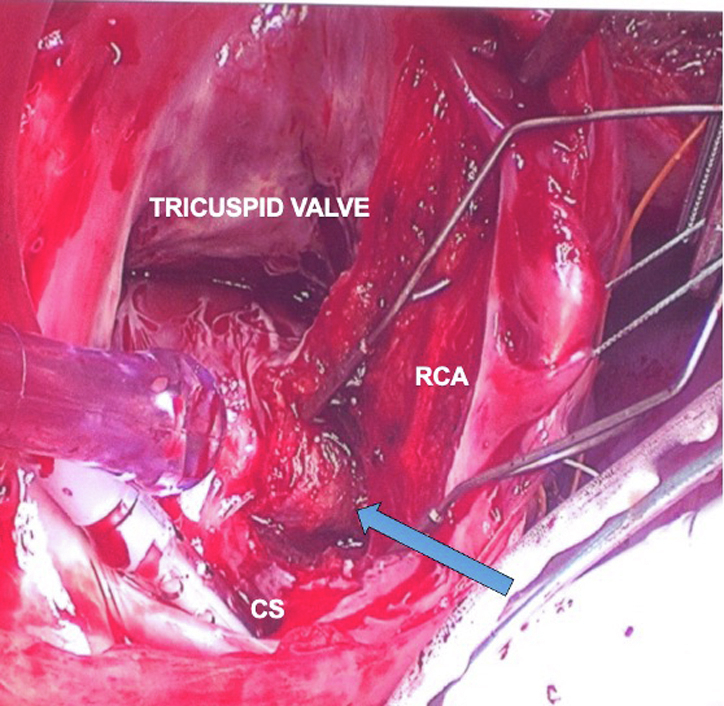
Dissection of RCA into pyramidal space exposes the site of accessory pathway.
Central Message.
This article describes, in detail, the techniques of surgical ablation for Wolff-Parkinson-White syndrome. Surgery is now rarely performed for this indication but may be necessary in refractory cases.
First performed by Sealy in 1968, surgical ablation of an accessory pathway was the gold standard for curative treatment of Wolff-Parkinson-White syndrome (WPW).1 However, in the early 1990s, the safety and efficacy of catheter radiofrequency ablation replaced the surgical procedure.2,3 In most patients, catheter ablation will provide a cure for WPW; however, in select, refractory cases there is still a role for surgical intervention. Here we present a case and the technical details for the surgical correction of WPW. Consent was obtained from the patient to publish the images and the data (IRB approval No. HM13607; July 29, 2013).
Presurgical History
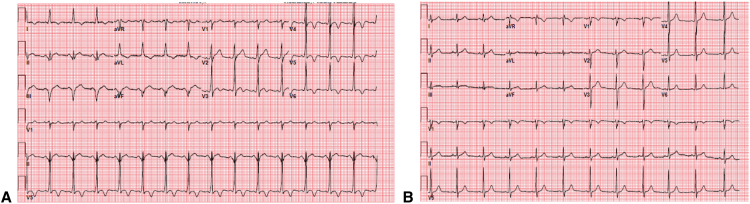
The patient is a 27-year-old man with history of WPW and 2 prior unsuccessful catheter ablation attempts who presented with cardiac arrest due to ventricular fibrillation. He was successfully resuscitated, and after recovery was noted to have an ejection fraction of 20% with an electrocardiogram showing a short PR interval and a delta wave (Figure 1, A). Electrophysiology mapping indicated a posterior-septal accessory pathway. Multiple attempts to ablate the accessory pathway endocardially and via the middle cardiac vein were unsuccessful. One additional attempt at catheter ablation via both endocardial and epicardial mapping and ablation was unsuccessful. In view of resuscitation from ventricular fibrillation due to rapid atrial fibrillation conducting rapidly over the accessory pathway, the patient was referred for surgical ablation.
Figure 1.
A, Preoperative electrocardiogram showing short PR interval and preexcitation (delta wave). B, Five-year postoperative electrocardiogram: Normal PR interval and absence of preexcitation.
Surgical Approach
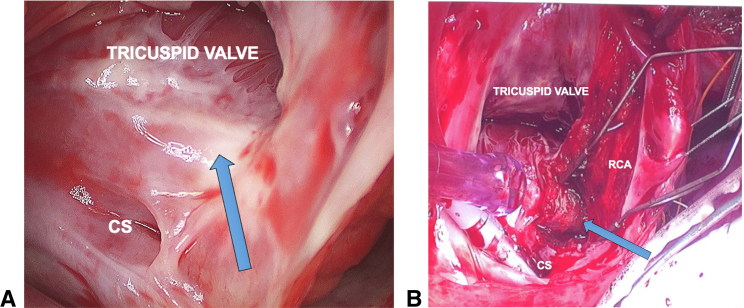
The procedure was performed through a median sternotomy under cardiopulmonary bypass but without cardioplegic arrest. The beating heart allowed real-time electrophysiologic testing during the procedure. An incision was made through the right ventricular epicardial fat a few millimeters lateral to the atrioventricular junction and over the right coronary artery (RCA). The epicardial fat was then dissected away from the RCA in an attempt to divide the accessory pathway.4 This resulted in no change in preexcitation. The right atrium was then opened with a free wall incision and the tricuspid valve was exposed. Sites of previous catheter ablation could be clearly seen by endocardial scarring (Figure 2, A). An incision was made approximately 5 mm from the tricuspid annulus starting at the 1 o’clock position and carried down to the coronary sinus. The dissection was extended through the endocardium, exposing the RCA from the inside and carried into the pyramidal space (Figure 2, B). At this point (blue arrow in Figure 2, B) of dissection into the pyramidal space the preexcitation disappeared—consistent with division of a subendocardial posterior-septal pathway.4 Adenosine was then administered for atrioventricular nodal blockade, and we could not demonstrate antegrade or retrograde conduction across the accessory pathway. Further ablation was performed by radiofrequency and cryoablative techniques along the endocardial dissection plane.
Figure 2.
A, Figure showing area of scar from previous catheter ablation. B, Dissection in the area. CS, Coronary sinus; RCA, right coronary artery.
In the operating room, 1 hour after the procedure, we still could not demonstrate accessory pathway conduction and QRS remained narrow with no preexcitation. At outpatient follow up 5 years after this procedure, the patient remains asymptomatic with narrow QRS and no preexcitation (Figure 1,B).
Discussion
With the excellent outcomes achieved by catheter ablation, the role of surgery for WPW has become historical. However, there are patients who have accessory pathways that are in the pyramidal space, a notoriously complex anatomical location, who might have failed catheter ablation due to presence of fat pad that insulates the accessory pathways from ablation energy.5 The knowledge of surgical techniques has faded. Our aim is to highlight specific surgical techniques. Our operative technique was a combination of both the endocardial approach pioneered by Sealy, Cox, and Gallagher and the epicardial approach developed by Guiraudon.1, 3, 4 An additional thoracoscopic approach has been described by Tanoue and colleagues,6 but only allows for ablation of epicardial pathways. Additionally, given our patient's posterior-septal pathway and the need for both endocardial and epicardial mapping, this approach would likely not be feasible.
This surgical technique report is presented to demonstrate the need for knowledge of surgical technique to appropriately care for rare patients who require surgical ablation for WPW.
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
References
- 1.Sealy W.C., Hattler B.G., Jr., Blumenschein S.D., Cobb F.R. Surgical treatment of Wolff-Parkinson-White syndrome. Ann Thorac Surg. 1969;8:1–11. doi: 10.1016/s0003-4975(10)66402-8. [DOI] [PubMed] [Google Scholar]
- 2.Cox J.L. Cardiac surgery for arrhythmias. Pacing Clin Electrophysiol. 2004;27:266–282. doi: 10.1111/j.1540-8159.2004.00426.x. [DOI] [PubMed] [Google Scholar]
- 3.Cox J.L., Gallagher J., Cain M.E. Experience with 118 consecutive patients undergoing operation for the Wolff-Parkinson-White syndrome. J Thorac Cardiovasc Surg. 1985;90:490–501. [PubMed] [Google Scholar]
- 4.Guiraudon G.M. Surgical treatment of Wolff-Parkinson-White syndrome: a “retrospectroscopic” view. Ann Thorac Surg. 1994;58:1254–1261. doi: 10.1016/0003-4975(94)90524-x. [DOI] [PubMed] [Google Scholar]
- 5.Menasche P. Anatomic bases of the surgical division of Kent bundles in the posterior septal area of the heart. Surg Radiol Anat. 1986;8:109–114. doi: 10.1007/BF02421377. [DOI] [PubMed] [Google Scholar]
- 6.Tanoue M., Sakamoto S., Miyauchi Y., Usuda J., Nitta T. Treatment of Wolff-Parkinson-White syndrome with a thoracoscopic surgical procedure. Ann Thorac Surg. 2015;100:e11–e13. doi: 10.1016/j.athoracsur.2015.04.081. [DOI] [PubMed] [Google Scholar]