Abstract
Undesirable postoperative tissue adhesions remain among the most common complications after surgery. Apart from pharmacological antiadhesive agents, various physical barriers have been developed in order to prevent postoperative tissue adhesions. Nevertheless, many introduced materials suffer from shortcomings during in vivo application. Thus, there is an increasing need to develop a novel barrier material. However, various challenging criteria have to be met, so this issue pushes the research in materials to its current limits. Nanofibers play a major role in breaking the wall of this issue. Due to their properties, such as a large surface area for functionalization, tunable degradation rate, or the possibility of layering individual nanofibrous materials, it is feasible to create an antiadhesive surface while maintaining biocompatibility. There are many ways to produce nanofibrous material; electrospinning is the most used and versatile technique. This review reveals the different approaches and puts them into context.
1. Introduction
Almost 30 years have passed since the founding of the multidisciplinary field called “tissue engineering”, and there is still a growing interest in its products. The attention stems from the tendency to effectively increase the quality of medical care, to offer patients new treatment options, and, above all, to address deficient organs for transplantation. The growing interest has naturally resulted in new possibilities for the application of nanofibrous scaffolds, which are, however, subjected to ever-increasing requirements.
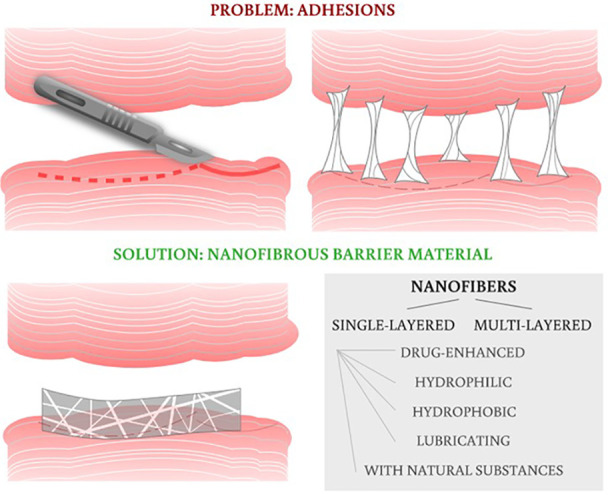
One of the urgent needs of the past decade is to develop a functional biomaterial to successfully prevent undesirable postoperative tissue adhesion (PA). Tissue adhesions are fibrous bands that commonly occur after surgery, infection, trauma, or radiation. These fibrous structures are formed between tissues and organs that are physiologically unconnected. Clinically, this problem is most serious in abdominal surgery, where adhesions between intraperitoneal organs and inner surfaces of the abdominal wall are formed,1 and also in musculoskeletal surgery, where adhesions between tendons and tendon sheaths are especially undesirable.2,3 They present postoperatively in a wide range of complications, from mild problems like abdominal discomfort to severe abdominal pains and gastrointestinal obstructions in abdominal surgery4 or significant limitation of limb mobility when tendon adhesions appear.2 Many of these conditions need to be treated again surgically. Unfortunately, these postoperative complications are not rare occurrences but commonly affect patients’ lives after pelvic, gynecological, peritoneal, tendon, and gastrointestinal surgeries.
The lack of commercially available materials for effective and safe PA prevention has driven research to the development of novel biomaterials. This review summarizes the attempts in the development of antiadhesive nanofibrous materials, including a description of the most commonly used fabrication method—electrospinning. The goal is to offer a comprehensive overview based mainly on literature research combined with gained knowledge in this field.
2. Methods
The mini-review was prepared on the basis of a careful search of the scientific literature in the Scopus and Web of Science databases. The search was based on a combination of keywords and Boolean operators. Suitable research articles were selected after entering the following combinations:
biomaterial AND (organ adhesion OR tissue adhesion) AND (nanomaterial OR nanofib*)
biomaterial AND adhesion AND (organ OR tissue) AND (nanomaterial OR nanofib*)
biomaterial AND adhesion AND (organ OR tissue) AND (nanomaterial OR nanofib*) AND (prevent OR avert OR preclude)
3. Tissue Adhesions
Undesirable PAs remain among the most frequent complications following a surgical operation. The formation of PA occurs in the early postoperative period, the most critical period being 3–5 days after the operation. Postoperative adhesions are the result of the organism’s reaction to a surgical intervention.5 During operations, the epithelial layers of tissues are intentionally damaged to ensure the surgical procedure itself. The healing process counts on tissue adhesion in a desired location, for example at the suture line. However, it can also lead to the formation of these tight fibrous connections in surrounding tissues and organs. These PAs can be a cause of pain, blockage of the small intestine, or even infertility in women. These need to addressed, often surgically, and they can significantly affect the quality of a patient’s life.4 The frequency of peritoneal adhesions in abdominal surgery ranges around 55–66%,6 with even higher numbers (90%) for patients who have undergone previous surgeries.7 The incidence rates (7–9%) of pericardial adhesions are observed after cardiac operations, and tendon surgeries are also frequently complicated by adhesions (16–27%).6
Adhesions arise from the activity of fibroblasts and their proliferation, coagulation, and fibrin deposition, mainly in places where bleeding or inflammation has occurred. During blood clotting, protein fibers called “fibrin” are formed. Subsequently, in the postoperative period, the fibrin is degraded due to fibrinolysis. However, when the fibrinolysis is not complete, the fibrin may create so-called “bridges” between normally unconnected tissues, and severe adhesions occur. These fibrous connections can become very firm in the weeks following surgery, and blood vessels and nerve fibers can be formed inside them. Since this review aims to focus more on the material part of the issue, we recommend readers to check other articles focusing more on the adhesion formation mechanism for better understanding of this phenomenon.6,8,9
Treatment of already formed adhesions is costly and time-consuming, leading to prolonged hospital stays and potentially impaired quality of life for patients. Understandably, attention is thus given to methods for preventing the formation of PA, with the resulting focus on the development of novel materials.8
One of the new approaches to the prevention of unwanted peritoneal adhesions arose at the beginning of this century together with the development of nanotechnology and especially the use of nanofibers for medical purposes. Planar nanofibrous layers can be used as barrier materials that form an interface between the damaged/operated on organ and the surrounding tissue in the human body. The indisputable advantage of nanofibrous materials is not only their potential biodegradability, which leads to the elimination of additional operations regarding the removal of the material, but also their similarity to the extracellular matrix. Nanofibrous materials can also support the healing of damaged tissue, which can be further accelerated by the incorporation of active substances into the nanofibrous layers. On the basis of the presented review, it is clear that several approaches to the preparation of nanofibers have been tried. One example is the application of biodegradable nanofibers to gastrointestinal anastomoses. Figure 1 depicts the documentation of the author’s group research, in which the nanofibrous patches were applied around intestinal anastomoses in porcine models to prevent undesirable peritoneal adhesions and anastomotic leakage.
Figure 1.
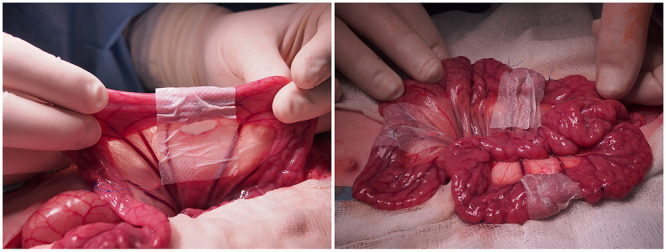
Application of nanofibrous patches around the gastrointestinal anastomoses in pig models. Author’s own photographs, previously unpublished data. Research is further documented in refs (5−7).
4. Fabrication of the Nanofibrous Membranes
Since the following literature investigation compares nanofibrous materials fabricated via different techniques, we decided to introduce and describe the commonly used approaches for more convenient reading, understanding, and orientation in the provided review.
Nanofibrous scaffolds for biomedical applications can be fabricated via several methods, including electrospinning, drawing, phase separation, template synthesis, freeze-drying, and self-assembly.13 Among these, electrospinning represents the most widely used approach. The reasons behind its popularity are its potential for controlled large-scale production, economic aspects, and simplicity, together with the variability of the process. Several natural and synthetic polymers, their blends, and mixtures with active substances can be processed in an electric field. In addition, the market offers various devices for laboratory research as well as equipment for industrial production.14
4.1. Principles of the Electrospinning Method
4.1.1. The Laboratory Electrospinning Method
Lab-scale electrospinning consists of three basic components, namely a DC high voltage source, a spinneret, and a collector. The most commonly used laboratory setups are needle (Figure 2A) and rod (Figure 2B) electrospinning. The process of fiber formation begins by applying a high electric voltage to the spinning electrode, thereby creating an electrostatic field between the electrode and the grounded/oppositely charged collector. The spinneret is commonly represented via a needle. A polymeric solution is dosed from a syringe at a controlled rate until a droplet is formed at the end of the needle. Due to the external electric field, a charge is induced on the surface of the polymer droplet. This charge has the same polarity as the high voltage source and is therefore attracted to the collector. Particles of the same charge repel each other, but at the same time the liquid tries to keep its shape due to surface tension. As a result, the hemispherical shape of the drop at the end of the needle is formed into a conical shape called a “Taylor cone”. After further increase of the electric field, the so-called “critical voltage” is reached. The surface tension is overcome, and a stream of solution is formed from the Taylor cone and directed toward the lower potential region (collector). The solution stream then passes through stable and unstable phases. During instability, the stream is stretched and narrowed and the solvent is rapidly evaporated, leaving behind dry nanofibers on the substrate. The clear disadvantages of the process are the low production rate, needle clogging, and inhomogeneities, together with environmental conditions that may be difficult or impossible to control.15
Figure 2.
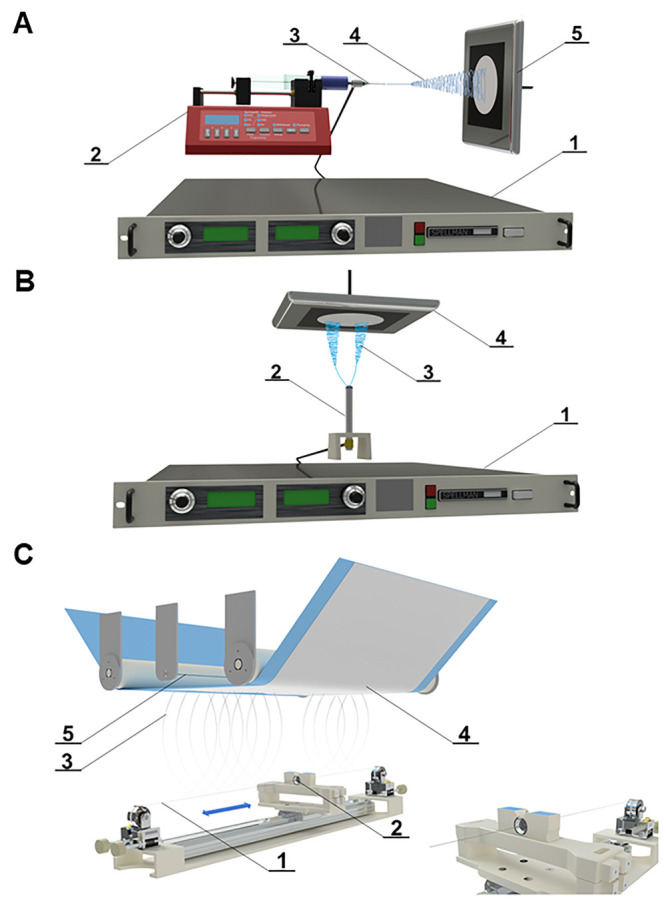
(A) Scheme of needle electrospinning: 1, high voltage source; 2, pump; 3, steel needle as a positive electrode; 4, fiber formation; 5, grounded steel collector. Nanofibers are collected on the black paper. (B) Scheme of rod electrospinning: 1, high voltage source; 2, steel rod as a positive electrode; 3, fiber formation; 4, grounded steel collector. Nanofibers are collected on the black paper. (C) Scheme of needleless nanospider technology: 1, steel wire used as a positive electrode; 2, steel orifice with polymer solution reservoir; 3, fiber formation; 4, nanofibrous layer collected on the supportive textile (e.g., blue polypropylene spunbond); 5, steel wire used as negative electrode.
4.1.2. Industrial-Scale Production
The large-scale production of nanofibers is carried out from an open liquid surface (Figure 2C). The commonly used needleless method is called “Nanospider technology”, developed at the Technical University of Liberec in the Czech Republic in 2003. The first generation of the device consisted of a rotary cylinder as the spinneret. The cylinder was partially immersed in the polymeric solution bath, causing a thin layer of the polymeric solution to be constantly created on its surface.15 This approach was implemented in industrial production by Elmarco s.r.o. (Liberec, Czech Republic). However, it was overtaken by the second generation, which solved several shortcomings of the first. In the second-generation setup, the cylindrical spinneret was replaced with a thin steel wire. This approach overcame disadvantages such as high consumption of polymer solution, evaporation of solvents from the bath before spinning, and an inferior spinning process with some polymers due to the round shape of the spinneret (electrical charge is concentrated around sharp points). The polymer solution is dosed to a moving cartridge, which applies a thin layer of the solution around the whole wire. Due to the electrostatic field, Taylor cones are formed on the entire surface of the wire. Thus, electrospinning is far more productive compared to a single needle electrode. The resulting fibrous layer is usually deposited on a substrate (e.g., nonwoven fabric). The substrate moves according to a predefined rewinding speed. The specific weight of the nanofibrous material can thus be easily influenced. Moreover, the temperature and air humidity can be easily controlled via a climate unit.
The difference between the manufacturability of nanofibers using laboratory techniques and semi-industrial production is shown in Figure 3. The images in Figure 3 depict not only the difference in productivity but also—perhaps even more fundamentally, and not only for medical research—in the homogeneity of the final material. Although, even during needle manufacturing, places with large defects can be macroscopically found, the overall homogeneity of the surface may not always correspond to the selected places shown in the scanning electron microscopy (SEM) images. As can be seen in Figure 3, the nanofibers look more or less the same in all the presented SEM images. However, this is not conclusive, as the macroscopic structures of the materials clearly differ (e.g., the specific weight is clearly inconsistent across the surface, and areas with lower layer thickness are visible). The SEM images represent only a small portion of the overall material properties and can thus be misleading. Moreover, most research articles present only the morphological SEM images and methodology, whereas the complete material dimensions are not evident. The same biodegradable polymer was fabricated via needleless and needle electrospinning (Figure 3). The needleless production of material A on the left (size 45 × 106 cm) took 30 min, the needle production of material B (4 × 15 cm) lasted 15 min, and material C (10 × 16 cm) was fabricated in 60 min. The size of the fibrous layer further limits the characterization methods that can be used to understand the material’s properties (limited number of tested samples, limited number of methods), but it also limits the medical research. Only a small amount of material with unclear parameters is available for further in vivo evaluation, which may lead to distorted or inaccurate results.
Figure 3.
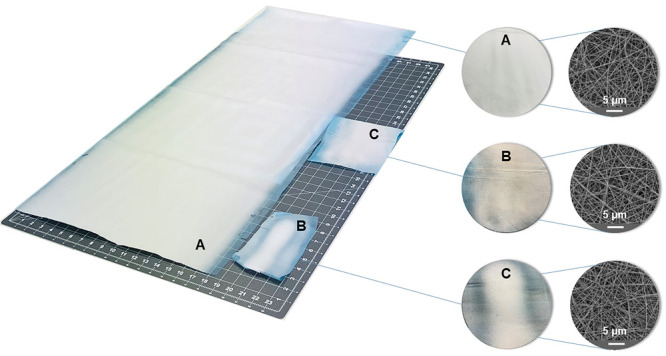
Difference between needleless electrospinning (A) and needle electrospinning nanofibrous products (B and C). Comparison of the homogeneity on the macroscopic (inhomogeneous) and microscopic views (homogeneous). The microscopy (SEM) images show similar structures in all products without revealing the specific weight/thickness inconsistency across the whole surface.
5. Types of Antiadhesive Nanofibrous Materials
During our literature review, we found that the developed nanofibrous materials could be divided into two major groups: (1) single-layered and (2) multilayered nanofibrous structures. Single-layered materials can be further divided into (a) pure untreated nanofibrous materials, (b) loaded nanofibers with chemical/natural substances, (c) hydrophilic structures, (d) hydrophobic structures, and (e) lubricating nanofibers. The multilayered materials were divided into (a) double-layered structures and (b) multilayered structures in this review. We were able to analyze more than 60 studies. From these, we decided to select specific studies representing different approaches, summarize the outcomes, and describe the connections.
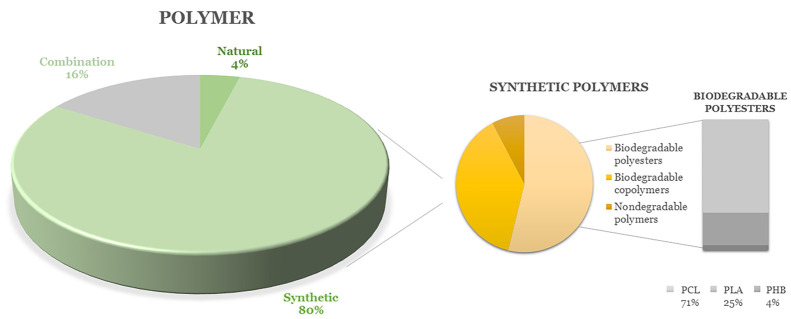
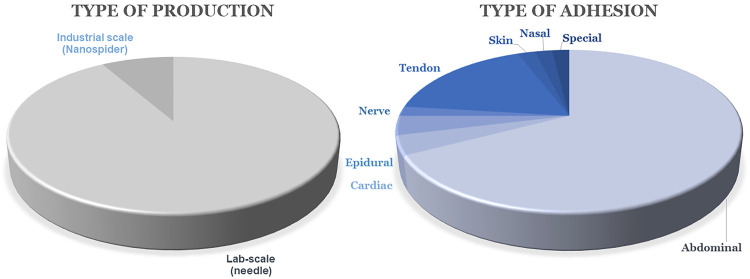
The breakthrough or pivotal results of these studies will be presented in more detail in the following sections; the figures below are used for clear and graphical analyses of the types of polymers used for the production of nanofibers for antiadhesive applications (Figure 4), whether these materials are produced on a large or laboratory scale (Figure 5, left), and which types of adhesion problems are solved (so far only preclinically) using nanofibers (Figure 5, right). It is clear from Figure 4 that synthetic polymers are used the most, and biodegradable polyesters are selected from among them, with polycaprolactone playing a leading role. Figure 5 shows that needle electrospinning is still the most widely used fabrication method. Of the reviewed articles, 92% used needle devices, despite certain limitations and the risk of low repeatability and homogeneity of the product, as explained above. It can be seen that the tendency to prevent abdominal postoperative adhesions is the most pronounced, in that 67% of the reviewed research focuses on complications after abdominal operations. Tendon adhesions are also intensively studied, with 17% of the research aiming to prevent them (Figure 5).
Figure 4.
Graphical overview of used polymers for antiadhesive nanofiber production.
Figure 5.
Methods for production of nanofibrous antiadhesive barriers (left). Prevented types of adhesions via nanofibrous materials (preclinical research only; right).
5.1. Single-Layered Nanofibrous Membranes
5.1.1. Pure Untreated Nanofibers
Some of the following studies considered pure untreated nanofibrous materials and compared different polymers with each other and with commercially available antiadhesive tools, such as Interceed.
Dinarvand et al.16 produced nanofibrous surfaces from biodegradable poly-ε-caprolactone (PCL), poly-l-lactide (PLLA), poly(lactic-co-glycolic acid) (PLGA), and nonabsorbable polyethylsulfone (PES) to prevent abdominal PA. The materials were fabricated via needle electrospinning. They compared the antiadhesive and anti-inflammatory properties of these surfaces with commercially used oxidized regenerated cellulose (Interceed). Mouse experiments were performed to determine the antiadhesive properties in various nanofibrous materials with similar fiber diameters and pore sizes. Considering the wound healing properties, nondegradable PES showed a high degree of inflammation and did not prevent tissue adhesion. Nanofibrous mats based on PLLA showed a better effect than the untreated group but a lower effect than Interceed. The PCL material was found to be as effective as Interceed and had a low rate of inflammation. The PLGA membrane had the lowest degree of inflammation compared to all other groups and showed the best antiadhesive results.16
Gholami et al.17 introduced nanofibrous membranes based on polyurethane (PU). Medical-grade polyurethane was dissolved in tetrahydrofuran and N,N-dimethylformamide in different concentrations (6, 8, 10, and 12% w/v). The nanofibers were created via needle electrospinning and collected on the aluminum foil wrapped around the collector (a rotating drum). The in vivo adhesion testing was performed via a rat cecal abrasion model. The adhesion efficacy of nanofibrous materials was compared with the control group and with commercial soft polypropylene nonabsorbable synthetic surgical mesh (PMS Stripsck). The antiadhesion behavior was evaluated at 3 and 5 weeks after a surgical operation macroscopically and histopathologically. The best adhesion inhibition score was reached by using the 8% polyurethane nanofibers. The study further investigated the degradation rates on the developed materials; the weight loss reached 86.23% by day 64 for the 8% PU nanofiber, meaning a faster degradation rate compared to the commercial surgical mesh.17
Our team investigated PCL and poly(l-lactide-co-ε-caprolactone) (PLCL) nanofibrous materials fabricated via the needleless electrospinning technique Nanospider. The homogeneous layers were sterilized via low-temperature ethylene oxide and applied in the size 2 × 5 cm to intestinal anastomoses during a large animal in vivo experiment. The application was found to be easy for surgeons, leading to fast manipulation without prolonging the operation time. Both materials promoted healing of the anastomoses in vivo; however, the study does not confirm any antiadhesive effect. Thus, further research was focused on the improvement of the current structure and development of the antiadhesive layer.10
5.1.2. Loaded Nanofibers with Chemical/Natural Substances
5.1.2.1. Antibiotics
The first reported attempt to test the antiadhesive behavior of nanofibers was found in the study by Zong et al. in 2004.18 The FDA-approved copolymer PLGA was electrospun via laboratory needle based equipment and used as the barrier material. In order to enhance the antiadhesive properties and prevent intra-abdominal infection, the antibiotic cefoxitin sodium was incorporated into the PLGA nanofibers. The presented results confirm the efficacy of the antibiotic-enhanced materials since the cecal adhesion was lower compared to the control and pure PLGA during in vivo studies on rat animal models.18
Bölgen et al.19 produced a nanofibrous surface via needle electrospinning to prevent postoperative abdominal adhesion and evaluated it in a rat model. Biodegradable PCL polymer was used as a nanofibrous drug delivery system, and an antibiotic (Biteral) was added to a group of samples to observe the antiadhesive effect. According to macroscopic and histological evaluations, it was observed the tissue adhesion decreased in both drug-loaded and unloaded groups; however, the antibiotic content in nanofibrous surfaces showed a significant effect in adhesion prevention and also contributed to healing.19
5.1.2.2. Ibuprofen
Liu et al.20 suggested that the degradable membranes could trigger inflammation during degradation, causing foreign body syndrome and leading to decreased antiadhesion behavior. Therefore, the study introduced the PLGA-based needle-electrospun membranes containing free ibuprofen (IBU) and a combination of ester-bound IBU and poly(hydroxyethyl methacrylate) (PIBU) for sustainable drug release. The aim of the material was to prevent unsuccessful back surgery syndrome after spine surgery. The electrospinning setup was based on laboratory needle spinning. According to the laminectomy rat model test results, PLGA–PIBU–IBU membrane shows an enhanced antiadhesive effect compared to the control group of drug-free membrane and membranes using only IBU or PIBU. According to the results of nerve function tests, PLGA–PIBU–IBU membranes reduce pain and neurological deficits.20
5.1.2.3. Mitomycin C
Zhao et al.21 aimed to solve the tendon adhesions with a hydrophobic PLLA nanofibrous layer releasing the antifibrotic mitomycin C. The sustainable drug release was achieved by incorporating mitomycin C into hyaluronic acid based hydrosols encapsulated in PLLA fibers. In vivo studies were performed on rat and rabbit Achilles tendon models; the results show well-healed lacerate tendon tissue with no presence of adhesions.21
5.1.2.4. Natural Substances
Shin et al.22 produced PLGA-based nanofiber membranes that provide a controlled release of polyphenol epigallocatechin-3-O-gallate (EGCG) to prevent tissue adhesion and accelerate the healing process. The PLGA was electrospun with EGCG from a solution via needle electrospinning. The efficacy of EGCG-releasing PLGA (E-PLGA) nanofiber membranes compared to those of pure PLGA and Interceed was tested in vivo in rat models. The results show that E-PLGA has a significant antiadhesive effect compared to the control and pure PLGA materials and that it gives values close to that of the commercial barrier Interceed.22
Chen et al.23 aimed to prevent peritendinous adhesion with PCL nanofibers grafted with chitosan. In the study, the PCL solution was electrospun and further treated with hydrophilic plasma grafted with chitosan molecules (CS). Chitosan is a biocompatible and biodegradable polysaccharide; moreover, its use as an antiadhesion agent had already been reported. In vitro cell culture experiments proved the PCL and PCL–CS surfaces to have a cytocompatible and antiadhesive surface. The efficacy of the materials was tested in vivo with a rabbit flexor digitorum profundus tendon model due to its analogous flexor mechanism to a human digit. The PCL–CS were compared with PCL and the commercially used Seprafilm. The developed material served as the best antiadhesive physical barrier according to extensive evaluation.23
Recently, Babadi et al.24 incorporated curcumin (CUR) into nanofibrous planar materials based on polycaprolactone. Their study first reports the use of curcumin-loaded nanofibers for antiadhesive applications. Needle electrospinning was used to fabricate the nanofibrous materials with a CUR concentration of 1:8 (CUR:PCL weight ratio). The impact of curcumin on peritoneal adhesion formation was evaluated via rat models 14 days after surgery. The control group remained untreated. The antiadhesion activity of nanofibrous materials was compared to that of polymeric films (PCL with CUR). The films showed better antiadhesive effects, probably due to less porous surfaces and better mechanical properties. Nanofibers loaded with CUR showed lower adhesion grades compared to blank PCL nanofibers. The authors suggest further investigation and long-term studies in vivo.24
5.1.3. Hydrophilic Nanofibers
Li et al.25 produced composite membranes by needle electrospinning to prevent postoperative abdominal adhesion. The material was based on PLGA. The high hydrophobicity of PLGA was reduced via poly(ethylene glycol) (PEG). Different concentrations of PEG in the final material were tested, namely 0, 5, 10, 15, 20, and 25% PEG. The cell attachment, proliferation, and penetration of the structures were prevented, and also these surfaces were biocompatible and biodegradable. Test results showed that a 5% PEG-containing surface provided the best antiadhesive effect on the repaired site of rat cecum mode.25
More recently, Klapstova et al.26 focused on the development of an antifibrotic nanofibrous layer by influencing the material’s structure and wettability rather than adding antifibrotic agents like mitomycin C. In their study, the nondegradable polyvinylidene fluoride (PVDF), with the addition of polyethylene oxide (PEO), could serve as a glaucoma drainage implant. In such applications, the long-term stability of the implant is highly desirable. The in vitro result with mouse fibroblasts shows inhibited cell growth due to the release of PEO. Further studies are necessary to prove the effect in vivo; however, the developed structure could serve as a promising antiadhesive material, especially in applications where nondegradability is required.26
5.1.4. Lubricating Nanofibers
Cheng et al.27 considered lubrication as the key factor in preventing adhesion and introduced lubricating nanofibrous membranes. The lubrication effect was performed on needle-electrospun PCL nanofibers via in situ grafting of a copolymer synthesized by dopamine methacrylamide (DMA) and 2-methacryloyloxyethyl phosphorylcholine (MPC). An in vivo rat animal study revealed the successful antiadhesion properties in tendon surgeries; this data was in correlation with their previous in vitro results with fibroblasts.27
Recently, Fan et al.28 published a study about superhydrophilic nanofibrous membranes to battle epidural adhesions. In the study, polyvinylpyrrolidone (PVP) was grafted to PLGA polymer, dissolved, and electrospun via a needle setup. The resulting fibers were mixed with phosphatidylcholine (PC), which was dissolved and electrospun from the opposite needle. The role of PC was to lubricate and smooth the membrane’s surface and thus prevent secondary damage. PC was previously used in abdominal operations to prevent adhesion. The rabbit animal models were used to perform a laminectomy and test and compare the developed material with Interceed. The nanofibrous membrane reduced the scar adhesion, and no side effects were observed.28
5.2. Multilayered Nanofibrous Membranes
The amount of research focusing on multilayered fibrous scaffolds is increasing. The reason for this interest lies primarily in the efforts to effectively mimic natural tissues since their complex structure cannot be replicated by using single-layered materials. Multilayered structures can also be very useful in adhesion prevention because the two sides of the material can have different properties. One side of the material can be adhesive and attach to the damaged tissue, while the other side can act as the antiadhesive agent and limit contact with the surrounding tissues. The current research on multilayered nanofibrous antiadhesive surfaces is summarized below.
Jiang et al.29 have developed a double-layered structure produced by electrospinning to investigate the abdominal antiadhesive activity. The inner layer of this membrane consists of PCL loaded with hyaluronic acid (HA), while the outer layer consists of PCL. The inner layer should be releasing the HA and thus maintaining tissue gliding. According to animal experiments on rats in a cecum abrasion mode, this double-layered structure showed a superior antiadhesive effect compared to the untreated control group and single-layer PCL group.29
In order to mimic the natural tendon structure, Deepthi et al.30 combined collagen–chitosan hydrogel with a PLLA electrospun nanofibrous membrane (three layers of nanofibers). The structure was further coated with alginate to enhance the antiadhesion by reducing protein adhesion to the alginate surface. The presented results proved the proper rate of degradation together with sufficient mechanical properties for flexor tendon regeneration. Preliminary studies have shown that alginate-coated collagen–chitosan-aligned PLLA nanofiber structures are suitable for flexor tendon regeneration and may prevent peritendinous adhesion.30
Shalumon et al.31 developed a multifunctional nanofiber structure with a needle coaxial electrospinning technique to prevent tissue adhesion after tendon surgery. They used silver nanoparticles (AgNPs) and ibuprofen to provide anti-infection and anti-inflammation properties together with HA for a lubricant effect. In the structure, the shell portion consisted of AgNP embedded PEG/PCL and the core portion was HA/IBU with PEO. According to a rabbit flexor tendon rupture model, the best antiadhesive result was shown by the structure with a PEG/PCL/Ag shell and an HA core containing 30% ibuprofen.31
In our research, we developed a double-layered material combining the advantages and different wettabilities of PCL and poly(vinyl alcohol) (PVA) polymers. Two types of PVA were chosen: PVA with a low degree of hydrolysis (soluble in water) and PVA with a high degree of hydrolysis (turns into hydrogel in an aqueous environment). The research solved the low stability of PVA in the aqueous environment since the PCL layer acted as a supportive structure maintaining the shape of the whole scaffold. The double-layered material was devoted to fortifying gastrointestinal anastomoses.32 During previous in vivo studies by Rosendorf et al., the single PCL layer showed appropriate behavior in vivo but did not prevent peritoneal adhesions.10 The new approach was built on the hypothesis of low cell adhesion due to the transformation of PVA nanofibers into hydrogels after direct contact with a wet tissue. The in vivo animal studies revealed the biocompatibility of the developed material; the anastomoses were covered without any adverse effects. However, the delamination of the PCL and PVA layers was observed in vivo, which is an undesirable effect. No signs of severe anastomotic leakage were observed after the operations; however, the materials did not exhibit any major effect on peritoneal adhesion formation.11
More recently, we fabricated a biocompatible hierarchical structure inspired by the antiadhesive behavior of natural lotus leaf. The desired structure was achieved by combining the needleless electrospinning and electrospraying of biodegradable PCL. Hydrophobic plasma treatment was used to enhance the antiadhesive properties, which were proven during mechanical testing ex vivo with the intestinal tissue. The material was noncytotoxic with stable plasma treatment during a 6 months period. A further in vivo study will be performed.33
6. Assessment of Antiadhesive Properties
Another issue related to the development of antiadhesive materials is the proper assessment of antiadhesive behavior. The most valuable outcomes come from in vivo evaluation; however, material characterization as well as in vitro analyses should be carried out before animal experiments. Most of the research analyzed wettability of produced materials, that is, measurement of the water contact angle (WCA). Interestingly, the outcomes of surface wettability are controversial in the studies, as seen from Figure 6. Additionally, adsorption of water or simulated intestine liquids could be analyzed as described, for example, in studies.23,33 Another material characterization used for prediction of antiadhesive properties ex vivo is mechanical assessment, as described in our previous study.33 The so-called “peel test” enables researchers to quantify mechanical interactions between a material and native tissue. However, the evaluation takes place ex vivo; thus, the results rather approximate the behavior of the material in the animal body and possibly help to reduce the number of animals used during in vivo trials.
Figure 6.
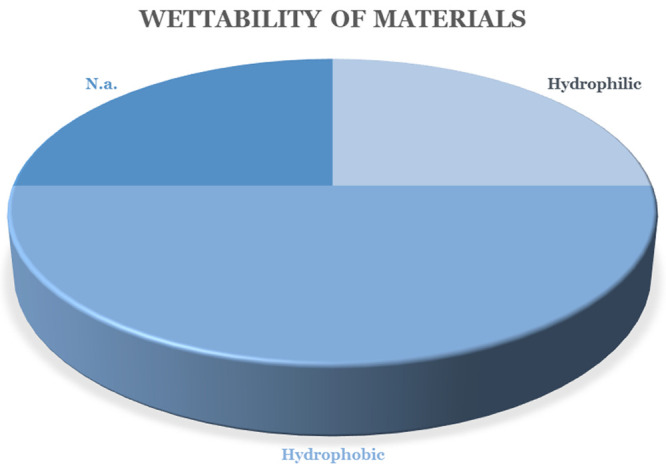
Wettability of the developed nanofibrous barrier materials.
Assessment of material characterization and in vitro experiments was provided, for example, in the study by Deepthi et al., in which protein adsorption on surfaces of materials was evaluated. It is expected that less protein adsorption means less cell attachment (in vitro/in vivo).30 On the other hand, such a prediction could be misleading since various types of proteins could be adsorbed on the surface, leading to further adhesion of cells. Moreover, conformation of bound proteins is very important for cell attachment, which could not be measured by such an experiment.
Another group of assessments of antiadhesive properties utilizes in vitro experiments. Most frequently, fibroblast cells are seeded on the material surface, and their proliferation is investigated using metabolic assays and/or fluorescent microscopical techniques with image analysis.7,21,23,26,28,31,32 When antiadhesion properties are studied in specific applications such as tendon, tenocytes could be used.30 Utilization of mesenchymal stem cells was also reported in the study of Dinarvand et al.16 More sophisticated in vitro assessment of antiadhesive properties utilizes a special double-chamber dish separated by a porous membrane, as described in the studies of Chen et al.23 and Shalumon et al.31 The upper chamber, containing tested material and a lower fetal bovine serum (FBS) concentration (2%), was inoculated with cells. The lower chamber contained higher FBS content (10%). Cell penetration to the lower chamber environment was analyzed by metabolic assay. It is presumed that, when cell penetration is reduced, the material tends to be antiadhesive.23,31
Last, but not least, in vivo experiments bring the most valuable outcomes. Some of the studies are performed on small animals, such as rats or mice.7,16,17,19−21,24,27,29 Tendon and epidural antiadhesion/adhesion properties were mostly tested on rabbit animal models, as in studies.23,28,31 Utilization of higher animals for the assessment of antiadhesive properties in abdominal surgeries was reported in our previous studies.10−12
Table 1 summarizes the evaluation of antiadhesive properties used in studies reported in the review. It is obvious that material characterization mostly leans on WCA measurement, whose correlation with antiadhesive properties is not straightforward. The peel test could be helpful for the prediction of adhesion properties of developed materials, but its utilization has been very limited to date. In vitro studies using fibroblasts of specific cell lines could serve as a good alternative to in vivo experiments in predicting adhesive behaviors of developed materials. The highest attention is paid to animal studies with utilization of small and higher animal models. The research should be focused also on proper material characterization predicting antiadhesion properties, which is very challenging due to the complexity of adhesion formation in vivo.
Table 1. Summary of Assessment of Antiadhesive Properties of Developed Materials.
| nanofibrous material | material characterization | in vitro assessment | in vivo assessment | ref |
|---|---|---|---|---|
| PCL + curcumin | WCA | rats (2 weeks) | (24) | |
| PCL + Biteral | rats (4, 30, 45, 60, 90 days) | (19) | ||
| PLLA (three layers) | protein adsorption | tenocytes (1, 3, 7 days) | (30) | |
| PCL | mesenchymal stem cells (12 h) | mice (1 week) | (16) | |
| PLGA + PVP + phosphatidylcholine | WCA, water absorption | fibroblasts (1, 3, 5 days) | rabbits (1, 2, 3, 4 weeks) | (28) |
| PU | rats (3, 5 weeks) | (17) | ||
| PCL + chitosan | WCA, water absorption | fibroblasts (1 day), penetration of fibroblasts | rabbits (2, 4, 8 weeks) | (23) |
| PCL + grafting of synthetic copolymer | WCA | fibroblasts (1, 3, 7 days) | rats (3 weeks) | (27) |
| PCL/HA (double layer) | rats (2 weeks) | (29) | ||
| PVDF + PEO | WCA | fibroblasts (1, 8 days) | (26) | |
| PCL/HMDSO | WCA, absorption of water/simulated intestine liquid, peel test | (33) | ||
| PCL/PVA (double layer) | WCA | fibroblasts (4, 8, 15, 21 days) | (32) | |
| chitosan + PLGA + PEO | fibroblasts (1, 3, 5, 7 days) | rats (4 weeks) | (7) | |
| PLGA + PEG | WCA | fibroblasts (1, 3, 5 days) | rats (2 weeks) | (25) |
| PLGA + ibuprofen | WCA | rats (4, 8 weeks) | (20) | |
| PCL | fibroblasts (3, 7, 14, 21 days) | pigs (3 weeks) | (12) | |
| PCL and PLCL | pigs (3 weeks) | (10) | ||
| PCL/PVA (double layer) | pigs (3 weeks) | (11) |
7. Connections and Outlook
According to the literature review, there are many existing scientific approaches to achieving a functional nanofibrous barrier material. This finding goes along with the fact that there is a lack of commercially available barrier materials that can be easily and safely used with a significant prevention effect. It is also evident that nanofibers are receiving much attention in this biomedical development. Due to their advantageous properties, they may form new barrier materials as the solution to several postoperative adhesion problems. Moreover, their use in surgical operations could be easy and fast without requiring any special equipment. On the other hand—despite many years of effort—the commercial market does not offer any nanofibrous antiadhesive solutions. With sincere respect to other colleagues, we critically analyze the possible causes of failure, which are also evident from Table 2, summarizing the selected approaches for their quick analysis and comparison.
Table 2. Overview of Developed Nanofibrous Materials and Their Measured Properties.
| polymer | electrospinning | type of adhesion | antiadhesive effect | combination of nanofibers with other structures/substances | degradability | wettability | comparison with commercial materials | ref |
|---|---|---|---|---|---|---|---|---|
| Single-Layered Materials | ||||||||
| PCL | lab scale | abdominal | yes, comparable to Interceed (small animal model) | no | yes | hydrophobic | Interceed | (16) |
| large scale | abdominal | no (large animal model) | no | yes | hydrophobic | no | (10) | |
| lab scale | abdominal | yes (small animal model) | antibiotic Biteral | yes | n.a. (PCL known to be hydrophobic) | no | (19) | |
| lab scale | tendon | yes (small animal model) | chitosan | yes | hydrophilic (due to chitosan) | Seprafilm | (23) | |
| lab scale | abdominal | yes | curcumin | yes | hydrophobic | no | (24) | |
| lab scale | tendon | yes (small animal model) | grafting of a copolymer synthesized by DMA and MPC | n.a. | hydrophobic | no | (27) | |
| PLLA | lab scale | abdominal | no, lower effect than Interceed (small animal model) | no | yes | hydrophobic | Interceed | (16) |
| lab scale | tendon | yes (small animal model) | mitomycin C | yes | hydrophobic | no | (21) | |
| PLGA | lab scale | abdominal | yes, higher than Interceed (small animal model) | no | yes | hydrophobic | Interceed | (16) |
| lab scale | abdominal | yes (small animal model) | antibiotics (cefoxitin sodium) | yes | n.a. | no | (18) | |
| lab scale | epidural | yes (small animal model) | two types of ibuprofen | yes | hydrophobic | no | (20) | |
| lab scale | abdominal | yes, close values to Interceed (small animal model) | polyphenol epigallocatechin-3-O-gallate | yes | n.a. | Interceed | (22) | |
| lab scale | abdominal | yes (small animal model) | PEG | yes | hydrophilic | no | (25) | |
| lab scale | epidural | yes (small animal model) | PVP + phosphatidylcholine | yes | hydrophilic | Interceed | (28) | |
| PES | lab scale | abdominal | no (small animal model) | no | no | hydrophobic | Interceed | (16) |
| PU | lab scale | abdominal | yes (small animal model) | no | yes | n.a. | PMS Stripsck | (17) |
| PLCL | large scale | abdominal | no (large animal model) | no | yes | hydrophobic | none | (10) |
| PVDF | lab scale | antifibrotic (glaucoma treatment) | yes (in vitro fibroblasts) | PEO | no | hydrophobic | none | (26) |
| Multilayered Materials | ||||||||
| PCL/HA | lab scale | abdominal | yes (small animal model) | no | yes | n.a. (HA known to be hydrophilic; PCL known to be hydrophobic) | none | (29) |
| PLLA (three layers) | lab scale | tendon | yes (in vitro model) | collagen, chitosan, alginate | yes | n.a. | none | (30) |
| PEG/PCL/HA | lab scale | tendon | yes (small animal model) | AgNPs + ibuprofen | yes | hydrophobic PEG/PCL sheath, hydrophilic HA | none | (31) |
| PVA/PCL | large scale | abdominal | no (large animal model) | no | yes | hydrophilic side (PVA)/hydrophobic side (PCL) | none | (11, 32) |
| PCL/HMDSO | large scale | abdominal | yes (ex vivo model) | PCL droplets | yes | hydrophobic | none | (33) |
The main drawback may be the method of preparation of nanofibers. In the majority of studies, only laboratory needle electrospinning was used (see Figure 5). Although this approach allows verifiability of the spinnability of the developed polymeric solutions, its yield and efficiency are very low. Moreover, most laboratory equipment does not enable the control of environmental conditions (e.g., temperature, humidity), which is essential for the electrospinning process. The outputs of nanofiber production on such devices are thus debatable because the process is not repeatable. Several scientific articles point to the great potential of the developed nanofibrous materials, but unfortunately, almost no scientific teams have continued to transfer the production to semi-industrial and industrial facilities.
The low production efficiency is related to the type of testing of nanofibrous materials for the prevention of adhesions. Here again we come across the low productivity of needle electrospinning. Most tests of antiadhesive behavior have only been performed on small animal models, where smaller material samples are sufficient. Although the knowledge of in vivo testing on small animals confirms the biocompatibility of the developed materials, their relevance and predictability for human patients are at least debatable.
Furthermore, it is worth noting that many authors implement the incorporation of active substances into nanofibrous systems and thus take advantage of their large surface areas and drug delivery potential. However, most authors present the results of nanofiber characterization before the sterilization process. In vivo testing strictly requires sterilized materials, and properties may change after sterilization. Materials are thus implanted into animal organisms without real knowledge of their behavior (contact angle, microscopic structure changes, etc.).
Finally, it is evident that the authors differ in their opinions on the required hydrophilicities/hydrophobicities of the materials. It is known that the surface properties and wettability of a material are crucial for cell adhesions, yet the optimal wettability value for the prevention of adhesions of individual tissues is not known. In the literature, we can find approaches for the prevention of postoperative adhesions using both hydrophilic and hydrophobic materials. Surprisingly, their success in preventing adhesions is not obvious, according to published data. For example, abdominal adhesions can be prevented using both types of material. In recent literature, attention has been focused on nanofibers with a lubricating effect. It is believed that cell attachment on the nanofibrous surfaces may be completely inhibited by the lubricating effect of the material. The lubricated surface might also reduce friction damage caused by tissue sliding. On the basis of the results of the current literature search in Figure 6, it is evident that, in a significant number of articles (25%), the contact angle of the developed nanofibrous barrier material was not measured or at least not stated (marked as “n.a.”, which means “not applicable”). Half of the research focused on hydrophobic nanofibrous materials, and 25% of articles used hydrophilic structures.
As can be seen in Table 2, some research approaches compared their outputs with those of commercially available materials (Interceed or Seprafilm), surprisingly revealing some shortcomings of these products. This knowledge indicates that we are constantly searching for effective barrier prevention, not only with the help of new fibrous materials but also with the help of traditionally established methodologies.
Regarding the outlook for research, it can be assumed that the development of effective barrier materials will continue. We assume that there will be an expansion of the semioperational capability for the production of nanofibers to more workplaces, which will naturally lead to a higher, more homogeneous, and consistent production of nanofibers, to which other consequences are linked, such as a more effective potential for testing materials (large animal models, etc.). Great emphasis should be placed on the biodegradability of the material; the material should prevent the formation of adhesions in the critical postoperative period but at the same time should not prevent the natural healing of tissues. The handling of the material should be easy and not prolong the surgical procedure, and the application technique should be quick and simple. Lastly, the price of the material is also important, and this could decrease in proportion to the implementation of nanofibrous materials in the healthcare sector.
8. Conclusion
This review offers an overview of the developed barrier antiadhesive nanofibrous materials and comparisons between them across published research. The wide spectrum of the research articles supports the importance and potential of nanofibrous structures as barrier materials to prevent severe postoperative adhesions. The ongoing intensive research is carried out by many scientific groups supported by doctors from clinical practice. The problem of the formation of postoperative adhesions remains unsolved, since the commercially available materials are not capable of sufficient prevention. The quality of life for patients after gastrointestinal, pelvic, tendon, heart, or gynecological surgeries is thus still threatened and, in many cases, permanently reduced. It was found that the research approaches to achieve functional nanofibrous barrier prevention bring plenty of novel information about the medical applications of nanofibers. On the other hand, the outcomes are inconsistent on certain points. This review aims to help indicate other research directions by means of a comprehensive overview of the previous approaches.
Acknowledgments
The research was supported by the project Czech Health Research Council (MZ ČR AZV) NU20J-08-00009 "Prevention of intestinal anastomotic leakage and postoperative adhesions by using nanofibrous biodegradable materials".
Biographies
Marketa Klicova is a Ph.D. candidate in materials and tissue engineering at the Technical University of Liberec. She mainly participates in the development of novel nanofibrous materials for the prevention of postoperative adhesions. She owns several awards for scientific breakthrough ideas. In 2018 she received the Falling Walls Lab award, which is awarded annually to young scientists and visionaries with unique ideas. She is the (co)author of 10 impactful publications and has several conference contributions. Her international experience includes an internship at the Wyss Institute for Biologically Inspired Engineering at Harvard Medical School, where she worked on the development of organs-on-a-chip.
Jachym Rosendorf is a clinician who participates in the research and development of nanofibrous coverings for the prevention of serious complications after operations of the gastrointestinal tract. Moreover, he is working on research regarding effective tissue decellularization. He is a member of a team led by Prof. Václav Liška at the Biomedical Center, Faculty of Medicine in Pilsen, Charles University. He is the author or coauthor of more than 20 impactful publications and numerous conference presentations.
Jakub Erben is a junior researcher at the Faculty of Textiles, Technical University of Liberec. For his research on thymic organoids based on micro- and nanofibers, he received the prestigious award Prix International Théophile Legrand de l’Innovation Textile in 2019. In addition to the development of micro- and nanofibrous scaffolds, he works on the preparation of new 3D printed biodegradable implants as well as the development and commercialization of fibrous sorbents for analytical chemistry. He participated in research at Harvard Medical School and Northeastern University, Boston, MA.
Jana Horakova is a senior scientist at the Technical University of Liberec, where she received the title of associate professor in 2023 after defending her habilitation thesis about the development of small-diameter nanofibrous scaffolds. She is the author of more than 25 impactful publications and worked at Michigan Technological University in the department of tissue engineering. In 2016, she received the prestigious award Prix International Théophile Legrand de l’Innovation Textile for global textile and material innovators.
The authors declare no competing financial interest.
References
- Schnüriger B.; et al. Prevention of postoperative peritoneal adhesions: A review of the literature. Am. J. Surg. 2011, 201, 111–121. 10.1016/j.amjsurg.2010.02.008. [DOI] [PubMed] [Google Scholar]
- Titan A. L.; Foster D. S.; Chang J.; Longaker M. T. Flexor Tendon: Development, Healing, Adhesion Formation, and Contributing Growth Factors. Plast. Reconstr. Surg. 2019, 144, 639e–647e. 10.1097/PRS.0000000000006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S. E.; Jha B.; Ross M. Rehabilitation following surgery for flexor tendon injuries of the hand. Cochrane Database Syst. Rev. 2021, 2021 (1), CD012479. 10.1002/14651858.CD012479.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor H. Consequences and complications of peritoneal adhesions. Colorectal Dis. 2007, 9 (s2), 25–34. 10.1111/j.1463-1318.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- Arung W.; Meurisse M.; Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J. Gastroenterol. WJG 2011, 17, 4545–4553. 10.3748/wjg.v17.i41.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Monsonís H.; Kearns S.; Kelly J.; Zeugolis D. I. Battling adhesions: From understanding to prevention. BMC Biomed. Eng. 2019, 1, 5. 10.1186/s42490-019-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J. E.; Ko Y.-G.; Kim W. I.; Kwon O. K.; Kwon O. H. Nanofiber mats composed of a chitosan-poly(d,l-lactic-co-glycolic acid)-poly(ethylene oxide) blend as a postoperative anti-adhesion agent. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1906–1915. 10.1002/jbm.b.33726. [DOI] [PubMed] [Google Scholar]
- Wu W.; et al. Advances in biomaterials for preventing tissue adhesion. J. Controlled Release 2017, 261, 318–336. 10.1016/j.jconrel.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Kheilnezhad B.; Hadjizadeh A. A review: Progress in preventing tissue adhesions from a biomaterial perspective. Biomater. Sci. 2021, 9, 2850–2873. 10.1039/D0BM02023K. [DOI] [PubMed] [Google Scholar]
- Rosendorf J.; et al. Experimental fortification of intestinal anastomoses with nanofibrous materials in a large animal model. Sci. Rep. 2020, 10, 1134. 10.1038/s41598-020-58113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendorf J.; et al. Double-layered nanofibrous patch for prevention of anastomotic leakage and peritoneal adhesions, experimental study. Vivo Athens Greece 2021, 35, 731–741. 10.21873/invivo.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendorf J.; et al. Reinforcement of colonic anastomosis with improved ultrafine nanofibrous patch: Experiment on pig. Biomedicines 2021, 9, 102. 10.3390/biomedicines9020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghoraibi I.; Alomari S.. Different methods for nanofiber design and fabrication. In Handbook of Nanofibers; Barhoum A., Bechelany M., Makhlouf A., Eds.; Springer International Publishing: 2018; pp 1–46. 10.1007/978-3-319-42789-8_11-2. [DOI] [Google Scholar]
- Li W.-J.; Shanti R. M.; Tuan R. S. Electrospinning technology for nanofibrous scaffolds in tissue engineering. Nanotechnologies Life Sci. 2007, 10.1002/9783527610419.ntls0097. [DOI] [Google Scholar]
- Partheniadis I.; Nikolakakis I.; Laidmäe I.; Heinämäki J. A Mini-review: Needleless electrospinning of nanofibers for pharmaceutical and biomedical applications. Processes 2020, 8, 673. 10.3390/pr8060673. [DOI] [Google Scholar]
- Dinarvand P.; et al. Function of poly (lactic-co-glycolic acid) nanofiber in reduction of adhesion bands. J. Surg. Res. 2012, 172, e1–9. 10.1016/j.jss.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Gholami A.; et al. Prevention of postsurgical abdominal adhesion using electrospun TPU nanofibers in rat model. BioMed. Res. Int. 2021, 2021, 9977142. 10.1155/2021/9977142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X.; et al. Prevention of postsurgery-induced abdominal adhesions by electrospun bioabsorbable nanofibrous poly(lactide-co-glycolide)-based membranes. Ann. Surg. 2004, 240, 910–915. 10.1097/01.sla.0000143302.48223.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölgen N.; Vargel I.; Korkusuz P.; Menceloğlu Y. Z.; Pişkin E. In vivo performance of antibiotic embedded electrospun PCL membranes for prevention of abdominal adhesions. J. Biomed. Mater. Res. 2007, 81B, 530–543. 10.1002/jbm.b.30694. [DOI] [PubMed] [Google Scholar]
- Liu S.; et al. Electrospun fibrous membranes featuring sustained release of ibuprofen reduce adhesion and improve neurological function following lumbar laminectomy. J. Control. Release Off. J. Control. Release Soc. 2017, 264, 1–13. 10.1016/j.jconrel.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Zhao X.; et al. Optimization of intrinsic and extrinsic tendon healing through controllable water-soluble mitomycin-C release from electrospun fibers by mediating adhesion-related gene expression. Biomaterials 2015, 61, 61–74. 10.1016/j.biomaterials.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Shin Y. C.; et al. PLGA nanofiber membranes loaded with epigallocatechin-3-O-gallate are beneficial to prevention of postsurgical adhesions. Int. J. Nanomedicine 2014, 9, 4067–4078. 10.2147/IJN.S68197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.-H.; Chen C.-H.; Fong Y. T.; Chen J.-P. Prevention of peritendinous adhesions with electrospun chitosan-grafted polycaprolactone nanofibrous membranes. Acta Biomater. 2014, 10, 4971–4982. 10.1016/j.actbio.2014.08.030. [DOI] [PubMed] [Google Scholar]
- Babadi D.; Rabbani S.; Akhlaghi S.; Haeri A. Curcumin polymeric membranes for postoperative peritoneal adhesion: Comparison of nanofiber vs. film and phospholipid-enriched vs. non-enriched formulations. Int. J. Pharm. 2022, 614, 121434. 10.1016/j.ijpharm.2021.121434. [DOI] [PubMed] [Google Scholar]
- Li J.; et al. Prevention of intra-abdominal adhesion using electrospun PEG/PLGA nanofibrous membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 988–997. 10.1016/j.msec.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Klapstova A.; et al. A PVDF electrospun antifibrotic composite for use as a glaucoma drainage implant. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111637. 10.1016/j.msec.2020.111637. [DOI] [PubMed] [Google Scholar]
- Cheng L.; et al. Hydration-enhanced lubricating electrospun nanofibrous membranes prevent tissue adhesion. Research 2020, 2020, 4907185. 10.34133/2020/4907185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q.; Wu H.; Kong Q. Superhydrophilic PLGA-graft-PVP/PC nanofiber membranes for the prevention of epidural adhesion. Int. J. Nanomedicine 2022, 17, 1423–1435. 10.2147/IJN.S356250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S.; Wang W.; Yan H.; Fan C. Prevention of intra-abdominal adhesion by bi-layer electrospun membrane. Int. J. Mol. Sci. 2013, 14, 11861–11870. 10.3390/ijms140611861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepthi S.; Nivedhitha Sundaram M.; Deepti Kadavan J.; Jayakumar R. Layered chitosan-collagen hydrogel/aligned PLLA nanofiber construct for flexor tendon regeneration. Carbohydr. Polym. 2016, 153, 492–500. 10.1016/j.carbpol.2016.07.124. [DOI] [PubMed] [Google Scholar]
- Shalumon K. T.; et al. Multi-functional electrospun antibacterial core-shell nanofibrous membranes for prolonged prevention of post-surgical tendon adhesion and inflammation. Acta Biomater. 2018, 72, 121–136. 10.1016/j.actbio.2018.03.044. [DOI] [PubMed] [Google Scholar]
- Klicova M.; et al. Novel double-layered planar scaffold combining electrospun PCL fibers and PVA hydrogels with high shape integrity and water stability. Mater. Lett. 2020, 263, 127281. 10.1016/j.matlet.2019.127281. [DOI] [Google Scholar]
- Klicova M.; et al. Biomimetic hierarchical nanofibrous surfaces inspired by superhydrophobic lotus leaf structure for preventing tissue adhesions. Mater. Des. 2022, 217, 110661. 10.1016/j.matdes.2022.110661. [DOI] [Google Scholar]