Significance
This study identifies a cell type predominantly localized to the lower airways of the developing human lung. These cells are defined by coexpression of SCGB3A2/SFTPB/CFTR, and functional studies show that they give rise to pulmonary neuroendocrine cells and a subpopulation of multiciliated cells, thereby shaping the cellular landscape of the lower airways.
Keywords: lung organoid, bud tip progenitor, basal cell, multiciliated cell, neuroendocrine cell
Abstract
Using scRNA-seq and microscopy, we describe a cell that is enriched in the lower airways of the developing human lung and identified by the unique coexpression of SCGB3A2/SFTPB/CFTR. To functionally interrogate these cells, we apply a single-cell barcode-based lineage tracing method, called CellTagging, to track the fate of SCGB3A2/SFTPB/CFTR cells during airway organoid differentiation in vitro. Lineage tracing reveals that these cells have a distinct differentiation potential from basal cells, giving rise predominantly to pulmonary neuroendocrine cells and a subset of multiciliated cells distinguished by high C6 and low MUC16 expression. Lineage tracing results are supported by studies using organoids and isolated cells from the lower noncartilaginous airway. We conclude that SCGB3A2/SFTPB/CFTR cells are enriched in the lower airways of the developing human lung and contribute to the epithelial diversity and heterogeneity in this region.
For decades, scientists have relied on morphology, histologic approaches, and low throughput methods to visualize a limited number of proteins or mRNA in human tissues. Identification and characterization of specialized cell types within human organs has been improved by recent technological advances in single-cell genomic and high-resolution spatial transcriptomic approaches to interrogate tissue composition, cellular heterogeneity, and organization (1–21). Over the past few years, troves of single-cell and spatial transcriptomic data within the lung field have described the vast cellular heterogeneity in the adult human lung at homeostasis (2, 9, 13, 22–24), or in various pathologic states, including (but not limited to) asthma (24), pulmonary fibrosis (12, 14, 17), cystic fibrosis (5), and infections such as SARS-CoV-2 (7, 25). Several studies have also revealed the complexities of the developing human lung (1, 11, 18, 20, 21, 26). This new information has shed light on cellular heterogeneity within the human lung, including how cells change fate throughout the process of development, and has identified interesting new cell types or states that are uniquely human with no obvious correlate in the well-studied murine lung (9, 22). However, studying human organs and tissue has inherent limitations based on ethical considerations and limited access to tissue, often making functional follow-up studies difficult.
Our group recently published a single-cell RNA sequencing (scRNA-seq) dataset characterizing the human fetal lung epithelium from 8 to 21 wk postconception (1). During our prior analysis, we identified a cohort of cells expressing a unique combination of genes encoding secreted proteins including SCGB3A2 and SFTPB. Recently, a population of cells with a similar expression profile were identified in the terminal respiratory bronchioles of adult human lungs (9, 22); however, human fetal SCGB3A2/SFTPB cells also express CFTR, which was not described in these adult cells. SCGB3A2/SFTPB/CFTR cells in fetal lung appear to lack genes canonically associated with other secretory (i.e., club, goblet), alveolar (i.e., alveolar epithelial type II cells), or ionocyte cell types. Based on their specificity to lung development, anatomic location, and progenitor-like capabilities described here, we refer to this population as SCGB3A2+ Lower Airway Progenitor (LAP) cells.
The goal of the current work is to characterize the LAP cell population spatially and temporally, and to understand the role of this population in the developing human lung. In situ analyses show that LAP cells arise during branching morphogenesis and are enriched in lower small cartilaginous and noncartilaginous airways (NCA) of the fetal lung. Organoids from the upper airway (trachea) and NCA show that LAP cells are only present in NCA organoids. In vitro lineage tracing and sorting experiments using organoids suggest that LAP cells are an airway progenitor, distinct from the basal cell lineage, predominantly giving rise to pulmonary neuroendocrine cells (PNECs) and a subset of multiciliated cells defined by C6 expression, while basal cells predominantly give rise to club cells and multiciliated cells defined by MUC16 expression. In vivo, MUC16 is enriched in multiciliated cells of the upper airway while C6 is enriched in multiciliated cells of the lower airways, supporting the lineage predictions from in vitro experiments. Collectively, this study reveals complex cellular heterogeneity across the proximal–distal axis of the lung driven by regionally defined progenitor cells within the fetal lung epithelium.
Results
scRNA-seq Identifies an Epithelial Cell during Development Defined by Coexpression of SCGB3A2/SFTPB/CFTR.
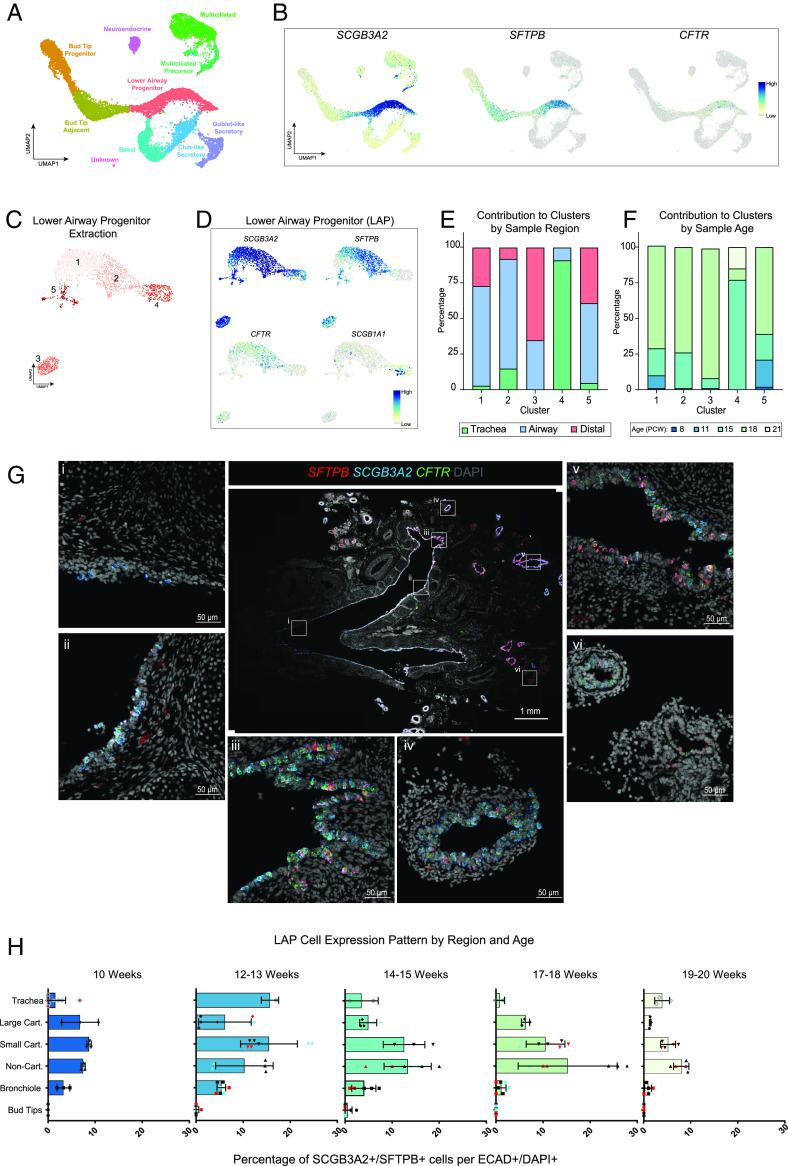
Original scRNA-seq data from our group (1) composing of 8 to 21 post conception weeks (PCW) human lung tissue dissections of trachea, proximal airway, and distal airway, was reanalyzed to interrogate epithelial heterogeneity (Fig. 1A). The data were analyzed using Seurat (27–30); epithelial populations were identified using Louvain clustering (31) and visualized via UMAP (32, 33) (Fig. 1A). Cell populations were annotated using gene lists generated by differential gene expression that correlate with previously published cell signatures (1) (SI Appendix, Fig. S1A). This analysis identified many of the previously characterized populations of lung epithelial cell types including bud tip progenitors (BTPs), bud tip adjacent (BTA) cells, basal cells, secretory cells including club and goblet cells, multiciliated cells, and PNECs (Fig. 1A and SI Appendix, Fig. S1A). We also identified a robust population of cells that we call LAP cells, that was positioned on the UMAP embedding between the BTA cluster, ‘basal cell’ cluster, and ‘club-like secretory’ cluster (Fig. 1A, pink cluster). This cluster has a unique gene expression profile that included coexpression of SCGB3A2, SFTPB, and CFTR (Fig. 1B). In addition to SCGB3A2/SFTPB/CFTR expression, these cells also have enriched expression for CYTL1, MUC1, SLC4A4, and STEAP4 (SI Appendix, Fig. S1A). Consistent with our findings, a SCGB3A2/CYTL1 population was also recently described in an independent set of data (21). Given that SCGB3A2 expression has been associated with club cells, SFTPB with alveolar type II cells, and CFTR with ionocytes and other cells in the adult airway (5, 13, 34), we further interrogated the LAP cell population for genes expressed in these cell types (SI Appendix, Fig. S1 A and B). LAP cells did not express high levels of club cell markers SCGB1A1 or SCGB3A1, or alveolar markers SFTPC and SFTPA1. The ionocyte marker FOXI1 was undetectable in the LAP cell cluster. When examining coexpression of SCGB3A2, SCGB3A1, and SCGB1A1 by in situ hybridization across several tissue specimens (SI Appendix, Fig. S1C), abundant SCGB3A1+ and SCGB1A1+ cells were present in the trachea, with some cells coexpressing SCGB3A2; fewer coexpressing cells were found in the bronchi, small cartilaginous, and NCA. Within the bronchi, small cartilaginous and NCA, abundant SCGB3A2+/SCGB3A1―/SCGB1A1― cells were present, with few cells expressing SCGB3A1 or SCGBA1 at all. We further investigated this heterogeneity by computationally extracting and reclustering the LAP cells (Fig. 1C). Reclustering predicted five subclusters, with subcluster 2 possessing the highest coexpression of SCGB3A2/SFTPB/CFTR, as well as the lowest expression of the club cell marker SCGB1A1 (Fig. 1 C and D and SI Appendix, Fig. S1D). Notably, RNASE1 was also enriched in subcluster 2 which has recently been reported in an SCGB3A2+/SFTPB+/SCGB1A1- cell population in the adult (9). Additional canonical secretory genes were interrogated and found to be minimally expressed in subcluster 2 (SI Appendix, Fig. S1D). To expand our analysis, we analyzed the contribution of each sample to the LAP cell subclusters by sample age and lung region (Fig. 1 E and F and SI Appendix, Fig. S1 E–G). When we analyzed the contribution to each LAP cell subcluster, we observed that the majority (>85%) of cells contributing to subclusters 1, 2, 3, and 5 are from airway and distal samples, and that cluster 4 is predominantly comprised of cells from the trachea (91%). By age, subclusters 1, 2, 3, and 5 are mostly comprised of cells from the 11, 15, and 18 PCW samples (>90%) while subcluster 4 (predominantly derived from trachea cells) is populated by the 15 and 21 PCW samples. SCGB1A1+ cells found in subcluster 4 have a secretory profile including MUC5B and SCGB3A1 and are distinct from bona fide LAP cells.
Fig. 1.
scRNA-sequencing identifies a SCGB3A2+/SFTPB+/CFTR+ progenitor cell in the lower airways of the developing human lung. (A) UMAP cluster plot of scRNA-seq data from human fetal lung epithelium. 6 biologically distinct samples were sequenced at PCW ages 8, 11, 15, 18, 19, 21 from distinct regions of trachea, airway, or distal lung. Each dot represents a single cell, and cells were computationally clustered based on transcriptional similarities. Clusters are colored and labeled by cell type, which were determined based on expression of canonical cell-type markers displayed in the dot plots in SI Appendix, Fig. S1. (B) UMAP feature plots of highly expressing genes SCGB3A2, SFTPB, and CFTR, which are coexpressed predominantly in the LAP cluster from Fig. 1A. (C) UMAP cluster plot of LAP cluster from Fig. 1A computationally extracted and reclustered. (D) Feature plots of SCGB3A2, SFTPB, CFTR, and SCGB1A1. SCGB3A2, SFTPB, and CFTR are coexpressed predominantly in subcluster 2. SCGB1A1 is highly expressed only in subcluster 4. (E) Quantification of tissue samples’ contributions to LAP cell subclusters are reported as percentages by the lung region. (F) Quantification of tissue samples’ contributions to LAP cell subclusters are reported as percentages by sample age (PCW). (G) Representative fluorescence in situ hybridization (FISH) images of 12 PCW lung for LAP cell markers SCGB3A2, SFTPB, and CFTR. Boxed regions within middle panel correspond to representative regions shown including trachea i), bronchus ii, iii), small cartilaginous v), noncartilaginous iv) and distal bud tip vi). (H) Quantification of SCGB3A2+/SFTPB+ expressing cells among 100 ECAD+/DAPI+ cells imaged across distinct lung regions (trachea, large cartilaginous, small cartilaginous, noncartilaginous, bronchioles, bud tips) and ages (10 to 20 PCW). Each symbol in the bar chart corresponds to quantifications from separate biological replicates at each timepoint.
To identify LAP cells in tissue, we performed fluorescence in situ hybridization (FISH) for SCGB3A2, SFTPB, and CFTR in human fetal lung samples ranging from 8 to 20 PCW. Abundant SCGB3A2+/SFTPB+/CFTR+ coexpressing cells were identified at all stages examined. Notably, in tissue sections, we observed SCGB3A2+, SFTPB+, or SCGB3A2+/SFTPB+ expressing cells also coexpressed CFTR, indicating that LAP cells could be identified by coexpression of SCGB3A2 plus SFTPB, but not by either marker alone. Representative low- and high-magnification images of human lung tissue sections from trachea, bronchi, small cartilaginous, noncartilaginous, and distal bud tip regions are shown (Fig. 1G). To support scRNA-seq and FISH data, we also identified LAP cells using SCGB3A2/SFTPB/ECAD coimmunofluorescence (co-IF) across several ages and airway regions and quantified the spatiotemporal location of LAP cells within the epithelium (Fig. 1H). We observed changes in abundance across time and region that are consistent with our scRNA-seq analysis (Fig. 1 E and F and SI Appendix, Fig. S1 E–G). In 10 PCW samples, 27% of epithelial cells coexpressed SCGB3A2 and SFTPB. LAP cells were most abundant (comprising 53% of epithelial cells) in 12 to 13 PCW samples, with the number of cells decreasing to 19% of epithelial cells coexpressing SCGB3A2/SFTPB at 20 PCW (38% in 13 to 14 PCW, 33.8% in 17 to 18 PCW). The regional distribution of cells demonstrated that SCGB3A2+/SFTPB+ cells are most abundant in the small cartilaginous (52.4%) and NCA (54.2%) with far fewer cells present in more proximal (25.3% trachea, 25.5% large cartilaginous) or distal (14% bronchiole, 0% bud tip) regions (Fig. 1H). Together, this analysis suggests LAP cells are enriched in the lower small cartilaginous and NCA of the developing lung, peak in abundance at 12 to 13 PCW and decrease by 20 PCW.
An In Vitro Bud Tip Organoid Differentiation Model to Functionally Interrogate LAP Cells.
To generate LAP cells for functional studies in vitro, we used primary human bud tip organoids (BTOs) (1, 35, 36). Under maintenance conditions, these organoids are a homogenous population of SOX2+/SOX9+ BTPs (1, 35) and can be differentiated into airway using a 21-d “dual SMAD activation/inhibition” (DSA/DSI) airway differentiation paradigm, which includes 3 d of DSA followed by 18 d of DSI (SI Appendix, Fig. S2A, see Methods, and refs. 1 and 37). This protocol robustly induces airway cell types including basal, multiciliated, neuroendocrine, and secretory cells, and prior scRNA-seq suggested that at 21-d, induced airway organoids possess abundant LAP cells (1, 37). However, when LAP cells emerge during airway differentiation, and their perdurance across time in organoid culture was not interrogated in prior studies. To better understand when LAP cells may begin to differentiate during in vitro airway differentiation, we performed a time course using RT-qPCR on BTOs throughout the DSA/DSI protocol (SI Appendix, Fig. S2B). BTOs were collected at 30 min, 3 h, 24 h, and 3 d during DSA, and 30 min, 3 h, 24 h, 11 d, and 17 d during DSI. Consistent with our previous reports, TP63, a marker of early airway differentiation, increased during the first 24 h and remained elevated (1); LAP cell markers (SCGB3A2, SFTPB) increased after switching to DSI media, and expression remained elevated throughout the remainder of the time series (SI Appendix, Fig. S2B). FISH with co-IF on 21-d airway organoids confirmed the presence of SCGB3A2+/SFTPB+/CFTR+ LAP cells (SI Appendix, Fig. S2C) and reanalysis of previously published scRNA-seq data from 21-d airway organoids (1) identified a large proportion of cells coexpressing the LAP cell markers SCGB3A2/SFTPB/CFTR (SI Appendix, Fig. S2D). Finally, to confirm the identity of LAP cells present in organoids, we performed reference-based mapping of organoid scRNA-seq data to scRNA-seq data of the human fetal lung (SI Appendix, Fig. S2 E, Left plot). Of the 1,083 cells in the 21-d airway organoid we identified as LAP cells, 93.8% of cells map to the fetal epithelial LAP cell cluster, 5.3% of cells map to the BTA cluster, and 0.9% to the club cell cluster. Altogether, these data show that the 21-d airway organoid differentiation paradigm is a robust model that can be used to functionally investigate LAP cells in vitro.
Single-Cell Barcode Lineage Tracing Suggests That LAP Cells Are Progenitors for PNECs and Multiciliated Cells.
We leveraged the induced airway organoid model to determine if LAP cells have the potential to give rise to other airway epithelial cells during airway differentiation. To do this, we implemented a barcode-based lineage tracing technique, called CellTagging (38), which utilizes a complex lentiviral library of unique and heritable barcodes affixed to the 3’ UTR of GFP enabling us to tag and track clones of individual cells using scRNA-seq. 21-d induced airway organoids were transduced with the lentiviral CellTagging V1 library and replated in Matrigel to reform organoids (Fig. 2A, SI Appendix, Fig. S2A, and see Methods). GFP expression, introduced by the CellTag library, could be detected within 24 h and was highly expressed on day 7 (SI Appendix, Fig. S2F). RT-qPCR of infected organoids and uninfected controls demonstrated no differences in expression of canonical lung epithelial markers (SI Appendix, Fig. S2G) suggesting viral transduction had no effect on cell-type composition of the organoids. On day 7, infected cultures were dissociated into a single-cell suspension and subjected to fluorescence-activated cell sorting (FACS) to isolate GFP+ cells, demonstrating that approximately 15% of live cells were GFP+ (SI Appendix, Fig. S2H, average of three experiments). We also used a well-established cell surface marker of BTPs, CPM, to remove any undifferentiated BTPs from the culture (Fig. 2A and SI Appendix, Fig. S2H). To establish clonal organoids after FACS, single cells were replated in Matrigel at low density to encourage clonal growth, so that the majority of organoids should derive from a single uniquely tagged cell (Fig. 2A). Organoids reformed and were allowed to expand for 30 d at which point they were collected for scRNA-seq (Fig. 2A).
Fig. 2.

Barcoding-based lineage tracing identifies separate lineages from LAP cells and basal cells in vitro. (A) Schematic displaying experimental design for lineage-tracing BTOs using CellTagging. BTOs were differentiated using the 21-d airway differentiation paradigm (SI Appendix, Fig. S2A). At 21 d, induced airway organoids were transduced with the CellTagging lentiviral-based library of barcoded GFP plasmids. After 7 d, GFP+ cells were sorted and remaining BTPs were removed using CPM. Single clones were replated and grown for 30 d, then submitted for scRNA-sequencing. (B) UMAP feature plots of cell-type scoring annotation for predominant cell types on CellTagged airway organoids. Cells most aligning to the scoring set are in orange, with little to no scoring represented in blue. (C) UMAP cluster plot of sequenced 58-d CellTagged airway organoids. Each dot represents a single cell, and cells were computationally clustered based on transcriptional similarities. Clusters were colored and labeled by cell type, which were determined based on expression of ‘Cell-type score’ (Fig. 2B and SI Appendix, Fig. S2J and Table S2) derived from fetal epithelial dataset. (D) UMAP plots of CellTagged airway organoids overlaid with cells labeled from LAP-rooted or basal-rooted clones. (E) UMAP plot of LAP-rooted and basal-rooted clones overlaid. (F) Quantification of cell types within LAP- or basal-rooted clones. Number of cells per cell type was divided by total number of cells of -rooted clone sample to normalize and report as percentage. (G) UMAP cluster plot of multiciliated clone extraction from 58-d CellTagged airway organoids. CellTag-expressing cells within the multiciliated cluster in Fig. 2C were computationally extracted and subclustered resulting in three subclusters. Cells from LAP-rooted or basal-rooted clones are shown as red dots in Top Middle and Right panels. Bottom panels are UMAP feature plots of multiciliated marker FOXJ1, and enriched genes C6 and MUC16. (H) Dot plot for expression of C6 and MUC16 between LAP-rooted and basal-rooted clones within multiciliated clone extraction (Fig. 2G). (I) Hypothesized model of airway differentiation cellular hierarchy from CellTagging predictions.
Following scRNA-seq, cell-type clusters within the CellTagging dataset were identified using a gene module-based cell scoring method where the top 50 genes enriched in each epithelial cell type in scRNA-seq from fetal epithelium (Fig. 1A) were used to annotate the identity of each cell in the CellTagging data (Fig. 2B and SI Appendix, S1A and S2 J and K). The cell types identified in this organoid model shared a high degree of transcriptional similarity to in vivo basal cells, secretory cells, multiciliated precursors (deuterosomes), multiciliated cells, PNECs, and LAP cells (Fig. 2 B and C and SI Appendix, Table S2). One cluster remained unidentified by the cell-type scoring; however, given that this cluster expressed early markers of PNEC differentiation (ASCL1HIGH) (37, 39–42) and its proximity to the PNEC cluster, we designated this cluster as a PNEC precursor (pink cluster).
To further validate the gene-module-based scoring (Fig. 2B), LAP cells from the CellTagging experiment were also queried against the fetal epithelial reference data using reference-based mapping (SI Appendix, Fig. S2 E, Right plot). Of note, 85.6% of LAP cells from the 58 d CellTagging experiment mapped to the fetal LAP cell cluster while 12.4% of cells mapped to the area between LAP cells and basal cells. 1.7% of cells mapped to the club cell clusters.
Next, we investigated the CellTag clone distributions to determine if there were general trends within the clonal relationships of cell types. Of 8,256 total cells sequenced that passed quality control parameters, 8,165 (98.9% of sequenced cells) contained at least one CellTag read (barcode). Subsequent filtering steps, such as requiring all CellTags to be read twice and considering only cells with ≥2 and ≤20 CellTags for clone calling left 5,789 tagged cells (70.1%). Clone calling identified 280 distinct clones containing 3,701 tagged cells and further classification of high frequency clones (>10 cells/clone) identified 43 clones containing 2,895 cells (35.1%), which were called ‘CellTagged’ cells. Each cluster contained a similar percentage (30 to 50%) of cells that were called ‘CellTagged’ cells using these metrics, suggesting the presence of a CellTag did not alter cell fate (SI Appendix, Fig. S2I). We interrogated clones containing LAP cells and noted that in the vast majority of cases these clones were devoid of basal and secretory cells, suggesting LAP cells may comprise a lineage distinct from basal-secretory. Based on this, clones enriched for LAP or basal cells were combined into groups: If a clone had ≥80% LAP cells, they were classified as “LAP-rooted”, whereas clones containing ≥80% basal cells were classified as “basal-rooted” (Fig. 2D). Of the 43 clones analyzed, there were 24 LAP-rooted clones (n = 955 cells) and 13 basal-rooted clones (n = 932 cells). Among the LAP-rooted and basal-rooted clones, cells occupy distinct regions of the UMAP (Fig. 2E) suggesting they represent distinct cell lineages. We quantified the distribution of cells attributed to LAP-rooted and basal-rooted clones (Fig. 2F) and observed that even with the 80% threshold, a very small portion of LAP cells are present in basal-rooted clones and vice versa (all LAP-rooted clones contain 1.6% basal cells, while all basal-rooted clones contain 3.3% LAP cells). Striking trends are found among the remaining cell types: PNECs and the ASCL1+ PNEC precursors are predominantly present within LAP-rooted clones (19% of cells in LAP-rooted clones are PNECs or PNEC precursors, compared to only 2% of cells in basal-rooted clones), while secretory cells make up 3% of cells in LAP-rooted clones compared to 37% of cells in basal-rooted clones. Finally, both populations contained a similar percentage of multiciliated and multiciliated precursor cells (21.8% of cells in LAP-rooted, 21% of cells in basal-rooted). Collectively, these data suggest that PNECs/PNEC precursors and multiciliated cells/multiciliated precursors share a cellular origin with LAP cells, while secretory and multiciliated cells/multiciliated precursors share a cellular origin with basal cells.
LAP Cells and Basal Cells Contribute to Multiciliated Cell Heterogeneity.
We noted that despite the difference in potential for LAP and basal cells to give rise to PNEC and secretory cells, respectively, in our assay, both LAP and basal cells shared contribution to the multiciliated cell lineage. However, within the multiciliated cluster, cells from LAP- and basal-rooted clones occupied distinct regions, suggesting that multiciliated cells are transcriptionally distinct based on cell of origin (Fig. 2E). To further explore transcriptional heterogeneity among multiciliated cells during in vitro airway differentiation, we computationally extracted and subclustered the multiciliated and multiciliated precursor clones from the organoid data (Fig. 2G). Subclustering predicted three subclusters within the extracted multiciliated cells. Among these cells, the LAP-rooted and basal-rooted clones occupy distinct subclusters: LAP-rooted clones predominate cluster 3 (blue) and a subset of cluster 1 (pink); basal-rooted clones occupy cluster 2 (green) and a subset of cluster 1 (pink) (Fig. 2G). Differential gene expression across clusters (SI Appendix, Table S3) allowed us to identify two candidate genes that distinguish multiciliated cells based on cell of origin. The complement component C6 is enriched within the LAP-rooted subset while MUC16, which was recently identified as a multiciliated marker in the adult trachea (5), is specific to the basal-rooted subset (Fig. 2 G and H).
As a result of the suggested lineage relationships from the CellTagging experiment, we proposed a revised model of airway differentiation within the developing human lung, where BTPs give rise to both LAP and basal cells, which then give rise to distinct differentiated cell types including subsets of molecularly distinct multiciliated cells (Fig. 2I). To test this model, and to determine if multiciliated cell heterogeneity is observed in vivo, we investigated the multiciliated cell population in the developing human lung epithelium using scRNA-seq data (Fig. 3 A and B and SI Appendix, Fig. S3A). In scRNA-seq data, C6 was indeed enriched in the multiciliated cluster while MUC16 was seen in the multiciliated cell, multiciliated precursor, and club-like secretory cell clusters at lower expression levels. Similar to the organoid data (Fig. 2), within the multiciliated cell cluster, these genes occupied distinct areas in the UMAP embedding (Fig. 3A). To interrogate this heterogeneity further, we computationally extracted and reclustered the multiciliated cluster from the human epithelial data (Fig. 3B and SI Appendix, Fig. S3A). Reclustering predicted seven clusters; three clusters (1, 2, 6) expressed the highest levels of FOXJ1, and had very low levels of other secretory markers (i.e. SCGB1A1, SCGB3A2, MUC5B). Cluster 2 was FOXJ1+/ MUC16 HIGH/C6 LOW, while cluster 1 was FOXJ1+/MUC16 LOW/C6 HIGH. This analysis shows that there are transcriptionally distinct MUC16 HIGH/C6 LOW and MUC16 LOW/C6 HIGH multiciliated cells within the human fetal lung.
Fig. 3.
Developing human lung exhibits regionally specific cellular heterogeneity along the proximal–distal axis. (A) UMAP feature plots of C6 and MUC16 on fetal lung epithelium UMAP from Fig. 1A, reintroduced here. (B) UMAP cluster plot of multiciliated cell extraction from fetal epithelium data. The multiciliated cell cluster was computationally extracted and reclustered resulting in seven subclusters. The plot is colored and numbered by cluster. Feature plots for C6 and MUC16 on multiciliated cell extraction plot. (C) FISH with co-IF staining on paraffin sections of 12-PCW fetal lung representative for multiciliated cell markers C6, MUC16, and FOXJ1, across different lung regions ranging from trachea, large cartilaginous, small cartilaginous and NCA. (D) Quantification of C6+ and MUC16+ expressing cells as a percentage of FOXJ1+ expressing cells among the four regions defined in C. Each dot represents quantification of one image; entire dataset contains six biologic replicates. (E) Schematic of regional airway organoid model derived from primary tissue including representative brightfield images of trachea- and noncartilaginous airway (NCA)-derived organoids after passage 2. (F) FISH with co-IF staining on paraffin sections of representative trachea- or NCA-derived organoids for basal cell markers (TP63, KRT5), LAP cell markers (SCGB3A2, CFTR, SFTPB), multiciliated cell markers (MUC16, C6, FOXJ1), and PNEC markers (CHGA, SYN) after passage 2. Insets on select images highlight RNA/protein expression in specific cells. Large images (Scale bar is 50 µm.), all Insets (Scale bar is 10 µm.) (G) RT-qPCR data comparing expression of various lung epithelial cell type markers including basal, LAP, and other differentiated cell types (multiciliated, PNEC, secretory, goblet) between trachea- and NCA-derived organoids after passage 2. Each dot represents a technical replicate, with the shape defining its biologic replicate. This quantification was performed on three technical replicates from four biological replicates (separately derived organoids lines). Error bars represent SEM. Statistical tests were performed by Welch’s t test (unpaired, two-tailed). P-values are (*) <0.05, (**) <0.01, (***) <0.001, (****) <0.0001.
Given that basal cells are more abundant in the proximal airways, and LAP cells are more abundant in the lower airways (Fig. 1), we hypothesized that the subpopulations of multiciliated cells observed in scRNA-seq data may represent heterogeneity across the proximal–distal axis of the lung. To test this idea, we carried out FISH for C6 and MUC16 with co-IF for FOXJ1 on human lung samples across the proximal-distal of the airway (trachea, large cartilaginous, small cartilaginous airway, NCA) and quantified the percentage of FOXJ1+ cells expressing C6 or MUC16 within these regions (Fig. 3 C and D). MUC16 and C6 expression were inversely correlated across the proximal–distal axis of the airway. That is, we observed that MUC16+ cells are most abundant in the trachea with decreasing cell numbers in the more distal airways, while C6+ cells were least abundant in the trachea and became more abundant in the distal airways. Given the clonal relationship between LAP cells, multiciliated cells/multiciliated precursors and PNEC/PNEC precursors in the CellTagging data (Fig. 2), we also examined primary lung tissue sections to determine if we could identify LAP cells coexpressing markers of multiciliated and PNEC cells (SI Appendix, Fig. S3 B and C). We carried out FISH with co-IF for SCGB3A2/FOXJ1/C6 to identify cells with both LAP and multiciliated cell markers (SI Appendix, Fig. S3B) and SCGB3A2/SFTPB/CHGA to identify cells with both LAP and PNEC markers. In both cases, cells coexpressing LAP cell markers with multiciliated cell (FOXJ1+) or PNEC (CHGA+) markers, respectively, were readily observed (SI Appendix, Fig. S3C). Collectively, these data suggest that multiciliated cells are heterogeneous, with regionally distinct gene profiles that are correlated with anatomical enrichment of LAP cells in the lower airways or basal cells in the upper airways and trachea.
Airway Organoids Retain Regional Patterning and Cellular Heterogeneity.
To further investigate regional heterogeneity within the airway, we established separate organoid lines using microdissected tissue from the trachea and NCA regions of primary fetal lung tissue (Fig. 3E) (see Methods). Organoids were collected after passage 2 to validate their regional heterogeneity with both protein (IF) and RNA expression (FISH, RT-qPCR) (Fig. 3 F and G and SI Appendix, Fig. S3 D and E). By examining IF/FISH in tissue sections and RT-qPCR, trachea organoids were enriched for basal cell markers (TP63, F3, KRT5, EGFR) as well as MUC16. By FISH, trachea organoids possessed SCGB3A1+ cells and SCGB1A1+ cells but lacked SCGB3A2+ cells (SI Appendix, Fig. S3D) and were entirely devoid of SCGB3A2+/SFTPB+/CFTR+ LAP cells (Fig. 3 F and G). In contrast, NCA organoids had few, sporadic TP63+ cells, but abundant SCGB3A2+/SFTPB+/CFTR+ LAP cells (Fig. 3F). NCA organoids contain significantly higher expression of several genes enriched in LAP cells when compared to trachea organoids (SCGB3A2, SFTPB, MUC1, SLC4A4, STEAP4) and had enriched expression of the multiciliated marker C6, and PNEC markers (ASCL1, CHGA, SYN). NCA organoids possess some SCGB1A1+ and SCGB3A1+ cells, which do not coexpress SCGB3A2 (SI Appendix, FIg. S3D). Using IF, we also observed that NCA organoids did not express alveolar markers such as SFTPC or ABCA3, confirming that these organoids were enriched for airway tissue derived from the NCA region.
Functional Assessment of Basal and LAP Cell Lineages In Vitro.
Given that NCA organoids had some TP63+ cells, as well as LAP cells, we used FACS to isolate sporadic basal and abundant LAP cells from the NCA organoids, expand these isolated cells into new organoids and then assess the cell types present in the organoids derived from basal or LAP cells (Fig. 4). To isolate basal cells, we sorted using antibodies that recognize EGFR and F3 (Fig. 4B), which we have previously shown as a robust method to isolate basal cells from the fetal lung (1). To isolate LAP cells, we sorted for MUC1+ cells (Fig. 4B), which we identified as an enriched gene in LAP cells using the scRNA-seq data, and validated to have cell surface expression with commercially available antibodies (SI Appendix, Figs. S1A and S4A). MUC1 is also expressed in alveolar cells (43), bud tip progenitor cells, and BTA cells (SI Appendix, Fig. S1A); however, NCA organoids do not possess these cell types (SI Appendix, Fig. S3E). Protein staining confirmed that the only MUC1+ cell types present in NCA organoids are SCGB3A2+/SFTPB+ LAP cells, and the occasional multiciliated cell (SI Appendix, Fig. S4A). Notably, TP63+ cells do not express MUC1 in NCA organoids (SI Appendix, Fig. S4A). After FACS, the basal cell and LAP cell fractions were collected and reembedded separately in Matrigel. Seven days after sorting, half of the samples were collected, and RT-qPCR confirmed that MUC1-sorting enriched for LAP cells, while F3/EGFR enriched for basal cells (SI Appendix, Fig. S4B).
Fig. 4.

LAP-enriched organoids give rise to distinct cell types from basal-enriched organoids. (A) Schematic of experimental design for separating basal and LAP cells in vitro. NCA organoids are sorted using fluorescence-activated cell sorting (FACS) by F3/EGFR for basal cells and MUC1 for LAP cells. Sorted basal cells and LAP cells were replated to form organoids and analyzed after an additional 4 wk. (B) Representative gating plot for FACS of NCA organoids, sorting F3+/EGFR+ cells for basal organoids separate from MUC1+ cells for LAP organoids. (C) RT-qPCR data comparing expression of LAP cell (SCGB3A2, SFTPB, CFTR), and basal cell (TP63, KRT5) markers between F3/EGFR-sorted (teal bar) and MUC1-sorted organoids (pink bar) 4 wk postsorting. Each dot represents a technical replicate with its shape defining the biological replicate. This quantification was performed on three technical replicates from three biological replicates (separately derived organoid lines). Error bars represent SEM. Statistical tests were performed by Welch’s t test (unpaired, two-tailed). P-values are (*) <0.05. (D) FISH and co-IF stains on paraffin sections of representative F3/EGFR- and MUC1-derived organoids collected 4 wk postsort for various cell types, left-to-right: basal and secretory, LAP, multiciliated, PNEC. Insets on select images highlight RNA/protein expression in specific cells. Large images (Scale bar is 50 µm.), all Insets have (Scale bar of 20 µm.) (E) Quantification of cell types in F3/EGFR-derived organoids (teal bars) or MUC1-derived organoids (pink bars) from images like those shown in D based on the following marker expression: multiciliated defined as C6 (solid bars) or MUC16-expressing (hashed) FOXJ1+ cells, SCGB3A2+ for LAP cells, TP63+ basal cells, and SYN+ PNECs. Additional details on quantification can be found in Methods. Each dot represents one quantified image which contains ≥1 organoid(s). This quantification contains n = 3 biologic replicates per stain from n = 2 experimental replicates. Error bars represent SEM. Statistical tests were performed by Welch’s t test (unpaired, two-tailed). P-values are (*) <0.05, (**) <0.01.
New organoids were established and expanded for 4 wk (Fig. 4A). RT-qPCR of F3/EGFR-derived and MUC1-derived organoids showed that basal cell markers (TP63, KRT5) were enriched in F3/EGFR-organoids compared to MUC1-organoids, whereas LAP cell markers (SCGB3A2, SFTPB, CFTR) were enriched in MUC1-organoids compared to F3/EGFR-organoids (Fig. 4C). We further analyzed F3/EGFR-organoids and MUC1-organoids by FISH/co-IF (Fig. 4 D and E). Organoids from three independent biological specimens were stained (Fig. 4D) and quantified to characterize the cell types present (Fig. 4E). We found that F3/EGFR-organoids had abundant TP63+ cells, and possessed SCGB1A1 and FOXJ1+ cells, but were devoid of LAP cells (SCGB3A2/CFTR/SFTPB) and PNECs (Fig. 4 D, Top row, 4E). In contrast, MUC1-organoids were enriched for LAP cells, possessed FOXJ1+ cells and PNECs, alongside a few basal cells and SCGB1A1 cells (Fig. 4 D, Bottom row, 4E, and SI Appendix, Fig. S4C). F3/EGFR-organoids contained a significantly higher percentage of MUC16/FOXJ1 cells compared to C6/FOXJ1 cells, whereas the MUC1-organoids possessed a significantly higher proportion of C6/FOXJ1 cells compared to MUC16/FOXJ1 cells (Fig. 4E ). These trends were supported by RT-qPCR data (SI Appendix, Fig. S4E). There were no significant differences in SCGB1A1 or MUC5B expression in these cultures (SI Appendix, Fig. S4 C and D). Taken together, this analysis demonstrates that F3/EGFR-organoids give rise to basal cell-derived organoids that are devoid of LAP cells and PNECs, whereas MUC1-organoids give rise to LAP cell-derived organoids possessing PNECs, and multiciliated cells.
Discussion
With widespread use of single-cell genomic technologies, the field has been able to study cellular diversity of the human lung at an unprecedented level, leading to the identification of new cell types and cell states (1, 2, 5, 7, 9, 11–15, 17, 18, 20, 22–25, 44). Functional assessment of these new cell types and states is essential to understand how these cells contribute to the unique physiology of the lung. Given the uniquely human biology being uncovered, animal models are not always appropriate for functional follow-up studies, generating increased interest in the use of in vitro human models. Here, we characterize the LAP cell within the developing human lung defined by the coexpression of SCGB3A2/SFTPB/CFTR. Using in situ analysis on tissue sections and an in vitro organoid model system coupled with single-cell-barcoding lineage tracing techniques, we demonstrate that LAP cells are a progenitor cell in the airway that give rise to PNECs and a subset of multiciliated cells within the lung marked by the expression of complement component C6, and comprise a cell lineage distinct from the basal cell lineage, which gives rise to secretory cells and multiciliated cells defined by MUC16.
The heterogeneity of multiciliated cells has recently been explored in adult lungs, which also defined a MUC16 + subpopulation in the trachea (5). Our work demonstrates that this heterogeneity may represent similar cells in different regions of the lung. Additionally, an interesting finding from the current work is that basal cells are enriched in the trachea and large cartilaginous airways while LAP cells are enriched in the small cartilaginous and NCA. Moreover, it is well established that cells in the trachea are specified early during development while the lower regions of the airway are formed later during branching morphogenesis (45). It is interesting to speculate that in the human lung, basal cells and LAP cells are specified at different developmental times and are responsible for tuning the distinct cell-type composition of airways along the proximal–distal axis. Consistent with this idea, large animal studies as well as studies in humans have defined functionally distinct subsets of multiciliated cells in different anatomical regions of the lung that have different ciliary beat frequencies (46–48). It is also possible that the changing cellular landscape of the airway epithelium along the proximal-to-distal axis provides an evolving defense mechanism, with a strong immune response (notably C6+ cells) becoming enriched before reaching the alveoli. Additional functional assessments of basal and LAP-derived multiciliated cell subtypes in the future will help to understand the importance of this heterogeneity.
The cell of origin for PNECs also remains unclear, with evidence suggesting that both BTPs and basal cells give rise to PNECs (1, 40, 42, 49–53). It is possible that PNECs are derived from both cell lineages but in different contexts (development versus injury repair). Furthermore, recent data suggest there may be additional PNEC heterogeneity that has yet to be functionally resolved (11). PNECs are believed to be the earliest specified cells in airway development (54), and it is also possible that our findings are capturing this earliest stage of PNEC differentiation during development, while basal cell–derived PNECs are more representative of a homeostatic adult lung. Given the importance of NOTCH signaling in both multiciliated and PNEC differentiation in the mouse lung (55–59), it will be interesting to carry out follow-up studies to understand how LAP-derived PNEC cells are regulated. Further understanding of PNECs within the human lung shed light on this unique cell type and its origin.
One interesting finding from our data is that F3/EGFR-derived organoids are completely devoid of LAP cells and PNECs; while on the other hand MUC1-derived organoids possess basal cells. It is possible that LAP cells give rise to basal cells; however, we cannot with certainty rule out that this result is an artifact of sorting. In addition, while there was a clear association between basal cells and MUC16 + multiciliated cells, and LAP cells and C6 + multiciliated cells, both F3/EGFR-organoids and MUC1-organoids possess MUC16 + and C6 + multiciliated cells. These data are consistent with in vivo staining data and trachea organoids, where the trachea and trachea organoids possess both MUC16 + and C6 + populations of cells. On the other hand, the NCA in vivo and NCA organoids are highly enriched for C6 + multiciliated cells, while sorted MUC1-organoids gave rise to both MUC16 + and C6 + multiciliated cells. One possible explanation for these data is that the culture conditions do not represent a physiological niche mimicking the anatomical environment from which the organoids were derived; therefore the in vitro niche environment may be influencing gene expression or heterogeneity during differentiation. Additional work will be needed to identify culture conditions and in vitro niche cues that are tailored to mimic the trachea or NCA.
While the existence of a LAP cell has not been demonstrated in mice, it is likely that there are different airway progenitors that populate murine lungs during development. It is well accepted that basal cells act as a stem/progenitor cell aiding in homeostasis and injury repair in mice; however, there is also evidence that basal cells are not required for airway development. For example, in the lungs of P63-KO mice, which lack basal cells and die shortly after birth, both secretory and multiciliated cells are still present during development, supporting the hypothesis that nonbasal cells can populate the airway (51, 60, 61). The presence of cells expressing Scgb3a2+ have also been identified during murine lung development (62, 63). It is not clear whether these cells are analogous to LAP cells, and future work comparing mouse and human lung development will help answer this question; however, existing evidence suggests functional differences. For example, Scgb3a2+ cells in mice predominantly give rise to club and multiciliated cells, but not the PNEC lineage (63), while our results suggest that LAP cells give rise to the PNEC lineage, but not the club lineage.
The developmental potential of the LAP cell also warrants further investigation. The presence of SCGB3A2+/SFTPB+/SCGB1A1- cells in the adult terminal respiratory bronchiole has recently been characterized, referred to as terminal respiratory bronchiolar stem cells (TRB-SC) or respiratory airway secretory cells (RASC) (9, 22). It is unclear if LAP cells are the fetal equivalent to this cell; however both TRB-SC and RASCs were shown to give rise to alveolar cell types, while LAP cells give rise to airway cell types, at least under the conditions tested here. The relationship between LAP cells and these populations with similar transcriptional profiles in adult remains unclear until the dynamics of these populations in later development and early in postnatal life are better known.
Taken together, the current work identifies LAP cells as an important cell type during human lung development which broadens our understanding of development of the human airway epithelium and challenged how we approach differentiation in this organ. Understanding the potential contribution of LAP cells to human lung disease and the importance of the unique composition of human lower airways are areas for future investigation with direct relevance to human respiratory health. More broadly, our results indicate that specific localization of progenitors may be a commonly employed strategy to establish region-specific cell-type composition within developing organs.
Materials and Methods
Cell Lines and Culture Conditions.
BTO line establishment, culture, and airway differentiation.
BTO lines were cultured as previously described (1); a technical overview can be found in ‘SI Appendix, Supplemental Materials and Methods.’ Airway differentiation was carried out as previously described (1); a technical overview can be found in ‘SI Appendix, Supplemental Materials and Methods.’
Regional airway organoid establishment and culture.
Proximal tracheal tissue or NCA tissue was dissected from tissue using a scalpel and forceps and subsequently minced for 2 min into small chunks (<1 mm3). Tissue was washed with 1× phosphate buffered saline (PBS), spun down at 300 g for 3 min at 4 °C, resuspended in Matrigel, replated in 20 µL droplets and allowed to solidify. Cultures were fed with 0.5 mL DSI media (see above) for 1 wk, changing media every 3 to 4 d, after which they were passaged using TrypLE (Invitrogen, Cat#12605010), for 30 min with mechanical dissociation every 10 min. Dissociation was quenched using DMEM/F-12 (Corning, Cat#10-092-CV) after which cells were spun down at 300 g for 3 min at 4 °C, resuspended in Matrigel and fed with 0.5 mL DSI media.
Tissue Processing and Staining.
Tissue processing.
Tissue processing was carried out as previously described (1, 37); a technical overview can be found in ‘SI Appendix, Supplemental Materials and Methods.’
FISH and immunofluorescence (IF) protein staining on paraffin sections.
FISH and IF staining were carried out as previously described (1, 37); a technical overview can be found in ‘SI Appendix, Supplemental Materials and Methods.’ Tissue slides were rehydrated in Histo-Clear II (National Diagnostics, Cat#HS-202), followed by serial rinses through an ethanol series. Antigen retrieval was performed using 1× Sodium Citrate Buffer [100 mM trisodium citrate (Sigma, Cat#S1804), 0.5% Tween 20 (Thermo Fisher, Cat#BP337), pH 6.0], for 20 min. Slides were incubated for 1 h with blocking solution [5% normal donkey serum (Sigma, Cat#D9663) in PBS with 0.1% Tween 20] followed by primary antibody diluted in blocking solution at 4 °C overnight. Slides were then incubated with secondary antibody with DAPI (1 µg/mL) diluted in blocking solution for 1 h, washed and mounted. All primary antibody concentrations are listed in SI Appendix, Table S4. Secondary antibodies, raised in donkey, were purchased from Jackson Immuno and used at a dilution of 1:500. A list of FISH probes and reagents can be found in SI Appendix, Table S4.
Organoid Tissue Prep for scRNA-seq.
Organoid prep for scRNA-seq was carried out as previously described (37); a technical overview can be found in ‘SI Appendix, Supplemental Materials and Methods.’ Briefly, organoids were digested to single cells using 0.5 mL TrypLE (Invitrogen, Cat#12605010) after which they were quenched with 1× Hank's Buffered salt solution (HBSS) and passed through a 40-µm filter (Bel-Art Flowmi, Cat#136800040). Approximately 100,000 cells were put on ice and single-cell libraries were immediately prepared at the 10× Chromium at the University of Michigan Sequencing Core with a target of 10,000 cells per sample.
RNA Extraction, cDNA, and RT-qPCR.
These methods were carried out as previously described (1, 37); a technical overview can be found in ‘SI Appendix, Supplemental Materials and Methods.’ mRNA was isolated using the MagMAX-96 Total RNA Isolation Kit (Thermo Fisher, Cat#AM1830). cDNA synthesis was performed using 100 ng RNA per sample with the SuperScript VILO cDNA Kit (Thermo Fisher, Cat#11754250). RT-qPCR was performed on a Step One Plus real-time PCR system (Thermo Fisher, Cat#42765592R) using the QuantiTect SYBR Green PCR Kit (Qiagen, Cat#204145). Primer sequences can be found in SI Appendix, Table S4. Gene expression as a measure of arbitrary units was calculated relative to housekeeping gene (GADPH or ECAD) using the following equation:
Gene expression as a measure of fold change (SI Appendix, Fig. S4B) was performed by calculating the average arbitrary units for all unsorted cultures and dividing the arbitrary units for either F3/EGFR- or MUC1-sorted samples by this unsorted average.
Quantification, Statistical Analysis.
Quantification of IF images (Fig. 4E) was performed using Nikon Elements General Analysis 3 (GA3), where algorithms were developed within the GA3 software to detect cells of interest based on specific markers. Briefly, thresholds were set to identify features based on marker expression (i.e., nuclei for DAPI+, TP63+, FOXJ1+ nuclei, cytoplasmic staining for SCGB1A1, SCGB3A2; puncta for C6, MUC16). Cells were defined as a region encasing 4 µm diameter around a DAPI+ nucleus (or FOXJ1+ nucleus for multiciliated cell count). Using a parent-child function, cells that contained defined markers were counted and then output as raw counts; final quantification is reported as a percentage of nuclei/DAPI+ staining noted in each graph. PNECs were counted by hand based on SYN+ expression. For Fig. 1H, 100 DAPI+ cells within the epithelium were counted for each airway region per sample, and results were plotted as percentage of SCGB3A2+/SFTPB+ cells per 100 DAPI+ cells.
For RT-qPCR analysis, biologic replicates containing three wells of organoids containing 10 to 60 organoids (technical replicates) were collected. All statistical analysis were performed in GraphPad Prism Software. See Figure Legends for the number of replicates used, statistical test performed, and the p-values used to determine the significance for each separate analysis.
CellTagging Library Prep and Lentiviral Transduction into Organoids.
CellTagging plasmid library preparation.
The CellTag V1 plasmid library was purchased from Addgene (‘CellTag A’, Cat#124591) and transformed using Stellar Competent Escherichia coli (Takara Biosciences, Cat#636763) at an efficiency of 1.79 × 109 cfus/µg. Plasmid library was isolated from 500 mL transformed E. coli using the Plasmid Plus Mega Kit (Qiagen, Cat#12981). High-throughput DNA sequencing assessed library complexity, resulting in 13,963 unique tags in the 90th percentile for frequency. Lentiviral packaging was performed by the University of Michigan Vector Core.
Lentiviral transduction of organoids.
A technical overview can be found in ‘SI Appendix, Supplemental Materials and Methods.’ Briefly, 21-d differentiated BTOs were needle passaged by two passes through a 27G needle, spun down and resuspended in 1 mL DSI media + 6 µg/mL polybrene for 15 min at 37 °C. Cells were again spun down and resuspended in 300 µL virus + DSI. Cells were incubated at 37 °C for 6 h with agitation every hour. Following transduction, cells were spun down again at 300 g for 3 min, resuspended in Matrigel, and fed with 0.5 mL DSI media.
Limited dilution plating following FACS.
To ensure LAP and basal cells descended from the same tagged BTP did not confound our analysis, we started cultures for CellTagging analysis by diluting the culture seed so that it was limited relative to the total number of unique CellTag combinations in the total population. This thereby reduces the probability of LAP and basal cells sharing a CellTag being used to seed the population for analysis. Using this approach, we estimated that based on the number of clones generated during viral transduction and the number of cells seeded in cultures after FACS isolation, the probability of cultures seeded after FACS to contain cells from the same clone were estimated to be 10% as determined by the probability of coincidences function in R.
FACS and Flow Cytometry.
FACS was carried out as previously described (37); a technical overview can be found in ‘SI Appendix, Supplemental Materials and Methods.’ Briefly, tissue was digested to single cells and passed through a 70-µm cell strainer. Then, 105 cells were placed into new FACS tubes for all controls, and all remaining cells were centrifuged and resuspended in FACS buffer for a concentration of 106 cells/100 µL. Primary antibodies were incubated for 30 min on ice. Secondary antibodies were incubated for 30 min on ice. Conjugated antibodies were incubated for 10 min on ice. FACS was performed using a Sony MA900 cell sorter or ThermoFisher Bigfoot cell sorter and accompanying software. All primary and conjugated antibody concentrations are listed in SI Appendix, Table S4.
Bioinformatics/sc-RNAseq Analysis.
Quality control, preprocessing and integration, dimension reduction and clustering, cluster annotation and cell scoring, and normalization for visualization and differential gene expression were carried out as previously described (37), and details can be found in ‘SI Appendix, Supplemental Materials and Methods.’
Quantification of LAP cell clusters.
To calculate each sample’s contribution to the LAP cell clustering (Fig. 1 E and F), the number of cells per sample was divided by all cells in the subclustering to calculate a normalization ratio, which was used to calculate a normalized value of cells/cluster for each sample. These values were then normalized by the total cells/cluster for each sample and finally the percentages of each sample (broken down by age and region) were combined, respectively, for the plots to be displayed either by sample age or sample region.
Analysis of CellTagged airway organoids.
Using the CellTagR package (https://github.com/morris-lab/CellTagR) CellTags were extracted from processed single-cell RNA-sequencing BAM files to generate a matrix of cell barcodes, unique molecular identifiers, and CellTags. This matrix was filtered for cell barcodes corresponding to cells as determined by the CellRanger pipeline and subjected to CellTag sequencing error correction using Starcode (https://github.com/gui11aume/starcode). Whitelisting was performed to remove tags not detected during assessment of CellTag library complexity. For clone calling, cells expressing ≤ 2 or ≥ 20 unique CellTags were removed and Jaccard analysis was performed to calculate pairwise similarity coefficients between combinations of CellTags. Cells with CellTag combinations having similarity scores better than 0.7 were called as clones. For scRNA-seq analysis and visualization the standard Seurat workflow was performed including cell filtering by number of features (<500, >10,000 removed), percentage of mitochondrial reads (>10% removed), normalization, variable feature selection (n = 500), dimensional reduction (10 principle components), and Louvain clustering (resolution = 0.3). Clonal identities were appended as metadata in a Seurat object by cell barcode using Seurat’s AddMetaData function. To ensure clones were sufficiently sampled, we limited our lineage analysis to clones containing ≥10 cells.
Reference-based mapping.
We followed Seurat’s recommended pipeline to perform single-cell reference mapping using 21-d airway organoid LAP cells or 60-d CellTagged airway organoid LAP cells as the query data and the fetal lung epithelium cells as the reference data (SI Appendix, Fig. S2E). PCAs are first performed on reference and query data. Then a set of anchors are identified and filtered based on the default setting of the function FindTransferAnchors. With the computed anchors, reference.reduction parameter set to PCA, and reduction.model set to UMAP, the function MapQuery returns the projected UMAP coordinates of the query cells mapped onto the reference UMAP. We then integrate the projected UMAP (colored in red) and the reference UMAP (colored in light gray) to visualize the result of our reference-based mapping in 2D.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We would like to thank the following University of Michigan core facilities: Advanced Genomics, Microscopy, Viral Vector, and Flow Cytometry. We thank Lindy K. Brastrom for her careful technical editing of the manuscript. We would also like to thank the University of Washington Laboratory of Developmental Biology. This work was supported in part by grant CZF2019-002440 from the Chan Zuckerberg Initiative Donor-advised fund, an advised fund of the Silicon Valley Community Foundation, and by the National Heart, Lung, and Blood Institute (NHLBI; R01HL119215) to J.R.S. A.S.C. is supported by the T32 Michigan Medical Scientist Training Program (5T32GM007863-40) and by a Ruth L. Kirschstein Predoctoral Individual National Research Service Award (NIH-NHLBI F30HL156474). T.F. is supported by a NIH Tissue Engineering and Regenerative Medicine Training Grant (NIH-NIDCR T32DE007057). P.P.H. is supported by the Rogel Cancer Center Fellowship, Judith Tam ALK NSCLC Research Initiative. A.J.M. was supported by a Ruth L. Kirschstein Predoctoral Individual National Research Service Award (NIH-NHLBI F31HL142197). R.F.C.H. was supported by a NIH Tissue Engineering and Regenerative Medicine Training Grant (NIH-NIDCR T32DE007057) and by a Ruth L. Kirschstein Predoctoral Individual National Research Service Award (NIH-NHLBI F31HL152531). E.M.H. was supported by a Ruth L. Kirschstein Predoctoral Individual National Research Service Award (NIH-NHBLI F31HL146162). I.G. and the University of Washington Laboratory of Developmental Biology was supported by the NIH award (NICHD-5R24HD000836) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Author contributions
A.S.C., T.F., P.P.H., A.J.M., and J.R.S. designed research; A.S.C., T.F., P.P.H., K.K., M.S.D., R.F.C.H., A.J.M., Y.-H.T., A.W., E.M.H., and A.A. performed research; A.S.C., Z.X., P.P.H., A.J.M., and I.G. contributed new reagents/analytic tools; A.S.C., T.F., Z.X., P.P.H., A.J.M., P.K.L.M., P.R.T., and J.R.S. analyzed data; P.K.L.M. and P.R.T. provided insight and feedback on the study and manuscript; A.S.C., T.F., P.P.H., and J.R.S. wrote the paper; and all authors edited and approved the manuscript.
Competing interests
A.J.M. and J.R.S. hold intellectual property pertaining to lung organoid technologies.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Sequencing data used in this study are deposited at EMBL-EBI ArrayExpress: human fetal lung (ArrayExpress: E-MTAB-8221, ArrayExpress: E-MTAB-10662) (1, 36), CellTagged organoids (ArrayExpress: E-MTAB-12753, this study). Code used to process data can be found at https://github.com/jason-spence-lab/Conchola_2022.
Supporting Information
References
- 1.Miller A. J., et al. , In vitro and in vivo development of the human airway at single-cell resolution. Dev. Cell 53, 117–128.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travaglini K. J., A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature 587, 619–625 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brazovskaja A., Treutlein B., Camp J. G., High-throughput single-cell transcriptomics on organoids. Curr. Opin. Biotechnol. 55, 167–171 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Yu Q., et al. , Charting human development using a multi-endodermal organ atlas and organoid models. Cell 184, 3281–3298. e22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carraro G., et al. , Transcriptional analysis of Cystic Fibrosis airways at single cell resolution reveals altered epithelial cell states and composition. Nat. Med. 27, 806 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haniffa M., A roadmap for the human developmental cell atlas. Nature 597, 196–205 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang A., et al. , Single-cell multiomic profiling of human lungs reveals cell-type-specific and age-dynamic control of SARS-CoV2 host genes. Elife 9, 1–28 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyser R. C. V., Single-cell transcriptomic characterization of a gastrulating human embryo. Nature 600, 285–289 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murthy P. K. L., Human distal lung maps and lineage hierarchies reveal a bipotent progenitor. Nature 604, 111–119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holloway E. M., et al. , Mapping development of the human intestinal niche at single-cell resolution. Cell Stem Cell 28, 568–580.e4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moghieb A., et al. , Time-resolved proteome profiling of normal lung development. Am. J. Physiol. Lung Cellular Mol. Physiol. 315, L11–L24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyfman P. A., et al. , Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am. J. Respir Crit Care Med. 199, 1517–1536 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plasschaert L. W., et al. , A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560, 377–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams T. S., Single Cell RNA-seq reveals ectopic and aberrant lung resident cell populations in Idiopathic Pulmonary Fibrosis. Sci. Adv. 6, eaba1983 (2020), 10.1101/759902. Accessed 30 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danopoulos S., Bhattacharya S., Mariani T. J., Al Alam D., Transcriptional characterisation of human lung cells identifies novel mesenchymal lineage markers. Eur. Respir. J. 55, 1900746 (2020). 10.1183/13993003.00746-2019. Accessed 30 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCauley K. B., et al. , Single-cell transcriptomic profiling of pluripotent stem cell-derived SCGB3A2+ airway epithelium. Stem Cell Rep. 10, 1579–1595 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y., et al. , Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1, e90558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo M., et al. , Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat. Commun. 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y., Beyond fibroblast heterogeneity: What single-cell RNA sequencing tells us. Am. J. Respir Cell Mol. Biol. 61, 7–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Treutlein B., et al. , Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509, 371–375 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sountoulidis A., et al. , A topographic atlas defines developmental origins of cell heterogeneity in the human embryonic lung. Nat. Cell Biol. 25, 351–365 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Basil M. C., Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature 604, 120–126 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angelidis I., et al. , An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat. Commun. 10, 963 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieira Braga F. A., et al. , A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 25, 1153–1163 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Steuerman Y., et al. , Dissection of influenza infection in vivo by single-cell RNA sequencing. Cell Syst. 6, 679–691.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du Y., et al. , Integration of transcriptomic and proteomic data identifies biological functions in cell populations from human infant lung. Am. J. Physiol. Lung Cell Mol. Physiol 317, L347–L360 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hafemeister C., Satija R., Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 1–15 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler A., Hoffman P., Smibert P., Papalexi E., Satija R., Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 536, 411–420 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satija R., Farrell J. A., Gennert D., Schier A. F., Regev A., Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 533, 495–502 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao Y., et al. , Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blondel V. D., Guillaume J. L., Lambiotte R., Lefebvre E., Fast unfolding of communities in large networks. J. Stat. Mech. Theory Exp. 2008, P10008 (2008). [Google Scholar]
- 32.Becht E., Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 37, 38–44 (2018). [DOI] [PubMed] [Google Scholar]
- 33.McInnes L., Healy J., Saul N., Großberger L., UMAP: Uniform manifold approximation and projection. J. Open Source Softw. 3, 861 (2018). [Google Scholar]
- 34.Montoro D. T., et al. , A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature 560, 319–324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller A. J., et al. , In Vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Rep. 10, 101–119 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hein R. F. C., R-SPONDIN2+ mesenchymal cells form the bud tip progenitor niche during human lung development. Dev. Cell 57, 1598–1614.e8 (2022). 10.1016/J.DEVCEL.2022.05.010 (June 11, 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hein R. F. C., et al. , Stable iPSC-derived NKX2-1+ lung bud tip progenitor organoids give rise to airway and alveolar cell types. Development 149, dev200693 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biddy B. A., et al. , Single-cell mapping of lineage and identity in direct reprogramming. Nature 564, 219–224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto Y., Korogi Y., Hirai T., Gotoh S., A method of generating alveolar organoids using human pluripotent stem cells. Methods Cell Biol. 159, 115–141 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Mou H., et al. , Airway basal stem cells generate distinct subpopulations of PNECs. Cell Rep. 35, 109011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sui P., Pulmonary neuroendocrine cells amplify allergic asthma responses. Science 360, eaan8546 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song H., et al. , Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 109, 17531–17536 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jarrare J. A., et al. , MUC1 is a novel marker for the Type II pneumocyte lineage during lung Carcinogenesis1. Cancer Res. 58, 5582–5589 (1998). [PubMed] [Google Scholar]
- 44.He P., et al. , A human fetal lung cell atlas uncovers proximal-distal gradients of differentiation and key regulators of epithelial fates. Cell 185, 4841–4860.e25 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Yang Y., et al. , Spatial-temporal lineage restrictions of embryonic p63+ progenitors establish distinct stem cell pools in adult airways. Dev. Cell 44, 752–761.e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joki S., Saano V., Ciliary beat frequency at six levels of the rabbit and rat respiratory tract in cow, dog, guinea-pig, pig, rabbit and rat. Clin. Exp. Pharmacol. Physiol. 21, 427–434 (1994). [DOI] [PubMed] [Google Scholar]
- 47.Nawroth J. C., van der Does A. M., Ryan A., Kanso E., Multiscale mechanics of mucociliary clearance in the lung. Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clary-Meinesz C., et al. , Ciliary beat frequency in human bronchi and bronchioles. Chest 111, 692–697 (1997). [DOI] [PubMed] [Google Scholar]
- 49.Rawlins E. L., Clark C. P., Xue Y., Hogan B. L. M., The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells Development 136, 3741–3745 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rockich B. E., et al. , Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc. Natl. Acad. Sci. U.S.A. 110, E4456–E4464 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniely Y., et al. , Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am. J. Physiol. Cell Physiol. 287, C171–C181 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Dye B. R., et al. , In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4, e05098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zepp J. A., Morrisey E. E., Cellular crosstalk in the development and regeneration of the respiratory system. Nat. Rev. Mol. Cell Biol. 20, 551–566 (2019), 10.1038/s41580-019-0141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J., Yu H., Sun X., Less is more: Rare pulmonary neuroendocrine cells function as critical sensors in lung. Dev. Cell 55, 123–132 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Noguchi M., Sumiyama K., Morimoto M., Directed migration of pulmonary neuroendocrine cells toward airway branches organizes the stereotypic location of neuroepithelial bodies. Cell Rep. 13, 2679–2686 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Kiyokawa H., Morimoto M., Notch signaling in the mammalian respiratory system, specifically the trachea and lungs, in development, homeostasis, regeneration, and disease. Dev. Growth. Differ. 62, 67–79 (2019), 10.1111/dgd.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsao P. N., et al. , Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136, 2297–2307 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsao P. N., et al. , Epithelial Notch signaling regulates lung alveolar morphogenesis and airway epithelial integrity. Proc. Natl. Acad. Sci. U.S.A. 113, 8242–8247 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu K., Moghal N., Egan S. E., Notch signaling in Embryology and Cancer. Adv. Exp. Med. Biol. 727, 89–98 (2012). [DOI] [PubMed] [Google Scholar]
- 60.Vaughan A. E., et al. , Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 517, 621–625 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang A., et al. , p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718 (1999). [DOI] [PubMed] [Google Scholar]
- 62.Kiyokawa H., et al. , Airway basal stem cells reutilize the embryonic proliferation regulator, Tgfβ-Id2 axis, for tissue regeneration. Dev Cell 56, 1917–1929.e9 (2021). [DOI] [PubMed] [Google Scholar]
- 63.Guha A., et al. , Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc. Natl. Acad. Sci. U.S.A. 109, 12592–12597 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Sequencing data used in this study are deposited at EMBL-EBI ArrayExpress: human fetal lung (ArrayExpress: E-MTAB-8221, ArrayExpress: E-MTAB-10662) (1, 36), CellTagged organoids (ArrayExpress: E-MTAB-12753, this study). Code used to process data can be found at https://github.com/jason-spence-lab/Conchola_2022.