Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) is associated with incident chronic kidney disease (CKD). We aimed to investigate outcomes and risk factors of CKD progression and regression.
Methods
This is a longitudinal community-based cohort study of patients with NAFLD. Exclusion criteria included alcoholic liver diseases, sero-positive for hepatitis B surface antigen, sero-positive for hepatitis C virus antibodies, fatty liver index <60, individuals with only one year of data, missing data for fibrosis-4 score (FIB-4 score) and NAFLD fibrosis score (NFS), and advanced CKD at baseline. Main outcomes were stratified according to estimated glomerular filtration rate (eGFR) and albuminuria categories as state 1 (low risk), state 2 (moderately increased risk), and state 3 (high-risk/very-high risk of progression). The multi-state Markov model was used for outcome analysis.
Results
This study included 1628 patients with NAFLD with a median follow-up of 3.4 years. State 2 CKD was found in 9.3% of patients at 5 years (95% CI, 8.1%–10.6%). Most patients with state 2 CKD recovered to state 1 (69%; 95% CI, 63.7%–74%), while 17.6% progressed to state 3 (95% CI, 13.4%–22.7%). Advanced liver fibrosis was found to be associated with the risk of transitioning from state 1 to state 2 ((FIB-4 score) ≥1.3; hazard ratio (HR), 1.42; 95% CI, 1.02–2.00), and reduced recovery from state 2 to state 1 (NFS≥−1.455; HR, 0.56; 95% CI, 0.34–0.91).
Conclusion
NAFLD severity is associated with CKD, which may be reversible before becoming high-risk. Controlling metabolic risk factors and preventing advanced liver fibrosis are recommended.
Keywords: Nonalcoholic steatohepatitis, Liver fibrosis, Multistate model, Fatty liver, Risk factors, Renal function
At a glance commentary
Scientific background on the subject
Patients with non-alcoholic fatty liver disease (NAFLD) are associated with incident chronic kidney disease (CKD). However, long-term outcomes and risk factors for those patients with incident CKD are needed to identify. Here, we aimed to investigate CKD outcomes and risk factors for CKD progression and regression.
What this study adds to the field
In this longitudinal study of 1628 patients with NAFLD followed up for a median of 3.4 years, most moderate to advanced CKD (69% participants) recover, while 17.6% progress to severer stages. After controlling metabolic factors, advanced liver fibrosis is associated with risks of developing CKD and reduced recovery from CKD.
Non-alcoholic fatty liver disease (NAFLD) is one of most common liver diseases worldwide, with an estimated global prevalence of approximately 25%–30% [[1], [2], [3]]. NAFLD is a spectrum of liver diseases, including hepatic steatosis, nonalcoholic steatohepatitis (NASH) with or without fibrosis, and cirrhosis [1,4]. Once advanced fibrosis or liver cirrhosis occurs, the risk for hepatocellular carcinoma and liver-related mortality increase markedly. Further, NAFLD is not only associated with liver-related mortality and morbidity, but it has also recently been recognized as a multisystem disease that relates to other medical comorbidities, such as metabolic syndrome (MetS), insulin resistance, cardiovascular diseases, and chronic kidney disease (CKD). Moreover, cumulative evidence indicates that the presence and severity of NAFLD is strongly associated with an increased incidence of CKD [[4], [5], [6]]. Recent studies have revealed that both NAFLD and CKD will continue to play a major role in the global disease burden [2,7,8].
Although the presence and severity of NAFLD has been associated with the development of incident CKD independent from traditional cardio-renal risk factors, it has yet to be determined if this is a causal relationship [4,6]. In fact, NAFLD and CKD share multiple risk factors, such as age, morbid obesity, insulin resistance, dyslipidemia, metabolic syndrome(MetS), diabetes, and hypertension. Currently, the well-known existence of mechanistic pathways linking the liver and kidneys is supported by the presence of hepato-renal syndrome, which can develop in patients with cirrhosis and portal hypertension. However, scanty studies investigate whether the NAFLD related CKD is reversible when controlling metabolic risk factors. Previous longitudinal studies [5,6] define time to event outcome of incident CKD as the eGFR < 60 ml/min/1.73 m2 or overt proteinuria (by urinary dipstick). Such analyses compromise clinicians to unravel whether incident CKD is reversible and the probability of CKD progression to advanced stages as well as its prognostic factors.
In this cohort study, we aimed to investigate the transition between stages of CKD among patients with NAFLD using the multi-state Markov model and investigate the risk factors for each transition. In addition, we investigate the effects of the severity of NAFLD on CKD disease dynamics.
Materials and methods
Study design
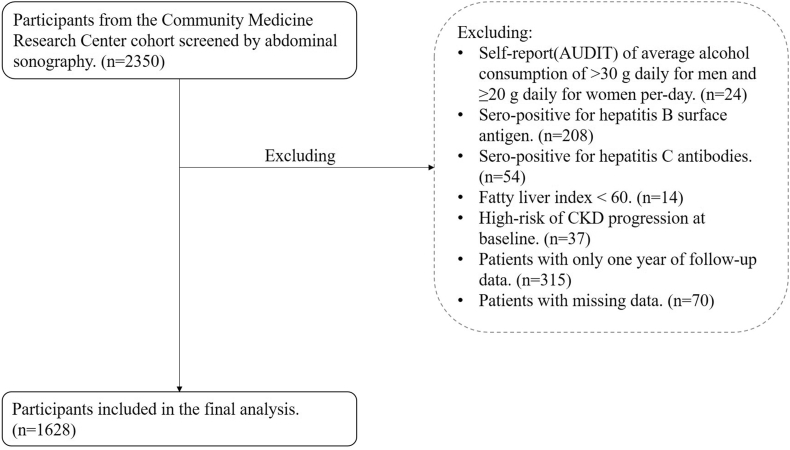
We analyzed the data of a community-based cohort study that was conducted from August 2013 to March 2019 in Taiwan (Northeastern Taiwan Community Medicine Research Cohort (NTCMRC); ClinicalTrials.gov Identifier: NCT04839796) [9]. In this cohort study, we recruited independent volunteer samples rather than family-based samples. The study included 2350 patients who underwent abdominal ultrasonography. We defined individuals with NAFLD of fatty liver index ≥60 [10]. Exclusion criteria included alcohol consumption (>30 g daily for men and ≥20 g daily for women; n = 24), sero-positive for hepatitis B surface antigen (n = 208) or sero-positive for hepatitis C antibodies (n = 54), fatty liver index <60 (n = 14) [11], and patients with only one year of follow-up data (n = 315). We also excluded patients with missing longitudinal data required to calculate the fibrosis-4 score (FIB-4 score) or NAFLD fibrosis score (NFS) and missing laboratory tests included in the univariable and multivariable analyses (n = 70). In addition, individuals with a high-risk or very high risk of CKD progression at baseline were excluded (n = 37) according to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 clinical practice guidelines for the evaluation and management of CKD [Supplementary Table 1] [12,13]. Finally, 1628 patients were included in the final analysis [Fig. 1].
Fig. 1.
Participants flow diagram.
Data collection
Patient's demographic characteristics, alcohol consumption, and medical history were collected during each visit via standardized questionnaires. Blood pressure, height, weight, body mass index (BMI) and laboratory tests were obtained at each visit. Obesity was defined as BMI ≥27 kg/m2 based on the definition provided by the Department of Health in Taiwan [14]. MetS was defined using a modified version of the Adult Treatment Panel III criteria [15]. Participants with any 3 of the 5 criteria were classified as having MetS. Insulin resistance was quantified by the homeostatic model assessment of insulin resistance (HOMA-IR) [16].
All ultrasound examinations were performed by experienced physicians using the same equipment. The degree of fatty liver disease identified on ultrasound was defined as none/mild, moderate, or severe, based on the discrepancy of the echogenicity between the liver and kidney, degree of posterior attenuation, visibility of the vessels, fogginess of the gallbladder wall, and an obscure diaphragm. The gastroenterologists confirmed the final reports for all participants. We calculated the NFS to assess the severity of fibrosis and to classify participants into two groups: high-intermediate and low probability of advanced fibrosis [17]. We also stratified participants according to the FIB-4 [18]. We used the cut-off value of −1.455 for NFS and 1.3 for FIB-4 according to its ability to exclude liver fibrosis in validation studies [17,18].
Outcomes
We calculated estimated glomerular filtration rate (eGFR in ml/min/1.73 m2) using the Chronic Kidney Disease-Epidemiology Collaboration equation [19]. CKD was defined as an eGFR <60 mL/min/1.73 m2 or the presence of proteinuria on two separate occasions three months apart. According to the clinical practice guidelines for the evaluation and management of CKD, we stratified the CKD outcomes by eGFR and albuminuria categories [Supplementary Table 1] [12,13]. eGFR categories were defined as: G1 = normal or high, G2 = mildly decreased, G3a = mildly to moderately decreased, G3b = moderately to severely decreased, G4 = severely decreased, and G5 = kidney failure. The albumin-to-creatinine ratio (ACR) was used to determine albuminuria categories, which were defined as: A1 = ACR <30 mg/g, A2 = 30 mg/g ≤ ACR ≤300 mg/g, and A3 = ACR >300 mg/g. We categorized outcomes into three states according to the risk of CKD progression as follows: state 1 = low-risk group, state 2 = moderately increased risk group, and state 3 = high-risk/very high-risk group [12,13].
Management of patients with CKD
Standardized, multidisciplinary educational sessions were periodically conducted in the community according to clinical guidelines for CKD [13]. Case-management nurses performed educational activities using standardized materials and were evaluated by independent experts to minimize inter-personal variability on knowledge delivery. The lectures introduced knowledge on healthy renal function, risk factors, and clinical manifestations of CKD. The case-management nurses also emphasized the importance of nutrition, lifestyle modification, nephrotoxin avoidance, dietary principles, and pharmacological regimens for the preservation of renal function. Further, the case-management nurse contacted the patients to ensure regular follow-up. Patients with moderate to advanced CKD (>G3a stage or > A2 stage) were referred to nephrology early to ensure a timely intervention.
Statistical analysis
Multi-state Markov model
As the stage of CKD could vary over time, we used a continuous time multi-state Markov model to describe the clinical transitions between the three states [20,21]. Such models have traditionally been used to study disease modeling for many chronic diseases and have a natural interpretation in terms of staged progression, which accounted for interval-censor data. The multistate model describes the transient states of individuals [Fig. 2] and allows for the estimation of both transition intensities (TIs) and transition probabilities (TPs) from one state to another. Initially, an unadjusted multistate model was used to summarize the TIs with confidence intervals (CIs). Next, we constrained the state 1 to state 3 TI to zero in the multivariable models to investigate covariate effects due to low probability for patients with CKD progression from state 1 to state 3. We performed multivariable models incorporating constant and time-varying characteristics of individuals. Covariate effects for a particular TI were investigated by modeling the intensity as a function of these variables using a form of a proportional hazards model described by Marshall and Jones [22]. We also conducted model comparisons using likelihood ratio test to compare homogeneous continuous-time Markov model and non-homogeneous continuous-time Markov models (See Appendix for statistical details).
Fig. 2.
Multi-state Markov model. State 1 CKD can progress to state 2 and state 2 can progress to state 3 or recover to state 1.
Directed acyclic graphs
Randomized controlled trials that investigate the effects of NAFLD on CKD are impractical and unethical. Therefore, methodologists, epidemiologists, and statisticians describe a range of systematic representations of hypothetical causal relationships between variables that facilitate statistical analyses [23]. Such systematic representations of causal relationships are widely credited with introducing directed acyclic graphs (DAGs) to the field of epidemiology for causal inference using observational data [24,25]. Thus, to investigate the effects of NAFLD on the transition between CKD stages, we constructed DAGs to conduct variable selection for multivariable analyses (Supplemental Fig. 1). By testing such implications statistically, we also investigate the testable implications indicated by DAGs, which is consistent with the dataset [Supplementary Table 2]. Consequently, we identified the 2 minimal sufficient adjustment sets from the DAG to investigate the total effects of NAFLD on the transition between CKD stages using the back-door criteria [25]. Moreover, we also constructed the exposure-outcome relationship of all of the DAGs in an equivalence class, significantly strengthening the validity of these sets [Supplemental Fig. 1] [24].
Baseline demographics and clinical characteristics were compared using the chi-square test or Wilcox rank-sum test for categorical and continuous variables, respectively. Statistical significance was set at p < 0.05. All analyses were performed with R version 4.0.2 software (R Foundation for Statistical Computing, Vienna, Austria) with the “msm” [20], “dagitty” [24], and “ggdag” packages.
Results
Of the 1628 individuals included in this study, 1491 were classified as state 1 and 147 as state 2 at baseline [Table 1]. There were no significant differences in sex, total cholesterol, aspartate aminotransferase (AST), and alkaline phosphatase (ALK-P) between patients in state 1 and those in state 2. Patients were followed for a median of 3.4 years (interquartile range (IQR): 2.1–4.3 years). Overall, 5831 longitudinal data items were included in the multi-state Markov model analysis. The number of transitions and the TIs in the unadjusted multistate Markov model are summarized in Table 2. Overall, 194 patients transitioned from state 1 to state 2, 10 patients transitioned from state 1 to state 3, 142 from state 2 to state 1, and 35 from state 2 to state 3. Patients in state 2 were more likely to transition to state 1 (TI: 0.436) than to state 3 (TI: 0.092). The ratio of TI to regression from state 2 to state 1 and progression from state 2 to state 3 was 4.76 (95% CI; 3.38–6.68). At 5 years, the TP from state 1 to state 2 was 9.3%, from state 1 to state 3 was 3.1%, from state 2 to state 1 was 69%, and from state 2 to state 3 was 17.6%. The TP for remaining in state 1 was 87.6% while the TP for remaining in state 2 was 13.4% [Table 3]. We stratified diabetes and non-diabetes cohorts to investigate the effect of diabetes on TP. For the diabetes cohort at 5 years, the TP from state 1 to state 2 was 21.5%, from state 1 to state 3 was 8.8%, from state 2 to state 1 was 47.5%, and from state 2 to state 3 was 27.8%. For the non-diabetes cohort at 5 years, the TP from state 1 to state 2 was 7.6%, from state 1 to state 3 was 2%, from state 2 to state 1 was 74%, and from state 2 to state 3 was 13.6%. These results indicate the diabetes cohort is more prone to CKD progression and has less chance to recover from advanced states compared to the non-diabetes cohort at each time. We summarized the observed sample size of individuals in each state at each time in the Supplementary Table 3.
Table 1.
Baseline patient demographics.
| Variables | State 1: Low risk of progression of CKD (n = 1491) | State 2: Moderately increased risk of progression of CKD (n = 137) | p value |
|---|---|---|---|
| FIB-4 score, median (IQR) | 1.05 (0.77, 1.39) | 1.13 (0.80, 1.65) | 0.007 |
| FIB-4 ≥1.3, No. (%) | 453 (30.4) | 61 (44.5) | 0.001 |
| NFS, median (IQR) | −2.62 (−3.34, −1.88) | −2.04 (−2.97, −1.18) | <0.001 |
| NFS ≥−1.455, No. (%) | 221 (14.8) | 42 (30.7) | <0.001 |
| Echo | |||
| No fatty liver, No. (%) | 509 (34.1) | 26 (19.0) | <0.001 |
| Mild fatty liver, No. (%) | 510 (34.2) | 45 (32.8) | |
| Moderate fatty liver, No. (%) | 390 (26.2) | 47 (34.3) | |
| Severe fatty liver, No. (%) | 82 (5.5) | 19 (13.9) | |
| Age, median (IQR) | 56 (48, 63) | 60 (50, 69) | <0.001 |
| Age ≥65 years, No. (%) | 279 (18.7) | 50 (36.5) | <0.001 |
| Sex, Female, No. (%) | 470 (31.5) | 43 (31.4) | 1 |
| BMI, kg/m2, median (IQR) | 24.16 (21.98, 26.61) | 25.83 (23.50, 28.62) | <0.001 |
| BMI ≥27 kg/m2, No. (%) | 335 (22.5) | 51 (37.2) | <0.001 |
| Waist, cm, median (IQR) | 79 (73, 86) | 84.5 (77, 91) | <0.001 |
| Systolic blood pressure, mmHg, median (IQR) | 128 (115, 141) | 140 (126, 152) | <0.001 |
| Diastolic blood pressure, mmHg, median (IQR) | 77 (69, 85) | 83 (74, 91) | <0.001 |
| HOMA-IR | 1.48 (0.99, 2.30) | 1.93 (1.21, 3.49) | <0.001 |
| Fasting glucose, mg/dl, median (IQR) | 95 (90, 103) | 98 (91, 109) | 0.002 |
| Insulin, mU/L, median (IQR) | 6.10 (4.30, 9.07) | 7.39 (5.04, 12.09) | <0.001 |
| HbA1C %, median (IQR) | 5.6 (5.4, 5.9) | 5.7 (5.4, 6.1) | 0.027 |
| Triglyceride, mg/dl, median (IQR) | 96 (67, 143) | 117 (79, 170) | 0.001 |
| HDL, mg/dl, median (IQR) | 56 (46.85, 66.45) | 52.10 (43.9, 63.2) | 0.016 |
| LDL, mg/dl, median (IQR) | 125.30 (105.7, 148.6) | 120.1 (96.8, 141.9) | 0.016 |
| Total cholesterol, mg/dl, median (IQR) | 209 (185, 234) | 212 (184, 233) | 0.674 |
| Metabolic syndrome, No. (%) | 262 (17.6) | 47 (34.3) | <0.001 |
| AST, U/L, median (IQR) | 22 (18, 25) | 22 (19, 27) | 0.079 |
| ALT, U/L, median (IQR) | 20 (16.0, 26.5) | 22 (18, 27) | 0.024 |
| ALK-P, U/L, median (IQR) | 63 (53, 76) | 66 (56, 78) | 0.23 |
| GGT, pg/mL, median (IQR) | 16 (13, 24) | 19 (14, 32) | <0.001 |
| Uric acid, mg/dl, median (IQR) | 5.2 (4.4, 6.2) | 5.9 (4.9, 7.0) | <0.001 |
Abbreviations: CKD: chronic kidney disease; IQR: interquartile range; FIB-4: score: fibrosis-4 score; NFS: non-alcoholic fatty liver disease fibrosis score; BMI: body mass index; HOMA-IR: homeostatic model assessment of insulin resistance; HbA1C: glycated hemoglobin; HDL: high-density lipoprotein; LDL: low-density lipoprotein; AST: aspartate aminotransferase; ALT: alanine transaminase; ALK-P: alkaline phosphatase; GGT: gamma-glutamyltransferase.
Table 2.
Number of transitions and transition intensities in unadjusted multistate model between states of chronic kidney diseases.
| To state |
|||
|---|---|---|---|
| State 1: Low risk of progression of CKD |
State 2: Moderately increased risk of progression of CKD |
State 3: High/Very high risk of progression of CKD |
|
| n; TI (95% CI); |
n; TI (95% CI) |
n; TI (95% CI) |
|
| From state | |||
| State 1a: Low risk | 3597; −0.059d (−0.068 to −0.051) |
194; 0.059 (0.051–0.068) |
10; 0e |
| State 2b: Moderately increased risk | 142; 0.436 (0.367–0.517) |
203; −0.527d (−0.613 to −0.454) |
35; 0.092c (0.068–0.123) |
| State 3: High/Very high risk | 0 | 0 | 0 |
Abbreviations: TI: transition intensity; CI: confidence interval.
The average length of stay in state 1 before transitioning to state 2 was 17 years (95% CI; 14.7–19.7).
The average length of stay in state 2 before transition either back to state 1 or to state 3 was 1.9 years (95% CI; 1.6–2.2).
The ratio of transition intensity to regression from state 2 to state 1 and progression from state 2 to state 3 (q 2,1/q 2,3) was 4.76 (95% CI; 3.38–6.68).
The diagonals of the transition intensity matrix were the sum of rows with negative signs in the TI matrix.
We make a constraint for State 1 to State 3 transition owing to rare events.
Table 3.
Transition probabilities from individual states at various follow-up times.
| TP (95% CI) | At the1st year | At the 2 nd year | At the 3rd year | At the 4th year | At the 5th year |
|---|---|---|---|---|---|
| All cohorts | |||||
| State 1 to State 2 | 0.044 (0.039–0.050) | 0.069 (0.060–0.078) | 0.082 (0.071–0.094) | 0.089 (0.077–0.103) | 0.093 (0.081–0.106) |
| State 1 to State 3 | 0.002 (0.002–0.003) | 0.008 (0.005–0.010) | 0.015 (0.011–0.020) | 0.022 (0.017–0.030) | 0.031 (0.023–0.041) |
| State 2 to State 1 | 0.329 (0.283–0.376) | 0.511 (0.458–0.564) | 0.611 (0.553–0.664) | 0.663 (0.605–0.716) | 0.690 (0.637–0.740) |
| State 2 to State 3 | 0.072 (0.054–0.094) | 0.115 (0.087–0.152) | 0.143 (0.106–0.188) | 0.162 (0.124–0.212) | 0.176 (0.134–0.227) |
| Diabetes cohort | |||||
| State 1 to State 2 | 0.100 (0.075–0.133) | 0.158 (0.117–0.207) | 0.190 (0.141–0.247) | 0.207 (0.152–0.270) | 0.215 (0.157–0.283) |
| State 1 to State 3 | 0.006 (0.003–0.011) | 0.021 (0.012–0.037) | 0.041 (0.023–0.071) | 0.064 (0.036–0.109) | 0.088 (0.050–0.147) |
| State 2 to State 1 | 0.222 (0.154–0.318) | 0.350 (0.249–0.473) | 0.421 (0.306–0.553) | 0.458 (0.339–0.588) | 0.475 (0.353–0.603) |
| State 2 to State 3 | 0.095 (0.055–0.157) | 0.161 (0.095–0.260) | 0.209 (0.126–0.332) | 0.247 (0.148–0.383) | 0.278 (0.168–0.424) |
| Non-diabetes cohort | |||||
| State 1 to State 2 | 0.036 (0.031–0.042) | 0.056 (0.048–0.065) | 0.067 (0.057–0.078) | 0.073 (0.061–0.086) | 0.076 (0.064–0.090) |
| State 1 to State 3 | 0.001 (0.001–0.002) | 0.005 (0.003–0.007) | 0.009 (0.006–0.014) | 0.014 (0.010–0.021) | 0.020 (0.013–0.029) |
| State 2 to State 1 | 0.349 (0.300–0.407) | 0.543 (0.481–0.612) | 0.651 (0.586–0.715) | 0.710 (0.647–0.769) | 0.740 (0.679–0.794) |
| State 2 to State 3 | 0.056 (0.038–0.082) | 0.090 (0.062–0.130) | 0.112 (0.077–0.159) | 0.126 (0.086–0.180) | 0.136 (0.093–0.194) |
State 1 = Low risk of progression of CKD; State 2 = Moderately increased risk of progression of CKD; State 3 = High/Very High risk of progression of CKD. TP: transition probability; CKD: chronic kidney disease.
Univariable multi-state Markov models
In the univariable analysis, the FIB-4 score (≥1.3 vs. <1.3; HR, 1.64 [95% CI, 1.22–2.20]), NFS (≥−1.455 vs. <−1.455; HR, 1.71 [95% CI, 1.23–2.38]), severity of the echogenicity of the fatty liver (moderate vs. none/mild fatty liver; HR, 1.54 [95% CI, 1.12–2.11]; severe vs. none/mild fatty liver; HR, 2.52 [95% CI, 1.51–4.19]), age (≥65 yeas vs. < 65 years; HR, 1.99 [95% CI, 1.47–2.69]), uric acid (UA) (every mg/dL increase; HR, 1.16 [95% CI, 1.05–1.29], obesity (BMI ≥27 vs. BMI <27; HR, 1.54 [95% CI, 1.13–2.11]), MetS (yes vs. no; HR, 2.30 [95% CI, 1.68–3.15]), glycated hemoglobin (HbA1C) (≥7% vs. <7%; HR, 2.85 [95% CI, 1.71–4.75]), hypertension (yes vs. no; HR, 1.44 [95% CI, 1.05–1.96]), and HOMA-IR (every point increase; HR, 1.11 [95% CI, 1.06–1.16]) were significantly associated with a transition from state 1 to state 2. Moreover, the FIB-4 score (≥1.3 vs. <1.3; HR, 0.70 [95% CI, 0.50–0.99]), NFS (≥−1.455 vs. <−1.455; HR, 0.44 [95% CI, 0.29–0.67]), age (≥65 years vs. < 65 years; HR, 0.54 [95% CI, 0.37–0.78]), UA (every mg/dL increase; HR, 0.83 [95% CI, 0.74–0.94], and hypertension (yes vs. no; HR, 0.48 [95% CI, 0.34–0.69]) were significantly associated with a reduced risk of a transition from state 2 to state 1. Additionally, NFS (≥−1.455 vs. <−1.455; HR, 1.87 [95% CI, 1.04–3.38]) and age (≥65 yeas vs. < 65 years; HR, 2.77 [95% CI, 1.48–5.17]) were statistically significantly associated with a transition from state 2 to state 3.
Multivariable multi-state Markov models
The TIs in the adjusted models using FIB-4 score, NFS, and severity of fatty liver by echogenicity for NAFLD in two adjustment sets were similar to those in the adjusted model [Supplementary Table 4]. In adjustment set 1 and adjustment 2, higher FIB-4 scores were significantly associated with the transition from state 1 to state 2, while higher NFS was significantly associated with a reduced risk of transition from state 2 to state 1 [Table 4; Supplementary Tables 5–10]. The severity of NAFLD by echogenicity was significantly associated with the transition from state 1 to state 2 only in the adjustment set 1. In addition to FIB-4 and severity of fatty liver by echogenicity, older age, higher HbA1C, MetS, hyperuricemia, and higher HOMA-IR were significantly associated with the transition from state 1 to state 2. Excluding NFS, older age, hypertension, and hyperuricemia were significantly associated with a reduced risk of transition from state 2 to state 1. Older age was significantly associated with the transition from state 2 to state 3 in all models. The Pearson type goodness-of-fit test revealed no significant results for model fit among six models [Supplementary Table 11]. Results of model comparisons also preferred homogeneous continuous-time Markov models [Supplementary Table 11].
Table 4.
Hazard ratios from the adjusted multistate Markov model.
| Adjustment set 1 |
Adjustment set 2 |
|||
|---|---|---|---|---|
| State 1 to State 2 | State 2 to State 1 | State 1 to State 2 | State 2 to State 1 | |
| FIB-4 (≥1.3 vs. <1.3) | 1.42 (1.02, 2.00) | 0.93 (0.61, 1.43) | 1.44 (1.03, 2.01) | 0.94 (0.62, 1.43) |
| NFS (≥−1.455 vs. <−1.455) | 1.07 (0.72, 1.57) | 0.56 (0.34, 0.91) | 1.02 (0.69, 1.50) | 0.47 (0.29, 0.78) |
| Echo | ||||
| None/mild fatty liver | Reference | Reference | Reference | Reference |
| Moderate fatty liver | 1.12 (0.78, 1.60) | 1.16 (0.77, 1.77) | 1.12 (0.79, 1.60) | 0.95 (0.63, 1.43) |
| Severe fatty liver | 1.80 (1.00, 3.22) | 1.61 (0.83, 3.12) | 1.72 (0.98, 3.00) | 1.15 (0.61, 2.15) |
Data are shown as hazards ratio (95% confidence interval).
The bold text indicates statistical significance.
Adjustment set 1: Age, sex, hypertension, metabolic syndrome, obesity, uric acid, and glycated hemoglobin.
Adjustment set 2: Age, sex, dyslipidemia, metabolic syndrome, obesity, insulin resistance, and glycated hemoglobin.
FIB-4: fibrosis-4 score; NFS: non-alcoholic fatty liver disease fibrosis score.
Discussion
There are a few previously published studies investigating the association of NAFLD and incident CKD, and most of these studies have reported time-to-event analyses that do not explore the consequence of incident CKD [5,6]. Whether or not NAFLD has a causal effect on CKD is unknown as well [4]. Using epidemiological tools and the multi-state model, we created a framework to determine the effects of the severity of NAFLD on the risk of CKD development and progression as well as disease dynamics for patients with NAFLD, CKD and found that NAFLD and advanced liver fibrosis may be associated with incident CKD and reduced CKD recovery.
Although KDIGO defines CKD as elevated ACR or decreased eGFR persisting for at least three months, most reports on this topic are based on an elevated ACR or decreased eGFR obtained at one time point, which does not meet the KDIGO definitions of chronicity [7,12]. Such definitions in these studies compromise the analyses of the consequences of disease burden. In addition, some studies included hospital-based cohorts that may have had acute kidney injuries. In contrast, our community-based cohort study includes healthy volunteers who were followed up annually for at least two years. Moreover, we routinely referred patients with moderate to advanced CKD (>G3a or > A2 stage) to the nephrology department. Education regarding lifestyle changes, diet, and reduction of metabolic risk factors was also provided. Therefore, the present study was designed to reflect the real–world association of NAFLD and CKD as well as the disease dynamics in a community-based cohort.
The well-known risk factors for CKD are age, hypertension, MetS, obesity, hyperuricemia, and diabetes. However, age, hypertension, hyperuricemia, and advanced liver fibrosis were found to be risk factors for a reduced risk of transition from state 2 to state 1 in the multivariable analyses. These results are consistent with evidence-based guidelines that advocate intensive control of metabolic risk factors including hypertension, hyperuricemia, and diabetes to avoid end organ damage [7,12]. In addition, a recent cohort study indicates that portal inflammation predicts renal dysfunction in patients with NAFLD. Thus, the treatment of NAFLD to avoid advanced fibrosis or cirrhosis is also important to prevent from CKD [1]. Futher, experts have suggested that the putative mechanisms linking NAFLD with CKD include type 2 diabetes, MetS, dysbiosis, platelet activation, ageing, adipokines, and genetic effects. Further investigations on the pathophysiology of NAFLD related CKD (including the alteration of renal blood flow, such as hepato-renal syndrome or through renal fibrosis due to liver fibrosis) are strongly recommended.
Our findings suggest NAFLD related CKD is reversible before advancing to advanced CKD stages. The underlying mechanisms might be modifications of metabolic risk factors and health educations on lifestyle changes in our community-based cohort. Similar findings are also noted in the literature [26]. Recently, NAFLD is suggested to be replaced as metabolic (dysfunction) associated fatty liver disease (MAFLD) [27]. MAFLD is diagnosed in patients when they have both hepatic steatosis and any one of the following three metabolic conditions: overweight/obesity, diabetes mellitus, or metabolic dysregulations in lean individuals. This novel concept and criteria may enable clinicians to identify more patients at risk of adverse outcomes in clinical practice. In addition, clinicians may prevent patients from progressing to adverse outcomes by modifying these risk factors.
Although hyperuricemia is a well-known risk factor for CKD, its pathophysiology is poorly understood. Recently, the temporal relationship between hyperuricemia and insulin resistance and its impact on the risk of hypertension were reported in a large longitudinal study [28,29]. In another study of two longitudinal cohorts, the temporal relationship between hyperuricemia and obesity and its association with the risk of type 2 diabetes were also reported [30]. Moreover, a recent review suggested that hyperuricemia plays important roles in cardiac-kidney-vascular system diseases and MetS [31]. In summary, advanced pathological mechanisms for injuries induced by hyperuricemia may lead to hypertension, atrial fibrillation, insulin resistance, obesity, hyperlipidemia, and NAFLD through different mechanistic pathways [31]. Such findings in previous reports were consistent with our DAGs.
Our study used stringent methodologies including the multi-state Markov model to investigate CKD disease dynamics [20,24,32,33]. However, this study is not without limitations. First, we did not perform a liver biopsy to diagnosis NAFLD, severity of NAFLD, or advanced fibrosis. Instead, we used validated non-invasive biomarkers and abdominal ultrasonography for large-scale screening [17,[34], [35], [36], [37], [38]]. Most published studies have also used these methods; therefore, our results are comparable to previously published studies. Second, a renal biopsy was not performed to diagnosis the etiology of CKD, and therefore, the cause of CKD could not be identified as NAFLD or other metabolic factors such as hypertension, hyperuricemia, or diabetes. Third, age is a common risk factor for NAFLD and CKD. Further, age was used to calculate the FIB-4, NFS, and eGFR. Therefore, if theassociation of NAFLD and CKD is driven by age, this could not be identified. Fourth, owing to the small number of patients in State 3, we did not conduct a multivariable analysis for the patients transited from State 2 to State 3. So that is another limitation of this study. Finally, we did not include medication information or the use of traditional Chinese medicine. Therefore, we could not exclude drug-induced CKD.
Conclusions
In summary, the severity of NAFLD is associated with CKD, and CKD may be reversible in its early stages. Controlling metabolic risk factors and preventing advanced liver fibrosis are strongly recommended to prevent the progression of CKD. Further studies to explore the mechanisms linking NAFLD and CKD and the pathophysiology of CKD are necessary.
Funding
This research is supported by the Chang Gung Memorial Hospital Research Projects CMRPG2H0342, CRRPG2H0064, CRRPG2F0012, and CLRPG2L0052.
Compliance with ethical standards
All contents have compliance with ethical standards.
Ethical approval
This study was approved by the Institutional Review Board of Chang Gung Foundation (201600379B0, 201800270B0) and was carried out in accordance with the guidelines of the 2013 Declaration of Helsinki. All study participants provided informed consent prior to recruitment into this cohort study.
Conflicts of interest
All authors declare that they do not have any relevant conflict of interest or other financial disclosures.
Acknowledgements
We want to thank Ms. Shin-Ying Lin to help the English editing of this manuscript.
Footnotes
Peer review under responsibility of Chang Gung University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bj.2022.04.003.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Sheka A.C., Adeyi O., Thompson J., Hameed B., Crawford P.A., Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175–1183. doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 2.Paik J.M., Golabi P., Younossi Y., Mishra A., Younossi Z.M. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology. 2020;72:1605–1616. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 3.Estes C., Chan H.L.Y., Chien R.N., Chuang W.L., Fung J., Goh G.B., et al. Modelling NAFLD disease burden in four Asian regions-2019-2030. Aliment Pharmacol Ther. 2020;51(8):801–811. doi: 10.1111/apt.15673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne C.D., Targher G. NAFLD as a driver of chronic kidney disease. J Hepatol. 2020;72:785–801. doi: 10.1016/j.jhep.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Sinn D.H., Kang D., Jang H.R., Gu S., Cho S.J., Paik S.W., et al. Development of chronic kidney disease in patients with non-alcoholic fatty liver disease: a cohort study. J Hepatol. 2017;67(6):1274–1280. doi: 10.1016/j.jhep.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A., Zaza G., Byrne C.D., Lonardo A., Zoppini G., Bonora E., et al. Nonalcoholic fatty liver disease increases risk of incident chronic kidney disease: a systematic review and meta-analysis. Metabolism. 2018;79:64–76. doi: 10.1016/j.metabol.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 7.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younossi Z.M., Tampi R., Priyadarshini M., Nader F., Younossi I.M., Racila A. Burden of illness and economic model for patients with nonalcoholic steatohepatitis in the United States. Hepatology. 2019;69(2):564–572. doi: 10.1002/hep.30254. [DOI] [PubMed] [Google Scholar]
- 9.Chen L.W., Huang P.R., Chien C.H., Lin C.L., Chien R.N. A community-based study on the application of fatty liver index in screening subjects with nonalcoholic fatty liver disease. J Formos Med Assoc. 2020;119(1 Pt 1):173–181. doi: 10.1016/j.jfma.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Fedchuk L., Nascimbeni F., Pais R., Charlotte F., Housset C., Ratziu V., et al. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40(10):1209–1222. doi: 10.1111/apt.12963. [DOI] [PubMed] [Google Scholar]
- 11.Bedogni G., Bellentani S., Miglioli L., Masutti F., Passalacqua M., Castiglione A., et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin A., Stevens P.E. Summary of KDIGO 2012 CKD Guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int. 2014;85(1):49–61. doi: 10.1038/ki.2013.444. [DOI] [PubMed] [Google Scholar]
- 13.Andrassy K.M. Comments on KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;84:622–623. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 14.Chu N.F. Prevalence of obesity in Taiwan. Obes Rev. 2005;6(4):271–274. doi: 10.1111/j.1467-789X.2005.00175.x. [DOI] [PubMed] [Google Scholar]
- 15.Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 16.Levy J.C., Matthews D.R., Hermans M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 17.Angulo P., Hui J.M., Marchesini G., Bugianesi E., George J., Farrell G.C., et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45(4):846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 18.Shah A.G., Lydecker A., Murray K., Tetri B.N., Contos M.J., Sanyal A.J., et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey A.S., Stevens L.A. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–627. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson C. Multi-state models for Panel data: the msm package for R. 2011. J Stat Softw. 2011;38:1–29. [Google Scholar]
- 21.Sweeting M.J., Farewell V.T., De Angelis D. Multi-state Markov models for disease progression in the presence of informative examination times: an application to hepatitis C. Stat Med. 2010;29(11):1161–1174. doi: 10.1002/sim.3812. [DOI] [PubMed] [Google Scholar]
- 22.Marshall G., Jones R.H. Multi-state models and diabetic retinopathy. Stat Med. 1995;14(18):1975–1983. doi: 10.1002/sim.4780141804. [DOI] [PubMed] [Google Scholar]
- 23.Hernán M.A., Robins J.M. 1st ed. Chapman & Hall/CRC; Boca Raton: 2020. Causal inference: what if. [Google Scholar]
- 24.Textor J., Hardt J., Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22:745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 25.Pearl J. An introduction to causal inference. Int J Biostat. 2010;6(5) doi: 10.2202/1557-4679.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilar-Gomez E., Calzadilla-Bertot L., Friedman S.L., Gra-Oramas B., Gonzalez-Fabian L., Villa-Jimenez O., et al. Improvement in liver histology due to lifestyle modification is independently associated with improved kidney function in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2017;45(2):332–344. doi: 10.1111/apt.13860. [DOI] [PubMed] [Google Scholar]
- 27.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 28.Han T., Lan L., Qu R., Xu Q., Jiang R., Na L., et al. Temporal relationship between hyperuricemia and insulin resistance and its impact on future risk of hypertension. Hypertension. 2017;70(4):703–711. doi: 10.1161/HYPERTENSIONAHA.117.09508. [DOI] [PubMed] [Google Scholar]
- 29.Dawson J., Wyss A. Chicken or the egg? Hyperuricemia, insulin resistance, and hypertension. Hypertension. 2017;70(4):698–699. doi: 10.1161/HYPERTENSIONAHA.117.09685. [DOI] [PubMed] [Google Scholar]
- 30.Han T., Meng X., Shan R., Zi T., Li Y., Ma H., et al. Temporal relationship between hyperuricemia and obesity, and its association with future risk of type 2 diabetes. Int J Obes(Lond) 2018;42(7):1336–1344. doi: 10.1038/s41366-018-0074-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang H., Zhang H., Sun L., Guo W. Roles of hyperuricemia in metabolic syndrome and cardiac-kidney-vascular system diseases. Am J Transl Res. 2018;10(9):2749–2763. [PMC free article] [PubMed] [Google Scholar]
- 32.Textor J., van der Zander B., Gilthorpe M.S., Liśkiewicz M., Ellison G.T. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol. 2016;45(6):1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 33.Boucquemont J., Heinze G., Jager K.J., Oberbauer R., Leffondre K. Regression methods for investigating risk factors of chronic kidney disease outcomes: the state of the art. BMC Nephrol. 2014;15:45. doi: 10.1186/1471-2369-15-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallet-Pichard A., Mallet V., Pol S. FIB-4: a simple, inexpensive and accurate marker of fibrosis in HCV-infected patients. Hepatology. 2006;44(3):769. doi: 10.1002/hep.21334. author reply 769-70. [DOI] [PubMed] [Google Scholar]
- 35.Huh J.H., Kim J.Y., Choi E., Kim J.S., Chang Y., Sung K.C. The fatty liver index as a predictor of incident chronic kidney disease in a 10-year prospective cohort study. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0180951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilar-Gomez E., Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68(2):305–315. doi: 10.1016/j.jhep.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 37.European Association for the Study of the Liver(EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 38.McPherson S., Stewart S.F., Henderson E., Burt A.D., Day C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.