Abstract
Objective
To characterize health care resource utilization (HCRU), health care costs, and adverse events (AEs) among patients with systemic lupus erythematosus (SLE) initiating oral corticosteroids (OCS) versus patients without OCS use.
Methods
In this retrospective cohort study (GSK Study 213061), eligible patients (aged ≥5 years at first OCS claim) with SLE from the IQVIA Real‐World Data Adjudicated Claims‐US database (January 2006 to July 2019) had continuous enrollment during the 6‐month preindex (baseline) and 12‐month postindex (observation) periods and one or more inpatient or emergency department SLE diagnosis codes or two or more outpatient SLE diagnosis codes during baseline. The “OCS‐initiator cohort” comprised patients with one or more OCS pharmacy claims during the study period and no evidence of preindex OCS use and was classified into three exposure categories based on the number of 6‐month periods of more than 5 mg/day of OCS use (0, 1, 2). The “no‐OCS‐use cohort” comprised patients without OCS claims, although patients may have received OCS prior to the study period. Clinical and economic outcomes were reported over the observation period.
Results
Adjusted health care costs differed significantly ($6542 [95% confidence interval (CI): $5761‐$7368], $19,149 [95% CI: $16,954‐$21,471], $28,985 [95% CI: $25,546‐$32,885]). HCRU incidence rates were significantly greater for all OCS‐initiator exposure categories (n = 16,216) versus the no‐OCS‐use cohort (n = 11,137; adjusted incidence rate ratios [95% CI]: 1.22 [1.19‐1.24], 1.39 [1.34‐1.43], 1.66 [1.60‐1.73]). OCS‐related AEs were experienced by 67.1% to 74.1% of patients with OCS initiation, most commonly affecting the immune system.
Conclusion
Within 12 months of OCS initiation, patients with SLE experienced substantial clinical and economic burden, which may imply a need to minimize OCS use.
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by autoantibody production and abnormal B cell function (1). Clinical manifestations of SLE are diverse; flares are common (2), and cutaneous, renal, or musculoskeletal involvement is frequently reported among patients with SLE (1, 3). The goals of SLE management are prevention of flares and organ damage, reduction of disease activity and symptoms, improvement of long‐term survival, and optimization of patient health‐related quality of life (3, 4).
Corticosteroids are a cornerstone of SLE therapy because of their antiinflammatory and immunosuppressive properties (4). However, cumulative exposure to corticosteroids is associated with increased risk of infections, adverse events (AEs), and irreversible damage to organ systems (5, 6, 7, 8, 9). The relationship between corticosteroid use as standard therapy and the occurrence of AEs is also well documented in other therapeutic areas, including asthma, chronic obstructive pulmonary disease, inflammatory bowel disease, and ocular conditions (8, 10, 11, 12, 13, 14, 15, 16, 17, 18). Patients receiving corticosteroids are at particular risk of treatment‐related acute and chronic AEs affecting the musculoskeletal, metabolic, gastrointestinal, cardiovascular, and central nervous systems (19). Furthermore, higher doses and long‐term use of corticosteroids are associated with a greater risk of toxicity (20).
The economic implications of using corticosteroids in patients with SLE are also substantial. Long‐term corticosteroid use, particularly at high doses, is associated with increased health care resource utilization (HCRU) and greater health care costs (19, 21, 22). A study in newly diagnosed patients initiating oral corticosteroids (OCS) found that the long‐term use of OCS, even in low prednisone‐equivalent doses (≤5 mg/day), incurs significantly greater costs and HCRU compared with no OCS use (21).
Guidelines and task force recommendations for the management of SLE recommend reducing corticosteroids to the lowest possible dose: no more than 7.5 mg/day (prednisone equivalent) for maintenance treatment and ideally less than 5 mg/day or withdrawal altogether (4, 23, 24). Despite these recommendations, a US retrospective claims database analysis (2012‐2018) found that patients with SLE initiating OCS therapy had an average dose of 19 mg/day in the first 12 months of use (25).
Given the increasing focus on treating to targets of remission or low disease activity with minimal corticosteroid intake (24, 26), outcomes among patients initiating OCS are of special interest.
Our study assessed clinical and economic outcomes associated with OCS use in patients with SLE in the United States during the first year after OCS initiation. Objectives of this study were to compare all‐cause HCRU, health care costs, and OCS‐related AEs among patients with OCS initiation at different lengths of exposure to more than 5 mg/day (prednisone equivalent) against patients with no OCS use and to describe OCS treatment patterns.
MATERIALS AND METHODS
Study design
This retrospective cohort study (GSK Study 213061) used medical and pharmacy claims data from the IQVIA Real‐World Data Adjudicated Claims‐US database spanning from January 1, 2006, to July 31, 2019, to identify patients with SLE.
The study design is shown in Figure 1. Patients were categorized into one of two cohorts: the OCS‐initiator cohort or the no‐OCS‐use cohort. The index date for the OCS‐initiator cohort was defined as the date of the first OCS pharmacy claim; for the no‐OCS‐use cohort, the index date was imputed based on the distribution of time between the first SLE claim in the continuous eligibility period and the index date for the OCS‐initiator cohort. The baseline period was defined as the 6 months preceding the index date, and the observation period was defined as the 12 months following the index date.
Figure 1.
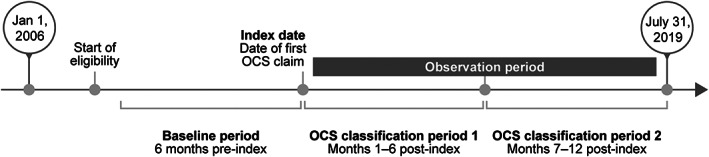
Study design. The index date for patients with no OCS pharmacy claims was imputed based on the distribution of time between the first claim with a systemic lupus erythematosus diagnosis in the continuous eligibility period and the index date in the OCS‐initiator cohort. OCS, oral corticosteroid.
Ethical approval and informed consent
The IQVIA Real‐World Data Adjudicated Claims‐US database is deidentified in compliance with the patient confidentiality requirement of the Health Insurance Portability and Accountability Act; therefore, no institutional board review or informed consent was required for this study.
Study population
Eligible patients were 5 years of age or older at the index date, had a diagnosis of SLE (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] codes 710.0x or International Classification of Diseases, Tenth Revision, Clinical Modification [ICD‐10‐CM] codes M32.0, M32.1x, M32.8, M32.9; SLE diagnosis on two or more outpatient medical claims or one or more inpatient or emergency department claims during the baseline period), and had continuous enrollment in a health plan for 6 months or more prior to the index date and 12 months or more following the index date.
Patients included in the OCS‐initiator cohort had one or more OCS pharmacy claims during the study period and no evidence of baseline OCS use. The OCS‐initiator cohort was divided into three exposure categories according to the number of 6‐month periods (0, 1, or 2 periods) of average daily prednisone‐equivalent OCS use greater than 5 mg/day during the observation period. Patients included in the no‐OCS‐use cohort had no OCS pharmacy claims at any time during the study period.
Variables and outcomes
Patient year of index, demographic characteristics (sex, age at index, geographic region, and health plan type), and clinical characteristics (physician specialty recording the SLE diagnosis, comorbidities [identified using ICD‐9‐CM and ICD‐10‐CM diagnostic codes], concomitant medications commonly prescribed for SLE, SLE disease severity and flares, and all‐cause health care costs) were captured during the baseline period. SLE disease severity and flares were identified using previously published algorithms (27, 28, 29) and classified as mild, moderate, or severe. SLE disease severity and flare episodes were also assessed during the observation period.
OCS exposure (list of included OCS medications can be found in the Supplementary Material) during the observation period was estimated from information extracted from pharmacy claims. The average daily dose (ADD) (observation) was based on the number of total days in the observation period and calculated using the following formula:
For the sensitivity analyses, ADD (days’ supply) was based on the number of days of supply of OCS dispensed and calculated using the following formula:
OCS doses were converted to prednisone‐equivalent strengths for the calculation of average daily OCS dose.
All‐cause HCRU was reported during the observation period and comprised outpatient visits, inpatient stays, emergency department visits, and other encounters (eg, ambulance, assisted living facilities, comprehensive rehabilitation facilities, custodial care facilities, hospice/home care services, intermediate care facilities, psychiatric facilities, and skilled nursing facilities).
OCS treatment patterns were evaluated during each 6‐month period of the observation period and consisted of cumulative OCS dose, ADD, and proportion of patients with OCS use greater than 5 mg/day.
Health care costs were reported during the baseline and observation periods. All‐cause health care costs comprised medical costs (outpatient, inpatient, emergency department visits, and other encounters) and pharmacy costs.
The incidence of OCS‐related AEs (including cardiovascular, metabolic and endocrine, central nervous system, bone and muscle, infections, ophthalmologic, gastrointestinal, dermatologic, and hematologic/oncologic AEs) were reported during the observation period. The AEs were chosen based on package inserts for frequently prescribed corticosteroids or systematic literature searches and have been previously shown to be associated with corticosteroid use (8, 15, 19).
Statistical analysis
For patient demographics and clinical characteristics, categorical variables were summarized using relative frequencies and proportions, whereas continuous variables were summarized as means and SDs. For statistical comparisons between cohorts, chi‐square tests and Wilcoxon rank sum tests were used for categorical and continuous variables, respectively.
To compare HCRU outcomes between OCS exposure categories within the OCS‐initiator cohort and the no‐OCS‐use cohort, adjusted incidence rate ratios (IRRs) were estimated using a generalized linear regression model with a negative binomial distribution and log link to account for overdispersion.
To further assess treatment patterns across the three OCS exposure groups, ADD was calculated for two stratified age groups, 5‐17 years and 18 years or older, where the Wilcoxon rank sum test was used for comparison between age groups.
Health care costs were reported in US dollars (USD) and adjusted using the 2018 US Medical Care Consumer Price Index to account for inflation. A two‐part model was applied to estimate adjusted mean differences in health care costs between the OCS‐initiator cohort and the no‐OCS‐use cohort, adjusting for baseline covariates (sex, age on index date, geographic region, total health care costs, Charlson Comorbidity Index, SLE medications [nonsteroidal antiinflammatory drugs (NSAIDs), antimalarials, immunosuppressants, and biologics], disease severity, and flares) (30, 31). The two‐part modeling approach involved fitting a logistic regression model for the probability of observing a positive cost and fitting a generalized linear regression model with a gamma distribution and log link among patients with nonzero health care costs. Final calculated cost differences represented the full population. Nonparametric bootstrap procedures with 999 replications were applied to determine 95% confidence intervals (CIs) and P values.
Two sensitivity analyses were conducted to estimate adjusted mean differences of health care costs using alternative definitions for OCS use. The first used ADD (days’ supply) rather than ADD (observation). The second used 7.5 mg/day rather than 5 mg/day for the OCS dose cutoff point.
Adjusted risk ratios (RRs) for developing each OCS‐related AE were estimated using a modified Poisson regression model. To ensure only initiator AEs were captured, analyses of each AE were limited to patients who had no claims for that AE during the 6‐month baseline period.
RESULTS
Baseline demographic and clinical characteristics
A total of 399,000 patients with at least one SLE claim were identified. Of these, 16,216 patients were included in the OCS‐initiator cohort, and 11,137 patients were included in the no‐OCS‐use cohort (Supplementary Figure 1).
In the OCS‐initiator cohort, 70.5% (n = 11,438) had no 6‐month periods of more than 5 mg/day OCS use (ie, their ADD was ≤5 mg/day throughout the 12‐month observation period), 18.8% (n = 3048) had one 6‐month period of more than 5 mg/day OCS use, and 10.7% (n = 1730) had two 6‐month periods of more than 5 mg/day OCS use.
Most patients were female (no‐OCS‐use cohort: 89.3%; OCS‐initiator cohort: 90.7%). Overall, the no‐OCS‐use cohort was older than the OCS‐initiator cohort, with a mean (SD) age of 47.3 (13.8) years versus 44.9 (13.4) years, respectively. The no‐OCS‐use cohort also had a lower mean (SD) Quan–Charlson Comorbidity Index score compared with the OCS‐initiator cohort (1.5 [1.1] vs. 1.6 [1.2], respectively; P < 0.01) and had lower concomitant medication use (antimalarials: 42.4% vs. 48.5%; NSAIDs: 19.2% vs. 28.5%; immunosuppressants/biologics: 12.3% vs. 17.4%; all: P < 0.01) (Table 1).
Table 1.
Patient baseline demographics, disease characteristics, and disease severity (N = 27,353)
| No OCS use (n = 11,137) | OCS initiation (n = 16,216) | P value | |
|---|---|---|---|
| Age, mean (SD), y | 47.3 (13.8) | 44.9 (13.4) | <0.01 |
| Age category, n (%), y | |||
| 5‐17 | 192 (1.7) | 488 (3.0) | <0.01 |
| ≥18 | 10,945 (98.3) | 15,728 (97.0) | <0.01 |
| Sex, n (%) a | |||
| Female | 9942 (89.3) | 14,705 (90.7) | <0.01 |
| Region, n (%) | |||
| South | 3623 (32.5) | 6196 (38.2) | <0.01 |
| Northeast | 2735 (24.6) | 3641 (22.5) | <0.01 |
| Midwest | 2394 (21.5) | 3675 (22.7) | 0.02 |
| West | 2276 (20.4) | 2567 (15.8) | <0.01 |
| Unknown | 108 (1.0) | 137 (0.8) | 0.28 |
| Insurance type, n (%) b | |||
| Commercial | 6672 (59.9) | 9461 (58.3) | 0.01 |
| Medicare | 330 (3.0) | 286 (1.8) | <0.01 |
| Medicaid | 1040 (9.3) | 1291 (8.0) | <0.01 |
| Self‐insured | 3076 (27.6) | 5216 (32.2) | <0.01 |
| Other/unknown | 67 (0.6) | 76 (0.5) | 0.13 |
| Year of index date, n (%) | |||
| 2006‐2007 | 1325 (11.9) | 2060 (12.7) | 0.05 |
| 2008‐2009 | 1911 (17.2) | 2993 (18.5) | <0.01 |
| 2010‐2011 | 1616 (14.5) | 2572 (15.9) | <0.01 |
| 2012‐2013 | 1803 (16.2) | 2631 (16.2) | 0.94 |
| 2014‐2015 | 2284 (20.5) | 3246 (20.0) | 0.32 |
| 2016‐2017 | 1871 (16.8) | 2419 (14.9) | <0.01 |
| 2018 | 327 (2.9) | 295 (1.8) | <0.01 |
| Physician specialty, n (%) | |||
| Primary care c | 2374 (21.3) | 3605 (22.2) | 0.07 |
| Rheumatologist | 2422 (21.7) | 3552 (21.9) | 0.76 |
| Quan–Charlson Comorbidity Index, mean (SD) | 1.5 (1.1) | 1.6 (1.2) | <0.01 |
| Comorbidities, n (%) b , d | |||
| Cardiovascular disease | 4194 (37.7) | 6221 (38.4) | 0.24 |
| Hypertension | 3803 (34.1) | 5580 (34.4) | 0.65 |
| Cerebrovascular disease | 574 (5.2) | 813 (5.0) | 0.60 |
| Congestive heart failure | 410 (3.7) | 625 (3.9) | 0.46 |
| Peripheral vascular disease | 387 (3.5) | 543 (3.3) | 0.57 |
| Myocardial infarction | 168 (1.5) | 276 (1.7) | 0.21 |
| Stroke | 202 (1.8) | 251 (1.5) | 0.09 |
| Infections e | 4207 (37.8) | 6553 (40.4) | <0.01 |
| Immunoinflammation related | 1471 (13.2) | 2492 (15.4) | <0.01 |
| Rheumatoid arthritis | 1192 (10.7) | 2050 (12.6) | <0.01 |
| Thyroiditis | 194 (1.7) | 292 (1.8) | 0.72 |
| Inflammatory bowel disease | 128 (1.1) | 224 (1.4) | 0.09 |
| Renal disease | 956 (8.6) | 1600 (9.9) | <0.01 |
| Diabetes | 1212 (10.9) | 1526 (9.4) | <0.01 |
| Osteoporosis | 784 (7.0) | 921 (5.7) | <0.01 |
| Concomitant medications, n (%) b , f | |||
| Antimalarials | 4718 (42.4) | 7865 (48.5) | <0.01 |
| NSAIDs | 2133 (19.2) | 4614 (28.5) | <0.01 |
| Immunosuppressants/biologics | 1368 (12.3) | 2816 (17.4) | <0.01 |
| Disease severity, n (%) | |||
| Mild | 7362 (66.1) | 9091 (56.1) | <0.01 |
| Moderate | 2686 (24.1) | 5248 (32.4) | <0.01 |
| Severe | 1089 (9.8) | 1877 (11.6) | <0.01 |
| SLE flares | |||
| Patients with ≥1 SLE flare, n (%) | 7348 (66.0) | 12,683 (78.2) | <0.01 |
| ≥1 mild SLE flare | 2022 (18.2) | 4389 (27.1) | <0.01 |
| ≥1 moderate SLE flare | 6148 (55.2) | 10,330 (63.7) | <0.01 |
| ≥1 severe SLE flare | 503 (4.5) | 1760 (10.9) | <0.01 |
| Number of any SLE flares per patient, mean (SD) | 1.4 (0.6) | 1.6 (0.7) | <0.01 |
| Health care costs, mean (SD) 2018 USD | |||
| All‐cause total | 12,547 (31,744) | 17,472 (42,039) | <0.01 |
| Medical | 11,087 (31,017) | 15,501 (41,327) | <0.01 |
| Pharmacy | 1460 (5047) | 1970 (5121) | <0.01 |
Abbreviations: NSAID, nonsteroidal antiinflammatory drug; OCS, oral corticosteroid; SLE, systemic lupus erythematosus; USD, United States dollars.
One patient with unknown sex was excluded from subsequent multivariate analysis.
Patients could have more than one value.
Primary care included general practitioner/family practitioner, nurse practitioner, and internal medicine physician.
Identified using International Classification of Diseases, Ninth Revision, Clinical Modification and International Classification of Diseases, Tenth Revision, Clinical Modification codes.
Infections included fungal infections, pneumonia, tuberculosis, urinary tract infection, varicella infection, and sepsis.
Identified using Generic Product Identifier and Healthcare Common Procedure Coding System codes.
The majority of patients were classified as having mild SLE during the baseline period (no‐OCS‐use cohort: 66.1%; OCS‐initiator cohort: 56.1%). However, the proportion of patients with moderate or severe SLE was lower in the no‐OCS‐use cohort than in the OCS‐initiator cohort (moderate SLE: 24.1% vs. 32.4%; severe SLE: 9.8% vs. 11.6%, respectively; both: P < 0.01) (Table 1).
The proportion of patients experiencing one or more flares during the baseline period was lower in the no‐OCS‐use cohort than in the OCS‐initiator cohort overall (66.0% vs. 78.2%, respectively; P < 0.01) and across all flare severities (mild: 18.2% vs. 27.1%; moderate: 55.2% vs. 63.7%; severe: 4.5% vs. 10.9%, respectively; all: P < 0.01) (Table 1).
During the baseline period, mean all‐cause health care costs in the no‐OCS‐use cohort were lower than in the OCS‐initiator cohort ($12,547 vs. $17,472, respectively; P < 0.01), driven by total medical costs (Table 1).
Clinical outcomes and HCRU during the 12‐month observation period
Disease severity and flares
In unadjusted analyses, the no‐OCS‐use cohort had milder disease versus the OCS‐initiator cohort when measured by disease severity and incidence of flare; 35.2% of patients had moderate or severe disease versus 96.5%, and 72.4% experienced one or more SLE flares versus 100% (P < 0.01), respectively (Table 2).
Table 2.
Disease severity and flare occurrence (N = 27,353) during the 12‐month observation period
| No OCS use | OCS initiation | P value | |
|---|---|---|---|
| (n = 11,137) | (n = 16,216) | ||
| Disease severity, n (%) | |||
| Mild | 7214 (64.8) | 567 (3.5) | <0.01 |
| Moderate | 2640 (23.7) | 11,527 (71.1) | <0.01 |
| Severe | 1283 (11.5) | 4122 (25.4) | <0.01 |
| SLE flares | |||
| Patients with ≥1 SLE flare, n (%) | 8066 (72.4) | 16,210 (100.0) | <0.01 |
| ≥1 mild SLE flare | 2560 (23.0) | 7105 (43.8) | <0.01 |
| ≥1 moderate SLE flare | 7047 (63.3) | 15,530 (95.8) | <0.01 |
| ≥1 severe SLE flare | 391 (3.5) | 3835 (23.6) | <0.01 |
| ≥1 moderate/severe SLE flare | 7151 (64.2) | 15,954 (98.4) | <0.01 |
| Number of any SLE flares per patient, mean (SD) | 2.1 (1.1) | 3.3 (1.5) | <0.01 |
| Number of moderate/severe SLE flares per patient | 1.9 (0.9) | 2.7 (1.3) | <0.01 |
Abbreviations: OCS, oral corticosteroid; SLE, systemic lupus erythematosus.
HCRU
This pattern continued when examining HCRU; the no‐OCS‐use cohort had lower all‐cause HCRU, outpatient visits, inpatient stays, and emergency department visits than the OCS‐initiator cohort in all exposure categories (Table 3). However, almost all patients (≥98%), regardless of OCS use, had one or more outpatient visit during the 12‐month observation period (Table 3).
Table 3.
HCRU incidence rates during the 12‐month observation period for the OCS‐initiator cohort exposure categories and the no‐OCS‐use cohort (N = 27,352)
| All‐cause HCRU, n (%) | Incidence per person‐year | Adjusted IRR (95% CI) a vs. the no‐OCS‐use cohort | P value | |
|---|---|---|---|---|
| No OCS use (n = 11,136) | ||||
| Any visits | 10,917 (98.0) | 25.03 | — | — |
| Outpatient | 10,880 (97.7) | 20.38 | — | — |
| Inpatient | 1367 (12.3) | 0.19 | — | — |
| Emergency department | 2305 (20.7) | 0.47 | — | — |
| Other b | 2856 (25.6) | 3.99 | — | — |
| No 6‐month periods of OCS use >5 mg/day (n = 11,438) | ||||
| Any visits | 11,410 (99.8) | 29.95 | 1.22 (1.19‐1.24) | <0.01 |
| Outpatient | 11,386 (99.5) | 24.74 | 1.23 (1.20‐1.25) | <0.01 |
| Inpatient | 1904 (16.6) | 0.26 | 1.43 (1.33‐1.53) | <0.01 |
| Emergency department | 3430 (30.0) | 0.86 | 1.74 (1.63‐1.85) | <0.01 |
| Other b | 3229 (28.2) | 4.08 | 1.07 (0.98‐1.17) | 0.12 |
| One 6‐month period of OCS use >5 mg/day (n = 3048) | ||||
| Any visits | 3044 (99.9) | 38.84 | 1.39 (1.34‐1.43) | <0.01 |
| Outpatient | 3040 (99.7) | 31.49 | 1.42 (1.38‐1.46) | <0.01 |
| Inpatient | 1114 (36.5) | 0.65 | 2.65 (2.42‐2.90) | <0.01 |
| Emergency department | 874 (28.7) | 0.82 | 1.42 (1.29‐1.56) | <0.01 |
| Other b | 999 (32.8) | 5.88 | 1.19 (1.04‐1.36) | 0.01 |
| Two 6‐month periods of OCS use >5 mg/day (n = 1730) | ||||
| Any visits | 1729 (99.9) | 47.28 | 1.66 (1.60‐1.73) | <0.01 |
| Outpatient | 1727 (99.8) | 36.07 | 1.61 (1.55‐1.68) | <0.01 |
| Inpatient | 779 (45.0) | 0.92 | 3.66 (3.30‐4.06) | <0.01 |
| Emergency department | 586 (33.9) | 1.07 | 1.71 (1.52‐1.93) | <0.01 |
| Other b | 655 (37.9) | 9.22 | 1.98 (1.67‐2.35) | <0.01 |
Note: One patient with unknown sex in the OCS nonuser cohort was excluded from the adjusted analyses.
Abbreviations: CI, confidence interval; HCRU, health care resource use; IRR, incidence rate ratio; OCS, oral corticosteroid.
IRRs and the respective 95% CIs and P values were calculated using generalized linear models with a negative binomial distribution and log link, controlling for the baseline covariates sex, age on index date, geographic region, baseline total health care costs, baseline Charlson Comorbidity Index, baseline use of prescription drugs, baseline disease severity, and baseline flares.
Including ambulance, assisted living facilities, comprehensive rehabilitation facilities, custodial care facilities, hospice/home care services, intermediate care facilities, psychiatric facilities, and skilled nursing facilities.
After adjusting for baseline covariates, incidence rates per person‐year were significantly lower for the no‐OCS‐use cohort compared with patients in each of the OCS‐initiator exposure categories for all‐cause HCRU, outpatient visits, inpatient stays, and emergency department visits (Table 3). Adjusted IRRs (95% CI) across any HCRU visit were 1.22 (1.19‐1.24), 1.39 (1.34‐1.43), and 1.66 (1.60‐1.73) for the exposure categories of 0, 1, and 2 periods of OCS initiation at more than 5 mg/day, respectively (all P < 0.01), versus no OCS use. The largest IRR was observed for inpatient stays for the exposure category with two 6‐month periods of more than 5 mg/day OCS initiation. Adjusted IRRs (95% CI) were 1.43 (1.33‐1.53), 2.65 (2.42‐2.90), and 3.66 (3.30‐4.06) for the exposure categories of 0, 1, and 2 periods of OCS initiation at more than 5 mg/day, respectively (all P < 0.01) (Table 3).
OCS treatment patterns: OCS‐initiator cohort
Cumulative OCS dose
Cumulative OCS dose per 6‐month period increased with the number of periods of OCS initiation at more than 5 mg/day. Patients with 0, 1, and 2 periods of OCS initiation had cumulative mean (SD) doses of 197.8 (163.2), 1316.4 (1905.4), and 3158.8 (9832.4) mg/6‐month period, respectively.
ADD
Mean (SD) ADD (observation) increased with the number of periods of OCS initiation at more than 5 mg/day (1.1 [0.9] mg/day, 7.2 [10.4] mg/day, and 17.3 [53.7] mg/day, respectively for patients with 0, 1, and 2 periods of OCS initiation) (Supplementary Figure 2). A similar trend was observed when ADD (days’ supply) was calculated (Supplementary Figure 2).
Patients in the OCS‐initiator cohort aged 5 to 17 years had significantly higher mean (SD) ADD (observation) than patients aged 18 years or older in the exposure categories with 1 or 2 periods of OCS initiation at more than 5 mg/day (11.0 [6.2] mg/day vs. 7.0 [10.6] mg/day and 25.1 [62.6] mg/day vs. 16.4 [52.6] mg/day for patients with 1 and 2 periods of OCS initiation, respectively; both P < 0.01). For patients with 0 periods of OCS initiation, the mean (SD) ADD (observation) was similar between patients aged 5 to 17 years and patients aged 18 years or older (1.2 [1.1] mg/day vs. 1.1 [0.9] mg/day).
Health care costs
During the observation period, the no‐OCS‐use cohort had lower unadjusted all‐cause total health care costs than the OCS‐initiator cohort in all exposure categories (Figure 2A). The exposure category with two 6‐month periods of OCS initiation at more than 5 mg/day incurred the highest costs across all medical and pharmacy categories evaluated, with mean (SD) all‐cause total health care costs of $51,354 ($74,031).
Figure 2.
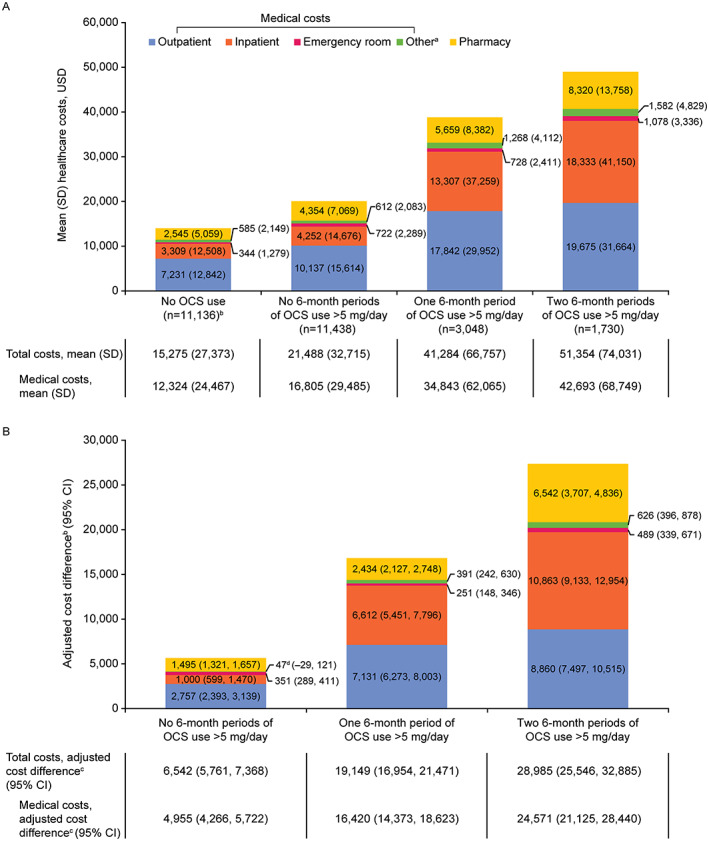
Unadjusted health care costs (A) and adjusted cost differences (B) for the OCS‐initiator cohort exposure categories compared with the no‐OCS‐use cohort as the reference group (N = 27,352). aOther visits include ambulance, assisted living facilities, comprehensive rehabilitation facilities, custodial care facilities, hospice/home care services, intermediate care facilities, psychiatric facilities, and skilled nursing facilities. bOne patient with unknown sex in the OCS nonuser cohort was excluded from the adjusted analyses. c P < 0.01 for adjusted all‐cause total cost, medical cost (all categories), and pharmacy cost comparisons between each OCS use exposure category and the no‐OCS‐use cohort. d P = 0.22 for adjusted other medical cost comparison between patients in the no 6‐month periods of more than 5 mg/day OCS use exposure category and the no‐OCS‐use cohort. CI, confidence interval; OCS, oral corticosteroid; USD, United States dollars.
After adjusting for baseline covariates, all‐cause total health care costs, medical costs, and pharmacy costs were significantly lower in the no‐OCS‐use cohort than in the OCS‐initiator cohort in all exposure categories (Figure 2B).
Sensitivity analyses using ADD (days’ supply) rather than ADD (observation) showed a similar pattern of results to the main analysis, although cost differences were smaller (Supplementary Figure 2), wherein costs were significantly lower in the no‐OCS‐use cohort than all the OCS‐initiator exposure categories. Sensitivity analyses using a higher OCS use cutoff point of 7.5 mg/day rather than 5 mg/day again showed a similar pattern of results to the main analysis, but cost differences were larger (Supplementary Figure 3).
Risk of OCS‐related AEs
OCS‐related AEs were reported frequently, with more than half of the no‐OCS‐use cohort (56.3%) and OCS‐initiator cohort (67.1%‐74.1%) experiencing one or more OCS‐related AEs during the observation period (Table 4). The risk of experiencing any OCS‐related AE was significantly lower in the no‐OCS‐use cohort compared with the OCS‐initiator cohort in each of the exposure categories (Table 4). Adjusted RRs (95% CI) for any OCS‐related AE were 1.15 (1.10‐1.20), 1.23 (1.15‐1.31), and 1.32 (1.22‐1.43) for patients with 0, 1, and 2 periods of OCS initiation at more than 5 mg/day, respectively (all P < 0.01). Similarly, RR statistical significance was observed for OCS‐related AEs across all organ‐domain level categories, except dermatologic AEs in patients with 0 or 2 periods of OCS initiation at more than 5 mg/day and ophthalmologic AEs in patients with 0 periods of OCS initiation at more than 5 mg/day. The greatest associations were seen in hematologic/oncologic AEs and cardiovascular AEs (Table 4). The individual AE that showed the greatest association with OCS initiation was pneumonia; adjusted RRs (95% CI) were 1.98 (1.73‐2.26), 2.54 (2.13‐3.04), and 4.03 (3.34‐4.87) for the exposure categories of 0, 1, and 2 periods of OCS initiation at more than 5 mg/day, respectively.
Table 4.
Risk of OCS‐related AEs by organ domain during the 12‐month observation period in the OCS‐initiator cohort exposure categories and the no‐OCS‐use cohort (N = 27,352)
| No OCS use (n = 11,136) | Number of exposure categories of OCS use >5 mg/day | |||
|---|---|---|---|---|
| 0 (n = 11,438) | 1 (n = 3048) | 2 (n = 1730) | ||
| Any OCS‐related AE | ||||
| Incidence, % | 56.3 | 67.1 | 70.5 | 74.1 |
| Adjusted RR (95% CI) | — | 1.15 (1.10‐1.20) | 1.23 (1.15‐1.31) | 1.32 (1.22‐1.43) |
| P value | — | <0.01 | <0.01 | <0.01 |
| Bone and muscle | ||||
| Incidence, % | 21.5 | 28.3 | 28.0 | 31.7 |
| Adjusted RR (95% CI) | — | 1.28 (1.21‐1.35) | 1.30 (1.19‐1.41) | 1.50 (1.36‐1.64) |
| P value | — | <0.01 | <0.01 | <0.01 |
| Immune system related | ||||
| Incidence, % | 18.5 | 26.1 | 32.1 | 37.6 |
| Adjusted RR (95% CI) | — | 1.36 (1.29‐1.44) | 1.61 (1.49‐1.74) | 1.88 (1.71‐2.05) |
| P value | — | <0.01 | <0.01 | <0.01 |
| Central nervous system | ||||
| Incidence, % | 18.3 | 25.0 | 23.9 | 24.9 |
| Adjusted RR (95% CI) | — | 1.31 (1.23‐1.39) | 1.25 (1.14‐1.37) | 1.30 (1.16‐1.46) |
| P value | — | <0.01 | <0.01 | <0.01 |
| Metabolic and endocrine | ||||
| Incidence, % | 18.0 | 20.6 | 22.2 | 26.0 |
| Adjusted RR (95% CI) | — | 1.13 (1.06‐1.21) | 1.23 (1.12‐1.35) | 1.44 (1.29‐1.60) |
| P value | — | <0.01 | <0.01 | <0.01 |
| Cardiovascular | ||||
| Incidence, % | 15.4 | 18.1 | 23.1 | 31.3 |
| Adjusted RR (95% CI) | — | 1.16 (1.08‐1.25) | 1.51 (1.36‐1.67) | 2.11 (1.89‐2.37) |
| P value | — | <0.01 | <0.01 | <0.01 |
| Gastrointestinal | ||||
| Incidence, % | 10.7 | 16.0 | 21.1 | 22.6 |
| Adjusted RR (95% CI) | — | 1.42 (1.32‐1.53) | 1.66 (1.50‐1.84) | 1.65 (1.46‐1.86) |
| P value | — | <0.01 | <0.01 | <0.01 |
| Ophthalmologic | ||||
| Incidence, % | 7.9 | 7.7 | 8.7 | 11.0 |
| Adjusted RR (95% CI) | — | 1.05 (0.95‐1.15) | 1.30 (1.14‐1.49) | 1.78 (1.53‐2.08) |
| P value | — | 0.36 | <0.01 | <0.01 |
| Dermatologic | ||||
| Incidence, % | 2.6 | 3.1 | 4.0 | 4.4 |
| Adjusted RR (95% CI) | — | 1.12 (0.96‐1.31) | 1.25 (1.01‐1.56) | 1.21 (0.94‐1.57) |
| P value | — | 0.15 | 0.04 | 0.15 |
| Hematologic/oncologic | ||||
| Incidence, % | 1.7 | 2.7 | 5.1 | 6.3 |
| Adjusted RR (95% CI) | — | 1.56 (1.30‐1.87) | 2.56 (2.05‐3.20) | 3.10 (2.40‐4.02) |
| P value | — | <0.01 | <0.01 | <0.01 |
Notes: One patient with unknown sex in the OCS nonuser cohort was excluded from the adjusted analyses. AEs with incidence of ≥3% that are included in the organ‐level analyses and analyzed individually are back pain, osteoporosis, fractures, muscle weakness, varicella/herpes zoster, urinary tract infection, sepsis, fungal infections, pneumonia, depression, sleep disturbance, migraine, dyslipidemia, obesity, diabetes mellitus, hyperglycemia, hypertension, nausea/vomiting, gastrointestinal bleeds/ulcers, cataracts, and glaucoma. AEs with incidence <3% that are included in the organ domain‐level analyses but not analyzed individually are bursitis, avascular necrosis, loss of muscle mass, myopathy, myocardial infarction, atrial fibrillation/flutter, congestive heart failure, stroke, bipolar disorder, steroid psychosis, akathisia, acne, hirsutism, erythema, dyspepsia, acute pancreatitis, abdominal distension, bladder cancer, epistaxis, leukocytosis, tuberculosis, metabolic syndrome, Cushing syndrome, and drug‐induced adrenocortical insufficiency. Modified Poisson regression models, controlling for the baseline covariates sex, age on index date, geographic region, baseline total health care costs, baseline Charlson Comorbidity Index, baseline use of prescription drugs, baseline disease severity, and baseline flares, were used to calculate RRs; the respective P values and 95% CIs were estimated with the robust variance estimator.
Abbreviations: AE, adverse event; CI, confidence interval; OCS, oral corticosteroid; RR, risk ratio.
DISCUSSION
This retrospective cohort study of a large US claims database found that compared with patients with no OCS use, patients with OCS initiation had significantly higher all‐cause HCRU, health care costs, and risk of OCS‐related AEs during the 12 months immediately following OCS initiation. The health care burden was most pronounced in patients with 2 periods of OCS initiation at more than 5 mg/day, although fewer periods of OCS initiation were also associated with substantial HCRU and costs. These results remained robust after adjustment for baseline covariates, including disease severity, prescriptions, and flares. Notably, patients receiving low‐dose OCS still had an adjusted medical cost difference of nearly $5000 compared with those patients who received no OCS, as displayed in Figure 2B. Therefore, patients receiving low‐dose OCS initiation had higher HCRU than the no‐OCS‐use cohort; previous studies emphasized the effect of high‐dose OCS on HCRU (21).
Patients with OCS initiation had higher disease severity and more SLE flares and experienced a higher severity of flares than patients with no OCS use. It is interesting to note that calculated ADDs were higher when using the days’ supply definition versus the observation definition, suggesting that OCS may have been administered in frequent high‐dose bursts to manage flares. These findings suggest that SLE was inadequately controlled despite ongoing high‐dose OCS use.
Patients with OCS initiation incurred higher HCRU and health care costs compared with patients with no OCS use, largely driven by outpatient visits and inpatient stays. In the 6 months before initiating OCS, the OCS‐initiator cohort had more concomitant medication use and higher health care costs than the no‐OCS‐use cohort, suggesting they represent a group of patients with potentially more severe disease requiring additional care. HCRU and health care costs in the OCS‐initiator cohort remained significantly higher than in the no‐OCS‐use cohort during the observation period, even after adjustment for baseline variables, including baseline total health care costs. These findings are consistent with results from previous studies. For example, a cross‐sectional study found that higher OCS doses in patients with SLE were associated with higher annual health care costs (22). A study of OCS‐naive patients newly diagnosed with SLE showed that subsequent initiation and long‐term exposure to OCS was associated with significantly higher HCRU and total health care costs than no OCS use in the third year after SLE diagnosis (21). This was the case even among patients with low dose exposure (≤5 mg/day for 2 years); total all‐cause health care costs increased with each additional mg of average daily OCS dose (21). Our study extends these findings by demonstrating that compared with patients with no OCS use, both pharmacy and medical costs were higher among patients with any OCS initiation, and the difference increased with greater OCS use.
Higher overall HCRU and costs among patients receiving OCS may be explained, in part, by the additional risk of OCS‐related AEs compared with patients not receiving OCS (32). Patients with OCS initiation had a higher risk of experiencing any OCS‐related AE as well as experiencing AEs across the majority of organ‐domain level categories explored. Previous studies have demonstrated that an increased risk of AEs, such as cardiovascular events, requires more frequent monitoring and assessment because the occurrence of AEs may lead to additional outpatient care or even hospitalization (33, 34). Furthermore, patients with SLE receiving high‐dose OCS require more urgent care visits than patients receiving low‐dose OCS or patients not receiving OCS (21, 22). Estimated mean costs attributable to OCS‐related AEs range between $2400 and $9800 per year (19), representing a substantial proportion of the additional costs incurred in the OCS‐initiator cohort described here. In particular, the occurrence of organ damage in SLE is associated with substantial clinical and economic burden, with all‐cause costs increasing by approximately 70% following the diagnosis of organ damage (32). Corticosteroid‐related complications and associated costs are similarly seen for other diseases, such as asthma, with costs driven by inpatient and outpatient visits increasing with dose (14, 17, 18). The relationship between prolonged or high‐dose OCS use, underlying poor health, severity of SLE, and OCS‐related AEs merits further study.
Guidelines for the management of SLE currently recommend maintaining average prednisone‐equivalent corticosteroid doses below 7.5 mg/day to prevent irreversible organ damage (4, 23, 35). Despite these recommendations, this study identified more than 3400 (12.5%) patients who received OCS with an average dose greater than 7.5 mg/day for at least 6 months of the 12‐month observation period. Similar use of high OCS doses in the year following the first OCS claim was reported by Birt et al (25). Furthermore, results of this study, in which two of the OCS use categories had a mean ADD of less than 7.5 mg/day, suggest that even low doses of OCS incur substantial clinical and economic burden. Taken altogether, the evidence that even low doses of OCS are associated with additional health care costs and HCRU supports the introduction of a lower prednisone‐equivalent corticosteroid dose threshold in the treatment of SLE.
Several limitations of this study that are common to all observational studies based on retrospective claims data should be noted. With pharmacy claims data, it is not possible to confirm whether the medication was taken as prescribed, and claims data do nor does it capture use of over‐the‐counter medications, drug samples or medications received during inpatient stays. The analysis may also be vulnerable to coding inaccuracies (eg, potential misclassification of codes, data entry errors). Additionally, potential confounders, such as ethnicity, certain clinical biomarkers, and disease activity measures (including SLE Disease Activity Index), were unavailable, although the current study attempted to address these confounders by adjusting for baseline disease and flare severity. Patients with dual coverage or supplemental health insurance or patients who received care outside of a managed care population, such as Medicare or Medicaid patients, may not have had their HCRU and associated costs fully captured.
The determination of OCS‐related AEs was based on previous literature as well as package inserts using the previous diagnostic codes in the claims data. Although these AEs are of interest, it is not possible to determine direct causation between OCS exposure and AEs.
OCS usage and rate of flare are closely linked because OCS are often used to provide rapid relief during flares (4). Therefore, it is not possible to determine the costs solely associated with OCS use rather than in combination with the incidence of flare. However, this limitation does not greatly reduce the impact of these data because it highlights the unmet need for more effective treatment regimens to reduce OCS use rather than implying that OCS are the direct and sole cause of increased HCRU.
Nevertheless, this retrospective cohort study has several key strengths, including the use of a large US claims database, which enabled rapid identification of a large geographically diverse group of patients with SLE in a real‐world setting who were either new users or nonusers of OCS over a 13‐year period and enabled investigation of their longitudinal economic and clinical outcomes. Associations between OCS use and health care burden were robust and remained after adjusting for baseline characteristics, when using different definitions to calculate ADD, and after applying alternative thresholds for OCS use.
In conclusion, OCS initiation in patients with SLE is associated with greater HCRU, higher health care costs, and increased risk of OCS‐related AEs compared with no OCS use during the 12 months immediately following OCS initiation. The highest burden was seen in patients receiving average doses of more than 5 mg/day. Improving adherence to current guidelines by minimizing the dose and duration of OCS use may reduce the clinical and economic burden of SLE management. To enable the improvement of outcomes in patients with SLE, effective and well‐tolerated treatment regimens that do not require prolonged OCS use are needed.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
DerSarkissian, Gu, Duh, Benson, Huang, Averell, Vu, Wang, Bell.
Acquisition of data
DerSarkissian, Gu, Duh, Benson, Huang, Vu, Wang, Bell.
Analysis and interpretation of data
DerSarkissian, Gu, Duh, Benson, Huang, Averell, Vu, Wang, Bell.
ROLE OF THE STUDY SPONSOR
Medical writing and submission support were provided by Olivia Hill, MPharmacol, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK. The authors independently collected the data, interpreted the results, and had the final decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by GSK.
Supporting information
Disclosure form
Appendix S1. Supplementary Information
This study (GSK Study 213061) was supported by GSK.
Author disclosures are available online at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11550.
REFERENCES
- 1. Justiz Vaillant AA, Goyal A, Varacallo M. Systemic lupus erythematosus. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 2. McElhone K, Abbott J, Hurley M, et al. Flares in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2021;60:3262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun 2019;96:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019;78:736–45. [DOI] [PubMed] [Google Scholar]
- 5. Bruce IN, O'Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort. Ann Rheum Dis 2015;74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Herrinton LJ, Liu L, Goldfien R, et al. Risk of serious infection for patients with systemic lupus erythematosus starting glucocorticoids with or without antimalarials. J Rheumatol 2016;43:1503–9. [DOI] [PubMed] [Google Scholar]
- 7. Sciascia S, Mompean E, Radin M, et al. Rate of adverse effects of medium‐ to high‐dose glucocorticoid therapy in systemic lupus erythematosus: a systematic review of randomized control trials. Clin Drug Investig 2017;37:519–24. [DOI] [PubMed] [Google Scholar]
- 8. Yasir M, Goyal A, Sonthalia S. Corticosteroid adverse effects. Treasure Island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 9. Chen HL, Shen LJ, Hsu PN, et al. Cumulative burden of glucocorticoid‐related adverse events in patients with systemic lupus erythematosus: findings from a 12‐year longitudinal study. J Rheumatol 2018;45:83–9. [DOI] [PubMed] [Google Scholar]
- 10. Manson SC, Brown RE, Cerulli A, et al. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med 2009;103:975–94. [DOI] [PubMed] [Google Scholar]
- 11. Bloechliger M, Reinau D, Spoendlin J, et al. Adverse events profile of oral corticosteroids among asthma patients in the UK: cohort study with a nested case‐control analysis. Respir Res 2018;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walters JA, Tan DJ, White CJ, et al. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2018:CD006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort [published erratum appears in PLoS One 2018;13:e0197341]. PLoS One 2016;11:e0158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lefebvre P, Duh MS, Lafeuille MH, et al. Acute and chronic systemic corticosteroid‐related complications in patients with severe asthma. J Allergy Clin Immunol 2015;136:1488–95. [DOI] [PubMed] [Google Scholar]
- 15. Sarnes E, Crofford L, Watson M, et al. Incidence and US costs of corticosteroid‐associated adverse events: a systematic literature review. Clin Ther 2011;33:1413–32. [DOI] [PubMed] [Google Scholar]
- 16. Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ 2017;357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dalal AA, Duh MS, Gozalo L, et al. Dose‐response relationship between long‐term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm 2016;22:833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lefebvre P, Duh MS, Lafeuille MH, et al. Burden of systemic glucocorticoid‐related complications in severe asthma. Curr Med Res Opin 2017;33:57–65. [DOI] [PubMed] [Google Scholar]
- 19. Shah M, Chaudhari S, McLaughlin TP, et al. Cumulative burden of oral corticosteroid adverse effects and the economic implications of corticosteroid use in patients with systemic lupus erythematosus. Clin Ther 2013;35:486–97. [DOI] [PubMed] [Google Scholar]
- 20. Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol 2013;9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kabadi S, Yeaw J, Bacani AK, et al. Healthcare resource utilization and costs associated with long‐term corticosteroid exposure in patients with systemic lupus erythematosus. Lupus 2018;27:1799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen SY, Choi CB, Li Q, et al. Glucocorticoid use in patients with systemic lupus erythematosus: association between dose and health care utilization and costs. Arthritis Care Res (Hoboken) 2015;67:1086–94. [DOI] [PubMed] [Google Scholar]
- 23. Franklyn K, Lau CS, Navarra SV, et al. Definition and initial validation of a lupus low disease activity state (LLDAS). Ann Rheum Dis 2016;75:1615–21. [DOI] [PubMed] [Google Scholar]
- 24. Van Vollenhoven RF, Bertsias G, Doria A, et al. 2021 DORIS definition of remission in SLE: final recommendations from an international task force [published erratum appears in Lupus Sci Med 2022;9:e000538corr1]. Lupus Sci Med 2021;8:e000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birt JA, Wu J, Griffing K, et al. Corticosteroid dosing and opioid use are high in patients with SLE and remain elevated after belimumab initiation: a retrospective claims database analysis. Lupus Sci Med 2020;7:e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doria A, Gatto M, Zen M, et al. Optimizing outcome in SLE: treating‐to‐target and definition of treatment goals. Autoimmun Rev 2014;13:770–7. [DOI] [PubMed] [Google Scholar]
- 27. Garris C, Jhingran P, Bass D, et al. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ 2013;16:667–77. [DOI] [PubMed] [Google Scholar]
- 28. Narayanan S, Wilson K, Ogelsby A, et al. Economic burden of systemic lupus erythematosus flares and comorbidities in a commercially insured population in the United States. J Occup Environ Med 2013;55:1262–70. [DOI] [PubMed] [Google Scholar]
- 29. Ruperto N, Hanrahan L, Alarcón G, et al. International consensus for a definition of disease flare in lupus. Lupus 2011;20:453–62. [DOI] [PubMed] [Google Scholar]
- 30. Honeycutt AA, Segel JE, Hoerger TJ, et al. Comparing cost‐of‐illness estimates from alternative approaches: an application to diabetes. Health Serv Res 2009;44:303–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diehr P, Yanez D, Ash A, et al. Methods for analyzing health care utilization and costs. Annu Rev Public Health 1999;20:125–44. [DOI] [PubMed] [Google Scholar]
- 32. Bell CF, Ajmera MR, Meyers J. An evaluation of costs associated with overall organ damage in patients with systemic lupus erythematosus in the United States. Lupus 2022;31:202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petri M, Bechtel B, Dennis G, et al. Burden of corticosteroid use in patients with systemic lupus erythematosus: results from a Delphi panel. Lupus 2014;23:1006–13. [DOI] [PubMed] [Google Scholar]
- 34. Mosca M, Tani C, Aringer M, et al. European League Against Rheumatism recommendations for monitoring patients with systemic lupus erythematosus in clinical practice and in observational studies. Ann Rheum Dis 2010;69:1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kostopoulou M, Ugarte‐Gil MF, Pons‐Estel B, et al. The association between lupus serology and disease outcomes: a systematic literature review to inform the treat‐to‐target approach in systemic lupus erythematosus. Lupus 2022;31:307–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form
Appendix S1. Supplementary Information


