Abstract
The cerebellar cortex is an important system for relating neural circuits and learning. Its promise has reflected the longstanding idea that it contains simple, repeated circuit modules with only a few cell types and a single plasticity mechanism that mediates learning according to classical Marr-Albus models. However, emerging data have revealed surprising diversity in neuron types, synaptic connections, and plasticity mechanisms, both locally and regionally within the cerebellar cortex. In light of these findings, it is not surprising that attempts to generate a holistic model of cerebellar learning across different behaviors have not been successful. While the cerebellum remains an ideal system for linking neuronal function with behavior, it is necessary to update the cerebellar circuit framework to achieve its great promise. In this review, we will highlight recent advances in our understanding of cerebellar-cortical cell types, synaptic connections, signaling mechanisms, and forms of plasticity that enrich cerebellar processing.
Keywords: Cerebellar interneurons, Purkinje cell, ephaptic signaling, climbing fiber, cerebellar circuit, motor learning
I. Introduction
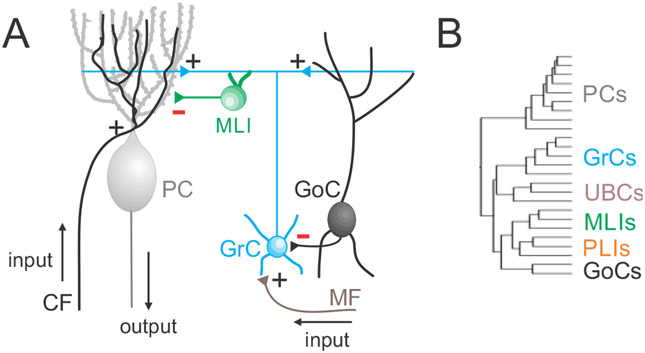
The apparent simplicity and regularity of cerebellar circuit organization has played a key role in establishing it as a model system for linking neuronal activity with behavior and learning. The cerebellar cortex contains a central trisynaptic arc: primary input from mossy fibers (MFs) excites granule cells (GrCs), which in turn excite Purkinje cells (PC) that provide the sole output synapses of the cerebellar cortex (Figure 1A). In addition, local interneurons termed Golgi cells (GoCs) and molecular layer interneurons (MLIs) inhibit GrCs and PCs respectively, while climbing fibers (CFs) powerfully excite PCs. This striking ‘crystalline’ cellular architecture is repeated throughout the cerebellar cortex, and has inspired extensive theoretical work to explain how this circuit generates learning.
Figure 1. Basic circuitry and cell types of the cerebellar cortex.
A. Simplified circuit of the cerebellar cortex. B. Dendrogram of the neurons based on RNAseq data reveals additional types and subtypes of neurons in the cerebellar cortex (Kozareva et al., 2021). Abbreviations: climbing fiber (CF), mossy fiber (MF), granule cell (GrC), Golgi cell (GoC), molecular layer interneuron (MLI), Purkinje cell (PC), unipolar brush cells (UBCs), and Purkinje layer interneurons (PLIs).
Cerebellar research has been largely guided by a conceptual framework referred to as the “Marr-Albus model” (Albus, 1971; Ito, 1972; Marr, 1969). This classical view has evolved over time, but is based on the premise that the cerebellar cortex utilizes a small number of cell types organized into repeated modules in order to 1) separate incoming sensorimotor information provided by MFs into unique GrC firing patterns, and 2) selectively modify GrC to PC connections to alter motor output. According to Marr-Albus models, the first goal is achieved in the GrC layer. Here, MFs from diverse sources synapse onto a much larger pool of GrCs (Raymond and Medina, 2018), and random input mixing and strong inhibition establishes sparse, decorrelated GrC activity patterns. Learning requires that GrCs uniquely represent each sensorimotor context carried by MFs. For example, in cerebellar-dependent associative learning tasks such as eyelid conditioning, GrCs carry information about the conditioned stimulus that predicts a noxious corneal airpuff (Steinmetz et al., 1989). GrCs must encode the conditioned stimulus with a unique population response to distinguish it from other stimuli. In the Marr-Albus model, sparse coding, in which very few GrCs (<5%) respond to any given stimulus, reduces overlap and aids in pattern separation. To achieve the second goal, hundreds of thousands of GrCs converge onto each PC. In turn, PCs generate predictive motor outputs that are refined when climbing fibers (CFs) instruct long-term synaptic depression (LTD) of GrC-to-PC synapses in response to motor errors.
It is increasingly clear, however, that the cerebellar cortex is far more complex than the simple framework envisioned by Marr-Albus models. Recent work has revealed considerable diversity in cell types (Figure 1B), regional circuit specializations, plasticity mechanisms and other physiological processes. At the same time, behaviors associated with cerebellar processing have expanded widely across both motor and nonmotor domains, challenging the idea of a homogenous cerebellar circuit function. The goal of this review is to highlight advances in our understanding of the cerebellar cortex, with a particular focus on new cell types and connections, to provide a more comprehensive framework for evaluating cerebellar computation.
Emerging Principles:
The cerebellar cortex has far greater molecular, anatomical and functional diversity within ‘cell types’ than was previously appreciated.
New connections and regional specializations have been identified that alter the previously accepted cerebellar circuit diagram.
New synaptic and intrinsic plasticity mechanisms have been identified that could support learning.
New roles have been identified for previously recognized cell types and synapses
New modulatory mechanisms and population dynamics have been revealed that could flexibly alter behavior and learning
II. Granule Cell Layer
The granule cell layer utilizes multiple mechanisms to process incoming MF input. Current evidence suggests that this processing can generate GrC activity that deviates from the sparse levels predicted by Marr-Albus models (Giovannucci et al., 2017; Knogler et al., 2017; Lanore et al., 2021; Ozden et al., 2012; Sylvester et al., 2017) (Figure 2A), and has suggested new roles for GrC layer processing (Wagner and Luo, 2020). Here we will review what is known about the GrC layer, and highlight recent advances that update classical views of its role in cerebellar function.
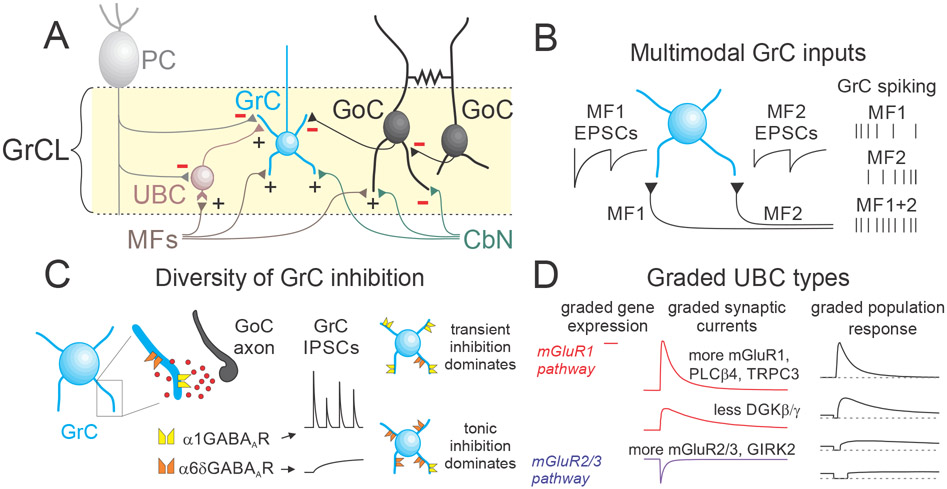
Figure 2. Circuit specializations of the input layer of the cerebellar cortex.
A. There are additional cell types and connections in the GrC layer that are neglected in simplified models of the cerebellar cortex: inhibitory feedback from the CbN, UBCs, gap junction coupling between GoCs, and PC feedback to GrCs and UBCs. B. MFs have disparate properties that evoke spiking in GrCs with diverse temporal properties (Chabrol et al., 2015). In this example MF1 has a high initial probability of release and depresses, whereas MF2 has a low initial probability of release and facilitates. GrC spiking evoked by activation of MF1 alone, MF2 alone and coactivation of MF1 and MF2. C. Specialized GABAA receptors in GrCs mediate inhibition with a conventional fast component (α1 subunit containing). and a slow tonic component (α6δ subunit containing). D. Cerebellar UBCs express elements of the mGluR1 excitatory pathway and the mGluR2 inhibitory pathway in inverse gradients to generate a continuum of temporal responses.
A. Mossy Fibers
Mossy fibers are the major excitatory inputs to the cerebellar cortex. They are predominantly glutamatergic, but can also release other neurotransmitters such as acetylcholine (Fore et al., 2020). Numerous regions with different functional roles provide MF inputs (Sillitoe et al., 2012): the pontine nucleus and the tegmental pontine reticular nucleus are involved in vision, planning and executing movement; the spinal cord and cuneate provide proprioceptive signals; the vestibular nucleus and primary vestibular inputs are involved in balance, posture and the control of eye movement; the lateral reticular nucleus and red nucleus are involved in coordinating movement, and the cerebellar nuclei (CbN, also sometimes referred to as deep cerebellar nuclei, or DCN) that can provide feedback corollary discharge signals to enhance associative learning (Gao et al., 2016; Houck and Person, 2014). Remarkably, these diverse sources all give rise to MFs within the granular layer that have the same basic ultrastructure (Billings et al., 2014; Eccles et al., 1967). MF boutons are large, contain multiple active zones, and make synapses with many tens of GrCs. Each MF bouton and its GrC dendritic targets are ensheathed by glia within specialized structures known as glomeruli. MFs also activate GoCs, UBCs, and candelabrum cells (Kanichay and Silver, 2008; Mugnaini et al., 2011; Osorno et al., 2021).
The glomerular organization confers specialized signaling properties. Within a glomerulus, neurotransmitters can pool, accumulate and persist to activate receptors on many cells. For example, single vesicle fusion at a MF active zone causes rapid stochastic excitation of target GrCs, and the collective release of many vesicles leads to glutamate pooling and reliable excitation of multiple GrCs (DiGregorio et al., 2002). Neurotransmitter pooling also allows MFs to suppress release from GoCs, and GoCs to inhibit release from MFs (Mitchell and Silver, 2000a, b), thereby allowing transmitters from different sources to provide both positive and negative feedback to GrCs.
MFs are specialized to favor fast and reliable excitatory synaptic transmission that may contribute to the dense GrC responses that have recently been observed in vivo. MFs have ultrafast action potentials, tight coupling of calcium channels to vesicle release, rapid exocytosis, an extremely large readily-releasable vesicle pool, rapid endocytosis and vesicle replenishment to sustain transmission, and fast AMPA receptors that recover rapidly from desensitization (Delvendahl and Hallermann, 2016; Hallermann et al., 2010; Ritzau-Jost et al., 2014; Saviane and Silver, 2006; Xu-Friedman and Regehr, 2003). Single MF action potentials can be translated to postsynaptic spikes when GrC inhibition is low (Chadderton et al., 2004). However, this powerful transmission is counteracted by synaptic inhibition onto GrCs that can impose a requirement for either multiple MFs (Jorntell and Ekerot, 2006) or bursting in single MFs (Rancz et al., 2007) to drive GrC spiking.
MFs originating from different sources can synapse onto a single GrC (Huang et al., 2013). Different MF pathways vary in their initial synaptic strength and short-term dynamics, which range from strong depression to pronounced facilitation. Consequently, the same presynaptic firing pattern in different types of MFs evokes GrC responses with distinct temporal dynamics, allowing decorrelation in the time domain (Chabrol et al., 2015) ( Figure 2B). The logic of MF input mixing is only beginning to be elucidated (Shuster et al., 2021), and is a key challenge in our understanding of GrC layer computation. Together, input mixing and input diversity can provide a rich repertoire of signal transformations that decorrelates incoming inputs and enhances pattern separation.
B. Granule cells
GrCs have a small soma with an average of four short dendrites (Eccles et al., 1967). They have a high input resistance, making them sensitive to small changes in synaptic conductance. While classical models assume that GrCs are homogenous, recent studies suggest that there are three molecularly separable GrC subtypes located preferentially in different cerebellar regions (Kozareva et al., 2021). In addition, GrCs within a given region are not uniform (Straub et al., 2020). Thus, while their functional differences are not known, it seems likely that GrCs are specialized to differentially contribute to processing across regions, and their local differences may help decorrelate MF inputs.
Transgenic animals have provided a means to assess the contribution of the GrC layer to cerebellar-dependent behaviors. For example, suppressing the output of the GrC layer by eliminating P-type calcium channels in most GrCs impairs motor learning without affecting overall motor performance (Galliano et al., 2013). However, manipulations with temporal and regional specificity will be necessary for more detailed analysis of granule cell layer computation.
C. Golgi Cells
GoCs have profuse axons within the GrC layer that can span several millimeters to inhibit thousands of GrCs by releasing GABA, and in some cases glycine. GoCs inhibit GrCs via two distinct types of GABAA receptors: low affinity α1 subunit-containing receptors that mediate inhibitory postsynaptic currents (IPSCs) lasting several milliseconds, and high-affinity, non-desensitizing α6δ subunit-containing GABAA receptors that mediate a very slow tonic current on a timescale of seconds (Brickley et al., 1996; Rossi and Hamann, 1998)(Figure 2C). This inhibition can elevate the threshold for GrC spiking in response to MF input (Brickley et al., 1996; Chadderton et al., 2004).
GoCs fire spontaneously at around 5Hz and continuously release GABA to tonically inhibit GrCs. GoCs provide highly variable phasic and tonic inhibition to different GrCs (Crowley et al., 2009) (Figure 2C). Such diversity may arise from differences in the number of GoC inputs per glomerulus (Jakab and Hamori, 1988), the number of dendrites per GrC (Palay and Chan-Palay, 1974), the diversity of GoC subtypes (Geurts et al., 2001; Kozareva et al., 2021; Neki et al., 1996; Simat et al., 2007), and differences in GABA receptor expression (Wall, 2002). In addition, the magnitude of GrC tonic inhibition is regulated by bidirectional neuromodulation of GoC firing rates (Fleming and Hull, 2019; Fore et al., 2020), dynamic regulation of GoC firing via intrinsic plasticity (Hull et al., 2013), and by altering the sensitivity of δ-containing GABAA receptors (Rudolph et al., 2020). These many ways of regulating tonic GrC inhibition provide a means of increasing processing flexibility within the GrC input layer.
GoCs are excited by MFs to evoke feedforward inhibition, and by GrCs to evoke feedback inhibition. The role of GoC feedforward inhibition is currently unclear, as it is weaker and is more temporally variable than other feedforward circuits (Duguid et al., 2015; Kanichay and Silver, 2008; Pouille and Scanziani, 2001). GoCs are also excited by many GrCs, which likely contributes to their broad stimulus tuning, and may generate feedback inhibition that is proportional to the average ongoing GrC population activity. In other systems, such broadly tuned feedback inhibition can enhance dynamic range and sharpen tuning, but it is not known if this is the case for GrCs, where tuning can be remarkably precise and may be determined primarily by subthreshold MF input (Chen et al., 2017).
There are several intriguing aspects of GoC signaling that should be considered in evaluating their role in cerebellar processing. First, GoCs are gap-junction coupled to each other, primarily on dendrites in the molecular layer, allowing them to share synaptic input from the parallel fibers (Vervaeke et al., 2012). Second, GoC cells inhibit other GoCs (Hull and Regehr, 2012), which may enrich the diversity of GoC responses and spiking in their target GrCs. Third, there are two different subtypes of GoCs based on the differential gene expression, including Gjd2 that encodes connexin 36 (Kozareva et al., 2021). And finally, in a departure from feedforward models of cerebellar processing, both glutamatergic and glycinergic/GABAergic neurons of the CbN project back to GoCs (Ankri et al., 2015; Batini et al., 1989; Houck and Person, 2015). The inhibitory projections extensively innervate a subpopulation of exclusively GABAergic GoCs with distinctive firing properties (Ankri et al., 2015). This raises the possibility that inhibitory CbN feedback could ultimately decrease GrC inhibition, and promote the flow of signals within targeted regions of the cerebellar cortex.
We are only beginning to understand how GoCs contribute to cerebellar processing. Tonic inhibition can increase the signal-to-noise of sensory responses by reducing spontaneous GrC spiking (Duguid et al., 2012), and regulate the gain of GrC spiking to extend the dynamic range over which GrCs can read out MF input (Mitchell and Silver, 2003). This may help GrCs linearly encode vestibular inputs over a wide range (Arenz et al., 2008). There are also many poorly understood aspects of GoC signaling, including the behavioral importance of GoC inhibition. It has long been hypothesized that inhibition from GoCs is necessary for pattern separation, and hence specificity of cerebellar associative learning, but this has yet to be tested. Further, selective deletion of the δ-subunit of GABAA receptors in GrCs strongly attenuates tonic inhibition and increases GrC excitability, but surprisingly does not impair motor performance or motor learning, instead only influencing nonmotor behaviors (Rudolph et al., 2020). This suggests that the cerebellum may utilize extensive compensatory mechanisms to overcome chronic reductions of inhibitory tone, and that restricting GrC layer excitability is an essential feature of cerebellar processing.
D. Unipolar Brush Cells
Unipolar brush cells (UBCs) are excitatory interneurons in the GrC layer that can transform brief MF inputs into long-lasting changes in firing, and are important for temporal processing (Mugnaini et al., 2011). UBCs are typically excited by a single MF or by another UBC. In turn, they excite several hundred GrCs, and other UBCs, enabling GrC layer responses that greatly outlast MF inputs (Mugnaini et al., 2011).
UBCs have traditionally been divided into ON and OFF subtypes based on their response to MF inputs, but there is growing evidence that UBCs have a continuum of properties (Figure 2D). In traditional OFF-UBCs, a MF burst suppresses firing for hundreds of milliseconds by activating inhibitory group II metabotropic glutamate receptors (mGluR2/3) coupled to inwardly rectifying potassium channels (Borges-Merjane and Trussell, 2015; Knoflach and Kemp, 1998; Russo et al., 2008). In traditional ON-UBCs, MF activation increases firing primarily due to a prolonged glutamate signal and long-lasting AMPA receptor activation (Kinney et al., 1997; Rossi et al., 1995; van Dorp and De Zeeuw, 2014; Zampini et al., 2016). However, in many UBCs, metabotropic glutamate receptors also dominate excitatory responses (mGluR1 coupled to TRPC3). snRNAseq experiments revealed inversely correlated expression gradients in the mGluR1 and mGluR2/3 signaling pathways in UBCs, and electrophysiological studies found that MF bursts evoke continuously varying responses in different UBCs (Guo et al., 2020; Kozareva et al., 2021). In this way, graded molecular variations across components of metabotropic signaling pathways generates a diverse continuum of cell-intrinsic synaptic responses that is suited for temporal learning over multiple timescales.
There is a high degree of specificity in the MF (Balmer and Trussell, 2019) and inhibitory connections made onto different types of UBCs. Inhibition by both GoCs and PCs shapes UBC responses, and the contributions of different neurotransmitters and receptors are specialized for different types of UBCs (Dugue et al., 2005; Guo et al., 2021; Kim et al., 2012). This complexity and specificity of connections is a enriches the diversity of GrC population responses, and is a departure from the traditional view that global synaptic inhibition regulates the GrC layer.
Most studies of UBCs have focused on vestibular regions, where UBCs transform sinusoidally modulated inputs into responses with different phase shifts and amplitudes (Guo et al., 2020; Zampini et al., 2016). As a result, GrCs fire with diverse phases that are useful for cerebellar learning. mGluR2-mediated outward currents generate phase inversion, and phase delays reflect slow AMPAR recovery from desensitization. Although there are pronounced regional differences in UBC densities, they are present in all regions of the cerebellar cortex. Because a single UBC can influence many GrCs, even a low density of UBCs will likely play an important role in regions outside of the vestibulocerebellum.
III. The Molecular Layer
Following integration and processing in the GrC layer, information is conveyed to the molecular layer via GrC axons. These axons have an ascending branch that then bifurcates to give rise to parallel fibers, so named for their dense parallel arrangement along the mediolateral axis of the cerebellar cortex. Here, GrCs release glutamate via en passant synapses on both their ascending axon and parallel fibers onto the dendrites of PCs, MLIs, GoCs, and at least some types of PC layer interneurons (PLIs) (Figure 3A). In addition, each PC receives a single CF fiber input. Studies of the molecular layer have centered on CF-instructed plasticity, but recent studies suggest additional complexity and processing in this layer.
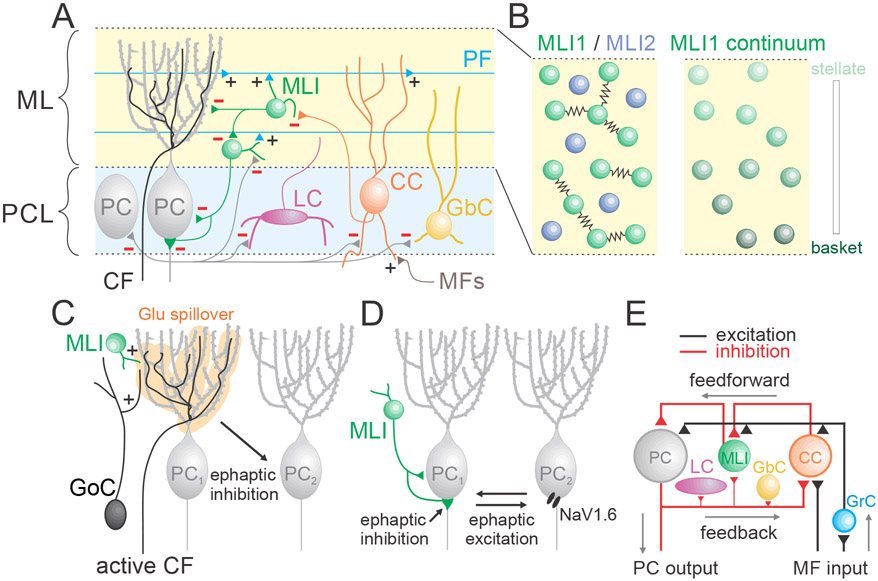
Figure 3. Additional circuit elements and signaling mechanisms in the molecular layer (ML) and the PC layer (PCL).
A. Schematic showing additional cell types and connections between cells that are not considered in the simplified circuit of the cerebellar cortex (Figure 1). Abbreviations for types of PLIs: Lugaro cell (LC), candelabrum cell (CC), and globular cell (GbC). Known inhibitory (−) and excitatory (+) synapses are shown. B. (left) An expanded view of the ML showing intermingled molecularly and functionally distinct subtypes of MLIs. MLI1s are gap junction coupled with each other, but MLI2s are not (right). The MLI1 population displays a molecular and anatomical gradient of properties. C. CFs, in addition to powerfully and directly exciting PCs, produce a glutamate signal that spills over to excite MLIs and GoCs. CFs also generate extracellular ephaptic signals that suppress firing in neighboring PCs. D. Extracellular ephaptic signals allow MLIs to rapidly inhibit and PCs to rapidly excite neighboring signals. E. PLIs are usually disregarded in considering the circuitry of the cerebellar cortex. PCs powerfully inhibit LCs, GbCs and CCs, and CCs primarily inhibit MLIs leading to disinhibition of PCs.
A. Molecular Layer Interneurons
Molecular layer interneurons are the most abundant inhibitory interneurons in the cerebellar cortex. They are spontaneously active (~ 10 Hz), and their dendrites and axons are confined to the parasagittal plane (orthogonal to the parallel fibers). MLIs are directly and powerfully excited by GrC parallel fibers, and are excited by a spillover of glutamate from CF to PC synapses (Jorntell and Ekerot, 2003; Szapiro and Barbour, 2007). MLIs in turn inhibit other MLIs and PCs, and some MLIs are inhibited by PCs (Witter et al., 2016). Interestingly, MLIs do not inhibit GoCs despite their close proximity (Hull and Regehr, 2012).
MLIs have previously been subdivided into stellate cells and basket cells based on their position and morphology. Stellate cells are located in the distal two thirds of the molecular layer, and make conventional inhibitory synapses onto both MLIs and PCs. Basket cells are located in the inner third of the molecular layer, and in additional to conventional synapses, they form specialized pinceau structures on the initial segment of PCs that inhibit PC firing through extremely rapid ephaptic signals (Blot and Barbour, 2014).
snRNAseq studies have identified two molecularly distinct types of MLIs, MLI1 and MLI2, that surprisingly do not correspond to stellate cells and basket cells (Kozareva et al., 2021) (Figure 3B, left). These subtypes are intermingled throughout the molecular layer, and their morphologies of both depend upon their layer position. For both types of MLIs, neurons located in the top two thirds of the molecular layer look like stellate cells, while those in the inner third of the molecular layer look superficially like basket cells, extending axons to the PC layer (although MLI1s in the inner third of the molecular layer have a more clearly defined pinceau). MLI1 and MLI2 neurons are physiologically distinct. MLI1s have higher rates of spontaneous activity, are less sensitive to depolarization, and are electrically coupled, whereas MLI2s are not electrically coupled. MLI1s also exhibit considerable molecular diversity that is dependent upon distance from the PC layer, with Grm8 (mGluR8) and other genes expressed at much higher levels in MLI1s near the PC layer (Figure 3B, right). It is not clear whether MLI1s are two discrete subtypes (MLI1_1 and MLI1_2), or a single population with continuously varying properties.
New insights are emerging regarding the contribution of MLIs to behavior. Measurements of MLI activity with genetically encoded calcium indicators have revealed large coordinated changes in MLI activity that correlate with movement rate (Gaffield and Christie, 2017). In addition, MLIs can regulate the magnitude and extent of CF-evoked calcium signals by regulating the excitability of PC dendrites, thereby controlling GrC to PC synaptic plasticity and possibly motor learning (Rowan et al., 2018). Moreover, a conditional genetics approach based on birth date has allowed selective suppression of either stellate cell or basket cell synapses (Brown et al., 2019), and revealed that suppression of stellate cell synapses increases simple spike regularity, while suppression of basket cell synapses increases simple spike frequency.
Currently, the roles of molecularly defined MLI subtypes are not known, and it cannot be assumed that the inputs and outputs of MLI1 and MLI2 are the same. Thus, clarification of their connectivity could provide important insight into their function. Because previous in vivo studies were performed prior to the identification of MLI1 and MLI2 subtypes, it will be important to determine whether there are differences in the activity of these populations, or in their influences on postsynaptic targets and behavior.
B. Climbing Fibers
Neurons in the inferior olive give rise to glutamatergic CFs that form extensive synaptic contacts onto PC dendrites, and each PC receives a single CF input. CFs powerfully depolarize PCs, activating voltage-activated calcium channels to produce both a dendrite-wide regenerative calcium spike and a brief flurry of sodium-based simple spikes in the soma and axon. This distinctive combined response is known as a “complex spike”. CF activation instructs long-term depression (LTD) of GrC to PC synapses (Ito, 1989). However, recent work suggests that CFs have additional roles.
Even though CFs do not directly contact MLIs or GoCs, glutamate released from CF to PC synapses spills out to excite nearby MLIs and GoCs (Nietz et al., 2017; Szapiro and Barbour, 2007) (Figure 3C). When CFs excite nearby MLIs, they can generate feedforward inhibition of more distant MLIs (Coddington et al., 2013). Hence, CF spillover can lead to both inhibition and disinhibition of nearby PCs (Arlt and Hausser, 2020). Similarly ,CF spillover can activate both excitatory and inhibitory glutamate receptors on GoCs (Nietz et al., 2017), and can therefore have a complex influence on processing in the GrC layer.
At present, the role of such CF activity in behavior and learning remains unclear. While considerable evidence supports a role for CFs in signaling supervised learning according to Marr-Albus models, recent evidence also suggests a broader role CFs, which may include forms for reinforcement learning for some behaviors (Hull, 2020). Hence, it will be important to understand the relationship between the different cellular plasticity and signaling mechanisms mediated by CFs and their diverse roles in behavior and learning.
IV. The Purkinje Cell Layer
Once viewed as containing only a homogenous pool of PC somata, the PC layer has undergone perhaps the largest revision in the cerebellar cortex in terms of identified cell types and connections. Here we will review this newfound complexity, highlighting the importance of previously underappreciated interneurons and recurrent synapses.
A. Purkinje cell layer interneurons
Interneurons whose cell bodies are located in or near the PC layer are known collectively as Purkinje layer interneurons (PLIs). Previous studies suggested a subdivision of PLIs into three types: Lugaro cells, globular cells and candelabrum cells (Figure 3A). However, the properties of PLIs have been incompletely characterized, and these subdivisions should be considered tentative. Consequently, their functional roles remain obscure, and they are not included in most models of the cerebellar cortex.
Lugaro cells are GABAergic/glycinegic PLIs with a characteristic fusiform soma that are inhibited by PCs, that locally inhibit GoCs and MLIs, and that send long-range axons to distant targets in the cerebellar cortex (Laine and Axelrad, 1996; Miyazaki et al., 2020; Palay and Chan-Palay, 1974; Sahin and Hockfield, 1990; Simat et al., 2007). Globular cells are glycinegic cells located near or below the PC layer that are inhibited by PCs (Hirono et al., 2012; Laine and Axelrad, 2002). Finally, there are candelabrum cells, which were identified in 1994 based solely on their distinctive light-level morphology (Laine and Axelrad, 1994). Until recently, candelabrum cells were the most enigmatic neuron of the cerebellar cortex.
Molecular characterization of the adult cerebellar cortex using snRNAseq has provided important insights by identifying three types of PLIs that may correspond to Lugaro cells, globular cells and candelabrum cells (Kozareva et al., 2021). These three types of PLIs are present in all regions of the cerebellar cortex, and together the PLIs are more numerous than GoCs.
Molecular characterization, identification of a transgenic mouse that labels candelabrum cells, electrophysiological recordings, and serial EM reconstructions have led to a major clarification of candelabrum cells within the cerebellar cortical circuitry (Osorno et al., 2021). These data revealed that MFs and GrCs excite candelabrum cells, and that PCs inhibit them. Candelabrum cells in turn primarily inhibit MLIs, leading to disinhibition of PCs (Figure 3E). The ability of candelabrum cells to weigh inputs to the cerebellar cortex (MFs), outputs from the cortex (PCs), and activity within the cortex (GrCs) to ultimately regulate PC excitability indicates their function is distinct from that of MLIs and GoCs.
Based on their prevalence, ubiquitous distribution, and unique circuit properties, PLIs must be considered important interneurons of the cerebellar cortex that need to be more fully characterized and then incorporated into circuit models to gain a full understanding of cerebellar processing.
B. Purkinje Cells
Purkinje cells fire spontaneous action potentials at high frequency (~20-100 Hz), and utilize a great many voltage and calcium-activated channels to generate diverse firing patterns. Despite this capacity for generating complex, nonlinear responses, there is a linear relationship between the number of active GrC inputs and the firing rate of the target PC (Walter and Khodakhah, 2009). It is possible, however, that when enough GrC inputs are activated, non-linearities can be generated by local dendritic spiking accompanied by a burst of simple spikes and subsequent pauses in firing (Zang and De Schutter, 2021).
PCs are organized into approximately 200 μm wide parasagittal ‘microzones’ that are thought to constitute discrete processing modules, and that can exhibit differential activity patterns during behavior (Heffley et al., 2018; Kostadinov et al., 2019; Tsutsumi et al., 2019). Microzones are innervated by CFs from discrete subdivisions of the inferior olive, and make specific contacts within the subregions of the cerebellar nuclei (Apps et al., 2018). Thus, microzones establish functional loops between the cerebellar cortex, CbN and IO.
PCs also have diverse molecular and physiological properties that correlate with microzonal organization. Molecular specialization is apparent in the differential expression pattern of Aldolase C (Aldoc, or “zebrin II”) that gives rise to the parasagittal zebrin stripes of the cerebellar cortex (Hawkes and Gravel, 1991). Aldoc− PCs express more TRPC3, fire at higher frequencies (Zhou et al., 2014), are more vulnerable to excitotoxity (Slemmer et al., 2007), express different glutamate transporters and are more susceptible to LTD (Wadiche and Jahr, 2005), and project to more rostrodorsal regions of the cerebellar nuclei (Fujita et al., 2014; Sugihara and Shinoda, 2007). This likely accounts for differential effects on cerebellar behaviors in PC-specific TRPC3 KO mice, in which eyelid conditioning is defective (in Aldoc− regions) and eye movement adaptation is unaffected (Aldoc + region) (Wu et al., 2019). RNAseq data indicate that PCs can be further subdivided into seven Aldoc+ and two Aldoc− subtypes that are differentially distributed within the cerebellar cortex (Kozareva et al., 2021), thought how these different PC subtypes are functionally specialized is not known.
Importantly, PCs do not simply convey output from the cerebellar cortex, as they have extensive axonal collaterals confined to a parasagittal plane that feed back to influence processing within the cerebellar cortex (Witter et al., 2016). PCs strongly inhibit other PCs, candelabrum cells, Lugaro cells, globular cells, some MLIs, GrCs in some regions and a subset of UBCs (Guo et al., 2021; Guo et al., 2016; Hirono et al., 2012; Orduz and Llano, 2007; Witter et al., 2016). PC collateral feedback could have several functional roles. In very young animals, collaterals propagate patterned waves of PC spiking that participate in the developmental refinement of downstream circuits (Watt et al., 2009). In adults, PC feedback could be a gain control mechanism that allows elevated PC output to suppress elements within the cerebellar cortex to maintain cerebellar cortical activity within an optimal range. PC feedback could also control the timing of firing of many elements of the cerebellar cortex (de Solages et al., 2008; Witter et al., 2016), though additional studies are needed to test such predictions.
VI. Mechanisms and Principles of Cerebellar Circuit Processing
In this section we highlight new advances and non-classical mechanisms of neuronal signaling likely to play a major role in cerebellar circuit processing.
A. Ephaptic Signaling
Ephaptic signaling has recently been shown to powerfully influence PC firing (Figure 3C-D). This form of signaling occurs when current flow across the neuronal membrane generates extracellular signals large enough to alter the firing of neighboring neurons (Anastassiou and Koch, 2015). Ephaptic signaling regulates PC firing in three ways: First, basket cell axons regulate sodium channels in PC axons (Figure 3D). Basket cells have a characteristic presynaptic specialization known as a pinceau that surrounds the proximal axon of PCs and lacks chemical synapses (Iwakura et al., 2012). When an action potential invades a pinceau, it opens potassium channels that produce a depolarizing extracellular signal. In turn, this extracellular depolarization almost instantly inhibits the activation of voltage-activated sodium channels in the axon of its associated PC (Blot and Barbour, 2014).
Second, ephaptic coupling also occurs between PCs (Figure 3D). When an action potential in a PC opens sodium channels in its own initial segment, it generates a hyperpolarizing extracellular signal that activates sodium channels in the axons of neighboring PCs to rapidly initiate spiking (Han et al., 2018). Thus, while opposite in effect, ephaptic signals from both basket cells and other PCs directly influence the site of PC action potential generation by locally regulating voltage-activated sodium channels.
PC firing is also regulated by a third form ephaptic signaling. CF synapses evoke a large intracellular depolarization of a PC dendrite that is accompanied by an extracellular hyperpolarization (Figure 3C). This extracellular hyperpolarization locally excites neighboring PC dendrites, but these dendrites lack the high density of sodium channels present in the axon. However, the extracellular hyperpolarization is sufficiently large and widespread that it also passively hyperpolarizes the somas and initial segments of nearby PCs to inhibit firing. This generates the surprising property that a powerful excitatory synapse almost instantly inhibits firing in neighboring PCs (Han et al., 2020).
Ephaptic signaling has several functional consequences in the cerebellum. Ephaptic inhibition provided by basket cells is almost a millisecond faster than chemical inhibition (Blot and Barbour, 2014). Consequently, direct GrC synaptic excitation and disynaptic MLI ephaptic inhibition of PCs occur roughly simultaneously, perhaps allowing GrCs to produce a net inhibition of PC spiking. In addition, ephaptic coupling between PCs could promote synchronous PC firing that is very rapid, with the firing of neighboring PCs having a dip at 0 latency and a peak at ±0.6 ms latency (Han et al., 2018). This mechanism may contribute to very fast PC layer oscillations observed in vivo (~200 Hz)(de Solages et al., 2008). In contrast, CF-mediated ephaptic inhibition of nearby PCs may briefly pause their firing (Han et al., 2020). Unfortunately, in contrast to other types of signaling that can be manipulated either pharmacologically or molecularly, it is not possible to selectively manipulate ephaptic signaling, making it challenging to directly determine its role in behavior and learning.
B. Spontaneous firing
Unlike most cortical areas, the cerebellar cortex contains many cell-types that fire action potentials spontaneously, including PCs, GoCs, and MLIs. The biophysical basis of such pacemaking has been well described for most cerebellar neurons (Hausser et al., 2004; Khaliq et al., 2003; Raman and Bean, 1999). For PCs, the unusually high rate of spontaneous firing (~20-100 Hz) comes at considerable energetic cost that likely makes them susceptible to cell death. This suggests that high spontaneous firing rates must have computational advantages. One possibility is that this property allows changes in PC firing to rapidly and bidirectionally influence the firing of CbN neurons. Multiple disorders ranging from ataxias to Autism Spectrum Disorders have been linked to inappropriate firing rates in PCs, further indicating that PC outputs must be finely calibrated to maintain normal cerebellar function (De Zeeuw et al., 2011; Tsai et al., 2012). It has also been suggested that changes in the precision of PC firing independent of changes in the spike rate can lead to cerebellar disfunction (Walter et al., 2006), although how this affects firing in the CbN sufficiently to disrupt cerebellar function is not understood.
C. Population Synchrony
There is considerable evidence that the cerebellar cortex can utilize a rate code for PC signaling to downstream CbN neurons to control behavior (Chen et al., 2016; Herzfeld et al., 2015; Payne et al., 2019). However, recent findings also suggest a role for temporal coding mediated by synchronous firing within different neuronal populations (Person and Raman, 2012). Accordingly, emerging evidence has suggested that there are multiple circuit mechanisms that allow certain classes of cerebellar neurons to synchronize their spiking.
i. Mechanisms of Cerebellar synchrony
In addition to ephaptic signaling discussed above, gap junctions are thought to play a key role synchronizing neuronal firing, with IO neurons (the source of CFs) and subpopulations of GoCs and MLI1s all expressing connexin 36. These gap junctions pass an attenuated and filtered action potential consisting of a small depolarization followed by a prominent slow AHP (Condorelli et al., 1998; Dugue et al., 2009; Llinas et al., 1974; Sotelo et al., 1974; van Welie et al., 2016; Vervaeke et al., 2010). This combined, passive sequence of depolarization followed hyperpolarization can act much like a traditional synaptic feedforward inhibitory circuit to enforce integration time windows and contribute to population synchrony (Hoehne et al., 2020).
Gap junction coupling of GoC dendrites (Vervaeke et al., 2012) has been shown to synchronize their firing and produce low frequency oscillations (5-30 Hz) in the GrC layer during periods of quiet wakefulness (Dugue et al., 2009). While the behavioral role of GrC layer oscillations remains unclear, they may support timing computations in the GrC layer by establishing narrow time windows for GrC spiking, and help bind activity in the cerebellum and neocortex during periods of motor preparation or passive expectancy (Courtemanche and Lamarre, 2005; O'Connor et al., 2002). GoC gap junctions are also thought to diversify their responses to allow both broad, relatively homogenous population activity on the timescale of seconds, as well as more heterogeneous, task specific activity on faster time scales (Gurnani and Silver, 2021).
Gap junctions can also synchronize MLI firing (Mann-Metzer and Yarom, 1999), such that the firing of two MLIs is correlated with a dip at 0 ms and peak firing offset by 1.7 ms (Han et al., 2018). MLI dendrites are confined to a parasagittal plane, and the extent of electrical coupling is strongest in MLI1s near the PC layer (Alcami and Marty, 2013; Kozareva et al., 2021). This suggests that electrical coupling could synchronize MLI1 basket cell firing within parasagittal planes (Hoehne et al., 2020), and may synchronize PC firing within parasagittal planes (Wise et al., 2010). Calcium imaging data suggests that MLI activity is correlated during movement (Gaffield and Christie, 2017), but this has not been addressed with high-temporal measurements (such as electrophysiology), and the role of electrical coupling is not known.
Electrical coupling can also promote synchronous firing of neighboring IO neurons with a precision of several milliseconds by synchronizing subthreshold membrane potential oscillations (Leznik and Llinas, 2005; Llinas and Yarom, 1986; Long et al., 2002). This results in synchronous CF activation during behaviors (Blenkinsop and Lang, 2006). While the role of such synchrony is debated, it has been hypothesized that it coordinates the pauses that follow complex spikes in many PCs, and can regulate CF-dependent associative learning (Kitazawa and Wolpert, 2005).
Synaptic inhibition can also promote synchronous firing. Feedforward inhibitory synapses can regulate spike timing by restricting the integration time window of their targets (Pouille and Scanziani, 2001). Such effects have been demonstrated in the cerebellum for feedforward connections from MLIs to PCs (Mittmann et al., 2005), where GrC inputs are integrated in a brief window due to feedforward MLI inhibition. Recurrent inhibition can also promote synchrony. In particular, recurrent inhibitory connections between PCs (Witter et al., 2016), between MLIs (Palay and Chan-Palay, 1974) and between GoCs (Hull and Regehr, 2012) could help promote population synchrony (Bartos et al., 2007).
ii. Synchrony and Cerebellar Output
Synchrony of PC firing that arises from recurrent inhibition, ephaptic signaling, and shared excitatory inputs, can have important consequences for cerebellar output. In vitro studies have shown that for synapses between PCs and the excitatory projection neurons of the CbN, a combination of convergence (Person and Raman, 2011), ultrafast IPSC kinetics (Najac and Raman, 2015), CbN intrinsic properties (Najac and Raman, 2015), and presynaptic neurotransmitter release properties (Turecek et al., 2016, 2017) allow synchronous PC firing to entrain CbN neuron spiking. Synchronous spiking among even a modest percentage (~ 10%) of the many PC inputs that converge onto a single CbN neuron can transiently reduce synaptic inhibition, both entraining and elevating CbN spiking (Person and Raman, 2011). In vivo, there is considerable evidence that PCs can achieve millisecond synchrony (Person and Raman, 2012), and indications that synchronous PC firing can entrain CbN firing (Brown and Raman, 2018; Sarnaik and Raman, 2018).
Although most attention has focused on synchronous PC firing, brief synchronous suppression of PC firing is also a highly effective means of promoting firing in the CbN (Han et al., 2020). This has been shown in vitro with dynamic clamp and in vivo by optogenetically suppressing PC firing for several milliseconds. Remarkably, brief suppression of a fraction of PCs converging onto a CbN neuron can lead to large, short latency (~ 1 ms), precise (~ 2 ms) increases in the firing of CbN neurons (10% PC suppression more than doubles CbN neuron firing). Synchronized suppression of PC firing could occur during synchronized CF-induced pauses, when a CF suppresses firing of nearby PCs, or when MLIs inhibiting many PCs.
Many aspects of cerebellar synchrony are also not understood. For example, it is unclear how PCs transition to and from states of broad population synchrony, allowing them to differentially influence their CbN neuron targets. It is also unclear how rate codes and population synchrony may differentially contribute to downstream processing and behavior (Hong et al., 2016).
D. Distributed Sites of Long-Term Plasticity
According to Marr-Albus models, and essentially all models of cerebellar function, cerebellar learning is achieved by modifying GrC to PC synapses (Albus, 1971; Ito, 1972; Marr, 1969). Most attention has focused on postsynaptic long-term depression (LTD) of GrC to PC synapses that are activate immediately prior to CF activation (Ekerot and Kano, 1985; Ito et al., 1982; Sakurai, 1987). However, GrC-PC LTD is not always required for learning, because chronic impairment of GrC to PC LTD (Schonewille et al., 2011), or a lack of CF activity (Kimpo et al., 2014) do not disrupt some forms of cerebellum-dependent learning. Thus, other types of long-term plasticity must be able to mediate some types of cerebellar learning. Importantly, this does not mean that the central role of synapse-specific plasticity of GrC to PC synapses needs to be abandoned. GrC to PC synapses exhibit multiple mechanisms of long-term plasticity, including presynaptic LTP (Salin et al., 1996) and LTD (Hoxha et al., 2016), and postsynaptic LTP (Belmeguenai and Hansel, 2005). Thus, the observation that some forms of cerebellar dependent leaning do not rely on postsynaptic LTD is still compatible with plasticity of the GrC to PC synapses being central to cerebellar-dependent learning.
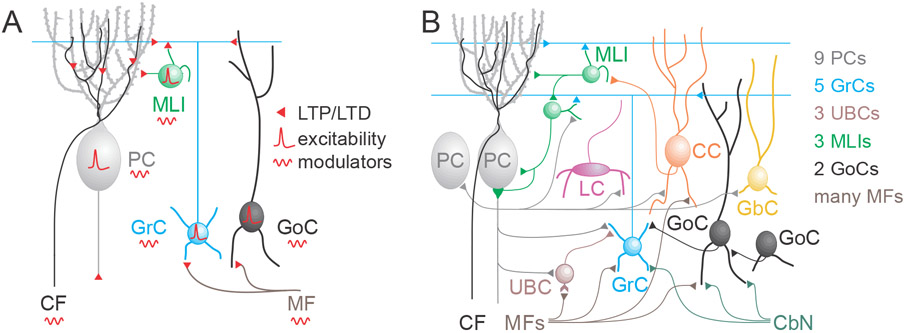
There are also many other forms of long-term plasticity in the cerebellum (Figure 4). These can be broadly categorized into long-term synaptic plasticity, and long-term changes in excitability (Figure 4A). LTD and LTP are both present at MF to GrC synapses (D'Angelo et al., 1999; D'Errico et al., 2009; Gall et al., 2005), MF and GrC to GoCs synapses (Locatelli et al., 2021; Robberechts et al., 2010), MLIs (Jorntell and Ekerot, 2003; Liu and Cull-Candy, 2000; Rancillac and Crepel, 2004; Soler-Llavina and Sabatini, 2006), and output synapses onto either CbN neurons (Aizenman et al., 1998; Pugh and Raman, 2006) or vestibular nucleus neurons (McElvain et al., 2010). Moreover, intrinsic plasticity mechanisms can also modify the firing and excitability of GoCs (Hull et al., 2013), MLIs (Alexander and Bowie, 2021), PCs (Belmeguenai et al., 2010), GrCs (Armano et al., 2000), CbN neurons (Aizenman and Linden, 2000) and vestibular nucleus neurons (Nelson et al., 2003). There are also likely to be intrinsic and synaptic plasticity for recently identified cell types and their connections (Figure 4B).
Figure 4. Cell types, synapses, circuitry and sites of plasticity.
A. Known sites of plasticity in the cerebellar cortex, showing synapses that undergo LTP and LTD, cell types where excitability and spontaneous activity can be altered, and sites that are known targets of modulators. B. Updated circuit showing the cells and known connections of the cerebellar cortex that could all undergo plasticity. The number of known subtypes of cells are indicted in the inset.
Importantly, numerous mechanisms can regulate the induction of long-term plasticity. The ability of CFs to induce GrC-to-PC LTD is regulated by MLI inhibition of PCs (Gaffield et al., 2018; Rowan et al., 2018), by presynaptic CF inhibition (Carey and Regehr, 2009), and by CF-LTD (Hansel and Linden, 2000). There is also evidence that the timing rules for LTD induction are highly precise and regionally dependent (Suvrathan et al., 2016), although it is not known how the signaling mechanisms that underlie LTD produce such temporal precision. CF input is also not binary, and CF burst firing can prolong the duration of complex spikes (number of spikelets) (De Gruijl et al., 2012; Lang et al., 2014), and enhance the postsynaptic calcium signal in PC dendrites (Gaffield et al., 2019; Roh et al., 2020), consistent with the observation that the number of spikelets in a complex spike correlates with learning on a single trial basis (Yang and Lisberger, 2014). Finally, behavioral context likely regulates plasticity and learning (Albergaria et al., 2018; Lawrenson et al., 2016), likely by mechanisms such as neuromodulation that can alter transmission and plasticity (Carey and Regehr, 2009; Dieudonne and Dumoulin, 2000; Fleming and Hull, 2019; Fore et al., 2020; Prestori et al., 2013).
With so many sites and forms of plasticity in the cerebellar cortex, it is hard to believe that CF-gated postsynaptic GrC to PC LTD mediates all cerebellar learning. However, it is equally difficult to imagine that all plasticity mechanisms play a direct role in cerebellar learning. The anatomical arrangement of MFs, GrCs, and PCs strongly implicate GrC to PC synapses as crucial sites of plasticity and learning. Thus, a key requirement for understanding cerebellar learning will be to determine how other forms of plasticity may complement the plasticity of GrC to PC synapses. For example, decreasing GrC to PC synaptic strength cannot inhibit PC firing, so regulation of MLI synapses and/or firing must also be involved in learning. It will also be necessary to assess other roles of plasticity, apart from a direct contribution to learning. For example, some forms of plasticity likely fine tune the cerebellar circuity during development, or according to behavioral context to optimize circuit function.
VII. Conclusions
Together, these recently described cell types, circuit connections, plasticity and signaling mechanisms, and other features of the cerebellar cortex not previously incorporated in classical Marr-Albus models vastly enrich our understanding of how this structure processes information. Newfound complexities, such as subtypes of MLIs and PLIs that are ubiquitously present, must be integrated into the canonical circuit. In addition, while the cerebellar cortex consists of repeated modules of the same basic circuit, it has also exhibits strong local and regional differences that allow specialized processing and learning. These new discoveries require major revisions of cerebellar circuit models. With such revisions, the cerebellum will remain an ideal system to relate neural circuit function and plasticity with behavior and learning. Such efforts will enable a more complete understanding of cerebellar function, and inform how it can contribute to behaviors ranging from motor control to social and cognitive tasks.
Future Issues
Recent advances have shown that the previous view of the cerebellar cortex was an oversimplification, but newly discovered features have not yet been incorporated into a new model of the cerebellar cortex.
What are the functional roles of newly discovered cell types and subtypes of the cerebellar cortex?
What is the logic of mossy fiber input to granule cells?
How does granule cell layer inhibition differentially contribute across behaviors and learning paradigms?
How do the molecular specializations within microzones tailor them for unique cerebellar computations?
What is the role of PC synchrony in behavior and learning, and how do PCs transition into and out synchrony?
How do different forms of ephaptic signaling contribute to behavior and learning?
How do diverse forms of plasticity combine to enable different forms of cerebellar learning?
Acknowledgements
We would like to thank Stephen Lisberger, Christopher Chen, and Lindsey Glickfeld for their input on this manuscript.
Terms and definitions
- MF
Mossy fiber
- CF
Climbing fiber
- GrC
Granule cell
- UBC
Unipolar brush cell
- GoC
Golgi cell
- CC
Candelabrum cell
- LC
Lugaro cell
- GlC
Globular cell
- MLI
Molecular layer interneuron
- MLI1
MLI type 1
- MLI2
MLI type 2
- PC
Purkinje cell
- CbN
Cerebellar Nuclei
Literature Cited
- Aizenman CD, and Linden DJ (2000). Rapid, synaptically driven increases in the intrinsic excitability of cerebellar deep nuclear neurons. Nat Neurosci 3, 109–111. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Manis PB, and Linden DJ (1998). Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron 21, 827–835. [DOI] [PubMed] [Google Scholar]
- Albergaria C, Silva NT, Pritchett DL, and Carey MR (2018). Locomotor activity modulates associative learning in mouse cerebellum. Nat Neurosci 21, 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albus JS (1971). A theory of cerebellar function. Mathematical Biosciences 10, 25–61. [Google Scholar]
- Alcami P, and Marty A (2013). Estimating functional connectivity in an electrically coupled interneuron network. Proc Natl Acad Sci U S A 110, E4798–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander RPD, and Bowie D (2021). Intrinsic plasticity of cerebellar stellate cells is mediated by NMDA receptor regulation of voltage-gated Na(+) channels. J Physiol 599, 647–665. [DOI] [PubMed] [Google Scholar]
- Anastassiou CA, and Koch C (2015). Ephaptic coupling to endogenous electric field activity: why bother? Curr Opin Neurobiol 31, 95–103. [DOI] [PubMed] [Google Scholar]
- Ankri L, Husson Z, Pietrajtis K, Proville R, Lena C, Yarom Y, Dieudonne S, and Uusisaari MY (2015). A novel inhibitory nucleo-cortical circuit controls cerebellar Golgi cell activity. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Hawkes R, Aoki S, Bengtsson F, Brown AM, Chen G, Ebner TJ, Isope P, Jorntell H, Lackey EP, et al. (2018). Cerebellar Modules and Their Role as Operational Cerebellar Processing Units: A Consensus paper [corrected]. Cerebellum 17, 654–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenz A, Silver RA, Schaefer AT, and Margrie TW (2008). The contribution of single synapses to sensory representation in vivo. Science 321, 977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt C, and Hausser M (2020). Microcircuit Rules Governing Impact of Single Interneurons on Purkinje Cell Output In Vivo. Cell Rep 30, 3020–3035 e3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armano S, Rossi P, Taglietti V, and D'Angelo E (2000). Long-term potentiation of intrinsic excitability at the mossy fiber-granule cell synapse of rat cerebellum. J Neurosci 20, 5208–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer TS, and Trussell LO (2019). Selective targeting of unipolar brush cell subtypes by cerebellar mossy fibers. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, and Jonas P (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8, 45–56. [DOI] [PubMed] [Google Scholar]
- Batini C, Buisseret-Delmas C, Compoint C, and Daniel H (1989). The GABAergic neurones of the cerebellar nuclei in the rat: projections to the cerebellar cortex. Neurosci Lett 99, 251–256. [DOI] [PubMed] [Google Scholar]
- Belmeguenai A, and Hansel C (2005). A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J Neurosci 25, 10768–10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Hosy E, Bengtsson F, Pedroarena CM, Piochon C, Teuling E, He Q, Ohtsuki G, De Jeu MT, Elgersma Y, et al. (2010). Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci 30, 13630–13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings G, Piasini E, Lorincz A, Nusser Z, and Silver RA (2014). Network structure within the cerebellar input layer enables lossless sparse encoding. Neuron 83, 960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkinsop TA, and Lang EJ (2006). Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci 26, 1739–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot A, and Barbour B (2014). Ultra-rapid axon-axon ephaptic inhibition of cerebellar Purkinje cells by the pinceau. Nat Neurosci 17, 289–295. [DOI] [PubMed] [Google Scholar]
- Borges-Merjane C, and Trussell LO (2015). ON and OFF unipolar brush cells transform multisensory inputs to the auditory system. Neuron 85, 1029–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, and Farrant M (1996). Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol 497 (Pt 3), 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Arancillo M, Lin T, Catt DR, Zhou J, Lackey EP, Stay TL, Zuo Z, White JJ, and Sillitoe RV (2019). Molecular layer interneurons shape the spike activity of cerebellar Purkinje cells. Sci Rep 9, 1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ST, and Raman IM (2018). Sensorimotor Integration and Amplification of Reflexive Whisking by Well-Timed Spiking in the Cerebellar Corticonuclear Circuit. Neuron 99, 564–575 e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MR, and Regehr WG (2009). Noradrenergic control of associative synaptic plasticity by selective modulation of instructive signals. Neuron 62, 112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrol FP, Arenz A, Wiechert MT, Margrie TW, and DiGregorio DA (2015). Synaptic diversity enables temporal coding of coincident multisensory inputs in single neurons. Nat Neurosci 18, 718–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, and Hausser M (2004). Integration of quanta in cerebellar granule cells during sensory processing. Nature 428, 856–860. [DOI] [PubMed] [Google Scholar]
- Chen S, Augustine GJ, and Chadderton P (2016). The cerebellum linearly encodes whisker position during voluntary movement. Elife 5, e10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coddington LT, Rudolph S, Vande Lune P, Overstreet-Wadiche L, and Wadiche JI (2013). Spillover-mediated feedforward inhibition functionally segregates interneuron activity. Neuron 78, 1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condorelli DF, Parenti R, Spinella F, Trovato Salinaro A, Belluardo N, Cardile V, and Cicirata F (1998). Cloning of a new gap junction gene (Cx36) highly expressed in mammalian brain neurons. Eur J Neurosci 10, 1202–1208. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, and Lamarre Y (2005). Local field potential oscillations in primate cerebellar cortex: synchronization with cerebral cortex during active and passive expectancy. J Neurophysiol 93, 2039–2052. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Fioravante D, and Regehr WG (2009). Dynamics of fast and slow inhibition from cerebellar golgi cells allow flexible control of synaptic integration. Neuron 63, 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E, Rossi P, Armano S, and Taglietti V (1999). Evidence for NMDA and mGlu receptor-dependent long-term potentiation of mossy fiber-granule cell transmission in rat cerebellum. J Neurophysiol 81, 277–287. [DOI] [PubMed] [Google Scholar]
- D'Errico A, Prestori F, and D'Angelo E (2009). Differential induction of bidirectional long-term changes in neurotransmitter release by frequency-coded patterns at the cerebellar input. J Physiol 587, 5843–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gruijl JR, Bazzigaluppi P, de Jeu MTG, and De Zeeuw CI (2012). Climbing Fiber Burst Size and Olivary Sub-threshold Oscillations in a Network Setting. PLOS Computational Biology 8, e1002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Solages C, Szapiro G, Brunei N, Hakim V, Isope P, Buisseret P, Rousseau C, Barbour B, and Lena C (2008). High-frequency organization and synchrony of activity in the purkinje cell layer of the cerebellum. Neuron 58, 775–788. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoebeek FE, Bosman LW, Schonewille M, Witter L, and Koekkoek SK (2011). Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci 12, 327–344. [DOI] [PubMed] [Google Scholar]
- Delvendahl I, and Hallermann S (2016). The Cerebellar Mossy Fiber Synapse as a Model for High-Frequency Transmission in the Mammalian CNS. Trends Neurosci 39, 722–737. [DOI] [PubMed] [Google Scholar]
- Dieudonne S, and Dumoulin A (2000). Serotonin-driven long-range inhibitory connections in the cerebellar cortex. J Neurosci 20, 1837–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGregorio DA, Nusser Z, and Silver RA (2002). Spillover of glutamate onto synaptic AMPA receptors enhances fast transmission at a cerebellar synapse. Neuron 35, 521–533. [DOI] [PubMed] [Google Scholar]
- Dugue GP, Brunel N, Hakim V, Schwartz E, Chat M, Levesque M, Courtemanche R, Lena C, and Dieudonne S (2009). Electrical coupling mediates tunable low-frequency oscillations and resonance in the cerebellar Golgi cell network. Neuron 61, 126–139. [DOI] [PubMed] [Google Scholar]
- Dugue GP, Dumoulin A, Triller A, and Dieudonne S (2005). Target-dependent use of co-released inhibitory transmitters at central synapses. J Neurosci 25, 6490–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid I, Branco T, Chadderton P, Arlt C, Powell K, and Hausser M (2015). Control of cerebellar granule cell output by sensory-evoked Golgi cell inhibition. Proc Natl Acad Sci U S A 112, 13099–13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid I, Branco T, London M, Chadderton P, and Hausser M (2012). Tonic inhibition enhances fidelity of sensory information transmission in the cerebellar cortex. J Neurosci 32, 11132–11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J, Ito M, and Szentágothai J (1967). The Cerebellum as a Neuronal Machine (Berlin: Springer; ). [Google Scholar]
- Ekerot CF, and Kano M (1985). Long-term depression of parallel fibre synapses following stimulation of climbing fibres. Brain Res 342, 357–360. [DOI] [PubMed] [Google Scholar]
- Fleming E, and Hull C (2019). Serotonin regulates dynamics of cerebellar granule cell activity by modulating tonic inhibition. J Neurophysiol 121, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fore TR, Taylor BN, Brunel N, and Hull C (2020). Acetylcholine Modulates Cerebellar Granule Cell Spiking by Regulating the Balance of Synaptic Excitation and Inhibition. J Neurosci 40, 2882–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Aoki H, Ajioka I, Yamazaki M, Abe M, Oh-Nishi A, Sakimura K, and Sugihara I (2014). Detailed expression pattern of aldolase C (Aldoc) in the cerebellum, retina and other areas of the CNS studied in Aldoc-Venus knock-in mice. PLoS One 9, e86679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield MA, Bonnan A, and Christie JM (2019). Conversion of Graded Presynaptic Climbing Fiber Activity into Graded Postsynaptic Ca(2+) Signals by Purkinje Cell Dendrites. Neuron 102, 762–769 e764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield MA, and Christie JM (2017). Movement Rate Is Encoded and Influenced by Widespread, Coherent Activity of Cerebellar Molecular Layer Interneurons. J Neurosci 37, 4751–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield MA, Rowan MJM, Amat SB, Hirai H, and Christie JM (2018). Inhibition gates supralinear Ca(2+) signaling in Purkinje cell dendrites during practiced movements. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall D, Prestori F, Sola E, D'Errico A, Roussel C, Forti L, Rossi P, and D'Angelo E (2005). Intracellular calcium regulation by burst discharge determines bidirectional long-term synaptic plasticity at the cerebellum input stage. J Neurosci 25, 4813–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano E, Gao Z, Schonewille M, Todorov B, Simons E, Pop AS, D'Angelo E, van den Maagdenberg AM, Hoebeek FE, and De Zeeuw CI (2013). Silencing the majority of cerebellar granule cells uncovers their essential role in motor learning and consolidation. Cell Rep 3, 1239–1251. [DOI] [PubMed] [Google Scholar]
- Gao Z, Proietti-Onori M, Lin Z, Ten Brinke MM, Boele HJ, Potters JW, Ruigrok TJ, Hoebeek FE, and De Zeeuw CI (2016). Excitatory Cerebellar Nucleocortical Circuit Provides Internal Amplification during Associative Conditioning. Neuron 89, 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts FJ, Timmermans J, Shigemoto R, and De Schutter E (2001). Morphological and neurochemical differentiation of large granular layer interneurons in the adult rat cerebellum. Neuroscience 104, 499–512. [DOI] [PubMed] [Google Scholar]
- Giovannucci A, Badura A, Deverett B, Najafi F, Pereira TD, Gao Z, Ozden I, Kloth AD, Pnevmatikakis E, Paninski L, et al. (2017). Cerebellar granule cells acquire a widespread predictive feedback signal during motor learning. Nat Neurosci 20, 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Huson V, Macosko E, and Regehr WG (2020). Graded heterogeneity of metabotropic signaling underlies a continuum of cell-intrinsic temporal responses. bioRxiv, 2020.2012.2027.424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Rudolph S, Neuwirth ME, and Regehr WG (2021). Purkinje cell outputs selectively inhibit a subset of unipolar brush cells in the input layer of the cerebellar cortex. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Witter L, Rudolph S, Elliott HL, Ennis KA, and Regehr WG (2016). Purkinje Cells Directly Inhibit Granule Cells in Specialized Regions of the Cerebellar Cortex. Neuron 91, 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnani H, and Silver RA (2021). Multidimensional population activity in an electrically coupled inhibitory circuit in the cerebellar cortex. Neuron 109, 1739–1753 e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallermann S, Fejtova A, Schmidt H, Weyhersmuller A, Silver RA, Gundelfinger ED, and Eilers J (2010). Bassoon speeds vesicle reloading at a central excitatory synapse. Neuron 68, 710–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KS, Chen CH, Khan MM, Guo C, and Regehr WG (2020). Climbing fiber synapses rapidly and transiently inhibit neighboring Purkinje cells via ephaptic coupling. Nat Neurosci 23, 1399–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KS, Guo C, Chen CH, Witter L, Osorno T, and Regehr WG (2018). Ephaptic Coupling Promotes Synchronous Firing of Cerebellar Purkinje Cells. Neuron 100, 564–578 e563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C, and Linden DJ (2000). Long-term depression of the cerebellar climbing fiber--Purkinje neuron synapse. Neuron 26, 473–482. [DOI] [PubMed] [Google Scholar]
- Hausser M, Raman IM, Otis T, Smith SL, Nelson A, du Lac S, Loewenstein Y, Mahon S, Pennartz C, Cohen I, and Yarom Y (2004). The beat goes on: spontaneous firing in mammalian neuronal microcircuits. J Neurosci 24, 9215–9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R, and Gravel C (1991). The modular cerebellum. Prog Neurobiol 36, 309–327. [DOI] [PubMed] [Google Scholar]
- Heffley W, Song EY, Xu Z, Taylor BN, Hughes MA, McKinney A, Joshua M, and Hull C (2018). Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions. Nat Neurosci 21, 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld DJ, Kojima Y, Soetedjo R, and Shadmehr R (2015). Encoding of action by the Purkinje cells of the cerebellum. Nature 526, 439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M, Saitow F, Kudo M, Suzuki H, Yanagawa Y, Yamada M, Nagao S, Konishi S, and Obata K (2012). Cerebellar globular cells receive monoaminergic excitation and monosynaptic inhibition from Purkinje cells. PLoS One 7, e29663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehne A, McFadden MH, and DiGregorio DA (2020). Feed-forward recruitment of electrical synapses enhances synchronous spiking in the mouse cerebellar cortex. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Negrello M, Junker M, Smilgin A, Thier P, and De Schutter E (2016). Multiplexed coding by cerebellar Purkinje neurons. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck BD, and Person AL (2014). Cerebellar loops: a review of the nucleocortical pathway. Cerebellum 13, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck BD, and Person AL (2015). Cerebellar Premotor Output Neurons Collateralize to Innervate the Cerebellar Cortex. J Comp Neurol 523, 2254–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxha E, Tempia F, Lippiello P, and Miniaci MC (2016). Modulation, Plasticity and Pathophysiology of the Parallel Fiber-Purkinje Cell Synapse. Front Synaptic Neurosci 8, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, Nelson SB, and Hantman AW (2013). Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells. Elife 2, e00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C. (2020). Prediction signals in the cerebellum: beyond supervised motor learning. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, and Regehr WG (2012). Identification of an inhibitory circuit that regulates cerebellar Golgi cell activity. Neuron 73, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull CA, Chu Y, Thanawala M, and Regehr WG (2013). Hyperpolarization induces a long-term increase in the spontaneous firing rate of cerebellar Golgi cells. J Neurosci 33, 5895–5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. (1972). Neural design of the cerebellar motor control system. Brain Res 40, 81–84. [DOI] [PubMed] [Google Scholar]
- Ito M. (1989). Long-term depression. Annu Rev Neurosci 12, 85–102. [DOI] [PubMed] [Google Scholar]
- Ito M, Sakurai M, and Tongroach P (1982). Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol 324, 113–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura A, Uchigashima M, Miyazaki T, Yamasaki M, and Watanabe M (2012). Lack of molecular-anatomical evidence for GABAergic influence on axon initial segment of cerebellar Purkinje cells by the pinceau formation. J Neurosci 32, 9438–9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, and Hamori J (1988). Quantitative morphology and synaptology of cerebellar glomeruli in the rat. Anat Embryol (Berl) 179, 81–88. [DOI] [PubMed] [Google Scholar]
- Jorntell H, and Ekerot CF (2003). Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J Neurosci 23, 9620–9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorntell H, and Ekerot CF (2006). Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J Neurosci 26, 11786–11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanichay RT, and Silver RA (2008). Synaptic and cellular properties of the feedforward inhibitory circuit within the input layer of the cerebellar cortex. J Neurosci 28, 8955–8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Gouwens NW, and Raman IM (2003). The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J Neurosci 23, 4899–4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Sekerkova G, Mugnaini E, and Martina M (2012). Electrophysiological, morphological, and topological properties of two histochemically distinct subpopulations of cerebellar unipolar brush cells. Cerebellum 11, 1012–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpo RR, Rinaldi JM, Kim CK, Payne HL, and Raymond JL (2014). Gating of neural error signals during motor learning. Elife 3, e02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney GA, Overstreet LS, and Slater NT (1997). Prolonged physiological entrapment of glutamate in the synaptic cleft of cerebellar unipolar brush cells. Journal of Neurophysiology 78, 1320–1333. [DOI] [PubMed] [Google Scholar]
- Kitazawa S, and Wolpert DM (2005). Rhythmicity, randomness and synchrony in climbing fiber signals. Trends Neurosci 28, 611–619. [DOI] [PubMed] [Google Scholar]
- Knoflach F, and Kemp JA (1998). Metabotropic glutamate group II receptors activate a G protein-coupled inwardly rectifying K+ current in neurones of the rat cerebellum. The Journal of physiology 509, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knogler LD, Markov DA, Dragomir EI, Stih V, and Portugues R (2017). Sensorimotor Representations in Cerebellar Granule Cells in Larval Zebrafish Are Dense, Spatially Organized, and Non-temporally Patterned. Curr Biol 27, 1288–1302. [DOI] [PubMed] [Google Scholar]
- Kostadinov D, Beau M, Blanco-Pozo M, and Hausser M (2019). Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells. Nat Neurosci 22, 950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozareva V, Martin C, Osorno T, Rudolph S, Guo C, Vanderburg C, Nadaf N, Regev A, Regehr W, and Macosko E (2021). A transcriptomic atlas of the mouse cerebellum reveals regional specializations and novel cell types. Nature in press, 2020.2003.2004.976407. [Google Scholar]
- Laine J, and Axelrad H (1994). The candelabrum cell: a new interneuron in the cerebellar cortex. J Comp Neurol 339, 159–173. [DOI] [PubMed] [Google Scholar]
- Laine J, and Axelrad H (1996). Morphology of the Golgi-impregnated Lugaro cell in the rat cerebellar cortex: a reappraisal with a description of its axon. J Comp Neurol 375, 618–640. [DOI] [PubMed] [Google Scholar]
- Laine J, and Axelrad H (2002). Extending the cerebellar Lugaro cell class. Neuroscience 115, 363–374. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Tang T, Suh CY, Xiao J, Kotsurovskyy Y, Blenkinsop TA, Marshall SP, and Sugihara I (2014). Modulation of Purkinje cell complex spike waveform by synchrony levels in the olivocerebellar system. Frontiers in Systems Neuroscience 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanore F, Cayco-Gajic NA, Gurnani H, Coyle D, and Silver RA (2021). Cerebellar granule cell axons support high-dimensional representations. Nat Neurosci 24, 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson CL, Watson TC, and Apps R (2016). Transmission of Predictable Sensory Signals to the Cerebellum via Climbing Fiber Pathways Is Gated during Exploratory Behavior. J Neurosci 36, 7841–7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leznik E, and Llinas R (2005). Role of gap junctions in synchronized neuronal oscillations in the inferior olive. J Neurophysiol 94, 2447–2456. [DOI] [PubMed] [Google Scholar]
- Liu SQ, and Cull-Candy SG (2000). Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature 405, 454–458. [DOI] [PubMed] [Google Scholar]
- Llinas R, Baker R, and Sotelo C (1974). Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol 37, 560–571. [DOI] [PubMed] [Google Scholar]
- Llinas R, and Yarom Y (1986). Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol 376, 163–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli F, Soda T, Montagna I, Tritto S, Botta L, Prestori F, and D'Angelo E (2021). Calcium Channel-Dependent Induction of Long-Term Synaptic Plasticity at Excitatory Golgi Cell Synapses of Cerebellum. J Neurosci 41, 3307–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Deans MR, Paul DL, and Connors BW (2002). Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci 22, 10898–10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann-Metzer P, and Yarom Y (1999). Electrotonic coupling interacts with intrinsic properties to generate synchronized activity in cerebellar networks of inhibitory interneurons. J Neurosci 19, 3298–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. (1969). A theory of cerebellar cortex. J Physiol 202, 437–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElvain LE, Bagnall MW, Sakatos A, and du Lac S (2010). Bidirectional plasticity gated by hyperpolarization controls the gain of postsynaptic firing responses at central vestibular nerve synapses. Neuron 68, 763–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, and Silver RA (2000a). GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. Journal of Neuroscience 20, 8651–8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, and Silver RA (2000b). Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature 404, 498–502. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, and Silver RA (2003). Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38, 433–445. [DOI] [PubMed] [Google Scholar]
- Mittmann W, Koch U, and Hausser M (2005). Feed-forward inhibition shapes the spike output of cerebellar Purkinje cells. J Physiol 563, 369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Yamasaki M, Tanaka KF, and Watanabe M (2020). Compartmentalized Input-Output Organization of Lugaro Cells in the Cerebellar Cortex. Neuroscience. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Sekerkova G, and Martina M (2011). The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res Rev 66, 220–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najac M, and Raman IM (2015). Integration of Purkinje cell inhibition by cerebellar nucleo-olivary neurons. J Neurosci 35, 544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neki A, Ohishi H, Kaneko T, Shigemoto R, Nakanishi S, and Mizuno N (1996). Metabotropic glutamate receptors mGluR2 and mGluR5 are expressed in two non-overlapping populations of Golgi cells in the rat cerebellum. Neuroscience 75, 815–826. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Krispel CM, Sekirnjak C, and du Lac S (2003). Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron 40, 609–620. [DOI] [PubMed] [Google Scholar]
- Nietz AK, Vaden JH, Coddington LT, Overstreet-Wadiche L, and Wadiche JI (2017). Non-synaptic signaling from cerebellar climbing fibers modulates Golgi cell activity. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor SM, Berg RW, and Kleinfeld D (2002). Coherent electrical activity between vibrissa sensory areas of cerebellum and neocortex is enhanced during free whisking. J Neurophysiol 87, 2137–2148. [DOI] [PubMed] [Google Scholar]
- Orduz D, and Llano I (2007). Recurrent axon collaterals underlie facilitating synapses between cerebellar Purkinje cells. Proc Natl Acad Sci U S A 104, 17831–17836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorno T, Rudolph S, Nguyen T, Kozareva V, Nadaf N, Macosko EZ, Lee W-CA, and Regehr WG (2021). Candelabrum cells are molecularly distinct, ubiquitous interneurons of the cerebellar cortex with specialized circuit properties. bioRxiv, 2021.2004.2009.439172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozden I, Dombeck DA, Hoogland TM, Tank DW, and Wang SS (2012). Widespread state-dependent shifts in cerebellar activity in locomoting mice. PLoS One 7, e42650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, and Chan-Palay V (1974). Cerebellar cortex (New York: Springer; ). [Google Scholar]
- Payne HL, French RL, Guo CC, Nguyen-Vu TB, Manninen T, and Raymond JL (2019). Cerebellar Purkinje cells control eye movements with a rapid rate code that is invariant to spike irregularity. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, and Raman IM (2011). Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature 481, 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, and Raman IM (2012). Synchrony and neural coding in cerebellar circuits. Front Neural Circuits 6, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, and Scanziani M (2001). Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163. [DOI] [PubMed] [Google Scholar]
- Prestori F, Bonardi C, Mapelli L, Lombardo P, Goselink R, De Stefano ME, Gandolfi D, Mapelli J, Bertrand D, Schonewille M, et al. (2013). Gating of long-term potentiation by nicotinic acetylcholine receptors at the cerebellum input stage. PLoS One 8, e64828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh JR, and Raman IM (2006). Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron 51, 113–123. [DOI] [PubMed] [Google Scholar]
- Raman IM, and Bean BP (1999). Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci 19, 1663–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancillac A, and Crepel F (2004). Synapses between parallel fibres and stellate cells express long-term changes in synaptic efficacy in rat cerebellum. J Physiol 554, 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancz EA, Ishikawa T, Duguid I, Chadderton P, Mahon S, and Hausser M (2007). High-fidelity transmission of sensory information by single cerebellar mossy fibre boutons. Nature 450, 1245–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, and Medina JF (2018). Computational Principles of Supervised Learning in the Cerebellum. Annu Rev Neurosci 41, 233–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzau-Jost A, Delvendahl I, Rings A, Byczkowicz N, Harada H, Shigemoto R, Hirrlinger J, Eilers J, and Hallermann S (2014). Ultrafast action potentials mediate kilohertz signaling at a central synapse. Neuron 84, 152–163. [DOI] [PubMed] [Google Scholar]
- Robberechts Q, Wijnants M, Giugliano M, and De Schutter E (2010). Long-term depression at parallel fiber to Golgi cell synapses. J Neurophysiol 104, 3413–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh SE, Kim SH, Ryu C, Kim CE, Kim YG, Worley PF, Kim SK, and Kim SJ (2020). Direct translation of climbing fiber burst-mediated sensory coding into post-synaptic Purkinje cell dendritic calcium. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Alford S, Mugnaini E, and Slater NT (1995). Properties of transmission at a giant glutamatergic synapse in cerebellum: the mossy fiber-unipolar brush cell synapse. J Neurophysiol 74, 24–42. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, and Hamann M (1998). Spillover-mediated transmission at inhibitory synapses promoted by high affinity alpha6 subunit GABA(A) receptors and glomerular geometry. Neuron 20, 783–795. [DOI] [PubMed] [Google Scholar]
- Rowan MJM, Bonnan A, Zhang K, Amat SB, Kikuchi C, Taniguchi H, Augustine GJ, and Christie JM (2018). Graded Control of Climbing-Fiber-Mediated Plasticity and Learning by Inhibition in the Cerebellum. Neuron 99, 999–1015 e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph S, Guo C, Pashkovski SL, Osorno T, Gillis WF, Krauss JM, Nyitrai H, Flaquer I, El-Rifai M, Datta SR, and Regehr WG (2020). Cerebellum-Specific Deletion of the GABAA Receptor delta Subunit Leads to Sex-Specific Disruption of Behavior. Cell Rep 33, 108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MJ, Yau H-J, Nunzi M-G, Mugnaini E, and Martina M (2008). Dynamic metabotropic control of intrinsic firing in cerebellar unipolar brush cells. Journal of neurophysiology 100, 3351–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M, and Hockfield S (1990). Molecular identification of the lugaro cell in the cat cerebellar cortex. The Journal of Comparative Neurology 301, 575–584. [DOI] [PubMed] [Google Scholar]
- Sakurai M. (1987). Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J Physiol 394, 463–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, and Nicoll RA (1996). Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron 16, 797–803. [DOI] [PubMed] [Google Scholar]
- Sarnaik R, and Raman IM (2018). Control of voluntary and optogenetically perturbed locomotion by spike rate and timing of neurons of the mouse cerebellar nuclei. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saviane C, and Silver RA (2006). Fast vesicle reloading and a large pool sustain high bandwidth transmission at a central synapse. Nature 439, 983–987. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Gao Z, Boele HJ, Veloz MF, Amerika WE, Simek AA, De Jeu MT, Steinberg JP, Takamiya K, Hoebeek FE, et al. (2011). Reevaluating the role of LTD in cerebellar motor learning. Neuron 70, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster SA, Wagner MJ, Pan-Doh N, Ren J, Grutzner SM, Beier KT, Kim TH, Schnitzer MJ, and Luo L (2021). The relationship between birth timing, circuit wiring, and physiological response properties of cerebellar granule cells. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe RV, Fu Y, and Watson C (2012). Cerebellum. 360–397. [Google Scholar]
- Simat M, Parpan F, and Fritschy JM (2007). Heterogeneity of glycinergic and gabaergic interneurons in the granule cell layer of mouse cerebellum. J Comp Neurol 500, 71–83. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Haasdijk ED, Engel DC, Plesnila N, and Weber JT (2007). Aldolase C-positive cerebellar Purkinje cells are resistant to delayed death after cerebral trauma and AMPA-mediated excitotoxicity. Eur J Neurosci 26, 649–656. [DOI] [PubMed] [Google Scholar]
- Soler-Llavina GJ, and Sabatini BL (2006). Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells. Nat Neurosci 9, 798–806. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Llinas R, and Baker R (1974). Structural study of inferior olivary nucleus of the cat: morphological correlates of electrotonic coupling. J Neurophysiol 37, 541–559. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, and Thompson RF (1989). Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse 3, 225–233. [DOI] [PubMed] [Google Scholar]
- Straub I, Witter L, Eshra A, Hoidis M, Byczkowicz N, Maas S, Delvendahl I, Dorgans K, Savier E, Bechmann I, et al. (2020). Gradients in the mammalian cerebellar cortex enable Fourier-like transformation and improve storing capacity. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I, and Shinoda Y (2007). Molecular, topographic, and functional organization of the cerebellar nuclei: analysis by three-dimensional mapping of the olivonuclear projection and aldolase C labeling. J Neurosci 27, 9696–9710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvrathan A, Payne HL, and Raymond JL (2016). Timing Rules for Synaptic Plasticity Matched to Behavioral Function. Neuron 92, 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester SJG, Lee MM, Ramirez AD, Lim S, Goldman MS, and Aksay ERF (2017). Population-scale organization of cerebellar granule neuron signaling during a visuomotor behavior. Sci Rep 7, 16240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szapiro G, and Barbour B (2007). Multiple climbing fibers signal to molecular layer interneurons exclusively via glutamate spillover. Nat Neurosci 10, 735–742. [DOI] [PubMed] [Google Scholar]
- Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, and Sahin M (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488, 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Hidaka N, Isomura Y, Matsuzaki M, Sakimura K, Kano M, and Kitamura K (2019). Modular organization of cerebellar climbing fiber inputs during goal-directed behavior. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecek J, Jackman SL, and Regehr WG (2016). Synaptic Specializations Support Frequency-Independent Purkinje Cell Output from the Cerebellar Cortex. Cell Rep 17, 3256–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecek J, Jackman SL, and Regehr WG (2017). Synaptotagmin 7 confers frequency invariance onto specialized depressing synapses. Nature 551, 503–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp S, and De Zeeuw CI (2014). Variable timing of synaptic transmission in cerebellar unipolar brush cells. Proceedings of the National Academy of Sciences 111, 5403–5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Welie I, Roth A, Ho SS, Komai S, and Hausser M (2016). Conditional Spike Transmission Mediated by Electrical Coupling Ensures Millisecond Precision-Correlated Activity among Interneurons In Vivo. Neuron 90, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervaeke K, Lorincz A, Gleeson P, Farinella M, Nusser Z, and Silver RA (2010). Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input. Neuron 67, 435–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervaeke K, Lorincz A, Nusser Z, and Silver RA (2012). Gap junctions compensate for sublinear dendritic integration in an inhibitory network. Science 335, 1624–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadiche JI, and Jahr CE (2005). Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci 8, 1329–1334. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, and Luo L (2020). Neocortex-Cerebellum Circuits for Cognitive Processing. Trends Neurosci 43, 42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ (2002). Furosemide reveals heterogeneous GABA(A) receptor expression at adult rat Golgi cell to granule cell synapses. Neuropharmacology 43, 737–749. [DOI] [PubMed] [Google Scholar]
- Walter JT, Alvina K, Womack MD, Chevez C, and Khodakhah K (2006). Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci 9, 389–397. [DOI] [PubMed] [Google Scholar]
- Walter JT, and Khodakhah K (2009). The advantages of linear information processing for cerebellar computation. Proc Natl Acad Sci U S A 106, 4471–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Cuntz H, Mori M, Nusser Z, Sjostrom PJ, and Hausser M (2009). Traveling waves in developing cerebellar cortex mediated by asymmetrical Purkinje cell connectivity. Nat Neurosci 12, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise AK, Cerminara NL, Marple-Horvat DE, and Apps R (2010). Mechanisms of synchronous activity in cerebellar Purkinje cells. J Physiol 588, 2373–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter L, Rudolph S, Pressler RT, Lahlaf SI, and Regehr WG (2016). Purkinje Cell Collaterals Enable Output Signals from the Cerebellar Cortex to Feed Back to Purkinje Cells and Interneurons. Neuron 91, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Blot FG, Wong AB, Osório C, Adolfs Y, Pasterkamp RJ, Hartmann J, Becker EB, Boele H-J, De Zeeuw CI, and Schonewille M (2019). TRPC3 is a major contributor to functional heterogeneity of cerebellar Purkinje cells. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, and Regehr WG (2003). Ultrastructural contributions to desensitization at cerebellar mossy fiber to granule cell synapses. J Neurosci 23, 2182–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, and Lisberger SG (2014). Purkinje-cell plasticity and cerebellar motor learning are graded by complex-spike duration. Nature 510, 529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampini V, Liu JK, Diana MA, Maldonado PP, Brunel N, and Dieudonne S (2016). Mechanisms and functional roles of glutamatergic synapse diversity in a cerebellar circuit. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Lin Z, Voges K, Ju C, Gao Z, Bosman LW, Ruigrok TJ, Hoebeek FE, De Zeeuw CI, and Schonewille M (2014). Cerebellar modules operate at different frequencies. Elife 3, e02536. [DOI] [PMC free article] [PubMed] [Google Scholar]