Abstract
Background
Recent studies mention a need to investigate partnership roles and dynamics within patient and public involvement and engagement (PPIE) in health research, and how impact and outcomes are achieved. Many labels exist to describe involvement processes, but it is unknown whether the label has implications on partnerships and outcomes. This rapid review investigates how roles between patients, relatives and researchers in a broad variety of PPIE activities in health research are described in peer reviewed papers and explores what enables these partnerships.
Methods
Rapid review of articles published between 2012 and February 2022 describing, evaluating, or reflecting on experiences of PPIE in health research. All research disciplines and research areas were eligible. Four databases (Medline, Embase, PsychInfo and CINAHL) were searched between November 2021 and February 2022. We followed PRISMA guidelines and extracted descriptive factors: year, origin, research area and discipline, study focus, framework used and co-authorship. On a selection of articles, we performed a narrative analysis of partnership roles using Smits et al.’s. Involvement Matrix. Lastly, we performed a meta synthesis of reported enablers and outcomes of the partnerships. Patients and Relatives (PRs) have been involved in the whole rapid review process and are co-authors of this article.
Results
Seventy articles from various research disciplines and areas were included. Forty articles were selected for a narrative analysis of the role description of PRs and researchers, and a meta synthesis of enablers and outcomes. Most articles described researchers as decision-makers throughout the research cycle. PRs most often were partners when they were included as co-authors; they were mostly partners in the design, analysis, write-up, and dissemination stages. Enablers of partnerships included: PR training, personality of PRs and communication skills, trust, remuneration and time.
Conclusions
Researchers’ decision-making roles gives them control of where and when to include PRs in their projects. Co-authorship is a way of acknowledging patients’ contributions which may lead to legitimation of their knowledge and the partnership. Authors describe common enablers, which can help future partnership formation.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40900-023-00448-z.
Keywords: Patient and public involvement, Coproduction, Partnerships, Evaluation, Rapid review, Co-authorship, Health research
Plain language summary
This article investigates how other articles describe the roles patients, relatives and researchers have in patient and public involvement activities in health research. It also investigates which factors are supportive of creating these research partnerships. We searched four health research databases and found 70 relevant articles which somehow evaluated patient involvement activities in research. From these 70 articles we chose 40 which we closely investigated for descriptions of roles in the partnerships between researchers and patients and relatives. For this, we used a tool called the Involvement Matrix which uses five different roles: Listener (who is given information), Co-thinker (who is asked to give opinion), Advisor (who gives (un)solicited advice), Partner (who works as an equal partner) and Decision-maker (Who takes initiative and (final) decisions). We found that it is often researchers who take on the role of Decision-maker and that involvement often happens on their terms. We noticed that patients and relatives most often had the role of partner, when they were listed as co-authors of the article. This shows co-authorship as an authorization of their work during patient and public involvement activities. We found that patient and relative training, patients’ and relatives’ personality and communication skills, trust, financial reimbursement, and time were mentioned most often as enablers of good research partnerships.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40900-023-00448-z.
Dansk lægmandssresumé
Denne artikel undersøger, hvordan andre artikler beskriver de roller patienter, pårørende og forskere har i patientinvolveringsaktiviteter i sundhedsforskning. Den undersøger også, hvilke faktorer, der virker understøttende for disse forskningspartnerskaber. Vi søgte i fire databaser for sundhedsforskning og fandt 70 artikler, der evaluerer patientinvolvering i forskning. Vi udvalgte 40 ud af de 70 artikler og undersøgte dem for, hvordan de beskrev patienternes, de pårørendes og forskernes roller i forskerpartnerskaberne. Til dette brugte vi en involverings-model, der deler mulige roller op i: Lytter (en der får information), Medtænker (en, der bliver spurgt om en holdning), Rådgiver (en, der giver råd), Partner (en, der arbejder som ligeværdig partner) og Beslutningstager (en, der tager initiativ og kan træffe endelige beslutninger). Vores undersøgelse viste, at det i høj grad er forskerne, der er Beslutningstagere i forskningspartnerskaberne og at inddragelse sker på deres foranledning. Patienter og pårørende var oftest Partnere, når de også var medforfattere på artiklerne og dermed bliver medforfatterskab en bekræftelse af patienternes arbejde. Vi så også efter elementer, der kan understøtte partnerskaberne og fandt at træning af patienter og pårørende, personlige- og kommunikative evner, tillid, dækning af udgifter eller betaling, samt tid var de faktorer, der oftest blev nævn som understøttende for gode samarbejder.
Contributions by patient partners
This rapid review was conducted with a group of patients and relatives (PRs) (AKS, KB, KEB and TA), each of them are / have been a patient or carer to a patient in Denmark. Table 1 and Additional file 1: Appendix D have been developed by AKS and AWK. AKS has been part of the development of study objectives, protocol, and discussions on synthesis strategy. She has read 11 articles [1–11]; she used those to populate the tables as well as provide analytical reflections. Patient partners KB, KEB and TA read 3 Danish articles [6–8] and provided analytical reflections on these. AWK developed an open question matrix to support their work with the articles. All patient partners actively contributed to the development of the column “Patient partner observations” and brought insights to the analysis and discussion below (If the reader is interested in more information on our collaborative processes, see Additional file 1: Appendix B for a GRIPP2 short form and overview of our involvement roles using the Involvement Matrix).
Table 1.
Overview of included articles
| Author | Country | Design | Area | Cycle | Focus of article | Partner group | Framework for inclusion | Themes | Co-author-ship |
|---|---|---|---|---|---|---|---|---|---|
| Abrehart [12] | UK | Feasibility study | Pediatrics | Whole | Development of a medical imaging test for children with constipation | Children aged 8–18 | GRIPP2 | Relations through shared endeavor/ Learning/ Direct impact/ Consistency/ Impact on children’s development/ Building rapport | Yes |
| *Alexander [13] | USA | Mixed methods | Pediatrics | Whole | Childhood obesity—Evaluation of a childhood obesity treatment pilot program | Parents of obese children | CBPR | Facilitating/ Definition of success/ Training/Skills used in everyday life/ From stakeholder to PR | No |
| Anang [14] | Canada | Qualitative | Mental health | Whole | Suicide prevention among Inuit Youth | Youth living in suicide risk communities | CBPR | Community partnership/ Training/ Employment/ Sense of ownership/ Vulnerable group | No |
| *Barn [15] | Canada | Evaluation | Pulmonology | Agendas, Governance | Asthma and COPD—evaluation of a PR group as resource for several research projects | Patients with Asthma and COPD | iKT | Confidence to contribute/Training/Peer support/PEIRS-22 evaluation tool | Yes |
| Beeker [16] | Germany | Mixed methods | Mental health | Whole (after protocol) | Qualitative description of a process evaluations of innovative models of psychiatric care in Germany | Mental health service users | Not specified | Structural organization/ Routines/ Supervision/ Tandeming/ Heterogeneity/ Conflicts/ Power/ Personal growth | Yes |
| *Beighton [17] | UK | Register study | Health Services | Outcome measures, Analysis, Dissemi-nation | Data analysis of effectiveness of annual health checks for adults with intellectual disabilities | Adults with intellectual disabilities, carers | Participatory approach | Longstanding relationships/ Authenticity/ Legitimacy/ Challenges/ Dissemination/ Increased confidence | No |
| Birch [18] | Europe | Mixed methods | Rheumatology | Predetermined project areas | Multi-site European study on biomarkers for early detection of arthritis to predict development | Patients with arthritis and relatives | Not specified | Involvement in specific tasks/ Impact on study process/ Training/ Feedback/ Impact on dissemination/ Inability to contribute | Yes |
| *Bourque [19] | Canada | Mixed methods | Oncology | Whole | Survivor needs of adolescent and young adults with brain tumors | Parents of—and youth with prior brain tumors | The Nesting Dolls Design | ‘Sherpas’ as stakeholders/ Empowerment/ Inclusion in whole process/ Capability to participate and facilitation of this/ Adapting and sharing knowledge | Yes |
| Brutt [20] | Germany | Review | Mental health | Outcomes, Analysis, Lay Summary | PPIE in a systematic review on metacognitive interventions | Mental health service users | Not specified | Focus group discussions/ Predefined protocol/ Remuneration | No |
| *Burrows [21] | UK | Mixed methods | Digital health | Design | Evaluation of collaboration in a large digital development study on home health technology | Not specified/ Advisory group | Gradinger et al.’s value systems/ GRIPP2 | Advisory groups/ Systematic approach to involvement/ Value systems | No |
| *Carr [22] | Canada | Survey | Rheuma-tology | Whole (after topic selection) | Co-design of patient experience survey | Patients with arthritis | PED framework | Peer-to peer with PaCER facilitators/ Online meetings/ Recommendations to research group | Yes |
| Castensøe-Seidenfaden [23] | Denmark | Mixed methods | Digital health | Whole | Development of app for self-management of type 1 diabetes mellitus | Young people with type 1 diabetes | Participatory Design | Conflict solving/ Value of diverse teams/ Separate steering group and PRs | No |
| *Chiu [24] | USA | Mixed methods | Oncology | Whole | Psychosocial impact of participating in activities (dragon boating) for breast cancer survivors | Breast cancer survivors | Participatory research | Development of survey/ Power sharing/ Mutual benefit/ Reciprocity | No |
| *Cook [25] | UK | Service design | Mental health | Whole (after topic selection) | Creating a Mindfulness-based course to support parent carers | Parent carers for adults with learning disabilities | Action Research/ Participatory Health research | Communicative spaces/ Impact: researchers, project | No |
| *Dawson [26] | UK | Qualitative | Minority health | Whole | PPIE in a doctoral project on inclusion of minority groups in PPI | BAME | INVOLVE | Relationship development/ PR reflections/ Researcher reflections/ Mapping research values | Yes |
| Dennehy [27] | Ireland | Qualitative | Digital health | Whole | Evaluation of the work an advisory group of young people did in a qualitative research project | Young people at risk of cyber bullying | Lundy’s Model of Participation | Voice/ Influence/ Right to be included | No |
| *Devonport [28] | UK | Intervention | Obesity and binge eating | Design, Analysis | Reflections on an intervention study on emotional eating with patients and practitioners | Patients with binge eating disorders/ weight management | INVOLVE | Group dynamics/ Development of relationships/ Difference in knowledge and what is shared/ Rights-based approach | No |
| Dewa [29] | UK | Qualitative | Mental health | Whole | Reflections on co-production of a qualitative interview study | 7 Young people with mental health difficulties | McPin Foundation Priority Setting/ James Lind Alliance | Describing the study against principles of coproduction/ Recommendations/ Coproducing interviews and analysis | Yes |
| *de Wit [30] | Nether-lands | Evaluation | Rheuma-tology | Predeter-mined project areas | Evaluation of a pilot study model for structural involvement in rheumatology research | Patients with arthritis | FIRST model | Challenges/ Guidance/ Researcher and patient needs/ structural partnerships | No |
| Dovey-Pearce [31] | UK | Service design | Health Services | Unclear | Reflections on a 5-year longitudinal health research program supporting young people transition to adulthood | People with intellectual disabilities and carers | INVOLVE/ GRIPP | Longstanding relationships/ Learning outcomes | Yes |
| *Faulkner [32] | UK | Qualitative | Mental health | Whole | Building capacity to support mental health service users’ experiences of hate crimes | Survivors of hate crime due to disabilities | Survivor research/ INVOLVE | Description of methods and process to demonstrate value of the approach/ User-led research/ Legitimacy/ Program development/ | Yes |
| *Frankena [33] | Europe | Case study | Several areas | Whole over several projects | Evaluation of 4 European case studies in health research including people with intellectual disabilities | People with intellectual disabilities | Rohlfing’s integrative framework | Partnership development/ Outcomes for all partners and projects/ Legitimacy | Yes |
| *Froggatt [34] | UK | Mixed methods | Health services | Design, Delivery | Qualitative evaluation of PR involvement in an evaluation on health promotion in primary care | Care home residents | APPROACH | Benefits/ Relationships/ PR management | No |
| *Gammon [35] | Norway | Service design | Mental health | Idea, Design | Case study evaluation of service user involvement in the design phase of an online tool for self help | People with mental illness | CBPR | Benefits for study and participants/ Partnerships/ Mutual learning/ Legitimacy | Yes |
| Grant [36] | UK | HTA | Digital health | Design | Developing E-mental health platforms for school children | Young school children | Not specified | Impact: project/ Focus groups | No |
| Grundry [37] | UK | Qualitative | Mental health | Whole | PRO-measures on quality of life in mental health service users | Mental health service users | Not specified | Impact/ Evaluation | Yes |
| Gupta [38] | UK | Case study | Mental health | Dissemination | Case study of the development of dissemination materials on mental health research to the public | Mental health service users | Not specified | User-researcher interactions/ Benefits/ Challenges/ Dissemination/ Empowerment/ Low- and middle-income countries | No |
| *Hitchen [39] | UK | Service design | Mental health | Whole (after topic selection) | Integrating user and carer views on implementation of self-directed support | Mental health service users, carers | Action Research | Professional talk/ Trust/ Legitimacy/ Empowerment/ Benefits/ Shared learning/ Safe spaces/ Remuneration | No |
| *Hoekstra [40] | USA | Qualitative | Neurology | Design, Analysis, Dissemination | Qualitative investigation of patients and researchers’ experiences participating in spinal cord research | Spinal cord injury patients | iKT | Dissemination/ Building knowledge together/ Researcher and participant reasons to PPIE/ Building relationships/ Valuing perspectives/ Role models | Yes |
| *Honey [41] | Australia | Evaluation | Mental health | Whole | Evaluation of a consumer-led evaluation of a mental health program | Prior mental health patients | Collaborative auto-ethnography | Reflexivity and its impact on partnerships/ Academics with history of mental illness | Yes |
| *Hutchinson [42] | UK | Qualitative | Mental health | Whole | Qualitative research on mental health users’ experiences being co-researchers in an IPA study | Mental health service users | PAR | Empowerment/ Transformation/ Impact: personal/ Reframing a narrative/ User-researchers | No |
| *Jewell [43] | UK | Register study | Mental health | Predete-mined project areas | Evaluation of a service user and carer advisory group for mental health data linkage research | Mental service users | GRIPP | Advisory boards as point of contact for researchers/ Researcher behavior/ Impact: projects, researchers/ Training | No |
| *Jørgensen [6] | Denmark | Service design | Oncology | Whole | Evaluation of PPIE in a project focusing on empowerment and development of PRO.measures | Patients with cancer | GRIPP2 | Discussion of methods and practice/ Views and experiences of researchers and patients from the study/ Type of patients involved/ Local context | No |
| Kara [44] | UK | Review | Mental health | Whole | Coproduced literature search and evaluation of a mental health carers research reference group | Carers of mental health service users | Participatory evaluation | PR-lead research/ Advisory groups in research/ Impact: projects/ Organizational space for advisory groups/ Benefits | No |
| *Kearns [45] | UK | Survey | Neurology | Design | Development of a questionnaire for future use in aphasia rehabilitation | People with aphasia | INVOVLE | Group dynamics/ Experiences/ Development of discussions/ Goals/ Researcher self-reflection | No |
| Lammons [46] | UK | RCT | Pediatrics | Design | Evaluation of PPI in first phase of an RCT study on preterm nutritional care | Former neonatal intensive care patients and parents | Not specified | PR view on RCT process/ Legitimacy from emotions/ Researcher hesitations | No |
| Leese [47] | Canada | Qualitative | Rheumatology | Whole over several projects | Co-produced study on PRs experience of PR-researcher relationships in health research | Patients with arthritis | Not specified | Patient experience/ Being heard/ Legitimacy/ Co-building social relations/ Hard work for both parties/ Ethics/ PR-led research | Yes |
| Liabo [48] | UK | Qualitative | Various | Whole over several projects | Qualitative co-produced self-evaluation of three public involvement health research groups | Patients, carers, members of public involvement groups | Not specified | Values in practice/ Principles of involvement in practice/ Challenges | Yes |
| Lincoln [49] | USA | Qualitative | Mental health | Whole | Qualitative interview study of needs when transitioning from child to adulthood with mental health issues | Young adults with a history of mental illness | CBPR | Training/ Relationships/ Impact: project/ Remuneration/ Giving voice to underrepresented groups | No |
| *Lindblom [11] | Sweden | Qualitative | Rehabilitation | Design | Co-design of person-centered transition from hospital to home | Patients with stroke and relatives | Arnstein’s ladder/Human-centered approach | Roles and power/ Shared understanding/ Participation via interaction/ Flexibility/ Types of researchers | No |
| Locock [50] | UK | Qualitative | Mental health | Analysis | Evaluation of PR involvement in analysis of qualitative interview study on quality improvement | Mental health service users and stroke patients | Not specified | Empowerment/ Impact: researchers, PRs, project/ Training | Yes |
| *Marks [3] | UK | Mixed methods | Nephrology | Whole (after topic selection) | Personal reflection on participation in a patient experience improvement study in the renal field | A patient with a renal condition | GRIPP2/ INVOLVE | Difference between advisory group and co-researcher/ Reflections on different stages of research cycle/ Role as co-researcher | Yes |
| *Melchior [51] | Nether-lands | Qualitative | Palliative care | Whole | Qualitative study on PPIE processes in 10 studies | Patients, caregivers not further specified | PAR | Participation cultures: Relationship, task and control/ Impacts of culture/ Relationships | Not clear |
| *Miah [1] | Europe | Mixed methods | Dementia Research | Whole | Qualitative evaluation of PPIE impact in a multi-site dementia research program | People with dementia and their carers | GRIPP 2 | Impact: project, PRs, researchers, personal/ Training/ Resources/ Multi-national project | No |
| Minouge [2] | UK | Service design | Health Services | Whole | Development of a training package for PPIE in health research | Not specified | Not specified | PR-led research/ Experiential knowledge/ Partnership dynamics/ Structural changes | No |
| Mjøsund [52] | Norway | Qualitative | Mental health | Whole | Description and evaluation of analysis methodology in a mental health promotion project | Mental health service users or their carers | Not specified | PR improves quality of research/ Role of PR-team/ Using interpretive phenomenological analysis/ Power of multiple perspectives/ Skills | Yes |
| Mockford [5] | UK | Qualitative | Dementia Research | Whole | Development of service user-led recommendations around discharge from acute care to community care | Patients with Alzheimer | Not specified | Structural changes/ Organizational culture/ Training/ Remuneration/ Motivation | Yes |
| *Nichols [53] | UK | Feasibility study | Neurology | Whole | Chronic headache management—Evaluation of a self-management program development | Patients with chronic headaches | GRIPP2 | PPIE in whole process/ Equality/ Using skills for right tasks/ Roles/ Rules of engagement/ Building relationship/ Remuneration | No |
| *Nierse [10] | Nether-lands | Agenda setting | Nephrology | Agenda | Research agenda setting with patients in a patient organization | Patients with chronic kidney disease | Responsive methodology | Group dynamics/ Dialogue/ Researchers as facilitators/ Empowerment / Building bridges between science and society | Yes |
| *Nissen [9] | Denmark | Feasibility study | Oncology | Predetermined project areas | Cancer rehabilitation—a feasibility study of a psychosocial mindfulness intervention | Patients with breast and prostate cancer | INVOLVE | Impact on project/Structural organization of PPIE / Challenges | No |
| Noyes [54] | UK | Service design | Health Services | Whole | Report co-productive strategies for a qualitative evaluation of a new soft opt-out system on organ donation | Family members of deceased donors | Not specified | Outcomes: preset measures/ PPIE as response to methodological challenges/ Vulnerable groups | Yes |
| *Nöstlinger [55] | Nether-lands | Prevalence study | Epidemiology | Whole | Epidemiological study testing the prevalence of HIV amongst immigrants | People living with HIV | CBPR | Partnerships/Team leadership/PR training as researchers/Ways of contributing | No |
| Olding [56] | Canada | Survey | Substance abuse | Design | Co-development of a patient-reported experience questionnaire for people who use drugs | Drug users | Not specified | Graphic facilitation/ Identifying unmet needs/ Ethical considerations/ Remuneration | No |
| *Ostrach [57] | USA | Qualitative | Health services | Design | Evaluation of the process of the development outpatient women’s health screening tools | At risk women | Human-Centered Design | Timing and trust/ Historical mistrust/ Relationships/PR perspective | No |
| *Pallesen [58] | Ireland | Intervention | Health Services | Design | Stakeholder evaluation of co-designing a leadership intervention to health care teams | Not specified | Experience based Co-design | Sharing experiences/ Legitimacy/ Relationships/ Feedback/ Sharing power/ Storytelling | No |
| Pinfold [59] | UK | Service design | Mental health | Proposal, Analysis, Dissemi-nation | Evaluation of a study on personalization in mental health policy | People with mental health problems | Not specified | Team building/ Lack of remuneration and training had impact on involvement level/ Inequalities/ Lived vs research experience | Yes |
| *Pomey [4] | Canada | HTA | Cardiology | Design | Evaluation of a project creating recommendations for cardiac defibrillator replacement | Patients with cardiac defibrillators | Own | Value of PPIE/ PPIE in literature review/ Co-construction of results/ Impact: researchers and project/ Challenges | Yes |
| *Rayment [60] | UK | RCT | Pediatrics | Design | Pilot trial examining the effects of probiotics during pregnancy and risk of preterm birth | Mothers from populations at risk of preterm birth | Nominal group technique for discussion groups | Discussion groups/ Advisory team throughout the RCT/ Impact: project/ Process tailored to the needs of PRs | No |
| Ruff [61] | USA | Survey | Health Services | Design, Implemen-tation | Designing and implementing a survey on mental needs of children in foster care transitioning into adulthood | Young adults with a background on foster care | CBPR | Building relationships/ Vulnerable groups/ PR consultants as bridge to target group/ Limitations/ Agency | No |
| *Seeralan [62] | Germany | RCT | Mental health | Design | Development of patient-targeted feedback intervention in primary practice | Patients with experience of depression | INVOLVE | PR-led workshops/ Importance of structure and researcher skills/ Impact: project/ Remuneration | No |
| *Sharmil [63] | Australia | Qualitative | Minority health | Whole | Aboriginal health research related to consumption of drugs and alcohol to improve health service delivery | Aboriginals at risk of substance abuse | PAR combined with aboriginal traditions | Researcher and PR joined forces/ Researcher learnings from subject population/ Adjusting research process to subject needs/ Incorporate PR knowledge | Yes |
| *Simpson [64] | UK | HTA | Health Services | Whole | Creation of an early awareness and alert system and related webpage | Not specified | INVOLVE | MedTech involvement/ Impact: product/ Various methods in one project/ Social Media/ Challenges/ Knowledge sharing | No |
| *Skovlund [8] | Denmark | Intervention | Oncology | Whole | Clinical controlled intervention trial on effects of using PRO before metastatic melanoma consultations | Patients with or with history of melanoma | INVOLVE | Focus on analysis/ Training/ Structural and emotional challenges/ Remuneration/ Building relationships/ Skills | Yes |
| Springs [65] | USA | Review | Health services | Whole | Evidence synthesis on integrating arts-based interventions in health care | Various patients, artists | PCORI | Dissemination/ Training helps legitimacy/ Confidence | Yes |
| Stocker [66] | UK | Qualitative | Health services | Analysis | Critical reflection on collaborative data analysis in a care home-study | Relatives of care home residents | Not specified | Multiple professions/ Role play/ Steering choices/ Lack of practical guidance/ PR Interest group | No |
| Thomas [67] | UK | Qualitative | Mental health | Whole | Mental health research—a reflection on a study exploring deprivation as a trigger for mental stress | People at risk of developing mental health issues | Not specified | Lack of equality/ Importance of socializing/ Power dynamics/ Trust/ Relational work/ Underserved communities | Yes |
| Tremblay [68] | Canada | Qualitative | Minority health | Whole | Evaluation of developing a design for including indigenous patients in research partnerships | Indigenous Canadian tribes | Not specified | Training/ PPI as validation of results/ Recruitment/ Trusting relationships/ Capacity building/ Historical context | Yes |
| *Vat [69] | Canada | Evaluation | Several areas | Whole, several projects | Co-designing an evaluation of 11 projects with PPIE | Not specified | Several local patient and public evaluation tool kit | Human resources needed from both parties/ Tokenism/ Learning as legitimacy/ Integrating experiential knowledge | Yes |
| *Vogsen [7] | Denmark | Clinical trial | Oncology | Design, Analysis, Dissemi-nation | Evaluation of PPIE impact on retention and recruitment in a clinical trial | Women with prior breast cancer | GRIPP2 | Researcher hesitation/ Involvement in Patient-related activities/Involvement increased over time/Dissemination impact in community | Yes |
| *Worsley [70] | UK | Health services | Mental health | Proposal | Development of a public-led research proposal on improving quality of therapeutic relations | Users of mental health services | James Lind Alliance | Writing grant applications/ Validating lived experience/ Remuneration/ Equality and respect/ Marginalization within the group/ Structural issues | Yes |
BAME = Black, Asian and Minority Ethnics, RCT = Randomized Controlled Trial, HTA = Health Technology Assessment, PPIE Patient and Public Involvement and Engagement, GRIPP2 = Guidance for Reporting Involvement of Patients and the public, PRO = Patient Reported Outcomes, PR = Patient or Relative, CBPR = Community-based Participatory Research, COPD = Chronic Obstructive Pulmonary Disease, PAR = Participatory Action Research, iKT = integrated Knowledge Translation, PCORI = Patient-Centered Outcomes Research Institute. * = Part of narrative analysis in Involvement Matrix (by reference number) and metasynthesis. Note: Barn 2021 and Nichols 2021 are not represented in Matrix, as these articles did not describe roles
Background
In the last decades, involving patients in developing and conducting health research projects has become a way of achieving high quality and efficient integration of health care [71] as well as improving the overall quality of health research [72]. When PRs are involved not as research subjects but as research partners in the health research process, it can lead to: “meaningful change in patient outcomes and health systems, and realigning both research processes and outcomes to be patient-centered” [73]. PR involvement has become a demand for many funding programmes and journals as well as a health policy prioritisation [74]. Despite increasing numbers of projects involving PRs, studies have highlighted unclear definitions of involvement and describe tensions when trying to validate experience-based knowledge in the medical field [75].
Many different labels exist to describe involvement processes: co-production, co-design, collaboration, involvement, engagement, patient and public involvement, community based participatory research, participatory action research and others in research reports. These concepts and methods are rooted in traditions of different research disciplines and contexts [76], but whether the involvement method or label used to describe this, has implications on the partnerships and outcomes is unclear [77]. Recent literature has looked at partnership practices and found that: role definition and partner expectations are necessary prerequisites for the partnership to be successful. Respect, equitable power, trust, transparency, shared and collaborative decision making has been described as foundational principles for research partnerships; and patients taking on different roles during research partnerships, such as: members of research teams, advisory groups, steering committees and working groups, consultation, and specific research tasks [73, 74, 78]. These authors recommended further detailed analysis of partnership role characteristics and what impact they have. Others concluded that we lack knowledge on how impact and outcomes are achieved in these collaborative partnerships [79] and how these partnerships might be similar or different [80]. It should be noted that the role of the researchers in research partnerships has been investigated much less than the role of patients [81].
Objectives of this study
We studied peer-reviewed articles describing PPIE activities for the roles researchers and PRs fulfil in different types of involvement activities, the factors that enable involvement, and how PRs’ knowledge is utilised. As part of that:
How do patients and relatives establish themselves as knowledgeable?
What roles do researchers, patients, and relatives have in enabling partnerships?
The term Patient and Public Involvement/Engagement (PPIE), which has previously been described as internationally representative to cover the wide range of involvement activities and methods [76], will be used in this article.
Methods
This study used a rapid review approach to obtain a systematic overview of articles describing PPIE in health research followed by a narrative analysis and meta synthesis of selected articles. Rapid reviews are a newer form of review and are described as an: “assessment of what is already known about a policy or practice issue, by using systematic review methods to search and critically appraise existing research” [82]. By omitting or simplifying the systematic review process, it has the benefit of providing an overview without requiring substantial resources and time and thereby helps closing the gap between decision making and evidence generation [83]. It has an explorative character and is suited to investigate new trends as it gives an overall quality or direction of the literature available [82].
Our methods were inspired by Haby [83], Dobbins [84] and Boden et al. [85]. As there is no set way of conducting a rapid review, we highlight the steps altered from a systematic review:
development of a high specificity search string,
limitation of databases to four,
no use of grey literature,
time limit on publications,
main screening performed by the first author,
no systematic quality assessment,
in-depth analysis limited to a selected group of included articles.
See Additional file 1: Appendix C for a justification of each of these choices. The selection process of included papers follows the PRISMA guidelines [86] (See Fig. 1), and the reporting uses the PRISMA 2020 checklist [86] where items are applicable according to the used rapid review methodology.
Fig. 1.
PRISMA Flowchart
In accordance with this study’s overarching principles of accessibility, transparency and reciprocity as basis for research partnerships, the protocol for this rapid review was co-developed and registered with the Open Science Framework on Nov 25th 2021 [87] https://doi.org/10.17605/OSF.IO/QMWVK). Alterations to the protocol can be found in Additional file 1: Appendix C; main changes included only performing narrative and meta analysis on a selection of included article.
Eligibility criteria
Inclusion criteria were: Published articles presenting practical examples and reflections, case studies, interviews, ethnographies, or evaluations of research partnerships between PRs and researchers in any type of qualitative or quantitative studies (see Additional file 1: Appendix C for detailed inclusion and exclusion criteria). The Guidance for Reporting Involvement of Patients and Public (GRIPP) guidelines, a checklist for reporting PPIE activities in health research first published in 2012 [88] (and the in 2017 revised GRIPP 2 [89]) first offered a comparable framework to describe and report involvement practices. Therefore, we excluded papers published prior to 2012. The population was defined as patients of any age with any (past or present) medical condition, relatives or caregivers participating as stakeholders, panellists, co-designers etc. at any point of the research cycle.
Search strategy
Searches were run in four databases (Medline, Embase, PsycInfo and CINAHL) in November 2021 and rerun in February 2022. It has been noted previously how reporting on patient involvement activities varies: sometimes not mentioned in the title or abstract [90] and challenging to capture in (standard) search terms [80]. Therefore, we used the quality-tested patient involvement search string developed by Rogers et al. [90] and Cooke & Smiths’ SPIDER-tool [91] incorporating search blocks on study design and research type for higher specificity. The search string was developed for Medline and translated with the assistance of a research librarian to match the other databases. See Additional file 1: Appendix A for SPIDER-tool (Additional file 1: Table S1) and Medline search string (Additional file 1: Fig. S1).
Data analysis
To provide an overview of study characteristics of included articles as well as an in-depth analysis of roles, enablers and outcomes, the results were synthesized as follows:
Overview of study characteristics of all eligible papers reporting on PR/researcher partnerships using a matrix to extract descriptive information.
Narrative analysis of partnership roles of selected articles using the Involvement Matrix.
Meta-synthesis of PPIE enablers of selected articles.
Study characteristics We conducted a descriptive summary of all 70 papers extracting data on geographical origin of project, research area and design, format of PR group, focus of article, philosophy for inclusion, themes discussed and PR co-authorship. This information was extracted from all parts of the papers; a thorough read and reread for each paper was required. Our goal was to create a searchable overview of relevant practical PPIE examples readily available for interested readers (Table 1). As such, we aimed to deliver towards one of the rapid review’s functions of providing clarity and accessibility of research evidence [83].
Narrative analysis As we found more eligible papers than anticipated we discussed how best to give an overview of available evidence in the timeframe available. We selected 40 papers which we considered most comparable (depicted with an asterisk in Table 1), according to the following rationale:
partnerships with adult patients and relatives (as research indicates that extra steps have to be taken to enable participation of children and youth [92]),
articles that reported the framework used to account for and or support their involvement activities either in the background or methods section (as we aimed to investigate different types of partnerships, we found it useful to understand the framework behind the PPIE activities),
Excluding systematic reviews (as detailed Cochrane guidelines on PR involvement in systematic reviews are available [93] and we considered this a readily available aid for researchers).
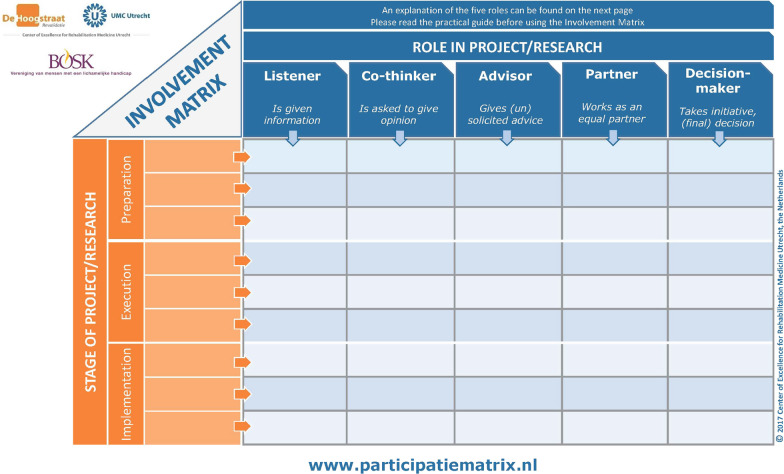
We used Smits and colleagues’ Involvement Matrix to perform the narrative analysis (see Fig. 2). The matrix was designed as a conversation-tool to discuss roles and expectations to support PPIE in research [94]. We have used the matrix to also describe researchers’ roles, as to our knowledge no model for analysis of researcher roles in partnerships with patients exists. We scrutinized the full papers for descriptions of roles to populate the matrix. This information was scattered throughout the papers; sometimes it was found in the methods section, but most often in designated PPIE-headlined sections or in the contributions or acknowledgement section. Authorship requires substantial contributions to the research process and article write up as recommended by the International Committee of Medical Journal Editors [95]; therefore, to understand different levels of partnership, we stratified the 40 articles in PR co-authored papers (n = 16) and non-PR co-authorship (n = 24). All authors discussed the extracted data.
Fig. 2.
Involvement Matrix (reprinted with permission)
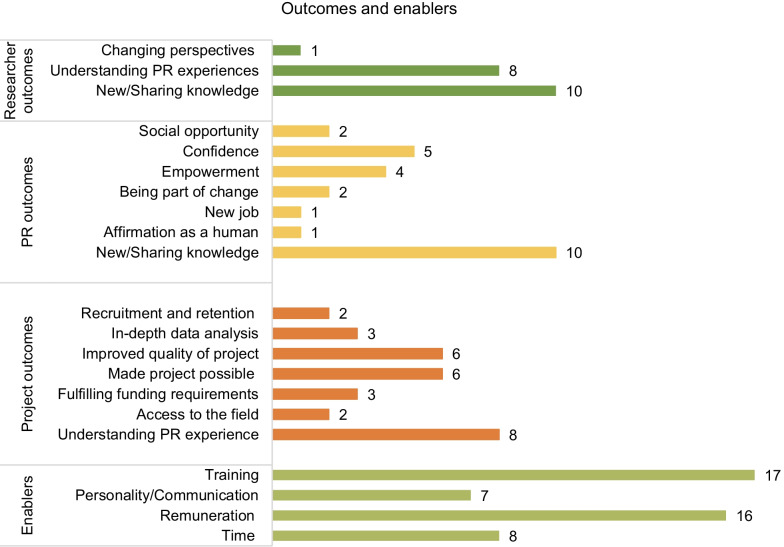
Meta-synthesis Finally, a meta-synthesis of enablers and outcomes described in the 40 articles was performed. Papers were analysed for value creation and outcomes related to and enabling actions in the partnerships. This information was found under findings, evaluations, self-reflections, or discussion sections depending on the scope of the article. Those sections were thoroughly read and information was extracted using a purposefully-developed matrix. The information was then synthesised and reported in Fig. 5.
Fig. 5.
Overview of enablers and outcomes for PR partnerships in included articles (n = 40)
Results
A total of 5261 potential hits were collected in Endnote 20 2.01 and transferred to Covidence 2.0 (Covidence.org) for screening and full text analysis. After deduplication, 3667 records were screened for title and abstract. Twenty-five percent of the records (918) were independently screened by two researchers (AWK and MLK) and inclusions compared for disagreement (15%). Disagreements were discussed and resolved by an external referee. The remainder of articles were screened by one researcher (AWK), who also screened the 285 articles eligible for full text reading. Seventy articles were included for analysis. A searchable full list is available at the Open Science Framework and will be available on the website for Center for Research with Patients and Relatives at Odense University Hospital [96].
Descriptive overview
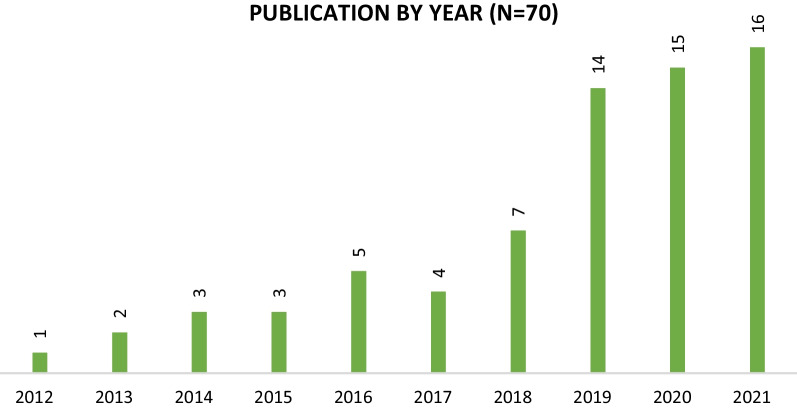
We found 70 articles eligible for inclusion; the total number of articles reflecting on and evaluating PPIE activities increased progressively between 2012 and 2021 (see Fig. 3).
Fig. 3.
Articles grouped per publication year
The geographic origin of studies is polarized predominantly between the UK (n = 32), Canada (n = 9), and the USA (n = 7). Other industrialized countries account for minor contributions: Denmark (n = 5), Netherlands (n = 4), Germany and European collaborations (n = 3), Norway, Ireland and Australia (n = 2) and Sweden (n = 1). Different research areas are represented (see Table 1) with mental health leading with 21 publications, followed by health services research (n = 11), and oncology (n = 6). A qualitative research design was most frequently used (n = 21), but more traditional biomedical research designs such as clinical trials, randomized controlled trials (RCTs), health technology assessments (HTA), register studies, surveys and reviews were also represented (see Additional file 1: Appendix D Table S2 for visualization of results). The included articles cover a wide range of involvement formats from advisory boards, being consulted once on a project [97], to full coproduction at all stages of a project [1]. All steps in the research cycle are represented: from research agenda generation to dissemination activities. Thirty-eight of 70 papers reported including patients in the whole research cycle; if patients were involved in just one step, the design phase (n = 11) was most frequently reported. Using authorship listing and information in the contribution and affiliation sections, 33 articles were identified as being co-authored by PRs. For one article we were unable to determine whether PRs had been co-authors, and thus classified this [51] as non-co-authored. Articles described inviting PRs with lived experience of the condition or service under investigation, yet in 5 articles the authors did not report any details on who the PRs were or reasons for selecting them [2, 21, 58, 64, 69].
Articles mentioned a total of 21 different frameworks for involving PRs, yet a large group of articles (n = 19) did not mention a specific framework guiding the collaborative processes. Geographical differences in the frameworks used can be seen with only European and UK-based articles referring to INVOLVE guidelines and/or the GRIPP reporting tool—both originating from the UK. In articles with no frameworks specified, 12 out of 19 had PRs listed as co-authors.
Authors described both positive experiences and challenges related to PPIE activities. However, all 70 articles report that the involvement activities ultimately resulted in positive changes in the projects, ranging from researchers gaining new perspectives on their project [12], reformulation of questions in questionnaires [37], changing the intervention design [35], and collaboratively developing guidelines [4]. A few articles mentioned how the researchers were worried that the PPIE in their research project would decrease scientific rigour [69], not be taken seriously [32] or fail to obtain legitimacy amongst clinicians [35].
Narrative analysis of partnership roles
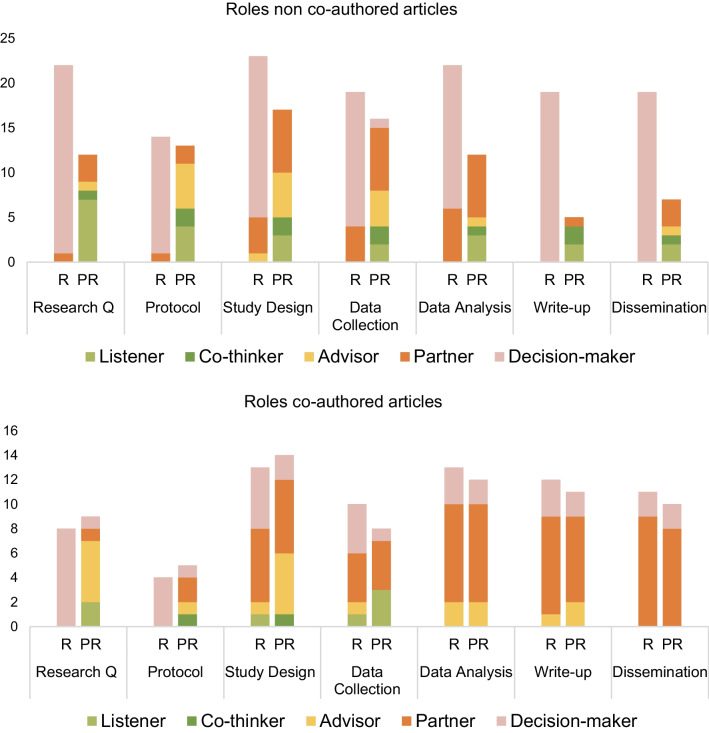
The role of both researchers and PRs in the partnerships was determined using Smits et al., Involvement Matrix in the 40 selected articles marked with an asterisk in Table 1. The results show that PR roles in non-co-authored articles are: listener, co-thinker, advisor, and partner. One article mentioned that PRs had decision-making authority [42]. See Fig. 4 and (Additional file 1: Table S3 in appendix for data details). PRs’ roles changed during the projects throughout the research cycle: In the earlier stages of research question and protocol development, PRs’ role is most often described as listener or advisor. This was difficult to determine as few articles reported clearly on PRs' roles in the early stages of the research cycle. During the design stage PRs most often had the role of advisor or partner. The role of partner was often described where PRs had been involved in the design, data collection and analysis stage. When PRs were involved in several steps of the research cycle they were more frequently described as partners in the project. Few articles described PRs being involved in the write-up and dissemination stage [6, 24, 42]. The most common role for the researchers was ‘Decision-maker’ (the one who takes initiative and/or makes final decisions). This role did not change during the research cycle.
Fig. 4.
Visualization of roles within non-co-authored and PR co-authored articles using the Involvement Matrix. Research Q = Research Question, R = Researcher, PR = Patient & Relative
PR role in PR co-authored articles was most frequently described as partner—especially at the execution and implementation stages. In the early stages of the research cycle, PRs had the role of advisor or listener. Again, few papers described the early stages of the projects clearly, therefore information is missing, and results should be treated with caution. After research question development, researcher role in PR co-authored articles was also most frequently described as partner. Their role seemed more flexible and change as the project progressed through the research cycle and several of the projects described PR and researcher roles as dynamic and shifting between both parties taking the lead and partnering.
Meta-synthesis of partnership enablers and outcomes
Included articles described several enablers that make the partnership or make it possible. An overview is presented in Fig. 5.
Training Several articles reported that researchers had provided training for the PRs to be able to contribute to the projects [1, 6, 13, 15, 22, 26, 34, 35, 42, 43, 55, 62, 69]. PRs also mentioned that they needed training and support to gain confidence to contribute at the same level as the researchers [1, 13, 15, 30, 43, 58]. A few articles [15, 30, 34, 69] mentioned training for researchers in PPIE as it was a novel territory for them, and one article [63] described that the PRs taught the researcher in their understanding of health and disease.
Personality and communication skills Finding a common language stripped from medical jargon was described as important; one paper mentioned that PRs made researchers aware when this happened [35]. PR personality and communication skills were described as important in the partnerships: being “pleasant collaborators” [9], to be “able to reflect their experience in a wider context” [4], “having the physiological and psychological means to contribute” [7], having “interpersonal skills to facilitate collaboration” [4], being able to “expresses him/herself clearly and simply” [4], and one patient mentioned “communication is my key skill” [3]. Two articles mentioned that researchers can also have strong personalities [11, 58] which can hindrance to the partnership.
Remuneration Six out of 24 non-co-authored articles [9, 24, 39, 42, 55, 70], and 9 out of 16 co-authored articles [3, 4, 7, 8, 15, 26, 32, 35, 41] mentioned remuneration or travel reimbursement as important. A few PRs declined [4, 6, 9] explaining it would change the relationship and bring more responsibility, or accepted remuneration, but felt as a volunteer [3].
Time The more steps the PRs are involved in, the more the influence of the PRs on the project and partnerships was reported as strong [9, 39, 51, 53] suggesting a longitudinal causality. Time was also mentioned as a factor in shorter partnerships as time spent on informal talks pre- and post-meeting helped build the relationship [15] and allowed for time spent together to reflect during the process [41]. However, time could also be a challenge, for example due to time pressure of other competing research activities [62]. Activities were less successful when “substantial” time and efforts were needed to organize and plan meetings [51] and when PRs had to spend time on activities in between meetings [9]. It was also mentioned that moving beyond consultation with PRs required extra time and workload [8].
Trust When PRs felt that they “were actually listened to” [17, 32] it helped the collaboration and created trust between the parties. Some PR co-authored articles described as PPIE intrinsic to the research project as PRs’ knowledge and perspectives actually made the research possible [19, 26, 32, 35, 40, 41, 55, 63]. In these papers PRs were described as: partners to the researchers in facilitating and conducting interviews [19], shared decision-makers when “working alongside researchers to coproduce interpretations” [32], essential to the research because they were gate-keepers to the community, a critical friend and [26, 55], the ones who accepted the researcher [63] and validated and consolidated the researchers’ point of view [35]. The researchers were trying to obtain legitimacy and gain PRs’ trust by initiating partnerships on the PRs’ terms. The researchers did so by focusing on creating supportive environments and levelling out power differences and by actively seeking acceptance of their suggestions.
The outcomes of PPIE
The outcomes reported for both co-authored and non-co-authored articles focus on impact for the research project (see Fig. 5). Authors mentioned: increased recruitment and retention in the studies as a result of the PPIE [7, 34], more in-depth data analysis [6, 39, 42], improved quality of the project [6, 11, 17, 33, 45, 58, 98], creation of new knowledge and exchange of knowledge of and aspects of a disease [1, 25, 28, 30, 42, 53, 55, 57, 62, 64], fulfilling funding requirements [6, 9, 43], and gaining access to the field of study [26, 55]. Personal accounts of PRs were persuasive [35] and changed researchers’ perspectives [19]. A few articles mentioned that the goal of collaborating with PRs was to create the research project around the PRs’ experiences. The PR groups here included black, Asian and minority ethnics [26], aboriginals [63], abuse survivors [32], mental health services users [39, 42, 70], people with spinal cord Injuries [40], and people with aphasia [45]. The value of working with PRs in those partnerships was described as “to enable service users to find voice and freedom” [32], to create research which makes a change in the lives of people [40], a way of bringing disadvantaged groups into research [63]. Articles reporting on personal value for PRs mentioned benefits as: a social opportunity to meet fellow experiencers [15, 45], to gain confidence [17, 28, 30, 33, 55] and feel empowered [17, 28, 39, 55], to be part of change or improvement [8, 33], to find a new job [39], and to get affirmation as a human being [42].
This project’s patient and relative partners’ observations and reflections
We, the patient and relative panel, selected 11 of the 70 articles to read and analyse. We selected Danish articles (n = 5) because we are from Denmark and sought to find a certain familiarity with the research and learn what is happening in Denmark on PPIE, and some international articles (n = 6) which had a focus on treatment and care for illnesses identical or similar to what we have experienced as patients and relatives [1–11]. The selected articles mostly described the involvement well and thoroughly. We found that the researchers had done a lot of work and focused on hearing what patients needed. Thus, it was difficult for some of us in the group to critically reflect on the researchers’ work, because we don’t know what challenges they faced or what considerations were behind the choices they made.
We would like to comment on the themes training and recruitment as reported in the articles. Pomey et al. [4] mentioned the importance of the right recruitment process and patient match, and Miah et al. [1] described not having minority groups represented in their study as a weakness. We believe it is important that researchers consider carefully how they can find PRs that have true lived experience within the area of research as well as taking care to seek diversity. Many of the groups were homogeneous, and we believe that a group must be diverse to deliver different views. If researchers would look for more diversity, they need to be more flexible in meeting time and place and recruitment processes. We believe it is important to consider whether all PRs need the same training—different people will also have different needs for training.
We also found that the selected articles described a power difference between PRs and researchers; it is important to be aware of the power balance between researchers and PRs. Lindblom et al., described that the PRs felt inferior to the health professionals during the research process [11] and Pomey et al. described how PRs were more comfortable having meetings without the researchers [4]. We consider the number of PRs partaking in a project as crucial in this matter. We think that the fewer PRs involved in a project, the more training and support they will likely need to be able to contribute on equal terms.
Researchers can inadvertently get the reply they are looking for if the PRs are only consulted briefly in one part of the research project. In the 11 articles we read, when researchers and PRs worked in the same group, a reciprocal learning dynamic was described. Therefore, having workshops or meetings with only patients or only researchers, it seems much learning between the two could be lost.
Some of us found it challenging to read the articles, due to reading academic papers in a foreign language and being unfamiliar with the structure of research articles. We had made a support tool for what to look for in the articles, but even then, we needed considerable time as we had to go over the articles several times. It has been interesting to get an insight into how eager and engaged researchers were to involve and collaborate with PRs. We would like future articles to report more on the outcomes of the involvement as experienced by the PRs: what do they gain from the different partnerships?
Discussion
We searched for papers that reported on researcher and PR partnerships as part of their description and or evaluation of the PPIE process. We found 3667 hits, briefly described the 70 eligible papers for inclusion, and performed a more in-depth analysis of the partnerships using the Involvement Matrix on 40 papers. We see that researchers predominantly took on the role of decision-maker and the PPIE tasks were often described as predetermined by the researchers. As such, researchers defined the PRs’ role in most partnerships. Some of the articles pointed out that this was important to mention at onset and clear role boundaries were perceived as positive by researchers and PRs. The yielded outcomes of PPIE were described as positive, often growing beyond initial expectations; so perhaps the full potential of a partnership is hard to reach when setting clear boundaries from the beginning. The most frequently described enabler was PR training, and both researchers and PRs felt training increased their ability to contribute. Others found offering systematic PR training builds patient capacity for engagement and helped legitimize their role [99]. Green et al. found that when members of the public fulfilled a designated role, they needed training and other support to equip them for this role and fit in a preexisting research structure [100]. Jones and Pietilä (2020) report how this results in PRs aligning themselves with health care professionals and adopting professional language to obtain legitimacy [75]. Our findings suggests, as per existing studies, that often what PRs bring to research projects are filtered lived experiences, tailored to meet dominant hermeneutical framework and adjusted to be compatible with existing research structures. Generally, our findings suggest that involved PRs were a homogenous group, and that active steps were taken to make sure that the PRs were pleasant collaborators with a constructive attitude who could express themselves clearly and simply. We need to be mindful that if only a selected group of patients are heard sharing filtered lived experiences, we risk excluding other perspectives whilst additionally creating a new norm(al) which will alienate other patients from their own (and perhaps different) experience [101], contributing to what Miranda Fricker [102] labels as hermeneutical injustice in PPIE. PPIE has the potential to decrease epistemic injustices in health care by helping mutual understanding between clinicians and patients [103], care should be taken to find breath in the PRs invited and allow them to contribute without having to make attempts to fit the hermeneutical and structural framework of the research world.
Overall, all articles reported positive about their PPIE practices which could indicate a reporting bias as negative experiences may be less pleasant to acknowledge, formulate and publish. Others have published in the past on potential negative consequences of PPIE such as abandonment of research ideas [104], and we believe it is important to continuously have honest conversations about both negative and positive aspects of PPIE. For example, are there cases where PPIE does not lead to changes, and if so, how do we as researchers navigate informing PRs (and funders) about this? Some researchers were concerned for the quality of the research and the opinions of fellow researchers when PRs were to be involved. Others have examples of researchers who omitted that PRs were involved out of fear of having the project rejected [100]. In this review, new knowledge and sharing knowledge were the most frequently reported positive outcomes of PR partnerships, and articles reported that PPIE improved the overall quality. Hence, concerns about experiential knowledge negatively impacting a project seem unwarranted and could benefit a more detailed analysis in the future.
Our analysis showed that when PRs were co-authors, they were more frequently described as partners through most steps of the research cycle and in this way, co-authorship legitimized the PR contribution. There is sparse literature addressing authorship in PPIE research; Richards et al., offered co-authorship as a way of giving credit to someone who made “important intellectual contributions” in a co-production process [105]. Despite of rigorous definitions of co-authorship (i.e. by ICMJE [95], co-authorship attribution is not always in line with these guidelines.. In this article we used PR co-authorship as a variable in our analysis; this provided us with insight into the PPIE practices behind PR-co-authored articles. Our findings showed that PRs earned their authorship by being partners in most parts of the research process.
In a review of reviews, Hoekstra et al. [80] found that partnership descriptions largely depended on research area and country origin of first author. We had similar findings and saw no clear association between PPIE labels or frameworks used and actual partnership practices. This review showed the importance of communication and personal skills, and as per existing literature, these findings suggest that to understand the gains of PPIE activities/practices, we cannot look solely at labels or frameworks; we must also investigate interpersonal relationships and partnership dynamics. As reported, both researchers and PRs can be “strong or difficult personalities” [58]; illustrative of how interpersonal skills are perhaps paramount these elements were only reported as recruitment criteria for PRs and not as a theme for training of researchers. Interpersonal skills of researchers should be an area for future focus.
Strengths and limitations
This review offers an overview of 70 articles reporting on PPIE activities as well as in depth analysis of a selection of those. The Involvement Matrix may not provide a full representation of partnership roles, as a few articles couldn’t be mapped on the matrix as they lacked thorough descriptions of the PR-involvement throughout all stages. This may have skewed the mapping results, but the mapped papers showed a trend which we feel is representative of our data.
The format of a rapid review is intended to explore the current trends and knowledge on a subject. It is meant to be a quick process (commonly less than 6 months [83]) to assess current knowledge about a policy or practice [82]. Perhaps as reporting on PPIE experiences is a relatively new field, with a lack of agreement on key definitions, this review did not unfold as rapid as intended with much scrutiny needed to extract data from each included article. We tried to alleviate work for future reviews by including a ‘patient partner contributions’ section making their contributions clear. PPIE activities are commonly found to be under-reported and under-reflected [106]. We found especially that research purpose and preparatory stages like origin of research idea and development of protocol were generally under-reported. GRIPP 1 and 2 do not provide a uniform reporting style as anticipated in our inclusion criteria. The IMRAD format for journal publications may limit PPIE reporting [107] and more openness to report personal outcomes for both PRs and researchers could perhaps alleviate this. Until this becomes mainstream, a future focus on actual activities and relations rather than terminology can help shed light on outcomes and impact of PPIE in health research.
Conclusions
In research partnerships between PRs and researchers, researchers most often have decision-making roles, which gives them control of where, when, and how to involve PRs in their projects. As PR-researcher collaborations seem to evolve during the projects, their full potential may not be reached if fully planned from the start. Co-authorship is can be an acknowledgement and legitimization of PR contributions—and should be used as such, yet, currently happens most often when PRs have had the role of partner in several parts of the research cycle even though they might have made a significant contribution to the research. Across a variety of involvement activities and frameworks, common partnership enablers were found; these include training, interpersonal skills, remuneration, time and trust. Reported PPIE outcomes included: overall improved quality of research and new learning for all parties involved. Care should be taken to include a variety of PRs, and consideration of individual PR needs may create the conditions to invite a more varied group of people into health research.
Supplementary Information
Additional file 1. Consisting of appendix A, B, C, D with extra information on search terms, PPIE activities, inclusion and exclusion criteria, alterations from the protocol and further tables and figures supporting the presentation of our findings.
Acknowledgements
We would like to thank Jacob Dennis Larsen for acting as referee in the screening process. We would also like to thank Professor Birgitte Nørgaard and Sally Wulff Jørgensen for input on tables and script content.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- GRIPP2
Guidance for reporting involvement of patients and the public
- HTA
Health technology assessment
- iKT
Integrated knowledge translation
- PAR
Participatory action research
- PCORI
Patient-centered outcomes research institute
- PPIE
Patient and public involvement and engagement
- PRO
Patient reported outcomes
- PR
Patient and relative
- RCT
Randomized controlled trial
Author contributions
Study concept was developed by AWK, AJ, MK, MJR and AKS. Searches were conducted by AWK and MLK. Analysis of data were conducted by AWK, AKS, KB, KEB, TA, MLK and AJ. Refinement of analysis and discussion were conducted by AWK, MJR, MK and AJ. Writing of this manuscript were conducted by AWK, AKS, KB, KEB and TA and supervised by MJR, MK and AJ. All authors read and approved the final manuscript.
Funding
The review is part of AWK’s Ph.D. project funded by Odense University Hospital and University of Southern Denmark. The funders have no influence on design of study, collection, analysis and interpretation of data or writing of this manuscript. No separate funding was received for this article.
Availability of data and materials
The protocol for this study is available at: https://doi.org/10.17605/OSF.IO/QMWVK. The datasets generated and analysed during the current study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Author information.
AWK is a nurse and PhD Student at the unit of user perspectives and community-based interventions at the University of Southern Denmark. She currently focuses her work on patient and public involvement in health research.
MJR is an Associate Professor in Diabetes Care, Patient and Public Involvement and Technology at Steno Diabetes Center Odense, Odense University Hospital and University of Southern Denmark. She is interested in diabetes care, clinical research and user involvement, and she is acknowledged within the scientific tradition of Participatory design in Health Science.
MK is an Associate Professor in Pediatric Rehabilitation at the University Medical Center Utrecht and De Hoogstraat Rehabilitation in the Netherlands. Her research interests include family-centered services, and the role of the family in the development of children with disabilities. Involving patients and families in all stages and aspects of research is one of the key principles in her research.
AKS is a patient partner in AWK’s PhD project. She is also a patient and relative representative at Odense University Hospital and a patient guest lecturer at University of Southern Denmark.
KB is a patient partner in AWK’s PhD project and serves as a patient representative in several regional councils of Southern Denmark and at Odense University Hospital.
KEB is a patient partner in AWK’s PhD project and serves as a patient representative in several councils at Odense University Hospital.
TA is a partner in AWK’s PhD project and a patient and relative representative in various employment committees at Odense University Hospital. He also occasionally teaches new staff about the experiences of being a relative.
MLK is a PhD student at University of Southern Denmark. She has a basic education as a trained nurse and a master’s degree in health sciences. Her research interest includes existential and spiritual care, missed nursing care and user involvement in healthcare research.
AJ is a Professor in Patient and Public Participation at Utrecht University Medical Center, Utrecht University (Netherlands), and University of Southern Denmark. She is a health service researcher with a passion and longstanding commitment to issues of democratisation, participation and service user involvement in health research and health education. She holds honorary positions with Exeter University Medical School (UK) and Odense University Hospital (Denmark).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miah J, Parsons S, Starling B, Lovell K, Leroi I, Dawes P. Impact of involving people with dementia and their care partners in research: A qualitative study. BMJ Open. 2020;10(10):e039321. doi: 10.1136/bmjopen-2020-039321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minogue V, Donskoy A-L. Developing a training package. Int J Health Care Qual Assur. 2017;30:458–466. doi: 10.1108/IJHCQA-06-2016-0084. [DOI] [PubMed] [Google Scholar]
- 3.Marks S, Mathie E, Smiddy J, Jones J, Da Silva-Gane M. Reflections and experiences of a co-researcher involved in a renal research study. Res Involv Engagem. 2018;4(1):1–10. doi: 10.1186/s40900-018-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pomey M-P, Brouillard P, Ganache I, Lambert L, Boothroyd L, Collette C, et al. Co-construction of health technology assessment recommendations with patients: an example with cardiac defibrillator replacement. Health Expect. 2020;23(1):182–192. doi: 10.1111/hex.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mockford C, Murray M, Seers K, Oyebode J, Grant R, Boex S, et al. A SHARED study-the benefits and costs of setting up a health research study involving lay co-researchers and how we overcame the challenges. Res Involv Engagem. 2016;2:1–12. doi: 10.1186/s40900-016-0021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jørgensen CR, Eskildsen NB, Johnsen AT. User involvement in a Danish project on the empowerment of cancer patients—experiences and early recommendations for further practice. Res Involv Engagem. 2018;4:1–13. doi: 10.1186/s40900-018-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogsen M, Geneser S, Rasmussen ML, Hørder M, Hildebrandt MG. Learning from patient involvement in a clinical study analyzing PET/CT in women with advanced breast cancer. Res Involv Engagem. 2020;6(1):1–8. doi: 10.1186/s40900-019-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skovlund PC, Nielsen BK, Thaysen HV, Schmidt H, Finset A, Hansen KA, et al. The impact of patient involvement in research: a case study of the planning, conduct and dissemination of a clinical, controlled trial. Res Involv Engagem. 2020;6:1–16. doi: 10.1186/s40900-020-00214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nissen ER, Bregnballe V, Mehlsen MY, Muldbjerg AKØ, O’Connor M, Lomborg KE. Patient involvement in the development of a psychosocial cancer rehabilitation intervention: evaluation of a shared working group with patients and researchers. Res Involv Engagem. 2018;4(1):1–16. doi: 10.1186/s40900-018-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nierse CJ, Schipper K, Van Zadelhoff E, Van De Griendt J, Abma TA. Collaboration and co-ownership in research: dynamics and dialogues between patient research partners and professional researchers in a research team. Health Expect. 2012;15(3):242–254. doi: 10.1111/j.1369-7625.2011.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindblom S, Flink M, von Koch L, Ytterberg C, Elf M, Laska AC. The manifestation of participation within a co-design process involving patients, significant others and health-care professionals. Health Expect. 2021;24(3):905–916. doi: 10.1111/hex.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrehart N, Frost K, Harris R, Wragg A, Stewart D, Sharif H, et al. “A little (PPI) MAGIC can take you a long way”: involving children and young people in research from inception of a novel medical device to multi-centre clinical trial Roald Dahl, James and the Giant Peach (1961) Res Involv Engagem. 2021;7(1):1–15. doi: 10.1186/s40900-020-00243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander R, Estabrooks P, Brock D-JP, Hill JL, Whitt-Glover MC, Zoellner J. Capacity development and evaluation of a parent advisory team engaged in childhood obesity research. Health Promot Pract. 2021;22(1):102–11. doi: 10.1177/1524839919862251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anang P, Naujaat Elder EH, Gordon E, Gottlieb N, Bronson M. Building on strengths in Naujaat: the process of engaging Inuit youth in suicide prevention. Int J Circumpolar Health. 2019;78(2):1508321. doi: 10.1080/22423982.2018.1508321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barn P, Rideout KL, Lo W, Josey D, Vint Z, Sha C, et al. Better together: launching and nurturing a community Stakeholder committee to enhance care and research for asthma and COPD. Chest. 2021;161:382–388. doi: 10.1016/j.chest.2021.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Beeker T, Gluck RK, Ziegenhagen J, Goppert L, Janchen P, Krispin H, et al. Designed to clash? reflecting on the practical, personal, and structural challenges of collaborative research in psychiatry. Front Psych. 2021;12:701312. doi: 10.3389/fpsyt.2021.701312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beighton C, Victor C, Carey IM, Hosking F, DeWilde S, Cook DG, et al. ‘I'm sure we made it a better study…’: experiences of adults with intellectual disabilities and parent carers of patient and public involvement in a health research study. J Intell Disabil. 2019;23(1):78–96. doi: 10.1177/1744629517723485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birch R, Simons G, Wähämaa H, McGrath CM, Johansson EC, Skingle D, et al. Development and formative evaluation of patient research partner involvement in a multi-disciplinary European translational research project. Res Involv Engagem. 2020;6(1):1–14. doi: 10.1186/s40900-020-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourque CJ, Bonanno M, Dumont E, Gaucher N, Lacoste-Julien A, Gomez-Tyo M, et al. The integration of resource patients in collaborative research: a mixed method assessment of the nesting dolls design. Patient Educ Couns. 2020;103(9):1830–1838. doi: 10.1016/j.pec.2020.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Brutt AL, Meister R, Bernges T, Kriston L, Moritz S, Harter M, et al. Patient involvement in a systematic review: Development and pilot evaluation of a patient workshop. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen. 2017;127–128:56–61. doi: 10.1016/j.zefq.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Burrows A, Meller B, Craddock I, Hyland F, Gooberman-Hill R. User involvement in digital health: working together to design smart home health technology. Health Expect. 2019;22(1):65–73. doi: 10.1111/hex.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carr ECJ, Patel JN, Ortiz MM, Miller JL, Teare SR, Barber CEH, et al. Co-design of a patient experience survey for arthritis central intake: an example of meaningful patient engagement in healthcare design. BMC Health Serv Res. 2019;19(1):1–10. doi: 10.1186/s12913-019-4196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castensøe-Seidenfaden P, Husted G, Teilmann G, Hommel E, Olsen B, Kensing F. Designing a self-management app for young people with type 1 diabetes: methodological challenges, experiences, and recommendations. JMIR Mhealth Uhealth. 2017;5(10):1–15. doi: 10.2196/mhealth.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu CG, Mitchell TL, Fitch MI. From patient to participant: enhancing the validity and ethics of cancer research through participatory research. J Cancer Educ. 2013;28(2):237–246. doi: 10.1007/s13187-013-0464-2. [DOI] [PubMed] [Google Scholar]
- 25.Cook T, Noone S, Thomson M. Mindfulness-based practices with family carers of adults with learning disability and behaviour that challenges in the UK: Participatory health research. Health Expect. 2019;22(4):802–812. doi: 10.1111/hex.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson S, Ruddock A, Parmar V, Morris R, Cheraghi-Sohi S, Giles S, et al. Patient and public involvement in doctoral research: reflections and experiences of the PPI contributors and researcher. Res Involv Engagem. 2020;6(1):1–13. doi: 10.1186/s40900-020-00201-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennehy R, Cronin M, Arensman E. Involving young people in cyberbullying research: the implementation and evaluation of a rights-based approach. Health Expect. 2019;22(1):54–64. doi: 10.1111/hex.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devonport TJ, Nicholls W, Johnston LH, Gutteridge R, Watt A. It's not just 'What' you do, it's also the 'Way' that you do it: patient and public involvement in the development of health research. Int J Qual Health Care. 2018;30(2):152–156. doi: 10.1093/intqhc/mzx177. [DOI] [PubMed] [Google Scholar]
- 29.Dewa LH, Lawrence-Jones A, Crandell C, Jaques J, Pickles K, Lavelle M, et al. Reflections, impact and recommendations of a co-produced qualitative study with young people who have experience of mental health difficulties. Health Expect. 2021;24(S1):134–146. doi: 10.1111/hex.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wit MPT, Elberse JE, Broerse JEW, Abma TA. Do not forget the professional-The value of the FIRST model for guiding the structural involvement of patients in rheumatology research. Health Expect. 2015;18(4):489–503. doi: 10.1111/hex.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dovey-Pearce G, Walker S, Fairgrieve S, Parker M, Rapley T. The burden of proof: the process of involving young people in research. Health Expect. 2019;22(3):465–474. doi: 10.1111/hex.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faulkner A, Carr S, Gould D, Khisa C, Hafford-Letchfield T, Cohen R, et al. 'Dignity and respect': an example of service user leadership and co-production in mental health research. Health Expect. 2021;24:10–19. doi: 10.1111/hex.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frankena TK, van Schrojenstein Lantman-de Valk H, Cardol M, van der Cruijsen A, Jansen H, Leusink G, et al. Contributing to inclusive research policy and practice: a synthesis of four inclusive (health) research projects. J Policy Pract Intell Disabil. 2019;16(4):352–60. [Google Scholar]
- 34.Froggatt K, Morbey H, Goodman C, Davies SL, Dickinson A, Masey H, et al. Public involvement in research within care homes: benefits and challenges in the APPROACH study. Health Expect. 2016;19(6):1336–1345. doi: 10.1111/hex.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gammon D, Strand M, Eng LS. Service users' perspectives in the design of an online tool for assisted self-help in mental health: a case study of implications. Int J Ment Heal Syst. 2014;8(1):1–8. doi: 10.1186/1752-4458-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant C, Widnall E, Cross L, Simonoff E, Downs J. Informing the development of an E-platform for monitoring wellbeing in schools: involving young people in a co-design process. Res Involv Engagem. 2020;6(1):1–11. doi: 10.1186/s40900-020-00219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grundy A, Keetharuth AD, Barber R, Carlton J, Connell J, Taylor Buck E, et al. Public involvement in health outcomes research: lessons learnt from the development of the recovering quality of life (ReQoL) measures. Health Qual Life Outcomes. 2019;17(1):1–10. doi: 10.1186/s12955-019-1123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta E, Roberts B. User and researcher collaborations in mental health in low and middle income countries: a case study of the EMPOWER project. BMC Res Notes. 2014;7(1):1–6. doi: 10.1186/1756-0500-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hitchen SA, Williamson GR. A stronger voice: action research in mental health services using carers and people with experience as co-researchers. Int J Health Care Qual Assur. 2015;28(2):211–222. doi: 10.1108/IJHCQA-10-2014-0101. [DOI] [PubMed] [Google Scholar]
- 40.Hoekstra F, Schaefer L, Athanasopoulos P, Gainforth HL. Researchers' and research users' experiences with and reasons for working together in spinal cord injury research partnerships: a qualitative study. Int J Health Policy Manag. 2021;82:666–674. doi: 10.34172/ijhpm.2021.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honey A, Berry B, Hancock N, Scanlan J, Schweizer R, Waks S. Using systematic collaborative reflection to enhance consumer-led mental health research. British J Occupat Therapy. 2019;82(11):666–674. [Google Scholar]
- 42.Hutchinson A, Lovell A. Participatory action research: moving beyond the mental health 'service user' identity. J Psychiatr Ment Health Nurs. 2013;20(7):641–649. doi: 10.1111/jpm.12001. [DOI] [PubMed] [Google Scholar]
- 43.Jewell A, Pritchard M, Barrett K, Green P, Markham S, McKenzie S, et al. The Maudsley biomedical research centre (BRC) data linkage service user and carer advisory group: creating and sustaining a successful patient and public involvement group to guide research in a complex area. Res Involv Engagem. 2019;5:1–10. doi: 10.1186/s40900-019-0152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kara H. The value of carers in mental health research. J Public Ment Health. 2016;15(2):83–92. [Google Scholar]
- 45.Kearns A, Kelly H, Pitt I. Rating experience of ICT-delivered aphasia rehabilitation: co-design of a feedback questionnaire. Aphasiology. 2020;34(3):319–342. [Google Scholar]
- 46.Lammons W, Moss B, Modi N, Battersby C, Cornelius V, Babalis D. Incorporating parent, former patient and clinician perspectives in the design of a national UK double-cluster, randomised controlled trial addressing uncertainties in preterm nutrition. BMJ Paediatrics Open. 2021;5(1):e001112. doi: 10.1136/bmjpo-2021-001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leese J, Macdonald G, Kerr S, Gulka L, Hoens AM, Lum W, et al. ‘Adding another spinning plate to an already busy life'. Benefits and risks in patient partner-researcher relationships: a qualitative study of patient partners' experiences in a Canadian health research setting. BMJ Open. 2018;8(8):e022154. doi: 10.1136/bmjopen-2018-022154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liabo K, Boddy K, Bortoli S, Irvine J, Boult H, Fredlund M, et al. Public involvement in health research: what does ‘good’ look like in practice? Res Involv Engagem. 2020;6(1):1–12. doi: 10.1186/s40900-020-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lincoln AK, Borg R, Delman J. Developing a community-based participatory research model to engage transition age youth using mental health service in research. Fam Community Health. 2015;38(1):87–97. doi: 10.1097/FCH.0000000000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Locock L, Kirkpatrick S, Brading L, Sturmey G, Cornwell J, Churchill N, et al. Involving service users in the qualitative analysis of patient narratives to support healthcare quality improvement. Res Involv Engagem. 2019;5:1–11. doi: 10.1186/s40900-018-0133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melchior I, van der Heijden A, Stoffers E, Suntjens F, Moser A. Patient and public involvement cultures and the perceived impact in the vulnerable context of palliative care: a qualitative study. Health Expect Int J Public Particip Health Care Health Policy. 2021;24(2):456–467. doi: 10.1111/hex.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mjosund NH, Eriksson M, Espnes GA, Haaland-Overby M, Jensen SL, Norheim I, et al. Service user involvement enhanced the research quality in a study using interpretative phenomenological analysis—the power of multiple perspectives. J Adv Nurs. 2017;73(1):265–278. doi: 10.1111/jan.13093. [DOI] [PubMed] [Google Scholar]
- 53.Nichols V, Pearce G, Ellard DR, Evans S, Haywood K, Norman C, et al. Patient and public involvement in a UK national institute for health research programme grant for applied research: experiences from the chronic headache education and self-management study (CHESS) Primary Health Care Res Develop. 2021;22:1–9. doi: 10.1017/S1463423621000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noyes J, McLaughlin L, Morgan K, Roberts A, Stephens M, Bourne J, et al. Designing a co-productive study to overcome known methodological challenges in organ donation research with bereaved family members. Health Expect. 2019;22:824–35. doi: 10.1111/hex.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nostlinger C, Loos J. Involving lay community researchers in epidemiological research: experiences from a seroprevalence study among sub-Saharan African migrants. AIDS Care. 2016;28(Suppl 1):119–123. doi: 10.1080/09540121.2016.1146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olding M, Hayashi K, Pearce L, Bingham B, Buchholz M, Gregg D, et al. Developing a patient-reported experience questionnaire with and for people who use drugs: A community engagement process in Vancouver’s Downtown Eastside. Int J Drug Policy. 2018;59:16–23. doi: 10.1016/j.drugpo.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Ostrach B. Human-centered design for a women's health screening tool: participant experiences. South Med J. 2020;113(10):469–474. doi: 10.14423/SMJ.0000000000001157. [DOI] [PubMed] [Google Scholar]
- 58.Pallesen KS, Rogers L, Anjara S, De Brun A, McAuliffe E. A qualitative evaluation of participants' experiences of using co-design to develop a collective leadership educational intervention for health-care teams. Health Expect. 2020;23(2):358–367. doi: 10.1111/hex.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinfold V, Szymczynska P, Hamilton S, Peacocke R, Dean S, Clewett N, et al. Co-production in mental health research: reflections from the people study. Ment Health Rev J. 2015;20(4):220–231. [Google Scholar]
- 60.Rayment J, Lanlehin R, McCourt C, Husain SM. Involving seldom-heard groups in a PPI process to inform the design of a proposed trial on the use of probiotics to prevent preterm birth: a case study. Res Involv Engagem. 2017;3:1–10. doi: 10.1186/s40900-017-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruff SC, Harrison K. "Ask Me What I Want": Community-based participatory research to explore transition-age foster Youth's use of support services. Child Youth Serv Rev. 2020;108:1–9. [Google Scholar]
- 62.Seeralan T, Harter M, Koschnitzke C, Scholl M, Kohlmann S, Lehmann M, et al. Patient involvement in developing a patient-targeted feedback intervention after depression screening in primary care within the randomized controlled trial get.Feedback.Gp. Health Expectations. 2020;24:95–112. [DOI] [PMC free article] [PubMed]
- 63.Sharmil H, Kelly J, Bowden M, Galletly C, Cairney I, Wilson C, et al. Participatory action research-dadirri-ganma, using yarning: methodology co-design with Aboriginal community members. International Journal for Equity in Health. 2021;20(1):160. doi: 10.1186/s12939-021-01493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simpson S, Cook A, Miles K. Patient and public involvement in early awareness and alert activities: an example from the United Kingdom. Int J Technol Assess Health Care. 2018;34(1):10–17. doi: 10.1017/S0266462317004421. [DOI] [PubMed] [Google Scholar]
- 65.Springs S, Rofeberg V, Brown S, Boudreau S, Hey SP, Baruch J. Community-engaged evidence synthesis to inform public health policy and clinical practice: a case study. Med Care. 2019;57:253–258. doi: 10.1097/MLR.0000000000001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stocker R, Brittain K, Hanratty B, Spilsbury K. Patient and public involvement in care home research: reflections on the how and why of involving patient and public involvement partners in qualitative data analysis and interpretation. Health Expect. 2021;24(4):1349–1356. doi: 10.1111/hex.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thomas F, Hansford L, Wyatt K, Byng R, Coombes K, Finch J, et al. An engaged approach to exploring issues around poverty and mental health: a reflective evaluation of the research process from researchers and community partners involved in the destress study. Health Expect. 2020;24:113–121. doi: 10.1111/hex.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tremblay M-C, Bradette-Laplante M, Berube D, Briere E, Moisan N, Niquay D, et al. Engaging indigenous patient partners in patient-oriented research: lessons from a one-year initiative. Res Involv Engagem. 2020;6:1–11. doi: 10.1186/s40900-020-00216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vat LE, Warren M, Goold S, Davidge EB, Porter N, Schuitmaker-Warnaar TJ, et al. Giving patients a voice: a participatory evaluation of patient engagement in Newfoundland and Labrador Health Research. Res Involv Engagem. 2020;6:1–14. doi: 10.1186/s40900-020-00206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Worsley JD, McKeown M, Wilson T, Corcoran R. A qualitative evaluation of coproduction of research: 'If you do it properly, you will get turbulence'. Health Expectations. 2021;1–9. [DOI] [PMC free article] [PubMed]
- 71.Fusco F, Marsilio M, Guglielmetti C. Co-production in health policy and management: a comprehensive bibliometric review. BMC Health Serv Res. 2020;20:1–16. doi: 10.1186/s12913-020-05241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen S, Doyle-Thomas KAR, Beesley L, Karmali A, Williams L, Tanel N, et al. How and why should we engage parents as co-researchers in health research? A scoping review of current practices. Health Expect. 2017;20(4):543–554. doi: 10.1111/hex.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bird M, Ouellette C, Withmore C, Li L, Nair K, MH M, et al. Preparing for patient partnership: A scoping review of patient partner engagement and evaluation in research. Health Expectations. 2020;23:523–39. [DOI] [PMC free article] [PubMed]
- 74.Harrison JD, Auerbach AD, Anderson W, Fagan M, Carnie M, Hanson C, et al. Patient stakeholder engagement in research: a narrative review to describe foundational principles and best practice activities. Health Expect. 2019;22(3):307–316. doi: 10.1111/hex.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jones M, Pietilä I. Alignments and differentiations: people with illness experiences seeking legitimate positions as health service developers and producers. Health. 2020;24:223–240. doi: 10.1177/1363459318800154. [DOI] [PubMed] [Google Scholar]
- 76.Locock L, Boaz A. Drawing straight lines along blurred boundaries: qualitative research, patient and public involvement in medical research, co-production and co-design. Evid Policy. 2019;15(3):409–421. [Google Scholar]
- 77.Ocloo J, Matthews R. From tokenism to empowerment: progressing patient and public involvement in healthcare improvement. BMJ Qual Saf. 2016;25(8):626–632. doi: 10.1136/bmjqs-2015-004839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCarron TL, Clement F, Rasiah J, Moran C, Moffat K, Gonzalez A, et al. Patients as partners in health research: a scoping review. Health Expect. 2021;24(4):1378–1390. doi: 10.1111/hex.13272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Staley K. ‘Is it worth doing?’ Measuring the impact of patient and public involvement in research. Res Involv Engagem. 2015;1(1):1–10. doi: 10.1186/s40900-015-0008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoekstra F, Mrklas KJ, Khan M, McKay RC, Vis-Dunbar M, Sibley KM, et al. A review of reviews on principles, strategies, outcomes and impacts of research partnerships approaches: a first step in synthesising the research partnership literature. Health Res Policy Syst. 2020;18:1–23. doi: 10.1186/s12961-020-0544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mortensen N, Brix J, Krogstrup H. Reshaping the Hybrid Role of public servants: identifying the opportunity space of co-production and the enabling skills required by professional co-producers. In: Sullivan H, Dickinson H, Henderson H, editors. The Palgrave Handbook of the Public Servant. Switzerland: Palgrave Macmillan; 2020. pp. 1–17. [Google Scholar]
- 82.Grant M, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26:91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 83.Haby M, Chapman E, Clark R, Barreto J, Reveiz LJ. What are the best methodologies for rapid reviews of the research evidence for evidence-informed decision making in health policy and practice: a rapid review. Health Res Policy Syst. 2016;14:1–12. doi: 10.1186/s12961-016-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dobbins M. Rapid review guidebook - steps for conducting a rapid review. Canada: The National Collaboration Centre for Methods and Tools 2017.
- 85.Boden C, Edmonds A, Porter T, Bath B, Dunn K, Gerrard A, et al. Patient partners' perspectives of meaningful engagement in synthesis reviews: a patient-orientet rapid review. Health Expect. 2021;24:1056–1071. doi: 10.1111/hex.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Page M, McKenzie J, Bossuyt PM, Boutron I, TC H, Mulrow C, The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karlsson A, Kragh-Sørensen A, Rothmann M, Ketelaar M, Janssens A. Partnerships within coproduced research - a rapid review protocol. Open Science Framework. 2021.
- 88.Staniszewska S, Brett J, Mockford C, Barber R. The GRIPP checklist: Strengthening the quality of patient and public involvement reporting in research. Int J Technol Assess Health Care. 2011;27(4):391–399. doi: 10.1017/S0266462311000481. [DOI] [PubMed] [Google Scholar]
- 89.Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Res Involv Engagem. 2017;3(1):1–11. doi: 10.1186/s40900-017-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rogers M, Bethel A, Boddy K. Development and testing of a medline search filter for identifying patient and public involvement in health research. Health Info Libr J. 2017;34(2):125–133. doi: 10.1111/hir.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cooke A, Smith D, Booth A. Beyond PICO - The SPIDER Tool for Qualitative Evidence Synthesis. Qual Health Res. 2012;22(10):1435–1443. doi: 10.1177/1049732312452938. [DOI] [PubMed] [Google Scholar]
- 92.Bailey S, Boddy K, Briscoe S, Morris C. Involving disabled children and young people as partners in research: a systematic review. Child Care Health Develop. 2015;41(4):505–14. doi: 10.1111/cch.12197. [DOI] [PubMed] [Google Scholar]
- 93.Cochrane Training. Consumer involvement: The Cochrane Collaboration; 2022 [Available from: https://training.cochrane.org/online-learning/consumer-involvement.
- 94.Smits D-W, Van Meeteren K, Klem M, Alsem M, Ketelaar M. Designing a tool to support patient and public involvement in research projects: the Involvement Matrix. Res Involv Engagem. 2020;6(1):1–7. doi: 10.1186/s40900-020-00188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.ICMJE. Defining the Role of Authors and Contributors: International Committee of Medical Journal Editors; 2022 [Available from: https://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html.
- 96.Center for Research with Patients and Relatives. Forskning i ForSa-P Odense, Denmark: Odense University Hospital; 2022 [Available from: https://ouh.dk/til-patienter-og-parorende/odense/centre/center-for-forskning-sammen-med-patienter-og-parorende/forskning-i-forsa-p
- 97.Hernandez ND, Dorsey J, Glass DM, Pope E, Worthy N, Blasingame E, et al. Community-engaged approaches to address the ethical concerns of maternal mental health disparities research. J Health Care Poor Underserved. 2019;30(4S):12–20. doi: 10.1353/hpu.2019.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nicholson L, Colyer M, Cooper SA. Recruitment to intellectual disability research: a qualitative study. J Intellect Disabil Res. 2013;57(7):647–656. doi: 10.1111/j.1365-2788.2012.01573.x. [DOI] [PubMed] [Google Scholar]
- 99.Shklarov S, Marshall DA, Wasylak T, Marlett NJ. “Part of the Team”: Mapping the outcomes of training patients for new roles in health research and planning. Health Expect. 2017;20(6):1428–1436. doi: 10.1111/hex.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Green G, Johns T. Exploring the Relationship (and power dynamics) between researchers and public partners working together in applied health research teams. Front Sociol. 2019;4:1–10. doi: 10.3389/fsoc.2019.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pascal J, Sagan O. Cocreation or collusion: the dark side of consumer narrative in qualitative health research. Illn Crisis Loss. 2018;26(4):251–269. [Google Scholar]
- 102.Fricker M. Testimonial Injustice. Epistemic Injustice: Power and the Ethics of Knowing. online edition ed. UK: Oxford Academic; 2007. p. 9–29.
- 103.Carel H, Kidd I. Epistemic Injustice in Medicine and Healthcare. In: Kidd I, Medina J, Pohlhaus G, editors. The Routledge Handbook of Epistemic Injustice. Online: Routledge; 2017. pp. 336–346. [Google Scholar]
- 104.Boote JD, Dalgleish M, Freeman J, Jones Z, Miles M, Rodgers H. ‘But is it a question worth asking?’ A reflective case study describing how public involvement can lead to researchers’ ideas being abandoned. Health Expect. 2014;17(3):440–451. doi: 10.1111/j.1369-7625.2012.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Richards DP, Birnie KA, Eubanks K, Lane T, Linkiewich D, Singer L, et al. Guidance on authorship with and acknowledgement of patient partners in patient-oriented research. Res Involv Engagem. 2020;6(1):38. doi: 10.1186/s40900-020-00213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Price A, Schroter S, Snow R, Hicks M, Harmston R, Staniszewska S, et al. Frequency of reporting on patient and public involvement (PPI) in research studies published in a general medical journal: a descriptive study. BMJ Open. 2018;8(3):e020452. doi: 10.1136/bmjopen-2017-020452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malterud K, Elvbakken KT. Patients participating as co-researchers in health research: A systematic review of outcomes and experiences. Scandinavian J Public Health. 2020;48(6):617–628. doi: 10.1177/1403494819863514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Consisting of appendix A, B, C, D with extra information on search terms, PPIE activities, inclusion and exclusion criteria, alterations from the protocol and further tables and figures supporting the presentation of our findings.
Data Availability Statement
The protocol for this study is available at: https://doi.org/10.17605/OSF.IO/QMWVK. The datasets generated and analysed during the current study are included in this published article and its supplementary information files.