Abstract
Background
Ultrasound (US)-guided percutaneous core needle biopsy (PCNB) has been used to diagnose subpleural lung lesions with high diagnostic performance and acceptable complication rates. However, with regard to the role of US-guided needle biopsy for the diagnosis of small (≤2 cm) subpleural lesions, limited information is available.
Methods
From April 2011 to October 2021, a total of 572 US-guided PCNBs in 572 patients were retrospectively reviewed. The lesion size, pleural contact length (PCL), lesion location, and operator’s experience were analyzed. Computed tomography features including peri-lesional emphysema, air-bronchogram, and cavitary change were also included in image analysis. The patients were divided into three groups according to lesion size (lesions ≤2 cm vs. 2 cm< lesions ≤5 cm vs. lesions >5 cm). The sample adequacy, diagnostic success rate, diagnostic accuracy, and complication rate was calculated. For statistical analysis, one-way ANOVA, Kruskal-Wallis test, or the chi-square test were used.
Results
The overall sample adequacy, diagnostic success rate, and diagnostic accuracy were 96.2%, 82.9%, and 90.4%, respectively. In the subgroup analysis, sample adequacy (93.1% vs. 96.1% vs. 96.9%, P=0.307), diagnostic success rate (75.0% vs. 81.6% vs. 85.7%, P=0.079), and diagnostic accuracy (84.7% vs. 90.8% vs. 90.5%, P=0.301) were not significantly different. Operator’s experience (OR, 0.64; 95% CI: 0.49–0.80; P<0.001), lesion size (OR, 0.68; 95% CI: 0.54–0.83; P<0.001), PCL (OR, 0.68; 95% CI: 0.52–0.84; P=0.001), and presence of air-bronchogram (OR, 14.36; 95% CI: 4.18–48.53; P<0.001) were independently associated with complication rate.
Conclusions
US-guided PCNB performed by an experienced radiologist could be an effective and safe diagnostic approach for subpleural lesions, even in small lesions.
Keywords: Biopsy, ultrasound, complication
Highlight box.
Key findings
• US-guided percutaneous core needle biopsy (PCNB) can be an effective and safe diagnostic approach for subpleural lung lesions, including small lesions (≤2 cm), with high diagnostic performance and acceptable complication rates.
What is known and what is new?
• Compared to CT-guided PCNB, US-guided PCNB may provide comparable or superior diagnostic yield with lower complication rates.
• This study focuses on safety and efficacy of US-guided PCNB for small subpleural lung lesions compared with larger lesions.
What is the implication, and what should change now?
• US-guided PCNB is a feasible and accurate diagnostic method for subpleural lung lesions, even in small lesions.
Introduction
With the widespread use of computed tomography (CT) in the last decades, the indeterminate lung nodules’ detection has been increasing (1,2). According to one lung cancer screening trial using low-dose CT, approximately 80% of detected lung cancer were small (≤2 cm) lung nodules (3). These incidental pulmonary nodules’ management requires a decision to proceed with biopsy when imaging tests cannot confirm that a nodule is benign. For lung lesion diagnosis, imaging-guided percutaneous core needle biopsy (PCNB) is well established as an accurate and safe method (4-6). However, compared to larger lesions, PCNB of small lesions (≤2 cm) has been technically challenging with a varying and lower diagnostic accuracy ranging from 75% to 92% (5,7-10).
Prior studies have reported that small nodules, particularly located in subpleural lung, are technically difficult to sample due to the influence of rib and/or respiratory movement (11-14). For subpleural lesions, ultrasound (US) has advantages for guiding PCNB, including lack of radiation exposure and real-time visualization of the biopsy needle and target lesion during the procedure. Several investigators reported that for subpleural lung lesions, the diagnostic yield and complication rate of US-guided PCNB was similar or superior to those of CT-guided PCNB with lower complication rate (15-17). In addition, the procedure time has been reported to be shorter under US guidance as compared with CT-guidance (17). However, with regard to the role of US-guided needle biopsy for the diagnosis of small (≤2 cm) subpleural lesions, limited information is available (18).
This present study’s aim is to evaluate the diagnostic performance and complications of US-guided PCNB for small (≤2 cm) subpleural lung lesions compared with larger lesions. We present this article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1546/rc).
Methods
Study population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Kyungpook National University Chilgok Hospital (KNUCH 2022-02-033) and individual consent for this retrospective analysis was waived. In this retrospective study, 850 consecutive patients who underwent US-guided biopsy for subpleural lesions at Kyungpook National University Chilgok Hospital from April 2011 to October 2021 were included. The exclusion criteria included the following: (I) patients with US-guided PCNB for pleural lesions (n=246), (II) unclearly diagnosed patients followed up for less than 2 years (n=9), (III) patients without a pathologic report (n=1), (IV) those with multiple subpleural lesions impossible to correlate with CT and US-image (n=7), (V) patients who underwent US-guided aspiration (n=15). In patients with repeated US-guided subpleural lung biopsy, only the first biopsy was only included in the analysis. Finally, for the study, a total of 572 patients with 572 US-guided subpleural lung biopsy were included. For the analysis, referring to previous studies (17,18) the patients were divided into three groups according to lesion size measured on preprocedural CT: lesions ≤2 cm (small lesions), 2 cm < lesions ≤5 cm (medium lesions), lesions >5 cm (large lesions) (Figure 1).
Figure 1.
Flow chart of the study population with exclusion criteria. US, ultrasound; CT, computed tomography.
US-guided PCNB for subpleural lung lesions
Three certified radiologist (KMS, SHJ, BP, with more than 11, 2, and 2 years of experience for lung biopsy, respectively) and one radiology resident (less than 1 year of experience for lung biopsy) performed the procedures, and the majority were performed by certified radiologists (563/572, 98%). Operator’s experience was quantified by years of experience with biopsies, which was calculated based on the time when each operator performed the procedure.
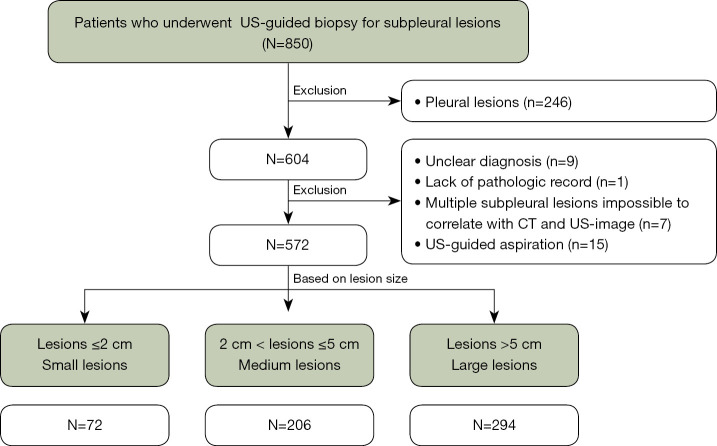
To ensure sterilization, the US transducer was cleaned for at least 2 minutes with 10% iodopovidone. The patient position was decided according to lesion location and the best way to reach it. US-guided biopsy was performed with two US system (iU22; Philips Healthcare, Bothell, WA, USA and EPIQ Elite; Philips Healthcare, Bothell, WA, USA). According to patient’s body habitus and for the lesion’s best visualization, low-frequency (1–5 MHz) or high-frequency (10–12 MHz) transducers were selected. To correlate the lesion with CT image, anatomical structures adjacent to the lesion such as the scapula, nipple, liver, or spleen were used as landmark (Figure 2).
Figure 2.
A 68-year-old male with a 1.3 cm subpleural nodule diagnosed with adenocarcinoma. Enhanced axial CT (A) and USG (B) images show a subpleural nodule located adjacent to the liver. The liver was used as a landmark to locate the lesion on USG. CT, computed tomography; USG, ultrasonography.
After lesion localization correlated with preprocedural CT image, real-time color Doppler was used to prevent vessel injury during the procedure. After local anesthesia, the biopsy needle was inserted and then advanced to the lesion with free hand technique (Videos 1,2). For lesions obscured by the ribs during quiet respiration, the patients were instructed to forced inspirate or expirate to reveal the lesion. One to five pieces of the sample was obtained depending on the specimens’ quality and the patient’s tolerance. All specimens were immediately fixed in 10% formalin and sent for histopathological examination. All procedures were performed using an 18-gauge automated core biopsy needle (Acecut; TSK Laboratory, Tochigi, Japan) without a coaxial approach. 1.1 cm throw lengths were used at the radiologists’ discretion.
Video 1.
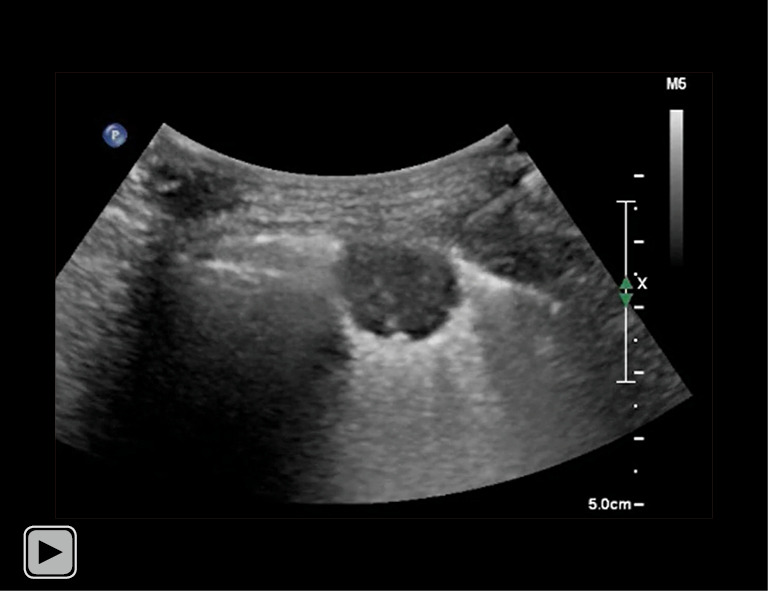
Operator performed local anesthesia with 23-gauge needle. Lidocaine was injected from the skin to the pleura during monitoring the location of the needle tip in real time.
Video 2.
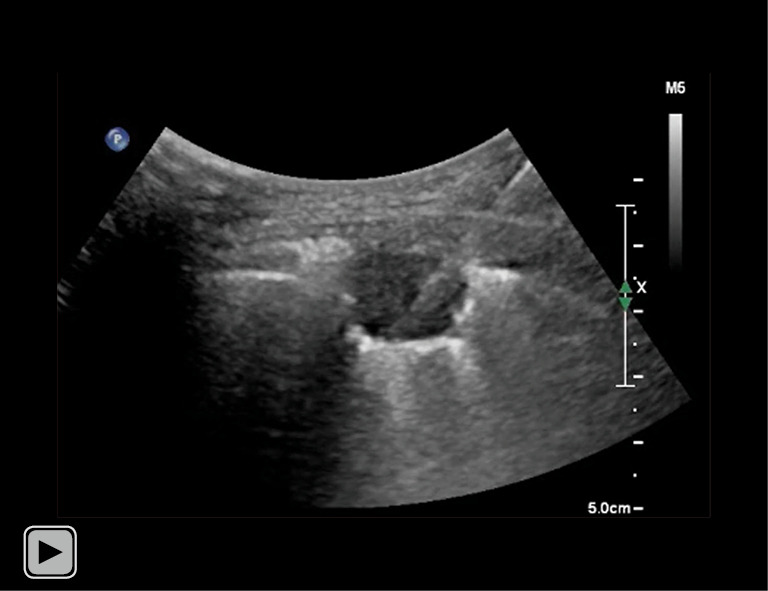
Operator performed core needle biopsy for subpleural lung nodule. After advancing needle tip to outer margin of lesion, operator pressed firing trigger to thrust inner trocar forward and confirmed that needle tip was within lesion, accompanied by advancing outer cutting cannula to extract tissue specimen (throw length: 11 mm).
Post-procedural management
Immediately after the procedure, US was performed to evaluate acute complication such as pneumothorax or active bleeding. After that, a chest radiograph was performed to determine the post-procedural pneumothorax or parenchymal hemorrhage within 3 hours. Twenty-four hours after biopsy, further follow-up chest radiographs were conducted.
Analysis of preprocedural CT images
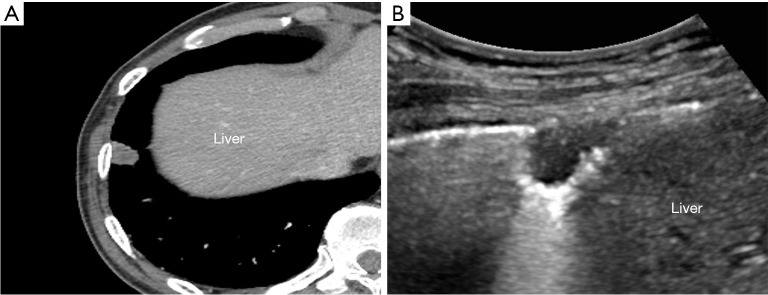
One radiologist (BP, with more than 6 years of experience for chest CT interpretation) evaluated all preprocedural CT images with mediastinal setting [width, 440 Hounsfield units (HU); level, 45 HU]. Subpleural lung lesion was defined as peripheral lung lesion abutting the pleura with variable lengths. In case of an unclear definition of the subpleural location, consensus was obtained with another radiologist (KMS, more than 15 years of experience for chest CT interpretation). The lesion size was measured at the level of the largest tumor diameter on axial CT image. All preprocedural CT was scanned within 30 days of US-guided subpleural biopsy. Pleural contact length (PCL) was measured with preprocedural CT and defined as the longest pleural length contacting with the mass as described in the previous study (17) (Figure 3).
Figure 3.
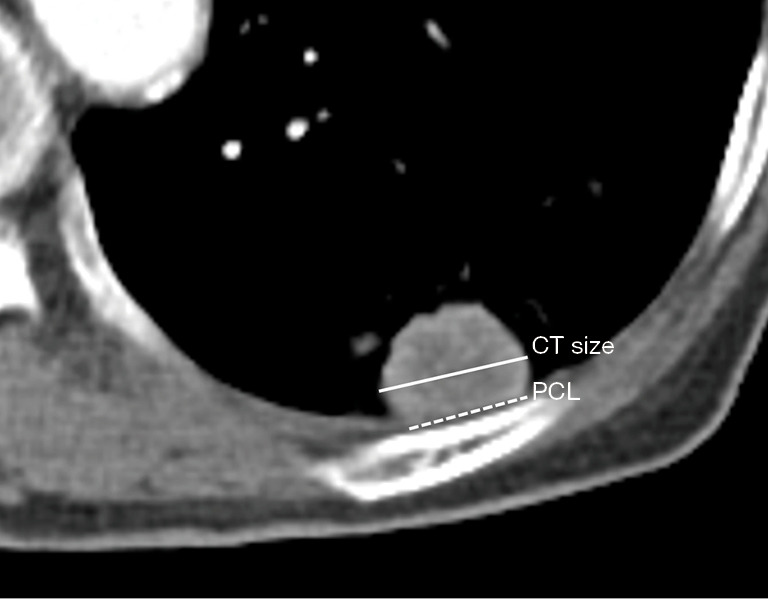
Measurement of the lesion size and PCL. The lesion size (solid line) was measured at the level of largest tumor diameter on axial CT image. PCL (dashed line) was defined as the longest pleural length contacting with the mass. CT, computed tomography; PCL, pleural contact length.
The lesion location was classified as follows: lesions at upper lobes (U), right middle lobe and left lingular segment (M), lower lobes (L). For imaging analysis, peri-lesional emphysema, peri-lesional honeycombing change, cavitary change, and presence of air-bronchogram were also included.
Procedure time was defined as time interval between the first US scanning to locate the lesion and the last image to evaluate acute post-procedural complication.
Diagnostic assessment
Diagnoses were classified as four categories: malignant lesion, specific benign lesion (e.g., benign tumor such as hamartoma or specific infection, such as tuberculosis and fungal infection), nonspecific benign inflammation (e.g., nonspecific inflammation or chronic inflammation) (19), and nondiagnostic. Among the lesions diagnosed with insufficient specimen such as fibromuscular tissue or skeletal muscle from US-guided PCNB, lesions without final diagnosis were classified as nondiagnostic. For each lesion, the reference standard was made by reviewing the medical records and follow-up images. Malignant and specific benign diagnoses at histopathological result of the initial US-guided PCNB or surgical pathology were considered as final diagnoses. Nonspecific benign inflammation diagnosed at initial US-guided PCNB is considered as final diagnosis only when subpleural lung lesions were improved or not changed for 2 years. A repeat US- or CT-guided biopsy, or a video-assisted thoracoscopic surgery, was performed in uncertain diagnosis cases.
If result of samples obtained from US-guided PCNB was nondiagnostic, the samples was regarded as inadequate. Otherwise, the samples were considered adequate.
Diagnostic success was defined as malignancy or specific benign lesions diagnosed by pathologic review of samples obtained by US-guided PCNB. Diagnostic failure was defined as nonspecific benign inflammation or nondiagnostic result such as insufficient specimens, fibromuscular tissue, or skeletal muscle.
A true positive diagnosis was defined as malignant lesion initially diagnosed by US-guided PCNB with malignancy. A true negative diagnosis was defined as specific benign lesions or nonspecific benign inflammation which is diagnosed by initial US-guided PCNB and subsequently improved or without change for more than 2 years. A false-positive diagnosis was defined as a lesion that was initially diagnosed as benign by US-guided PCNB but was finally diagnosed as malignant or nondiagnostic by US-guided PCNB. A false-positive diagnosis was defined as a lesions that was initially diagnosed as malignancy by US-guided PCNB but was finally diagnosed as benign. True positive and true negative diagnosis were considered as accurate diagnosis.
Statistical analysis
All statistical analyses were performed using the SPSS statistical software version 22.0 (IBM, Chicago, IL, USA) and R software package (R version 4.0.3, The R Foundation for Statistical Computing).
Continuous variables were presented as mean ± standard deviation and were analyzed using one-way analysis of variance or Kruskal-Wallis test. Categorical variables were presented as frequency and percentages and were compared between groups using the chi-square test. As a post hoc analysis, Tukey test with the Bonferroni correction was applied.
Factors which are independently associated with biopsy related complication were identified using odds ratio (OR) and confidence interval (CI) from the multivariable logistic analysis. In the univariable analysis, variables with a P value <0.1 were included as input variables for multivariable logistic regression analysis using the backward stepwise method. For all statistical analyses, the significance level was set at values of P<0.05.
Results
Characteristics of study population according to lesion size
Five hundred seventy-two lesions with 572 patients (age, 74.1±10.8 years) consisting of 143 females and 429 males were included in this study. The mean lesion size and PCL was 5.4±2.9 and 4.7±2.8 cm, respectively. 71 (12.5%) of the 572 lesions were benign, and 497 (87.5%) were malignant.
According to the lesion size, 72 of 572 lesions were classified as small, 206 as medium lesions, and 294 as large lesions. The mean lesion size of small, medium, and large lesions were 1.6±0.4 cm, 3.6±0.9 cm, and 7.7±2.2 cm, respectively. The mean PCL of small, medium, and large lesions were 1.3±0.4 cm, 3.0±1.1 cm, and 6.7±2.5 cm, respectively.
The lesion distribution and malignancy rate of final diagnosis were significantly different in large lesions compared with small or medium lesions (P<0.05 for all). The number of transthoracic needle passage for US-guided PCNB was significantly higher in large lesions than in small or medium lesions (small: 1.8±0.2 vs. medium: 1.9±0.7 vs. large 2.1±0.9, P=0.003). Between groups, other factors including age, sex, and experience of operator were not significantly different. The characteristics of study population and lesions were summarized in Table 1 and Table S1.
Table 1. Clinical characteristics of study population and subpleural lung lesions.
| Variable | Total (N=572) | Small (N=72) | Medium (N=206) | Large (N=294) | P |
|---|---|---|---|---|---|
| Sex | 0.452 | ||||
| Female | 143 (25.0) | 20 (27.8) | 56 (27.2) | 67 (22.8) | |
| Age, years | 74.1±10.8 | 72.3±10.3 | 73.8±11.4 | 74.8±10.5 | 0.184 |
| Number of transthoracic passage | 2.0±0.7 | 1.8±0.2 | 1.9±0.7 | 2.1±0.9 | 0.003* |
| Experience, years | 4.6±2.9 | 4.7±2.9 | 4.4±2.8 | 4.7±3.0 | 0.519 |
| Lesion size, cm | 5.4±2.9 | 1.6±0.4 | 3.6±0.9 | 7.7±2.2 | <0.001* |
| PCL, cm | 4.7±2.8 | 1.3±0.4 | 3.0±1.1 | 6.7±2.5 | <0.001* |
| Final diagnosis | <0.001* | ||||
| Malignancy | 497 (87.5) | 53 (74.6) | 173 (84.8) | 271 (92.5) | |
| Benign | 71 (12.5) | 18 (25.4) | 31 (15.2) | 7.5 (7.5) | |
| Location | <0.001* | ||||
| L | 352 (61.5) | 52 (72.2) | 144 (69.9) | 156 (53.1) | |
| M | 44 (7.7) | 4 (5.6) | 17 (8.3) | 23 (7.8) | |
| U | 176 (30.8) | 16 (22.2) | 45 (21.8) | 115 (39.1) |
Except where otherwise indicated, data are numbered with percentage in parentheses, continuous variables were presented as mean ± standard deviation variables. *, statistically significant. PCL, pleural contact length; L, lower lobes; M, right middle lobe and left lingular segment; U, upper lobes.
The histopathologic result of US-guided PCNB and final diagnosis
Five hundred seventy-two histopathological results of US-guided subpleural lung biopsy showed 453 malignant lesions, 21 specific benign lesions, 68 nonspecific benign inflammatory lesions, eight atypical cells, and 22 nondiagnostic lesions. Furthermore, the final diagnoses were 497 malignant lesions, 28 specific benign lesions, 43 nonspecific benign inflammatory lesions, and four nondiagnostic. Table 2 summarizes the pathological results of initial US-guided subpleural PCNB and final diagnoses.
Table 2. The diagnostic classifications of US-guided PCNB.
| Diagnosis | US-guided biopsy | Final diagnosis |
|---|---|---|
| Malignant lesions | 453 | 497 |
| Lung cancer | 404 | 444 |
| Metastasis | 41 | 44 |
| Lymphoma | 8 | 9 |
| Benign lesions | 97 | 71 |
| Tuberculosis | 13 | 16 |
| Fungal infection | 3 | 4 |
| NTM infection | 0 | 1 |
| Sarcoidosis | 1 | 1 |
| Hamartoma | 2 | 2 |
| Amyloidosis | 1 | 1 |
| Thymoma | 1 | 1 |
| Actinomycosis | 0 | 2 |
| Nonspecific inflammation | 68 | 43 |
| Atypical cell | 8 | 0 |
| Nondiagnostic | 22 | 4 |
US, ultrasound; PCNB, percutaneous core needle biopsy; NTM, non-tuberculous mycobacteria.
Among the 22 nondiagnostic results from the US-guided PCNB, nine lung cancer, two metastases, two specific benign disease (actinomycosis, tuberculosis), five nonspecific benign inflammation, and four nondiagnostic were finally diagnosed. Of the 68 nonspecific benign inflammatory lesions, 24 lung cancers, one metastasis, five specific benign disease (fungal infection, actinomycosis, two tuberculosis, one non-tuberculous mycobacterial infection), and 38 nonspecific benign inflammation were finally diagnosed. Seven of the eight lesions diagnosed as atypical cells were finally diagnosed as lung cancer and one as lymphoma.
Diagnostic performances and procedure time according to lesion size
The overall sample adequacy, diagnostic success rate, and diagnostic accuracy of lesions of all sizes were 96.2%, 82.9%, and 90.4%, respectively. In subgroup analysis according to lesion size, there was no statistically significant difference in sample adequacy (small: 93.1% vs. medium: 96.1% vs. large: 96.9%, P=0.307) and diagnostic success rate (small: 75.0% vs. medium: 81.6% vs. large: 85.7%, P=0.079) of small lesions compared with medium or large lesions. Also, diagnostic accuracy of small lesions was not significantly different from those of medium or large lesions (small: 86.1% vs. medium: 91.7% vs. large: 90.5%, P=0.366) (Table 3). Details of diagnostic performance of US-guided PCNB is shown in Table 4. The mean procedure time was 10.0±4.7 minutes and significantly shortened with increasing lesion size (small: 14.8±6.1 minutes vs. medium: 9.8±4.5 minutes vs. large: 8.9±3.7 minutes, P<0.001) (Table S1 and Table 3).
Table 3. Diagnostic yield, procedure time, and complication rate of US-guided PCNB for subpleural lung lesions.
| Variable | Total (N=572) | Small (N=72) | Medium (N=206) | Large (N=294) | P |
|---|---|---|---|---|---|
| Sample adequacy | 0.307 | ||||
| Adequate | 550 (96.2) | 67 (93.1) | 198 (96.1) | 285 (96.9) | |
| Inadequate | 22 (3.8) | 5 (6.9) | 8 (3.9) | 9 (3.1) | |
| Success rate | 0.079 | ||||
| Success | 474 (82.9) | 54 (75.0) | 168 (81.6) | 252 (85.7) | |
| Fail | 98 (17.1) | 18 (25.0) | 38 (18.4) | 42 (14.3) | |
| Accuracy | 0.366 | ||||
| Accurate | 517 (90.4) | 62 (86.1) | 189 (91.7) | 266 (90.5) | |
| Inaccurate | 55 (9.6) | 10 (13.9) | 17 (8.3) | 28 (9.5) | |
| Procedure time, min | 10.0±4.7 | 14.8±6.1 | 9.8±4.5 | 8.9±3.7 | <0.001* |
| Complication | 27 (4.7) | 8 (11.1) | 13 (6.3) | 6 (2.0) | <0.001* |
| Pneumothorax | 19 (3.3) | 6 (8.3) | 10 (4.9) | 3 (1.0) | |
| Hemorrhagic | 8 (1.4) | 2 (2.8) | 3 (1.4) | 3 (1.7) |
Except where otherwise indicated, data are numbered with percentage in parentheses. *, statistically significant. US, ultrasound; PCNB, percutaneous core needle biopsy.
Table 4. Diagnostic performance of US-guided PCNB.
| Biopsy result | Final result | Total | |
|---|---|---|---|
| Malignancy | Benign | ||
| Malignancy | 453 (79.2%) | 0 | 453 |
| Benign | 55 (9.6%) | 64 (11.2%) | 119 |
| Total | 508 | 64 | 572 |
Non-diagnostic results of US-guided PCNB were considered as false positives, resulting in discrepancy of malignancy rate of final diagnosis. US, ultrasound; PCNB, percutaneous core needle biopsy.
Influencing factors for complication rate
Twenty-seven of the 572 patients who underwent US-guided PCNB had post-procedural complications (19 pneumothoraces, seven hemoptysis, and three parenchyma hemorrhages); 17 of 19 pneumothoraces were detected by ultrasound performed immediately after procedure. All complications were self-limited, which did not require chest tube insertion or other specific management. Complications more frequently occurred in small subpleural lesions than large subpleural lesions (small: 11.1% vs. large: 2.0%, P<0.001) but there was no difference in comparison with medium subpleural lesions (small: 11.1% vs. medium: 6.3%, P=0.858) (Table 3 and Table S1).
Univariate logistic regression analysis revealed that operator’s experience, lesion size, and PCL were inversely associated with complication development. Upper lobe location, peri-lesional emphysema, and presence of air-bronchogram were also significantly associated with complication rate.
For multivariate analysis, two models were built, one that excluded lesion size and the other that excluded PCL due to the high collinearity between these two variables (correlation coefficient =0.910, R2=0.829, P<0.001) (Figure S1).
On the model with the lesion size, operator’s experience (OR, 0.64; 95% CI: 0.49–0.80; P<0.001), lesion size (OR, 0.68; 95% CI: 0.54–0.83; P<0.001) and presence of air-bronchogram (OR, 14.36; 95% CI: 4.18–48.53; P<0.001) were independently associated with complication rates. On the model with PCL, operator’s experience (OR, 0.66; 95% CI: 0.52–0.82; P<0.001), PCL (OR, 0.68; 95% CI: 0.52–0.84; P=0.001), and presence of air-bronchogram (OR, 17.35; 95% CI: 4.99–55.95; P<0.001) independently associated with complication rates (Table 5).
Table 5. Univariable and multivariable logistic regression analysis to identify clinical and radiologic features of lesions associated with post-procedural complication.
| Characteristics | Univariable logistic regression analysis | Multivariable logistic regression analysis (with lesion size) | Multivariable logistic regression analysis (with PCL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |||
| Age | 0.98 | 0.95, 1.02 | 0.356 | ||||||||
| Sex | 0.144 | ||||||||||
| Female | Reference | ||||||||||
| Male | 0.55 | 0.25, 1.27 | |||||||||
| Number of transthoracic passage | 0.66 | 0.37, 1.12 | 0.140 | ||||||||
| Operator’s experience† | 0.67 | 0.52, 0.82 | <0.001* | 0.64 | 0.49, 0.80 | <0.001* | 0.66 | 0.52, 0.82 | <0.001* | ||
| Lesion size† | 0.7 | 0.57, 0.84 | <0.001* | 0.68 | 0.54, 0.83 | <0.001* | |||||
| PCL† | 0.7 | 0.55, 0.85 | <0.001* | 0.68 | 0.52, 0.84 | 0.001* | |||||
| Final diagnosis | 0.419 | ||||||||||
| Benign | Reference | ||||||||||
| Malignancy | 0.7 | 0.31, 1.75 | |||||||||
| Location | |||||||||||
| L | Reference | ||||||||||
| U† | 0.29 | 0.07, 0.85 | 0.047* | Stepwise eliminated | Stepwise eliminated | ||||||
| M | 1.66 | 0.47, 4.66 | 0.367 | ||||||||
| Peritumoral emphysema† | 0.34 | 0.10, 0.90 | 0.049* | Stepwise eliminated | Stepwise eliminated | ||||||
| Peritumoral honeycombing | 1.01 | 0.06, 5.15 | 0.993 | ||||||||
| Cavitary change | 1.54 | 0.50, 3.91 | 0.397 | ||||||||
| Air-bronchogram† | 10.84 | 3.55, 30.08 | <0.001* | 14.36 | 4.18, 48.53 | <0.001* | 17.35 | 4.99, 55.95 | <0.001* | ||
†, variables with P<0.1 on univariable analysis were included in multivariable analysis. *, statistically significant. OR, odds ratio; CI, confidence interval; PCL, pleural contact length.
Discussion
In the current study, the results demonstrated that US-guided PCNB is an effective procedure with no significant deterioration in diagnostic accuracy for diagnosing small (≤2 cm) subpleural lung lesions. Complications more frequently occurred in small (≤2 cm) subpleural lesions than larger lesions, but all were minor complications and self-limited cases. The procedure time of US-guided PCNB for small (≤2 cm) subpleural lesions was also within reasonable range. These findings suggest that even in small subpleural (≤2 cm) lesions, US-guided PCNB is an appropriate diagnostic method.
Sample adequacy of US-guided PCNB for subpleural lung lesions has been reported to range from 81.7% to 98% (17,18,20-23). Regarding the diagnostic accuracy for malignancy diagnosis, US-guided PCNB yielded accuracy ranging from 59% to 81.8% in diverse design studies (18,21,24). Although several factors may affect the diagnostic efficacy of US-guided PCNB, the lesion size (17,21) and PCL (23,24) are the most important determining factors in US-guided PCNB diagnostic accuracy and sample adequacy. In the current study, the diagnostic accuracy and sample adequacy of small lesions based on lesion size (≤2 cm) were also lower than those of medium size (2 cm< lesions ≤5 cm) and large-size lesions (>5 cm), but these differences did not reach statistical significance (84.7% vs. 90.8% vs. 90.5%, P=0.301 and 93.1% vs. 96.1% vs. 96.9%, P=0.307, respectively). This study’s results of diagnostic accuracy and sample adequacy for small lesions (≤2 cm) were higher than those of the recent study for lung nodule less than 2 cm (84.7% vs. 81.4%, 93.1% vs. 83.0%) (18). Additionally, given that the mean lesion size included in the previous studies was from 35 to 36 mm (17,20,21,23,24), the results show higher diagnostic accuracy over the previous studies, which have ranged from 59% to 81.8%. In this study, higher proportion of malignant lesions (87.5%) may have contributed to higher diagnostic accuracy. According to the previous series, compared with benign lesion, malignant lesion was associated with higher diagnostic yield (19,25).
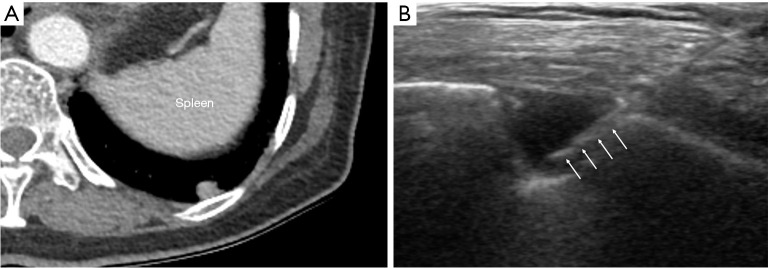
For small subpleural lung lesions, PCNB have been technically challenging due to the impact of overlying rib and/or respiratory movement (11-14). Especially, lower lobar location of small subpleural lesions is more susceptible to respiratory movement, which may lead to diagnostic failure and pneumothorax occurrence. According to previous studies, the target lesion’s lower lobar location was associated with diagnostic failure and post-procedural pneumothorax (26-28). In this study, the relationship between the lobar location of small subpleural lesions and diagnostic yield or complications was not found. Although target lesions located in lower lobes are more affected by respiratory movement, real-time visualization of the lesions under US guidance could provide more flexibility to reach small subpleural lesions, even in lesions located adjacent to the diaphragm that is strongly affected by respiratory motion (Figure 4).
Figure 4.
A 75-year-old female with a 0.8 cm subpleural nodule diagnosed with adenocarcinoma. (A) Axial enhanced CT image shows small subpleural nodule adjacent to diaphragm in the left lower lobe. (B) Procedural USG image shows hypoechoic subpleural nodule with core biopsy needle traversing the lesion. The needle path appeared hyperechoic (arrows). CT, computed tomography; USG, ultrasonography.
In terms of post-procedural complications, the predominant complication was pneumothorax (3.7%), and hemorrhagic complication was rare (1.7%). All cases were self-limited and improved with conservative treatment. Reported pneumothorax incidence and hemorrhagic complications after US-guided PCNB have ranged from 2% to 15% (17,18,20,23,24) and 2% to 8% (18,20,21,23,24,29), respectively. Major pneumothorax requiring chest tube insertion was from 0.6% to 5% (17,20,24). This complication rate’s variability is related to various factors, including lesion size, needle size, patient cooperation, and operator’s experience (18,21,24). For pneumothorax, needle and lesion size and PCL were identified in the recent series to be determining factors (24), which are consistent with the results that lesion size and PCL were independently associated with post-procedural pneumothorax. In contrast with other imaging-guided PCNB, US-guided biopsy is only applicable to subpleural lung lesions; thus, the cause of pneumothorax development is different. Pneumothorax during or after US-guided PCNB is mainly related to the guidance deviation, poor sonic window due to patient’s respiratory movement, and gas reverberation artifact. Therefore, pneumothorax occurs easily in small lesions or lesions with small PCL.
In our study, pneumothorax rate (8.3%) was also higher for small lesions (≤2 cm) than those of medium and large-size lesions. However, it was within a range similar to that of reported US-guided PCNB (20,21,24), and all cases were recorded as minor pneumothorax that improved with conservative management.
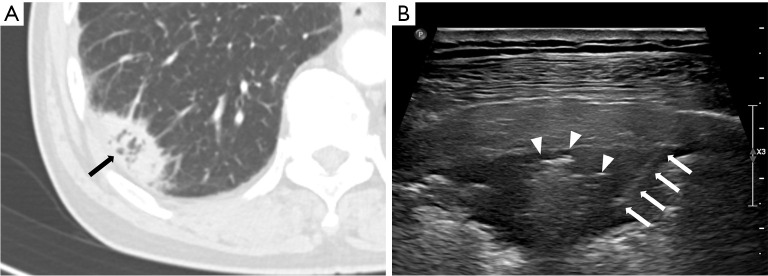
Regarding CT features of US-guided PCNB-related complications, Zhang et al. (30) reported that 20 of 209 patients had post-procedural hemoptysis, and air-bronchogram on CT was a risk factor for hemoptysis of US-guided PCNB. In this study, only one of 20 patients (5%) with air-bronchogram on CT had post-procedural hemoptysis. In the previous study, the operators were pulmonologist who had little acquaintance with air-bronchogram US imaging and the relationship between air-bronchogram and hemoptysis. In this study, the operators were radiologists, and they tried to avoid needle passage through the air-bronchogram to prevent hemoptysis (Figure 5), which may decrease the likelihood of hemoptysis.
Figure 5.
A 73-year-old male with 3.1 cm subpleural lung mass diagnosed with adenocarcinoma. (A) Axial chest CT image shows subpleural lung mass with air-bronchogram (arrow) in the right lower lobe. (B) USG image during the US-guided percutaneous core needle biopsy shows subpleural mass with central hyperechogenecity suggesting air-bronchogram. Biopsy needle (arrows) traverses periphery of the tumor to avoid needle passage through the air-bronchogram (arrow heads). CT, computed tomography; USG, ultrasonography; US, ultrasound.
In this study, operator’s experience was found to be associated with complication rate of US-guided PCNB for subpleural lesions. This result is inconsistent with a recent series by Lemieux et al. (24) that operator’s experience was not associated with post-procedural complications. Generally, mastering US-guided biopsy requires longer training period than other imaging modality. Further study is needed to elucidate the relationship of the degree of operator’s experience and complication development.
Compared with CT, US-guided PCNB procedure time is time saving (17) and has been reported to range from 5 to 31 minutes (15,17,20). The procedure time could be affected by lesion size, anesthesia type, sedation time, operator’s experience, and patient’s cooperation. In this study, the mean procedure time was 10.0±4.7 min, which was shorter than the reported data (17,20). There is larger lesion size in this study in comparison with the previous study (5.4±2.9 vs. 3.5±2.2 and 4.8±2.6 cm), and only local anesthesia use without sedation may be attributable to shorter procedure time.
US-guided PCNB procedure time for small lesions (≤2 cm) was significantly longer than those of medium- or large-size lesions (8.9 vs. 9.8 vs. 14.8 min). For smaller lesions, it is difficult to locate the lesion on US due to overlying bony structures and subjection to respiratory movement. Moreover, deliberated needle guiding is required to sample small lesions, which leads to longer procedure time. Although, specific PCNB procedure time for small subpleural lesion has not been reported, considering the procedure time (18.8 min) of cone-beam CT-guided biopsy for small lesions (≤2 cm) (31), this study’s procedure time is reasonable in clinical practice.
The current study has several limitations. First, it was a single-center retrospective study with a small sample size and unintended selection bias. Second, US-guided PCNB was performed by three certified radiologists and one radiology resident. As US guidance is operator dependent, the results may not be generally applied. Third, post-procedural complications were evaluated by post-procedural USG and chest radiograph; therefore, compared with CT-guided PCNB, complication rate may be underestimated. However, pneumothorax not detected on chest radiograph is negligible in terms of safety.
Conclusions
US-guided PCNB diagnostic yield for subpleural lung lesions including sample adequacy, diagnostic success rate, and diagnostic accuracy were not significantly different among groups classified by lesion size. Although complications more frequently occurred in small subpleural lesions, there were no complications requiring chest tube insertion or other specific management. Therefore, US-guided PCNB performed by an experienced radiologist could be an effective and safe diagnostic approach for subpleural lung lesions, even in small lesions.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Kyungpook National University Chilgok Hospital (KNUCH 2022-02-033) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1546/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1546/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-1546/coif). The authors have no conflicts of interest to declare.
References
- 1.Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 2002;222:773-81. 10.1148/radiol.2223010490 [DOI] [PubMed] [Google Scholar]
- 2.Henschke CI. Early lung cancer action project: overall design and findings from baseline screening. Cancer 2000;89:2474-82. [DOI] [PubMed] [Google Scholar]
- 3.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. 10.1016/S0140-6736(99)06093-6 [DOI] [PubMed] [Google Scholar]
- 4.Manhire A, Charig M, Clelland C, et al. Guidelines for radiologically guided lung biopsy. Thorax 2003;58:920-36. 10.1136/thorax.58.11.920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiraki T, Mimura H, Gobara H, et al. CT fluoroscopy-guided biopsy of 1,000 pulmonary lesions performed with 20-gauge coaxial cutting needles: diagnostic yield and risk factors for diagnostic failure. Chest 2009;136:1612-7. 10.1378/chest.09-0370 [DOI] [PubMed] [Google Scholar]
- 6.Geraghty PR, Kee ST, McFarlane G, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology 2003;229:475-81. 10.1148/radiol.2291020499 [DOI] [PubMed] [Google Scholar]
- 7.Ohno Y, Hatabu H, Takenaka D, et al. CT-guided transthoracic needle aspiration biopsy of small (< or = 20 mm) solitary pulmonary nodules. AJR Am J Roentgenol 2003;180:1665-9. 10.2214/ajr.180.6.1801665 [DOI] [PubMed] [Google Scholar]
- 8.Kothary N, Lock L, Sze DY, et al. Computed tomography-guided percutaneous needle biopsy of pulmonary nodules: impact of nodule size on diagnostic accuracy. Clin Lung Cancer 2009;10:360-3. 10.3816/CLC.2009.n.049 [DOI] [PubMed] [Google Scholar]
- 9.Heck SL, Blom P, Berstad A. Accuracy and complications in computed tomography fluoroscopy-guided needle biopsies of lung masses. Eur Radiol 2006;16:1387-92. 10.1007/s00330-006-0152-2 [DOI] [PubMed] [Google Scholar]
- 10.Hwang HS, Chung MJ, Lee JW, et al. C-arm cone-beam CT-guided percutaneous transthoracic lung biopsy: usefulness in evaluation of small pulmonary nodules. AJR Am J Roentgenol 2010;195:W400-7. 10.2214/AJR.09.3963 [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Krishnamurthy S, Broemeling LD, et al. Small (≤2-cm) Subpleural Pulmonary Lesions: Short- versus Long-Needle-Path CT-guided Biopsy—Comparison of Diagnostic Yields and Complications. Radiology 2005;234:631-7. 10.1148/radiol.2342031423 [DOI] [PubMed] [Google Scholar]
- 12.Yeow KM, Su IH, Pan KT, et al. Risk factors of pneumothorax and bleeding: multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest 2004;126:748-54. 10.1378/chest.126.3.748 [DOI] [PubMed] [Google Scholar]
- 13.Moore EH. Technical aspects of needle aspiration lung biopsy: a personal perspective. Radiology 1998;208:303-18. 10.1148/radiology.208.2.9680552 [DOI] [PubMed] [Google Scholar]
- 14.Yeow KM, See LC, Lui KW, et al. Risk factors for pneumothorax and bleeding after CT-guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol 2001;12:1305-12. 10.1016/S1051-0443(07)61556-5 [DOI] [PubMed] [Google Scholar]
- 15.Sconfienza LM, Mauri G, Grossi F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology 2013;266:930-5. 10.1148/radiol.12112077 [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto N, Watanabe T, Yamada K, et al. Efficacy and safety of ultrasound (US) guided percutaneous needle biopsy for peripheral lung or pleural lesion: comparison with computed tomography (CT) guided needle biopsy. J Thorac Dis 2019;11:936-43. 10.21037/jtd.2019.01.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MH, Lubner MG, Hinshaw JL, et al. Ultrasound Guidance Versus CT Guidance for Peripheral Lung Biopsy: Performance According to Lesion Size and Pleural Contact. AJR Am J Roentgenol 2018;210:W110-7. 10.2214/AJR.17.18014 [DOI] [PubMed] [Google Scholar]
- 18.Huang W, Ye J, Qiu Y, et al. Ultrasound-Guided Percutaneous Core Needle Biopsy of Peripheral Pulmonary Nodules ≤ 2 cm: Diagnostic Performance, Safety and Influence Factors. Front Oncol 2021;11:671884. 10.3389/fonc.2021.671884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KH, Lim KY, Suh YJ, et al. Nondiagnostic Percutaneous Transthoracic Needle Biopsy of Lung Lesions: A Multicenter Study of Malignancy Risk. Radiology 2019;290:814-23. 10.1148/radiol.2018181482 [DOI] [PubMed] [Google Scholar]
- 20.Mychajlowycz M, Alabousi A, Mironov O. Ultrasound- Versus CT-Guided Subpleural Lung and Pleural Biopsy: An Analysis of Wait Times, Procedure Time, Safety, and Diagnostic Adequacy. Can Assoc Radiol J 2021;72:883-9. 10.1177/0846537120939073 [DOI] [PubMed] [Google Scholar]
- 21.Guo YQ, Liao XH, Li ZX, et al. Ultrasound-Guided Percutaneous Needle Biopsy for Peripheral Pulmonary Lesions: Diagnostic Accuracy and Influencing Factors. Ultrasound Med Biol 2018;44:1003-11. 10.1016/j.ultrasmedbio.2018.01.016 [DOI] [PubMed] [Google Scholar]
- 22.Khan RA, Kumar V, Taimur M, et al. Diagnostic Yield of Ultrasound-guided Trucut Biopsy in Diagnosis of Peripheral Lung Malignancies. Cureus 2019;11:e4802. 10.7759/cureus.4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon KN, Bae K, Park MJ, et al. US-guided transthoracic biopsy of peripheral lung lesions: pleural contact length influences diagnostic yield. Acta Radiol 2014;55:295-301. 10.1177/0284185113494984 [DOI] [PubMed] [Google Scholar]
- 24.Lemieux S, Kim T, Pothier-Piccinin O, et al. Ultrasound-guided transthoracic needle biopsy of the lung: sensitivity and safety variables. Eur Radiol 2021;31:8272-81. 10.1007/s00330-021-07888-9 [DOI] [PubMed] [Google Scholar]
- 25.Tongbai T, McDermott S, Kiranantawat N, et al. Non-Diagnostic CT-Guided Percutaneous Needle Biopsy of the Lung: Predictive Factors and Final Diagnoses. Korean J Radiol 2019;20:1515-26. 10.3348/kjr.2019.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang MD, Weng HH, Hsu SL, et al. Accuracy and complications of CT-guided pulmonary core biopsy in small nodules: a single-center experience. Cancer Imaging 2019;19:51. 10.1186/s40644-019-0240-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshita J, Masago K, Kato R, et al. CT-guided fine-needle aspiration and core needle biopsies of pulmonary lesions: a single-center experience with 750 biopsies in Japan. AJR Am J Roentgenol 2015;204:29-34. 10.2214/AJR.14.13151 [DOI] [PubMed] [Google Scholar]
- 28.Hiraki T, Mimura H, Gobara H, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. AJR Am J Roentgenol 2010;194:809-14. 10.2214/AJR.09.3224 [DOI] [PubMed] [Google Scholar]
- 29.Liao WY, Chen MZ, Chang YL, et al. US-guided transthoracic cutting biopsy for peripheral thoracic lesions less than 3 cm in diameter. Radiology 2000;217:685-91. 10.1148/radiology.217.3.r00dc21685 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, He L, Zhou X, et al. Hemoptysis complicating ultrasound-guided transthoracic needle lung biopsy: air bronchial sign is a risk predictor. J Thorac Dis 2020;12:3167-77. 10.21037/jtd-20-1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi JW, Park CM, Goo JM, et al. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of small (≤ 20 mm) lung nodules: diagnostic accuracy and complications in 161 patients. AJR Am J Roentgenol 2012;199:W322-W330. 10.2214/AJR.11.7576 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as