Abstract

Wildfire smoke penetrates indoors, and polycyclic aromatic hydrocarbons (PAHs) in smoke may accumulate on indoor materials. We developed two approaches for measuring PAHs on common indoor materials: (1) solvent-soaked wiping of solid materials (glass and drywall) and (2) direct extraction of porous/fleecy materials (mechanical air filter media and cotton sheets). Samples are extracted by sonication in dichloromethane and analyzed with gas chromatography–mass spectrometry. Extraction recoveries range from 50–83% for surrogate standards and for PAHs recovered from direct application to isopropanol-soaked wipes, in line with prior studies. We evaluate our methods with a total recovery metric, defined as the sampling and extraction recovery of PAHs from a test material spiked with known PAH mass. Total recovery is higher for “heavy” PAHs (HPAHs, 4 or more aromatic rings) than for “light” PAHs (LPAHs, 2–3 aromatic rings). For glass, the total recovery range is 44–77% for HPAHs and 0–30% for LPAHs. Total recoveries from painted drywall are <20% for all PAHs tested. For filter media and cotton, total recoveries of HPAHs are 37–67 and 19–57%, respectively. These data show acceptable HPAH total recovery on glass, cotton, and filter media; total recovery of LPAHs may be unacceptably low for indoor materials using methods developed here. Our data also indicate that extraction recovery of surrogate standards may overestimate the total recovery of PAHs from glass using solvent wipe sampling. The developed method enables future studies of accumulation of PAHs indoors, including potential longer-term exposure derived from contaminated indoor surfaces.
Introduction
Wildfire events are occurring with increasing frequency and severity all over the world1 and are impacting both outdoor and indoor air quality.2 Wildfire smoke is a source of polycyclic aromatic hydrocarbons (PAHs), organic compounds with two to seven fused benzene rings. During a wildfire event, regional populations are exposed to PAHs.3 Understanding the pathways and magnitudes of exposure to PAHs is important because of their known mutagenicity and carcinogenicity4 and impacts on human development,5 including the respiratory6 and reproductive systems.7 Sixteen PAHs are designated high priority by the Environmental Protection Agency (EPA) because of their toxicity and prevalence.8 Studies show that PAHs not currently identified as priority pollutants are also suspected to be toxic or possibly carcinogenic.9
PAHs exist across a wide range of volatilities, and in outdoor air, they are present in the gas phase and partitioned to ambient particles.10,11 Partitioning behavior is a function of many factors, including ambient temperature and humidity, aerosol and surface properties, and the physicochemical properties of the specific PAH.12 Indoors, there exists high material surface area compared to outdoors, where deposition and chemical interactions occur;13,14 semi-volatile compounds, including PAHs, can partition with indoor materials and dust and particles deposited on material surfaces.15
Studies show PAHs can accumulate on indoor surfaces. For example, Schick et al.16 studied the fate of 16 PAHs present in cigarette smoke and showed the majority of PAHs released during smoking are deposited on surfaces. Pan et al.17 studied accumulation of PAHs on indoor glass window surfaces over a 40-day period. They show light PAHs rapidly reach a steady state while heavy PAHs have a nonlinear accumulation. Van Loy et al.18 investigate the partitioning of phenanthrene with carpet in a controlled chamber, observing modest uptake to the carpet and noting the need for further study of sorption and desorption of semi-volatile organic compounds (SVOCs) to indoor surfaces. Singer et al.19 report sorption/desorption coefficients of three PAHs in a model furnished room, finding stronger uptake for PAHs than other, more volatile compounds studied.
Prior studies of wildfire smoke and indoor PAHs exist in the literature, though they do not specifically address accumulation on real indoor materials following a wildfire smoke event. Kohl et al.20 show that PAH levels in house dust in 64 homes were similar to or lower than homes not impacted by fire after 14 months. Ghetu et al.21 deployed low-density polyethylene passive samplers to indoor environments in the Pacific Northwest region of the United States during and after wildfire events; results implied consistently higher PAH concentrations in indoor air than outdoor air, including during wildfire periods. Prior studies examine accumulation of PAHs on firefighters’ skin and equipment to assess their exposure during simulated and real fire events.22−30 Ruokojärvi et al.31 show accumulation of PAHs on indoor surfaces during simulated house fires. These studies imply it is plausible that wildfire smoke plumes are loading indoor surfaces with PAHs, though questions remain regarding abundance and persistence on indoor materials.
Given the increasing frequency of regional smoke events, new methods are needed to complement airborne PAHs sampling31 with approaches that recover PAHs from indoor materials that may act as a reservoir for PAHs post-smoke events. Solvent wipes of materials followed by solvent extraction is an effective, nondestructive approach for quantification and identification of surface-associated PAHs.22−36 The use of the wipe soaked in isopropanol is applied broadly, from windows to clothes or even human skin.23−30,34,35 Passive sampling devices are also employed to measure PAHs.21 Passive samplers, however, are distinct media from indoor material and must be deployed in advance of a smoke event; direct sampling of indoor materials may offer a compelling complement to passive deployments. PAHs are also measured from indoor dust, allowing estimation of the PAHs deposition on surfaces.37,38 However, dust residence time indoors differs from that of indoor surfaces and likely has distinct material-PAHs interactions (i.e., sorption parameters). Multiple studies have examined the PAH concentration on indoor surfaces; however, some analytical challenges remain and need to be addressed, specifically on the sampling process when applied to real indoor materials beyond glass surfaces. This study aims to test and critically evaluate analytical methods for analysis of PAHs on four common indoor materials.
To understand the abundance, retention, and chemistry of PAHs on indoor surfaces, we propose and evaluate sampling and extraction methods that use sonication-assisted extraction of solvent wipes or indoor materials directly, followed by GC/MS analysis. Methods to characterize surface-associated PAHs will support studies of dermal and/or longer-term PAH exposure pathways after a smoke event clears. Such a method will also enable the study of the dynamics and partitioning of PAHs to indoor surfaces.
Experimental Section
Reagents
Sixteen PAHs (certified reference material, TraceCERT, Sigma-Aldrich Co., LLC) with a nominal concentration of 2000 μg/mL for each component in dichloromethane (DCM) were used for preparation of the calibration standard and spiking solution. Naphthalene-D8 and chrysene-D12 (certified reference materials, TraceCERT, Sigma-Aldrich Co., LLC) at 2000 μg/mL each in DCM were used as stock solutions for preparation of the mixture of surrogate standards. Pyrene-D10 (98 atom % D, Sigma-Aldrich Co., LLC), used to prepare a stock solution at 2000 μg/mL in DCM, and Phenanthrene-D10 (certified reference materials, TraceCERT, Sigma-Aldrich Co., LLC), at 2000 μg/mL in DCM, were used as internal standards. Stock solutions were stored in amber vials at −20 °C. Dichloromethane Optima (99.9%, Fisher Scientific) was used as a sampling, extraction, and analysis solvent. Isopropanol (IPA) 99.5% was used as a sampling solvent.
Studied PAHs
Sixteen PAHs were studied based on a review of the US EPA priority list:39 naphthalene (Nap), 2-bromonaphthalene (BrNap), fluorene (Flu), phenanthrene (Phe), acenaphthene (Ace), acenaphthylene (Acy), anthracene (Ant), fluoranthene (Flt), benz[a]anthracene (BaA), chrysene (Chr), pyrene (Pyr), benzo[a]pyrene (BaP), benzo[b]fluoranthene (BbF), dibenz[a,h]anthracene (DahA), benzo[ghi]perylene (BghiP), and indeno[1,2,3-cd]pyrene (IcdP). Table S1 in the supporting information shows the name and detailed information of the PAHs in this study. PAHs can be divided into two groups: “light” PAHs (LPAHs) consisting of 2–3 aromatic rings and “heavy” PAHs (HPAHs) consisting of 4 or more aromatic rings.40
Tested Materials
Four common household materials were studied: glass, painted drywall, cotton, and mechanical air cleaner filters. Petri dishes (100 mm × 10 mm, Pyrex, 7740 borosilicate glass) were used as glass materials, with PAHs applied over 39 cm2. Gypsum drywall wallboard (10 cm × 20 cm, USG SHEETROCK) was painted five years prior to the experiments with a low VOC paint (Emerald, Interior Acrylic Latex Paint). PAHs were applied to 49 cm2 of painted area. Squares of 25 cm2 were cut from a bedsheet (Mellanni, 400 thread count organic cotton) and from an unused filter (MERV13, Tex-Air filters, Air Relief Technologies, Inc.).
Spiking Procedure
A clean, dry wipe (Kimwipes, Kimtech, 11 cm × 21 cm) was used to remove dust on glass and painted drywall prior to spiking. Each material was spiked with the mixture of 16 PAHs diluted in DCM (500 μL at 0.2 ng/μL, i.e., 100 ng on each surface). Material loadings were 20–40 μg/m2. The spiking solution was uniformly applied on the surface of each material using a glass syringe.
Sampling Procedure
The sampling, extraction, and analysis processes were based on Stec et al.,25 with minor adjustments. Clean wipes (11 cm × 21 cm, Kimwipes, Kimtech) were folded in half three times and soaked with 2 mL of solvent (DCM or IPA) in a petri dish, covered to limit evaporation. For square samples (painted drywall), one side of the wipe was used to wipe the surface from left to right ten times, and another side of the wipe was used top to bottom ten times. For round samples (glass), one side of the wipe was used to wipe in a circle from the edge to center 10 times and another side from the center to edge 10 times. Wipes were individually placed into 40 mL amber vials. The tweezers were cleaned with DCM between each sample to avoid cross-contamination. Cotton and filter samples were directly extracted following the same procedure as the wipes. Field blanks for sampling wipes were prepared identically to sample wipes, except that field blank wipes did not contact surfaces. Field blanks for cotton and filters consisted of clean material with the same area as the spiked samples and were directly extracted without being spiked. Cotton and filters were directly extracted in DCM; therefore, there is no variable of sampling solvent to consider.
Extraction Method
100 μL of the solution of surrogate standards (naphthalene-D8 and chrysene-D12 at 5 ng/μL) was added directly in each amber vial containing the sample (wipe, cotton, or filter), and 15 mL of DCM was added to cover the sample. A laboratory blank was prepared by placing the same amount of surrogate solution and DCM in an empty amber vial. We choose DCM as the extraction solvent for multiple reasons: (1) DCM has been widely used as a solvent for PAHs because of the solubility of PAHs in DCM, (2) the boiling point of DCM (39.6 °C) is much lower than that of IPA (82.5 °C), allowing for a nitrogen blowdown evaporation at lower temperatures, limiting the evaporation of targeted compounds (PAHs), and (3) it has been shown that there are no significant differences between solvents during ultrasonic extraction.41 The amber vials were placed in an ultrasonic bath for 30 min held between 20–22 °C. After sonication, wipes were squeezed and removed from amber vials using clean tweezers. The vials containing the extracted samples and solvent were centrifuged at 1600 rpm for 20 min. The samples were transferred from the amber vials to 3 mL Reacti-Vials, and the solvent was evaporated under a gentle stream of nitrogen in a nitrogen blowdown apparatus (Reacti-Therm III, Pierce) maintained at 33–35 °C (below the boiling point of DCM, 39.6 °C) until complete evaporation of the solvent. The sample was reconstituted with 90 μL of DCM and 10 μL of solution of internal standards (phenanthrene-D10 and pyrene-D10, 50 ng/μL) before transfer to GC/MS vials with 300 μL fixed insert. Samples were stored at −20 °C until analysis, which occurred within 2 days (see stability test results in Table S2 in the Supporting Information).
Analysis
Samples, field blanks, laboratory blanks, and calibration standards were analyzed using a gas chromatograph (model 7890 A, Agilent Technologies) with a DB-5MS column (30 m length × 0.25 mm i.d. × 0.25 μm film thickness, Agilent J&W) coupled to a mass selective detector (model 5975 C, Agilent Technologies). 1 μL of solution was injected in a split/splitless injector kept at 300 °C. The injector was in splitless mode with a split vent after 1 min and a purge flow of 100 mL/min and a gas saver at 15 mL/min after 6 min. Helium was used as the carrier gas at a constant flow of 1 mL/min. The oven temperature started at 50 °C for 2 min, was then raised at 20 °C/min up to 180 °C, held for 0.5 min, raised again at 10 °C/min up to 300 °C, and kept isothermal for 5 min. The mass spectrometer conditions were: transfer line at 300 °C, ion source at 300 °C, and electron ionization (EI) voltage at 70 eV. Data were recorded in selected ion monitoring (SIM) mode with a quantifier ion corresponding to the molecular mass of the compound and a qualifier ion corresponding to, in general, half of the molecular mass of the parent compound (see Table S3 of the Supporting Information). Examples of chromatograms are presented in Figure S1 in the Supporting Information. The GC/MS method detection limit (MDL) and limit of quantification (LOQ) for each compound were calculated and are shown in Tables S4 and S5, respectively, of the Supporting Information.
Calculations
Peak integration of the quantifier ion was performed using Agilent ChemStation software. A five-point linear calibration curve was used to calculate the concentrations of target PAHs and the two surrogate standards using an internal standard normalized response factor. The range of the calibration was 0.05–5 ng/μL. Total recovery percentages are defined as recovery of sampling and extraction, shown in eq 1
| 1 |
where R% is the total recovery (%), Cmeas is the measured PAH concentration (ng/μL), and Cspiked is the spiked concentration of a PAH, calculated from prepared standards (ng/μL).
Extraction recovery percentages are recovery of only the extraction process28,34,36 and were calculated similar to eq 1 but using measured and spiked surrogate standards, which were applied post-sampling into the amber vial. Uncertainty in recovery is the propagated error on eq 1, calculated from the standard deviation of triplicate measurements and 1% uncertainty in spiked mass (Gastight syringe, Hamilton).
Results and Discussion
Prior Studies Measuring PAH Surface Accumulation
We summarize a noncomprehensive review of the recent (since 2000) literature quantifying PAHs on a variety of surfaces such as skin, windows, or filter materials (Tables S6 and S7 of the supporting information). Previous studies use a wipe soaked in IPA23−30,34,35 and also directly extract PAHs from the material.21,37,41−44 Extraction typically occurs in dichloromethane27,32,33,35 using an ultrasonic bath for 30 min.22,23,25,27,29,30,36,41 This method enables a relatively simple, inexpensive, and rapid extraction of PAHs with lower solvent consumption compared to other methods.31−35,37 Accelerated solvent extraction is possible with specialized equipment.42,44 Prior to analysis, filtration to remove particles is typical.22,23,27−30 However, the presence of a filter may decrease recovery of PAHs. Therefore, centrifugation is sometimes used to separate particles.25,29,30,36
Nitrogen blowdown is the most common evaporation process, as it is gentle and limits analyte loss.27,29,30,32,35−37,41,43,44 Gas chromatography with mass spectrometry (GC/MS) in selected ion monitoring (SIM) mode is the most common analysis technique.24−26,28,31,32,34−37,41,43,44 The column is usually a DB-5MS or equivalent,24−37,41,43,44 which is a nonpolar phenyl arylene polymer (equivalent to (5%-phenyl)-methylpolysiloxane). This column can handle high temperatures with low bleeding; the resulting high sensitivity makes it well suited for the study of PAHs at trace levels. The program temperature of the oven will depend on the mixture of target PAHs. Most methods start at 70 or 80 °C;27,29,30,34,35 if naphthalene analysis is necessary, the start temperature may need to be lower than 70 °C.37,41,43,44 Studies reviewed here generally end the method with temperature ≥300 °C, holding this temperature for several minutes to allow elution of heavier PAHs.27,29,30,34−36,41,43,44 The surrogate standard recovery percentage of the method is generally ∼70% or greater on average with the recovery being proportional to the molecular weight of the PAHs.23,28,33−36,44 However, we note that 12 of 19 studies reviewed do not communicate surrogate standard recovery, and no studies present or discuss the potential for analyte loss during the sampling step.
Extraction Recovery
Figure 1 shows the extraction recovery percentages of the 16 PAHs that were spiked directly onto a solvent-soaked wipe; results are shown for a DCM-wipe (orange bars, Figure 1) and an IPA-wipe (blue bars, Figure 1). A known concentration of surrogate standards, naphthalene-D8 and chrysene-D12, is spiked on the wipes before extraction once placed in the amber vials. IPA-wipes show better extraction recovery percentages (average ± standard deviation = 66 ± 18%, range from 21 to 86%) than DCM-wipes (44 ± 25%, range from 1 to 70%), particularly for LPAHs. A t-test shows significant differences between recovery percentages of IPA-wipes and recovery percentages of DCM-wipes for LPAHs (0.002 ≤ p ≤ 0.03), except for naphthalene (p = 0.07). We hypothesize that LPAHs will more strongly favor partitioning to the gas phase with the DCM-wipe than the IPA-wipe because of the order of magnitude higher vapor pressure of DCM compared to IPA; we expect from theory (e.g., Raoult’s law) that the overall vapor pressure of a PAH-solvent mixture to be greater for those wipe samples containing IPA than DCM. Naphthalene recovery percentages are low (≤20%) for both sampling solvents; we believe this comparatively volatile PAH is lost during the nitrogen blowdown process that occurred at temperatures slightly above ambient conditions (33–35 °C). A way to improve the recovery of naphthalene and other LPAHs using this method may be to perform the nitrogen blowdown at ambient temperature. The recovery percentage increases with the increasing aromatic rings: LPAHs show recoveries in IPA-wipes ranging 48–69% while recoveries for HPAHs range from 74–86%, except for benzo[b]fluoranthene (51%). A similar trend is observed for DCM-wipe samples with lower percentages, especially for LPAHs. Recovery percentages of deuterated compounds were similar to their associated nondeuterated compounds.
Figure 1.
Mean extraction recovery percentages with increasing aromatic ring number for (A) 16 PAHs applied to solvent-soaked wipes and (B) the surrogate standards of deuterated compounds. Solutions of PAHs were applied to wipes soaked in IPA (blue) or DCM (orange).
Total Recovery: Glass and Painted Drywall
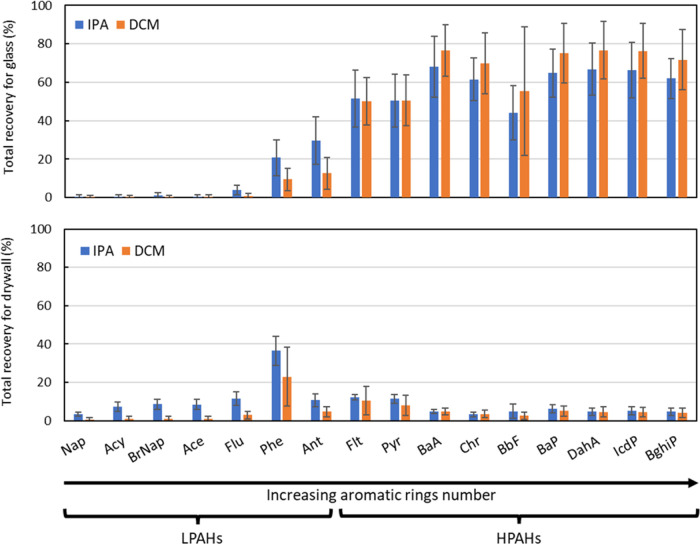
We define total recovery as the recovery of a spiked mass of PAH applied to a material; total recovery is distinct from recoveries shown in Figure 1, as it includes losses from the sampling process. Figure 2 shows the total recovery of the 16 PAHs from two materials: glass and painted drywall. A known concentration of the PAHs mixture was evenly loaded on each material prior to sampling. Figure 2 shows the total recovery achieved using an IPA-wipe (blue bars) or a DCM-wipe (orange bars).
Figure 2.
Mean total recovery percentages for the 16 PAHs extracted from IPA-wipes (blue) or DCM-wipes (orange).
On the glass material, sampling with an IPA-wipe showed a total recovery that averaged 61 ± 20% (range: 44–77%) for HPAHs and 9.4 ± 10% (range: 0–30%) for LPAHs. Sampling with DCM-wipe showed similar results averaging 68 ± 28% (range: 50–77%) for HPAHs and 3 ± 5% (range: 0–13%) for LPAHs. The substantially lower total recoveries for LPAHs than for extraction recoveries shown in Figure 1 can be explained by the higher volatility of these compounds that are expected to readily partition to the gas phase before or during sampling, as well as losses occurring during the nitrogen blowdown evaporation; as such, recoveries of LPAHs determined here may be considered lower limits of true total recoveries. We note that differences between extraction (Figure 1) and total recoveries (Figure 2) for LPAHs from glass show sampling losses are meaningful and should be addressed in campaigns using IPA or DCM solvent-wipes to measure LPAHs on materials.
On painted drywall material, total recovery percentages are <20% for all compounds, except for phenanthrene, regardless of sampling with IPA- or DCM-wipes. We believe these results can be explained by the complex surface of the paint vs glass. A dry paint film is porous45 and composed of diverse chemicals.46,47 We speculate PAHs are sorbed to the surface of the paint and diffuse through the porous media into the bulk of the paint film and cannot be recovered by wipe sampling. In addition, both IPA and DCM-wipes appeared to damage the surface by removing a thin film of paint; this likely created a complex matrix in our extracted sample. We conclude that wipe-based sampling of paint is not viable with this method.
Total Recovery: Cotton and Air Filter
Cotton and mechanical air filter media were spiked with a known concentration of the PAHs mixture. These fleecy and fibrous materials were directly extracted. Figure 3 shows the total recovery percentages of the 16 PAHs from MERV13 filter material (yellow bars, Figure 3) and from cotton (gray bars, Figure 3). The average recovery percentage of HPAHs was 57 ± 15% (ranging 36–67%) and 41 ± 12% (ranging 19–57%) for filters and cotton, respectively. For LPAHs, the recovery percentages were lower than for HPAHs: 20 ± 10% (ranging 1–51%) and 22 ± 9% (ranging 2–46%). We observe higher recoveries of LPAHs for filters and cotton than for glass. These improved, though still low, LPAH recoveries for direct extraction imply some LPAH losses from glass may be occurring during the sampling process. This might be explained by (1) incomplete transfer from material to wipe and (2) altered fluid mechanics during the wiping process that will reduce mass transfer resistances from the surface to the air. In contrast, recoveries of HPAHs for filters and cotton are lower than for glass; this may be a result of retention on fibers within the material bulk and/or in material pores.
Figure 3.
Mean total recovery percentages for the 16 PAHs directly extracted from mechanical air filter media (MERV13) (yellow) and cotton (gray).
Surrogate Standards
Surrogate standards are generally used to estimate the recovery percentage of the extraction method and apply correction factors to concentrations to reflect analyte loss. However, this does not consider the recovery percentage of the sampling method itself. By spiking the materials directly, we were able to estimate the total recovery percentage that accounts for both the sampling and the extraction method for each compound and each indoor material studied.
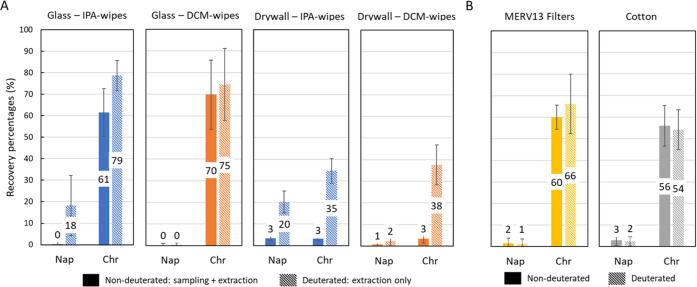
In Figure 1, we reported extraction recoveries of surrogate standards that are similar to their nondeuterated counterparts directly applied to a wipe. This implies the surrogate recovery accurately estimates extraction recovery from wipes. In contrast, Figure 4A shows that extraction recoveries of the surrogate standards are higher than total extraction recoveries of their associated nondeuterated compounds applied directly to glass and drywall. For example, for glass using IPA-wipes, chrysene-D12 recovery (i.e., extraction recovery) is 79%, while chrysene recovery (i.e., total recovery) is 61%. Similarly, for glass using IPA-wipes, recovery is 18% for naphthalene-D8 and 0.1% for naphthalene. Differences in extraction and total recovery demonstrate that the use of surrogate standards introduced post-sampling and preextraction may overestimate the total recovery capability of wipe sampling.
Figure 4.
Mean total (solid) and extraction (hatched) recovery percentages from (A) glass and drywall with IPA-wipes (blue) or DCM-wipes (orange) and from (B) direct extraction of MERV13 filters (yellow) and cotton (gray).
In contrast to the results for wipe-based sampling, materials undergoing direct extraction have similar recoveries for surrogate standards (Figure 4B) and nondeuterated counterparts. For example, in the case of cotton, recoveries are 54% for chrysene-D12 and 56% for chrysene. Other comparisons shown in Figure 4B demonstrate general agreement when comparing total and extraction recoveries. We conclude that for samples directly extracted, recovery of surrogate standards is a suitable proxy of total analyte loss, while additional correction may be necessary to address analyte loss during a wipe sampling procedure. This comparison of total and extraction recoveries supports the previous speculation that there exists some retention of PAHs on the sampling wipe itself, the surface being sampled, or there exist sampling losses due to changes at the material–air interface.
Viability of the Method
The extraction method used is efficient for PAHs, with most compounds having extraction recoveries >60% and <120%, in line with EPA standard method TO-13a.48 We note from our review of the literature that acceptable surrogate recoveries depend on the context (extraction method, matrices, etc.) and the goal of the study and that extraction error may be corrected using surrogate recoveries. Our study also demonstrates that surrogate recoveries may overestimate total recovery. To our knowledge, our study is the first to characterize the potential for analyte loss at the sampling stage and so prior estimates of total recovery thresholds are not available for comparison. Ideally, total recovery should be similar to extraction recovery to limit overall analyte loss.
Our total recovery data show that a common indoor material, glass, is a promising material for evaluating the accumulation and retention of PAHs on a realistic indoor surface. However, we note that our study shows additional corrections for sampling recovery may be required to improve absolute estimates of HPAHs recovered from glass. The magnitude of corrections necessary to account for observed total recoveries for LPAHs from glass is substantial and may introduce unacceptable uncertainty using this method. The method also does not appear suitable to extract PAHs from more complex indoor materials such as painted drywall. Future work should further develop methods for PAH recovery from painted surfaces, as they are a substantial fraction of indoor surface area. For materials that can be directly extracted, such as cotton or filters, the method is successful at recovering HPAHs (generally > 50% total recovery). The total recovery percentages presented herein can be used subsequently as a criteria and/or correction for using this developed method as a function of the materials studied and target PAHs.
Conclusions
A method of sampling and extracting surface-associated PAHs was developed and tested on common indoor materials. This study provides quantitative data showing (1) how to sample common indoor materials and (2) extraction and total recoveries of the approaches. This study introduces a metric of total recovery, calculated by spiking materials themselves with known quantities of PAHs and comparing the theoretical mass applied to the measured mass recovered. Results show important differences between total recovery vs extraction recovery for wipe-based sampling of glass materials. Correction for analyte loss during the wipe sampling process itself appears important for absolute quantification of surface-associated PAHs. Our results show these losses should not be neglected at the risk of underestimation of the material-associated concentration of the PAHs studied.
Acknowledgments
This article was developed under Assistance Agreement No. R840238 awarded by the U.S. Environmental Protection Agency to Elliott Gall, Portland State University. It has not been formally reviewed by EPA. The views expressed in this document are solely those of Aurélie Laguerre and Elliott Gall and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication. This work was partially supported by a seed grant from the Sloan Surface Consortium for Chemistry of Indoor Environments (SURF-CIE).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c01184.
List of the 16 PAHs studied and their properties (Table S1); t-test results for the stability tests (Table S2); quantifier and qualifier ions used for each compound in the SIM mode method in GC/MS (Table S3); examples of chromatograms (Figure S1); method detection limits (MDL) (Table S4); limit of quantification (LOQ) (Table S5); summary of studies using solvent-wipe sampling for PAHs analysis (Table S6); and summary of studies using direct extraction for PAHs analysis (Table S7) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Jones M. W.; Abatzoglou J. T.; Veraverbeke S.; Andela N.; Lasslop G.; Forkel M.; Smith A. J. P.; Burton C.; Betts R. A.; van der Werf G. R.; Sitch S.; Canadell J. G.; Santín C.; Kolden C.; Doerr S. H.; Le Quéré C. Global and Regional Trends and Drivers of Fire Under Climate Change. Rev. Geophys. 2022, 60, e2020RG000726 10.1029/2020RG000726. [DOI] [Google Scholar]
- Messier K. P.; Tidwell L. G.; Ghetu C. C.; Rohlman D.; Scott R. P.; Bramer L. M.; Dixon H. M.; Waters K. M.; Anderson K. A. Indoor versus Outdoor Air Quality during Wildfires. Environ. Sci. Technol. Lett. 2019, 6, 696–701. 10.1021/acs.estlett.9b00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. N.; Tao S.; Dou H.; Zhang T. W.; Zhang X. L.; Dawson R. Exposure of Traffic Police to Polycyclic Aromatic Hydrocarbons in Beijing, China. Chemosphere 2007, 66, 1922–1928. 10.1016/j.chemosphere.2006.07.076. [DOI] [PubMed] [Google Scholar]
- Kim K.-H.; Jahan S. A.; Kabir E.; Brown R. J. C. A Review of Airborne Polycyclic Aromatic Hydrocarbons (PAHs) and Their Human Health Effects. Environ. Int. 2013, 60, 71–80. 10.1016/j.envint.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Margolis A. E.; Herbstman J. B.; Davis K. S.; Thomas V. K.; Tang D.; Wang Y.; Wang S.; Perera F. P.; Peterson B. S.; Rauh V. A. Longitudinal Effects of Prenatal Exposure to Air Pollutants on Self-Regulatory Capacities and Social Competence. J. Child Psychol. Psychiatry 2016, 57, 851–860. 10.1111/jcpp.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Shafy H. I.; Mansour M. S. M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. 10.1016/j.ejpe.2015.03.011. [DOI] [Google Scholar]
- Xia Y.; Zhu P.; Han Y.; Lu C.; Wang S.; Gu A.; Fu G.; Zhao R.; Song L.; Wang X. Urinary Metabolites of Polycyclic Aromatic Hydrocarbons in Relation to Idiopathic Male Infertility. Hum. Reprod. 2009, 24, 1067–1074. 10.1093/humrep/dep006. [DOI] [PubMed] [Google Scholar]
- Hussar E.; Richards S.; Lin Z.-Q.; Dixon R. P.; Johnson K. A. Human Health Risk Assessment of 16 Priority Polycyclic Aromatic Hydrocarbons in Soils of Chattanooga, Tennessee, USA. Water Air Soil Pollut. 2012, 223, 5535–5548. 10.1007/s11270-012-1265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J. T.; Achten C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycyclic Aromat. Compd. 2015, 35, 330–354. 10.1080/10406638.2014.991042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankow J. F. Review and Comparative Analysis of the Theories on Partitioning between the Gas and Aerosol Particulate Phases in the Atmosphere. Atmospheric Environ. 1967 1987, 21, 2275–2283. 10.1016/0004-6981(87)90363-5. [DOI] [Google Scholar]
- Pankow J. F.; Bidleman T. F. Effects of Temperature, TSP and per Cent Non-Exchangeable Material in Determining the Gas-Particle Partitioning of Organic Compounds. Atmos. Environ., Part A 1991, 25, 2241–2249. 10.1016/0960-1686(91)90099-S. [DOI] [Google Scholar]
- Lima A. L. C.; Farrington J. W.; Reddy C. M. Combustion-Derived Polycyclic Aromatic Hydrocarbons in the Environment—A Review. Environ. Forensics 2005, 6, 109–131. 10.1080/15275920590952739. [DOI] [Google Scholar]
- Manuja A.; Ritchie J.; Buch K.; Wu Y.; Eichler C. M. A.; Little J. C.; Marr L. C. Total Surface Area in Indoor Environments. Environ. Sci.: Processes Impacts 2019, 21, 1384–1392. 10.1039/C9EM00157C. [DOI] [PubMed] [Google Scholar]
- Ault A. P.; Grassian V. H.; Carslaw N.; Collins D. B.; Destaillats H.; Donaldson D. J.; Farmer D. K.; Jimenez J. L.; McNeill V. F.; Morrison G. C.; O’Brien R. E.; Shiraiwa M.; Vance M. E.; Wells J. R.; Xiong W. Indoor Surface Chemistry: Developing a Molecular Picture of Reactions on Indoor Interfaces. Chem 2020, 6, 3203–3218. 10.1016/j.chempr.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W.; Dassonville C.; Sivanantham S.; Gregoire A.; Mercier F.; Le Bot B.; Malingre L.; Ramalho O.; Derbez M.; Mandin C. Semivolatile Organic Compounds in French Schools: Partitioning between the Gas Phase, Airborne Particles and Settled Dust. Indoor Air 2021, 31, 156–169. 10.1111/ina.12724. [DOI] [PubMed] [Google Scholar]
- Schick S. F.; Farraro K. F.; Perrino C.; Sleiman M.; Vossenberg G.; van de Trinh M. P.; Hammond S. K.; Jenkins B. M.; Balmes J. Thirdhand Cigarette Smoke in an Experimental Chamber: Evidence of Surface Deposition of Nicotine, Nitrosamines and Polycyclic Aromatic Hydrocarbons and de Novo Formation of NNK. Tob. Control 2014, 23, 152–159. 10.1136/tobaccocontrol-2012-050915. [DOI] [PubMed] [Google Scholar]
- Pan S.-H.; Li J.; Lin T.; Zhang G.; Li X.-D.; Yin H. Polycyclic Aromatic Hydrocarbons on Indoor/Outdoor Glass Window Surfaces in Guangzhou and Hong Kong, South China. Environ. Pollut. 2012, 169, 190–195. 10.1016/j.envpol.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Van Loy M. D.; Riley W. J.; Daisey J. M.; Nazaroff W. W. Dynamic Behavior of Semivolatile Organic Compounds in Indoor Air. 2. Nicotine and Phenanthrene with Carpet and Wallboard. Environ. Sci. Technol. 2001, 35, 560–567. 10.1021/es001372a. [DOI] [PubMed] [Google Scholar]
- Singer B. C.; Revzan K. L.; Hotchi T.; Hodgson A. T.; Brown N. J. Sorption of Organic Gases in a Furnished Room. Atmos. Environ. 2004, 38, 2483–2494. 10.1016/j.atmosenv.2004.02.003. [DOI] [Google Scholar]
- Kohl L.; Meng M.; de Vera J.; Bergquist B.; Cooke C. A.; Hustins S.; Jackson B.; Chow C.-W.; Chan A. W. H. Limited Retention of Wildfire-Derived PAHs and Trace Elements in Indoor Environments. Geophys. Res. Lett. 2019, 46, 383–391. 10.1029/2018GL080473. [DOI] [Google Scholar]
- Ghetu C. C.; Rohlman D.; Smith B. W.; Scott R. P.; Adams K. A.; Hoffman P. D.; Anderson K. A. Wildfire Impact on Indoor and Outdoor PAH Air Quality. Environ. Sci. Technol. 2022, 56, 10042–10052. 10.1021/acs.est.2c00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent K. W.; Eisenberg J.; Snawder J.; Sammons D.; Pleil J. D.; Stiegel M. A.; Mueller C.; Horn G. P.; Dalton J. Systemic Exposure to PAHs and Benzene in Firefighters Suppressing Controlled Structure Fires. Ann. Occup. Hyg. 2014, 58, 830–845. 10.1093/annhyg/meu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent K. W.; Alexander B.; Roberts J.; Robertson S.; Toennis C.; Sammons D.; Bertke S.; Kerber S.; Smith D.; Horn G. Contamination of Firefighter Personal Protective Equipment and Skin and the Effectiveness of Decontamination Procedures. J. Occup. Environ. Hyg. 2017, 14, 801–814. 10.1080/15459624.2017.1334904. [DOI] [PubMed] [Google Scholar]
- Keir J. L. A.; Akhtar U. S.; Matschke D. M. J.; Kirkham T. L.; Chan H. M.; Ayotte P.; White P. A.; Blais J. M. Elevated Exposures to Polycyclic Aromatic Hydrocarbons and Other Organic Mutagens in Ottawa Firefighters Participating in Emergency, On-Shift Fire Suppression. Environ. Sci. Technol. 2017, 51, 12745–12755. 10.1021/acs.est.7b02850. [DOI] [PubMed] [Google Scholar]
- Stec A. A.; Dickens K. E.; Salden M.; Hewitt F. E.; Watts D. P.; Houldsworth P. E.; Martin F. L. Occupational Exposure to Polycyclic Aromatic Hydrocarbons and Elevated Cancer Incidence in Firefighters. Sci. Rep. 2018, 8, 2476 10.1038/s41598-018-20616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir J. L. A.; Akhtar U. S.; Matschke D. M. J.; White P. A.; Kirkham T. L.; Chan H. M.; Blais J. M. Polycyclic Aromatic Hydrocarbon (PAH) and Metal Contamination of Air and Surfaces Exposed to Combustion Emissions during Emergency Fire Suppression: Implications for Firefighters’ Exposures. Sci. Total Environ. 2020, 698, 134211 10.1016/j.scitotenv.2019.134211. [DOI] [PubMed] [Google Scholar]
- Beitel S. C.; Flahr L. M.; Hoppe-Jones C.; Burgess J. L.; Littau S. R.; Gulotta J.; Moore P.; Wallentine D.; Snyder S. A. Assessment of the Toxicity of Firefighter Exposures Using the PAH CALUX Bioassay. Environ. Int. 2020, 135, 105207 10.1016/j.envint.2019.105207. [DOI] [PubMed] [Google Scholar]
- Cherry N.; Galarneau J.-M.; Kinniburgh D.; Quemerais B.; Tiu S.; Zhang X. Exposure and Absorption of PAHs in Wildland Firefighters: A Field Study with Pilot Interventions. Ann. Work Exposures Health 2021, 65, 148–161. 10.1093/annweh/wxaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks A. P. W.; Thai P.; Engelsman M.; Wang X.; Osorio A. F.; Mueller J. F. Characterising the Exposure of Australian Firefighters to Polycyclic Aromatic Hydrocarbons Generated in Simulated Compartment Fires. Int. J. Hyg. Environ. Health 2021, 231, 113637 10.1016/j.ijheh.2020.113637. [DOI] [PubMed] [Google Scholar]
- Banks A. P. W.; Wang X.; Engelsman M.; He C.; Osorio A. F.; Mueller J. F. Assessing Decontamination and Laundering Processes for the Removal of Polycyclic Aromatic Hydrocarbons and Flame Retardants from Firefighting Uniforms. Environ. Res. 2021, 194, 110616 10.1016/j.envres.2020.110616. [DOI] [PubMed] [Google Scholar]
- Ruokojärvi P.; Aatamila M.; Ruuskanen J. Toxic Chlorinated and Polyaromatic Hydrocarbons in Simulated House Fires. Chemosphere 2000, 41, 825–828. 10.1016/S0045-6535(99)00549-4. [DOI] [PubMed] [Google Scholar]
- Diamond M. L.; Gingrich S. E.; Fertuck K.; McCarry B. E.; Stern G. A.; Billeck B.; Grift B.; Brooker D.; Yager T. D. Evidence for Organic Film on an Impervious Urban Surface: Characterization and Potential Teratogenic Effects. Environ. Sci. Technol. 2000, 34, 2900–2908. 10.1021/es9906406. [DOI] [Google Scholar]
- Gingrich S. E.; Diamond M. L.; Stern G. A.; McCarry B. E. Atmospherically Derived Organic Surface Films along an Urban-Rural Gradient. Environ. Sci. Technol. 2001, 35, 4031–4037. 10.1021/es010699o. [DOI] [PubMed] [Google Scholar]
- Unger M.; Gustafsson Ö. PAHs in Stockholm Window Films: Evaluation of the Utility of Window Film Content as Indicator of PAHs in Urban Air. Atmos. Environ. 2008, 42, 5550–5557. 10.1016/j.atmosenv.2008.03.030. [DOI] [Google Scholar]
- Melymuk L.; Bohlin-Nizzetto P.; Vojta Š.; Krátká M.; Kukučka P.; Audy O.; Přibylová P.; Klánová J. Distribution of Legacy and Emerging Semivolatile Organic Compounds in Five Indoor Matrices in a Residential Environment. Chemosphere 2016, 153, 179–186. 10.1016/j.chemosphere.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Su P.; Zhang W.; Hao Y.; Tomy G. T.; Yin F.; Chen L.; Ding Y.; Li Y.; Feng D. Polycyclic Aromatic Hydrocarbon Contaminations along Shipping Lanes and Implications of Seafarer Exposure: Based on PAHs in Ship Surface Films and a Film-Air-Water Fugacity Model. Sci. Total Environ. 2020, 731, 138943 10.1016/j.scitotenv.2020.138943. [DOI] [PubMed] [Google Scholar]
- Yassin M. F.; Alhajeri N. S.; Kassem M. A. Polycyclic Aromatic Hydrocarbons Collected from Indoor Built Environments on Heating, Ventilation and Air Conditioning Dust Filters. Indoor Built Environ. 2016, 25, 137–150. 10.1177/1420326X14541557. [DOI] [Google Scholar]
- Wang M.; Jia S.; Lee S. H.; Chow A.; Fang M. Polycyclic Aromatic Hydrocarbons (PAHs) in Indoor Environments Are Still Imposing Carcinogenic Risk. J. Hazard. Mater. 2021, 409, 124531 10.1016/j.jhazmat.2020.124531. [DOI] [PubMed] [Google Scholar]
- Priority Pollutants List 2014https://www.epa.gov/sites/default/files/2015-09/documents/priority-pollutant-list-epa.pdf. (accessed 2021-Nov-25).
- Leroy-Cancellieri V.; Cancellieri D.; Leoni E. Characterization of PAHs Trapped in the Soot from the Combustion of Various Mediterranean Species. Atmosphere 2021, 12, 965. 10.3390/atmos12080965. [DOI] [Google Scholar]
- Flasch M.; Kistler M.; Kirchsteiger B.; Kasper-Giebl A.. Solvent Effects on Extraction of Polycyclic Aromatic Hydrocarbons in Ambient Aerosol Samples, In E3S Web of Conferences; EDP Sciences, 2016; p 00021. 10.1051/e3sconf/20161000021. [DOI]
- Sadiktsis I.; Nilsson G.; Johansson U.; Rannug U.; Westerholm R. Removal of Polycyclic Aromatic Hydrocarbons and Genotoxic Compounds in Urban Air Using Air Filter Materials for Mechanical Ventilation in Buildings. Sci. Technol. Built Environ. 2016, 22, 346–355. 10.1080/23744731.2016.1152155. [DOI] [Google Scholar]
- Paulik L. B.; Donald C. E.; Smith B. W.; Tidwell L. G.; Hobbie K. A.; Kincl L.; Haynes E. N.; Anderson K. A. Emissions of Polycyclic Aromatic Hydrocarbons from Natural Gas Extraction into Air. Environ. Sci. Technol. 2016, 50, 7921–7929. 10.1021/acs.est.6b02762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfouz M. M.; Hassan H. M.; Elobaid E. A.; Yigiterhan O.; Alfoldy B. PAH Concentrations and Exposure Assessment from House Dust Retained in Air-Conditioning Filters Collected from Greater Doha, Qatar. Environ. Geochem. Health 2019, 41, 2251–2263. 10.1007/s10653-019-00271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestarquit B. Geometry of a Paint Film: Basics Revisited. Prog. Org. Coat. 2016, 90, 200–221. 10.1016/j.porgcoat.2015.09.023. [DOI] [Google Scholar]
- Liu Z.; Ye W.; Little J. C. Predicting Emissions of Volatile and Semivolatile Organic Compounds from Building Materials: A Review. Build. Environ. 2013, 64, 7–25. 10.1016/j.buildenv.2013.02.012. [DOI] [Google Scholar]
- Algrim L. B.; Pagonis D.; de Gouw J. A.; Jimenez J. L.; Ziemann P. J. Measurements and Modeling of Absorptive Partitioning of Volatile Organic Compounds to Painted Surfaces. Indoor Air 2020, 30, 745–756. 10.1111/ina.12654. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . Compendium Method TO-13A: Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Air Using Gas Chromatography/Mass Spectrometry (GC/MS); EPA/625/R-96/010b; U.S. Environmental Protection Agency: Center for Environmental Research Information, 1999https://www.epa.gov/sites/default/files/2019-11/documents/to-13arr.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.