Abstract
Purpose
The purpose of this study was to evaluate the clinical usefulness of zero-echo-time (ZTE)–based magnetic resonance imaging (MRI) in planning the optimum surgical approach and applying ZTE for anatomical guidance during transcranial surgery.
Methods
Eleven of 26 patients who underwent transcranial surgery and carotid endarterectomy and in whom ZTE-based MRI and magnetic resonance angiography (MRA) data were obtained were analyzed by creating ZTE/MRA fusion images and 3D ZTE-based MRI models. We examined whether these images and models can be substituted for computed tomography imaging for neurosurgical procedures. Furthermore, the clinical usability of the 3D ZTE-based MRI models was evaluated by comparing them with actual surgical views.
Results
Zero-echo-time/MRA fusion images and 3D ZTE-based MRI models clearly illustrated the cranial and intracranial morphology without radiation exposure or the use of iodinated contrast medium. The models allowed determination of the optimum surgical approach to cerebral aneurysms, brain tumors near the brain surface, and cervical internal carotid artery stenosis by visualizing the relationship of lesions with adjacent bone structures. However, ZTE-based MRI did not provide useful information for surgery for skull base lesions such as vestibular schwannoma because bone structures of the skull base often include air components, which cause signal disturbance in MRI.
Conclusions
Zero-echo-time sequences on MRI allowed distinct visualization of not only bone but also vital structures around the lesion. This technology has low invasiveness for patients and was useful for preoperative planning and guidance of the optimum approach during surgery in a subset of neurosurgical diseases.
Keywords: Zero-echo-time sequence, new bone imaging, neurosurgical procedure, new three-dimensional modeling with magnetic resonance angiography
Introduction
Recently, magnetic resonance imaging (MRI) using the zero-echo-time (ZTE) sequence has been proposed as a new imaging method for bone identification.1–5 The conventional MRI sequence cannot be used to visualize bone components such as cortical bone and calcified structures. For information about bone components, computed tomography (CT) images are typically obtained to evaluate the relationship between bone and lesions including vital structures in the operative field, especially during skull base surgery. The ZTE sequence allows direct visualization of bone structures on MRI.1–5 In addition, the technology of magnetic resonance angiography (MRA) has been remarkably improved, and the combination of bone images obtained with ZTE and vascular images obtained with MRA enables clear and easy evaluation of the relationship between the skull and vasculature without the use of iodine contrast agents. 6 It has been demonstrated that three-dimensional (3D)-ZTE–based MRI models are very useful for visualization of vital structures and tumors around the sellar floor for endoscopic endonasal transsphenoidal surgery (ETSS) for pituitary tumors. 4 However, whether and how ZTE-based MRI is valuable for lesions treated by transcranial surgery or other methods have not been reported. Here, we present MR images of diseases that were treated with transcranial surgery or carotid endarterectomy (CEA) taken by using ZTE sequences. We investigated whether ZTE-based MRI and ZTE-based 3D MRI models are useful for providing preoperative orientation of critical structures, including bone structures, and how these images and models can be applied to achieve an exact roadmap for neurosurgical procedures.
Materials and methods
All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The present study was approved by the local ethics committee for clinical research at our institute (approval no. xxxx). Informed consent was obtained from all individual participants enrolled in the study, including for the surgical procedure and potential risks.
Experimental design
In this study, 11 patients who underwent transcranial surgery and CEA among 26 patients with data for ZTE-based MRI and MRA were analyzed by creating ZTE-based MRA fusion images and 3D ZTE-based MRI models (Table 1). The MR images were acquired by radiologists, and the 3D-reconstructed models were created by another radiologist in this research. We evaluated whether these fusion images and 3D models can provide usable quality imaging of both lesions and bone structures, and whether they can be used for transcranial surgery as guiding images.
Table 1.
Patient characteristics.
| Parameter | Value |
|---|---|
| No. of patients | 11 |
| Female gender (%) | 9 (81.8) |
| Age (years), median (range) | |
| Type of disease (%) | |
| Cervical internal carotid artery stenosis | 1 (9.1) |
| Unruptured aneurysm | 4 (36.4) |
| Brain tumor | 6 (54.5) |
| Type of brain tumor pathology (%) | |
| Glioblastoma | 2 (33.3) |
| Meningioma | 3 (50.0) |
| Vestibular neurinoma | 1 (16.7) |
| Disease of skull base lesion (%) | |
| Acquisition of zero-echo-time dataset (%) | 11 (100) |
| 3D MRI model construction (%) | 11 (100) |
No., number; 3D MRI, three-dimensional magnetic resonance imaging.
Constructed 3D ZTE-based MRI models
Prior to surgery, patients underwent MRI and MRA using a 3.0-T whole-body MR scanner (General Electric (GE) Healthcare, Waukesha, WI) with a forty eight-channel phased-array head coil. Patients were given an i.v. injection of gadopentetate dimeglumine, and a high-resolution anatomic dataset was established for each patient from 3D spoiled gradient recalled echo sequences (repetition time (TR), 15 ms; echo time (TE), 2.3 ms; flip angle, 10°; matrix, 256 × 320; field of view (FOV), 230 mm; and thickness, 0.9 mm). 3D time-of-flight MRA sequences (TR, 23 ms; TE, 3.4 ms; flip angle, 18°; matrix, 416 × 224; FOV, 230 mm; and thickness; 0.5 mm) were obtained for arterial information. Novel bone imaging based on proton density (PD)-weighted ZTE was newly obtained for this study. A 3D gradient-echo imaging technique with a very short TE and low flip angles were implemented for this purpose. This sequence involves inherent contrast without preparatory pulses, similar to PD. The pulse sequence incorporates a 3D radial center-out sampling scheme in which the terminations of each radial line follow a spiral trajectory in time. Isotropic voxels were obtained with a TE near 0 ms. The acquisition time was 393 s, the FOV was 12 cm (transaxial) and 26 cm (axial), and the resolution was 1.0 × 1.0 × 1.0 mm. Four excitations were obtained that had a flip angle of 1° and bandwidth of 62.5 kHz. 4 MRI data were analyzed with a 3D Advantage Workstation Volume Share 4 (GE Healthcare). 4 The acquired fusion image model data from MRI were saved in Digital Imaging and Communications in Medicine format. A slide presentation file consisting of digital images obtained at different stages of the simulated surgery was used for visual confirmation during the actual surgery. The locations of the tumor and vital structures according to the 3D model images were assessed and compared with the actual intraoperative microscopic and endoscopic views. These procedures were described in detail previously. 4
Statistical methods
Statistical analysis was performed using Fisher’s exact test for categorical variables and analysis of variance for continuous variables. Two-tailed tests were performed for each scenario, and the significance level was set at p < .05. All analyses were performed using Office Excel 2016 software (Microsoft, Redmond, WA).
Results
The diseases of the 11 patients included four unruptured cerebral aneurysms, two malignant gliomas, three meningiomas, one vestibular schwannoma, and one cervical internal carotid artery (ICA) stenosis (Table 1). Except for the patient with ICA stenosis, all remaining patients underwent transcranial surgery.
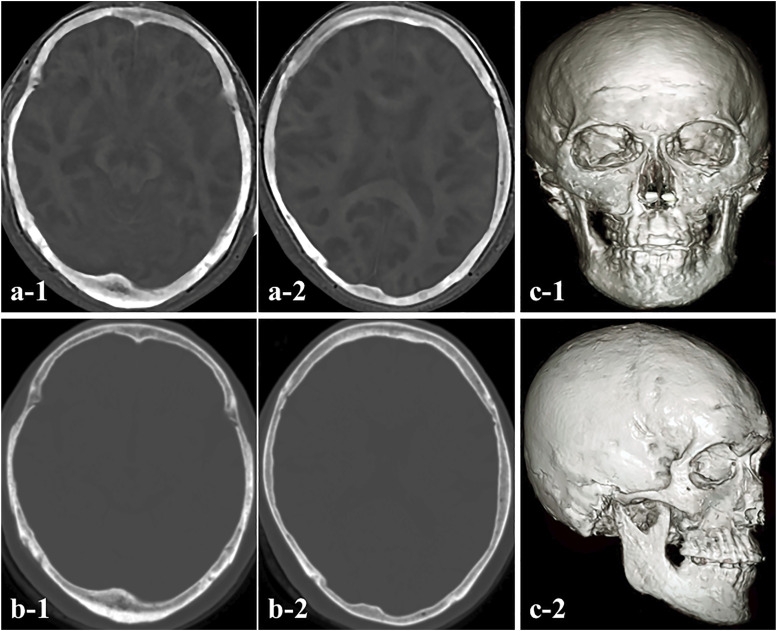
Zero-echo-time–based MRI data (PD image) were obtained from all patients. After acquiring the PD images, we converted these data into CT-like ZTE images to allow recognition of bone structures (Figure 1(a)). We observed almost no differences in the depiction of the bone edge of the skull between ZTE-based MRI and CT (Figure 1(b)), and also no imaging distortion of the bone edge on ZTE-based MRI. In addition, we successfully created ZTE-based 3D MRI models by combining these ZTE-based MRI datasets (Figure 1(c)). The models also permitted determination of the surgical procedure including the minimum marginal line of the craniotomy and optimum surgical approach by visualizing bone structures including the cranium and vital structures such as bridging veins and arteries near the lesions. With such 3D MRI models, the surgeon could access the intracranial area through the cranium in a safer manner without complications. The ZTE-based 3D MRI models can be viewed in the operating room during surgery as multi-slice images on a computer monitor. Compared to existing 3D-CT-based models or 3D-CT/MRI fusion models, the ZTE-based 3D MRI models were not inferior for understanding anatomical locations between lesions and bone structures. In addition, they had the advantages of no radiation exposure and no use of iodine contrast agent. However, ZTE-based MRI was not suitable for lesions at the skull base and those requiring precise microsurgery such as vestibular schwannoma.
Figure 1.
(a) Bone images obtained with zero-echo-time (ZTE)-based magnetic resonance imaging (MRI). (b) Computed tomography (CT) showing axial views of a typical dataset for the skull region. The edges of the cranium acquired with the ZTE sequence were almost matched to the images obtained with CT, and no limbal distortion was observed. (c) 3D ZTE-based MRI model demonstrating cranial bone.
Illustrative cases
Case 1
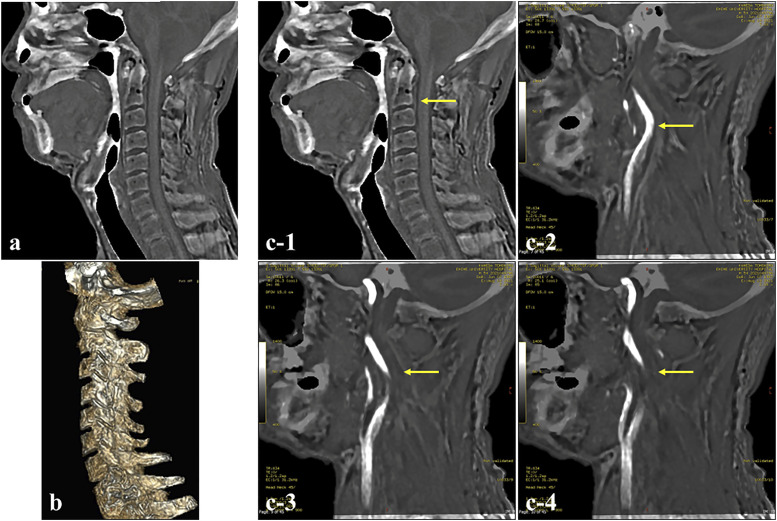
A 54-year-old man visited our department with repeated motor weakness in the left upper extremity. Cervical MRA and carotid artery echography revealed irregular stenosis in the right ICA. Evaluation of the level of vertebral bodies that corresponded to the distal site of the stenotic lesion was required for surgical intervention with CEA. We investigated whether ZTE-based MRI could determine the positional relationship between the stenotic lesion and level of vertebral bodies of the cervical spine. The ZTE sequence could be used to visualize the vertebral body of the cervical spine (Figures 2(a) and (b)). By fusing the MRA and ZTE sequences, we could produce images that were almost equivalent to computed tomographic angiography (CTA) (Figure 2(c)), and the positional relationship between the lesion and the vertebral body could be fully understood.
Figure 2.
Identification of vertebral body at the distal portion of the internal carotid artery (ICA) stenosis site (Case 1). (a) ZTE-based MRI, sagittal view. (b) 3D ZTE-based MRI model showing the cervical vertebral body. (c) Fusion images of ZTE-based MR image and magnetic resonance angiography (MRA) at the cervical portion. The image demonstrated a stenotic lesion of the ICA and the level of the vertebral body corresponding to the height at the distal end of the stenotic lesion (bold yellow arrow) (c-1: ZTE-based MRI, c-2, 3, 4: Fusion image).
Case 2
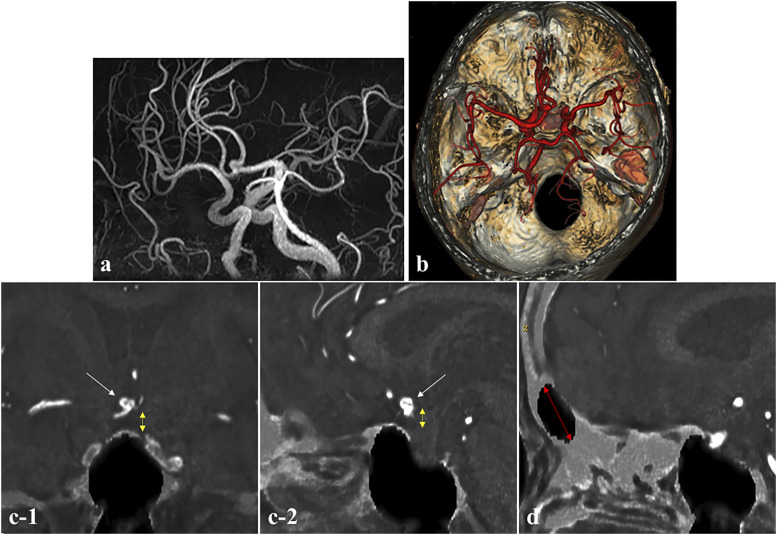
A 69-year-old woman was referred to our hospital for headache. MRA showed a cerebral aneurysm located on the anterior communicating artery (Figure 3(a)), and the 3D ZTE-based MRI model clearly depicted the aneurysm (Figure 3(b)). Thus, we assessed whether we could determine the appropriate surgical approach in this case using ZTE/MRA fusion images. As shown in Figure 3, a fusion image of MRA and ZTE-based MRI depicted the positional relationship between the aneurysm and the planum sphenoidale very well. Thus, the fusion image could be used to estimate the height of the aneurysm from the frontal base and determine the appropriate surgical approach (Figure 3(c)). In addition, because the size of the frontal sinus could be imaged to some extent, the fusion image was a guide for performing the craniotomy without opening the frontal sinus (Figure 3(d)).
Figure 3.
Information needed to determine the surgical approach for an anterior communicating artery aneurysm (Case 2). (a) MRA showing the anterior communicating artery aneurysm and (b) Fusion image of 3D ZTE-based model and MRA showing the positional relationship of the anterior communicating artery aneurysm and bone structure of the skull base. (c-1) Coronal and (c-2) sagittal fusion images of ZTE-based image and MRA, demonstrating the height of the aneurysm from the planum sphenoidale (white arrow: aneurysm, yellow double-headed dashed arrow: distance between aneurysm and planum sphenoidale) and the size of the frontal sinus (red double-headed dashed arrow). (d) Identification of the size and location of the frontal sinus.
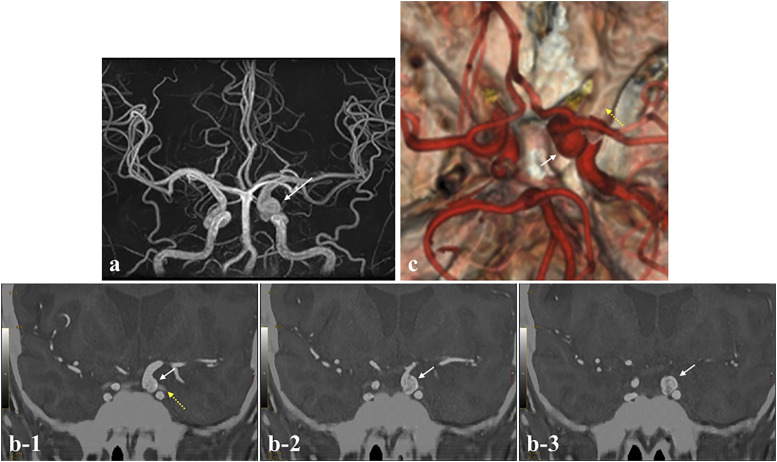
Case 3
A 43-year-old woman with headache was referred to our department. MRA revealed a cerebral aneurysm located on the left ICA (Figure 4(a)). In determining a surgical plan, it is important to grasp the accurate location of the aneurysm (intracranial or extracranial) and positional relationship between the aneurysm and the anterior clinoid process. So, the bone image was created with the ZTE-based MRI model and fused with MRA. This fusion image disclosed that the aneurysm was located intracranially (Figure 4(b)). In addition, the 3D model clearly displayed the positional relationship between the aneurysm and the anterior clinoid process (Figure 4(c)). Thus, the 3D ZTE-based MRI model could serve as an imaging guide for the safe treatment strategy of the aneurysm.
Figure 4.
Information needed to determine the surgical approach for a left ICA aneurysm (Case 3). (a) MRA showing the left ICA aneurysm (C3 portion). (b) The fusion image of ZTE-based image and MRA depicted clearly that the aneurysm was located intracranially. (c) Fusion image of 3D ZTE-based model and MRA showing the positional relationship between the aneurysm and anterior clinoid process (white arrow: aneurysm, yellow dashed arrow: anterior clinoid process).
Case 4
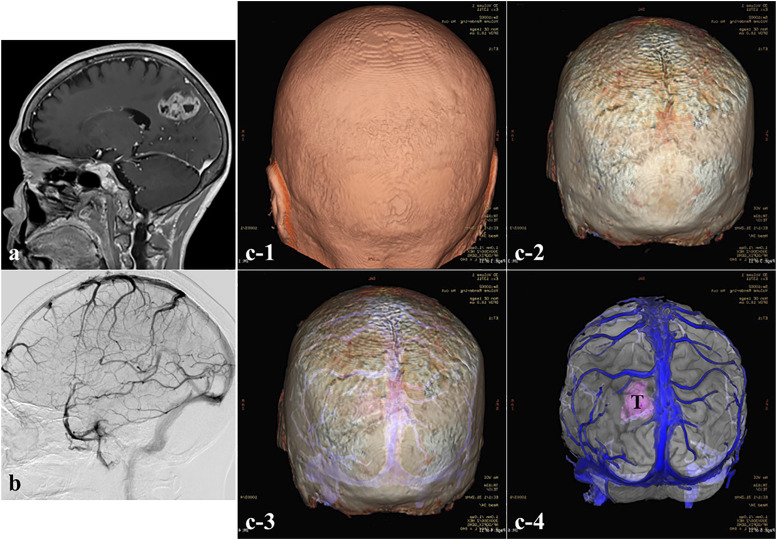
A 70-year-old woman with epilepsy was referred to our department on suspicion of glioblastoma (GBM). MRI showed a heterogeneously enhanced tumor with necrotic cysts in the right parietal lobe, and cerebral angiography showed a large cortical vein at the anterior border of the tumor (Figures 5(a) and (b)). The bone image was created with the 3D ZTE-based MRI model and fused with gadolinium (Gd)-weighted MRI. These MRI models clearly showed the positional relationships among the scalp, cranial bone, cortical veins, and tumor mass. Thus, the 3D ZTE-based MRI model could serve as an imaging guide for the optimum craniotomy, allowing safe and maximum tumor resection (Figure 5(c)).
Figure 5.
Operative approach in the case of a glioblastoma (GBM) using the ZTE sequence (Case 4). (a) Preoperative sagittal Gd-enhanced MRI and (b) cerebral angiography showing a heterogeneously enhanced tumor with necrotic cysts in the right parietal lobe and cortical veins in the anterior part of the tumor. (c1–c4) Preoperative 3D ZTE-based MRI model fused with Gd-enhanced MRI clearly revealed the location of the scalp, skull, cortical veins, and tumor. T, tumor. Endoscopic view of the reconstruction procedure after tumor removal. T, tumor; ON, optic nerve; ICA, internal carotid artery; MCA, middle cerebral artery; ACA, anterior cerebral artery.
Case 5
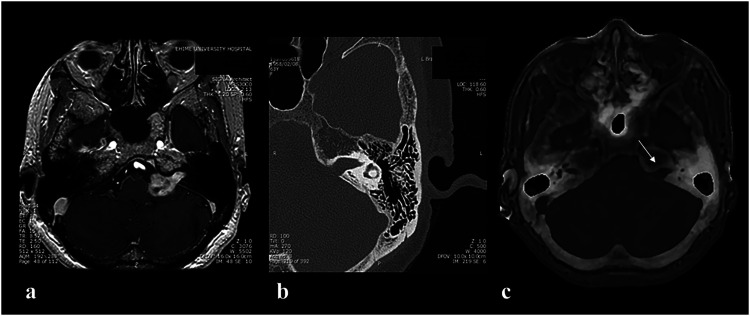
A 63-year-old woman was referred to our department because of left-sided hearing loss that began about 5 years ago. Gd-enhanced MRI showed an intensely Gd-enhanced tumor mass with tongue-like protrusion into the internal auditory canal (IAC) in the left cerebellopontine angle, which is equivalent to a vestibular schwannoma (Figure 6(a)). CT showed enlargement of the left IAC (Figure 6(b)). We investigated whether ZTE-based MRI could provide crucial information for performing surgery, similar to information obtained with CT. The information related to the bone structures included the following: Assessment of (1) the size of the enlarged IAC, (2) the degree of development of the mastoid air cells, and (3) the exact location of the semicircular canal. Although an enlarged IAC was confirmed (Figure 6(c)), evaluation of the development of the mastoid air cells compared with CT was difficult (Figure 7). In addition, for identification of the location of the semicircular canal, which is required for drilling of the IAC, ZTE-based MRI showed the presence of the semicircular canal, but the precise structures were not obvious (Figure 7).
Figure 6.
(a) Gd-enhanced MRI showing a tumor located at the left cerebellopontine angle cistern that was protruding into the left internal auditory canal (IAC) (Case 5). (b) CT with the condition of bone density showing an enlargement of the left IAC and the degree of development of mastoid air cells. (c) ZTE-based MRI revealing the expansion of the left IAC, but no clear depiction of mastoid air cells (white arrow: left IAC).
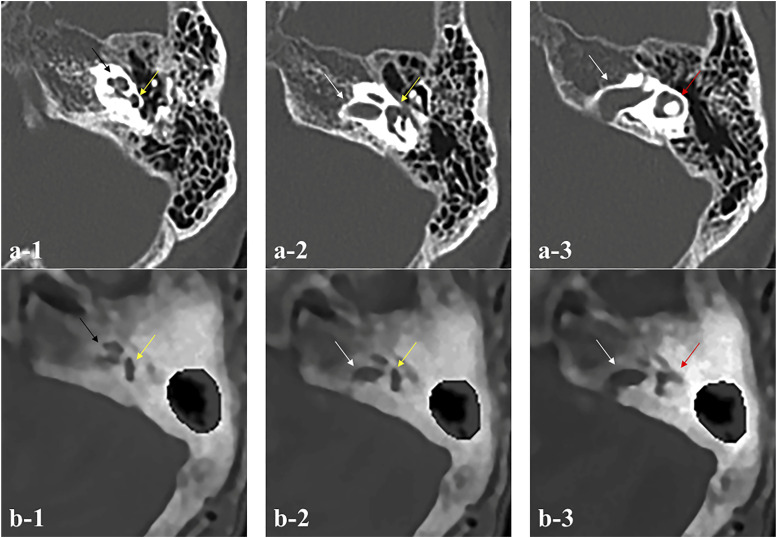
Figure 7.
Weakness of ZTE-based MRI compared with CT imaging. (a1-3) Detailed images of internal structures of pyramidal bone by CT and (b1-3) by the ZTE-based MRI in Case 5. Compared with CT imaging, exact identification of the fine structures in the inner ear including accurate location of the semicircular canal is difficult (black arrow: cochlea, yellow arrow: superior semicircular canal, white arrow: IAC, red arrow: lateral semicircular canal).
Discussion
By taking advantage of its zero TE MRA feature, silent technology “SILENT SCAN” developed by GE Healthcare is useful for pediatric examinations and is also used for vascular evaluation after coil embolization with stenting.5,7,8 However, in recent years, ZTE-based MR imaging has been applied for bone imaging.1–5
Computed tomography scans are the current gold standard technology to evaluate bone structures.1–5 Therefore, CT imaging is considered an essential tool for analyzing bone structures in neurosurgical practice. In addition, CTA and 3D-CT models are extremely important diagnostic methods for understanding the location of a lesion in relation to the skull and perilesional vasculature. These CT images can show the clear and exact orientation, thus playing a critical role as an anatomic indicator for both transcranial and endoscopic transsphenoidal surgery. Furthermore, preoperative surgical planning can be performed by simulation with these models according to the individual patient’s anatomy.9,10 However, clinical application of both CTA and 3D-CT–based models also has several disadvantages. Disadvantages are that patients often receive excessive radiation exposure to X-rays from CT examination, and iodine contrast agents for visualization of vascular structures sometimes cause a serious allergic reaction.9,10 Accordingly, a less invasive imaging method for evaluating bone structures and the relationship between bones and pathological structures is eagerly awaited.
In contrast, MRI is a non-invasive and highly diagnostic imaging method by which images with excellent soft tissue contrast and high resolution of anatomical details in the body can be obtained without exposure to radiation.1–5,11 However, conventional MR sequences cannot depict cortical bone tissue as a signal void because of the low PD and very short T2 relaxation time.3–5,12 Therefore, this modality is considered inappropriate for depicting bone structures including the cranium. Recently, GE Healthcare developed an investigational work-in-progress MR research package called ZTE for bone imaging, consisting of a pulse sequence technique designed to image cortical bone surfaces. 4 The ZTE sequence is a 3D radial sequence used for silent MRI, and the extremely short effective TE allows detection of the shortest T2 tissues, including cortical bone.1,3–5 To date, in neuroradiology, ZTE-based MRI has been gradually introduced to evaluate skull lesions in patients with head trauma. 3 We have reported that ZTE is a useful tool for providing a roadmap for surgical approaches to ETSS for pituitary adenomas. 4
In a previous study, it was shown that MRI using the ZTE sequence can be used to obtain CT-like bone imaging. 4 In addition, it was demonstrated that the bone images acquired from MRI were matched with those of CT. These results supported the use of ZTE-based MRI as guiding images in the navigation system (Figures 1(a) and (b)). However, whether ZTE-based MRI can be used in the field of neurosurgery in general as a useful diagnostic image and/or an assisting image for surgical procedures, particularly in transcranial and other surgeries, has remained an open question. Several problems have been reported for ZTE sequences.1,4,5 In previous reports, the boundary between bone and fluid as seen in the inner ear was a major problem because images depicted by ZTE-based MRI did not obviously show the boundary. Furthermore, the accuracy is insufficient at the base of the skull bone or around the paranasal sinuses. 1 Also, in the present study, a clear depiction of mastoid air cells was not obtained as shown in Figures 6 and 7. Unfortunately, because the patient number was very small, a cut-off value of the air volume could not be determined. Therefore, by using ZTE-based MRI, we obtained images of lesions including bony structures in various diseases treated with transcranial surgery, CEA, and ETSS, and analyzed for what types of diseases ZTE-based MRI can be used and the effectiveness of ZTE-based MRI.
3D MRI models created by combining ZTE-based MRI and MRA enabled us to determine the optimum surgical approach to the lesions. The models allowed full understanding of the positional relationship among the cranium, vertebral bodies, cortical veins, and lesions such as tumors, aneurysms, and arterial stenosis. In addition, fusing of ZTE-based MRI and MRA allowed us to obtain images similar to CTA without the use of iodinated contrast medium. According to the results of this study, the diseases and their situations for which ZTE sequences can be effectively used in place of CT include: (1) decision of the level of the vertebral body at the distal portion of ICA stenosis, (2) optimum approach to cerebral aneurysms including the positional relationship between aneurysms and bone structures of the skull base, and (3) resection of tumors such as GBMs located near the surface of the brain with large cortical veins over the tumor. In contrast, some concerns exist regarding lesions of the skull base including vestibular schwannoma because the presence of air in the bone disrupts the MR signal, resulting in inaccurate imaging. As we discovered in this study, grasping the skull in an area close the air is difficult. Certainly, bone depiction using the ZTE sequence is inferior to CT images, particularly images of fine bone structures of the skull base including the semicircular canal, IAC, cochlea, and mastoid air cells. Therefore, it is difficult to use ZTE sequence as a preoperative and intraoperative navigation image when performing skull base surgery, which requires more precise procedures. Although many issues remain to be addressed, the ZTE sequence is useful. The fact that bone structures can be determined with MRI is attractive because it is less invasive to the patient, and the patient is not exposed to radiation. Although ZTE-based MRI is thought to be an attractive MRI sequence as a surgical assistant image to provide bony information for not only ETSS but also transcranial and other surgeries, further experience with more cases will be needed to confirm the safety and effectiveness of this technique.
Conclusion
The ZTE-based MRI model provided surgeons with imaging of clear anatomical information of bone structures for transcranial surgery for unruptured aneurysms and brain tumors and CEA for ICA stenosis. The model can optimize preoperative surgical planning by identifying the individual anatomy of the patient. In addition, the greatest advantage of this model is the low invasiveness to patients. In contrast, the model did not depict clear and precise bone images in the skull base including the semicircular canal, IAC, cochlea, and mastoid air cells. Thus, the ZTE-based MRI model is thought to be not suitable for preoperative bone imaging for skull base surgery in which precise and fine microsurgery including bone drilling is required. This sequence provides critical anatomical information about bone-containing lesions and bone-affecting lesions prior to surgical procedures, allowing preoperative determination of the surgical strategy, and can provide anatomical guidance for smoother, safer surgery.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
ORCID iD
Akihiro Inoue https://orcid.org/0000-0002-7438-2369
References
- 1.Delso G, Wiesinger F, Sacolick LI, et al. Clinical evaluation of zero-echo-time MR imaging for the segmentation of the skull. J Nucl Med 2015; 56: 417–422. [DOI] [PubMed] [Google Scholar]
- 2.Wiesinger F, Sacolick LI, Menini A, et al. Zero TE MR bone imaging in the head. Magn Reson Med 2016; 75: 107–114. [DOI] [PubMed] [Google Scholar]
- 3.Cho SB, Baek HJ, Ryu KH, et al. Clinical feasibility of zero TE skull MRI in patients with head trauma in comparison with CT: a single-center study. AJNR Am J Neuroradiol 2019; 40: 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue A, Kohno S, Nishida N, et al. Clinical utility of new three-dimensional model using a zero-echo-time sequence in endoscopic endonasal transsphenoidal surgery. Clin Neurol Neurosurg 2020; 190: 105743. [DOI] [PubMed] [Google Scholar]
- 5.Ljungberg E, Damestani NL, Wood TC, et al. Silent zero TE MR neuroimaging: current state-of-the-art and future directions. Prog Nucl Magn Reson Spectrosc 2021; 123: 73–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo PH, Kanal E, Abu-Alfa AK, et al. Gadolinium based MR contrast agents and nephrogenic systemic fibrosis. Radiology 2007; 242: 647–649. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo-Hagiyama C, Watanabe Y, Tanaka H, et al. Comparison of silent and conventional MR imaging for the evaluation of Myelination in children. Magn Reson Med Sci 2017; 16: 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takano N, Suzuki M, Irie R, et al. Usefulness of non-contrast-enhanced MR angiography using a silent scan for follow-up after Y-Configuration stent-assisted coil embolization for Basilar tip aneurysms. AJNR Am J Neuroradiol 2017; 38: 577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue A, Ohnishi T, Kohno S, et al. Utility of three-dimensional computed tomography for anatomical assistance in endoscopic endonasal transsphenoidal surgery. Neurosurg Rev 2015; 38: 559–565. [DOI] [PubMed] [Google Scholar]
- 10.Inoue A, Ohnishi T, Kohno S, et al. Usefulness of an image fusion model using three-dimensional CT and MRI with indocyanine green fluorescence endoscopy as a multimodal assistant system in endoscopic transsphenoidal surgery. Int J Endocrinol 2015: 694273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blystad I, Warntjes JB, Smedby O, et al. Synthetic MRI of the brain in a clinical setting. Acta Radiol 2012; 53: 1158–1163. [DOI] [PubMed] [Google Scholar]
- 12.Du J, Carl M, Bydder M, et al. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J Magn Reson 2010; 207: 304–311. [DOI] [PubMed] [Google Scholar]