Abstract

Hyperphosphorylated nucleotide (p)ppGpp, synthesized by Rel protein, regulates the stringent response pathway responsible for biofilm and persister cell growth in mycobacteria. The discovery of vitamin C as an inhibitor of Rel protein activities raises the prospect of tetrone lactones to prevent such pathways. The closely related isotetrone lactone derivatives are identified herein as inhibitors of the above processes in a mycobacterium. Synthesis and biochemical evaluations show that an isotetrone possessing phenyl substituent at C-4 inhibit the biofilm formation at 400 μg mL–1, 84 h post-exposure, followed by moderate inhibition by the isotetrone possessing the p-hydroxyphenyl substituent. The latter isotetrone inhibits the growth of persister cells at 400 μg mL–1 f.c. when monitored for 2 weeks, under PBS starvation. Isotetrones also potentiate the inhibition of antibiotic-tolerant regrowth of cells by ciprofloxacin (0.75 μg mL–1) and thus act as bioenhancers. Molecular dynamics studies show that isotetrone derivatives bind to the RelMsm protein more efficiently than vitamin C at a binding site possessing serine, threonine, lysine, and arginine.
Introduction
Persister cells form a group of an antibiotic-tolerant bacterial cell population responsible for the resurgence of pathogenic infections upon withdrawal of antibiotics on infected host cells.1 Among other factors, the stringent response pathway regulates the persister cell emergence and the pathway is regulated heavily by hyperphosphorylated guanosine nucleotides, namely, (p)ppGpp.2−7 Sustained investigations show the intense inter-relationship of mycobacterial stringent response pathway, persister cell formation, and infection to the host cells by overcoming antibiotic treatments.8−12 The emergence of persister cells is often correlated to the synthesis of (p)ppGpp by Rel, which is a predominant bacterial enzyme. Under stress, such as, in starvation conditions, the Rel enzyme activates the (p)ppGpp synthesis, which, in turn, leads to persister cell growth, possessing altered metabolic processes. Targeting the stringent response pathway, and thus the (p)ppGpp synthesis, appears to be a promising approach to overcome persister cell-mediated infections to host cells.
Relacin and derivatives thereof have been developed as potent Rel inhibitors, although their in vivo inhibition activities occur in millimolar ranges.13−15 Among other potent inhibitors studied so far, vitamin C shows promising inhibitory activities by inhibition of the ppGpp synthesis.11 Vitamin C binds directly to the Rel enzyme and inhibits (p)ppGpp biosynthesis, thereby establishing the importance of vitamin C to prevent mycobacterial persister cell emergence. The inhibition of (p)ppGpp biosynthesis occurs at millimolar concentrations of vitamin C, although such high concentrations of vitamin C would not be an impediment to a normal course of administration to antibiotic-tolerant infected cells populated with persisters. Vitamin C has also been discovered recently as a potent inhibitor of persister cell growth in M. smegmatis and M. tuberculosis bacterial species.16−21 These discoveries prompt studies of newer chemical entities possessing the tetrone lactone scaffold. Vitamin C is a tetrone lactone22 and thus scaffold search of potential inhibitors with a varied substituent on the tetrone lactone is a viable approach. The present report demonstrates the potencies of newly formed C-4 modified isotetronic acids as inhibitors of M. smegmatis persister cell growth through a series of biochemical evaluations.
Results and Discussion
Chemical Synthesis of C-4-Modified Isotetronic Acids
α-hydroxy-γ-butyrolactones, namely, isotetrone lactones form as a scaffold for many important natural products of therapeutic importance.23−27 Many natural products possess the α-hydroxy-γ-butyrolactone scaffold.28−34 Early biosynthesis studies by Yamamoto and co-workers demonstrated that α-amino acids form the substrate for the formation of α-hydroxy-γ-butyrolactone metabolites, produced during the culture growth of fungus Aspergillus terreus IFO 8835 strain.35 The deamination of α-amino acids was presumed to form the first of several steps, leading to isotetrone formation. The deamination process led to the transformation of the α-amino acid to α-keto acid, namely, pyruvic acid. Pyruvic acid acts as an excellent nucleophile to aldol reactions with carbonyl electrophiles and in biosynthesis, and the reaction is mediated by a type II aldolase enzyme.36,37 Subsequent lactonization leads the resulting cross-aldol product to the isotetrone. Pyruvic acid as an important synthon is well-exploited in the chemical synthesis of many derivatives of isotetrone, with substitutions at C-3 to C-5 carbons.38−40 Enantioselective synthesis led to a paradigm shift and syntheses of stereochemically pure butyrolactones are also achieved.41−49 Recognizing the importance of deamination of amino acids to the corresponding substituted pyruvic acids, we undertook to synthesize isotetrones that possess variations of the substituent at C-4 carbon of the furanone scaffold.
A facile approach to deamination of L-amino acid is considered as an important strategy, in order to enable the incorporation of substituents at the C-4 position of isotetrone.50 The reaction of L-amino acids 1–3, leucine, phenylalanine, and tyrosine, respectively, with trifluoroacetic anhydride, at optimal conditions of 85 °C and 1.5 h duration, afforded oxazolones 4–6 (Scheme 1), in good to moderate yields, in addition to the formation of trifluoroacetyl amino acids.51,52 Oxazolones 4–6 were subjected to aq. alkaline hydrolysis at room temperature for 18 h and the corresponding pyruvic acids 7–9 formed, possessing either keto- or the enol ether functionality.53,54 Enolization was observed higher in products 8 and 9.
Scheme 1. Synthesis of Isotetrones 10–12, from a-Amino Acids 1–3, through Oxazolones 4–6 and C-3 Substituted Pyruvic Acid 7–9 Intermediates.
The reaction of pyruvic acids 7–9 with formalin for 4 h, in the presence of K2CO3, in THF, initiated the aldolization and subsequent lactonization of the cross-aldol intermediate during acidic work-up that led to the formation of isotetrones 10–12. When the reaction was left for a longer duration, a double aldolization product was also noticed in the crude reaction mixture, particularly, with pyruvic acid intermediate derived from isoleucine 1, whereas unreacted starting materials 4–6 remained when aldolization reactions were conducted for a shorter duration. The identities and structural homogeneities of intermediates 4–9 and final products 10–12 were ascertained by nuclear magnetic resonance (NMR) spectroscopies and mass spectrometry.
A similar reaction sequence was extended to valine, isoleucine, and tryptophan amino acids. Oxozolone formation, hydrolysis to the corresponding pyruvic acid derivatives,55−57 aldolization with formalin, in the presence of K2CO3 and subsequent lactonization led to the formation of isotetrones 13–15 (Figure 1). In the case of valine, intermediate α-keto-γ-butyrolactone was observed to undergo a further reduction in the presence of formalin so as to afford α-hydroxy-γ-butyrolactone 13, which is a pantolactone. Such a transformation was not observed with butyrolactone 14, derived from isoleucine. Additional hydroxymethylation also occurred at the indole–nitrogen of tryptophan-derived butyrolactone 15. Characterizations of products 13–15 were established by NMR spectroscopies and mass spectrometry.
Figure 1.
γ-Butyrolactones 13 and 14, and isotetronic acid 15 synthesized from valine, isoleucine, and tryptophan, respectively.
Each one of the isotetrones differ at the substituent nature at the C-4 carbon of the scaffold. Aromatic substituents possess variations, namely, phenolic, indole, and phenyl moieties as the substituent in one series. Aliphatic series possesses methyl, ethyl, and isopropyl substituents in a quaternary carbon. Isotetrone further was presented either in a keto or enol or a hydroxy functionality at the C-3 carbon. These functionalities represent the variations at each series of the scaffold.
Specific Isotetrones Interfere with Planktonic Growth of M. smegmatis
The studies were undertaken with fast-growing M. smegmatis acid-fast bacterium. Of the six C-4 modified isotetrones 10–15, preliminary screening of the effects on planktonic growth of the bacterium showed isotetrones 11 and 12 showed inhibitory properties and these derivatives were shortlisted for further studies. Isotetrone 11 showed significant growth inhibition at the early exponential and late exponential phases compared to the untreated controls (Figure 2a).
Figure 2.

M. smegmatis growth curves in the presence of varying concentrations of (a) 11; (b) 12; and (c) 10.
Further analysis showed that derivative 11 inhibited the bacterial planktonic growth in a dose-dependent manner, and at 400 μg mL–1, complete growth inhibition occurred up to 72 h. After this duration, regrowth of cells was observed. A moderate growth inhibition was observed for isotetrone 12 during the exponential growth phase of M. smegmatis cells in a concentration-dependent manner (Figure 2b). In this instance too, cells tended to recover from the early inhibition in the exponential phase and growth similar to untreated control was observed by 60–72 h, indicating a transient inhibitory effect of these isotetrones. Isotetrone 10 did not intervene in the growth profile of M. smegmatis cells across all concentrations when compared to the untreated control (Figure 2c). These differences in the growth profiles indicate that both the isotetrones 11 and 12 possessing an aromatic moiety at C-4 carbon act as transient bacterial growth inhibitors. Furthermore, the minimum inhibitory concentration (MIC) was checked for compound 11 and compound 12, and it was found to be 400 μg mL–1.
Isotetrones Showed a Differential Killing Pattern in M. smegmatis
The transient growth inhibition prompted us to verify the effects of isotetrones in combination with a known antimicrobial drug.58,59 A clinically relevant fluoroquinolone series drug, namely, ciprofloxacin (Cip), having a low MIC value against M. smegmatis, was chosen for this purpose. The initial determination of the MIC of Cip, in the presence of derivatives 10–12 (200 μg mL–1), showed the following trend: Cip: 0.25 μg mL–1; Cip + 10: 0.25 μg mL–1; Cip + 11: 0.125 2 μg mL–1; and Cip + 12: 0.5 μg mL–1.
In order to assess the efficacies of isotetrones to kill M. smegmatis WT cells, in MB7H9 media, a kill kinetics assay was conducted, either alone or in combination with ciprofloxacin. Isotetrone derivatives alone did not result in any drop in CFU mL–1, implying that the derivatives lacked activity to kill the cells (Figure S13). An alternating killing pattern emerged when the cells were treated with a combination of ciprofloxacin (2.5 μg mL–1) and different isotetrones (400 μg mL–1). The drug in combination with 10 did not lead to any appreciable change in the killing, whereas ciprofloxacin (2.5 μg mL–1) and isotetrone 11 and 12 combinations led to an antagonistic response, where the combinations led to a reduced killing at each plating time point (Figure 3). We presume the intrinsic growth inhibitory properties of 11 and 12 interfered with ciprofloxacin activity, leading to the differential killing of the replicating cells.
Figure 3.
Kill kinetics of M. smegmatis with ciprofloxacin (Cip) (2.5 μg mL–1) and Cip in the presence of derivatives 10–12 (400 μg mL–1).
Selective Isotetrones Affect the Biofilm Formation of M. smegmatis
Vitamin C is the first tetronic acid derivative demonstrated to exhibit inhibitory activity against biofilm-grown mycobacterium, occurring at a concentration of ∼10 mM.22 It was also assessed to interfere with the ppGpp biosynthesis in M. smegmatis and the biofilm growth.18,60−62 Isotetrones synthesized in the present work were assessed for the inhibition of biofilm formation at the maturation stage of M. smegmatis. The biofilm morphologies were monitored for 84 h post-exposure to 10–12. Among the derivatives, 11 showed the largest effect in a concentration-dependent manner (Figure 4). Derivative 12 showed moderate inhibition of the biofilm and derivative 10 did not show inhibition of biofilm, as that of the dense mature biofilm formed with untreated control (Figure 4).
Figure 4.

Images of M. smegmatis biofilms after 84 h, in the presence of varying concentrations of compounds 10–12 with untreated control.
Isotetronic Acids 11 and 12 Intercepts with Persister Population Regrowth and Resistant Mutant Enrichment at Low Ciprofloxacin Concentration
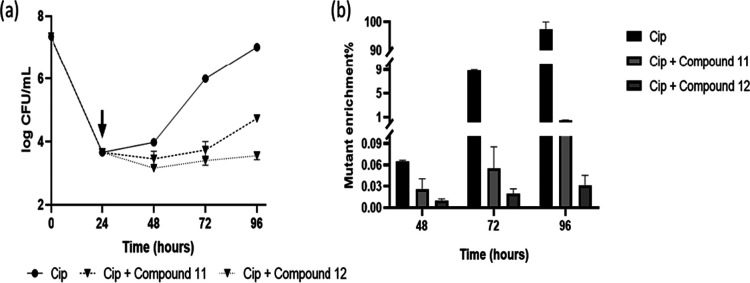
Earlier studies show that vitamin C targets the (p)ppGpp biosynthesis. The (p)ppGpp alarmone promotes the persister cell formation and plays a role in the antibiotic tolerance of the growing bacterium.64,65 Given this, further experiments were performed to identify whether selected isotetrones would intercept the persister population dynamics and resistant mutant enrichment at low ciprofloxacin concentration. In laboratory and clinical settings, antibiotic treatment often leads to a biphasic response, with a rapid killing phase followed by a plateau, which is represented by a 0.01–0.001% nongrowing persister population. These antibiotic-tolerant persister cells lead to a major threat to effective antibiotic therapy, due to a resuscitation of the persisters into normal growing cells at a point soon after the antibiotic treatment is terminated, which results in recurrent infection. It is thus of interest to identify the effect of the isotetronic acids, specifically that 11 and 12, if these derivatives would possess a restrictive effect on the regrowth of the persister-dominated antibiotic-survived population, in the presence of a lower concentration of ciprofloxacin (0.75 μg mL–1, which is 3 times higher than MIC). The lower concentration of the drug was chosen as our data indicated that the regrowth phenotype of drug-tolerant cells was very prominent at this concentration (Figure S14a) as well as genetic mutant enrichment (Figure S14b). Furthermore, the lower antibiotic concentration is very much relevant from a clinical perspective when optimum concentration could not be maintained. Next, the persister regrowth in the presence of isotetrones 11 and 12 was checked. In this assay, cells were treated first with the drug (0.75 μg mL–1) for 24 h, thereby achieving ∼99.99% killing of the cells, leaving a residual ∼0.01% persister population retained in the culture. We measured the ciprofloxacin activity after 24 h of killing by a growth bioassay where it is shown that the culture filtrate retains the same active ciprofloxacin concentration for up to 96 h (Figure S16). In order to make sure the residual 0.01% after 24 h of ciprofloxacin treatment are bonafide persisters and not resistant mutants, the plating was done in MB + Cipro plates and hardly any resistant mutants were detected. By patching individual colonies of ciprofloxacin survived population in a 5X ciprofloxacin plate, we confirmed ∼90% of the cells were persisters and ∼10% of the cells were resistant mutants (Figure S17). At this point, the culture was divided into three portions, added with either derivative 11 or 12 (400 μg mL–1) and an equal volume of sterile milli-Q water. The growth kinetics was subsequently monitored, a brief nongrowing phase of approximately 24–36 h was observed first, and then, antibiotic-survived cells started to grow, as evident by the increase in CFU mL–1 at later time points. The data suggested significant prevention of the regrowth phase and a general delayed resuscitation of tolerant cells, in the presence of isotetrones 11 and 12 (400 μg mL–1). As shown in the resuscitation growth curve (Figure 5a) between 48 and 72 h, the cells without isotetrones grew exponentially and reached to a cell population comparable to the time when drug treatment was initiated. Whereas, in the presence of derivatives 11 and 12, regrowth was prevented remarkably up to 72 h. Studies earlier reported on varied bacteria pointed out how persister cells could act as the predecessor of resistant mutants.66,67 In our assay since antibiotic-survived cells were able to grow in the presence of the same drug (ciprofloxacin), the possibility of emergence of resistant genetic mutants (ciproR) was estimated by plating parallelly in ciprofloxacin containing MB plates. A visible increase in ciproR mutants was observed during the plateau/nongrowing phase and, in the presence of isotetrones, a significant arrest of mutant enrichment occurred further. In the absence of isotetrones, namely, only in the presence of the drug, uninhibited resistant mutant enrichment over time occurred, with a remarkable ∼98% mutant population taking over within 48 h of the regrowth phase. Additionally, by patching individual colonies in a 5X ciprofloxacin plate at 96 h, we confirmed 100% of them are ciprofloxacin-resistant mutants (Figure S17). Both derivatives 11 and 12 significantly restricted resistant mutant enrichment for 48 h, presumably due to their inherent ability to slow regrowth of persisters and the subsequent transformation into mutants (Figure 5b). Our data further suggested that the nongrowing phase/plateau between 24 and 48 h in the assay is responsible for conversion of persisters into genetic mutants and this critical step could be successfully prevented by isotetrones. These observations tempt us to raise and prove that isotetrones 11 and 12, in combination with ciprofloxacin, act as bioenhancers and are therapeutically superior when administered together. The combination significantly inhibited regrowth of drug-tolerant population and arrested the resistant mutant enrichment. These findings implicate in the larger context of preventing resistance to antibiotics.
Figure 5.
M. smegmatis delayed addition time-kill kinetics profile. (a) Reduction in the regrowth of ciprofloxacin (0.75 μg mL–1) treated tolerant cells in the presence of compounds 11 and 12 (400 μg mL–1); (b) reduction of resistant mutant generation during ciprofloxacin (0.75 μg mL–1) treatment in the presence of 11 and 12 (400 μg mL–1). Black arrows depict the time of compound addition.
Experiments of kill kinetics, regrowth assay, and mutant enrichment assays were performed with ciprofloxacin + vitamin C (4 mM, MIC concentration) and ciprofloxacin + derivative 12 (400 μg mL–1). The experiments showed that the combination of ciprofloxacin (0.75 μg mL–1) + vitamin C (4 mM) did not show any altered kill kinetics (Figure S15) and was unable to inhibit the regrowth of the drug-tolerant population over 72 and 96 h time points (Figure S18a). Furthermore, the ciprofloxacin + vitamin C combination did not arrest the resistant mutant enrichment at 72 and 96 h (Figure S18b). At 96 h, although the combination appeared more efficacious, as compared to ciprofloxacin alone, still the mutant enrichment percentage was significantly high, as compared to the ciprofloxacin + derivative 12 combinations. The result suggests that the inhibition of regrowth of ciprofloxacin survived population and mutant enrichment at a lower concentration of the drug is only possible in the presence of selective isotetrone derivatives and not in the presence of vitamin C alone. In all the kill kinetics and mutant detection assays, the initial inoculum was adjusted to ∼1 × 107 CFU/mL, which is based on the ciprofloxacin resistance frequency value of M. smegmatis WT, i.e., 4.5 × 10–8,63 to avoid any spontaneous mutant in the beginning of the assay.
Isotetrone 12 Kills Cells under Stringent Conditions
In order to verify whether isotetrones would affect the survival kinetics under PBS starvation, cells in the PBS solution were incubated with the derivatives, at 400 μg mL–1 f.c., and CFU was estimated after 72 h for 2 weeks. The derivative 12 was able to inactivate cells under PBS starvation in PBS (Figure 6). The long-term survival in PBS-starved conditions is linked to (p)ppGpp stress alarmone proliferation and earlier studies showed that vitamin C interfered with (p)ppGpp biosynthesis.64,65 in M. smegmatis. Isotetrones 12 might possibly interfere with the stress alarmone biosynthesis similarly, and thus, a visible drop in CFU was observed even in the absence of an antibiotic under such stringent conditions. There are previous reports of vitamin C interacting with RelMsm enzyme65 and since our result also indicated a possible role of isotetrone 12 in regulating stringent response in M. smegmatis, we checked the possible interaction of compound 12 with RelMsm with molecular simulations.
Figure 6.
Plots of inhibition under long-term starvation of M. smegmatis in PBS (untreated) and in the presence of compound 12.
Molecular Dynamics Simulation Reveals Notable Interactions between Compound 11, 12, and RelMsm
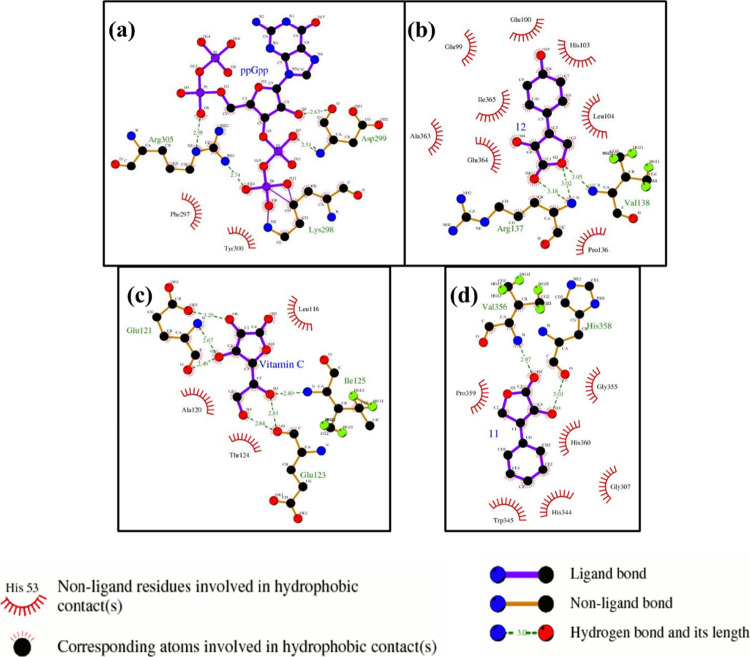
A systematic, molecular dynamics (MD) simulation study was undertaken to assess the binding interaction of the isotetrone derivative 12 and 11 with the RelMsm enzyme, with the knowledge that vitamin C binds directly to RelMsm enzyme and inhibits (p)ppGpp biosynthesis. Figure 7 shows the RelMsm–ligand complex at their most energetically preferable binding sites, found from the docking study. The corresponding binding affinity is tabulated in Table 1. The structures served as initial configurations for subsequent MD simulation study. The protein–ligand complex structures after 10 ns of simulation are depicted in Figure 8. It should be noted that we run short 10 ns long MD simulation to get the most realistic binding site before calculating binding energy. The binding sites obtained in the docking study are preserved during the MD simulation. The average binding energies (ΔHbind) for ppGpp, compound 11, 12, and vitamin C, obtained from the simulated trajectories, are presented in Table 1. It is evident that ppGpp binds to RelMsm protein most strongly, followed by compound 12, vitamin C, and compound 11. In fact, compound 11 detached from the RelMsm at the end of the simulation (Figure 8d). The binding energy trend is mostly in agreement with the docking results. Figure 9a presents the contributions of electrostatic and VdW components of the binding energy, for all the four ligands. For ppGpp, it can be easily seen that the protein–ligand binding is mostly driven by the electrostatic energy, whereas the contribution from the VdW interaction is not significant (Figure 9a). This can be attributed to the presence of the phosphate groups on the ppGpp molecule. In the case of compound 12 and 11, both the VdW and electrostatic energies significantly promoted the binding process (Figure 9a). Like ppGpp, vitamin C also binds to RelMsm through Coulombic interactions only, although the magnitude of the interaction was much smaller than the former (Figure 9a) because of the absence of any charged functional groups. An interesting observation is that in case of vitamin C, the electrostatic contribution to ΔH is higher than that of compound 12 (Figure 9a). Despite that, the binding affinity of vitamin C and RelMsm is weak, probably because of its higher solubility than compound 12. It is noteworthy that not all the residues of the Rel protein took part in the ligand binding phenomena. The per residue decomposition data of ΔGbind are depicted in Figure 9b–d. In corroboration with the previous observations, the significant contribution toward RelMsm-ppGpp binding came from the positively charged residues like Arg and Lys (Figure 9b). This is another evidence that the binding of ppGpp with RelMsm is dominated by the electrostatic interaction among the negatively charged phosphate groups present in ppGpp and the positively charged residues of the RelMsm. An interesting observation is that the contribution from ppGpp do not favor the protein–ligand binding (Figure 9b) due to a high positive change in the polar solvation energy upon binding (1364.06 ± 140.56 kcal/mol) to Rel, indicating the presence of an attractive Coulombic interaction between ppGpp and solvent molecules. For derivative 12 and vitamin C, no specific protein residues provided a major contribution toward the binding energy, rather significant contribution came from the ligands (Figure 9c,d). The absence of a charged group in 12 and vitamin C can be attributed as the probable reason behind this striking difference. A similar decomposition study was not performed on derivative 11, due to very weak binding.
Figure 7.
2D interaction plots of various protein–ligand complexes, obtained from docking studies. (a) RelMsm-ppGpp; (b) RelMsm-derivative 12; (c) RelMsm-vitamin C; and (d) RelMsm-derivative 11 complexes at their lowest energy conformations, predicted by molecular docking.
Table 1. Binding Affinity Obtained from the Docking Study and Average Binding Energy as Calculated from MD-Simulated Trajectories Using MM-GBSA.
| ligand | ΔH (kcal/mol) from docking | ΔH (kcal/mol) from MM-GBSA |
|---|---|---|
| ppGpp | –8.17 ± 0.33 | –38.58 ± 8.40 |
| compound 12 | –4.66 ± 0.34 | –9.74 ± 3.37 |
| vitamin C | –3.35 ± 0.33 | –4.01 ± 4.04 |
| compound 11 | –4.36 ± 0.18 | –3.88 ± 4.20 |
Figure 8.
(a) RelMsm-ppGpp, (b) RelMsm-12, (c) RelMsm-vitamin C, and (d) RelMsm-11 complexes after 10 ns simulation. The secondary structure of the protein has been represented with the color code: yellow: β sheet, pale blue: turns, and white: other residues. The ligands are presented using “licorice” representation scheme of VMD. Protein residues within a 5 Å distance from the ligands are presented using VdW spheres with a color code: White: nonpolar residues, blue: basic residues, red: acidic residues, and green: polar residues. Water molecules are removed for clarity.
Figure 9.

(a) Average electrostatic and VdW components of binding energy. Contribution of different residues in the binding energy for (b) RelMsm-ppGpp, (c) RelMsm-compound 12, and (d) RelMsm-vitamin C complexes. Nomenclature: Tyr Lac: compound 12; Vit C: vitamin C.
Apart from the electrostatic and VdW interactions, protein–ligand binding can also be mediated by the formation of hydrogen bonds (H-bonds) between the protein and ligand. We used a cutoff distance of 0.35 nm between donor–acceptor and a cutoff angle of 30° to define H-bonds. In the case of ppGpp and RelMsm, Lys298, Tyr300, and Arg305 residues were involved in H-bonding. For derivative 12, Glu100, His103, Arg137residues, and for vitamin C, Leu116, and Arg126 residues were found to form H-bonds with ligands. The average number of hydrogen bonds formed between ppGpp and RelMsm was found to be the highest (9.23 ± 1.68), followed by 12 (1.18 ± 0.85) and vitamin C (1.31 ± 1.24). This occurred due to the presence of more H-bond forming acceptors and donors in ppGpp, compared to derivative 12 or vitamin C. On the other hand, derivative 11 hardly formed any H-bonds (0.36 ± 0.51) with RelMsm protein. Our simulation study confirms the experimental observation of the highest binding affinity of ppGpp, followed by derivative 12 and vitamin C. The highest binding energy of ppGpp is attributed to the presence of the phosphate group and also to its ability to form more hydrogen bonds with RelMsm.
The maximum concentration tested for the isotertrones 10–12 against M. smegmatis is ∼2 mM in the present work. The isotetrone derivatives possess an isomeric scaffold of the tetrone, to which vitamin C belongs to. Vitamin C was demonstrated to have inhibition efficacy against mycobacteria at a concentration of 10 mM.58 The MIC values of vitamin C against M. smegmatis and M. tuberculosis are 8 and 1 mM, respectively.18 Vitamin C also possesses a bactericidal activity at 4 mM. Furthermore, the synthetic isotetrones are studied in the present study as adjuvants in order to enhance the inhibition efficacies of ciprofloxacin, by preventing the persister cell regrowth and mutant enrichment. Such an effect would not arise by monotherapy. In a related study involving acute and chronic infection models of M. tuberculosis in mice,68 it was shown that vitamin C at 1 mM concentration, in combination with rifampicin and isoniazid, potentiates in vivo killing by 1 log and that optimum concentration in serum can be achieved by i.p. infection.68 It is noted that vitamin C is a part of regular diet, and high doses of vitamin C stands to be safe69 to achieve a human plasma concentration of up to 49 mM.70,71 Considering the above aspects on tetrone scaffolds, we adjudge that the isotetrones studied herein, ∼200 and 400 μg mL–1 concentrations, are relatively lower and better when compared to vitamin C as inhibitors of not only the planktonic growth but also stationary phase processes. Combined with lesser cytotoxicities, these derivatives show a promise as scaffolds for inhibitor development for mycobacterial persister cells.
The present investigation focuses on advancing the scaffold, namely, tetrone and isotetrone in mycobacterium growth and stationary phase processes. The work follows the results on vitamin C, which was demonstrated earlier to possess inhibition activities for the above processes. The tetrone scaffold of vitamin C prompted us to investigate the isomeric isotetrone scaffold. The synthetic method was thus developed to prepare a number of isotetrones, each one differing at the substituent nature at the C-4 carbon of the scaffold. The variation in the substituent was chosen such that aliphatic and aromatic substituents were covered. Within these substitutions, functionalities were varied, so as to present the phenolic moiety, indole moiety, and phenyl moiety as the substituent in one series. In another series, methyl, ethyl, and isopropyl substituents in a quaternary carbon were prepared. The isotetrone further was presented either in a keto or enol or a hydroxy functionality at the C-3 carbon. These variations are representatives of different series that are functionalized on the scaffold. The focus was to identify the functionalities on the scaffold that would show promise for further involved biological and computational studies. These studies require the scaffold functionality that exhibits the most effect. As detailed above, the present scaffold functionalization search is covered with complementarity in the nature of functionalization. Aryl substituents at C-4 carbon in derivatives 11 and 12 show the most efficacy in both mycobacterial growth, biofilm inhibition, and inhibition of persister cells in combination therapy, as observed in this study. Derivatives 13–15 also did not exhibit effects neither on growth nor biofilm inhibitions. Results from these sets of derivatives provide a clue for further advancement, where the direct attachment of the aryl moiety on the tetrone scaffold would be the focus for further inhibitor diversification and SAR studies.
Conclusions
The work herein demonstrates (i) chemical synthesis of hither-to unknown C-4 modified isotetrones from α-amino acids, through the formation of pyruvic acids, their aldonization, and lactonization. (ii) Biochemical studies discover the role of these new isotetrones to interfere the growth of mycobacterium planktonic cells; inhibition of M. smegmatis biofilm formation is seen in a dose-dependent manner. (iii) Synergistic inhibitory effects are observed with well-known antibiotic ciprofloxacin in the presence of the selected isotetronic acids, wherein the MIC of ciprofloxacin is improved by twofold. (iv) Specific activities of isotetrones possessing aromatic substituents at C-4 carbon include the prevention of the regrowth of antibiotic-tolerant population in M. smegmatis. We observe that M. smegmatis cells survive without isotetrones, form nongrowing persisters, and resuscitate in the presence of lower concentrations of antibiotic ciprofloxacin, presumably due to enrichment of genetic mutants as it occurs in a clinical scenario. (v) Systematic MD simulations uncover the stabilities of RelMsm protein–inhibitor complexes, occurring through VdW, electrostatic, and hydrogen-bonding interactions. The study shows that the addition of isotetrones hamper the regrowth stage of ciprofloxacin-selected persister population. Recurrent infection is a challenge in current antibiotic treatment regimens. The present work demonstrates the complete occlusion of the generation of resistant mutants, thereby opening up the feasibility to administer fewer concentrations of antibiotics and minimize associated off-target effects.
Experimental Section
General Procedure
Formalin and K2CO3 were sequentially added to the solution of keto acid, derived from L-valine, L-leucine, L-isoleucine, L-phenylalanine, L-tyrosine, and L-tryptophan in THF, and stirred at room temperature for 3–15 h. The solution was evaporated in vacuo, and the reaction mixture was treated with aq. HCl (3 N), extracted with Et2O (3 × 15 mL), organic portion washed with water (10 mL), brine (10 mL), and dried over Na2SO4 and concentrated in vacuo.
3-Hydroxy-4-isopropylfuran-2(5H)-one (10)
Formalin (0.38 mL, 4.62 mmol) and K2CO3 (0.66 g, 4.84 mmol) were sequentially added to a stirred solution of 7(53) (0.5 g, 3.85 mmol) in THF (5 mL), stirred for 4 h at room temperature, and worked up as described in the general procedure. The crude product was purified by column chromatography (SiO2) (pet. Ether/EtOAc, linear gradient) to afford 10 (0.12 g, 34%) as a colorless oil. Rf (pet. Ether/EtOAc = 7:3) 0.4. 1H NMR (CDCl3, 400 MHz): δ 4.68 (s, 2 H, CH2O), 2.89 (m, 1 H, CH(CH3)2), 1.18 (d, J = 6.8 Hz, 6 H, CH(CH3)2); 13C NMR (CDCl3, 100 MHz): δ 171.7, 137.5, 135.6, 68.1, 25.5, 20.4. GCMS: m/z calcd. For C7H10O3: 142 [M+], found 142, with fragments at m/z at 97, 69 and 41.
3-Hydroxy-4-phenylfuran-2(5H)-one (11)
Formalin (0.09 mL, 1.08 mmol) and K2CO3 (0.14 g, 1.14 mmol) were added sequentially to a solution of 8(54) (0.15 g, 0.9 mmol) in THF (2.5 mL), and the reaction mixture was stirred for 4 h at room temperature and worked up as described in the general procedure. The residue was purified by column chromatography (SiO2) (pet. ether/EtOAc, linear gradient) to afford 11 (0.073 g, 56%) as a white solid. Mp 135.8 °C. Rf (pet. ether/EtOAc = 9:1) 0.7. 1H NMR (CD3OD, 400 MHz): δ 7.66 (d, J = 8 Hz, 2 H, C6H5), 7.36 (t, J = 8 Hz, 2 H, C6H5), 7.29–7.26 (m, 1 H, C6H5), 5.03 (s, 2H, CH2O); 13C NMR (CD3OD, 400 MHz): δ 172.3, 138.8, 134.01, 132.2, 130.7, 129.9, 129.7, 129.4, 127.6, 69.2. HRMS: m/z calcd. For C10H8O3: 177.0552 [M + H]+, found 177.0551.
3-Hydroxy-4-(4-hydroxyphenyl)furan-2(5H)-one (12)
Formalin (0.16 mL, 1.94 mmol) and K2CO3 (0.28 g, 2.04 mmol) were successively added to a solution of 9(54) (0.35 g, 1.94 mmol) in THF (5 mL), stirred for 4 h at room temperature, and worked up as described in the general procedure. Column chromatography (SiO2) (pet. ether/EtOAc, linear gradient) of the residue afforded 12 (0.127 g, 47%) as a white solid. Mp 144.9 °C. Rf (pet. ether/EtOAc = 3:2) 0.5. 1H NMR (CD3OD, 400 MHz): δ 7.59 (d, J = 8.8 Hz, 2 H, C6H5), 6.83 (d, J = 8.4 Hz, 2 H, C6H5), 5.06 (s, 2 H, CH2O); 13C NMR (CD3OD, 100 MHz): δ 172.8, 159.6, 136.5, 129.3, 128.8, 123.6, 116.6, 69.2. HRMS: m/z calcd. For C10H9O4: 193.0501 [M + H]+, found 193.0501.
3-Hydroxy-4,4-dimethyldihydrofuran-2-one (13)
Formalin (1.75 mL, 21.5 mmol) and K2CO3 (1.5 g, 10.75 mmol) were added successively to a solution of 2-oxoisovaleric acid55 (0.5 g, 4.3 mmol) in THF (5 mL); the reaction was stirred for 15 h at room temperature and worked up as described in the general procedure. Vacuum distillation was performed to remove the unreacted starting material and column chromatography (SiO2) (pet. ether/EtOAc, linear gradient) of the residue gave 13 (0.19 g, 34%) as a solid. Mp 84.3 ± 2 °C. Rf (pet. ether/EtOAc = 9:1) 0.8. 1H NMR (CDCl3, 400 MHz): δ 4.15 (s, 1 H, CHOH), 3.98 (d, J = 9.2 Hz, 1 H, CH2O), 3.92 (d, J = 9.2 Hz, 1 H, CH2O), 1.18 (s, 3 H, CH3), 1.03 (s, 3 H, CH3); 13C NMR (CDCl3, 100 MHz): δ 178.1, 76.4, 75.6, 40.7, 22.6, 18.7. GC–MS: m/z calcd. For C6H10O3: 131 [M + H], found 131, with fragments at m/z at 71, 57, 43 and 41.
4-Ethyl-4-methyl-dihydrofuran-2,3-dione (14)
Formalin (1.9 mL, 23.1 mmol) and K2CO3 (1.6 g, 11.55 mmol) were added successively to a solution of 3-methyl-2-oxopentanoic acid57 (0.5 g, 3.85 mmol) in THF (5 mL), stirred for 15 h, and worked up as described in the general procedure. The residue was purified by vacuum distillation to afford 14 (0.29 g, 54%) as a yellow liquid. (Rf) (pet. ether/EtOAc = 9:1) 0.8. 1H NMR (CDCl3, 400 MHz): δ 4.55 (d, J = 9.6 Hz, 1 H, CH2O), 4.39 (d, J = 9.6 Hz, 1 H, CH2O), 1.78–1.62 (m, 2 H, CH2CH3), 1.26 (s, 3 H, CH3), 0.92 (t, J = 7.2 Hz, 3 H, CH2CH3); 13C NMR (CDCl3, 100 MHz): δ 198.4, 161, 75.6, 45.4, 29.3, 19.4, 8.10. GCMS: m/z calcd. For C7H10O3: 142 [M+], found 142, with fragments at m/z at 85, 71, 69, 55 and 41.
3-Hydroxy-4-(1-hydroxymethyl)-1H-indol-3-yl)furan-2(5H)-one (15)
Formalin (0.22 mL, 2.71 mmol) and K2CO3 (0.38 g, 2.71 mmol) were added successively to a solution of indole-3-pyruvic acid56 (0.5 g, 2.46 mmol) in THF (5 mL) was treated with, and the reaction was stirred for 6 h at room temperature and worked up as described in the general procedure. The crude product was purified by column chromatography (SiO2) (pet. ether/EtOAc, linear gradient) to afford 15 (0.174 g, 33%) as a white solid. Mp 113.4 ± 2 °C. Rf (pet. ether/EtOAc = 3:1) 0.3. 1H NMR (CD3OD, 400 MHz): δ 7.96 (s, 1 H, CHNH), 7.82 (d, J = 8 Hz, 1 H, C6H5), 7.59 (d, J = 8 Hz, 1 H, C6H5), 7.28 (t, J = 8 Hz, 1 H, C6H5), 7.19 (t, J = 8 Hz, 1 H, C6H5), 5.63 (s, 2 H, CH2O), 5.29 (s, 2 H, CH2O); 13C NMR (CD3OD, 100 MHz): δ 171.2, 136.1, 133.1, 129.2, 126.4, 126.2, 122.3, 120.7, 120.5, 110.1, 107, 69, 68.1. HRMS: m/z calcd. For C13H11NO4: 246.0766 [M + H]+, found 246.0767.
Bacterial Growth and Culture Conditions
For all the assays, the M. smegmatis mc2155 strain was grown in MB7H9 media (HiMedia) containing 0.05% Tween-80 and 2% glucose; agar (1.6%, w/v) (Himedia) was used to make agar plates. Ciprofloxacin powder was obtained from Sisco Research Laboratories. Antibiotics were used at variable concentrations. Unless mentioned otherwise, strains were grown at 37 °C and 150 rpm.
Growth Inhibition Assay
For analyzing the effect of isotetrones on the growth of the M. smegmatis mc2155 strain, bacteria were grown till the mid-exponential phase, further inoculation was done in MB7H9 media containing 0.05% Tween-80 and 2% glucose to make the final OD ∼ 0.03. Such culture (3 mL) was taken in a sterile glass tube, and 30 μL of each of the respective isotetrones with varying final concentrations (100, 200, 300, and 400 μg mL–1) was added to the media at 0 h, and OD600 nm was measured. The bacterial growth was further monitored in equal intervals for 96 h by recording OD600 and plotted using Graph Pad Prism Software. The tube containing no compounds (only DMSO) served as the untreated positive control to observe and compare growth inhibition in the presence and absence of the compounds. This experiment was performed in a set of two experimental replicates.
Biofilm Formation Assay
The primary culture for the wild-type strain M. smegmatis mc2 155 was grown in MB7H9 medium at 37 °C, 150 rpm shaking. The cells were harvested at the stationary phase and washed with Sauton’s media (Himedia) twice, the cell pellet was resuspended in the Sauton’s media containing 2% glucose, and the final OD was adjusted to 0.05. Subsequently, 200 μL of culture was poured into a well of sterile 96-well microtiter plates supplemented with 2 μL respective isotetrones having a final concentration of 100, 200, 300, and 400 μg mL–1. The control wells included in the same plate were untreated control (only cells, no compounds to monitor uninhibited biofilm formation) and media control (only media, no cells to monitor contamination). The inoculated plates were sealed and kept in a 37 °C humidified incubator to avoid drying of media and incubated for a minimum of 72–96 h without external disturbance. The images were captured under white light at different time points. The experiment was performed in a set of three biological replicates.
MIC Determination
For analyzing if the isotetrones could increase the susceptibility to ciprofloxacin, we performed an MIC assay using the ciprofloxacin in combination with compounds (200 μg mL–1 f.c.) by broth microdilution assay using resazurin dye. Ciprofloxacin was 2-fold diluted in the 96-well microplate to achieve a broad range of final concentrations ranging from 16 to 0.03 μg mL–1. M. smegmatis cultures were grown till mid-exponential phase (OD600 of 0.6–1), diluted to OD600 of 0.01 in fresh MB7H9 medium, and 196 μL was added in each well of the 96-well plate containing 4 μL of different concentrations of ciprofloxacin. Depending on the series, cells were premixed with different isotetrones (200 μg mL–1 f.c.); in the no-compound series, an equal amount of sterile miliQ was added. Following the incubation of 36 h standing at 37 °C, 30 μL of 0.1 mg mL–1 of resazurin was added to each of the wells and again incubated for 6–8 h at 37 °C, and the dye reduction value was recorded.
Time-Kill Kinetics Assay
M. smegmatis cultures were grown till the late exponential/stationary phase (OD600 of 1.5–2.5), diluted 1:100 to start a secondary culture and grown till the mid-exponential phase (OD600 of 0.6–1), and further diluted to adjust to OD600 of 0.2 corresponding to ∼2 × 107 CFU mL–1 (CFU = colony-forming unit) in the fresh MB7H9 medium. The culture (3 mL) was added to a tube and 2.5 μg mL–1 (f.c.) ciprofloxacin (10X MIC) was added with or without isotetrones (400 μg mL–1) at T-0, and CFU mL–1 was estimated by spreading into the MB7H9 agar plate. Tubes were incubated at 37 °C in a shaker, and CFU estimations (plating) were done every 24 h up to 96 h. Plates were further incubated at 37 °C for 3–4 days for colony growth and subsequently counted. In order to measure the killing efficiency of the compounds alone (without ciprofloxacin), a similar kill kinetics assay was set up in parallel. All kill kinetics assays were performed with at least 3 biological replicates.
Delayed Addition of Isotetrones in Time-Kill Kinetics
M. smegmatis. Secondary culture was prepared as mentioned above, and then, 0.75 μg mL–1 of ciprofloxacin (3X MIC) was added to the media. After 24 h of incubation, the culture was divided into separate tubes and different isotetrones were added (400 μg mL–1 f.c.). CFU estimations (plating) were performed every 24 h up to 96 h. The tube containing no compounds (only DMSO) served as the untreated control to compare regrowth in the absence of the compounds. This experiment was performed in a set of three replicates.
Mutant Enrichment Determination
In parallel to the CFU estimation in kill kinetics, spotting/spreading was conducted in ciprofloxacin (1.25 μg mL–1) plates to enumerate resistant mutant population enrichment over time.
Kill Kinetics under Nutrient Starvation
M. smegmatis cultures were grown till the late exponential/stationary phase (OD600 of 1.5–2.5), diluted 1:100 to start a secondary culture, and grown till the mid-exponential phase (OD600 of 0.6–1). After that, the cells were washed twice with PBS and resuspended in an equal volume of PBS. T-0 CFU mL–1 estimation was done immediately, and the culture was divided into equal volumes in several glass tubes followed by the addition of selective isotetrones (400 μg mL–1 f.c.). In the untreated control tube, an equal volume of sterile MilliQ water was added. CFU estimation was done every 72 h for ∼3 weeks.
Acknowledgments
The authors thank the Department of Biotechnology (DBT), Government of India, for funding this work (Grant number: BT/PR33123/MED/29/1497/2020). Indian Institute of Science, Bangalore, is gratefully acknowledged for a research fellowship to K.B.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c00822.
Docking and molecular dynamics simulation protocol; NMR spectra of new compounds; and kill kinetics and persister regrowth (PDF)
Author Contributions
D.C., P.M., A.G. and N.J. contributed to the conception and design of the study. K.B., A.P., S.B. M.S. and A.G. performed the experiments. D.C., P.M., A.G. and N.J. participated in data analysis and interpretation. All authors participated in writing the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Harms A.; Maisonneuve E.; Gerdes K. Mechanisms of Bacterial Persistence During Stress and Antibiotic Exposure. Science 2016, 354, aaf4268 10.1126/science.aaf4268. [DOI] [PubMed] [Google Scholar]
- Chatterji D.; Ojha A. K. Revisiting the Stringent Response, ppGpp And Starvation Signaling. Curr. Opin. Microbiol. 2001, 4, 160–165. 10.1016/S1369-5274(00)00182-X. [DOI] [PubMed] [Google Scholar]
- Srivatsan A.; Wang J. D. Control of Bacterial Transcription, Translation and Replication by (p)ppGpp. Curr. Opin. Microbiol. 2008, 11, 100–105. 10.1016/j.mib.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Hengge R. High-Specificity Local and Global c-di-GMP Signaling. Trends Microbiol. 2021, 29, 993–1003. 10.1016/j.tim.2021.02.003. [DOI] [PubMed] [Google Scholar]
- Kushwaha G. S.; Oyeyemi B. F.; Bhavesh N. S. Stringent Response Protein as A Potential Target to Intervene Persistent Bacterial Infection. Biochimie 2019, 165, 67–75. 10.1016/j.biochi.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Sharma I. M.; Petchiappan A.; Chatterji D. Quorum Sensing and Biofilm Formation in Mycobacteria: Role of c-di-GMP and Methods to Study This Second Messenger. IUBMB Life 2014, 66, 823–834. 10.1002/iub.1339. [DOI] [PubMed] [Google Scholar]
- Hogg T.; Mechold U.; Malke H.; Cashel M.; Hilgenfeld R. Conformational Antagonism Between Opposing Active Sites in A Bifunctional RelA/SpoT Homolog Modulates (p)ppGpp Metabolism During the Stringent Response [corrected]. Cell 2004, 117, 57–68. 10.1016/S0092-8674(04)00260-0. [DOI] [PubMed] [Google Scholar]
- Page R.; Peti W. Toxin-Antitoxin Systems in Bacterial Growth Arrest and Persistence. Nat. Chem. Biol. 2016, 12, 208–214. 10.1038/nchembio.2044. [DOI] [PubMed] [Google Scholar]
- Syal K.; Joshi H.; Chatterji D.; Jain V. Novel pppGpp Binding Site at the C-Terminal Region of the Rel Enzyme from Mycobacterium smegmatis. FEBS J. 2015, 282, 3773–3785. 10.1111/febs.13373. [DOI] [PubMed] [Google Scholar]
- Liu S.; Wu N.; Zhang S. S.; Yuan Y. H.; Zhang W. H.; Zhang Y. Variable Persister Gene Interactions with (p)ppgpp for Persister Formation in Escherichia coli. Front Microbiol. 2017, 8, 1795. 10.3389/fmicb.2017.01795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C.; Semsey S.; Mitarai N. Synchronized Switching of Multiple Toxin–Antitoxin Modules by (p)ppGpp Fluctuation. Nucleic Acids Res. 2017, 45, 8180–8189. 10.1093/nar/gkx552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petchiappan A.; Chatterji D. Antibiotic Resistance: Current Perspectives. ACS Omega 2017, 2, 7400–7409. 10.1021/acsomega.7b01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H. W.; Lee C. R.; Cho S. H.; Song M. R.; Cashel M.; Choy H. E.; Seok Y. J.; Hong J. I. Selective Fluorescent Chemosensor for the Bacterial Alarmone (p)ppGpp. J. Am. Chem. Soc. 2008, 130, 784–785. 10.1021/ja0759139. [DOI] [PubMed] [Google Scholar]
- Wexselblatt E.; Kaspy I.; Glaser G.; Katzhendler J.; Yavin E. Design, Synthesis and Structure-Activity Relationship of Novel Relacin Analogs as Inhibitors of Rel Proteins. Eur. J. Med. Chem. 2013, 70, 497–504. 10.1016/j.ejmech.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Sureka K.; Ghosh B.; Dasgupta A.; Basu J.; Kundu M.; Bose I. Positive Feedback and Noise Activate the Stringent Response Regulator Rel in Mycobacteria. PLoS One 2008, 3, e1771 10.1371/journal.pone.0001771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syal K.; Bhardwaj N.; Chatterji D. Vitamin C Targets (p)ppGpp Synthesis Leading to Stalling of Long-Term Survival and Biofilm Formation in Mycobacterium smegmatis. FEMS Microbiol. Lett. 2017, 364, fnw282 10.1093/femsle/fnw282. [DOI] [PubMed] [Google Scholar]
- Vilchèze C.; Kim J.; Jacobs W. R. Jr. Vitamin-C Potentiates the Killing of Mycobacterium tuberculosis by the First-Line Tuberculosis Drugs Isoniazid and Rifampin in Mice. Antimicrob. Agents Chemother. 2018, 62, e02165-17 10.1128/AAC.02165-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchèze C.; Hartman T.; Weinrick B.; Jacobs W. R. Jr. Mycobacterium tuberculosis is Extraordinarily Sensitive to Killing by A Vitamin C-Induced Fenton Reaction. Nat. Commun. 2013, 4, 1881. 10.1038/ncomms2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren I.; Kaldalu N.; Spoering A.; Wang Y.; Lewis K. Persister Cells and Tolerance to Antimicrobials. FEMS Microbiol. Lett. 2004, 230, 13–18. 10.1016/S0378-1097(03)00856-5. [DOI] [PubMed] [Google Scholar]
- Peddireddy V.; Doddam S. N.; Ahmed N. Mycobacterial Dormancy Systems and Host Responses in Tuberculosis. Front. Immunol. 2017, 8, 84. 10.3389/fimmu.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjitha J.; Rajan A.; Shankar V. Features of the Biochemistry of Mycobacterium smegmatis, as a Possible Model for Mycobacterium tuberculosis. J. Infect. Public Health 2020, 13, 1255–1264. 10.1016/j.jiph.2020.06.023. [DOI] [PubMed] [Google Scholar]
- Princiotto S.; Jayasinghe L.; Dallavalle S. Recent Advances in the Synthesis of Naturally Occurring Tetronic Acids. Bioorg. Chem. 2022, 119, 105552 10.1016/j.bioorg.2021.105552. [DOI] [PubMed] [Google Scholar]
- Fischer P. M.; Lane D. P. Inhibitors of Cyclin-Dependent Kinases as Anti-Cancer Therapeutics. Curr. Med. Chem. 2000, 7, 1213–1245. 10.2174/0929867003374048. [DOI] [PubMed] [Google Scholar]
- Nishio K.; Ishida A.; Arioka H.; Kurokawa H.; Fukuoka K.; Nomoto T.; Fukumoto H.; Yokote H.; Saijo N. Antitumor Effects of Butyrolactone I, A Selective cdc2 Kinase Inhibitor, on Human Lung Cancer Cell Lines. Anticancer Res. 1996, 16, 3387–3395. [PubMed] [Google Scholar]
- Suzuki M.; Hosaka Y.; Matsushima H.; Goto T.; Kitamura T.; Kawabe K. Butyrolactone I Induces Cyclin B1 and Causes G2/M Arrest and Skipping of Mitosis in Human Prostate Cell Lines. Cancer Lett. 1999, 138, 121–130. 10.1016/S0304-3835(98)00381-4. [DOI] [PubMed] [Google Scholar]
- Parvatkar R. R.; D’Souza C.; Tripathi A.; Naik C. G. Aspernolides A and B, Butenolides from A Marine-Derived Fungus Aspergillus terreus. Phytochemistry 2009, 70, 128–132. 10.1016/j.phytochem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Haritakun R.; Rachtawee P.; Chanthaket R.; Boonyuen N.; Isaka M. Butyrolactones from the Fungus Aspergillus terreus BCC 4651. Chem. Pharm. Bull. 2010, 58, 1545–1548. 10.1248/cpb.58.1545. [DOI] [PubMed] [Google Scholar]
- Adpressa D. A.; Loesgen S. Bioprospecting Chemical Diversity and Bioactivity in A Marine Derived Aspergillus terreus. Chem. Biodiversity 2016, 13, 253–259. 10.1002/cbdv.201500310. [DOI] [PubMed] [Google Scholar]
- Dewi R. T.; Tachibana S.; Darmawan A. Effect On Α-Glucosidase Inhibition and Antioxidant Activities of Butyrolactone Derivatives from Aspergillus terreus MC751. Med. Chem. Res. 2014, 23, 454–460. 10.1007/s00044-013-0659-4. [DOI] [Google Scholar]
- Niu X.; Dahse H. M.; Menzel K. D.; Lozach O.; Walther G.; Meijer J.; Grabley S.; Sattler I. Butyrolactone I Derivatives from Aspergillus terreus Carrying an Unusual Sulfate Moiety. J. Nat. Prod. 2008, 71, 689–692. 10.1021/np070341r. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y.; Yoshida K.; Abe N.; Hirota A. Soybean Lipoxygenase Inhibitory and DPPH Radical-Scavenging Activities of Aspernolide A and Butyrolactones I and II. Biosci., Biotechnol., Biochem. 2010, 74, 881–883. 10.1271/bbb.90918. [DOI] [PubMed] [Google Scholar]
- Brachmann A. O.; Forst S.; Furgani G. M.; Fodor A.; Bode H. B. Xenofuranones A and B: Phenylpyruvate Dimers from Xenorhabdus szentirmaii. J. Nat. Prod. 2006, 69, 1830–1832. 10.1021/np060409n. [DOI] [PubMed] [Google Scholar]
- Ingerl A.; Justus K.; Hellwig V.; Steglich W. Syntheses of Retipolide E and Ornatipolide, 14-Membered Biaryl-ether Macrolactones from Mushrooms. Tetrahedron 2007, 63, 6548–6557. 10.1016/j.tet.2007.03.022. [DOI] [Google Scholar]
- Justus K.; Herrmann R.; Klamann J.-D.; Gruber G.; Hellwig V.; Ingerl A.; Polborn K.; Steffan B.; Steglich W. Retipolides – Unusual Spiromacrolactones from the Mushrooms Retiboletus retipes and R. ornatipes. Eur. J. Org. Chem. 2007, 2007, 5560–5572. 10.1002/ejoc.200700579. [DOI] [Google Scholar]
- Nitta K.; Fujita N.; Yoshimura T.; Arai K.; Yamamoto Y. Metabolic Products of Aspergillus Terreus. IX. Biosynthesis of Butyrolactone Derivatives Isolated from Strains IFO 8835 and 4100. Chem. Pharm. Bull. 1983, 31, 1528–1533. 10.1248/cpb.31.1528. [DOI] [Google Scholar]
- Machajewski T. M.; Wong C.-H. The Catalytic Asymmetric Aldol Reaction. Angew. Chem., Int. Ed. 2000, 39, 1352–1375. . [DOI] [PubMed] [Google Scholar]
- Allen S. T.; Heintzelman G. R.; Toone E. J. Pyruvate Aldolases as Reagents for Stereospecific Aldol Condensation. J. Org. Chem. 1992, 57, 426–427. 10.1021/jo00028a005. [DOI] [Google Scholar]
- Enders D.; Dyker H.; Leusink F. R. Enantioselective Synthesis of Protected Isotetronic Acids. Chem. – Eur. J. 1998, 4, 311–320. . [DOI] [Google Scholar]
- Dambruoso P.; Massi A.; Dondoni A. Efficiency in Isotetronic Acid Synthesis Via A Diamine–acid Couple Catalyzed Ethyl Pyruvate Homoaldol Reaction. Org. Lett. 2005, 7, 4657–4660. 10.1021/ol051809p. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Walleser P. M.; Tius M. A. Isotetronic Acids from an Oxidative Cyclization. Chem. Commun. 2015, 51, 10858–10860. 10.1039/C5CC04051E. [DOI] [PubMed] [Google Scholar]
- Mao B.; Fañanas-Mastral M.; Feringa B. L. Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones. Chem. Rev. 2017, 117, 10502–10566. 10.1021/acs.chemrev.7b00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhl K.; Gathergood N.; Jørgensen K. A. Catalytic Asymmetric Homo-Aldol Reaction of Pyruvate-A Chiral Lewis Acid Catalyst that Mimics Aldolase Enzymes. Chem. Commun. 2000, 2211–2212. 10.1039/b007125k. [DOI] [Google Scholar]
- Gathergood N.; Juhl K.; Poulsen T. B.; Thordrup K.; Jørgensen K. A. Direct Catalytic Asymmetric Aldol Reactions of Pyruvates: Scope and Mechanism. Org. Biomol. Chem. 2004, 2, 1077–1085. 10.1039/B316092K. [DOI] [PubMed] [Google Scholar]
- Vincet J. M.; Margottin C.; Berlande M.; Cavagnat D.; Buffeteau T.; Landais Y. A Concise Organocatalytic and Enantioselective Synthesis of Isotetronic Acids. Chem. Commun. 2007, 4782–4784. 10.1039/b711192d. [DOI] [PubMed] [Google Scholar]
- Roy B.; Das E.; Roy A.; Mal D. Ni(ii)-Catalyzed Vinylic C–H Functionalization of 2-Acetamido-3-Arylacrylates to Access Isotetronic Acids. Org. Biomol. Chem. 2020, 18, 3697–3706. 10.1039/D0OB00557F. [DOI] [PubMed] [Google Scholar]
- Chen P.; Wang K.; Zhang B.; Guo W.; Liu Y.; Li C. Water Enables an Asymmetric Cross Reaction of α-Keto Acids With α-Keto Esters for the Synthesis of Quaternary Isotetronic Acids. Chem. Commun. 2019, 55, 12813–12816. 10.1039/C9CC06356K. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Jiang Z.; Zhou X.; Lu S.; Li J.; Liu Y.; Li C. The Synthesis of Chiral Isotetronic Acids with Amphiphilic Imidazole/Pyrrolidine Catalysts Assembled in Oil-In-Water Emulsion Droplets. Angew. Chem., Int. Ed. 2012, 51, 13159–13162. 10.1002/anie.201206438. [DOI] [PubMed] [Google Scholar]
- Xu X. -Y.; Tang Z.; Wang Y. -Z.; Luo S. W.; Cun L. F.; Gong L. -Z. Asymmetric Organocatalytic Direct Aldol Reactions of Ketones with α-keto acids and their Application to the Synthesis of 2-Hydroxy-γ-Butyrolactones. J. Org. Chem. 2007, 72, 9905–9913. 10.1021/jo701868t. [DOI] [PubMed] [Google Scholar]
- Lee D.; Newman S. G.; Taylor M. S. Boron-Catalyzed Direct Aldol Reactions of Pyruvic Acids. Org. Lett. 2009, 11, 5486–5489. 10.1021/ol902322r. [DOI] [PubMed] [Google Scholar]
- Cooper A. J. L.; Ginos J. Z.; Meister A. Synthesis and Properties of the α-Keto Acids. Chem. Rev. 1983, 83, 321–358. 10.1021/cr00055a004. [DOI] [Google Scholar]
- Liu Y.-X.; Zhang P.-X.; Li Y.-Q.; Song H.-B.; Wang Q.-M. Design, Synthesis, and Biological Evaluation Of 2-Benzylpyrroles and 2-Benzoylpyrroles Based on Structures of Insecticidal Chlorfenapyr and Natural Pyrrolomycins. Mol. Diversity 2014, 18, 593–598. 10.1007/s11030-014-9515-9. [DOI] [PubMed] [Google Scholar]
- Gräßle S.; Vanderheiden S.; Hodapp P.; Bulat B.; Nieger M.; Jung N.; Bräse S. Solid Phase Synthesis of (Benzannelated) Six-Membered Heterocycles via Cyclative Cleavage of Resin-Bound Pseudo-Oxazolones. Org. Lett. 2016, 18, 3598–3601. 10.1021/acs.orglett.6b01609. [DOI] [PubMed] [Google Scholar]
- Furukawa K.; Inada H.; Shibuya M.; Yamamoto Y. Chemoselective Conversion from α-Hydroxy Acids to α-Keto Acids Enabled by Nitroxyl-Radical-Catalyzed Aerobic Oxidation. Org. Lett. 2016, 18, 4230–4233. 10.1021/acs.orglett.6b01964. [DOI] [PubMed] [Google Scholar]
- Yu J.; Li J.; Cao S.; Wu T.; Zeng S.; Zhang H.; Liu J.; Jiao Q. Chemoenzymatic Synthesis of L-3,4-Dimethoxyphenyl-Alanine and Its Analogues Using Aspartate Aminotransferase as A Key Catalyst. Catal. Commun. 2019, 120, 28–32. 10.1016/j.catcom.2018.10.033. [DOI] [Google Scholar]
- Endo Y.; Shudo K.; Itai A.; Hasegawa M.; Sakai S. I. Synthesis and Stereochemistry of Indolactam-V, An Active Fragment of Teleocidins. Structural Requirements for Tumor-Promoting Activity. Tetrahedron 1986, 42, 5905–5924. 10.1016/S0040-4020(01)96073-9. [DOI] [Google Scholar]
- Bergman J.; Lidgren G.; Gogoll A. Synthesis and Reactions of Oxazolones from L-Tryptophan and α-Haloacetic Anhydrides. Bull. Soc. Chim. Belg. 1992, 101, 643–660. 10.1002/bscb.19921010712. [DOI] [Google Scholar]
- Lin D. W.; Masuda T.; Biskup M. B.; Nelson J. D.; Baran P. S. Synthesis-Guided Structure Revision of the Sarcodonin, Sarcoviolin, and Hydnellin Natural Product Family. J. Org. Chem. 2011, 76, 1013–1030. 10.1021/jo102228j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikri K.; Duggal P.; Kumar C.; Batra S. D.; Vashist A.; Bhaskar A.; Tripathi K.; Sethi T.; Singh A.; Tyagi J. S. Multifaceted Remodeling by Vitamin C Boosts Sensitivity of Mycobacterium Tuberculosis Subpopulations to Combination Treatment by Anti-Tubercular Drugs. Redox Biol. 2018, 15, 452–466. 10.1016/j.redox.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khameneh B.; Bazzaz B. S. F.; Amani A.; Rostami J.; Vahdati-Mashhadian N. Combination of Anti-Tuberculosis Drugs with Vitamin C or NAC Against Different Staphylococcus Aureus and Mycobacterium Tuberculosis Strains. Microb. Pathog. 2016, 93, 83–87. 10.1016/j.micpath.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Gupta K. R.; Kasetty S.; Chatterji D. Novel Functions of (p)ppGpp and Cyclic di-GMP in Mycobacterial Physiology Revealed by Phenotype Microarray Analysis of Wild-Type and Isogenic Strains of Mycobacterium smegmatis. Appl. Environ. Microbiol. 2015, 81, 2571–2578. 10.1128/AEM.03999-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta K. R.; Baloni P.; Indi S. S.; Chatterji D. Regulation of Growth, Cell Shape, Cell Division, and Gene Expression by Second Messengers (p)ppGpp and Cyclic di-GMP in Mycobacterium smegmatis. J. Bacteriol. 2016, 198, 1414–1422. 10.1128/JB.00126-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petchiappan A.; Naik S. Y.; Chatterji D. RelZ-mediated Stress Response in Mycobacterium smegmatis: pGpp Synthesis and Its Regulation. J. Bacteriol. 2020, 202, e00444-19 10.1128/JB.00444-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A.; Ghosh A. c-di-AMP signaling plays important role in determining antibiotic tolerance phenotypes of Mycobacterium smegmatis. Sci. Rep. 2022, 12, 13127. 10.1038/s41598-022-17051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutte C. C.; Crosson S. Bacterial Lifestyle Shapes Stringent Response Activation. Trends Microbiol. 2013, 21, 174–180. 10.1016/j.tim.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syal K.; Flentie K.; Bhardwaj N.; Maiti K.; Jayaraman N.; Stallings C. L.; Chatterji D. Synthetic (p)ppGpp Analogue is an Inhibitor of Stringent Response in Mycobacteria. Antimicrob. Agents Chemother. 2017, 61, e00443-17 10.1128/AAC.00443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windels E. M.; Michiels J. E.; Fauvart M.; Wenseleers T.; Van den Bergh B.; Michiels J. Bacterial Persistence Promotes the Evolution of Antibiotic Resistance by Increasing Survival and Mutation Rates. ISME J. 2019, 13, 1239–1251. 10.1038/s41396-019-0344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. R.; Lobritz M. A.; Collins J. J. Microbial Persistence and the Road to Drug Resistance. Cell Host Microbe 2013, 13, 632–642. 10.1016/j.chom.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchèze C.; Hartman T.; Weinrick B.; Jain P.; Weisbrod T. R.; Leung L. W.; Freundlich J. S.; Jacobs W. R. Jr. Enhanced Respiration Prevents Drug Tolerance and Drug Resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 4495–4500. 10.1073/pnas.1704376114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathcock J. N.; Azzi A.; Blumberg J.; Bray T.; Dickinson A.; Frei B.; Jialal I.; Johnston C. S.; Kelly F. J.; Kraemer K.; Packer L.; Parthasarathy S.; Sies H.; Traber M. G. Vitamins E and C are Safe Across a Broad Range of Intakes. Am. J. Clin. Nutr. 2005, 81, 736–745. 10.1093/ajcn/81.4.736. [DOI] [PubMed] [Google Scholar]
- Hoffer L. J.; Levine M.; Assouline S.; Melnychuk D.; Padayatty S. J.; Rosadiuk K.; Rousseau C.; Robitaille L.; Miller W. H. Jr. Phase I Clinical Trial of i.v. Ascorbic Acid in Advanced Malignancy. Ann. Oncol. 2008, 19, 1969–1974. 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- Stephenson C. M.; Levin R. D.; Spector T.; Lis C. G. Phase I Clinical Trial to Evaluate the Safety, Tolerability, and Pharmacokinetics of High-Dose Intravenous Ascorbic Acid in Patients with Advanced Cancer. Cancer Chemother. Pharmacol. 2013, 72, 139–146. 10.1007/s00280-013-2179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.