Abstract

Verbascoside (VER) is a phenylethanoid glycoside compound found in Clerodendrum species and is an important part of traditional medicine. It is found in the leaves of Clerodendrum glandulosum, which is taken as a soup or vegetable and also utilized in traditional medicine by the people of Northeast India, especially against hypertension and diabetes. In the present study, VER was extracted from C. glandulosum leaves using ultrasound-assisted extraction through the solvent extraction method (ethanol–water, ethanol, and water). The ethanol extract had the highest phenolic and flavonoid contents, viz., 110.55 mg GAE/g and 87.60 mg QE/g, respectively. HPLC and LC–MS were used to identify the active phenolic compound, and VER was found to be the main component present in the extraction with a molecular weight of 624.59 g/mol. NMR (1H, 2D-COSY) analysis showed the presence of hydroxytyrosol, caffeic acid, glucose, and rhamnose in the VER backbone. Further, different antioxidant activities and antidiabetic and antihyperlipidemia enzyme markers’ inhibition against VER-enriched ethanol extract were evaluated. The results showed that ultrasound extraction of polyphenols using ethanol from C. glandulosum could be a promising technique for the extraction of bioactive compounds.
Highlights
-
1.
Verbascoside was extracted by ultrasound from Clerodendrum glandulosum leaves.
-
2.
HPLC revealed that verbascoside content was the highest (20.14%) for ethanol extracts.
-
3.
NMR analysis revealed the presence of hydroxytyrosol, caffeic acid, glucose, and rhamnose in verbascoside.
-
4.
Verbascoside exhibited antioxidant activities and different enzyme inhibitions.
1. Introduction
Polyphenols are secondary metabolites synthesized by plants to protect against pathogen invasion and hostile environmental conditions.1 Polyphenols are composed of phenylpropanoids that have an aromatic nucleus along with one or more −OH groups.2 It has a wide range of medical applications like lowering reactive species (oxygen and nitrogen), transferring free radicals, activating antioxidant enzymes, improving oxidative stress, and preventing diabetes and other cardiovascular diseases.3 Because of the high applicability in disease management, polyphenols have been continuously explored as a potential medicine against specific comorbid diseases. Plant metabolites are also rich in polyphenols and serve as healthier alternatives to artificial and synthetic antioxidants.4 Extraction of polyphenols from plants involves different techniques (viz., Soxhlet, maceration, percolation, etc.) that are effective for obtaining enough amounts of compounds. However, conventional techniques have different disadvantages as they require longer durations, higher solvent quantities, and lower extraction yields. Therefore, ultrasound-assisted extraction (UAE) has emerged as a nonconventional technique that extracts polyphenols from plants more feasibly and economically.5 UAE is an effective technique for enhancement of polyphenolic content by 32–36% as compared to conventional techniques using a low amount of solvent.6,7
Clerodendrum glandulosum (CG), belonging to the family Lamiaceae, is enriched with polyphenols and major marker compounds that exhibited effective therapeutic properties.8 CG serves as a food (as a soup or vegetable) and traditional medicine for the people of Northeast India.9 Verbascoside (VER), a phenylethanoid glycoside, is one of the most widespread and potent among the reported polyphenols.10 VER was reported to attenuate cytotoxicity induced by elevated glucose levels and to reduce oxidative stress by downregulation of PKC/HMGB1/RAGE/NFκβ signaling.11 Recently, VER isolated from Odontonema strictum in an aqueous medium showed effective free radical scavenging activities.12 Extraction of polyphenols by UAE has been reported from plants such as Jatropha dioica, Flourensia cernua, Turnera diffusa, and Eucalyptus camaldulensis by using ethanol.13 UAE enhanced the polyphenolic contents as compared to maceration and thermal extraction from Acacia confusa.(14) A similar result was reported in the case of polyphenol extraction from Eucalyptus marginata L. where higher phenolic contents were obtained in UAE (∼210 mg GAE/g DW) than maceration (∼150 mg GAE/g DW).15 Several studies have been conducted on VER from different leaf extracts of CG and found that it was an effective compound against hypertension and hyperlipidemia.16,17
Previous reports on CG have utilized conventional methods of extraction such as maceration, Soxhlet, and decoction. This study aims to understand the extraction of polyphenols from CG using ultrasound-assisted technology from a limited sample size and within a stipulated time period. Extraction was performed by different solvents to understand the importance of each solvent followed by characterization of the main polyphenol compound (VER) using HPLC, HPTLC, LC–MS, and NMR methods. Different antioxidant assessments (ABTS+•, DPPH, and phosphomolybdenum assays) and enzyme inhibition (α-amylase, α-glucosidase, and pancreatic-lipase) potentials of VER-enriched extracts were evaluated.
2. Materials and Methods
2.1. Chemicals
All the chemicals were procured from Sigma Aldrich (USA). All solvents and other reagents (HPLC grade) were purchased from Merck Limited (Mumbai, India).
2.2. Plant Collection and Identification
Fresh leaves of CG were collected from the medicinal plant garden of the Institute of Advanced Study in Science and Technology (IASST), Assam, India, situated between 25°43′ to 26°53′ north latitude and 90°39′ to 92°11′ east longitude. The plant specimen was authenticated by Dr. Chaya Deori, Scientist-D & HOD of the Botanical Survey of India (BSI), Laitumkhrah, Shillong. An herbarium specimen (No. BSI/ERC/Tech/2019/614) was submitted to the Life Sciences Division, IASST, Assam, India, for future reference. The collected leaves of CG were washed, shade dried, ground into a coarse powder, and kept in an airtight container for further use.
2.3. Preparation of Extracts
Dried CG leaf powder (50 g) was dissolved in different solvents (500 mL, 1:10 w/v) for 15 min in an ultrasonic bath (JSGW, India, model no. 21773-1236/3). Three solvent systems were selected for the study, namely, ethanol, ethanol–water (1:1), and water. The extract was filtered and dried in a rotary evaporator (BUCHI Labortechnik AG, catalog no. 1000170673) and subsequently in a lyophilizer (LABCONCO, catalog no. 710612070). The dried extract was stored at −20 ± 2 °C in the dark for further use.
2.4. Extraction Yield of Extracts
The yield of extract for three solvents was calculated using the formula described in ref (18):
where Y is for yield of extraction (%), M is the mass of the extract after the evaporation of the extraction solvent (mg), and m is the mass of the plant sample (mg).
2.5. Quantitative Phytochemical Analysis
2.5.1. Total Phenolic Content (TPC)
The total phenolic content was estimated using the Folin–Ciocalteu colorimetric method with slight modifications.19
2.5.2. Total Flavonoid Content (TFC)
The total flavonoid content of extracts was evaluated according to the earlier established method with slight modifications.20
2.5.3. Identification and Quantification of VER Using HPLC
Qualitative and quantitative analyses of polyphenols were conducted in a Waters Breeze QS manual injector HPLC system (Waters Corporation, Milford, Massachusetts, USA) equipped with Waters 1525 binary HPLC pumps connected to a Waters 2998 photodiode-array detector (PDA) and the Breeze software. Chromatographic analyses were carried out using a C18 column (80 Å, 5 μm, 4.6 × 250 mm) at 25 °C using 1% acetic acid in water (A) and methanol (B) as a mobile phase.21 The flow rate was maintained at 1 mL/min, and 20 μL of the sample was injected. Extracts were investigated for VER at 335 nm wavelength.
2.5.4. HPTLC Analysis of UAE Extracts
Plates were run on CAMAG HPTLC instruments, and data were collected using the VisionCATS software. Samples (5 μL) were applied on a clean TLC plate (10 × 10 cm) using CAMAG LINOMAT 5 under a flow of N2 gas with a dosage speed of 200 nL/s. The plate was then transferred to the TLC running chamber lined with a filter paper and presaturated with the mobile phase [ethyl acetate/water/acetic acid/formic acid (8.5:0.5:0.5:0.5)].22 After the run, the plate was removed from the mobile phase and air-dried for 5 min. The plates were visualized using the CAMAG TLC scanner 2 and analyzed by the CAMAG TLC scanner 4. The wavelength of maximum absorption (λmax) was determined after recording UV spectra from 200 to 700 nm with a scanning speed of 20 mm/s, slit width (4 × 0.3 mm), and data resolution of 100 mm/step. The specificity of the method was confirmed by comparing the Rf value and UV spectrum of the samples and the standard, VER. Subsequent analysis of the reference compound was performed at its λmax with the following conditions.
2.5.5. LC–MS Analysis
LC–MS analysis was performed using the ExActive Plus Ultimate 3000 UHPLC (Thermo Scientific) in negative ionization mode. A Hypersil Gold C18 column (150 × 2.1 mm, 1.9 μm particle size) was used for compound separation at 25 ± 2 °C temperature. Acetonitrile (A) and 0.1% (v/v) formic acid (B) was used as elution. The gradient was initiated at 5% A and 95% B to 25% A and 75% B (after 5 min) followed by 40% A (10 min), 60% B (60 min); 45% A (15 min), 55% B (15 min); and 5% A (20 min), 95% B (20 min). Solvent system B was injected with a flow rate of 0.2 mL/min. The mass spectrometer was operated in the range of 100–1000 m/z, and N2 gas was used as a nebulizer. Drying gas flow rate was 8 L/min at 325 °C and nebulizer gas at 25 psi with a fragmentor voltage of 150 V. The Xcalibur analysis software package (Thermo Fisher) was used for the analysis of the peak data, and detected compounds were validated based on the molecular formula, molecular mass, retention time, and m/z ratio.
2.5.6. NMR Analysis
1H and 2D COSY (1H-1H, correlated spectroscopy) NMR was performed for the ethanol extract containing verbascoside, and NMR spectra were recorded in an AVNeo 400 MHz spectrometer (Bruker, Germany) at IIT Guwahati, India. Dimethyl sulfoxide (DMSO) was used for internal reference. 1H NMR spectra of the sample were phased, baseline corrected, and characterized by the TopSpin software (4.1.4, 2022 Bruker BioSpin GmbH), and chemical shifts were recorded in δ units (ppm).
2.6. In Vitro Antioxidant Activity
2.6.1. DPPH Radical Scavenging Assay
The DPPH radical scavenging assay was adapted from an established method.23
2.6.2. ABTS•+ Radical Scavenging Assay
The ABTS•+ radical scavenging assay was performed according to an earlier reported method.24
2.6.3. Phosphomolybdenum Assay (PMD)
The antioxidant activity of VER enriched extract was evaluated by the PMD method according to a previous report.25
2.7. Enzyme Inhibition Assays
2.7.1. α-Amylase Inhibition Assay
Inhibition of α-amylase is an important therapeutic target for regulating postprandial increase in blood glucose in diabetic conditions. In vitro, the α-amylase inhibition assay was carried out using the 3,5-dinitrosalicylic acid (DNS) method using 1% soluble starch (Merck, catalog no. 101257, Darmstadt, Germany) as substrate with slight modifications.26
2.7.2. α-Glucosidase Inhibition Assay
The α-glucosidase inhibition assay was performed according to the established method with slight modifications.27
2.7.3. Pancreatic Lipase Inhibition Assay
The pancreatic lipase enzyme (EC 3.1.1.3) inhibition capacity of the extracts was estimated using an earlier report with minor modifications.28
3. Statistical Analysis
All the results are expressed in mean ± standard deviation. All statistical analyses were performed in the GraphPad Prism 5.0 software. One-way ANOVA was performed that was followed by Turkey’s multiple comparison tests.
4. Results and Discussion
4.1. Extraction Yield of Extracts
Extraction yield (mass of extract/mass of dry matter %) was used as an indicator of the efficiency of the extraction conditions. UAE yields of CG leaf extracts prepared using water, ethanol, and water–ethanol are shown in Figure 1C. Results showed that ethanol–water (17.52 ± 0.24%) and water (16.57 ± 0.14%) extracts had similar extraction yields followed by the ethanol extract (5.71 ± 0.11%). Similar results were reported by Dhanani et al. where Withania somnifera extracted by UAE had the lowest extraction yield for ethanol (3.17%) compared to water (10.27%) and ethanol–water (9.08%) extracts.29 Extraction yield is significantly dependent on the moisture content and nature of the plant material. The presence of water as a solvent might lead to higher extraction yields of the plant extract.29 The highest extraction yield (16.97 w/w %) was obtained from leaves of Prunus laurocerasus when water was used as a solvent.30 This result is consistent with the extraction yield of other medicinal plants such as Severinia buxifolia, Passiflora caerulea L., Physalis peruviana L. and Solanum muricatum.(31,32)
Figure 1.

Ultrasound-assisted extraction (UAE) of different bioactive compounds from CG leaves. (A) Total phenolic content (TPC, mg GAE/g), (B) total flavonoid content (TFC, mg QE/g), and (C) extraction yield (%) using three different extractions, viz., ethanol–water, ethanol, and water. Values are expressed as mean and SD (n = 3) with significant variation at **p < 0.005 and ***p < 0.001, respectively.
4.2. Quantitative Phytochemical Analysis
4.2.1. Total Phenolic Content (TPC)
Phenolics are a group of secondary plant metabolites produced under stressful conditions that play an important role in the prevention of certain illnesses. UAE extracts showed phenolic contents of 92.01 ± 3.52 (ethanol–water), 110.31 ± 1.92 (ethanol), and 45.71 ± 2.11 (water) mg GAE/g, respectively (Figure 1A). The ethanol concentration in UAE played a major role in extracting higher amounts of phenolic compounds. The ethanol extract showed the highest phenolic (110.31 ± 1.92 mg GAE/g) contents, and the extraction trend was ethanol > ethanol–water > water. Salih et al. reported higher TPC in ethanol compared to water in the seed extracts of Juniperus procera.(33) Another study reported lower polyphenolic contents in the water extract obtained by maceration and decoction of different parts of Passiflora caerulea L., Physalis peruviana L., and Solanum muricatum Aiton as compared to their hydroethanolic extracts.32 These results were attributed to the lower polyphenol extraction yield obtained when water was used. This may be due to the fact that water as a solvent is suitable for the extraction of highly polar bioactive compounds. On the other hand, ethanol or ethanol–water solvent is suitable for the extraction of compounds with a broad range of polarity.34 Seasonal variations of the CG leaf extracts for TPC levels were also performed, and higher TPC levels were obtained for the summer and monsoon seasons (Figure S1). The increase in phenolic content in the flowering period has been reported in several studies, irrespective of the solvent used.35,36 The high TPC observed in the ethanol extract of the preflowering and flowering stages might be a result of nutrient depletion in the soil as the plant approaches maturation.37 Phenolics increased significantly during the reproductive phase and were higher during inflorescence maturity, indicating that the synthesized phenolics play an active role in the flowering season.36 Castro et al. also reported high phenolic contents at the beginning of flowering periods in Baccharis myriocephala that declined thereafter.38
4.2.2. Total Flavonoid Content (TFC)
Flavonoids are a class of phenolic compounds that protect the plant from stresses such as UV damage, help in counteracting oxidative stress in plants, and also act as growth regulators.39,40 TFC from CG leaves using UAE was highest for the ethanol extract, 87.12 ± 0.45, followed by ethanol–water, 70.01 ± 0.44, and water, 27.09 ± 0.14 mg QE/g, extracts, respectively (Figure 1B). Similar to our findings, Backes et al. also reported maximum recovery of flavonoids (anthocyanins) from Ficus carica L. peel using UAE to yield 3.82 mg cyanidin 3-rutinoside/g from 100% ethanol extract.41 In the present study, TFC levels were 2.58 times higher for ethanol–water as compared to water extract. Ethanol extracts showed the highest TFC contents in the leaves and seeds of Juniperus procera.(33) Several studies confirmed that ethanol added to water when used as an extractant resulted in the extraction of the highest amounts of total flavonoids from different plants.32,42 It has been suggested that ethanol and ethanol–water at different proportions were often used for the extraction of polar flavonoids.43
4.2.3. HPLC Analysis
VER, a phenylpropanoid glycoside, is a hydrophilic compound that has potent biological activities such as antioxidant, anti-inflammatory, antineoplastic, wound healing, neuroprotective, etc.44 The standard for VER (Figure 2A), as well as the UAE extracts (Figure 2B–D) of CG, was analyzed by HPLC-PDA and successfully identified by this method with a retention time (Rt) = 3.6 min and λmax = 335 nm. UAE extracts showed VER contents of 107.37 (10.737%, ethanol–water), 201.41 (20.141%, ethanol), and 83.46 (8.346%, water) μg/mg, respectively. Lee et al. reported the highest VER contents in the 100% ethanol extract of Osmanthus fragrans flower (i.e., 13.86%). A total of 162.11 mg/g (i.e., 16.211%) of VER contents were reported from ethanolic extracts of Abeliophyllum distichum.(21) The retention time, UV spectra, and co-chromatography of the peak were all consistent with the VER standard. The chemical identity of VER was further confirmed by HPTLC, LC–MS, and NMR.
Figure 2.
HPLC analysis of different extracts using ultrasound-assisted extraction. (A) Standard (verbascoside), (B) ethanol–water, (C) ethanol, (D) water. The principal compound verbascoside had a retention time (Rt) of around 3.6 min.
4.2.4. HPTLC Analysis
The λmax obtained from the UV spectrum was 332 nm for VER. The plates developed after elution were observed under 366 nm with Rf = 0.382 as shown in Figure 3A. The 3D presentation of the TLC plate is shown in Figure 3B. VER contents of UAE extracts were quantified as 395.42 (ethanol–water), 433.71 (ethanol), and 319.71 (water) μg/10 mg. The yield of VER was the highest in the ethanol extract, i.e., 1.35 times that of the water extract, which had the lowest yield as confirmed from the HPTLC data. Gupta et al. reported that the amount of VER contents available in the leaves of CG extracted by maceration in methanol extracts ranged between 2.17 and 2.26% using the same HPTLC method.22 In the present study, VER contents ranged between 3.19 and 4.33%, indicating higher VER yields by UAE in comparison to maceration.
Figure 3.
Identification of active molecule present in the extract. (A) HPTLC analysis of extract where lanes 1 to 5 show different concentrations of pure verbascoside and L6 to L8 were ethanol–water, ethanol, and water (Rf = 0.382), respectively. (B) 3D graph of HPTLC analysis. (C) LC–MS/MS analysis of ethanol extract. The predominant ion at m/z 623.20 (at Rt = 11.84 min) was later designated as verbascoside. (D) 1H NMR spectra of the ethanol extract.
4.2.5. LC–MS Analysis
Tentative metabolite assignments were conducted via comparing molecular ions of [M – H]− in the negative ionization mode in addition to lower m/z fragment ions resulting from MS/MS fragmentation. VER, a phenylethanoid glycoside, is composed mainly of a sugar skeleton of β-glucose and rhamnose monosaccharides, whereas the caffeoyl and hydroxyl phenylethyl aglycons replaced the hydroxyl groups of C4 and C1 of β-glucose, respectively, as shown in the structure in Figure 3C. The total ionization chromatogram in the negative mode (TIC) of ethanol extracts revealed the presence of VER at an Rt of 11.84 min. Identification of VER through its fragmentation was observed in the presented fragmentation as shown in Figure 3C and the daughter ion spectrum as shown in Figure 3C. Spectroscopy data showed the presence of a predominant ion at m/z 623.20 (C29H35O15)− for VER [M – H]− that likely corresponds to the deprotonated molecule of VER and the corresponding ions at 179.03 [C9H7O4]−, a characteristic ion peak of the caffeic acid, and at m/z 461.15, representing [M – H – hexose sugar]− due to loss of a hexose sugar (−162 m/z), observed in the negative mode with Rt of 11.84 min. The ions m/z 461 and 179 along with m/z 623 have previously been reported in the LC–MS spectra of VER isolated from Euphrasia rostkoviana.(45) Similar characteristic ion peaks were observed by Attia et al. at m/z 623 for VER [M – H]− and its daughter ions m/z 161 [caffeic acid – H – H2O]− and m/z 461 [M – H – hexose sugar]− as characterized in Plantago psyllium and P. afra.(46)
4.2.6. NMR Analysis
NMR analysis (1H and 2D COSY) was used to determine the molecular identity and structure of VER. The UV spectrum of ethanol extract showed the hydroxytyrosol [δ: 6.69 (1H, d, J = 2.31 Hz), δ: 6.75 (1H, d, J = 8.4 Hz), δ: 6.51 (1H, dd, J = 1.8, 7.9), δ: 2.79 (2H, m), δ: (2H, 4.06, dd, J = 7.02, 14.32)], caffeic acid [δ: 7.06 (1H, d, J = 1.98), δ: 6.75 (1H, d, J = 8.42), δ: 6.99 (1H, dd, J = 1.71, 8.24), δ: 6.25 (d, J = 8.3)], glucose [δ: 4.36 (1H, d, J = 7.9), δ: 3.36 (1H, d, J = 7.9), δ: 3.83 (1H, t, J = 9.5), δ: 4.89 (1H, t, J = 9.16), δ: 3.82 (1H, m), δ: 3.68 (2H, m)], and rhamnose [δ: 5.05 (1H, d, J = 7.9), δ: 3.90 (1H, m), δ: 3.11 (1H, m), δ: 3.34 (1H, m), δ: 3.48 (1H, m), δ: 1.16 (1H, d, J = 7.0)] moieties (Figure 3D) as these are the main structural moieties of phenylethanoid glycoside VER. 1H NMR chemical shifts and the coupling constants of VER are shown in Table 1. NMR analysis of crude extracts for confirming the presence of active polyphenols has been reported previously in several studies.47,48 Zou et al. utilized 1H NMR to characterize the chemical profile of plant extracts of Cistanche deserticola and confirmed VER as one of the active marker compounds.49 Confirmation by NMR of phenylethanoids (VER) has been reported earlier from Rydingia michauxii extracts.47 NMR in combination with LC–MS data was adopted to elucidate the structural basis of different classes of polyphenols in case of a metabolomic study.471H and 2D-NMR spectra of crude methanolic extract of Stachys thracica led to the unambiguous assignment of 15 individual compounds including VER.48 In the present study, principal signals showing the 1H resonances and principal homonuclear correlations obtained by 2D-COSY spectra have led to the assignment of the different moieties of VER in the ethanol extract on the basis of previously published compound assignments.50−52
Table 1. 1H NMR of VER in the Ethanol Extracta.
| moiety | position | δH, mult. (J in Hz) | moiety | position | δH, mult. (J in Hz) |
|---|---|---|---|---|---|
| hydroxytyrosol | 1 | caffeic acid | 1″ | ||
| 2 | 6.69, d (2.31) | 2″ | 7.06, d (1.98) | ||
| 3 | 3″ | ||||
| 4 | 4″ | ||||
| 5 | 6.75, d (8.4) | 5″ | 6.75, d (8.42) | ||
| 6 | 6.51, dd (1.8, 7.9) | 6″ | 6.99, dd (1.71, 8.24) | ||
| 7 | 2.79, m | 7″ | not resolved | ||
| 8 | 4.06, dd (7.02, 14.32) | 8″ | 6.25, d (8.3) | ||
| glucose | 1′ | 4.36, d (7.9) | rhamnose | 1‴ | 5.05, d (7.9) |
| 2′ | 3.36, d (7.9) | 2‴ | 3.90, m | ||
| 3′ | 3.83, t (9.5) | 3‴ | 3.11, m | ||
| 4′ | 4.89, t (9.16) | 4‴ | 3.34, m | ||
| 5′ | 3.82, m | 5‴ | 3.48, m | ||
| 6′ | 3.68, m | 6‴ | 1.16, d (7.0) |
δH: chemical shift (in ppm) for 1H NMR, mult.: multiplicity (J in Hz), d: doublet, dd: double doublet, M: multiplet, T: triplet.
4.3. Antioxidant Assays
4.3.1. DPPH Radical Scavenging Assay
The DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical method is an antioxidant assay based on electron transfer that produces a violet solution in ethanol. These free radicals are stable at room temperature and reduced in the presence of an antioxidant molecule. The VER-enriched ethanol extract had higher DPPH radical scavenging activity (IC50 of 39.51 ± 0.51 μg/mL) than ethanol–water (IC50 of 50.62 ± 2.06 μg/mL) and water (IC50 of 52.51 ± 1.54 μg/mL) (Figure 4A). This is in correlation with the presence of higher VER contents in the ethanol extract, which showed 63.09 ± 1.47% DPPH radical scavenging at 50 μg. Similar results were reported where ethanol (100%) extract from Limnophila aromatica showed DPPH radical scavenging activity of 70.06 ± 1.0 μg/mL.53 Georgiev et al. reported VER to be an effective free radical scavenger.54 The scavenging ability of VER is due to the involvement in proton and/or electron transfer to/from the free radicals.55 Studies confirm that VER has a significant radical scavenging potential as shown by the following reports.56,57 Burgos et al. reported a higher DPPH radical scavenging ability (58.1 ± 0.6 μM) in VER compared to ascorbic acid (284.9 ± 1.2 μM).56 It was reported that VER isolated from Abeliophyllum distichum exhibited significant DPPH radical scavenging activity with an IC50 of 8.81 μg/mL.57 Therefore, the presence of higher amounts of VER in the ethanol extracts correlated significantly (p < 0.01) with the DPPH radical scavenging activities. VER metabolites are reported to enhance the activities of major antioxidant enzymes (catalase, glutathione peroxidase, and glutathione reductase) in model systems as compared to VER.58
Figure 4.
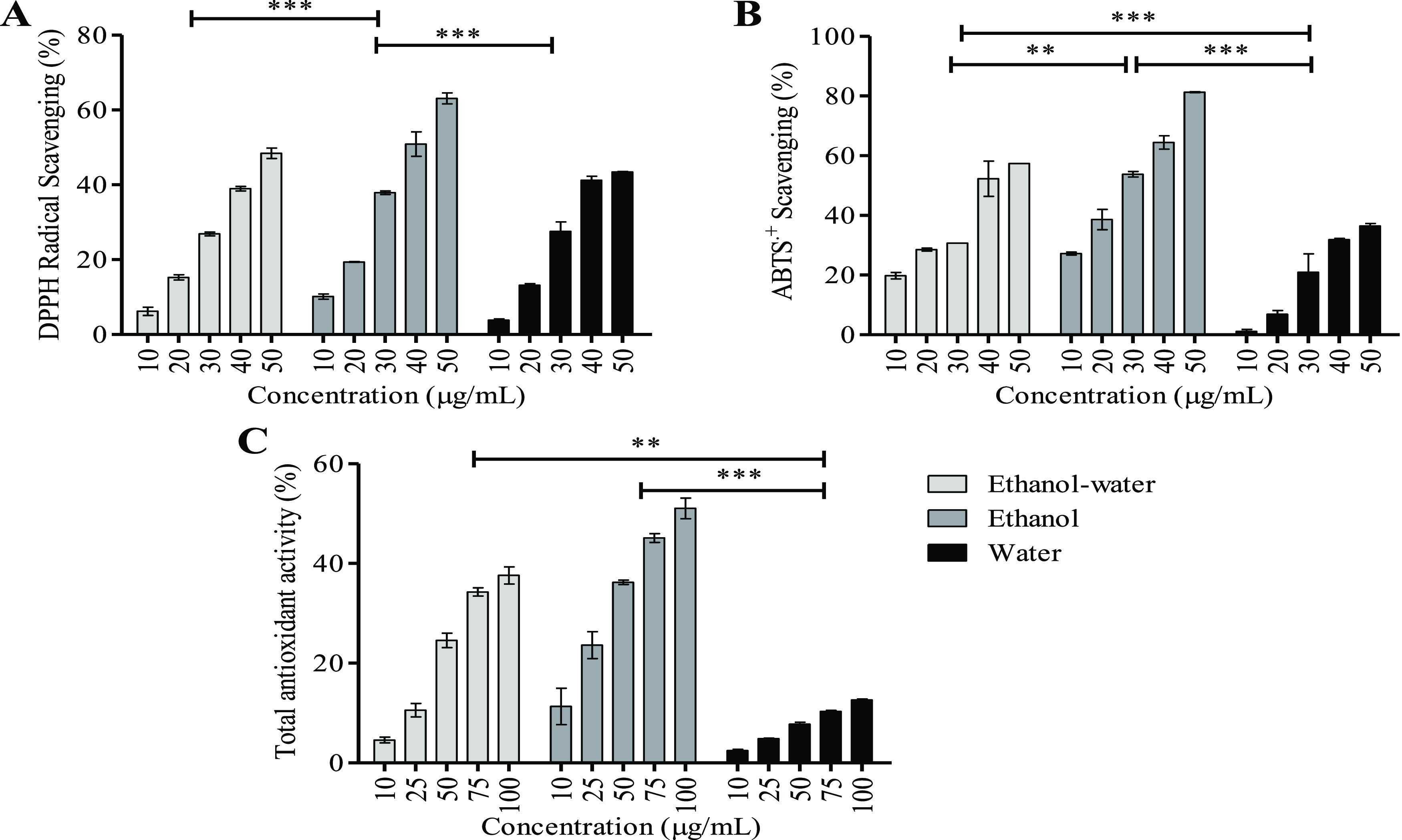
Antioxidant assay. (A) DPPH radical scavenging, (B) ABTS.+ scavenging, and (C) total antioxidant assay. Activities of three different extracts, viz., ethanol–water, ethanol, and water extracts, were evaluated. Values are expressed as mean and SD (n = 3) with significant variation at *p < 0.05, **p < 0.005, and ***p < 0.001, respectively.
4.3.2. ABTS•+ Scavenging Assay
The ABTS•+ [2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)] radical is generated by oxidation of ABTS with potassium persulfate and reduced in the presence of antioxidants.24 ABTS•+ scavenging activities of UAE ethanol extract showed a scavenging effect of 27.70 ± 0.61 μg/mL (IC50) followed by ethanol–water (IC50, 42.38 ± 0.54 μg/mL) and water (IC50, 61.55 ± 1.78 μg/mL) (Figure 4B). VER and its derivatives may undergo multiple pathways to exert their antioxidant action. These antioxidant pathways are involved in electron transport and H+ transfer but not by radical adduct formation.59 VER and its derivatives exhibited significant ABTS•+ scavenging activities in a concentration-dependent manner with an IC50 value of 12.5 ± 1.9 μM.59 Jang et al. reported that VER isolated from Abeliophyllum distichum exhibited ABTS•+ scavenging with IC50 of 6.47 μg/mL.57 Therefore, it was confirmed that the radical scavenging potential of the UAE extracts was highest for the VER-enriched ethanol extracts with a radical scavenging activity of 81.29 ± 0.19% for 50 μg.
4.3.3. PMD Assay
The PMD assay is an antioxidant assay that involves the reduction of Mo(VI) to Mo(V) by antioxidant compounds. The EC50 value of VER present in ethanol UAE was lowest at 90.58 ± 2.09 μg/mL, and it was highest for water (EC50, 433.71 ± 5.02 μg/mL) (Figure 4C). The antioxidant activities of VER are due to the sequential loss of proton to yield a single electron transfer known as the SPLET (sequential proton loss electron transfer) pathway. This pathway proceeds by deprotonation of VER (H+-transfer) yielding an anion that donates an electron to form a phenoxy radical. These phenoxy radicals impart the antioxidant activity through p-π conjugation.55 VER-rich Stachys germanica extracts showed PMD activity with an EC50 of 1.68 mg/mL, which was comparatively lower than this report.60 VER-enriched ethanol extracts showed 100% total antioxidant activity at a concentration of 207.81 μg.
4.4. Enzyme Inhibition Assays
4.4.1. α-Amylase Enzyme Inhibition Assay
α-Amylase enzyme inhibition is considered an important therapeutic target for the regulation of postprandial blood glucose increment in diabetic patients.61 CG leaves have been proven to have an effective antidiabetic potential earlier.62,63 In this context, it was observed that the VER-enriched ethanol extract exhibited significant α-amylase inhibition (IC50, 346.39 ± 5.04 μg/mL) compared to the standard α-amylase inhibitor (acarbose, IC50, 460.65 ± 2.38 μg/mL). Ethanol–water (IC50, 454.09 ± 29.43 μg/mL) extract inhibition was also comparable to acarbose. α-Amylase enzyme inhibition of CG leaf extracted by UAE is shown in Figure 5A. Earlier, a hydromethanolic extract of CG leaves obtained by maceration was reported for α-amylase inhibition with an IC50 of 876.98 ± 11.58 mg/mL.8 The VER-enriched extract from Marrubium vulgare showed an antidiabetic effect of around a 50% decrease in blood glucose at 100 mg/kg dose.64 Molehin et al. reported α-amylase inhibition with IC50 of 0.58 mg/mL against Clerodendrum volubile extract.65 We also investigated the seasonal variation of the α-amylase inhibitory activity of CG leaf and found that samples inhibited the activity, increasing in a concentration-dependent manner in winter and summer (Figure S3).
Figure 5.

Enzyme inhibition assay. (A) α-Amylase, (B) α-glucosidase, and (C) porcine pancreatic lipase. Activities of three different extracts, viz., ethanol–water, ethanol, and water extracts, were evaluated. Values are expressed as mean and SD (n = 3) with significant variations at *p < 0.05, **p < 0.005, and ***p < 0.001, respectively.
4.4.2. α-Glucosidase Enzyme Inhibition Assay
α-Glucosidase inhibitors block the enzymatic degradation of complex carbohydrates in the small intestine and lower postprandial glucose. Acarbose is one of the most used α-glucosidase inhibitors in T2DM therapy.66 In the present study, the VER-enriched ethanol extract showed α-glucosidase inhibitory activity with an IC50 of 195.13 ± 3.26 μg/mL, which indicates better inhibition potential compared to that of the standard inhibitor. All the extracts inhibited α-glucosidase in a concentration-dependent manner as shown in Figure 5B. Previously, CG leaf extract indicated α-glucosidase inhibitory activity (IC50 of 104.11 ± 0.36 mg/mL) as shown by Deb et al.8 VER isolated from Monochasma savatieri exhibited significant α-glucosidase inhibitory activity (IC50 of 0.5 ± 0.03 mM) compared to the standard (acarbose, IC50 of 14.4 ± 0.3 mM).67 In a report, VER from Cistanche tubulosa inhibited postprandial blood glucose in mice and increased glucose tolerance.68 VER isolated from the dried roots of Clerodendrum bungei exhibited stronger α-glucosidase inhibitory effects (IC50 of 0.5 ± 0.03 mmol/L; acarbose, IC50 of 14.4 ± 0.3 mmol/L).69
4.4.3. Pancreatic Lipase Enzyme Inhibition Assay
Pancreatic lipase is considered a target for the prevention and treatment of obesity as it is responsible for the hydrolysis of triglycerides to fatty acids and glycerol.70 VER-enriched ethanol and ethanol–water extracts exhibited significant lipase inhibition with an IC50 of 757.28 ± 13.22 and 734.80 ± 20.17 μg/mL as shown in Figure 5C. Docking-based studies showed that the numbers of hydrogen bonds and phenolic hydroxyl groups play an important role in the binding process of VER with lipase leading to its inhibition.71 Wu et al. reported the inhibitory activity of VER (IC50, 2.17 ± 0.13 μg/mL) on porcine pancreatic lipase isolated from leaves of Ligustrum purpurascens.71 A significant lowering of total lipid, triglyceride, and total cholesterol levels was observed in Wistar rats when treated with VER-rich Marrubium vulgare extracts.64 Phenolic hydroxyl groups present in VER had lipase inhibitory potentials based on the number and position present.72
5. Conclusions
Ultrasound-assisted extraction was effective for the extraction of polyphenols, mainly verbascoside, from C. glandulosum leaves. Among the three solvents used for extraction, ethanol was found to be better than the other solvents (ethanol–water and water). HPLC and LC–MS confirmed the presence of verbascoside among the main polyphenol compound. Different backbone moieties (hydroxytyrosol, glucose, and rhamnose) were confirmed by the proton NMR (1H) and 2D COSY (1H-1H, correlated spectroscopy) analysis. Results suggested that UAE of verbascoside using ethanol from C. glandulosum might be an ideal alternative source for large-scale extraction of verbascoside that exhibited efficient antioxidant and antidiabetic activities.
Acknowledgments
We would like to acknowledge the Bioinformatics Facility (BIF), Institute of Advanced Study in Science and Technology (IASST), and Department of Science and Technology (DST) under the Ministry of Science and Technology, Government of India, New Delhi, India, for providing the necessary support for the implementation and successful completion of the study.
Glossary
List of Abbreviations
- ABTS
2,2′-azinobis-3-ethylbenzenothiazoline-6-sulfonic acid
- CG
Clerodendrum glandulosum Lindl.
- COSY
correlated spectroscopy
- DNS
3,5-dinitrosalicylic acid
- DPPH
1-1-diphenyl-2-picrylhydrazyl radical
- EDTA
ethylenediaminetetraacetic acid
- GAE
gallic acid equivalent
- HMGB1
high mobility group box 1
- HPLC
high-performance liquid chromatography
- HPTLC
high-performance thin layer chromatography
- IC50
inhibitory concentration 50%
- LC–MS
liquid chromatography–mass spectrometry
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NMR
nuclear magnetic resonance
- PKC
protein kinase C
- PMD
phosphomolybdenum
- QE
quercetin equivalent
- RAGE
receptor for advanced glycation end products
- Rf
retention factor
- Rt
retention time
- SPLET
sequential proton loss electron transfer
- TPC
total phenolic content
- TFC
total flavonoid content
- UAE
ultrasound-assisted extraction
- VER
verbascoside
- UV
ultraviolet
- λmax
maximum wavelength
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c00173.
Phenolic content of CG extracts collected in different seasons (Figure S1), 2D-COSY NMR spectrum of the ethanol extract (Figure S2), IC50 values of enzyme inhibition assays (Figure S3, Table S1), and standard curve of verbascoside (Chart S1) (PDF)
Author Present Address
# Current Address: Crystallography & Molecular Biology Division, Saha Institute of Nuclear Physics, Kolkata 700064, West Bengal, India
Author Present Address
§ Current Address: Sophisticated Analytical Instrument Facility, Girjananda Chowdhury Institute of Pharmaceutical Science, Girjananda Chowdhury University, Guwahati 781017, Assam, India
The authors declare no competing financial interest.
Notes
Verbascoside (PubChem CID: 5459010).
Notes
This study did not involve human participants or animals.
Supplementary Material
References
- Cosme P.; Rodríguez A. B.; Espino J.; Garrido M. Plant Phenolics: Bioavailability as a Key Determinant of Their Potential Health-Promoting Applications. Antioxidants 2020, 9, 1–20. 10.3390/ANTIOX9121263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Lorenzo C.; Colombo F.; Biella S.; Stockley C.; Restani P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 1–30. 10.3390/NU13010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad M.; Kumar N. V. A.; Zucca P.; Varoni E. M.; Dini L.; Panzarini E.; Rajkovic J.; Valere Tsouh Fokou P.; Azzini E.; Peluso I.; Prakash Mishra A.; Nigam M.; el Rayess Y.; el Beyrouthy M.; Polito L.; Iriti M.; Martins N.; Martorell M.; Oana Docea A.; Setzer W. N.; Calina D. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol 2020, 11, 694. 10.3389/fphys.2020.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço S. C.; Moldão-Martins M.; Alves V. D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. 10.3390/molecules24224132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X.; Belwal T.; Cravotto G.; Luo Z. Sono-Physical and Sono-Chemical Effects of Ultrasound: Primary Applications in Extraction and Freezing Operations and Influence on Food Components. Ultrason. Sonochem. 2020, 104726 10.1016/j.ultsonch.2019.104726. [DOI] [PubMed] [Google Scholar]
- Maza M.; Álvarez I.; Raso J.. Thermal and Non-Thermal Physical Methods for Improving Polyphenol Extraction in Red Winemaking. Beverages 2019, 5 (), 10.3390/beverages5030047. [DOI] [Google Scholar]
- Chmelová D.; Škulcová D.; Legerská B.; Horník M.; Ondrejovič M. Ultrasonic-Assisted Extraction of Polyphenols and Antioxidants from Picea abies Bark. J. Biotechnol. 2020, 314-315, 25–33. 10.1016/J.JBIOTEC.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Deb P. K.; Khound P.; Bhattacharjee S.; Choudhury P.; Sarma H.; Devi R.; Sarkar B. Variation in Chemical Constituents, in-vitro Bioactivity and Toxicity Profile among Different Parts of Clerodendrum glandulosum Lindl. (C. Colebrookianum Walp.). S. Afr. J. Bot. 2021, 140, 50–61. 10.1016/j.sajb.2021.03.023. [DOI] [Google Scholar]
- Kalita J.; Singh S. S.; Khan M. L. Clerodendrum colebrookianum Walp.: A Potential Folk Medicinal Plant of North East India. Asian J. Pharm. Biol. Res. 2012, 2, 256–261. [Google Scholar]
- Kubica P.; Szopa A.; Kokotkiewicz A.; Miceli N.; Taviano M. F.; Maugeri A.; Cirmi S.; Synowiec A.; Gniewosz M.; Elansary H. O.; Mahmoud E. A.; El-Ansary D. O.; Nasif O.; Luczkiewicz M.; Ekiert H. Production of Verbascoside, Isoverbascoside and Phenolic Acids in Callus, Suspension, and Bioreactor Cultures of Verbena officinalis and Biological Properties of Biomass Extracts. Molecules 2020, 25, 5609. 10.3390/molecules25235609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P. F.; Yu C. C.; Chu P. M.; Hsieh P. L.. Verbascoside Protects Gingival Cells against High Glucose-Induced Oxidative Stress via Pkc/Hmgb1/Rage/Nfκb Pathway. Antioxidants 2021, 10 (), 10.3390/ANTIOX10091445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre Luhata L.; Usuki T. Free Radical Scavenging Activities of Verbascoside and Isoverbascoside from the Leaves of Odontonema strictum (Acanthaceae). Bioorg. Med. Chem. Lett. 2022, 59, 128528 10.1016/J.BMCL.2022.128528. [DOI] [PubMed] [Google Scholar]
- Wong Paz J. E.; Muñiz Márquez D. B.; Martínez Ávila G. C. G.; Belmares Cerda R. E.; Aguilar C. N. Ultrasound-Assisted Extraction of Polyphenols from Native Plants in the Mexican Desert. Ultrason. Sonochem. 2015, 22, 474–481. 10.1016/J.ULTSONCH.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Tung Y. T.; Chang W. C.; Chen P. S.; Chang T. C.; Chang S. T. Ultrasound-Assisted Extraction of Phenolic Antioxidants from Acacia confusa Flowers and Buds. J. Sep. Sci. 2011, 34, 844–851. 10.1002/jssc.201000820. [DOI] [PubMed] [Google Scholar]
- Hasni S.; Rigane G.; Ghazghazi H.; Riguene H.; Bouallegue A.; Khedher O.; Oueslati M. A.; Salem R. B. Optimum Conditions and LC-ESI-MS Analysis of Phenolic Rich Extract from Eucalyptus marginata L. under Maceration and Ultrasound-Assisted Extraction Methods Using Response Surface Methodology. J Food Qual 2021, 2021, 5591022. 10.1155/2021/5591022. [DOI] [Google Scholar]
- Dutta A. Evaluation of Mechanism for Antihypertensive Action of Clerodendrum colebrookianum Walp., Used by Folklore Healers in North-East India. J. Ethnopharmacol. 2012, 143, 207–212. 10.1016/j.jep.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Nath S. C.; Bordoloi D. N. Clerodendrum colebrookianum, a Folk Remedy for the Treatment of Hypertension in Northeastern India. Int. J. Pharm. 1991, 29, 127–129. 10.3109/13880209109082863. [DOI] [Google Scholar]
- Falleh H.; Ksouri R.; Chaieb K.; Karray-Bouraoui N.; Trabelsi N.; Boulaaba M.; Abdelly C. Phenolic Composition of Cynara cardunculus L. Organs, and Their Biological Activities. C R Biol 2008, 331, 372–379. 10.1016/J.CRVI.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Singleton V. L.; Orthofer R.; Lamuela-Raventós R. M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Zhao J.; Maitituersun A.; Li C.; Li Q.; Xu F.; Liu T. Evaluation on Analgesic and Anti-Inflammatory Activities of Total Flavonoids from Juniperus Sabina. Evidence-Based Complementary Altern. Med. 2018, 2018, 7965306. 10.1155/2018/7965306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H.; Seo S.-W.; Song S.-Y.; Oh D.-S.; Shim J.-H.; Yoon G.; Choi Y.-Y.; Kim D.-K.; Kim J.-S.; Yoon I.-S.; Cho S.-S. HPLC Analysis and Antioxidant Evaluation of Acteoside-Rich Osmanthus fragrans Extracts. J. Food Chem. 2020, 8851285. 10.1155/2020/8851285. [DOI] [Google Scholar]
- Gupta S.; Kumar S.; Saraf I.; Dhage A. H.; Upadhyay R.; Chatterjee A.; Singh I. P. Quantification of Acteoside in Clerodendrum colebrookianum Walp.by QNMR. HPLC and HPTLC. Trends Carbohydr. Res. 2016, 8, 15–23. [Google Scholar]
- Blois M. S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. 10.1038/1811199a0. [DOI] [Google Scholar]
- Re R.; Pellegrini N.; Proteggente A.; Pannala A.; Yang M.; Rice-Evans C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radical Biol. Med. 1999, 26, 1231–1237. 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Prieto P.; Pineda M.; Aguilar M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. 10.1006/ABIO.1999.4019. [DOI] [PubMed] [Google Scholar]
- Bendelow V. M. Modified Procedure for the Determination of Diastatic Activity and α-Amylase Activity. J. Inst. Brew. 1963, 69, 467–472. 10.1002/j.2050-0416.1963.tb01954.x. [DOI] [Google Scholar]
- Costantino H. R.; Brown S. H.; Kelly R. M. Purification and Characterization of an α-Glucosidase from a Hyperthermophilic Archaebacterium, Pyrococcus furiosus, Exhibiting a Temperature Optimum of 105 to 115°C. J. Bacteriol. 1990, 172, 3654–3660. 10.1128/jb.172.7.3654-3660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N.; Sharma V. K.; Seo S. Y. Screening of Some Medicinal Plants for Anti-Lipase Activity. J. Ethnopharmacol. 2005, 97, 453–456. 10.1016/J.JEP.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Dhanani T.; Shah S.; Gajbhiye N. A.; Kumar S. Effect of Extraction Methods on Yield, Phytochemical Constituents and Antioxidant Activity of Withania somnifera. Arabian Journal of Chemistry 2017, 10, S1193–S1199. 10.1016/J.ARABJC.2013.02.015. [DOI] [Google Scholar]
- Karabegović I. T.; Stojičević S. S.; Veličković D. T.; Todorović Z. B.; Nikolić N. Č.; Lazić M. L. The Effect of Different Extraction Techniques on the Composition and Antioxidant Activity of Cherry Laurel (Prunus laurocerasus) Leaf and Fruit Extracts. Ind. Crops Prod. 2014, 54, 142–148. 10.1016/j.indcrop.2013.12.047. [DOI] [Google Scholar]
- Truong D. H.; Nguyen D. H.; Ta N. T. A.; Bui A. V.; Do T. H.; Nguyen H. C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and in vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Quality 2019, 2019, 1. 10.1155/2019/8178294. [DOI] [Google Scholar]
- Lezoul N. E. H.; Belkadi M.; Habibi F.; Guillén F.. Extraction Processes with Several Solvents on Total Bioactive Compounds in Different Organs of Three Medicinal Plants. Molecules 2020, 25 (), 10.3390/molecules25204672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salih A. M.; Al-Qurainy F.; Nadeem M.; Tarroum M.; Khan S.; Shaikhaldein H. O.; Al-Hashimi A.; Alfagham A.; Alkahtani J.. Optimization Method for Phenolic Compounds Extraction from Medicinal Plant (Juniperus procera) and Phytochemicals Screening. Molecules 2021, 26 (), 10.3390/molecules26247454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C.; Wu Z.; Wang Z.; Zhang H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. J. Evidence-Based Complementary Altern. Med. 2015, 2015, 1. 10.1155/2015/595393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugna A. Z.; Gurni A. A.; Wagner M. L. Phenological Variations of Polyphenols in Smilax campestris (Smilacaceae). Turk. J. Botany 2013, 37, 350–354. 10.3906/bot-1112-15. [DOI] [Google Scholar]
- Adegbaju O. D.; Otunola G. A.; Afolayan A. J. Effects of Growth Stage and Seasons on the Phytochemical Content and Antioxidant Activities of Crude Extracts of Celosia argentea L. Heliyon 2020, 6, e04086 10.1016/j.heliyon.2020.e04086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimoh M. O.; Afolayan A. J.; Lewu F. B. Antioxidant and Phytochemical Activities of Amaranthus caudatus L. Harvested from Different Soils at Various Growth Stages. Sci. Rep. 2019, 9, 12965. 10.1038/s41598-019-49276-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro H. G.; Casali V. W. D.; Barbosa L. C. A.; Cecon P. R. Rendimento de Tanino Em Dois Acessos de Carqueja (Baccharis myriocephala DC), Em Diferentes Épocas de Colheita Em Viçosa-MG. Rev. Bras. Plant. Med. 1999, 1, 29–33. [Google Scholar]
- Pourcel L.; Routaboul J. M.; Cheynier V.; Lepiniec L.; Debeaujon I. Flavonoid Oxidation in Plants: From Biochemical Properties to Physiological Functions. Trends Plant Sci. 2007, 29–36. 10.1016/j.tplants.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Zoratti L.; Karppinen K.; Luengo Escobar A.; Häggman H.; Jaakola L.; Damiani F.; Yonekura-Sakakibara K.; Schmeda-Hirschmann G. Light-Controlled Flavonoid Biosynthesis in Fruits. Front. Plant Sci. 2014, 5, 534. 10.3389/fpls.2014.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backes E.; Pereira C.; Barros L.; Prieto M. A.; Genena A. K.; Barreiro M. F.; Ferreira I. C. F. R. Recovery of Bioactive Anthocyanin Pigments from Ficus carica L. Peel by Heat, Microwave, and Ultrasound Based Extraction Techniques. Food Res. Int. 2018, 113, 197–209. 10.1016/j.foodres.2018.07.016. [DOI] [PubMed] [Google Scholar]
- Mulinacci N.; Prucher D.; Peruzzi M.; Romani A.; Pinelli P.; Giaccherini C.; Vincieri F. F. Commercial and Laboratory Extracts from Artichoke Leaves: Estimation of Caffeoyl Esters and Flavonoidic Compounds Content. J. Pharm. Biomed. Anal. 2004, 34, 349–357. 10.1016/S0731-7085(03)00552-1. [DOI] [PubMed] [Google Scholar]
- Chaves J. O.; de Souza M. C.; da Silva L. C.; Lachos-Perez D.; Torres-Mayanga P. C.; Machado A. P. D. F.; Forster-Carneiro T.; Vázquez-Espinosa M.; González-de-Peredo A. V.; Barbero G. F.; Rostagno M. A. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 10.3389/fchem.2020.507887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alipieva K.; Korkina L.; Orhan I. E.; Georgiev M. I. Verbascoside - A Review of Its Occurrence, (Bio)Synthesis and Pharmacological Significance. Biotechnol. Adv. 2014, 32, 1065–1076. 10.1016/j.biotechadv.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Blazics B.; Alberti Á.; Béni S.; Kursinszki L.; Tölgyesi L.; Kéry Á. Identification and LC–MS–MS Determination of Acteoside, the Main Antioxidant Compound of Euphrasia rostkoviana, Using the Isolated Target Analyte as External Standard. J. Chromatogr. Sci. 2011, 49, 203–208. 10.1093/chrsci/49.3.203. [DOI] [Google Scholar]
- Attia Y. M.; El-Kersh D. M.; Wagdy H. A.; Elmazar M. M. Verbascoside: Identification, Quantification, and Potential Sensitization of Colorectal Cancer Cells to 5-FU by Targeting PI3K/AKT Pathway. Sci. Rep. 2018, 8, 16939. 10.1038/s41598-018-35083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sut S.; Tahmasebi A.; Ferri N.; Ferrarese I.; Rossi I.; Panighel G.; Lupo M. G.; Maggi F.; Karami A.; Dall’acqua S.. NMR, LC-MS Characterization of Rydingia michauxii Extracts, Identification of Natural Products Acting as Modulators of LDLR and PCSK9. Molecules 2022, 27 (), 10.3390/molecules27072256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovska D. I.; Zhiponova M. K.; Georgiev M. I.; Alipieva K.; Tsacheva I.; Simova S.; Yordanova Z. P.. Biological Activity and NMR-Fingerprinting of Balkan Endemic Species Stachys thracica Davidov. Metabolites 2022, 12 (), 10.3390/metabo12030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou P.; Song Y.; Lei W.; Li J.; Tu P.; Jiang Y. Application of 1H NMR-Based Metabolomics for Discrimination of Different Parts and Development of a New Processing Workflow for Cistanche deserticola. Acta Pharm. Sin. B 2017, 7, 647–656. 10.1016/j.apsb.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Tsao R.; Liu Z.; Liu S.; Yang R.; Young J. C.; Zhu H.; Deng Z.; Xie M.; Fu Z. Isolation and Purification of Acteoside and Isoacteoside from Plantago psyllium L. by High-Speed Counter-Current Chromatography. J. Chromatogr. A 2005, 1063, 161–169. 10.1016/j.chroma.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Zaki L. H.; Mohamed S. M.; Bashandy S. A. E.; Morsy F. A.; Tawfik K. M.; Shahat A. A. Hypoglycemic and Antioxidant Effects of Hibiscus rosa-sinensis L. Leaves Extract on Liver and Kidney Damage in Streptozotocin Induced Diabetic Rats. Afr. J. Pharm. Pharmacol. 2017, 11, 161–169. 10.5897/ajpp2017.4764. [DOI] [Google Scholar]
- Dirar A. I.; Wada M.; Watanabe T.; Devkota H. P. Phenolic Compounds from the Aerial Parts of Blepharis linariifolia Pers. and Their Free Radical Scavenging and Enzyme Inhibitory Activities. Medicines 2019, 6, 113. 10.3390/medicines6040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Q. D.; Angkawijaya A. E.; Tran-Nguyen P. L.; Huynh L. H.; Soetaredjo F. E.; Ismadji S.; Ju Y. H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiev M. I.; Ludwig-Müller J.; Alipieva K.; Lippert A. Sonication-Assisted Agrobacterium Rhizogenes-Mediated Transformation of Verbascum xanthophoeniceum Griseb. for Bioactive Metabolite Accumulation. Plant Cell Rep. 2011, 30, 859–866. 10.1007/s00299-010-0981-y. [DOI] [PubMed] [Google Scholar]
- López-Munguía A.; Hernandez-Romero Y.; Pedraza-Chaverri J.; Miranda-Molina A.; Regla I.; Martinez A.; Castillo E. Phenylpropanoid Glycoside Analogues: Enzymatic Synthesis, Antioxidant Activity and Theoretical Study of Their Free Radical Scavenger Mechanism. PLoS One 2011, 6, e20115 10.1371/journal.pone.0020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos C.; Muñoz-Mingarro D.; Navarro I.; Martín-Cordero C.; Acero N. Neuroprotective Potential of Verbascoside Isolated from Acanthus mollis L. Leaves through Its Enzymatic Inhibition and Free Radical Scavenging Ability. Antioxidants 2020, 9, 1–16. 10.3390/antiox9121207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang T. W.; Choi J. S.; Park J. H. Protective and Inhibitory Effects of Acteoside from Abeliophyllum distichum Nakai against Oxidative DNA Damage. Mol. Med. Rep. 2020, 22, 2076–2084. 10.3892/mmr.2020.11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirantes-Piné R.; Herranz-López M.; Funes L.; Borrás-Linares I.; Micol V.; Segura-Carretero A.; Fernández-Gutiérrez A. Phenylpropanoids and Their Metabolites Are the Major Compounds Responsible for Blood-Cell Protection against Oxidative Stress after Administration of Lippia citriodora in Rats. Phytomedicine 2013, 20, 1112–1118. 10.1016/J.PHYMED.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Li X.; Xie Y.; Li K.; Wu A.; Xie H.; Guo Q.; Xue P.; Maleshibek Y.; Zhao W.; Guo J.; Chen D.. Antioxidation and Cytoprotection of Acteoside and Its Derivatives: Comparison and Mechanistic Chemistry. Molecules 2018, 23 (), 10.3390/molecules23020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carev I.; Sarikurkcu C. LC-MS/MS Profiles and in vitro Biological Activities of Extracts of an Endemic Species from Turkey: Stachys cretica Ssp. Anatolica 2021, 10.3390/plants10061054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur N.; Kumar V.; Nayak S. K.; Wadhwa P.; Kaur P.; Sahu S. K. α-Amylase as Molecular Target for Treatment of Diabetes Mellitus: A Comprehensive Review. Chem. Biol. Drug Des. 2021, 539–560. 10.1111/cbdd.13909. [DOI] [PubMed] [Google Scholar]
- Sharma H. K.; Chhangte L.; Dolui A. K. Traditional Medicinal Plants in Mizoram, India. Fitoterapia 2001, 72, 146–161. 10.1016/S0367-326X(00)00278-1. [DOI] [PubMed] [Google Scholar]
- Jadeja R. N.; Thounaojam M. C.; Patel V. B. aProtective Effect of Clerodendron glandulosum Extract against Experimentally Induced Metabolic Syndrome in Rats. Pharm. Biol. 2010, 48, 1312–1319. 10.3109/13880201003739304. [DOI] [PubMed] [Google Scholar]
- Boudjelal A.; Henchiri C.; Siracusa L.; Sari M.; Ruberto G. Compositional Analysis and in vivo Anti-Diabetic Activity of Wild Algerian Marrubium vulgare L. Infusion. Fitoterapia 2012, 83, 286–292. 10.1016/j.fitote.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Molehin O. R.; Oloyede O. I.; Adefegha S. A. Streptozotocin-Induced Diabetes in Rats: Effects of White Butterfly (Clerodendrum volubile) Leaves on Blood Glucose Levels, Lipid Profile and Antioxidant Status. Toxicol. Mech. Methods 2018, 28, 573–586. 10.1080/15376516.2018.1479476. [DOI] [PubMed] [Google Scholar]
- Akmal M.; Wadhwa R.. Alpha Glucosidase Inhibitors. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557848/?report=printable. [PubMed] [Google Scholar]
- Liu Y. L.; He W. J.; Mo L.; Shi M. F.; Zhu Y. Y.; Pan S.; Li X. R.; Xu Q. M.; Yang S. L. Antimicrobial, Anti-Inflammatory Activities and Toxicology of Phenylethanoid Glycosides from Monochasma savatieri Franch. Ex Maxim. J. Ethnopharmacol. 2013, 149, 431–437. 10.1016/j.jep.2013.06.042. [DOI] [PubMed] [Google Scholar]
- Morikawa T.; Ninomiya K.; Imamura M.; Akaki J.; Fujikura S.; Pan Y.; Yuan D.; Yoshikawa M.; Jia X.; Li Z.; Muraoka O. Acylated Phenylethanoid Glycosides, Echinacoside and Acteoside from Cistanche tubulosa, Improve Glucose Tolerance in Mice. J Nat Med 2014, 68, 561–566. 10.1007/s11418-014-0837-9. [DOI] [PubMed] [Google Scholar]
- Liu Q.; Hu H. J.; Li P. F.; Yang Y. B.; Wu L. H.; Chou G. X.; Wang Z. T. Diterpenoids and Phenylethanoid Glycosides from the Roots of Clerodendrum bungei and Their Inhibitory Effects against Angiotensin Converting Enzyme and α-Glucosidase. Phytochemistry 2014, 103, 196–202. 10.1016/J.PHYTOCHEM.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Mermer A.; Demirci S.; Tatar G. Synthesis of Novel Pancreatic Lipase Inhibitors: Biological Investigation and in silico Studies. J. Biomol. Struct. Dyn. 2022, 40, 931–940. 10.1080/07391102.2021.1950573. [DOI] [PubMed] [Google Scholar]
- Wu X.; Feng Y.; Lu Y.; Li Y.; Fan L.; Liu L.; Wu K.; Wang X.; Zhang B.; He Z. Effect of Phenolic Hydroxyl Groups on Inhibitory Activities of Phenylpropanoid Glycosides against Lipase. J. Funct. Foods 2017, 38, 510–518. 10.1016/j.jff.2017.09.022. [DOI] [Google Scholar]
- Buchholz T.; Melzig M. F. Polyphenolic Compounds as Pancreatic Lipase Inhibitors. Planta Med. 2015, 81, 771–783. 10.1055/s-0035-1546173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




