Abstract
Background
Emerging evidence suggests preoperative opioid use may increase the risk of negative outcomes following orthopedic procedures. This systematic review evaluated the impact of preoperative opioid use in patients undergoing shoulder surgery with respect to preoperative clinical outcomes, postoperative complications, and postoperative dependence on opioids.
Methods
EMBASE, MEDLINE, CENTRAL, and CINAHL were searched from inception to April, 2021 for studies reporting preoperative opioid use and its effect on postoperative outcomes or opioid use. The search, data extraction and methodologic assessment were performed in duplicate for all included studies.
Results
Twenty-one studies with a total of 257,301 patients were included in the final synthesis. Of which, 17 were level III evidence. Of those, 51.5% of the patients reported pre-operative opioid use. Fourteen studies (66.7%) reported a higher likelihood of opioid use at follow-up among those used opioids preoperatively compared to preoperative opioid-naïve patients. Eight studies (38.1%) showed lower functional measurements and range of motion in opioid group compared to the non-opioid group post-operatively.
Conclusion
Preoperative opioid use in patients undergoing shoulder surgeries is associated with lower functional scores and post-operative range of motion. Most concerning is preoperative opioid use may predict increased post-operative opioid requirements and potential for misuse in patients.
Level of evidence
Level IV, Systematic review.
Keywords: opioid, preoperative, shoulder surgery, total shoulder arthroplasty, arthroscopy
Introduction
The opioid crisis is a major public health issue that has resulted in widespread overdose and addiction with recent estimates suggesting that no less than 289 million opioid prescriptions are filled yearly in the United States. 1 Recent studies suggest that at least 1.9 million Americans have developed opioid use disorder,2,3 and at least 530 people die weekly from opioid overdose. 4 Over 50% of opioid-related deaths occur despite acceptable prescription of the medications with respect to medical board guidelines. 5 Additionally, complications arising from opioid misuse and overuse result in significant healthcare expenses, estimated at an annual cost of $78.5 billion in US. 6
Optimal pain control for patients with shoulder disorders or following surgical intervention is an area of ongoing research. 7 Current practices of orthopedic surgeons are highly variable and opioids are commonly relied upon or prescribed without individualizing the needs of patients. 8 As many patients expect to be pain-free postoperatively and satisfaction of treatment is associated with such an expectation, orthopedic surgeons have been keen to achieve this goal, resulting in overreliance on discharge opioid prescriptions. This is reflected in the fact that orthopedic surgeons file 7.7% of all US opioid prescriptions, representing the third-largest provider of opioids among clinicians.9,10
Opioid medications have traditionally been widely prescribed as a non-operative management of chronic joint pain. However, emerging evidence has shown that preoperative opioid use is associated with an increased risk of negative outcomes and increased postoperative demand for opioids following total joint arthroplasties.11,12 As such the utility of preoperative opioid use in such patients has come under significant scrutiny.11,13,14 Preoperative opioid use has been shown to be associated with increased readmission rates and risk of early revision among patients undergoing total knee or hip arthroplasty. 12 Although increased revision rates and readmission were also evident in patients undergoing shoulder arthroplasty and rotator cuff repair,15,16 the effects of preoperative opioid use on revision surgery, readmission, and costs after shoulder surgeries are mixed. Some studies show no link between preoperative opioid use and complications, increased duration of stay, or readmission rates following shoulder arthroplasty.17,18
The objective of this systematic review is to evaluate currently available evidence on the association between preoperative opioid use in patients undergoing shoulder surgery with respect to perioperative clinical outcomes, postoperative complications, and postoperative dependence on opioids.
Materials and methods
The study was conducted according to the Cochrane Handbook for Systematic Reviews 19 and reported as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 20
Search strategy
EMBASE, MEDLINE, PubMed, Cochrane Central Register of Controlled Trials (CENTRAL), and Cumulative Index to Nursing and Allied Health Literature (CINAHL) database were searched from date of inception to April 15th, 2021.
The search strategy, adapted to each database, included terms representing preoperative opioid use and its association with postoperative outcomes or opioid use. MeSH and EMTREE terms were used, along with free text, in several combinations to increase search sensitivity. We consulted with experts in the field, manually reviewed the reference lists of articles that fulfilled the eligibility criteria and used the “related articles” feature in PubMed. The search strategy was adapted in PubMed to search for articles published online ahead of print (Appendix 1).
Eligibility criteria
In order to maximize potentially eligible data, no restrictions was made on publication date or follow-up. The criteria for inclusion were: (1) Studies on shoulder arthroplasty or scope procedures evaluating preoperative use of opioids; (2) Adult patients (3) Studies that reported at least one outcome related to shoulder arthroplasty or scope procedures; (4) Studies focused on postoperative opioid use; (5) any randomized or non-randomized studies were included.
The exclusion criteria were: (1) Studies that did not report on pre-operative opioid use; (2) Studies that included less than five patients; (3) Reviews and conference abstracts were excluded; (4) Studies without adequately reported functional outcomes or postoperative opioid use; (5) We excluded patients with functional pain syndromes. (6) Non-English language studies were excluded.
Study screening
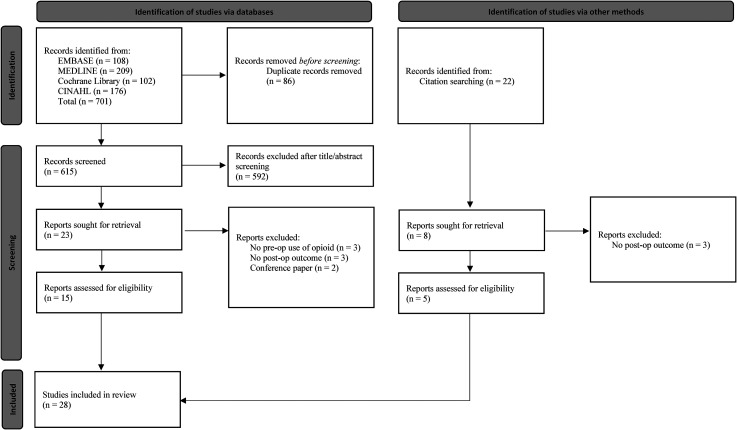
Study selection was performed by two authors (OAM, CP) using Covidence (Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia). The selection of studies was performed in a stepwise manner, first by title and abstract, then full-text review. A resolve-by-consensus strategy was utilized for all discrepancies. If consensus could not be reached, a third senior researcher (MK) was consulted. Manual screening of the references of included studies was done to identify additional articles which may have eluded the initial search strategy (Figure 1).
Figure 1.
PRISMA flow diagram.
Extraction of data
Data were extracted by two reviewers (OAM, CP) independently from each other into a collaborative pre-defined data abstraction web-based spreadsheet (Google Sheets, 2021. California, United States: Google LLC.). To ensure accuracy, the results were audited by both reviewers (OAM, CP) and reviewed by the principal investigator (MK).
Extracted data included information on basic study characteristics (authors, publication year, country, journal of publication, study type), demographic data (age, sex, sample size, diagnosis, intervention type), preoperative opioid use, period of follow-up, study outcomes, and complications. The authors of included studies were contacted if there were any uncertainties regarding the data.
Risk of bias and quality assessment
The Methodological Index for Non-Randomized Studies (MINORS) was used by two independent reviewers (OAM, CP) to assess risk of bias in the included studies. 21 The MINORS scale assigns a score of 0, 1, or 2 for a list of 8 and 12 questions in non-comparative, and comparative studies, respectively.
Statistical analysis
Descriptive statistics were calculated to reflect the frequency and percentage of abstracted study data. Using Cohen's Kappa (κ), inter-reviewer agreement at each stage of the screening process was calculated. Agreement was categorized a priori, as per Landis and Koch, 22 with k of 0.81 to 1.0 for near perfect agreement; k of 0.61 to 0.80 for substantial agreement; k of 0.41 to 0.60 for moderate agreement; and k of 0.21 to 0.40 as fair agreement. Interobserver agreement for methodologic quality assessment was calculated using the intraclass correlation coefficient (ICC); a value of ≥0.65 was considered adequate. 23
Results
Study characteristics
The search strategy retrieved 701 studies, of which 28 were identified to undergo full-text review. Twenty-one studies which met all of the inclusion criteria and were included in the final qualitative synthesis (Figure 1).15–18,24–40 There was substantial agreement for both title and abstract screening (Cohen's kappa = 0.71) and full-text review (Cohen's kappa = 0.72).
There was a near perfect level of agreement among quality assessment scores using the MINORS criteria (ICC, 0.93). The mean MINORS score for the included studies was 12 ± 0.7 out of 16 for non-comparative studies and 19.9 ± 1.3 out of 24 for comparative studies. Characteristics of included studies can be found in Table 1.
Table 1.
Characteristics of included studies.
| Study | Year | Study design | Level of evidence | Country | MINORS score | Age, mean ± SD | Sample size | Sex (female%) | BMI | Comorbid Conditions | Diagnosis | Operative Procedure | Follow-up preiod |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morris et al. | 2015 | Retrospective cohort study | III | US | 19 | 68.5 ± 9.5 | 68 | 39 (57.3) | 28.3 ± 7.35 | No differences were found between the preoperative opioid group and the nonopioid group in smoking status, BMI, history of chronic back pain, depression, diabetes, or heart disease. | Rotator cuff arthropathy | rTSA | 3.14 ± 1.7 |
| Morris et al. | 2016 | Retrospective cohort study | III | US | 18 | 66.5 ± 9.5 | 224 | 78 (34.8) | 30.5 ± 6.0 | No statistical differences were noted between the preoperative opioid group and the nonopioid group regarding diabetes, and heart disease, Patients with preoperative opioid use had a significantly higher prevalence of chronic back pain (52% vs. 30%; P = 0.003) and depression (13% vs. 4%; P = 0.04) and higher BMI (32 ± 7 kg/m2 vs. 29 ± 5 kg/m2; P = 0.01). | Glenohumeral joint osteoarthritis | aTSA | 3.3 ± 1.2 |
| Cuff et al. | 2016 | Prognostic case series | IV | US | 12 | 63.3 years (range, 22 to 77 years) | 181 | 79 (44) | NR | NR | Partial- or full-thickness rotator cuff tear | Arthroscopic rotator cuff surgery along with subacromial decompression | NR |
| Cheah et al. | 2017 | Retrospective cohort study | III | US | 19 | 67.3 | 262 | 139 (53) | Opioid group had a significantly greater number of patients with a BMI greater than or equal to 30 compared with the non–opioid user cohort (P = 0.016). | All other characteristics were similar, with a noted trend that the opioid cohort had a slightly higher Charlson Comorbidity Index (P = 0.057) | NR | 170 rTSA and 92 aTSA | 2 years |
| Berglund et al. | 2018 | Retrospective cohort study | III | US | 11 | 71 years (range, 30 to 90 years) | 490 | NR | NR | NR | OA without RCT, OA with RCT, Fracture sequelae, Avascular necrosis, Failed arthroplasty, Locked dislocation and Inflammatory arthritis | HA, aTSA, rTSA | 47 months; range, 24 to 124 months |
| Grace et al. | 2018 | Retrospective analysis of prospective cohort study | II | US | 21 | median age was 68 years (range, 29–89 years) | 119 | 56 (47.1) | 30.1 ± 5.4 | There was no significant difference in prevalence of depression (8.5% vs 5.6%, P = 0.53). | Osteoarthritis, Avascular necrosis, Proximal humeral fracture, Rotator cuff arthropathy | aTSA (37.0%), rTSA (63.0%). | NR |
| Menendez et al. | 2018 | Retrospective analysis of prospective cohort study | II | US | 21 | 69 ± 8 | 415 | 253 (61) | 30.8 ± 6.3 | Patients reporting severe postoperative pain were more likely to have more self-reported allergies (3.4 vs. 1.5, P < 0.001), diabetes (22% vs. 11%, P = 0.005), comorbid diagnosis of major depression (37% vs. 21%, P = 0.001), and an ASA score ≥ III (30% vs. 20%, P = 0.031). The most prevalent comorbidities were hypertension (60%), hypercholesterolemia (39%), and depression (25%). | osteoarthritis (69), rotator cuff arthropathy (22) | aTSA (29%), rTSA (71%) | NR |
| Rao et al. | 2018 | Retrospective cohort study | III | US | 12 | 84.1% (n = 3570) were aged ≥60 years | 3996 | 2201 (51.9) | 57.0% (n = 2176) had a BMI < 30 | Hypertension was the most prevalent medical comorbidity (n = 2574 [69.0%]), and depression was the most common opioid use-related comorbidity (n = 604 [17.2%]) | NR | Hemiarthroplasty/humeral head resurfacing 891 (21.0%), rTSA 923 (21.8%), aTSA 2429 (57.2%) | minimum of 1 year |
| Thompson et al. | 2019 | Retrospective cohort study | III | US | 18 | 61.0 ± 11.8 | 73 | 36 (49.3) | 32.2 ± 6.2 | No significant differences were noted between the groups with respect comorbidities including DM, smoking, chronic pain syndromes, or mood disorders (including depression and anxiety). | primary glenohumeral osteoarthritis | aTSA | 32.8 ± 10.3 months |
| Brock et al. | 2019 | Retrospective cohort study | III | US | 21 | 69.0 ± 8.6 | 22,524 | 10,170 (45.5) | NR | Depression increased the risk for chronic postoperative opioid use, as did intravenous drug use for RCR but not TSA. Smoking and chronic lung disease were not significant predictors. | diagnosis codes used for TSA and RCR | TSA and rotator cuff repair | NR |
| Curtis et al. | 2019 | Retrospective cohort study | III | US | 22 | 67.55 ± 11.15 | 138 | 73 (52.9) | NR | The opioid cohort included five patients with a medical history of myocardial infarction (versus 1 control subject; P = 0.014) and three with peptic ulcer disease (versus 0 control subject; P = 0.057). Differences between cohorts in all other comorbidities, were not statistically significant. | Osteoarthritis, Rotator cuff arthropathy, Chronic dislocation, fractures, AVN, Osteomyelitis, Periprosthetic infection, and Arthroplasty failure. | TSAs (69.1% reverse and 30.9% anatomic). | 393 ± 299 days |
| Mayer et al. | 2019 | Prognostic case-control study | III | US | 21 | 61.9 | 152 | 67 (44.0) | 33.7 | The chronic narcotic-use group had significantly more smokers than the non-narcotic group (P = 0.002); there were no other significant differences in comorbidities between groups. These comorbidities include: Asthma, COPD, Heart disease, HTN, DM, Depression/anxiety, OSA, and Kidney disease. | Osteoarthritis | aTSA | NR |
| Williams et al. | 2019 | Retrospective cohort study | III | US | 19 | 58.4 ± 10.1 | 200 | 96 (48.0) | 30.6 ± 6.1 | Notable statistically significant differences between groups included increased frequencies of back pain, depression, use of antidepressants or anxiolytics, or both, and degenerative joint disease in the preoperative opioid group. | Full-thickness or partial-thickness tears of the supraspinatus tendon | Arthroscopic Rotator Cuff Repair | 47.2 (15.1) |
| Khazi et al. | 2019 | Retrospective cohort study | III | US | 20 | Age < 25 yr = 2045 (42.6) | 4802 | 1384 (28.82) | BMI ≥ 30 kg/m2 = 346 (7.21) | 5.5% (n = 266) had a diagnosis of depression or anxiety, 7.1% (n = 339) had a diagnosis of fibromyalgia, and 0.3% (n = 15) had a diagnosis of Ehlers-Danlos syndrome | Shoulder instability | 4268 (88.9%) underwent arthroscopic stabilization; 298 (6.2%), open Bankart procedures; 114 (2.4%), Latarjet procedures; and 122 (2.5%), other open procedures | minimum of 1 year |
| Kolade et al. | 2020 | Retrospective cohort study | III | US | 13 | 68.6 ± 10.1 | 622 | 368 (59.5) | BMI was reported within bivariate analysis of average MME by patient characteristics for TSA patients with no differences between groups | Psychiatric disorders 217 (35); ASA II to IV 610 (98.1); Prior shoulder surgery 382 (62). | NR | rTSA (56%); TSA (43%); Revision rTSA (1.28%); Revision TSA (0.16%). | |
| Best et al. | 2020 | Prospective cohort study | II | US | 12 | 57 ± 5.6 | 5621 | 2375 (42) | NR | Charlson comorbidity index 0.33 ± 0.68 | NR | Primary TSA | NR |
| Sabesan et al. | 2020 | Retrospective cohort study | III | US | 18 | 68.1 ± 9.4 | 162 | 94 (58) | 30.2 ± 6.3 | ASA class II to IV, 11.7% smokers | NR | 60 aTSA; 22 rTSA (group 1) and 80 revision TSA patients (all had a revision to Reversed) (group 2). | |
| Jildeh et al. | 2020 | Retrospective cohort study | III | US | 19 | 26.3 ± 11.7 | 340 | 85 (25) | 27.5 ± 5.4 | NR | Biceps tenodesis 12 (3.5); SLAP tear 105 (30.9); ALPSA, GLAD, or HAGL 13 (3.8); Bankart lesion 242 (71.2); Hill-Sachs lesion 150 (44.1); Reverse Hill-Sachs lesion 9 (2.6); Instability events 21 (97.3). | Shoulder arthroscopy with capsulorrhaphy including Bankart repair, arthroscopic SLAP repair, and limited arthroscopic debridement. | |
| Peratikos et al. | 2020 | Retrospective cohort study | III | US | 21 | 61 (57 to 64) | 1387 | 550 (40) | NR | Charlson comorbidity index, median (IQR) 0 (0 to 1); Severe comorbidity 47 (3); History of mood or anxiety disorder 398 (29); History of substance use disorder 82 (6), History of personality or trauma and stressor disorder 38 (3); History of tobacco use 232 (17) | NR | TSA | NR |
| Lu et al. | 2020 | Retrospective cohort study | III | US | 21 | 56.82 ± 11.36 | 1242 | 505 (40.6) | 31.06 ± 7.38 | Depression 137 (11.0), Diabetes 113 (9.1), Heart disease 51 (4.1), Hypertension 313 (25.2), Degenerative joint disease 274 (18.1), Alcohol abuse 44, Smoking history 105 (6.5). | NR | Rotator cuff repair 298 (24.0); SLAP repair 25 (2.0); Capsulorraphy 102 (8.2); Biceps tenodesis 37 (3.0); Subacromial decompression 430 (34.6); Extensive debridement 220 (17.7); Limited debridement 130 (10.5). | minimum of 1 year |
| Farley et al. | 2020 | Retrospective cohort study | III | US | 20 | While statistically different, patient age breakdown was overall clinically similar among groups without an obvious trend. | 214,283 | 92,123 (43.0) | NR | Significant differences in every examined comorbidity at baseline although small, they were universally higher in the high opioid use groups (P < 0.001 for all comparisons). These include: Obesity, Chronic kidney disease, Alcohol use disorder, Tobacco use, Hypertension, Coronary artery disease, Congestive heart failure, Hyperlipidemia, Rheumatic disease, Diabetes, Depression. | NR | Arthroscopic Rotator Cuff Repair ± biceps tenodesis, subacromial decompression, distal clavicle resection, these were overall similar between groups. | NR |
The included studies were conducted between 2015 and 2020, with 17 (81.9%) studies published within 3 years of the search. Three of the studies were of level II evidence (n = 6155),17,30,34 one was level IV evidence (n = 181) and 38 17 were level III evidence, (n = 250,965). All of the included studies were performed in the United States.
This review includes a total of 257,301 patients. The median sample size was 378 participants (interquartile range: 167 to 2692). Of those, 110,771 (43.1%) were female (range: 39 to 92,123). The median age of the included participants was 66.9 (range: 22 to 90). Shoulder arthroplasty was evaluated in 14 studies, while shoulder arthroscopy was assessed in 6 studies. Brock et al. reported on outcomes of both types of procedures. 27 The follow-up was reported in 10 studies, with a mean of 2.3 years. Regarding body mass index (BMI), the median was 30.5 kg/m2 (range: 27.5 to 32.2).
Diagnosis
Partial- or full-thickness rotator cuff tears (RCTs), glenohumeral joint osteoarthritis with and without RCT, fracture sequelae, avascular necrosis (AVN), locked dislocation, inflammatory arthritis, osteomyelitis, and periprosthetic infection were reported pathology in the included studies. Seven studies did not report diagnosis of included patient populations.
Opioid use
Preoperative: Of the included studies, 51.5% (n = 132,524) of the patients reported pre-operative opioid use. Also, 8 studies reported the opioid types, and 10 studies reported doses expressed as Oral Morphine Equivalent (OME) (Table 2).
Table 2.
Preoperative opioid use.
| Study | Year | Pre-op opioid use | Opioid type | Opioid dose (OME) | Post-op opioid use |
|---|---|---|---|---|---|
| Morris et al. | 2015 | 32 (47.1) | NR | NR | NR |
| Morris et al. | 2016 | 60 (26.8) | NR | NR | NR |
| Cuff et al. | 2016 | 14 (8) | A prescription for 7.5 mg of hydrocodone and 325 mg of acetaminophen for postoperative pain management and was instructed to take 1 or 2 pills every 4 to 6 h as needed for pain during the first postoperative week | ||
| Cheah et al. | 2017 | 138 (52) | Short-acting opioids (oral hydrocodone, oxycodone, hydromorphone, or morphine) or long-acting opioids (extended release morphine, extended release oxycodone, methadone, or fentanyl patch) | Non–opioid users: 66.9 ± 41; Short-acting opioid users: 111.4 ± 127.3; Long-acting opioid users: 208.3 ± 135.3 | NR |
| Berglund et al. | 2018 | 174 (35.5) | NR | NR | Patients reporting preoperative narcotic use had a markedly higher incidence of opioid use at 1-year follow-up (29.1% versus 4.9%; OR, 8.320; 95% CI, 4.509 to 15.355; P < 0.001) and at final follow-up (35.1% versus 7.3%; OR, 6.877; 95% CI, 4.062 to 11.641; P < 0.001) compared with patients without preoperative use. |
| Grace et al. | 2018 | 47 (39.4) | Acetaminophen and hydrocodone (55.3%), tramadol (21.3%), oxycodone immediate release (IR) (12.8%), acetaminophen and oxycodone (8.5%), oxycodone extended release (6.4%), acetaminophen and codeine (4.3%), methadone (4.3%), morphine extended release (4.3%), and hydromorphone (2.1%) | Opioid users were found to have significantly higher opioid requirements on the first postoperative day (60 OMEs vs 45 OMEs, P = 0.01) and the day prior to discharge (42 OMEs vs 15 OMEs, P < 0.001) than non-opioid users. | A significantly higher percentage of opioid users still required opioids 6 weeks after surgery, when compared with non-opioid users (71.0% vs 9.1%, P < 0.001) |
| Menendez et al. | 2018 | 68 (16.4) | NR | NR | Patients with severe pain after surgery took more opioids (202 vs. 84 mg OMEs, P < 0.001; daily average: 73 vs. 42 mg OMEs, P < 0.001). |
| Rao et al. | 2018 | 3182 (75%) | The most common opioid type prescribed was oxycodone (37%), followed by morphine (18%) and hydromorphone. | NR | Postoperatively, 92.6% used opioids in the early recovery period, and 38% to 42% used opioids in the later rehabilitation period |
| Thompson et al. | 2019 | 26 (35.6) | NR | NR | NR |
| Brock et al. | 2019 | 3992 (17.7) | NR | NR | 1723 patients continued to have postoperative opioid use. Continuous chronic preoperative opioid use was the most significant risk factor for chronic postoperative use (odds ratio (OR) 4.84 to 39.75 for >6 M group, depending on the procedure). |
| Curtis et al. | 2019 | 50 (36.2) | Tramadol only 7 (7.95), Tramadol and an additional opioid 4 (8), Narcotic 50 (36.23), Oxycodone 15 (30), Hydrocodone 33 (66), Morphine 3 (6), Hydromorphone 1 (2), Fentanyl 1 (2). | NR | Patients using opioids for pain management preoperatively were 4.7 times as likely to be on an opioid at 3 months postarthroplasty (confidence interval, 1.96 to 11.29; P = 0.001). |
| Mayer et al. | 2019 | 27 (17.7) | NR | During the global postoperative period, chronic preoperative narcotic users had significantly higher cumulative OME compared to nonusers as inpatients and at 2 wk, 6 wk, and 12 wk (3209 Vs 1814; P = 0.003). | NR |
| Williams et al. | 2019 | 44 (22.0) | NR | NR | On average (log-transformed), patients in the preoperative opioid group received 1.91 (95% confidence interval, 1.31–2.78) times more opioids over a postoperative course of treatment that was 2.73 (95% confidence interval, 1.62–4.59) times longer than patients who did not take opioids preoperatively |
| Khazi et al. | 2019 | 1812 (37.7) | NR | NR | After the first postoperative month, patients in the preoperative opioid group had a significantly higher opioid prescription fill rate than patients in the N-OU group (P < 0.0001, Table 2) |
| Kolade et al. | 2020 | 122 (20) | NR | 47.4 (65.7) | Preoperative opioid use was correlated with higher inpatient opioid after TSA. Patients who received opioid prescriptions prior to surgery had 43% higher opioid consumption on average in the immediate postoperative period (P = 0.0013). The cohort of patients with preoperative opioid use had an average of 38.8 MMEs compared with 27.1 MMEs in the cohort of patients without a history of preoperative opioid use |
| Best et al. | 2020 | 1571 (28) | Hydrocodone Short Acting 939 (60); Oxycodone Short Acting 386 (25); Codeine 54 (3.4); Oxycodone Long Acting 46 (2.9); Fentanyl Long Acting 31 (2.0); Hydromorphone Short Acting 27 (1.7); Morphine Long Acting 23 (1.5); Other 65 (4.1) | Oxycodone Short Acting 112 ± 261; Hydrocodone Short Acting 92 ± 116 | Postoperative opioid use was found in 4424 (79) |
| Sabesan et al. | 2020 | In the primary arthroplasty cohort 19.5% patients were preoperatively dependent compared to 38.8% in the revision cohort. | NR | Mean preoperative TMEs of 115.2 (±245.6) for the revision arthroplasty group compared to the primary arthroplasty group (mean TMEs 31.5 ± 54.8). For the preoperatively naïve patients, the results were significantly lower, at 86.5 TMEs in the primary group and 115.1 for the revision group. | Postoperatively the revision group had significantly higher rates of postoperative dependence at 43.8% compared to 29.3% patients in the primary group. The OR for the type of surgery decreased after being adjusted for confounders, and its association with the outcome remained non-significant (Adjusted Odd Ratio (aOR) 1.27, 95%CI 0.59–1.74, P = 0.54). Preoperative dependence (aOR 6.44, 95%CI 2.89–14.4, P < 0.0001) was independently associated with postoperative opioid dependence ater adjustment. |
| Jildeh et al. | 2020 | Acute user 19 (5.6); Chronic user 32 (9.4); total 51 (15) | NR | NR | On average, chronic users filled 2.61 ± 3.53 prescription refills, acute opioid users filled 1.63 ± 2.09 prescription refills, and non-opioid users filled 0.57 ± 1.14 prescription refills. |
| Peratikos et al. | 2020 | 318 (23) | NR | from 0 to 100 | 45 days post op 1243 (90); 18 months post op 322 (23) |
| Lu et al. | 2020 | 184 | Hydrocodone 214 (86.6), Oxycodone 9 (3.6), Hydromorphone 9 (3.6), Fentanyl (transdermal) 2 (0.8), Codeine 11 (4.45). | Perioperative daily OME: 20.5 ± 15.9, Perioperative OME above the threshold of 723: 31 (12.3) | Preoperative opioid use was the greatest predictor of postoperative opioid use (OR, 21.29, 95% CI, 12.2–37.15, P < 0.001). |
| Farley et al. | 2020 | 120,569 (56.2) | Hydrocodone, oxycodone, oxymorphone, codeine, dihydrocodeine, morphine, hydromorphone, fentanyl, methadone, meperidine. | <1 OME (n = 16,468 [7.7%]); 1-<5 OMEs (n = 57,378 [26.8%]); 5-<10 OMEs; (n = 13,393 [6.3%]); >10 OMEs (n = 18,450 [8.6%]) | NR |
Postoperative: Fourteen studies (66.7%) reported a higher likelihood of opioid use at follow-up among those prescribed opioids preoperatively compared to preoperative opioid-naïve patients.
For instance, Grace et al. 17 reported that opioid users were found to have significantly higher opioid requirements on the first postoperative day (60 OMEs vs 45 OMEs, P = 0.01) and the day prior to discharge (42 OMEs vs 15 OMEs, P < 0.001), than non-opioid users. Curtis et al. 15 reported that patients using opioids for pain management preoperatively were 4.7 times as likely (95% CI 1.96 to 11.29; P = 0.001) to be on an opioid at 3 months post-arthroplasty. This was also found in a 1-year follow-up as per Berglund et al. in which patients reporting preoperative opioid use had a markedly higher incidence of opioid use (29.1% versus 4.9%; odds ratio, 8.320; P < 0.001). 40
Type of opioids
A wide variety of opioid medications were included, although they were reported in less than 50% of included studies. Cuff et al. 38 reported only postoperative opioid regimen as a prescription for 7.5 mg of hydrocodone and 325 mg of acetaminophen during the first postoperative week. On the other hand, 4 studies reported preoperative use of opioids which included acetaminophen and hydrocodone, tramadol, oxycodone immediate release, acetaminophen and oxycodone, oxycodone extended release, acetaminophen and codeine, methadone, morphine extended release, and hydromorphone.15,17,24,30
Comorbid conditions
In the majority of included studies reporting comorbidies, no statistical differences were noted between the preoperative opioid and non-opioid groups.15–17,29,36,37
However, Farley et al. 24 identified significant differences in every examined comorbidity at baseline as they were universally higher in the high opioid use groups (P < 0.001 for all comparisons). Two studies linked between preoperative opioid use and prevalence of one of the following comorbidities: chronic back pain, depression, obesity, self-reported allergies and an ASA score ≥ III.27,34 Three studies did not report comorbid conditions.28,38,40
Pain reporting, functional scores and range of motion (ROM)
Pain reporting and functional assessment scores were reported in eight studies (38.1%). The American Shoulder and Elbow Surgeons (ASES) score was the most commonly used (8 studies) followed by Visual Analogue Scale (VAS) for pain which was reported in 6 studies. Four studies assessed ROM at final follow up (Table 3).
Table 3.
Pain reporting, functional scores and range of motion.
| Study | Year | Functional assessment scores | Baseline ROM (active or passive) | ROM (active or passive) scores at final follow up | Functional scores (pre-op) | Functional scores (post-op) |
|---|---|---|---|---|---|---|
| Morris et al. | 2015 | Constant score, the American Shoulder and Elbow Surgeons (ASES) score, the Western Ontario Osteoarthritis Shoulder (WOOS) index, the Single Assessment Numeric Evaluation (SANE), and range of motion measurements. | Preoperative opioid group: Forward flexion 40 ± 37; Abduction 38 ± 36; External rotation 8 ± 16. Non opioid group: Forward flexion 43 ± 51; Abduction 42 ± 49; External rotation 9 ± 13. | Pre-oprative opioid group: Forward flexion 142 ± 30; Abduction 136 ± 39; External rotation 32 ± 16. Non opioid group: Forward flexion 147 ± 29; Abduction 145 ± 30; External rotation 27 ± 16. | Pre-oprative opioid group: total Constant score 14.9 ± 8.7; ASES 25.6 ± 13.1; WOOS 79.0 ± 14.0; SANE; 27.9 ± 26.4. Non opioid group: total Constant score 19.4 ± 13.3; ASES 37.4 ± 18.6; WOOS 67.4 ± 20.2; SANE; 27.9 ± 24.8. | Pre-oprative opioid group: total Constant score 58.8 ± 19.5; ASES 65.0 ± 26.4; WOOS 33.4 ± 31.6; SANE; 62.8 ± 32.9. Non opioid group: total Constant score 67.4 ± 18.5; ASES 75.8 ± 21.4; WOOS 22.3 ± 21.1; SANE; 62.1 ± 35.8 |
| Morris et al. | 2016 | Constant score, the American Shoulder and Elbow Surgeons (ASES) score, the Western Ontario Osteoarthritis Shoulder (WOOS) index, the Single Assessment Numeric Evaluation (SANE), and range of motion measurements. | Preoperative opioid group: Forward flexion 73 ± 39; Abduction 70 ± 39; External rotation 11 ± 14. Non opioid group: Forward flexion 89 ± 37; Abduction 82 ± 36; External rotation 11 ± 15. | Pre-oprative opioid group: Forward flexion 155 ± 32; Abduction 153 ± 32; External rotation 45 ± 13. Non opioid group: Forward flexion 164 ± 13; Abduction 164 ± 14; External rotation 46 ± 13. | Pre-oprative opioid group: total Constant score 21 ± 16; ASES 33 ± 19; WOOS 76 ± 13; SANE 32 ± 28. Non opioid group: total Constant score 32 ± 17; ASES 44 ± 18; WOOS 62 ± 18; SANE 32 ± 24. | Pre-oprative opioid group: total Constant score 73 ± 20; ASES 83 ± 20; WOOS 19 ± 25; SANE 67 ± 35. Non opioid group: total Constant score 82 ± 12; ASES 89 ± 15; WOOS 11 ± 16; SANE 71 ± 36. |
| Cuff et al. | 2016 | VAS | NR | NR | NR | NR |
| Cheah et al. | 2017 | VAS, ASES | NR | NR | VAS pain scores between groups were similar, with a trend of higher scores in long-acting opioid users; ASES shoulder scores between groups were similar, with a trend of higher scores in opioid users. | VAS pain scores were higher in opioid users (4.0 ± 1.8 for nonusers, 4.9 ± 1.9 for short-acting users, and 6.0 ± 1.5 for long-acting users; P < 0.001); ASES scores: Analysis of variance showed similar preoperative and 2-year postoperative ASES results between both non–opioid users and opioid users in the TSA and rTSA groups. |
| Berglund et al. | 2018 | NR | NR | NR | NR | NR |
| Grace et al. | 2018 | VAS and ASES score | NR | NR | Opioid users reported worse pain (7 vs 4, P = 0.007) and ASES (32.8 vs 46.0, P = 0.003) scores in the preoperative period compared with non-opioid users. | Opioid users again reported more pain in the operative shoulder than non-opioid users 6 weeks after surgery (1 vs 0, P = 0.036), the magnitude of improvement from the preoperative visit to 6 weeks after surgery was similar between the 2 cohorts (4 vs 3, P = 0.16). Both the 6-week postoperative ASES scores (51.6 vs 56.2, P = 0.32) and the magnitude of ASES score improvement from preoperatively to 6 weeks postoperatively (18.8 vs 10.2, P = 0.11) were similar between the 2 groups. |
| Menendez et al. | 2018 | Preoperative ASES score | NR | NR | 34.1 ± 16.5 | NR |
| Rao et al. | 2018 | NR | NR | NR | NR | NR |
| Thompson et al. | 2019 | ASES scores, VAS scores, ROM, strength | Preoperative opioid group: FE ROM 90 (130; 20 to 150), strength 4 (2; 3 to 5); ER ROM 30 (90; −10 to 80), strength 5 (1; 4 to 5); IR ROM 30 (85; 0 to 85), strength 5 (1; 4 to 5). Non opioid group: FE ROM 110 (170; 0 to 170), strength 5 (3; 2 to 5); ER ROM 30 (80; 0 to 80), strength 5 (2; 3 to 5); IR ROM 45 (120; 0 to 120), strength 5 (1; 4 to 5). | Preoperative opioid group: FE ROM 135 (130; 50 to 180), strength 5 (2; 3 to 5); ER ROM 45 (60;0 to 60), strength 5 (1; 4 to 5); IR ROM 60 (150; 0 to 150), strength 5 (1; 4 to 5). Non opioid group: FE ROM 160 (185; 85 to 270), strength 5 (1; 4 to 5); ER ROM 45 (60; 25 to 85), strength 5 (0; 4 to 5); IR ROM 60 (60; 30 to 90), strength 5 (0; 4 to 5). | Preoperative opioid group: ASES 32 (61; 0 to 61); VAS 6 (8; 2 to 10). Non opioid group: ASES 42 (75; 5 to 80); VAS 5 (9; 1 to 10). | Preoperative opioid group: ASES 61 (96; 0 to 96); VAS 2 (10; 2 to 10). Non opioid group: ASES 91.7 (43.4; 56.6 to 100); VAS 0 (3; 0 to 3). |
| Brock et al. | 2019 | NR | NR | NR | NR | NR |
| Curtis et al. | 2019 | Numeric Rating Scale (NRS) | NR | NR | Scores were not significantly different between cohorts. NRS at rest: (F = 1.26; P = 0.26). NRS with activity: (F = 0.01; P = 0.93). | Postoperatively, the opioid cohort demonstrated mean resting pain score of 3.0 6 2.6, which was 1.6 points higher than that of the nonopioid cohort, 1.4 6 1.9 (P < 0.001). |
| Mayer et al. | 2019 | VAS | NR | NR | No other significant differences in preop VAS between both groups (7.0 vs 6.0 P = 0.10) | At 2 wk postoperatively, there was no statistically significant difference in VAS scores between chronic preoperative narcotic users and nonusers, although there was a trend toward higher VAS scores among narcotic users. At 6 and 12 wk, however, chronic narcotic users had significantly higher VAS scores. |
| Williams et al. | 2019 | ASES, SST, VAS and ROM | Preoperative opioid group: Forward flexion 120 ± 36; Abduction 110 ± 37; External rotation (side) 56 ± 18; External rotation (90) 74 ± 18. Non opioid group: Forward flexion 134 ± 31; Abduction 126 ± 34; External rotation (side) 63 ± 14; External rotation (90) 78 ± 15. | Preoperative opioid group: Forward flexion 147 ± 22; Abduction 148 ± 19; External rotation (side) 65 ± 15; External rotation (90) 83 ± 15. Non opioid group: Forward flexion 153 ± 13; Abduction 151 ± 15; External rotation (side) 67 ± 8; External rotation (90) 83 ± 11. | Pre-oprative opioid group: total Constant score 48.3 ± 15.6; ASES 32.1 ± 16.1; SST 4.1 ± 2.5; VAS 6.7 ± 2.2. Non opioid group: total Constant score 60.1 ± 17.4; ASES 47.6 ± 19.6; SST 6.1 ± 3.3; VAS 4.9 ± 2.6. | Pre-oprative opioid group: total Constant score 81.3 ± 14.1; ASES 68.4 ± 27.8; SST 7.5 ± 3.7; VAS 3.2 ± 3.0. Non opioid group: total Constant score 60.1 ± 17.4; ASES 47.6 ± 19.6; SST 6.1 ± 3.3; VAS 4.9 ± 2.6. |
| Khazi et al. | 2019 | NR | NR | NR | NR | NR |
| Kolade et al. | 2020 | NR | NR | NR | NR | NR |
| Best et al. | 2020 | NR | NR | NR | NR | NR |
| Sabesan et al. | 2020 | NR | NR | NR | NR | NR |
| Jildeh et al. | 2020 | NR | NR | NR | NR | NR |
| Peratikos et al. | 2020 | NR | NR | NR | NR | NR |
| Lu et al. | 2020 | American Shoulder and Elbow Surgeo), SANE, Constant score, SF-12 MCS, SF-12 PCS, pain VAS, VR-12 MCS, VR-12 PCS, and Veterans RAND 6D | NR | NR | American Shoulder and Elbow Surgeon form (36.6 ± 16.8 vs 46.5 ± 17.5), SANE (25.10 ± 19.05 vs 32.64 ± 19.01), Constant–Murley score (10.9 ± 5.34 vs 12.5 ± 4.99), SF-12 MCS (51.1 ± 12.0 vs 55.2 ± 8.74), SF-12 PCS (31.1 ± 5.21 vs 35.5 ± 7.03), pain VAS (6.23 ± 1.91 vs 5.06 ± 2.13), VR-12 MCS (52.6 ± 11.2 vs 57.7 ± 8.32), VR-12 PCS (33.5 ± 5.7 vs 38.1 ± 7.46), and Veterans RAND 6D (59 ± 8 vs 65 ± 8), for the OU and NOU cohorts, respectively | Both the OU and NOU groups showed statistically significant improvement—at the P < 0.05 level—in all PROMs after shoulder surgery; however, the OU group had significantly worse absolute outcome scores on all PROM measures compared with the NOU group (all P < 0.001) |
| Farley et al. | 2020 | NR | NR | NR | NR | NR |
Grace et al. 17 reported that opioid users had worse VAS (7 vs 4, P = 0.007) and ASES (32.8 vs 46.0, P = 0.003) in the preoperative period compared with non-opioid users. Morris et al. 37 found that the non-opioid group had significantly greater preoperative forward flexion (average 16° greater; P = 0.005) and abduction (average 12° greater; P = 0.03). Although both groups significantly improved on ROM measurements from preoperative to most recent follow-up (P < 0.001), the opioid group demonstrated significantly lower ROM measurements, except for external rotation (P = 0.58), compared to the non-opioid group post-operatively.
Complications and revision surgery
Complications reported among included studies were of heterogeneous conclusion. Best et al. found that preoperative opioid use is independently associated with significantly greater odds of 90-day readmission following primary TSA (OR 2.56, 95% CI: 1.99, 3.29, p < 0.001) and with an increase in hospital length of stay of 0.13 days (p = 0.001). 30 On the other hand, Mayer et al. 18 found that the differences in complication rates and readmission rates were not statistically significant, and no difference in length of stay between both groups was seen.
Again, Best et al. 30 reported preoperative opioid use is independently associated with having revision surgery within 1-year (OR 2.35, 95% CI: 1.46, 3.79, p = 0.001), which is consistent with the report of Peratikos et al. who showed an 18-month surgical revision of 3% in the opioid group. 26 (Table 4).
Table 4.
Complications and revision surgery.
| Study | Year | Complications | Revision surgery | Study conclusion |
|---|---|---|---|---|
| Morris et al. | 2015 | NR | NR | Although Preoperative range of motion measurements did not differ significantly between the 2 groups, Preoperative opioid use was associated with significantly lower preoperative scores for ASES score (P = 0.004) and WOOS index (P = 0.008). Non group had significantly better post-operative outcome scores, including Constant–Total (P = 0.035), ASES (P = 0.005), and WOOS (P = 0.013) |
| Morris et al. | 2016 | NR | NR | Patients with preoperative opioid use have a significantly lower preoperative baseline and achieve significantly lower final outcome scores after TSA compared with patients without a history of preoperative opioid use |
| Cuff et al. | 2016 | NR | NR | Preoperative narcotic use was also significantly predictive (P = 0.010) of high pain scores on postoperative day 1 and day 7 (P = 0.019) |
| Cheah et al. | 2017 | With respect to perioperative outcomes, length of stay, and 90-day complication and readmission rates were similar regardless of preoperative opioid use. | Reoperation (90 d) was non significant between the two groups | Preoperative opioid use in shoulder arthroplasty patients is common and associated with increased postoperative pain and opioid consumption while preoperative opioid use was not found to be associated with increased perioperative complications including mobilization, length of stay, discharge to a skilled nursing facility, readmission, or reoperation within 90 days of shoulder arthroplasty. |
| Berglund et al. | 2018 | NR | NR | Patients taking preoperative opioids had five times greater incidence of opioid use postoperatively and was nearly seven times more likely to be using opioids at an average of 4 years later. Narcotic usage did not markedly change from 1-year follow-up to final follow-up. |
| Grace et al. | 2018 | NR | NR | Daily and total opioid regimens prescribed after primary shoulder arthroplasty were similar between prior opioid users and nonusers despite large differences in their inpatient opioid requirements. |
| Menendez et al. | 2018 | NR | NR | The predictors of severe postoperative pain were greater number of self-reported allergies, preoperative chronic opioid use, lower American Shoulder and Elbow Surgeons score, and depression. Patients reporting severe pain took more opioids, stayed longer in the hospital, used postacute inpatient rehabilitation services more frequently, and were more likely to be high-cost patients. |
| Rao et al. | 2018 | NR | NR | Opioid usage in patients undergoing SA is widespread at 1 year, with three-fourths of patients having been dispensed at least one Rx |
| Thompson et al. | 2019 | Four complications occurred in the narcotic group (14%) and one (2%) in the control group (P = 0.05). | No surgical intervention was performed for any complication encountered. | Postoperatively, significant differences were noted between the narcotic and nonnarcotic groups regarding American Shoulder and Elbow Surgeons scores and visual analog scale scores, as well as forward elevation, external rotation, and all strength measurements (P, 0.01). The nonnarcotic group had markedly higher American Shoulder and Elbow Surgeons scores, better overall range of motion and strength, and markedly lower visual analog scale scores than the narcotic group. |
| Brock et al. | 2019 | NR | NR | By far the most important risk factor for chronic postoperative opioid use was chronic preoperative opioid use; however, this risk was significantly lower when chronic opioid users did not fill an opioid prescription in the three months before surgery. |
| Curtis et al. | 2019 | NR | NR | Patients who take opioids preoperatively are at risk for increased postoperative pain, continued opioid use at 3 months, and longer duration of opioid use after shoulder arthroplasty compared with non-opioid users. |
| Mayer et al. | 2019 | The differences in complication rates and readmission rates were not statistically significant, and there was no difference in length of stay between groups. | NR | Patients using chronic preoperative narcotic pain medication had significantly higher VAS scores and narcotic requirements after anatomic TSA. |
| Williams et al. | 2019 | NR | NR | Patients taking opioids preoperatively required a significantly greater quantity and longer duration of postoperative opioid therapy and did not ultimately reach the same level of functionality, as indicated by final follow-up outcomes scores |
| Khazi et al. | 2019 | NR | NR | Patients who were prescribed opioids between 1 to 3 months before surgery had the highest risk of prolonged opioid use following surgery |
| Kolade et al. | 2020 | Intraoperative complications 27 (4) | NR | Preoperative opioid use was correlated with higher inpatient opioid after TSA. |
| Best et al. | 2020 | Preoperative opioid use is independently associated with significantly greater odds of 90-day readmission following primary TSA (OR 2.56, 95% CI: 1.99, 3.29, p < 0.001). Preoperative opioid use was associated with an increase in hospital length of stay by 0.13 days (p = 0.001) | Preoperative opioid use is independently associated with having revision surgery within 1-year (OR 2.35, 95% CI: 1.46, 3.79, p = 0.001). | Preoperative opioid use is associated with increased rates of readmission, revision surgery and higher healthcare costs following primary total shoulder arthroplasty. |
| Sabesan et al. | 2020 | NR | – | For the preoperatively dependent cohorts that were assessed our subanalyses demonstrated these patients had over 6 times increase in postoperative dependence regardless of primary or revision surgery. |
| Jildeh et al. | 2020 | NR | NR | preoperative opioid use correlates with postoperative opioid demand |
| Peratikos et al. | 2020 | Nonhome discharge 45 (3); 30-d hospital readmission 25 (2); 90-d surgical site infection 29 (2). | 18-mo surgical revision 46 (3). | Preoperative opioid users had longer length of stay, increased revision rates, higher spend, and persistent opioid use, which worsened with dose. |
| Lu et al. | 2020 | NR | Consumption of perioperative OME >723 was the strongest predictor of revision surgery (OR, 8.59, 95% CI, 2.12–34.78, P < 0.003) at 1 year | Patients with a history of preoperative opioid use can achieve significant improvements in patient-reported outcomes after arthroscopic shoulder surgery. However, preoperative opioid use negatively impacts patients’ level of satisfaction and is a significant predictor of pain and continued opioid usage |
| Farley et al. | 2020 | As average daily preoperative OMEs increased, so too did the odds of incurring a postoperative complication. Compared with opioid-naive patients, all opioid use groups had increased odds of postoperative emergency department visits and readmissions at 30 and 90 days. | Patients averaging 10 OMEs per day showed a 103% (odds ratio, 2.03 [95% CI, 1.62–2.54]; P < 0.001) increase in the odds of revision surgery compared with opioid-naive patients. | Preoperative opioid use was a risk factor for complications and revision surgery after arthroscopic RCR. We also observed a dose-dependent response between opioid use and postoperative complications |
Discussion
The association between preoperative opioid use and clinical outcomes is complex. The primary finding of this review is that the preoperative use of opioids is associated with inferior clinical outcomes and increased postoperative opioid use in patients undergoing shoulder surgeries.
It has been proposed that preoperative opioid use in orthopedic surgery increases the risk of chronic opioid use, thereby contributing to the ongoing “opioid crisis” in North America. 41 Indeed, opioid use has become a central focus of orthopedic research in recent years. 42 Preoperative opioid use has been identified as a predictor of prolonged postoperative use after many orthopedic procedures.43,44 Bartels et al. 45 and Kim et al. 46 identified multiple predictors of long-term opioid use during follow-up such as high in-hospital opioid use, procedure type, anesthesia type, age, and insurance type.
One of the problems that exists when analyzing the literature in this topic, is the lack of standardization of the definition of “chronic opioid use” leading to a wide variety in terminology used and outcome reporting suggesting the need of standardization. 47 Nevertheless, we noted that there is a higher likelihood of ongoing opioid use at follow-up among those prescribed opioids preoperatively compared to preoperative opioid-naïve patients. Most of the studies included in this review report that those patients given opioids prior to their shoulder surgery were more likely to continue using opioids after surgery and reported higher pain scores and lower functional outcomes. Opioid stewardship is very important as it seems likely that preoperative opioid use leads to opioid tolerance and a diminished analgesic effect which ultimately leads to worse postoperative pain and greater opioid consumption.48,49
Although our study suggests that prescribing patients an opioid before surgery is associated with ongoing long-term opioid use, we cannot exclude the possibility that this is an artifact arising from underlying patient characteristics (e.g. patients with chronic pain conditions or psychiatric disorders).50–53 In a large retrospective study involving 29,827 arthroscopic rotator cuff repair patients, Westermann et al. 54 found that those patients prescribed opioids preoperatively were more than 7 times more likely to continue using opioids after discharge. Other factors (e.g. psychiatric conditions and lower back pain) were also identified as contributing to ongoing opioid use. Although most of the studies showed no statistical differences were noted between the preoperative opioid and non-opioid groups regarding comorbidities, it is possible that the worst outcomes associated with preoperative use of opioids might be linked with certain comorbidities and/or the presence of chronic pain in this population, with the subsequent impact on clinical outcomes. However, there is an urgent need for standardized, evidence-based postoperative opioid prescribing protocols, 55 as well as the development of strategies to reduce prolonged opioid use following surgery that consider a multimodal analgesic approach. 56 This can be achieved by identifying patients preoperatively and try to use strategies to decrease their preoperative opioid use. 57
Goplen et al. 43 reported that total joint arthroplasty patients that received opioids preoperatively have worse overall pain and function than opioid-naïve patients. This was also found in patients undergoing ACL reconstruction, where the preoperative opioid group scored more poorly in patient-reported outcomes (at baseline and 1-year follow-up) and were more likely to fail to reach a patient acceptable symptomatic state. 58 Chronic opioid use before cervical arthrodesis has also been associated with worse functional outcomes following surgery. 59 In the context of shoulder surgery outcomes, most of the studies found that patients with preoperative opioid use had poor functional scores throughout the postoperative period. However, it is noteworthy that the preoperative opioid group tended to start with lower functional scores, so that the magnitudes of improvement did not differ greatly when comparing the preoperative opioid and non-preoperative opioid groups.18,26,30 In a subsequent work, Cozowicz et al. found that those patients with the most opioid prescriptions were at increased risk of treatment complications. 60 Although this review was carefully designed to focus on functional outcomes, these data were missing from many of the included studies. Nevertheless, these results highlight important points regarding functional outcomes and ROM in patients undergoing shoulder surgeries, which develops earlier work questioning the benefit of prescribing opioids preoperatively to orthopedic surgery patients.
Recently, there has been a trend towards the use of non-opioid pain regimens favoring multimodal analgesia schemes. In fact, opioids are no longer a part of standard procedures in the treatment of athletes,61,62 and have been proposed as the second or third-line pharmacological option for the management of chronic painful joint conditions such as osteoarthritis.63,64 Multimodal analgesia can improve pain control and lessen reliance on opioids.57,65 Also, it was recently reported that a multimodal pain protocol could improve recovery (patient-reported) following arthroscopic shoulder surgery, with reduced opioid use relative to a conventional opioid-receiving group. 66 Despite the importance that multimodal analgesia schemes might have in decreasing postoperative opioid use, future studies should ideally have as their key measure the improvements in patient-related outcomes instead of decreased opioid usage. 67
Limitations
There are several limitations in this review. First, we could not establish causality between preoperative opioid use and its effects on the outcomes of shoulder surgeries. This is due to many confounders, such as chronic pain, which were not adequately addressed in included studies. Furthermore, the indications of the preoperative opioid use were not mentioned since these medications might be taken for other causes of pain such as back pain or cancer pain.
Also, this review lacked any level I type of evidence. Because of this, some groups may have been imbalanced concerning gender, severity of back pain, mood disorders, and other chronic pain conditions.
Second, our analyses were constrained by the availability of published data as most of the studies included in this review did not include data on patient-reported outcomes, ROM, or strength. This was due to the older studies lacking access to the big data registries currently available, and the absence of certain information which was probably omitted during the surgeon's routine follow-up assessments. This limits the applicability of our results when shoulder surgeons have their preoperative counseling with their patients who are chronic users of opioids.
Despite these limitations, our findings are consistent with those reported in other orthopedics studies.43,68–70 Nevertheless, further research is required to better understand the influence that preoperative opioids have on postoperative outcomes in shoulder surgery.
Taken together, our findings agree with the previous orthopedic literature regarding preoperative use of opioids. This work and prior studies have identified major associations with preoperative opioid use: inferior outcomes, greater ongoing opioid use, depression, anxiety, and psychiatric conditions. Collectively, these data are likely to help guide clinical and surgical decision-making when planning orthopedic patient care protocols. Even if those patients given opioids preoperative are likely to experience an equal magnitude of improvement as those not given opioids. Also, postoperative programs and pain management counseling should be considered to help patients achieve adequate analgesia, thereby reducing the opioid burden.
Conclusion
Our review suggests that in patients undergoing shoulder surgery, the preoperative use of opioids is associated with inferior clinical outcomes and increased postoperative opioid use and misuse. It is unclear to what degree this influence is due to the opioid use itself and to what extent it is related to comorbidities, severity of the preoperative condition leading to surgery, and/or chronic pain prevalence. Future prospective studies controlling for comorbidities, severity of the pathology and chronic pain, and OME are needed.
Supplemental Material
Supplemental material, sj-docx-1-sel-10.1177_17585732211070193 for The role of preoperative opioid use in shoulder surgery—A systematic review by Omar A Al-Mohrej, Carlos Prada, Kim Madden, Harsha Shanthanna, Timothy Leroux, and Moin Khan in Shoulder & Elbow
Acknowledgements
None.
Footnotes
Contributorship: OAM: Study design, data analysis, processing, writing—original draft, and approval of final draft. CP: Data collection, processing and reviewing—original draft. KM: methodology reviewing—original draft and approval of final draft. HH: reviewing, editing and approval of final manuscript. LL: reviewing, editing and approval of final manuscript. MK: Data curation, methodology, validation, visualization, project administration, and approval of final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical approval: Not applicable.
Informed Consent: Not applicable.
ORCID iDs: Carlos Prada https://orcid.org/0000-0003-0088-1859
Moin Khan https://orcid.org/0000-0002-8237-8095
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Levy B, Paulozzi L, Mack KA, et al. Trends in opioid analgesic-prescribing rates by specialty, U.S., 2007–2012. Am J Prev Med 2015; 49: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Florence CS, Zhou C, Luo F, et al. The economic burden of prescription opioid overdose, abuse, and dependence in the United States, 2013. Med Care 2016; 54: 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirson NY, Shei A, Rice JB, et al. The burden of undiagnosed opioid abuse among commercially insured individuals. Pain Med 2015; 16: 1325–1332. [DOI] [PubMed] [Google Scholar]
- 4.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA 2013; 309: 657–659. [DOI] [PubMed] [Google Scholar]
- 5.Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med 2015; 372: 241–248. [DOI] [PubMed] [Google Scholar]
- 6.Jalali MS, Botticelli M, Hwang RC, et al. The opioid crisis: need for systems science research. Heal Res Policy Syst 2020; 18: 1–5. DOI: 10.1186/s12961-020-00598-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson T, Maltenfort M, Getz C, et al. Multimodal pain management protocol versus patient controlled narcotic analgesia for postoperative pain control after shoulder arthroplasty. Arch Bone Jt Surg 2018; 6: 196–202. [PMC free article] [PubMed] [Google Scholar]
- 8.Trasolini NA, McKnight BM, Dorr LD. The opioid crisis and the orthopedic surgeon. J Arthroplasty 2018; 33: 3379–3382. e1. [DOI] [PubMed] [Google Scholar]
- 9.Volkow ND, McLellan TA, Cotto JH, et al. Characteristics of opioid prescriptions in 2009. JAMA 2011; 305: 1299–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boddapati V, Padaki AS, Lehman RA, et al. Opioid prescriptions by orthopaedic surgeons in a medicare population: recent trends, potential complications, and characteristics of high prescribers. J Am Acad Orthop Surg 2021; 29: e232–e237. [DOI] [PubMed] [Google Scholar]
- 11.Kaidi AC, Lakra A, Jennings EL, et al. Opioid prescription consumption patterns after total joint arthroplasty in chronic opioid users versus opioid naive patients. J Am Acad Orthop Surg Glob Res Rev 2020; 4: e20.00066. DOI: 10.5435/JAAOSGlobal-D-20-00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weick J, Bawa H, Dirschl DR, et al. Preoperative opioid use is associated with higher readmission and revision rates in total knee and total hip arthroplasty. J Bone Joint Surg Am 2018; 100: 1171–1176. [DOI] [PubMed] [Google Scholar]
- 13.Kim SC, Choudhry N, Franklin JM, et al. Patterns and predictors of persistent opioid use following hip or knee arthroplasty. Osteoarthr Cartil 2017; 25: 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhary MA, Dalton MK, Koehlmoos TP, et al. Identifying patterns and predictors of prescription opioid use after total joint arthroplasty. Mil Med 2021; 186: 587–592. [DOI] [PubMed] [Google Scholar]
- 15.Curtis W, Rounds AD, Stone M, et al. Effect of preoperative opioid usage on pain after total shoulder arthroplasty. J Am Acad Orthop Surg 2019; 27: E734–E742. [DOI] [PubMed] [Google Scholar]
- 16.Thompson KM, Hallock JD, Smith RA, et al. Preoperative narcotic use and inferior outcomes after anatomic total shoulder arthroplasty: a clinical and radiographic analysis. J Am Acad Orthop Surg 2019; 27: 177–182. [DOI] [PubMed] [Google Scholar]
- 17.Grace TR, Khanna K, Choo KJ, et al. The influence of preoperative opioid use on inpatient opioid requirements and discharge prescriptions after primary shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27: 1572–1579. [DOI] [PubMed] [Google Scholar]
- 18.Mayer MN, Mulligan RP, Villarreal AD, et al. Effect of chronic narcotic use on episode-of-care outcomes following primary anatomic total shoulder arthroplasty. Curr Orthop Pract 2019; 30: 231–234. [Google Scholar]
- 19.Higgins JP, Thomas J, Chandler J, et al. (Editors). Cochrane handbook for systematic reviews of interventions. John Wiley & Sons, 2019. [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Br Med J 2009; 339: 332–336. [PMC free article] [PubMed] [Google Scholar]
- 21.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003; 73: 712–716. [DOI] [PubMed] [Google Scholar]
- 22.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33: 159. [PubMed] [Google Scholar]
- 23.Bobak CA, Barr PJ, O’Malley AJ. Estimation of an inter-rater intra-class correlation coefficient that overcomes common assumption violations in the assessment of health measurement scales. BMC Med Res Methodol 2018; 18: 1–11. DOI: 10.1186/s12874-018-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farley KX, Wilson JM, Spencer CC, et al. Preoperative opioid use is a risk factor for revision surgery, complications, and increased resource utilization after arthroscopic rotator cuff repair. Am J Sports Med 2020; 48: 3339–3346. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Beletsky A, Cohn MR, et al. Perioperative opioid use predicts postoperative opioid use and inferior outcomes after shoulder arthroscopy. Arthroscopy 2020; 36: 2645–2654. [DOI] [PubMed] [Google Scholar]
- 26.Peratikos MB, Weeks HL, Pisansky AJB, et al. Effect of preoperative opioid use on adverse outcomes, medical spending, and persistent opioid use following elective total joint arthroplasty in the United States: a large retrospective cohort study of administrative claims data. Pain Med 2020; 21: 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brock JL, Jain N, Phillips FM, et al. Postoperative opioid cessation rates based on preoperative opioid use: analysis of common orthopaedic procedures. Bone Joint J 2019; 101-B: 1570–1577. [DOI] [PubMed] [Google Scholar]
- 28.Jildeh TR, Taylor KA, Tramer JS, et al. Risk factors for postoperative opioid use in arthroscopic shoulder labral surgery. Arthroscopy 2020; 36: 1813–1820. [DOI] [PubMed] [Google Scholar]
- 29.Sabesan VJ, Grauer J, Stankard M, et al. Does revision shoulder arthroplasty increase your risk of opioid use and dependence? Semin Arthroplast JSES 2020; 30: 188–194. [Google Scholar]
- 30.Best MJ, Harris AB, Bansal A, et al. Preoperative opioid use is associated with increased readmission, revision surgery and cost after total shoulder arthroplasty. Semin Arthroplast JSES 2020; 30: 35–41. [Google Scholar]
- 31.Kolade OO, Ghosh N, Fernandez L, et al. Study of variations in inpatient opioid consumption after total shoulder arthroplasty: influence of patient- and surgeon-related factors. J Shoulder Elbow Surg 2020; 29: 508–515. [DOI] [PubMed] [Google Scholar]
- 32.Khazi ZM, Lu Y, Shamrock AG, et al. Opioid use following shoulder stabilization surgery: risk factors for prolonged use. J Shoulder Elbow Surg 2019; 28: 1928–1935. [DOI] [PubMed] [Google Scholar]
- 33.Williams BT, Redlich NJ, Mickschl DJ, et al. Influence of preoperative opioid use on postoperative outcomes and opioid use after arthroscopic rotator cuff repair. J Shoulder Elbow Surg 2019; 28: 453–460. [DOI] [PubMed] [Google Scholar]
- 34.Menendez ME, Lawler SM, Ring D, et al. High pain intensity after total shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27: 2113–2119. [DOI] [PubMed] [Google Scholar]
- 35.Rao AG, Chan PH, Prentice HA, et al. Risk factors for postoperative opioid use after elective shoulder arthroplasty. J Shoulder Elbow Surg 2018; 27: 1960–1968. [DOI] [PubMed] [Google Scholar]
- 36.Morris BJ, Laughlin MS, Elkousy HA, et al. Preoperative opioid use and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg 2015; 24: 11–16. [DOI] [PubMed] [Google Scholar]
- 37.Morris BJ, Sciascia AD, Jacobs CA, et al. Preoperative opioid use associated with worse outcomes after anatomic shoulder arthroplasty. J Shoulder Elbow Surg 2016; 25: 619–623. [DOI] [PubMed] [Google Scholar]
- 38.Cuff DJ, O’Brien KC, Pupello DR, et al. Evaluation of factors affecting acute postoperative pain levels after arthroscopic rotator cuff repair. Arthroscopy 2016; 32: 1231–1236. [DOI] [PubMed] [Google Scholar]
- 39.Cheah JW, Sing DC, McLaughlin D, et al. The perioperative effects of chronic preoperative opioid use on shoulder arthroplasty outcomes. J Shoulder Elbow Surg 2017; 26: 1908–1914. [DOI] [PubMed] [Google Scholar]
- 40.Berglund DD, Rosas S, Kurowicki J, et al. Preoperative opioid use among patients undergoing shoulder arthroplasty predicts prolonged postoperative opioid use. J Am Acad Orthop Surg 2019; 27: E691–E695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phillips JK, Ford MA, Bonnie RJ. National academies of sciences, engineering, and medicine. Trends in opioid use, harms, and treatment. In Pain Management and the opioid epidemic: balancing societal and individual benefits and risks of prescription opioid use 2017 Jul 13. National Academies. Press (US) 2017: 187–266. [PubMed] [Google Scholar]
- 42.Savannah S, Bido J, Jamie C, et al. Impact of preoperative opioid use on total knee arthroplasty outcomes. J Bone Joint Surg Am 2017; 99: 803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goplen CM, Verbeek W, Kang SH, et al. Preoperative opioid use is associated with worse patient outcomes after total joint arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord 2019; 20: 1–12. DOI: 10.1186/s12891-019-2619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicholson AD, Kassam HF, Steele JL, et al. Development of a clinical risk calculator for prolonged opioid use after shoulder surgery. J Shoulder Elbow Surg 2019; 28: 2225–2231. [DOI] [PubMed] [Google Scholar]
- 45.Bartels K, Mayes LM, Dingmann C, et al. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One 2016; 11: e0147972. DOI: 10.1371/journal.pone.0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim N, Matzon JL, Abboudi J, et al. A prospective evaluation of opioid utilization after upper-extremity surgical procedures: identifying consumption patterns and determining prescribing guidelines. J Bone Joint Surg Am 2016; 98: e89. [DOI] [PubMed] [Google Scholar]
- 47.Schoenfeld AJ. Special considerations in pain management in orthopaedic subspecialties. J Bone Joint Surg Am 2020; 102: 47–53. [DOI] [PubMed] [Google Scholar]
- 48.Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia—when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet 2019; 393: 1558–1568. [DOI] [PubMed] [Google Scholar]
- 49.Mercadante S, Arcuri E, Santoni A. Opioid-induced tolerance and hyperalgesia. CNS Drugs 2019; 33: 943–955. [DOI] [PubMed] [Google Scholar]
- 50.Braden JB, Sullivan MD, Ray GT, et al. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry 2009; 31: 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng J, Kahn RL, Yadeau JT, et al. The fibromyalgia survey score correlates with preoperative pain phenotypes but does not predict pain outcomes after shoulder arthroscopy. Clin J Pain 2016; 32: 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gil JA, Gunaseelan V, DeFroda SF, et al. Risk of prolonged opioid use among opioid-naïve patients after common shoulder arthroscopy procedures. Am J Sports Med 2019; 47: 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valencia C, Fillingim R, Bishop M, et al. Investigation of central pain processing in post-operative shoulder pain. J Pain 2012; 13: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westermann RW, Anthony CA, Bedard N, et al. Opioid consumption after rotator cuff repair. Arthroscopy 2017; 33: 1467–1472. [DOI] [PubMed] [Google Scholar]
- 55.Earp BE, Silver JA, Mora AN, et al. Implementing a postoperative opioid-prescribing protocol significantly reduces the total morphine milligram equivalents prescribed. J Bone Joint Surg Am 2018; 100: 1698–1703. [DOI] [PubMed] [Google Scholar]
- 56.Hah JM, Bateman BT, Ratliff J, et al. Chronic opioid use after surgery: implications for perioperative management in the face of the opioid epidemic. Anesth Analg 2017; 125: 1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shanthanna H, Ladha KS, Kehlet H, et al. Perioperative opioid administration: a critical review of opioid-free versus opioid-sparing approaches. Anesthesiology 2021: 645–659. [DOI] [PubMed] [Google Scholar]
- 58.Forlenza EM, Lavoie-Gagne O, Lu Y, et al. Preoperative opioid use predicts prolonged postoperative opioid use and inferior patient outcomes following anterior cruciate ligament reconstruction. Arthroscopy 2020; 36: 2681–2688. e1. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence JTR, London N, Bohlman HH, et al. Preoperative narcotic use as a predictor of clinical outcome: results following anterior cervical arthrodesis. Spine 2008; 33: 2074–2078. [DOI] [PubMed] [Google Scholar]
- 60.Cozowicz C, Olson A, Poeran J, et al. Opioid prescription levels and postoperative outcomes in orthopedic surgery. Pain 2017; 158: 2422–2430. [DOI] [PubMed] [Google Scholar]
- 61.Ekhtiari S, Yusuf I, AlMakadma Y, et al. Opioid use in athletes: a systematic review. Sports Health 2020; 12: 534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harle CA, Danielson EC, Derman W, et al. Analgesic management of pain in elite athletes: a systematic review. Clin J Sport Med 2018; 28: 417–426. [DOI] [PubMed] [Google Scholar]
- 63.Rees HW. Management of osteoarthritis of the hip. J Am Acad Orthop Surg 2020; 28: e288–e291. [DOI] [PubMed] [Google Scholar]
- 64.McGrory B, Weber K, Lynott JA, et al. The American academy of orthopaedic surgeons evidence-based clinical practice guideline on surgical management of osteoarthritis of the knee. J Bone Joint Surg Am 2016; 98: 688–692. [DOI] [PubMed] [Google Scholar]
- 65.McLaughlin DC, Cheah JW, Aleshi P, et al. Multimodal analgesia decreases opioid consumption after shoulder arthroplasty: a prospective cohort study. J Shoulder Elbow Surg 2018; 27: 686–691. [DOI] [PubMed] [Google Scholar]
- 66.Elkassabany NM, Wang A, Ochroch J, et al. Improved quality of recovery from ambulatory shoulder surgery after implementation of a multimodal perioperative pain management protocol. Pain Med 2019; 20: 1012–1019. [DOI] [PubMed] [Google Scholar]
- 67.Conti Mica M. Optimal designs for multicenter interventional trials in musculoskeletal pain alleviation. J Bone Joint Surg Am 2020; 102: 59–60. [DOI] [PubMed] [Google Scholar]
- 68.Bonner BE, Castillo TN, Fitz DW, et al. Preoperative opioid use negatively affects patient-reported outcomes after primary total hip arthroplasty. J Am Acad Orthop Surg 2019; 27: E1016–E1020. [DOI] [PubMed] [Google Scholar]
- 69.Ingall E, Klemt C, Melnic CM, et al. Impact of preoperative opioid use on patient-reported outcomes after revision total knee arthroplasty: a propensity matched analysis. J Knee Surg. 2021 May 15. Epub ahead of print 2021. DOI: 10.1055/s-0041-1729966. [DOI] [PubMed] [Google Scholar]
- 70.Singh V, Kugelman DN, Rozell JC, et al. Impact of preoperative opioid use on patient outcomes following primary total hip arthroplasty. Orthopedics 2021; 44: 77–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-sel-10.1177_17585732211070193 for The role of preoperative opioid use in shoulder surgery—A systematic review by Omar A Al-Mohrej, Carlos Prada, Kim Madden, Harsha Shanthanna, Timothy Leroux, and Moin Khan in Shoulder & Elbow