The aorta is dissected in the groove between the aorta, right ventricle, and pulmonary artery.
Central Message.
The aorta is dissected in the groove between the aorta, right ventricle, and pulmonary artery to identify fibers of the infundibular septum. An oblique incision is made in the aorta.
Although a transaortic septal myectomy (Morrow's procedure) is generally accepted as the standard surgical treatment for obstructive hypertrophic cardiomyopathy (HCM), it may be difficult to perform an adequate septal myectomy for midventricular and apical HCM.1 Aortic valve replacement is the ultimate treatment for severe aortic stenosis (AS). Aortic root enlargement (ARE) is used to facilitate the implementation of artificial valves of appropriate size to prevent patient–prosthesis mismatch. The Konno-Rastan operation is among the most common and traditional techniques for ARE. It also can be used to treat HCM.
Vouhe aortoventriculoplasty is another method for ARE. It mainly includes a partial dissection through the aorta and pulmonary artery to expand the surgical field without making any incisions on the aortic valves.
Recently, our department received a male patient with severe AS, midventricular septal hypertrophy, and obstructive HCM. We attempted the Vouhe operation to manage the issues in a single operation. Herein, we report the case with Vouhe aortoventriculoplasty in detail. Institutional review board approval was not required; the patient gave written consent for publication of study data.
Case Presentation
A 57-year-old man presented with a 30-year history of chest tightness and shortness of breath. The symptoms had been aggravated since last year. His previous history included psoriasis, thrombocytopenic purpura, and emphysema.
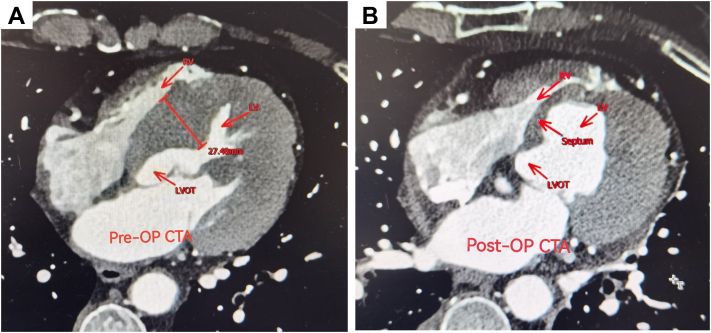
The physical exam revealed 4 to 5/6 systolic murmurs audible in the aortic auscultation area. Echocardiography revealed aortic bicolor malformation, severe AS with incomplete closure, left ventricular myocardial hypertrophy, severe left ventricular outflow obstruction (LVOT), and Sam signs (+). The preoperative Doppler echocardiography scan findings showed severe AS with a valve area of 0.8 cm2, peak velocity 5.9 m/sec, mean gradient 77 mm Hg, aortic regurgitation velocity 4.3 m/sec, and normal left ventricle size and function (56%). The aortic annulus diameter and ascending aorta measured 25 mm and 42 mm, respectively, with normal right ventricular pressure (tricuspid regurgitation velocity, 2.3 m/sec) (Table 1). Computed tomography angiography in the midventricular septum was hypertrophic, and the thickest part was 27.48 mm, with LVOT obstruction (Figure 1).
Table 1.
Preoperative and postoperative color Doppler echocardiography scan findings of the ventricular septum and surrounding structures
| Color Doppler Echocardiography Scan Findings | Preoperative | Postoperative |
|---|---|---|
| Thickness of ventricular septum (mm) | 27.48 | 7.00 |
| Peak aortic velocity (m/sec) | 5.90 | 1.86 |
| Mean gradient (mm Hg) | 75 | 14 |
| Aortic regurgitation velocity (m/sec) | 4.3 | 1.6 |
| Left ventricle size (mm) | 42 | 40 |
| Left ventricle function∗ (%) | Normal (56) | 51 |
| Sinus of Valsalva (mm) | 26 | 26 |
| Aortic valve annulus diameter (mm) | 19 | 25 |
| Ascending aorta diameter (mm) | 37 | 32 |
| Right ventricular pressure | Normal | Normal |
| Pulmonary valve regurgitation velocity (cm/sec) | 116 | 85 |
Ejection fraction.
Figure 1.
Preoperative (pre-op) and postoperative computed tomography angiography (CTA) findings of the ventricular septum and surrounding structures. The pre-op thickest part of ventricular septum was 27.48 mm (A). Postoperatively, it was 7.00 mm (B). RV, Right ventricular; LV, left ventricular; LVOT, left ventricular outflow obstruction.
Procedure Details
Thirteen days after admission, the operation was performed under general anesthesia, hypothermia, and cardiopulmonary bypass. Following successful administration of the anesthesia, an ultrasonic probe of the esophagus was placed, with a T-shaped, 10-cm longitudinal incision of the upper sternum followed by whole-body heparinization, pericardium, the ascending aorta, double vena cava, and right pulmonary vein intubation. This established extracorporeal circulation. The ascending aorta was blocked after the cut, the aortic root was opened at the left, right coronary artery perfusion cardiac arrest was initiated, and there was good heart relaxation.
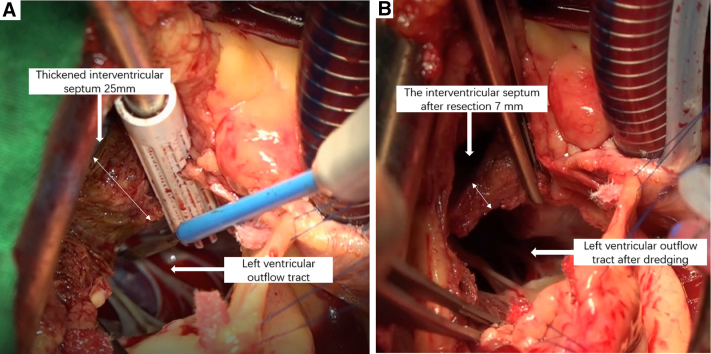
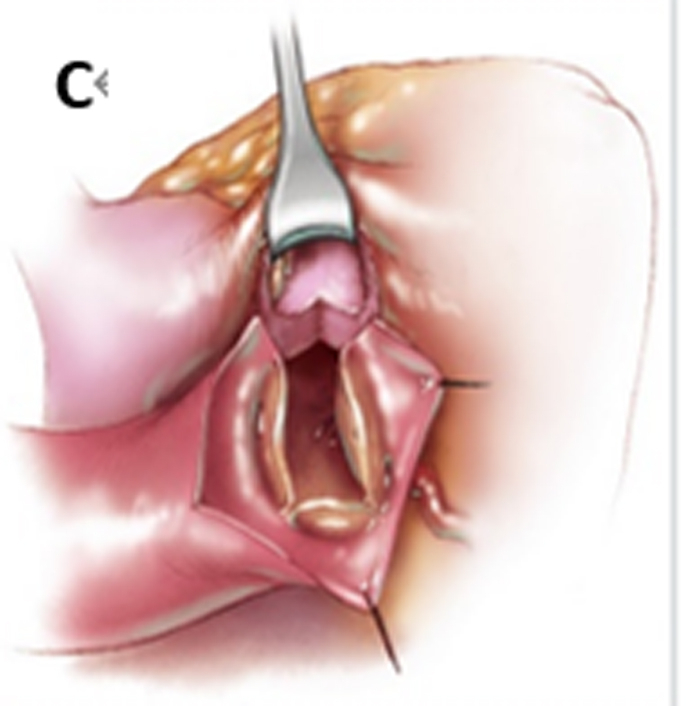
The aortic annulus measured 19 mm and it was difficult to remove the hypertrophic muscle from the middle of the ventricular septum during the operation. Hence, ARE was necessary and we followed the original plan. A 15-mm Vouhe incision was made at the junction of the left and right coronary sinus of the aorta (Figure 2), splitting the valve ring and dissecting into the interventricular septum for about 15 mm, resulting in exposure of the operative field, and the interventricular septum hypertrophic muscle was removed from the corresponding site (Figure E1). Further, the pulmonary artery and the aorta incisions were intermittently sutured and the No. 25 aortic valve mechanical valve was implanted in the aortic valve position followed by closure of the aortic incision (Video 1).
Figure 2.
Vouhe procedure. A, An oblique incision is made in the aorta. B, The aortic ring was opened along the junction of the left and right coronary sinus. C, A bovine pericardium patch was used to widen the aorta, reduce tension, and implant the valve. D, Operation to complete. (Figure used from Cardiac Surgery Safeguards and Pitfalls in Operative Technique, 2nd ed., P168).
Figure E1.
Intraoperative findings and different stages of surgical repair. The thickness of preoperative interventricular septum was 25 mm (A). The thickness of postoperative was 7 mm (B).
During the operation, bleeding was about 300 mL, and 2U red blood cells, 400 mL plasma, and 1U platelets were perfused. The time taken for extracorporeal circulation and aorta occlusion was 254 minutes and 190 minutes, respectively.
After operation, symptoms such as chest tightness and shortness of breath disappeared and the murmur in aortic valve area was not heard. Nine days later, an ultrasound scan showed that the ventricular septal thickness had reduced to 7 mm and the cardiac hemodynamic parameters were close to normal levels (Table 1 and Figure 1). At the time of reporting this case, 6 months have passed since his surgery without any complications.
Discussion
Morrow's procedure is the most common operation for hypertrophic ventricular septal myectomy. It passes through a patient's aortic valve orifice without any expansion or incision to try to remove the upper hypertrophic ventricular septum.1
There are 4 main techniques used to perform ARE: Nicks, Manouguian, Nunez (modified Manouguian), and the Kanno-Rastan procedures.2,3 Vouhe is a type of traditional aortoventriculoplasty4 in which an incision is made in the aorta and the pulmonary artery to obtain the largest surgical field without any incisions on aortic valves. We believe it is the best choice for patients with HCM in the mid- or apical-ventricular septum and with no valve problems.
Our patient had 2 obvious complications, including severe HCM in the mid-ventricular septum measuring 27.48 mm preoperatively along with severe AS. There were fewer chances of complications with the Vouhe surgery compared with the Kanno-Rastan procedure.
In a common Vouhe aortoventriculoplasty procedure, the aortic incision is extended into the commissure between the left and right coronary cusps of the aortic valve into the interleaflet triangle and the outflow chamber of the right ventricle is opened transversely. The thickness of the hypertrophied ventricular septum is reduced by resecting the left ventricle side of the septum to relieve the LVOT obstruction.4 Vouhe aortoventriculoplasty can significantly expand the visual field and accurately control the width and thickness of the resected ventricular septal muscle.
Conclusions
To surgically treat AS or HCM, a cardiac surgeon should be well skilled in common cardiac procedures. The Vouhe aortoventriculoplasty may be a better option for obstructive HCM and in patients with obvious distal ventricular septal hypertrophy without AS.
Footnotes
Disclosures: The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
The authors certify that they have obtained all appropriate patient consent of forms. In the forms the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published.
IRB approval was not required.
Supplementary Data
Vouhe aortoventriculoplasty for hypertrophy in the middle and lower ventricular septum and aortic valve stenosis (AS). Video available at: https://www.jtcvs.org/article/S2666-2507(23)00131-1/fulltext.
Appendix 1
References
- 1.Matsuno Y., Fujita A., Kurazumi H., Ikenaga S. Transmitral septal myectomy and mitral valve plasty for hypertrophic obstructive cardiomyopathy combined with organic mitral Regurgitation; report of a case. Kyobu Geka. 2020;73:1101–1104. [PubMed] [Google Scholar]
- 2.Yu W., Tam D.Y., Rocha R.V., Makhdoum A., Ouzounian M., Fremes S.E. Aortic root enlargement is safe and reduces the incidence of patient–prosthesis mismatch: a meta-analysis of early and late outcomes. Can J Cardiol. 2019;35:782–790. doi: 10.1016/j.cjca.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Massias S.A., Pittams A., Mohamed M., Ahmed S., Younas H., Harky A. Aortic root enlargement: when and how. J Card Surg. 2021;36:229–235. doi: 10.1111/jocs.15175. [DOI] [PubMed] [Google Scholar]
- 4.Dumani S., Likaj E., Dibra L., Beca V., Kuci S., Refatllari A. Aortic annulus enlargement: early and long-term results. Open Access Maced J Med Sci. 2017;5:23–26. doi: 10.3889/oamjms.2017.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vouhe aortoventriculoplasty for hypertrophy in the middle and lower ventricular septum and aortic valve stenosis (AS). Video available at: https://www.jtcvs.org/article/S2666-2507(23)00131-1/fulltext.