Abstract
A new species of spikemoss, Selaginelladensiciliata in S.subg.Heterostachyssect.Tetragonostachyae, China, is described from southeastern Xizang, based on morphological and molecular phylogenetic data. Morphologically, S.densiciliata is similar to S.repanda, S.subvaginata and S.vaginata, but the new species can be easily distinguished from them by having sterile leaves margins densely ciliate, symmetrical axillary leaves oblong ovate to ovate-triangular, and ovate dorsal leaves obviously carinate. Molecular phylogenetic analysis resolves S.densiciliata as sister to the clade comprised with S.vaginata and S.xipholepis, which confirms the recognition of the new species.
Key words: Medog, Selaginellavaginata , submonomorphic sporophylls, S. subg. Heterostachys
Introduction
Located in southeastern Xizang, Medog county and adjacent regions are one of the biodiversity hotspots in the world (Myers et al. 2000; Mittermeier et al. 2005), even harboring the highest species diversity of plants in China (Sun and Zhou 1996). According to Du et al. (2021), the most number of new species of plants have been discovered in Medog, among all the counties in China in 2020. From 2015 to 2022, most of the authors (Bo Xu, Liang Zhang, Xin-Mao Zhou and Zhao-Rong He) carried out several field investigations and collected a large number of specimens in Medog. Based on those collections, three fern species, Athyriumaberrans Liang Zhang & Li Bing Zhang, Hymenaspleniumtholiformis Liang Zhang, W.B. Ju & K.W. Xu, and Selligueawusugongii Liang Zhang, X.P.Fan & Li Bing Zhang have been discovered (Fan et al. 2021; Qiu et al. 2022a, b). When we studied the lycophytes from these collections, we found some materials of Selaginella belonging to the S.vaginata group, but differing from all recognized species in this group.
The Selaginellavaginata group, including at least three species, i.e., S.subvaginata X.C.Zhang & Shalimov, S.repanda (Desv. & Poir.) Spring, and S.vaginata Spring, represents a taxonomically difficult group in S.sect.Tetragonostachyae of S.subg.Heterostachys sensu Zhou and Zhang (2015). Another classification with seven subgenera in Selaginella was also proposed by Weststrand and Korall (2016a). However, a subsequent study has evidenced that Stachygynandrum Weststrand & Korall (2016a) (~600 species) was not monophyletic (Zhou et al. 2022). In this study, Zhou and Zhang (2015)’s classification was followed. The Selaginellavaginata group is characterized by generally small plants, nearly monomorphic sporophylls, and more or less ciliate margins of leaves (Zhang et al. 2013).
Our previous phylogenetic study of Selaginella firstly found that the S.vaginata (= S.compta Hand.-Mazz. in Zhou et al. 2016) group was not monophyletic and it clustered with those species with distinctly dimorphic sporophylls (e.g., S.albociliata P.S.Wang, S.ciliaris (Retz.) Spring, S.lutchuensis Koidz, S.xipholepis Baker) in the S.ciliaris clade (= “Asia” clade in Zhou et al. 2016) of S.sect.Tetragonostachyae (Zhou and Zhang 2015; Zhou et al. 2016). Also, S.vaginata (= S.compta) was paraphyletic in relation to S.xipholepis (Zhou et al. 2016). Subsequently, Zhang et al. (2020) confirmed the non-monophyly of the S.vaginata group and described a new species, S.subvaginata X.C. Zhang & Shalimovin. Selaginella vaginata is widely distributed in East, Southeast and South Asia, and its elevations range from 500 to 3,600 m according to Zhang et al. (2013) and our own field investigation.
Our further studies of the morphology, phylogeny, and spore morphology of those species related to the S.vaginata group confirm that materials from Medog represent a new species. We describe it here as Selaginelladensiciliata.
Materials and methods
Morphological study
Field observations were conducted in June (in 2015) and October (in 2017) respectively. The photos of plants, leaves, and strobili were taken in the field. All research materials were deposited at KUN and PYU (Index Herbarium: Thiers 2018). More details of morphology were observed and photographs were taken using SMZ1270 stereo microscope (Nikon, Japan). Megaspores and microspores were selected and attached to Carbon Adhesive Tape (CAT) using anatomical lens, then samples were coated with gold using the BAL-TEC SCD 005 Cool Sputter Coater (BAL-TEC AG., Liechtenstein) and visualized via QUANTA 200 Scanning Electron Microscope (SEM) (FEI Co., USA) at 25 kV at Yunnan University, Kunming, China. The morphological terminology of spore follows Tryon and Lugardon (1991) and Zhou et al. (2015).
DNA extraction, amplification and sequencing
Total genomic DNA of seven samples (one from Selaginelladianzhongensis X.C.Zhang, two from S.wuyishanensis K.W.Xu, X.M.Zhou & Y.F.Duan, and four from the new species) was extracted from silica-dried material using the TIANGEN plant genomic DNA extraction kit (TIANGEN Biotech., Beijing, China) following the manufacturers’ protocols. One nuclear locus (ITS) and one plastid gene (rbcL) were selected for amplification and sequencing. Primers and the PCR conditions followed Zhou et al. (2016). Amplified fragments were purified with TIANquick Mini Purification Kits (Tiangen Biotech, Beijing, China) and purified polymerase chain reaction (PCR) products were sequenced by Tsingke (Kunming, China). Fourteen sequences were newly generated in this study (7 5.8S+ITS2 and 7 rbcL) (Appendix 1). Newly generated sequences were edited and assembled using Sequencher v. 4.1.2 (Gene Codes Corporation, Ann Arbor, Michigan).
Phylogenetic analysis
Based on a previous phylogenetic study of Selaginella (Zhou et al. 2016; Weststrand and Korall 2016b), three species, S.bisulcata Spring, S.nipponica Franch. & Sav., and S.uncinata (Desv. ex Poiret) Spring from subg. Heterostachys sensu Zhou and Zhang (2015) were selected as outgroups. A total 102 accessions representing 58 species of Selaginella were sampled (Appendix 1). All sequences were aligned using MAFFT ver. 7 (Katoh and Standley 2013), followed by manual adjustment in BioEdit (Hall 1999). A matrix with 1702 characters (5.8S + ITS2: 425 and rbcL: 1277) was used for phylogeny study. The jModeltest2 (Darriba et al. 2012) was used to choose the best-fitting likelihood model. The AIC (Akaike information criterion) was used to select the best model (Akaike 1974), GTR+I+G, was chosen for the Maximum likelihood (ML) and Bayesian inference (BI) analysis for combined dataset. Maximum likelihood (ML) bootstrapping was performed with 1000 rapid bootstrap replicates (BS) analyses followed by a search for the best-scoring tree in a single run in RAxML v. 8 (Stamatakis et al. 2008). Bayesian inference (BI) was conducted using MrBayes ver. 3.2.7a (Ronquist and Huelsenbeck 2003) with two runs of four Markov chain Monte Carlo (MCMC) chains, each beginning with a random tree and sampling every 1000 generations for 10,000,000 generations. Convergence among runs and stationarity was assessed using Tracer ver. 1.4 (Rambaut and Drummond 2007), and the first 25% was discarded as burnin. The remaining trees were used to calculate a 50% majority-rule consensus topology and posterior probabilities (PP). ML and BI analyses were executed on Cipres (Miller et al. 2010).
Results and discussion
The aligned length of combined plastid gene (rbcL: 1277 bp) and nuclear loci (ITS: 425 bp) was 1702 bp, of which 1242 sites were identical, 389 characters were parsimony informative, and 71 variable characters were parsimony-uninformative.
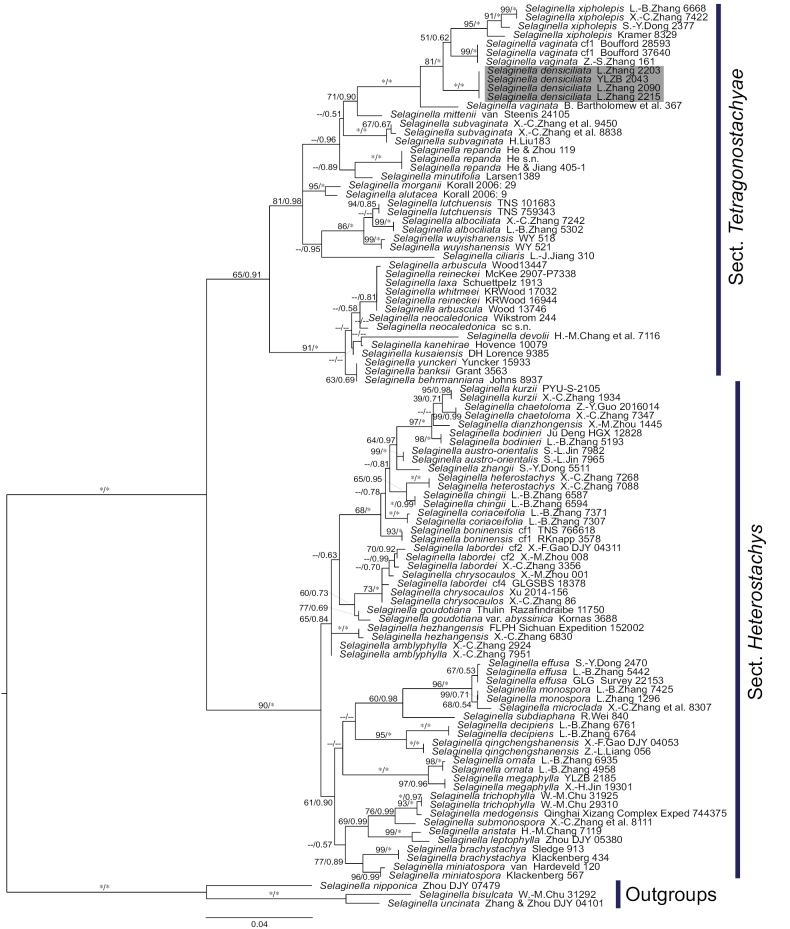
As our previous phylogenetic studies for Selaginella (Zhou et al. 2016, 2022), phylogeny showed that S.sect.Heterostachys (Baker) Li Bing Zhang & X.M.Zhou is sister to S.sect.Tetragonostachyae (Fig. 1). The trees from the ML and BI analyses revealed identical topologies, and four samples of the new species form a highly supported clade (MLBS=100; BIPP=1.00, Fig. 1). Selaginelladensiciliata is sister to a clade containing some samples of S.vaginata and all samples of S.xipholepis. Selaginelladensiciliata is a distant relative of its morphologically similar species (S.repanda, S.subvaginata and S.vaginata). As previous studies (e.g., Zhou et al. 2016; Zhang et al. 2020) have suggested, both the S.vaginata group (including S.repanda, S.subvaginata and S.vaginata) and S.vaginata itself are not monophyletic (Fig. 1) and two lineages were found. With extensive sampling, potentially, more new taxa will be detected and evidenced in the S.vaginata group.
Figure 1.
Maximum likelihood phylogeny of Selaginelladensiciliata and its allies in subg. Heterostachys. based on molecular data. The numbers associated with branches are maximum likelihood bootstrap support (MLBS) ≥ 50% and Bayesian posterior probability (BIPP) ≥ 0.50; the dash (--) indicates MLBS < 50% or BIPP < 0.50; the asterisk indicates MLBS = 100 or BIPP = 1.00; omitted support values indicate both MLBS < 50% and BIPP < 0.50. Sections followed Zhou and Zhang (2015)’s classification.
Comparison of morphological characters between Selaginelladensiciliata and its morphologically similar species is shown in Table 1. Selaginelladensiciliata is easily distinguished from other species in the S.vaginata morphological group by sterile leaves with margins densely ciliate at the lower parts (or at least at base) (Fig. 2H–J), ventral leaves falcate (Fig. 2C, E, J), dorsal leaves obvious carinate (Fig. 2D, I), axillary leaves symmetrical (Fig. 2C, E, H), and fine reticulation of megaspore surfaces (Fig. 2M, N).
Table 1.
Morphological comparison among Selaginelladensiciliata, S.repanda, S.subvaginata, and S.vaginata.
| Selaginelladensiciliata | S.repanda | S.subvaginata | S.vaginata | |
|---|---|---|---|---|
| Habit | Ascending to suberect | Suberect or ascending | Suberect | Creeping |
| Leaves margin | Not white-margined | White-margined | Not white-margined | White-margined |
| Axillary leave | Symmetrical, oblong-ovate to ovate-triangular | Symmetrical, ovate-lanceolate | Asymmetrical, ovate to ovate-triangular | Asymmetrical, ovate-triangular |
| Dorsal leave | Ovate, margins ciliate, apex aristate; carinate | Obliquely ovate, margins denticulate; slightly carinate | Ovate-lanceolate, inner margins ciliate; slightly carinate | Ovate-lanceolate or ovate-triangular, base margins ciliate; slightly carinate |
| Ventral leave | Oblong-falcate, basiscopic base margins denticulate | Ovate or obliquely ovate, basiscopic base margins ciliate | Oblong-falcate, basiscopic base margins denticulate | Ovate-lanceolate or ovate, basiscopic base margins denticulate |
| Dorsal sporophyll | Ovate, margins denticulate | Ovate-lanceolate, margins denticulate | Ovate, base margins ciliate | Ovate-lanceolate, base margins ciliate |
| Ventral sporophyll | Broadly ovate, margins denticulate | Broadly ovate, margins denticulate | Ovate, base margins ciliate | Ovate-lanceolate, margins denticulate |
| Megaspore | Fine reticulate | Verrucate | Fine reticulate | Verrucate |
| Microspore | Verructae and rugulate with spiny microstructure | Verructae and rugulate | Smooth | Verructae and rugulate |
Figure 2.
SelaginelladensiciliataA dorsal view of branches B ventral view of branches with strobili C ventral view of branches, showing rhizophores, axillary leaves, and ventral leaves D dorsal view of branches, showing dorsal leaves E ventral view of branches, showing axillary leaves, and ventral leaves F dorsal view of strobilus G ventral view of strobilus H axillary leaf on stems (left) and branches (right) I dorsal leaf J ventral leaf K dorsal sporophyll L ventral sporophyll M proximal surface of megaspore N distal surface of megaspore O proximal surface of microspore P distal surface of microspore (from the holotype: L. Zhang et al. 2215).
Submonomorphic sporophylls are similar dorsal and ventral sporophylls in morphology, but dorsal sporophylls are slightly larger than ventral ones. Submonomorphic sporophylls are only present in some species of S.sect.Heterostachys (e.g., S.monospora Spring) and S.sect.Tetragonostachyae (Hook. & Grev.) Hieron. & Sadeb. in S.subg.Heterostachys sensu Zhou and Zhang (2015). Submonomorphic sporophylls are derived from distinctly dimorphic sporophylls in S.subg.Heterostachys Baker sensu Zhou and Zhang (2015) (Fig. 1, Zhou et al. 2016).
Taxonomic treatment
. Selaginella densiciliata
X.M.Zhou, Liang Zhang & Bo Xu sp. nov.
B2761967-8FDB-5D36-BA91-5DC6E1EE8106
urn:lsid:ipni.org:names:77320720-1
Type.
China. Xizang: Medog County, Beibeng Township, on the way from A’niqiao to #3 bridge, in broad-leaved evergreen forest, 29°20'41.56"N, 95°9'56.99"E, elev. 1600 m, 15 Oct. 2017, Liang Zhang, Wen-Bin Ju & Heng-Ning Deng2215 (holotype: KUN-1572683!, isotypes: KUN-1572684!, PYU-02074721!, PYU-02074722!).
Diagnosis.
Selaginelladensiciliata is similar to S.repanda, S.subvaginata, and S.vaginata in having relatively small plants (Fig. 3E), base of stem with ventral leaves strongly curly and surrounding stem when dry (Fig. 3E), submonomorphic sporophylls (Fig. 2B, F, G), but the new species has sterile leaves margins densely ciliate at base (Fig. 2H–J), dorsal leaves obviously carinate (Fig. 2D, I), symmetrical axillary leaves oblong-ovate ovate-triangular (Fig. 2H), and megaspore surfaces fine reticulate (Fig. 2M, N).
Figure 3.
Type of SelaginelladensiciliataA dorsal view of branches with strobili B ventral view of branches with strobili C dorsal view of branches D ventral view of branches E holotype of Selaginelladensiciliata (L. Zhang et al. 2215).
Description.
Plants terrestrial, evergreen, ascending to suberect, 7.0–15.0 cm tall, without creeping rhizomes or stolons, without elongate tuber at base of stem (Fig. 3E). Rhizophores grow from the base to the middle of stem, borne on ventral side in axils of branches (Figs 2C, 3E), 0.2–0.3 mm in diam. Main stem branched upwards from near the base, pinnately branched (Figs 2A, 3), unbranched main stem is 0.5–3.5 cm tall, terete, glabrous, 0.4–0.6 mm in diam (Fig. 3). Branches 6–14 pairs, 2 or 3 times pinnately branched; adjacent main branches on main stem 3.1–5.0 mm apart, the terminal branches 2.6–5.2 mm wide (Fig. 3C–E). Sterile leaves four rows, leathery, margins densely ciliate on the lower parts or at least at base (Fig. 2H–J). Axillary leaves on main stems not larger than those on branches, symmetrical, oblong-ovate to ovate-triangular ovate-triangular, base not peltate, truncate; axillary leaves on branches symmetrical, oblong-ovate to ovate-triangular, base cordate, 1.6–2.1×0.8–1.2 mm, slightly carinate, margins densely ciliate in basal half to denticulate at apex, apex acute (Fig. 2C, E, H). Dorsal leaves asymmetrical, those on main stems strongly larger than those on branches; ovate, 1.2–1.9 × 0.5–1.0 mm, strongly carinate, base truncate, oblique, peltate, margins slightly densely ciliate at basal half, upward denticulate, apex shortly aristate (Fig. 2C, E, J). Ventral leaves asymmetrical, overlapping stem and branches, those on main stem strongly larger than those on branches, oblong-falcate, 2.0–2.7×1.0–1.5 mm, carinate, base round, peltate, apex acute; basiscopic margins slightly denticulate at base, upward subentire; acroscopic base margins densely ciliate at lower part, upward subentire (Fig. 2D, I). Strobili solitary, terminal, compact, quadrangular, 4.3–7.9 mm (Figs 2B, F, G, 3A, B). Sporophylls slightly dimorphic, dorsal sporophylls slightly longer than ventral sporophylls (Fig. 2B, F, G); dorsal sporophylls ovate, carinate, 1.4–1.5×0.7–0.9 mm, margins denticulate, base cuneate, not peltate, apex acuminate, without sporophyll-pteryx (Fig. 2K); ventral sporophylls broadly ovate, carinate, 1.23–1.47×0.69–0.81 mm, base truncate, not peltate, apex acuminate, margin denticulate (Fig. 2G). Megasporophylls in basal portion on lower side of strobilus. Megaspore white-yellow, oblate spheroid to subglobose, 225.6–280.2 μm in diam., prominent laesurae extend 2/3 of the distance to the equator; surface finely reticulate ornamentation (Fig. 2M, N). Microspore orange, hemispherical, 27.4–37.7 μm, surfaces with dense and large verrucate ornamentation covered with densely irregular granular microstructure (Fig. 2O, P).
Geographical distribution and habitat.
Selaginelladensiciliata is only known from Beibeng Township, Medog County, Xizang Province, China. It grows in humid places in evergreen broadleaved forests, at elevations of 1000–1600 m.
Additional specimens examined (paratypes).
China. Xizang: Nyingchi City, Medog county, Beibeng township. on the way from A’niqiao to Hanmi village, elev. ca. 1000 m, 29°20'14.40"N, 95°10'19.19"E, 4 Jun. 2015. Bo Xu & Xin-Mao Zhou YLZB2043 (CDBI, PYU); on the way from A’niqiao to Hanmi village, elev. 1530 m, 29°20'29.51"N, 95°10'12.74"E, 15 Oct. 2017, Liang Zhang, Wen-Bin Ju & Heng-Ning Deng 2090 (KUN, PYU); on the way from A’niqiao to Hanmi village, alt. 1120 m, 29°19'42.75"N, 95°10'36.47"E, 17 Oct. 2017, Liang Zhang, Wen-Bin Ju & Heng-Ning Deng 2203 (KUN, PYU).
Etymology.
The specific epithet “densiciliata” is a compound word derived from the Latin word “dense” which means dense and suffix “ciliata” which means ciliate. The specific epithet “densiciliata” refers to sterile leave (axillary leaves, dorsal leaves, and ventral leaves, Fig. 2H–J) margins with dense cilia at base.
Key to Selaginelladensiciliata and its relative species of the S.vaginata group
| 1 | Stem nearly creeping, only fertile parts (strobili) ascending | S.vaginata |
| – | Stem more or less suberect or ascending | 2 |
| 2 | Leaves distinctly white-margined | S.repanda |
| – | Leaves not obviously white-margined | 3 |
| 3 | Base of sterile leave margins sparsely ciliate or denticulate; axillary leaves asymmetrical; dorsal leaves ovate-lanceolate, slightly carinate; microspore surface smooth | S.subvaginata |
| – | Base of sterile leave margins densely ciliate; axillary leaves symmetrical; dorsal leaves ovate, obvious carinate; microspore surface verrucate | S.densiciliata |
Supplementary Material
Acknowledgements
The research was supported by the National Natural Science Foundation of China (NSFC) to X.M. Zhou (#31900186, #32260050). We thank the two anonymous reviewers for their valuable comments and suggestions.
Appendix 1
List of taxa sampled with information related to taxonomy, GenBank accession numbers (rbcL, ITS), references, and vouchers information. Herbarium codes follow Index Herbariorum (Thiers 2018).
S.albociliata P. S. Wang (1) L.-B. Zhang et al.et al. 5302 (CDBI), China (Guangxi), KT161379 (Zhou et al. 2016), KT161648 (Zhou et al. 2016); (2) X.-C. Zhang 7242 (PE), China (Guizhou), MH814882 (Shalimov et al. 2019), —. S.alutacea SpringKorall 2006-9 (S), Malaysia, KY022958 (Weststrand and Korall 2016b), —. S.amblyphylla Alston (1) X.-C. Zhang 2924 (PE), China (Yunnan), MH814883 (Shalimov et al. 2019), —; (2) X.-C. Zhang 7951 (PE), China (Yunnan), MH814884 (Shalimov et al. 2019), —. S.arbuscula Spring (1) Wood 13447 (PTBG), Hawaii (Kauai, Wainiha), KT161387 (Zhou et al. 2016), KT161656 (Zhou et al. 2016); (2) Wood 13746 (PTBG), Hawaii (Maui, Kipahulu), KT161388 (Zhou et al. 2016), KT161657 (Zhou et al. 2016). S.aristata SpringH.-M. Chang et al. 7119 (TAIE), MF313959 (Sheue et al. Unpublished), —. S.austro-orientulis H. J. Wei & X. M. Zhou (1) S.-L. Jin et al. 7965 (CSH, IBK, PYU), China (Jiangxi), OP690605 (Wei et al. 2023), OP683200 (Wei et al. 2023); (2) S.-L. Jin et al. 7982 (CSH), China (Jiangxi), OP690606 (Wei et al. 2023), OP683199 (Wei et al. 2023). S.banksii AlstonGrant 3563 (L), French Polynesia, KY022972 (Weststrand and Korall 2016b), —. S.behrmanniana HieronJohns 8937 (L), Indonesia, KY022973 (Weststrand and Korall 2016b), —. S.bisulcata SpringChu et al. 31292 (PYU), China (Yunnan), KT161404 (Zhou et al. 2016), KT161673 (Zhou et al. 2016). S.bodinieri Hieron. (1) Ju & Deng HGX12828 (CDBI), China (Sichuan), KT161414 (Zhou et al. 2016), KT161677 (Zhou et al. 2016); (2) L.-B. Zhang et al. 5193 (CDBI), China (Guangxi), KT161409 (Zhou et al. 2016), KT161680 (Zhou et al. 2016). S.boninensis Baker. (1) Knapp 3578 (P), China (Taiwan), MZ571146 (He et al. 2021), —; (2) TNS766618 (TNS), Japan (Tokyo), AB574642 (Ebihara et al. 2010), —. S.brachystachya (Hook. & Grev.) Spring (1) J. Klackenberg 434 (S), Sri Lanka, KY022980 (Weststrand and Korall 2016b), —; (2) W.A. Sledge 913 (L), Sri Lanka, KY022979 (Weststrand and Korall 2016b), —. S.chaetoloma Alston (1) Z.-Y. Guo 2016014 (PE), China (Guizhou), MH814888 (Shalimov et al. 2019), —; (2) X.-C. Zhang 7347 (PE), China (Guizhou), MH814889 (Shalimov et al. 2019), —. S.chingii Alston (1) L.-B. Zhang et al. 6587 (CDBI, MO, VNMN, PYU), Vietnam (Lang Son), KT161417 (Zhou et al. 2016), KT161683 (Zhou et al. 2016); (2) L.-B. Zhang et al. 6594 (CDBI, MO, VNMN, PYU), Vietnam (Lang Son), KT161416 (Zhou et al. 2016), KT161868 (Zhou et al. 2016). S.chrysocaulos (Hook. & Grev.) Spring (1) Xu et al. 2014-156 (CDBI), China (Sichuan), KT161427 (Zhou et al. 2016), KT161690 (Zhou et al. 2016); (2) X.-M. Zhou 001 (CDBI), China (Yunnan), MZ532020 (He et al. 2021), —; (3) X.-C. Zhang 86 (PE), China (Sichuan), MH814891 (Shalimov et al. 2019), —. S.ciliaris (Retz.) SpringJiang 310 (PYU, CDBI), China (Hainan), KT161428 (Zhou et al. 2016), KT161691 (Zhou et al. 2016). S.coriaceifolia X. M. Zhou, N. T. Lu & Li Bing Zhang (1) L.-B. Zhang et al. 7307 (CDBI, MO, VNMN), Vietnam (Quang Binh), MT386596 (He et al. 2021), MZ570596 (He et al. 2021); (2) L.-B. Zhang et al. 7371 (CDBI, MO, VNMN), Vietnam (Quang Binh), MT386598 (Ye et al. 2020), MT386595 (Ye et al. 2020). S.decipiens Warb. (1) L.-B. Zhang et al. 6761 (CDBI, MO, VNMN, PYU), Vietnam (Bac Kan), KT161439 (Zhou et al. 2016), KT161697 (Zhou et al. 2016); (2) L.-B. Zhang et al. 6764 (CDBI, MO, VNMN, PYU), Vietnam (Bac Kan), KT161438 (Zhou et al. 2016), KT161698 (Zhou et al. 2016). S.densiciliata Xin-Mao Zhou & Liang Zhang (1) L. Zhang et al. 2090 (KUN, PYU), China (Xizang), OQ723681 (this study), OQ728789 (this study); (2) L. Zhang et al. 2203 (KUN, PYU), China (Xizang), OQ723684 (this study), OQ728792 (this study); (3) L. Zhang et al. 2215 (KUN, PYU), China (Xizang), OQ723682 (this study), OQ728790 (this study); (4) B. Xu & X.-M. Zhou 2043 (CDBI, PYU), China (Xizang), OQ723683 (this study), OQ728791 (this study). S.devolii H. M. ChangH.-M. Chang et al. 7116 (TAIE), Unknown, MF313957 (Sheue et al. 2017), —. S.dianzhongensis X. C. ZhangX.-M. Zhou 1445 (PYU), China (Yunnan), OQ723685 (this study), OQ728793 (this study). S.effusa Alston (1) Dong 2470 (PYU), China (Guangdong), (Zhou et al. 2016), KT161705 (Zhou et al. 2016); (2) GLG Survey 22153 (GH), China, KY023020 (Weststrand and Korall 2016b), —; (3) L.-B. Zhang et al. 5442 (CDBI), China (Guangxi), KT161451 (Zhou et al. 2016), KT161707 (Zhou et al. 2016). S.goudotiana SpringM. Thulin and H. Razafindraibe 11750 (UPS), Madagascar, KY023039 (Weststrand and Korall 2016b), —. S.goudotianaSpringvar.abyssinica (Spring) BizzarriJ. Kornaś and A. MedweckaKornaś 3688 (BR), Zamb, KY023036 (Weststrand and Korall 2016b), —. S.heterostachys Baker (1) X.-C. Zhang 7088 (PE), China (Guizhou), MH814896 (Shalimov et al. 2019), —; (2) X.-C. Zhang 7268 (PE), China (Guizhou), MH814897 (Shalimov et al. 2019), —. S.hezhangensis P. S. Wang et X. Y. Wang (1) FLPH Sichuan Expedition 152002, China (Sichuan), OM864654 (Shalimov and Zhang 2022), —; (2) X.-C. Zhang 6830 (PE), China (Guizhou), OM864656 (Shalimov and Zhang 2022), —. S.kanehirae Alston (1) Hovence 10079 (PTBG), Hawaii, MZ571148 (He et al. 2021), —. S.kurzii Baker (1) X.-M. Zhou et al. PYU-S-2105 (PYU), China (Yunnan), MZ532022 (He et al. 2021), MZ570598 (He et al. 2021); (2) X.-C. Zhang 1934 (PE), Unknown, MH814898 (Shalimov et al. 2019), —. S.kusaiensis Hosok.D.H. Lorence 9385 (PTBG), Unknown, MT657911 (Nitta et al. 2020), —. S.labordei Hieron. ex Christ (1) Gao et al. DJY04311 (CDBI), China (Sichuan), KT161503 (Zhou et al. 2016), KT161751 (Zhou et al. 2016); (2) X.-M. Zhou 008 (CDBI), China (Sichuan), KT161505 (Zhou et al. 2016), —; (3) Gaoligong Shan Biodiversity Survey 18378 (GH), China (Yunnan), KY023061 (Weststrand and Korall 2016b), —; (4) X.-C. Zhang 3356 (PE), China (Hubei), MH814899 (Shalimov et al. 2019), —. S.laxa Spring (1) Schuettpelz 1913 (US), French Polynesia (Marquesas Islands), MT216111 (He et al. 2021), —. S.leptophylla BakerX.-M. Zhou et al. DJY05380 (CDBI), China (Sichuan), KT161513 (Zhou et al. 2016), KT161756 (Zhou et al. 2016). S.lutchuensis Koidz. (1) TNS101683 (TNS), Japan, MT680176 (Zhang et al. 2020), —; (2) TNS759343 (TNS), Japan (Okinawa), AB574648 (Ebihara et al. 2010), —. S.medogensis Ching et S. K. WuQinghai-Xizang Complex Exped 74-4375 (PE), China (Xizang), OK247696 (Shalimov and Zhang 2021), —. S.megaphylla Baker (1) X.-H. Jin 19301 (PE), Unknown, MH814901 (Shalimov et al. 2019), —; (2) X.- M. Zhou YLZB2185 (CDBI, PYU), China (Xizang), ON994456 (Xu et al. 2022), ON994203 (Xu et al. 2022). S.microclada BakerX.-C. Zhang et al. 8307 (PE), China (Yunnan), OK247702 (Shalimov and Zhang 2021), —. S.miniatospora (Dalzell) Baker (1) J. Klackenberg and R. Lundin 567 (S), India (Kerala), KY023081 (Weststrand and Korall 2016b), —; (2) C. van Hardeveld and H. H. van der Werff 120 (U), India (Tamil Nadu), KY023080 (Weststrand and Korall 2016b), —. S.minutifolia SpringLarsen et al. 1389 (S), Thailand, KY023082 (Weststrand and Korall 2016b), —. S.mittenii Bakervan Steenis 24105 (L), South Africa, KY023083 (Weststrand and Korall 2016b), —. S.monospora Spring (1) L.-B. Zhang et al. 7425 (CDBI), Vietnam (Quang Binh), MZ571145 (He et al. 2021), —; (2) L. Zhang 1296, China (Yunnan), MZ532023 (Xu et al. 2022), —. S.morganii ZeillerP. Korall 2006-29 (S), Peninsular Malaysia, KY023088 (Weststrand and Korall 2016b), —. S.neocaledonica Baker (1) sc s.n. Unknown, KY985453 (Klaus et al. 2017), —; (2) Wikstrom 244 (S), New Caledonia, KY023095 (Weststrand and Korall 2016b), —. S.nipponica Franch. & Sav. (1) Zhou et al. DJY07479 (CDBI), China (Sichuan), KT161542 (Zhou et al. 2016), KT161784 (Zhou et al. 2016). S.ornata Spring (1) Zhang 4958 (CDBI), China (Guizhou), KT161522 (Zhou et al. 2016), KT161768 (Zhou et al. 2016); (2) Zhang et al. 6935 (CDBI, MO, VNMN, PYU), Vietnam (Ha Giang), KT161526 (Zhou et al. 2016), KT161772 (Zhou et al. 2016). S.qingchengshanensis Li Bing Zhang & X. M. Zhou (1) Gao et al. DJY04053 (CDBI), China (Sichuan), KT161381 (Zhou et al. 2016), KT161649 (Zhou et al. 2016); (2) Z.-L. Liang & X. Pu 056 (CDBI, PYU), China (Sichuan), MZ532027 (He et al. 2021), MZ570603 (He et al. 2021). S.reineckei Hieron. (1) K.R. Wood 16944 (PTBG), Samoa (Savaii), MT657902 (Nitta et al. 2020), —; (2) McKee 2907 - P7338 (L), Samoa, KY023129 (Weststrand and Korall 2016b), —. S.repanda (Desv. ex Poir.) Spring (1) He & Jiang 405-1 (CDBI), China (Yunnan), KT161584 (Zhou et al. 2016), —; (2) Z.-R. He & X.-M. Zhou 119 (PYU, CDBI), China (Yunnan), KT161583 (Zhou et al. 2016), KT161816 (Zhou et al. 2016); (3) He s.n. China (Yunnan), KT161817 (Zhou et al. 2016). S.subdiaphana (Wall. ex Hook. et Grev.) Spring (1) Y.-D.Wu 840 (PE), China (Yunnan), OM864659 (Shalimov and Zhang 2022), —. S.submonospora Shalimov et X.-C. ZhangX.-C. Zhang et al. 8111 (PE), China (Yunnan), OM864660 (Shalimov and Zhang 2022), —. S.subvaginata Shalimov et X. C. Zhang (1) H. Liu 183 (PE), China (Sichuan), MT680178 (Zhang et al. 2020), —; (2) X.-C. Zhang et al. 8838 (PE), China (Sichuan), MT680179 (Zhang et al. 2020), —; (3) X.-C. Zhang et al. 9450 (PE), China (Sichuan), MT680181 (Zhang et al. 2020), —. S.trichophylla K. H. Shing (1) W.-M. Chu et al. 29310 (PYU), China (Yunnan), KT161622 (Zhou et al. 2016), KT161846 (Zhou et al. 2016); (2) Chu et al. 31925 (PYU), China (Yunnan), KT161621 (Zhou et al. 2016), KT161847 (Zhou et al. 2016). S.uncinata (Desv. ex Poir.) SpringZhang & Zhou DJY04101 (CDBI), China (Sichuan), KT161626 (Zhou et al. 2016), KT161852 (Zhou et al. 2016). S.vaginata Spring (1) D.E. Boufford et al. 28593 (A), China (Sichuan), KY023167 (Weststrand and Korall 2016b), —; (2) D.E. Bouff ord 37640 (A), China (Gansu), KY023168, (Weststrand and Korall 2016b), —; (3) B. Bartholomew et al. 367 (PE), China (Guizhou), MT680182 (Zhang et al. 2020), —; (4) Z.-S. Zhang 161 (PE), China (Shanxi), MH814907 (Shalimov et al. 2019), —. S.whitmeei BakerK.R. Wood 17032 (PTBG), Samoa (Savaii), MT657910 (Nitta et al. 2020), —. S.wuyishanensis K. W. Xu, X. M. Zhou & Y. F. Duan (1) K.-W. Xu WY518 (NF), China (Fujian), OQ723687 (this study), OQ728795 (this study); (2) K.-W. Xu WY521 (NF), China (Fujian), OQ723686 (this study), OQ728794 (this study). S.xipholepis Baker (1) S.-Y. Dong 2377 (PYU), China (Guangdong), KT161645 (Zhou et al. 2016), —; (2) K.U. Kramer et al. 8329 (U), China (Hong Kong), KY023179 (Weststrand and Korall 2016b), —; (3) L.-B. Zhang et al. 6668 (CDBI, MO, VNMN, PYU), Vietnam (Bac Kan), KT161646 (Zhou et al. 2016), KT161867 (Zhou et al. 2016); (4) X.-C. Zhang 7422 (PE), China (Guizhou), MH814908 (Shalimov et al. 2019), —. S.yunckeri AlstonYuncker 15933 (U), Tonga, KY023182 (Weststrand and Korall 2016b), —. S.zhangiiS. Y. DongDong 5511, China (Yunnan), MW316869 (Huang et al. 2022), —.
Citation
Fang S-L, Xu B, Zhang L, He Z-R, Zhou X-M (2023) Selaginella densiciliata (subg. Heterostachys, Selaginellaceae), a new spikemoss species from China based on morphological and molecular data. PhytoKeys 227: 135–149. https://doi.org/10.3897/phytokeys.227.101222
Funding Statement
National Natural Science Foundation of China (NSFC)
Contributor Information
Zhao-Rong He, Email: zhrhe@ynu.edu.cn.
Xin-Mao Zhou, Email: xinmao.zhou@ynu.edu.cn.
Additional information
Conflict of interest
No conflict of interest was declared.
Ethical statement
No ethical statement was reported.
Funding
No funding was reported.
Author contributions
Conceptualization: BX. Formal analysis: SLF. Funding acquisition: XMZ. Methodology: SLF. Project administration: XMZ. Resources: BX. Software: SLF. Supervision: XMZ. Writing - original draft: XMZ, SLF. Writing - review and editing: ZRH, BX, LZ.
Data availability
All of the data that support the findings of this study are available in the main text or Supplementary Information.
References
- Akaike H. (1974) A new look at the statistical model identification. Institute of Electrical and Electronics Engineers Transactions on Automatic Control 19(6): 716–723. 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) jModelTest 2: More models, new heuristics and parallel computing. Nature Methods 9(8): 772–772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du C, Liu J, Ye W, Liao S, Ge BJ, Liu B, Ma JS. (2021) New taxa of plants of China, new name 2020 Annual Report. Biodiversity (Nepean) 29(8): 1011–1020. 10.17520/biods.2021122 [DOI] [Google Scholar]
- Ebihara A, Nitta JH, Ito M. (2010) Molecular species identification with rich floristic sampling: DNA barcoding the pteridophyte flora of Japan. PLoS ONE 5(12): e15136. 10.1371/journal.pone.0015136 [DOI] [PMC free article] [PubMed]
- Fan XP, Zhang L, Zhang LB, Zhang L. (2021) Selligueawusugongii (Polypodiaceae), a new fern species from southeastern Xizang, China based on morphological and molecular evidence. Phytotaxa 480(1): 57–68. 10.11646/phytotaxa.480.1.5 [DOI] [Google Scholar]
- Hall TA. (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- He M, He ZR, Zhang LB, Zhou XM. (2021) Selaginellaqingchengshanensis (sect. Heterostachys; Selaginellaceae), a new species from Sichuan, China. Phytotaxa 522(4): 285–293. 10.11646/phytotaxa.522.4.2 [DOI] [Google Scholar]
- Huang L, Li SH, Dong SY. (2022) Two new species of the lycophyte genus Selaginella (Selaginellaceae) from China, with Notes on the phylogenetic positions of related species. Systematic Botany 47(1): 85–96. 10.1600/036364422X16442668423400 [DOI] [Google Scholar]
- Katoh K, Standley DM. (2013) MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30(4): 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus KV, Schulz C, Bauer DS, Stützel T. (2017) Historical biogeography of the ancient lycophyte genus Selaginella: Early adaptation to xeric habitats on Pangea. Cladistics 33(5): 469–480. 10.1111/cla.12184 [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE). 14 November 2010. New Orleans, LA, 1–8. 10.1109/GCE.2010.5676129 [DOI]
- Mittermeier RA, Hawkins F, Rajaobelina S, Langrand O. (2005) Wilderness conservation in a biodiversity hotspot. International Journal of Wilderness 11(3): 42–45. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J. (2000) Biodiversity hotspots for conservation priorities. Nature 403(6772): 853–858. 10.1038/35002501 [DOI] [PubMed] [Google Scholar]
- Nitta J, Steier J, Schuettpelz E. (2020) Biogeography of Pacific pteridophytes in a global context. [Unpublished]
- Qiu YL, Xu KW, Ju WB, Zhao WL, Zhang L. (2022a) Hymenaspleniumtholiformis (Aspleniaceae), a new fern species from southeastern Xizang, China based on morphological and molecular evidence. PhytoKeys 204: 43–56. 10.3897/phytokeys.204.85746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YL, Zhang L, Zhang LB, Zhou XM, Zhang L. (2022b) Athyriumaberrans (Athyriaceae), a new species of the lady ferns from southeastern Xizang, China, based on morphological and molecular evidence. Phytotaxa 533(3): 165–172. 10.11646/phytotaxa.533.3.2 [DOI] [Google Scholar]
- Rambaut A, Drummond AJ. (2007) Tracer 1.4. http://beast.bio.ed.ac.uk/Tracer [Accessed 15 Jan. 2022]
- Ronquist F, Huelsenbeck JP. (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19(12): 1572–1574. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Shalimov AP, Zhang XC. (2021) A taxonomic revision of Selaginellamonospora Spring (Selaginellaceae). Turczaninowia 24(3): 175–193. 10.14258/turczaninowia.24.3.14 [DOI] [Google Scholar]
- Shalimov AP, Zhang XC. (2022) Selaginellasubmonospora (Selaginellaceae), a new species from Yunnan. Turczaninowia 25(1): 153–165. 10.14258/turczaninowia.25.1.15 [DOI] [Google Scholar]
- Shalimov AP, Zhu YM, Zhang MH, Zhang XC. (2019) Selaginelladianzhongensis (Selaginellaceae), a new spikemoss from China. PhytoKeys 118: 75–87. 10.3897/phytokeys.118.30375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheue CR, Chesson P, Li SF, Liu JW, Chang HM, Valdespino I, Salazar N, Chesson C, Oppenheimer H, Bakutis A, Saenger P, Yong JWH, Das S, Adjie B, Kiew R, Nadkarni N, Dong SY, Kao MF, Huang CL. (2017) Recent chloroplast diversification in the ancient genus Selaginella. [Unpublished]
- Stamatakis A, Hoover P, Rougemont J. (2008) A rapid bootstrap algorithm for the RAxML Web servers. Systematic Biology 57(5): 758–771. 10.1080/10635150802429642 [DOI] [PubMed] [Google Scholar]
- Sun H, Zhou ZK. (1996) The characters and origin of the flora from the big bend gorge of Yalutsangpu (Brahmabutra) river, eastern Himalyas. Acta Botanica Yunnanica 18(2): 185–204. [Google Scholar]
- Thiers B. (2018) Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/ [Accessed 26 November 2018]
- Tryon AF, Lugardon B. (1991) Spores of the Pteridophyta. Springer-Verlag, New York, 156 pp. 10.1007/978-1-4613-8991-0 [DOI] [Google Scholar]
- Wei HJ, Chen B, Fang SL, Zhou XM. (2023) Selaginellaaustro-orientalis (Selaginellaceae), a new species from Southeast China. Phytotaxa 579(2): 87–97. 10.11646/phytotaxa.579.2.2 [DOI] [Google Scholar]
- Weststrand S, Korall P. (2016a) A subgeneric classification of Selaginella (Selaginellaceae). American Journal of Botany 103(12): 2160–2169. 10.3732/ajb.1600288 [DOI] [PubMed] [Google Scholar]
- Weststrand S, Korall P. (2016b) Phylogeny of Selaginellaceae: There is value in morphology after all! American Journal of Botany 103(12): 2136–2159. 10.3732/ajb.1600156 [DOI] [PubMed]
- Xu KW, Chen SF, Song Q, Zheng X, Li M, Fang YM, Wei HJ, Ding H, Zhou XM, Duan YF. (2022) Selaginellawuyishanensis (sect. Tetragonostachyae, Selaginellaceae), a new species from East China and its phylogenetic position based on molecular data. PhytoKeys 202(3): 107–119. 10.3897/phytokeys.202.85410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZY, Lu NT, Zhang L, Zhou XM, Zhang LB. (2020) Selaginellacoriaceifolia (sect. Heterostachys; Selaginellaceae), a new lycophyte species from central Vietnam. Phytotaxa 453(2): 121–129. 10.11646/phytotaxa.453.2.3 [DOI] [Google Scholar]
- Zhang XC, Nooteboom HP, Kato M. (2013) Selaginellaceae. In: Wu Z-Y, Raven PH, Hong D-Y. (Eds) Flora of China (Vol.2–3). Science Press, Beijing and Missouri Botanical Garden Press, St. Louis, 37–66.
- Zhang XC, Shalimov AP, Kang JS, Zhang MH. (2020) Selaginellasubvaginata (Selaginellaceae), a new spikemoss from China. Journal of Species Research 9(3): 221–232. 10.12651/JSR.2020.9.3.221 [DOI] [Google Scholar]
- Zhou XM, Zhang LB. (2015) A classification of Selaginella (Selaginellaceae) based on molecular (chloroplast and nuclear), macromorphological, and spore features. Taxon 64(6): 1117–1140. 10.12705/646.2 [DOI] [Google Scholar]
- Zhou XM, Jiang LJ, Zhang L, Gao XF, He ZR, Zhang LB. (2015) Spore morphology of Selaginella (Selaginellaceae) from China and its systematic signifcance. Phytotaxa 237: 001–067. 10.11646/phytotaxa.237.1.1 [DOI] [Google Scholar]
- Zhou XM, Rothfels CJ, Zhang L, He ZR, Le Péchon T, He H, Lu NT, Knapp R, Lorence D, He XJ, Gao XF, Zhang LB. (2016) A large‐scale phylogeny of the lycophyte genus Selaginella (Selaginellaceae: Lycopodiopsida) based on plastid and nuclear loci. Cladistics 32(4): 360–389. 10.1111/cla.12136 [DOI] [PubMed] [Google Scholar]
- Zhou XM, Zhao J, Yang JJ, Le Péchon T, Zhang L, He ZR, Zhang LB. (2022) Plastome structure, evolution, and phylogeny of Selaginella. Molecular Phylogenetics and Evolution 169: 107410. 10.1016/j.ympev.2022.107410 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data that support the findings of this study are available in the main text or Supplementary Information.