAC, right auditory cortex. *: P-value significant at <0.025 level for 2-sided analyses. Note that raw scores were converted to z-sores for statistical analyses.
Discussion
In this study, we sought to support the optimal development of RAP prelinguistic abilities by passively exposing 4-month infants for 6 weeks to nonspeech pairs of sounds separated by ISIs known to be in the range important for speech processing. Experience-dependent plasticity effects of the passive auditory exposure (PAE) seen in auditory cortices were characterized by decreases in inter-trial phase synchrony suggesting enhanced efficiency in processing syllabic content. Group differences in the left auditory source were seen at younger ages, whereas in the right source, differences were predominantly found at later ages. A correlational lateralization pattern dependent on the type of stimulus showed that at 7 months less theta phase synchrony for the STD representation in the left auditory source was associated with higher expressive language scores at 12 months, whereas at 9 months, less ITPL for DEV discrimination in both auditory sources was associated with higher expressive language at 18 months. Our results suggest that supporting emerging auditory perceptual abilities during early development, even with passive exposure, can positively influence later language outcomes.
One primary goal of our research is to explore ways to modify early auditory processing abilities in infants at higher risk for DLD, to facilitate the development of proficient language. Deficits in RAP abilities can be detected during the preverbal period and have been shown to be predictive of later language outcomes (Benasich and Tallal 2002; Tallal 2004; Guttorm et al. 2005; Benasich et al. 2006; Leppänen et al. 2010; Choudhury and Benasich 2011; Benasich and Choudhury 2012; Maitre et al. 2013; Cantiani et al. 2016, 2019). Supporting and optimizing RAP abilities in infancy has the potential to enhance cortical representation and discrimination of the linguistic elements basic to acquire language at the appropriate time, when infants are in the process of assembling them. Sensitive periods are crucial to consider when planning the timing of an intervention and the evaluation of outcomes as they provide “windows of opportunity” in which the effect of experience on brain development is remarkably powerful as an effective modulator of the emergent neural circuits (Ismail et al. 2017). Passive exposure to sounds has been proven sufficient to induce plastic representational changes in the rodent auditory cortex (Zhang et al. 2001; Schreiner and Polley 2014), strongly suggesting that auditory cortical maps can be fine-tuned by passive exposure. Our findings showed that less phase synchrony for syllable representation at 7 months in the LAC was correlated with higher expressive language scores at 12 months of age. Furthermore, at 9 months, less theta phase synchrony for syllable discrimination in both left and right auditory sources was associated with greater expressive language scores at 18 months. The predictive pattern of negative correlations between early phase synchrony and language measures found in this study is consistent with studies using ERP measures in school-age children that have shown that larger ERP responses to speech stimuli is concurrently correlated with poorer reading scores (Bruder et al. 2011; Hämäläinen et al. 2018; Azaiez et al. 2022). The smaller response observed in more proficient readers may reflect the maturity of the neural network. Our results confirm that indeed, supporting the development of preverbal perceptual skills at a younger age, even passively, is specifically correlated with increased syllabic processing efficiency and later linguistic abilities.
We examined longitudinally the trajectory of ITPL across age as a function of PAE. We confirmed a decrease in ITPL from 7 to 18 months in both LAC and RAC sources for syllabic representation. Across the cross-sectional control groups, we also found a decrease in ITPL in the older (NC18) as compared with the younger (NC07) group but only in the LAC source. A decrease in theta ITPL during the first year of life has been interpreted as an increase in processing efficiency and automatization achieved as infants mature (Musacchia et al. 2017; Hämäläinen et al. 2019) and interact with the linguistic environment (Ortiz-Mantilla et al. 2016, 2022). The decrease of neural activity during perceptual processing seen across maturation might reflect changes in the number of neurons recruited for processing, the strength of the involved synaptic activity, or because of enhanced tuning of cortical mapping (Albrecht et al. 2000; Mittag et al. 2021; Musacchia et al. 2017). We found that in normative development, examined cross-sectionally in the NC groups, proficiency effects were seen only in the left auditory source. However, plasticity-dependent effects of PAE across age were seen in both left and right auditory sources. The RAC is involved in sampling slow-rate information such as that contained in syllabic patterns (Poeppel 2003; Abrams et al. 2008; Giraud and Poeppel 2012; Ghitza 2013; Vanvooren et al. 2014; Hyafil et al. 2015). Thus, it seems likely that passive acoustic exposure facilitated more mature processing of slow-rate syllabic content in the RAC beyond what is seen in normative development, in addition to the expected increase in processing efficiency for syllable representation in the LAC.
A decrease in the magnitude of inter-trial phase synchrony might indicate less synaptic activity suggesting more automatized, efficient neural processing (Albrecht et al. 2000; Mittag et al. 2021) as a function of changes in sensory encoding after training (Mankel et al. 2022). In this study, however, the decrease in the magnitude of theta phase synchrony seen in the PEx infants was well above that seen in typical maturation in the nonexposed NC infants (Table 4). This result supports the premise that the PEx group, above and beyond the controls, needed to dedicate fewer neuronal resources to the processing of the STD and DEV. In short, as a function of passive acoustic exposure infants receiving PAE enhanced cortical representation and discrimination of the syllables via faster and more efficient processing.
The PAE modulatory effect on phase synchrony was seen at an earlier developmental time in the left than in the RAC, in line with the asymmetrical maturational sequence described in auditory areas during the first year of life (Adibpour et al. 2020; Dehaene-Lambertz et al. 2002; Deoni et al. 2011; Gervain et al. 2008; Gilmore et al. 2007; Paus et al. 2001; Pujol et al. 2006; Witelson and Pallie 1973). It is known that the processing of the acoustic elements of speech occurs in both auditory cortices (Hickok 2001; Poeppel 2003). Whereas the left highly specializes in temporal analysis of rapid presented cues such as those characteristic of phonemes (Zatorre and Belin 2001), the right is better suited for coding syllabic information represented by phase-locking to the speech envelope’s slow fluctuations, with larger responses seen in the right than in the LAC (Abrams et al. 2008). The modulatory effect of PAE on phase synchrony lateralization closely aligns with the asymmetrical maturational pattern described during normative development with experience-dependent effects in the left seen earlier than in the RAC.
One discrepancy was noted regarding correlations between early ITPL and later language: larger theta phase synchrony for the DEV syllable in the RAC at 7 months was correlated with a larger receptive language score at 12 months when all other correlations went in the opposite direction. A possible explanation for this exception to our pattern of findings is that this result may have been influenced by the language scores of 2 participants. Two infants in the exposed group scored below the normal range in the receptive measure but had expressive scores within the normative range. In these 2 cases, it was reported that their state was not ideal during the 12-month language test, which may have resulted in decreased attention and less than optimal performance. Another possible explanation might be related to the asymmetric maturation timeline of the left versus the right auditory cortical areas. As the RAC matures later than the left it could be that the developmental maturational lag impacted the more demanding DEV processing, which may be reflected in the magnitude of the ITPL response. Nevertheless, we believe that the strong pattern of correlations found is well in line with the literature reporting on older children.
Conclusions
Our results demonstrate that the experience-dependent effects of passive nonspeech exposure on syllabic processing are both significant and long-lasting, indicating that PEx can be a valuable method for inducing auditory plasticity at this age. Neural plasticity was characterized by modulation of inter-trial phase synchrony in the theta range reflecting increased processing efficiency of syllable representation and syllable discrimination. The magnitude of the theta phase synchrony at 7 and 9 months was negatively correlated with expressive language abilities at 12, and 18 months, thus supporting our premise that phase synchrony efficiency relates to better language outcome. These results clearly show that short but intensive passive exposure to nonspeech sounds that contain spectral-temporal modulation in the speech range can impact speech processing efficiency and later language in typically developing infants. This is an important finding in the context of constructing early interventions for infants at familial risk for developmental language impairments. The next logical step is to examine the effects of providing a similar passive auditory exposure to preverbal infants at higher risk for DLD.
Acknowledgments
We thank our colleagues at the Infancy Studies Laboratory at the Center for Molecular and Behavioral Neuroscience at Rutgers University-Newark for assistance during recruitment and data collection. Special thanks go to all the infants and families who participated in the study.
Contributor Information
Silvia Ortiz-Mantilla, Center for Molecular & Behavioral Neuroscience, Rutgers University Newark, NJ, USA.
Cynthia P Roesler, Center for Molecular & Behavioral Neuroscience, Rutgers University Newark, NJ, USA.
Teresa Realpe-Bonilla, Center for Molecular & Behavioral Neuroscience, Rutgers University Newark, NJ, USA.
April A Benasich, Center for Molecular & Behavioral Neuroscience, Rutgers University Newark, NJ, USA.
CRediT author statement
Silvia Ortiz-Mantilla (Data curation, Formal analysis, Methodology, Validation, Writing—original draft, Writing—review & editing), Cynthia P. Roesler (Investigation, Project administration, Writing—original draft, Writing—review & editing), Teresa Realpe-Bonilla (Data curation, Investigation, Project administration, Software, Validation), and April A. Benasich (Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing—review & editing)
Funding
The Elizabeth H. Solomon Center for Neurodevelopmental Research.
Conflict of interest statement
None declared.
References
- Abrams DA, Nicol T, Zecker S, Kraus N. Right-hemisphere auditory cortex is dominant for coding syllable patterns in speech. J Neurosc. 2008:28:3958–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adibpour P, Lebenberg J, Kabdebon C, Dehaene-Lambertz G, Dubois J. Anatomo-functional correlates of auditory development in infancy. Dev Cogn Neurosci. 2020:42:100752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahissar E, Nagarajan SS, Ahissar M, Protopapas A, Mahncke H, Merzenich MM. Speech comprehension is correlated with temporal response patterns recorded from auditory cortex. PNAS. 2001:98:13367–13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht R, Suchodoletz WV, Uwer R. The development of auditory evoked dipole source activity from childhood to adulthood. Clin Neurophysiol. 2000:111:2268–2276. [DOI] [PubMed] [Google Scholar]
- Aslin RN. Discrimination of frequency transitions by human infants. J Acoust Soc Am. 1989:86:582–590. [DOI] [PubMed] [Google Scholar]
- Azaiez N, Loberg O, Hämäläinen JA, Leppänen PHT. Brain source correlates of speech perception and reading processes in children with and without reading difficulties. Front Neurosci. 2022:16:921977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant and Toddler Development. 2nd ed. (Bayley-III) San Antonio (TX): Harcourt Assessment; 2006. [Google Scholar]
- Benasich AA, Choudhury N. Timing, information processing and efficacy: Early factors that impact childhood language trajectories. In: Benasich A, Fitch R, editors. Developmental dyslexia: early precursors, neurobehavioral markers and biological substrates (the extraordinary brain series). Baltimore (MD): Brookes Publishing; 2012. pp. 99–118. [Google Scholar]
- Benasich AA, Choudhury N, Friedman JT, Realpe Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006:44:396–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Choudhury N, Peters S, Ortiz-Mantilla S. Early screening and intervention of infants at high risk for developmental language disorders. In: The role of nonlinguistic rapid auditory processing. International Dyslexia Association (IDA), Perspectives on Language and Literacy; 2016:42:24–29. [Google Scholar]
- Benasich AA, Choudhury N, Realpe-Bonilla T, Roesler CP. Plasticity in developing brain: active auditory exposure impacts prelinguistic acoustic mapping. J Neurosc. 2014:34:13349–13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Thomas JJ, Choudhury N, Leppänen PHT. The importance of rapid auditory processing abilities to early language development: evidence from converging methodologies. Dev Psychobiol. 2002:40:278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 2002:136:31–49. [DOI] [PubMed] [Google Scholar]
- Bennett SH, Kirby AJ, Finnerty GT. Rewiring the connectome: evidence and effects. Neurosci Biobehav Rev. 2018:88:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoncini J, Mehler J. Syllables as units in infant speech perception. Inf Behav Dev. 1981:4:247–260. [Google Scholar]
- Bettoni R, Riva V, Cantiani C, Molteni M, Cassia VM, Bulf H. Infants’ learning of rule-based visual sequences predicts language outcome at 2 years. Front Psychol. 2020:11:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornfleth H, Cho JH, Spangler R. 2020. BESA statistics 2.1 user manual. https://www.besa.de/wp-content/uploads/2014/05/BESA-Statistics-2.1-User-Manual.pdf. (accessed on February 17, 2023).
- Bosseler AN, Clarke M, Tavabi K, Larson ED, Hippe DS, Taulu S, Kuhl PK. Using magnetoencephalography to examine word recognition, lateralization, and future language skills in 14-month-old infants. Dev Cog Neurosc. 2021:47:100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosseler AN, Taulu S, Pihko E, Mäkelä JP, Imada T, Ahonen A, Kuhl PK. Theta brain rhythms index perceptual narrowing in infant speech perception. Front Psychol. 2013:4:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder J, Leppänen PHT, Bartling J, Csépe V, Démonet JF, Schulte-Körne G. An investigation of prototypical and atypical within-category vowels and non-speech analogues on cortical auditory evoked related potentials (AERPs) in 9 year old children. Int J Psychophysiol. 2011:79:106–117. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998:21:149–186. [DOI] [PubMed] [Google Scholar]
- Cantiani C, Ortiz-Mantilla S, Riva V, Piazza C, Bettoni R, Musacchia G, Molteni M, Marino C, Benasich AA. Reduced left-lateralized pattern of event-related EEG oscillations in infants at familial risk for language and learning impairment. NeuroImage Clin. 2019:22:101778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiani C, Riva V, Piazza C, Bettoni R, Molteni M, Choudhury N, Marino C, Benasich AA. Auditory discrimination predicts linguistic outcome in Italian infants with and without familial risk for language learning impairment. Dev Cog Neurosci. 2016:20:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury N, Benasich AA. Maturation of auditory evoked potentials from 6 to 48 months: prediction to 3 and 4 year language and cognitive abilities. Clin Neurophysiol. 2011:122:320–338. [DOI] [PubMed] [Google Scholar]
- Cirelli LK, Spinelli C, Nozaradan S, Trainor L. Measuring neural entrainment to beat and meter in infants: effects of music background. Front Neurosci. 2016:10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MX. Analyzing neural time series data: theory and practice. In: Grafman J, editors. Issues in clinical and cognitive neuropsychology. Cambridge MA and London: The MIT Press Cambridge; 2014. pp. 16–21, 257–272. [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002:298:2013–2015. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007:27:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster D, Bick J, Johnson U, Montroy JJ, Landry S, Duncan AF. Nurturing the preterm infant brain: leveraging neuroplasticity to improve neurobehavioral outcomes. Pediatr Res. 2019:85:166–175. [DOI] [PubMed] [Google Scholar]
- Deoni SCL, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, Williams SCR, Murphy DGM. Mapping infant brain myelination with magnetic resonance imaging. J Neurosci. 2011:31:784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Simon JZ. Cortical entrainment to continuous speech: functional roles and interpretations. Front Hum Neurosci. 2014:8:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondena C, Riva V, Molteni M, Musacchia G, Cantiani C. Impact of early rhythmic training on language acquisition and electrophysiological functioning underlying auditory processing: feasibility and preliminary findings in typically developing infants. Brain Sci. 2021:11:1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers RE, Morse PA, Gavin WJ, Oller DK. Discrimination of voice onset time in infancy. J Acoust Soc Am. 1981:70:955–965. [DOI] [PubMed] [Google Scholar]
- Eimas PD. Segmental and syllabic representations in the perception of speech by young infants. J Acoust Soc Am. 1999:105:1901–1911. [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick SJ, Thal D, Bates E, Hartung JP, Pethick S, Reilly JS. Mac Arthur Communicative Development Inventories: User’s Guide and Technical Manual. San Diego (CA): Singular Publishing Group, Inc.; 1993. [Google Scholar]
- Freedman D, Lane D. A nonstochastic interpretation of reported significance levels. J Bus Econ Stat. 1983:1:292–298. [Google Scholar]
- Froemke RC, Jones BJ. Development of auditory cortical synaptic receptive fields. Neurosci Biobehav Rev. 2011:35:2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Neural plasticity of development and learning. Hum Brain Mapp. 2010:31:879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerry DW, Faux AL, Trainor LJ. Effects of Kindermusik training on infants’ rhythmic enculturation. Dev Sci. 2010:13:545–551. [DOI] [PubMed] [Google Scholar]
- Gerry D, Unrau A, Trainor LJ. Active music classes in infancy enhance musical, communicative and social development. Dev Sci. 2012:15:398–407. [DOI] [PubMed] [Google Scholar]
- Gervain J. Plasticity in early language acquisition: the effects of prenatal and early childhood experience. Curr Opin Neurobiol. 2015:35:13–20. [DOI] [PubMed] [Google Scholar]
- Gervain J, Macagno F, Cogoi S, Peña M, Mehler J. The neonate brain detects speech structure. Proc Natl Acad Sci U S A. 2008:105:14222–14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza O. The theta-syllable: a unit of speech information defined by cortical function. Front Psychol. 2013:4:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YSK, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007:27:1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud AL, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nat Neurosci. 2012:15:511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S, Kinsbury BED. The modulation spectrogram: In pursuit of an invariant representation of speech. Procedings of the ICASSP-97 International Conference on Acoustics, Speech, and Signal Processing, Munich, April, 1997. Vol.3. Institute of Electrical and Electronics Engineers, p. 1647–1650.
- Greenberg S. A syllable-centric framework for the evolution of spoken language. Beh Brain Sci. 1998:21:499–546. [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987:58:539–559. [PubMed] [Google Scholar]
- Gross J, Hoogenboom N, Thut G, Schyns P, Panzeri S, Belin P, Garrod S. Speech rhythms and multiplexed oscillatory sensory coding in the human brain. PLoS Biol. 2013:11:e1001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttorm TK, Leppänen PH, Poikkeus AM, Eklund KM, Lyytinen P, Lyytinen H. Brain event-related potentials (ERPs) measured at birth predict later language development in children with and without familial risk for dyslexia. Cortex. 2005:41:291–303. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Vernon DJ, Dwivedi P, Gruzelier JH, Baldeweg T. Event-related brain potential correlates of human auditory sensory memory-trace formation. J Neurosci. 2005:25:10494–10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P, Poeppel D. The infrastructure of the language-ready brain. In: Arbib MA, editors. Language, music, and the brain: a mysterious relationship. Cambridge (MA): MIT Press; 2013. pp. 233–255. [Google Scholar]
- Hämäläinen JA, Ortiz-Mantilla S, Benasich AA. Source localization of event related potentials to pitch change mapped onto age-appropriate MRIs at 6 months-of-age. NeuroImage. 2011:54:1910–1918. [DOI] [PubMed] [Google Scholar]
- Hämäläinen JA, Ortiz-Mantilla S, Benasich AA. Change detection to tone pairs during the first year of life – predictive longitudinal relationships for EEG-based source and time-frequency measures. NeuroImage. 2019:198:83–92. [DOI] [PubMed] [Google Scholar]
- Hämäläinen J, Landi N, Loberg O, Lohvansuu K, Pugh K, Leppänen PHT. Brain event-related potentials to phoneme contrasts and their correlation to reading skills in school-age children. Int J Beh Develop. 2018:42:357–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen J, Lohvansuu K, Ervast L, Leppänen PH. Event-related potentials to tones show differences between children with multiple risk factors for dyslexia and control children before onset of formal reading instruction. Int J Psychophysiol. 2015:95:101–112. [DOI] [PubMed] [Google Scholar]
- Hannon EE, Trehub SE. Tuning in to musical rhythms: infants learn more readily than adults. PNAS. 2005:102:12639–12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends Neurosci. 1997:20:44–49. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior: a neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- Hickok G. Functional anatomy of speech perception and speech production: psycholinguistic implications. J Psycholinguist Res. 2001:30:225–235. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends Cog Sci. 2000:4:131–138. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007:8:393–402. [DOI] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: a new method to study cortical oscillatory coupling. Brain Topogr. 2004:16:233–238. [DOI] [PubMed] [Google Scholar]
- Homma NY, Hullet PW, Atencio CA, Schreiner CE. Auditory cortical plasticity dependent on environmental noise statistics. Cell Rep. 2020:30:4445–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil A, Fontolan L, Kabdebon C, Gutkin B, Giraud AL. Speech encoding by coupled cortical theta and gamma oscillations. eLife. 2015:4:e06213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail FY, Fatemi A, Johnston MV. Cerebral plasticity: windows of opportunity in the developing brain. Eur J Paediatr Neurol. 2017:21:23–48. [DOI] [PubMed] [Google Scholar]
- Jin Y, Diaz B, Colomer M, Sebastián-Gallés N. Oscillation encoding of individual differences in speech perception. PLoS One. 2014:9:e100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusczyk PW. How infants begin to extract words from speech. Trends Cog Sci. 1999:3:323–328. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW, Houston DM, Newsome M. The beginnings of word segmentation in English-learning infants. Cogn Psychol. 1999:39:159–207. [DOI] [PubMed] [Google Scholar]
- Kalashnikova M, Peter V, Di Liberto GM, Lalor EC, Burnham D. Infant-directed speech facilitates seven-month-old infants’ cortical tracking of speech. Sci Rep. 2018:8:13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Ince RA, Panzeri S. Analysis of slow (theta) oscillations as a potential temporal reference frame for information coding in sensory cortices. PLoS Comput Biol. 2012:8:e1002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Harker A, Gibb R. Principles of plasticity in the developing brain. Dev Med Child Neurol. 2017:59:1218–1223. [DOI] [PubMed] [Google Scholar]
- Kudo T, Morohashi Y, Yazaki-Sugiyama Y. Early auditory experience modifies neuronal firing properties in the zebra finch auditory cortex. Front Neural Circuits. 2020:14:570174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuuluvainen S, Leminen A, Kujala T. Auditory evoked potentials to speech and nonspeech stimuli are associated with verbal skills in preschoolers. Dev Cogn Neurosci. 2016:19:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen PHT, Hämäläinen JA, Salminen HK, Eklund KM, Guttorm TK, Lohvansuu K, Puolakanaho A, Lyytinen H. Newborn brain event-related potentials revealing atypical processing of sound frequency and the subsequent association with later literacy skills in children with familial dyslexia. Cortex. 2010:46:1362–1376. [DOI] [PubMed] [Google Scholar]
- Liu L, Kager R. Statistical learning of speech sounds is most robust during the period of perceptual attunement. J Exp Child Psychol. 2017:164:192–208. [DOI] [PubMed] [Google Scholar]
- Luo H, Poeppel D. Phase patterns of neuronal responses reliably discriminate speech in human auditory cortex. Neuron. 2007:54:1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre NL, Lambert WE, Aschner JL, Key AP. Cortical speech sound differentiation in the neonatal intensive care unit predicts cognitive and language development in the first 2 years of life. Dev Med Child Neurol. 2013:55:834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankel K, Shrestha U, Tipirneni-Sajja A, Bidelman GM. Functional plasticity coupled with structural predispositions in auditory cortex shape successful music category learning. Front Neurosci. 2022:16:897239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG-and MEG-data. J Neurosci Methods. 2007:164:177–190. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Cristià A, Dupoux E. Cerebral lateralization and early speech acquisition: a developmental scenario. Dev Cogn Neurosci. 2011:1:217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag M, Larson E, Clarke M, Taulu S, Kuhl K. Auditory deficits in infants at risk for dyslexia during a linguistic sensitive period predict future language. Neuroimage Clin. 2021:30:102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag M, Larson E, Taulu S, Clarke M, Kuhl PK. Reduced theta sampling in infants at risk for dyslexia across the sensitive period of native phoneme learning. Int J Environ Res Public Health. 2022:19:1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchia G, Choudhury NA, Ortiz-Mantilla S, Realpe-Bonilla T, Roesler CP, Benasich AA. Oscillatory support for rapid frequency change processing in infants. Neuropsychologia. 2013:51:2812–2824. [DOI] [PubMed] [Google Scholar]
- Musacchia G, Ortiz-Mantilla S, Choudhury N, Realpe-Bonilla T, Roesler C, Benasich AA. Active auditory experience in infancy promotes brain plasticity in theta and gamma oscillations. Dev Cogn Neurosci. 2017:26:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchia G, Ortiz-Mantilla S, Realpe-Bonilla T, Roesler CP, Benasich AA. Infant auditory processing and event-related brain oscillations. J Vis Exp. 2015:101:e52420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R, Alho K. Mismatch negativity — the measure for central sound representation accuracy. Audiol Neurootol. 1997:2:341–353. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Winkler I. The concept of auditory stimulus representation in cognitive neuroscience. Psychol Bull. 1999:125:826–859. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007:118:2544–2590. [DOI] [PubMed] [Google Scholar]
- Nacar L, Guerrero-Mosquera C, Colomer M, Sebastian-Galles N. Evoked and oscillatory EEG activity differentiates language discrimination in young monolingual and bilingual infants. Sci Rep. 2018:8:2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Gorman TW. Adaptive tests of significance using permutations of residuals with R and SAS. Hoboken (NJ): Wiley; 2012. [Google Scholar]
- Obleser J, Herrmann B, Henry MJ. Neural oscillations in speech: don’t be enslaved by the envelope. Front Hum Neurosci. 2012:6:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Oever S, Sack AT. Oscillatory phase shapes syllable perception. PNAS. 2015:112:15833–15837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Hämäläinen JA, Benasich AA. Time course of ERP generators to syllables in infants: a source localization study using age-appropriate brain templates. NeuroImage. 2012:59:3275–3287. [DOI] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Hämäläinen JA, Musacchia G, Benasich AA. Enhancement of gamma oscillations indicates preferential processing of native over foreign phonemic contrasts in infants. J Neurosci. 2013:33:18746–18754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Hämäläinen JA, Realpe-Bonilla T, Benasich AA. Oscillatory dynamics underlying perceptual narrowing of native phoneme mapping from 6 to 12 months of age. J Neurosci. 2016:36:12095–12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Realpe-Bonilla T, Benasich AA. Early interactive acoustic experience with non-speech generalizes to speech and confers a syllabic processing advantage at 9 months. Cereb Cortex. 2019:29:1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Mantilla S, Roesler CP, Realpe-Bonilla T, Benasich AA. Modulation of theta phase synchrony during syllable processing as a function of interactive acoustic experience in infancy. Cereb Cortex. 2022:32:919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen E, Kujala T, Tervaniemi M, Huotilainen M. Prenatal music exposure induces long-term neural effects. PLoS One. 2013:8:e78946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul-Jordanov I, Hoechstetter K, Bornfleth H, Waelkens A, Rusiniak M, Cho J-H, Spangler R, Scherg M. 2021. BESA research 7.1 user manual. https://www.besa.de/wp-content/uploads/2021/11/BESA-Research-7.1-Tutorial.pdf. (accessed on February 17, 2023).
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: a review of magnetic resonance studies. Brain Res Bull. 2001:54:225–266. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Davis MH. Neural oscillations carry speech rhythm through to comprehension. Front Psychol. 2012:3:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Gross J, Davis MH. Phase-locked responses to speech in human auditory cortex are enhanced during comprehension. Cereb Cortex. 2013:23:1378–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppel D. The analysis of speech in different temporal integration windows: cerebral lateralization as “asymmetric sampling in time”. Speech Comm. 2003:41:245–255. [Google Scholar]
- Pujol J, Soriano-Mas C, Ortiz H, Sebastian-Galles N, Losilla JM, Deus J. Myelination of language-related areas in the developing brain. Neurol. 2006:66:339–343. [DOI] [PubMed] [Google Scholar]
- Räsänen O, Doyle G, Frank MC. Pre-linguistic segmentation of speech into syllable-like units. Cognition. 2018:171:130–150. [DOI] [PubMed] [Google Scholar]
- Reh RK, Dias BG, Nelson CA III, Kaufer D, Werker JF, Kolb B, Levine JD, Hensch TK. Critical period regulation across multiple timescales. PNAS. 2020:117:23242–23251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh RK, Hensch TK, Werker JF. Distributional learning of speech sound categories is gated by sensitive periods. Cognition. 2021:213:104653. [DOI] [PubMed] [Google Scholar]
- Riva V, Cantiani C, Benasich AA, Molteni M, Piazza C, Giorda R, Dionne G, Marino C. From CNTNAP2 to early expressive language in infancy: the mediation role of rapid auditory processing. Cereb Cortex. 2018:28:2100–2108. [DOI] [PubMed] [Google Scholar]
- Rivera-Gaxiola M, Silva-Pereyra J, Kuhl PK. Brain potentials to native and non-native speech contrasts in 7- and 11-month-old American infants. Dev Sci. 2005:8:162–172. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr Opin Neurobiol. 2009:19:188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassenhagen J, Draschkow D. Cluster-based permutation tests of MEG/EEG data do not establish significance of effect latency or location. Psychophysiology. 2018:56(6):e13335. [DOI] [PubMed] [Google Scholar]
- Scherg M, Von Cramon D. Two bilateral sources of the late AEP as identified by a spatio-temporal dipole model. Electroencephalogr Clin Neurophysiol. 1985:62:32–44. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Polley DB. Auditory map plasticity: diversity in causes and consequences. Curr Opin Neurobiol. 2014:24:143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013:207:3–34. [DOI] [PubMed] [Google Scholar]
- Tallal P. Improving language and literacy is a matter of time. Nat Rev Neurosci. 2004:5:721–728. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999:3:151–162. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase locked 40 Hz visual responses in humans. J Neurosci. 1996:16:4240–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor LJ, Marie C, Gerry D, Whiskin E, Unrau A. Becoming musically enculturated: effects of music classes for infants on brain and behavior. Ann N Y Acad Sci. 2012:1252:129–138. [DOI] [PubMed] [Google Scholar]
- Tsao FM, Liu HM, Kuhl PK. Speech perception in infancy predicts language development in the second year of life: a longitudinal study. Child Dev. 2004:75:1067–1084. [DOI] [PubMed] [Google Scholar]
- Vanvooren S, Poelmans H, Hofmann M, Ghesquière P, Wouters J. Hemispheric asymmetry in auditory processing of speech envelope modulations in prereading children. J Neurosc. 2014:34:1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker JF, Hensch TK. Critical periods in speech perception: new directions. Annu Rev Psychol. 2015:66:173–196. [DOI] [PubMed] [Google Scholar]
- Werker JF, Tees RC. Speech perception as a window for understanding plasticity and commitment in language systems of the brain. Dev Psychobiol. 2005:46:233–251. [DOI] [PubMed] [Google Scholar]
- White EJ, Hutka SA, Williams LJ, Moreno S. Learning, neural plasticity and sensitive periods: implications for language acquisition, music training and transfer across the lifespan. Front Syst Neurosci. 2013:7:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witelson SF, Pallie W. Left hemisphere specialization for language in the newborn. Neuroanatomical evidence of asymmetry. Brain. 1973:96:641–646. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Belin P. Spectral and temporal processing in human auditory cortex. Cereb Cortex. 2001:11:946–953. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001:4:1123–1130. [DOI] [PubMed] [Google Scholar]
- Zhao TC, Kuhl PK. Musical intervention enhances infants’ neural processing of temporal structure in music and speech. PNAS. 2016:113:5212–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale—4. Examiner’s Manual. San Antonio (TX): The Psycho al Corporation; 2002. [Google Scholar]