Abstract
Background
OSA has been linked to microaspiration, systemic inflammation, and suboptimal immune function.
Research Question
Is OSA prospectively associated with risk of hospitalization for pneumonia, respiratory, and total infections?
Study Design and Methods
Prospective cohort. Participants in the Atherosclerosis Risk in Communities (ARIC) study (N = 1,586) underwent polysomnography in 1996-1998 and were followed up through 2018 for infection-related hospitalizations. The apnea-hypopnea index (AHI; events/h) was used to categorize participants as having severe OSA (≥ 30), moderate OSA (15-29), mild OSA (5-14), or a normal breathing pattern (< 5). Cox regression was used to calculate hazard ratios (HRs) and 95% CIs.
Results
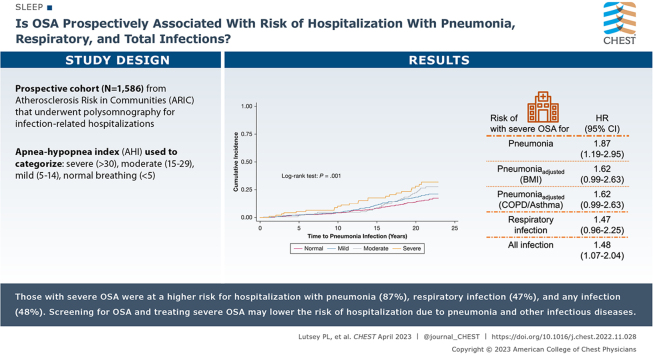
ARIC participants were on average 62.7 (SD = 5.5) years of age, and 52.8% were female. Severe OSA was present in 6.0%, moderate OSA in 12.7%, mild OSA in 30.0%, and normal breathing in 51.3%. A total of 253 hospitalizations with pneumonia occurred over a median 20.4 (max, 22.9) years’ follow-up. Participants with severe OSA were at 1.87 times (95% CI, 1.19-2.95) higher risk of hospitalization with pneumonia compared with those with a normal breathing pattern after adjustment for demographics and lifestyle behaviors. Results were attenuated modestly after adjustment for BMI (1.62 [0.99-2.63]), and prevalent asthma and COPD (1.62 [0.99-2.63]). A similar pattern existed for hospitalization with respiratory infection and composite infection (demographic and behavior-adjusted HRs: 1.47 [0.96-2.25] and 1.48 [1.07-2.04], respectively).
Interpretation
Severe OSA was associated with increased risk of hospitalizations with pneumonia in this community-based cohort. OSA patients may benefit from more aggressive efforts to prevent pneumonia and other infectious conditions.
Key Words: Atherosclerosis Risk in Communities (ARIC) study, infection, OSA, pneumonia, Sleep Heart Health Study (SHHS)
Graphical Abstract
Take-home Points.
Study Question: Is OSA prospectively associated with risk of hospitalization with pneumonia, respiratory, and total infections?
Results: Compared with individuals with a normal sleep breathing pattern, those with severe OSA were at 87% higher risk of hospitalization with pneumonia, 47% higher risk of hospitalization with respiratory infection, and 48% higher risk of hospitalization with any infection.
Interpretation: Screening for OSA and treating severe OSA in the primary care setting may lower the risk of hospitalization caused by pneumonia and other infectious diseases.
OSA is a common and chronic sleep disorder characterized by the collapse of the upper airway soft tissue during sleep, causing recurrent and intermittent hypoxia with hypercapnia resulting in frequent nocturnal awakening and sleep interruption.1 Although the exact prevalence of OSA is unknown, approximately 22% of adult men and 17% of adult women have at least some degree of OSA.1, 2, 3
Pneumonia is one of the most common infectious causes of hospitalization and death among adults in the United States.4, 5, 6 Emerging evidence suggests that patients with OSA may be more susceptible to developing pneumonia and more severe pneumonia outcomes. However, the few epidemiologic and clinical investigations that have explored the association have been limited by lack of systematic assessment of OSA, small sample sizes, clinical populations, or cross-sectional or retrospective study designs.7, 8, 9, 10, 11
Several hypothesized pathways link OSA with risk of pneumonia and other infections. From a mechanistic standpoint, OSA causes upper airway sensory dysfunction and excessive microaspirations, which can result in significant increases in bacterial organisms in the airway, leading to upper airway and laryngeal inflammation.7,12, 13, 14 Systemically, healthy sleep is believed to play an important role in the body’s inflammation control and immune system regulation.15,16 As additional supporting evidence, intermittent hypoxia and chronic sleep deprivation are common among OSA patients and are believed to cause increased oxidative stress and systemic inflammation.15, 16, 17, 18, 19 Prior work has also linked both reduced and prolonged habitual sleep durations to increased risk of pneumonia.20
In the context of the COVID-19 pandemic, OSA has been speculated to be a probable risk factor for severe COVID-19 because of shared pathophysiological pathways.21, 22, 23 OSA and COVID-19 also share several common risk factors, such as prevalent obesity, hypertension, diabetes, and cardiovascular disease. Additionally, OSA has been linked to increases in inflammatory markers that are implicated in more severe COVID-19 outcomes. Among the rapidly emerging studies,24, 25, 26, 27 an observational study of health care system records in Chicago reported patients with OSA had a greater risk of COVID-19 infection, hospitalization, and poorer outcomes than similar-age patients receiving the same care.27 However, this study and many of the prior studies did not examine undiagnosed OSA.
More research is needed to better understand whether OSA increases risk of infection, particularly pneumonia. Therefore, we investigated these associations using data from ∼2,000 participants of the Atherosclerosis Risk in Communities (ARIC) study who had polysomnography in 1996-1998 and were followed through 2018 for hospitalizations. We hypothesized that severe OSA would be associated with greater risk of incident hospitalization with pneumonia, respiratory infections, and any composite infections over 20 years of follow-up. Although this study cannot directly address COVID-19, insights regarding the association of OSA with the infections studied herein may have some relevance.
Study Design and Methods
Study Design
The ARIC study is a prospective cohort study that enrolled 15,792 participants, aged 45 to 64 years, from four communities in the United States from 1987 to 1989.28 Since its inception, participants have been followed up continuously for hospitalized outcomes, and several in-person clinic visits have taken place. Relevant to the current analysis, visit 4 took place in 1996-1998. Shortly after visit 4, participants from the suburban Minneapolis, MN, and Washington County, MD, sites were invited to take part in the Sleep Heart Health Study (SHHS), which conducted in-home polysomnography and sleep questionnaires.29 Local Institutional Review Boards approved the protocol, and all participants provided written informed consent.
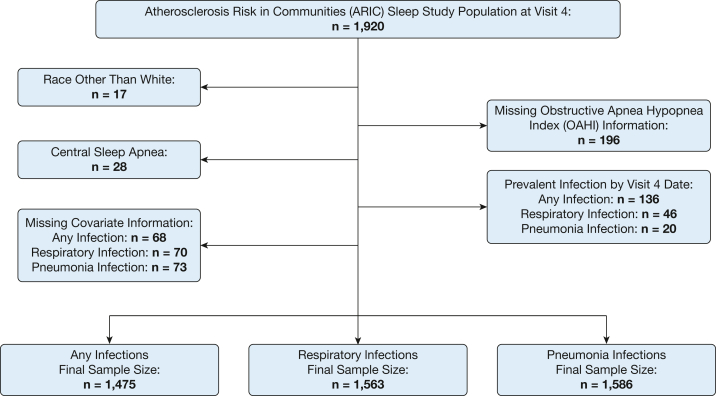
A total of 1,920 participants took part in both ARIC visit 4 and the SHHS and were potentially eligible for inclusion in this analysis. For this analysis, we further required that the participants have valid polysomnography data, self-identify as White (due to low numbers for participants who did not identify as White), not have central sleep apnea or missing data on key covariates, and not have the prevalent outcome of interest at the time of visit 4. An inclusion flow chart is provided in Figure 1. The final sample size varied slightly according to outcome of interest, ranging from 1,475 to 1,586, because of differences in the number of prevalent cases excluded.
Figure 1.
Participants flow chart.
Sleep Evaluation
Sleep evaluation was performed using in-home unattended polysomnography (PS-2 System; Compumedics Limited), as described in prior SHHS study publications.29,30 Apnea was defined as absence or near absence (< 25% of baseline) of airflow for a duration of > 10 s.29,30 Hypopnea was defined as a decrease in the amplitude of the airflow below 70% of baseline for ≥ 10 s and an oxyhemoglobin desaturation of at least 4%. The apnea-hypopnea index (AHI) was calculated as the number of obstructive apneas (regardless of the oxygen desaturation level) plus hypopneas (with a ≥ 4% decrease in oxygen saturation) per hour of sleep.30,31 AHI was used to categorize OSA into four categories as follows: normal, less than 5 events per hour; mild OSA, 5-14 events per hour; moderate OSA, 15-29 events per hour; and severe OSA, ≥ 30 events per hour.31 Additionally, we defined OSA severity according to a polysomnography-derived hypoxic burden (T90). Hypoxic burden captures the total amount of respiratory event-related hypoxemia over the sleep period. Hypoxic burden (T90) was defined as the proportion of the total sleep time with oxygen saturation < 90%, and participants were categorized by tertiles: < 1% (reference), 1% to < 5% (mild/moderate), and ≥ 5% (severe).
Hospitalizations With Infection
Since baseline, ARIC participants have been followed for incident hospitalizations through regular follow-up phone calls (annual before 2012, twice yearly thereafter), surveillance of local hospital patient lists, and through linkage with national and state death indexes (followed by proxy interviews in instances of death). International Classification of Disease (ICD) codes are collected for all hospitalizations.
For the current manuscript, the primary outcome of interest was hospitalization with pneumonia, given the potential that microaspirations associated with OSA may exacerbate risk of this outcome. Secondary outcomes were hospitalizations with a new diagnosis of pneumonia, respiratory infection, or any infection (composite). We used the same ICD-9 codes as in a prior ARIC publication,32 then cross-walked ICD-10 codes to ICD-9 codes (with evaluation for face-validity) to allow for expanded follow-up. The codes used are provided in e-Table 1. To increase the likelihood that we included infections that were meaningful, we required infections to be listed in the first five positions of the hospital discharge summary.
Covariates
Information on potential confounders was collected at ARIC clinic visits. Age, sex, and education were gathered at visit 1. Sports index was collected at visit 3. Smoking and drinking status were self-reported at visit 4. Asthma and COPD were self-reported at the SHHS visit. At visit 4, height (m) and weight (kg) were measured by trained staff. BMI was calculated as weight divided by height squared. Diabetes at visit 4 was defined by fasting glucose ≥ 126 mg/dL, nonfasting glucose ≥ 200 mg/dL, self-reported physician diagnosis, or current use of medications for diabetes. Using blood samples collected at visit 4, estimated glomerular filtration rate (eGFR) was calculated using both creatinine and cystatin-C. Also using samples from visit 4, high-sensitivity C-reactive protein (CRP) was measured using a nephelometric method on the Siemens Dade Behring BN II analyzer (Siemens Healthcare Diagnostics). Prevalent cardiovascular disease CVD (coronary heart disease, heart failure, or stroke) was ascertained using all information available in the cohort (self-reported prevalence, incidence from ICD codes before visit 4), as has been done previously in ARIC.33
Statistical Analysis
Descriptive statistics (means and proportions) are provided by OSA severity categories. Kaplan-Meier graphs are used to illustrate the cumulative incidence of infection outcomes between OSA categories over time. Cox proportional hazards regression was used to evaluate the association between OSA severity and risk of hospitalization with pneumonia, respiratory infection, and any infection. Person-time accrued from the date of the SHHS examination until hospitalization with an incident outcome of interest, loss to follow-up, death, or administrative censoring at December 31, 2018. A series of nested adjustments were conducted to account for potential confounders of the association between OSA and incident hospitalized infection. Model 1 adjusted for demographic factors, including age, sex, and center. Model 2 additionally adjusted for educational attainment and behaviors, including sports index, drinking status, and smoking status. Model 3 also adjusted for BMI, which has an established and strong association with OSA. Model 4 further adjusted for clinical lung disease conditions that may confound the association, specifically prevalent COPD, and prevalent asthma. Model 5 additionally adjusted for eGFR, prevalent diabetes, and prevalent cardiovascular disease. Lastly, model 6 also adjusted for CRP to explore whether adjustment for systemic inflammation as assessed by CRP attenuates the association. Multiplicative interactions by age (median split), sex, and BMI were tested by including cross-product terms in the models. The proportional hazards assumption was tested by evaluating the interaction between OSA categories and the natural log of person-time, and by visual inspection of graphs of the survival function vs survival time stratified by OSA categories. The proportioanl hazards assumption held in both instances. Finally, as a sensitivity analysis we accounted for competing risk of death, using the Fine-Gray method.34 All statistical analyses were done using SAS v 9.4 (SAS Inc.).
Results
Among 1,586 participants who were included in the analysis for the pneumonia outcome, 47.2% were male, and their median age was 62 years. Of this sample, 6.0% had severe OSA, 12.7% moderate OSA, 30.0% mild OSA, and 51.3% had a normal sleep breathing pattern. Participants with severe OSA tended to be male, be older, be former smokers, have a higher BMI, have higher CRP, have more prevalent CVD, and have lower eGFR (Table 1). Similar results were observed in the respiratory infections and any infections analytic samples (e-Tables 2, 3).
Table 1.
Participant Characteristics by OSA–Hypopnea Index (OAHI) Categories: The ARIC Study, 1996-1998
| Participant Characteristics | OSA Category |
P for Difference | |||
|---|---|---|---|---|---|
| Normal |
Mild |
Moderate |
Severe |
||
| < 5 Events/h | 5-14 Events/h | 15-29 Events/h | ≥ 30 Events/h | ||
| No. | 814 | 475 | 202 | 95 | |
| OAHI median, events/h | 1.8 | 8.4 | 19.9 | 43.9 | |
| Demographics | |||||
| Age, y | 61.9 ± 5.5 | 63.4 ± 5.3 | 63.5 ± 5.4 | 63.9 ± 5.4 | < .0001 |
| Male, No. (%) | 286 (35.1) | 260 (54.7) | 141 (69.8) | 61 (64.2) | < .0001 |
| Education, No. (%) | .20 | ||||
| Less than high school diploma | 72 (8.9) | 61 (12.8) | 27 (13.4) | 8 (8.4) | |
| High school graduate | 389 (47.8) | 218 (45.9) | 88 (43.6) | 50 (52.6) | |
| Some college/ graduate school | 353 (43.4) | 196 (41.3) | 87 (43.1) | 37 (39.0) | |
| Behavioral characteristics | |||||
| Drinking status, No. (%) | .37 | ||||
| Current | 568 (69.8) | 323 (68.0) | 125 (61.9) | 62 (65.3) | |
| Former | 158 (19.4) | 104 (21.9) | 53 (26.2) | 20 (21.1) | |
| Never | 88 (10.8) | 48 (10.1) | 24 (11.9) | 13 (13.7) | |
| Smoking status, No. (%) | .001 | ||||
| Current | 110 (13.5) | 31 (6.5) | 14 (6.9) | 7 (7.4) | |
| Former | 360 (44.2) | 245 (51.6) | 110 (54.5) | 50 (52.6) | |
| Never | 344 (42.3) | 199 (41.9) | 78 (38.6) | 38 (40.0) | |
| Sport index | 2.7 ± 0.8 | 2.7 ± 0.8 | 2.6 ± 0.8 | 2.5 ± 0.7 | .06 |
| Physiologic characteristics | |||||
| BMI | 27.3 ± 4.4 | 29.4 ± 4.9 | 31.4 ± 5.5 | 34.0 ± 5.4 | < .0001 |
| C-reactive protein, mg/La | 2.1 (3.5) | 2.2 (3.6) | 2.1 (3.6) | 4.0 (6.4) | .001 |
| Prevalent asthma, No. (%) | 51 (6.3) | 31 (6.5) | 20 (9.9) | 5 (5.3) | .28 |
| Prevalent COPD, No. (%) | 6 (0.7) | 4 (0.8) | 2 (1.0) | 0 (0) | … |
| Prevalent CVD, No. (%) | 84 (10.3) | 54 (11.4) | 29 (14.4) | 18 (19.0) | .05 |
| Prevalent diabetes, No. (%) | 65 (8.0) | 73 (15.4) | 27 (13.4) | 20 (21.1) | < .0001 |
| eGFR, mL/min/1.73 m2 | 86.6 ± 14.2 | 84.2 ± 15.1 | 83.8 ± 14.6 | 78.7 ± 17.1 | < .0001 |
| < 60 | 40 (4.9) | 33 (7.0) | 14 (6.9) | 9 (9.5) | .005 |
| 60-89 | 408 (50.1) | 253 (53.3) | 114 (56.4) | 62 (65.3) | |
| ➢ 90 | 366 (45.0) | 189 (39.8) | 74 (36.6) | 24 (25.3) | |
Data are shown as No. (percentage) for categorical variables and as mean ± SD for continuous variables. CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate.
Data are shown as median (interquartile range) for C-reactive protein because of skewness.
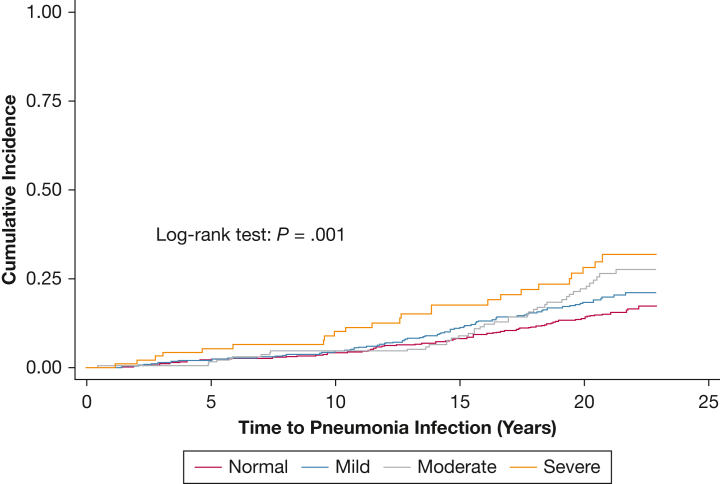
Over a median follow-up of 20.4 years, a total of 253 participants experienced hospitalization with pneumonia, yielding a crude incidence rate of 9.0 per 1,000 person-years. The crude association between OSA severity and hospitalization with pneumonia is depicted with a Kaplan-Meier plot (Fig 2). After adjustment for demographics and behaviors (model 2), participants with severe OSA had a 1.87 (95% CI, 1.19-2.95) times higher risk of hospitalization with pneumonia compared with participants without OSA (Table 2). The results were attenuated with additional adjustment for BMI (model 3, 1.62 [0.99-2.63]). Results were similar, with additional adjustment for comorbidities, eGFR, and CRP. Although there was some suggestion of dose response, mild and moderate OSA estimates were not statistically different from the null. No significant interactions were observed by age, sex, or BMI category. Results were similar in the sensitivity analysis, accounting for competing risk of death. In that analysis the subdistribution HR for model 3 was 1.66 (0.99-2.79) (e-Table 4).
Figure 2.
Kaplan-Meier plot - The crude association between OSA–hypopnea index categories and incident hospitalization with pneumonia: the ARIC study, 1996-1998 through 2018 (N = 1,586).
Table 2.
Association Between OSA–Hypopnea Index and Incident Hospitalization With Pneumonia Infection: The ARIC Study, 1996-1998 Through 2018 (N = 1,586)
| OSA Category |
P for Trend | ||||
|---|---|---|---|---|---|
| Normal |
Mild |
Moderate |
Severe |
||
| < 5 Events/h | 5-14 Events/h | 15-29 Events/h | ≥ 30 Events/h | ||
| No. | 814 | 475 | 202 | 95 | |
| Any incident hospitalization with pneumonia, n | 109 | 79 | 41 | 24 | |
| Person-y at risk | 14,700 | 8,289 | 3,471 | 1,535 | |
| Crude pneumonia infection incidence rate (per 1,000 person-y) | 7.4 | 9.5 | 11.8 | 15.6 | |
| Model 1 hazard ratio (95% CI) | 1 (reference) | 1.08 (0.81-1.45) | 1.32 (0.91-1.92) | 1.77 (1.13-2.76) | .01 |
| Model 2 hazard ratio (95% CI) | 1 (reference) | 1.19 (0.88-1.61) | 1.34 (0.92-1.95) | 1.87 (1.19-2.95) | .01 |
| Model 3 hazard ratio (95% CI) | 1 (reference) | 1.14 (0.84-1.55) | 1.24 (0.85-1.83) | 1.62 (0.99-2.63) | .05 |
| Model 4 hazard ratio (95% CI) | 1 (reference) | 1.13 (0.83-1.53) | 1.23 (0.83-1.80) | 1.62 (0.99-2.63) | .06 |
| Model 5 hazard ratio (95% CI) | 1 (reference) | 1.15 (0.85-1.56) | 1.25 (0.85-1.84) | 1.55 (0.95-2.51) | .07 |
| Model 6 hazard ratio (95% CI) | 1 (reference) | 1.15 (0.85-1.56) | 1.29 (0.87-1.90) | 1.54 (0.95-2.51) | 0.06 |
ARIC = Atherosclerosis Risk in Communities.
Model 1: Cox regression adjusted for age, sex, and center.
Model 2: Model 1 + adjustment for education, sports index, drinking status, and smoking status.
Model 3: Model 2 + adjustment for BMI.
Model 4: Model 3 + adjustment for prevalent asthma and prevalent COPD.
Model 5: Model 4 + adjustment for estimated glomerular filtration rate (eGFR), diabetes, and prevalent cardiovascular disease.
Model 6: Model 5 + adjustment for log(C-reactive protein).
Hospitalization with respiratory infection and any infection were secondary outcomes. Over follow-up, 330 participants experienced incident hospitalization with respiratory infections; the crude incidence rate was 12.1 per 1,000 person-years. A total of 589 participants were hospitalized with any infection, yielding a crude incidence rate of 24.8 per 1,000 person-years. Crude associations between OSA severity and incident respiratory and any infection are depicted with Kaplan-Meier plots (e-Figs 1, 2). We also found that participants with severe OSA had 1.47 (95% CI, 0.96-2.25) times higher risk of incident hospitalization with respiratory infection (Table 3, model 2) and 1.48 times (95% CI: 1.07-2.04) higher risk of incident hospitalization with any infection (Table 4, model 2), after adjustment for demographics, physical activity, alcohol use, and smoking. However, these results were attenuated with further adjustment in the remaining models. No significant interactions were observed by age, sex, or BMI category for respiratory infection. For any infection, there was a significant interaction (P = .03) by baseline age, whereas the association of severe OSA vs normal was stronger among younger (≤ 62 years; HR: 2.11 [1.26-3.54]) than older (> 62 years; HR: 0.89 [0.56-1.40]) individuals with model 3 adjustments. Results were similar in the sensitivity analysis accounting for competing risk of death; for respiratory infection (e-Table 5) the subdistribution HR for model 3 was 1.34 (0.83-2.17), whereas for any infection (e-Table 6) it was 1.15 (0.80-1.64).
Table 3.
Association Between OSA–Hypopnea Index and Incident Hospitalization With Respiratory Infection: The ARIC Study, 1996-1998 Through 2018 (N = 1,563)
| OSA Category |
P for Trend | ||||
|---|---|---|---|---|---|
| Normal |
Mild |
Moderate |
Severe |
||
| < 5 Events/h | 5-14 Events/h | 15-29 Events/h | ≥ 30 Events/h | ||
| No. | 803 | 467 | 199 | 94 | … |
| Incident hospitalization with respiratory infection, No. | 152 | 103 | 49 | 26 | … |
| Person-y at risk | 14,290 | 7,992 | 3,389 | 1,497 | … |
| Crude respiratory infection incidence rate, per 1,000 person-y | 10.6 | 12.9 | 14.5 | 17.4 | … |
| Model 1 hazard ratio (95% CI) | 1 (reference) | 1.03 (0.80-1.33) | 1.15 (0.83-1.60) | 1.40 (0.92-2.13) | .13 |
| Model 2 hazard ratio (95% CI) | 1 (reference) | 1.10 (0.85-1.43) | 1.19 (0.85-1.66) | 1.47 (0.96-2.25) | .07 |
| Model 3 hazard ratio (95% CI) | 1 (reference) | 1.08 (0.83-1.40) | 1.13 (0.80-1.59) | 1.34 (0.85-2.11) | .21 |
| Model 4 hazard ratio (95% CI) | 1 (reference) | 1.07 (0.82-1.39) | 1.11 (0.78-1.56) | 1.33 (0.85-2.10) | .25 |
| Model 5 hazard ratio (95% CI) | 1 (reference) | 1.07 (0.82-1.40) | 1.14 (0.80-1.61) | 1.26 (0.80-1.99) | .27 |
| Model 6 hazard ratio (95% CI) | 1 (reference) | 1.07 (0.82-1.40) | 1.15 (0.81-1.62) | 1.26 (0.80-1.97) | .27 |
ARIC = Atherosclerosis Risk in Communities.
Model 1: Cox regression adjusted for age, sex, and center.
Model 2: Model 1 + adjustment for education, sports index, drinking status, and smoking status.
Model 3: Model 2 + adjustment for BMI.
Model 4: Model 3 + adjustment for prevalent asthma and prevalent COPD.
Model 5: Model 4 + adjustment for estimated glomerular filtration rate (eGFR), diabetes, and prevalent cardiovascular disease.
Model 6: Model 5 + adjustment for log(C-reactive protein).
Table 4.
Association Between OSA–Hypopnea Index and Incident Hospitalized Infection: The ARIC Study, 1996-1998 Through 2018 (N = 1,475)
| OSA Category |
P for Trend | ||||
|---|---|---|---|---|---|
| Normal |
Mild |
Moderate |
Severe |
||
| < 5 Events/h | 5-14 Events/h | 15-29 Events/h | ≥ 30 Events/h | ||
| No. | 750 | 447 | 189 | 89 | … |
| Any incident hospitalization with infection, n | 274 | 191 | 78 | 46 | … |
| Person-y at risk | 12,419 | 7,073 | 3,012 | 1,280 | … |
| Crude infection incidence rate, per 1,000 person-y | 22.1 | 27.0 | 25.9 | 35.9 | … |
| Model 1 hazard ratio, 95% CI | 1 (reference) | 1.08 (0.89-1.31) | 1.05 (0.81-1.36) | 1.45 (1.05-1.99) | .07 |
| Model 2 hazard ratio, 95% CI | 1 (reference) | 1.13 (0.93-1.37) | 1.08 (0.83-1.40) | 1.48 (1.07-2.04) | .04 |
| Model 3 hazard ratio, 95% CI | 1 (reference) | 1.06 (0.87-1.29) | 0.95 (0.72-1.24) | 1.20 (0.85-1.69) | .58 |
| Model 4 hazard ratio, 95% CI | 1 (reference) | 1.05 (0.86-1.28) | 0.94 (0.72-1.23) | 1.20 (0.85-1.69) | .61 |
| Model 5 hazard ratio, 95% CI | 1 (reference) | 1.05 (0.86-1.28) | 0.95 (0.72-1.24) | 1.13 (0.80-1.59) | .76 |
| Model 6 hazard ratio, 95% CI | 1 (reference) | 1.04 (0.86-1.27) | 0.95 (0.73-1.25) | 1.12 (0.80-1.58) | .77 |
ARIC = Atherosclerosis Risk in Communities.
Model 1: Cox regression adjusted for age, sex, and center.
Model 2: Model 1 + adjustment for education, sports index, drinking status, and smoking status.
Model 3: Model 2 + adjustment for BMI.
Model 4: Model 3 + adjustment for prevalent asthma and prevalent COPD.
Model 5: Model 4 + adjustment for estimated glomerular filtration rate (eGFR), diabetes, and prevalent cardiovascular disease.
Model 6: Model 5 + adjustment for log(C-reactive protein).
In additional analyses, we explored the association between hypoxic burden (T90) and risk of hospitalized pneumonia and respiratory and composite infections. As shown in e-Tables 7 through 9, after accounting for demographics, behaviors, and BMI, severe hypoxic burden (> 5%) compared with normal (< 1%) was associated with elevated risk of incident hospitalized pneumonia (HR, 1.59 [95% CI, 1.12-2.24]), respiratory infection (HR, 1.53 [95% CI, 1.12-2.08]), and any infection (HR, 1.25 [95% CI, 0.98-1.59]).
Discussion
In a community-based sample of nearly 1,600 middle-aged and older adults, severe OSA was associated with an 87% greater risk of incident hospitalization with pneumonia. Hypoxic burden (T90) > 5% vs < 1% was associated with a 59% greater risk of hospitalized pneumonia. These associations persisted after accounting for demographics and lifestyle behaviors, including smoking. For OSA, there was also evidence of dose-response, with increasing risk of hospitalization with pneumonia observed with greater severity of OSA. The overall pattern of association was similar for hospitalization with respiratory infections and with any infection, although the magnitudes of association were somewhat smaller. These findings complement the growing evidence base linking OSA to chronic inflammation, adverse infectious disease outcomes, and specifically pneumonia. OSA is substantially underdiagnosed in the community, with an estimated 85% of individuals who meet the diagnostic criteria unaware of their status.35 Through appropriate OSA screening in primary care settings and subsequent treatment, it may be possible to reduce the rate of hospitalizations attributable to pneumonia and other respiratory infections, including COVID-19 infection.
Comparison With Prior Literature
Our findings supporting the association of severe OSA with greater risk of hospitalization with pneumonia align with prior literature. In a study by Chiner et al,7 among 82 patients with community-acquired pneumonia matched to 41 control subjects with other nonrespiratory infections, patients with severe OSA had three times higher odds for pneumonia (OR, 3.18; 1.11-11.56) compared with patients with normal breathing during sleep.7 Furthermore, evidence was seen of dose-response across AHI strata and numerous indexes of oxygen saturation, suggesting that as OSA severity increased, so did the odds of pneumonia. In a retrospective cohort analysis of the Taiwan National Health Insurance database, Su et al8 reported a modest (1.2-fold) greater risk of pneumonia among 6,186 OSA patients compared with 27,284 age-, sex-, and comorbidity-matched control subjects over 4.5 years of follow-up.8 Although the Chiner et al7 study used a polysomnography assessment of OSA similar to our study, in the Su et al8 study, OSA was defined by ICD-9 diagnostic codes. The high prevalence of undiagnosed OSA in the general population,1 which would not be captured by an exposure defined by OSA diagnosis, may have resulted in misclassification that could explain the difference in the magnitude of association between Su et al’s8 findings and our own results. In the current analysis, we leveraged polysomnography assessment of OSA in a nonclinical population and a prospective design to enhance causal inference regarding the association between OSA and risk of hospitalization with pneumonia.
Although not the focus of the current manuscript, studies evaluating whether pneumonia patients with prevalent clinically recognized OSA experience worse outcomes have yielded mixed findings.9,11 One of the highest-quality studies was a retrospective cohort of 250,907 pneumonia patients from 347 US hospitals; patients with concomitant OSA had higher rates of mechanical ventilation, intubation, transfer to the ICU, and longer hospital stays.9 In aggregate, data from numerous clinical and epidemiologic sources suggest OSA is associated with greater risk of pneumonia and poorer outcomes among individuals with pneumonia. Randomized clinical trials are needed to evaluate whether screening for and treatment of OSA could reduce the burden of pneumonia and other infectious conditions in the aging population.
Pathophysiology of OSA and Risk of Pneumonia and Other Infections
Mechanistic and systemic pathways may underlie the association between OSA and risk of pneumonia and other infections. In the current analysis, associations between OSA and risk of infections were attenuated after accounting for BMI. Although the observed pattern remained consistent with OSA associated with greater hospitalized infection risk, precision was poor, and some of the associations were not statistically significant after BMI adjustment. The strongest findings were for the pneumonia outcome, for which we observed a 54% greater risk of pneumonia among individual with OSA vs no OSA in our most adjusted model. Independence of the association between OSA and pneumonia risk suggests that OSA may act directly on risk of pneumonia, and not simply through the effect of obesity.
Mechanistically, OSA may lead to increased risk of respiratory infection via inflammation of the upper airway, episodes of gastroesophageal reflux, and excessive silent aspirations. Coordination between swallowing and breathing is essential to prevent aspiration of food, fluids, saliva, pharyngeal secretions, or gastric contents, which can lead to aspiration pneumonitis or aspiration pneumonia, along with other comorbidities,36, 37, 38 particularly among older indviduals.39,40 Excessive silent microaspirations may repeatedly expel significant quantities of bacterial organisms into the airway.7 Although microaspirations are experienced by healthy adults during sleep, some evidence suggests that they are more frequent in patients with OSA.41 Upper airway inflammation and laryngeal inflammation have been shown to be common among OSA patients.12 Furthermore, greater upper airway sensory impairment and a weak cough reflex are correlated with both the severity of inflammation and higher AHI.12, 13, 14 OSA has also been associated with dysphagia (difficulty swallowing), which makes OSA patients prone to aspiration.36 Finally, in a study conducted among COPD patients, intermittent hypoxia was associated with higher concentrations of hypoxia–inducible factor, which can upregulate the platelet-activating factor receptor on the airway epithelial surface and may facilitate respiratory bacterial infections.42 Taken together, the sensory and reflux impairments and repeated microaspirations commonly experienced in OSA patients may lead to changes in the oropharyngeal microflora, an inflammatory response in the lower airway, and dysregulation of the gut microbiome,43 which all may contribute to the development of lower respiratory infections, including pneumonia.
Systemically, patients with OSA experience frequent sleep disruptions and chronic sleep deprivation, which can impair immune function and increase inflammation, causing greater susceptibility to infection.15,16 Chronic sleep deprivation may impact immune response via alternation in cytokine production, decreased immune cell count, and impaired function and elevation of glucocorticoids.15,16,44 Both the sleep deprivation and intermittent hypoxia commonly experienced by OSA patients are believed to trigger systemic inflammation.15,45 Epidemiologic studies and mouse models support the connection between OSA and inflammation, with higher inflammatory markers, including interleukin 6, tumor necrosis factor alpha, and CRP reported among OSA patients,46,47 and inflammatory responses observed in mice with simulated OSA.48 These findings, together with mechanistic evidence, suggest OSA may exacerbate or cause systemic inflammation, impaired immune response, and increased risk of infection.
Strengths and Limitations
Strengths of the study include the prospective design, community-based (nonclinical) population, objective assessment of OSA, a substantial number of hospitalizations with infections, and information on key covariates such as BMI. The current study also has important limitations. Only White individuals from two US communities were included; therefore, generalizability in other populations is uncertain. Evaluation of OSA was done using in-home polysomnography rather than in-lab polysomnography, which is the gold standard diagnostic method.49 However, data from home sleep testing has been shown to be consistent with lab polysomnography.31,50 The indication for hospitalization may have been other noninflammatory conditions. However, to increase the likelihood that infections were clinically meaningful, we required them to be recorded in the first five positions of the hospitalization record. Unfortunately, our infection ICD code-based outcome definitions have not been validated. Also, most infections do not require inpatient treatment. Although our study did not capture milder infections, severe forms of infection are a clinically relevant outcome. Finally, the study is observational, so causal inference is limited.
Interpretation
Severe OSA and hypoxemia were independently associated with increased risk of hospitalization with pneumonia in a community-based sample of older adults. Findings for respiratory infection and hospitalization with any infection followed a similar pattern, though effect sizes were modestly lower. Recently, OSA has been linked to COVID-19 infection, hospitalizations, and respiratory failure.24,27,51 Given this emerging evidence, more research is needed to determine whether screening for OSA and treating severe OSA in the primary care setting will lower the risk of hospitalization resulting from pneumonia and other infectious diseases, including COVID-19.
Funding/Support
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005). The ARIC portion of the Sleep Heart Health Study (SHHS) was supported by National Heart, Lung, and Blood Institute (NHLBI) cooperative agreements U01 HL53934 (University of Minnesota) and U01 HL64360 (Johns Hopkins University). P. L. L. was also supported NHLBI K24 HL159246, K. M. F. by NHLBI T32 HL007779 and J. I. by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) K01 DK125616.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: P. L. L., I. Z., and K. M. drafted the manuscript. J. R. M. conducted data analyses. All authors interpreted the data and provided critical revision for scientific content. P. L. L. and J. R. M. are the guarantor of the paper, taking responsibility for the integrity of the work.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the staff and participants of the ARIC study for their important contributions.
Additional information: The e-Figures and e-Table are available online under “Supplementary Data.”
Supplementary Data
References
- 1.Veasey S.C., Rosen I.M. Obstructive sleep apnea in adults. N Engl J Med. 2019;380(15):1442–1449. doi: 10.1056/NEJMcp1816152. [DOI] [PubMed] [Google Scholar]
- 2.Heinzer R., Vat S., Marques-Vidal P., et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen X., Wang R., Zee P., et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain S., Self W.H., Wunderink R.G., et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes B.H., Haberling D.L., Kennedy J.L., Varma J.K., Fry A.M., Vora N.M. Burden of pneumonia-associated hospitalizations: United States, 2001-2014. Chest. 2018;153(2):427–437. doi: 10.1016/j.chest.2017.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kennedy J.L., Haberling D.L., Huang C.C., et al. Infectious disease hospitalizations: United States, 2001 to 2014. Chest. 2019;156(2):255–268. doi: 10.1016/j.chest.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiner E., Llombart M., Valls J., et al. Association between obstructive sleep apnea and community-acquired pneumonia. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su V.Y., Liu C.J., Wang H.K., et al. Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ. 2014;186(6):415–421. doi: 10.1503/cmaj.131547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindenauer P.K., Stefan M.S., Johnson K.G., Priya A., Pekow P.S., Rothberg M.B. Prevalence, treatment, and outcomes associated with OSA among patients hospitalized with pneumonia. Chest. 2014;145(5):1032–1038. doi: 10.1378/chest.13-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mok E.M., Greenough G., Pollack C.C. Untreated obstructive sleep apnea is associated with increased hospitalization from influenza infection. J Clin Sleep Med. 2020;16(12):2003–2007. doi: 10.5664/jcsm.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jean R.E., Gibson C.D., Jean R.A., Ochieng P. Obstructive sleep apnea and acute respiratory failure: an analysis of mortality risk in patients with pneumonia requiring invasive mechanical ventilation. J Crit Care. 2015;30(4):778–783. doi: 10.1016/j.jcrc.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Payne R.J., Kost K.M., Frenkiel S., et al. Laryngeal inflammation assessed using the reflux finding score in obstructive sleep apnea. Otolaryngol Head Neck Surg. 2006;134(5):836–842. doi: 10.1016/j.otohns.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen A.T., Jobin V., Payne R., Beauregard J., Naor N., Kimoff R.J. Laryngeal and velopharyngeal sensory impairment in obstructive sleep apnea. Sleep. 2005;28(5):585–593. doi: 10.1093/sleep/28.5.585. [DOI] [PubMed] [Google Scholar]
- 14.Kimoff R.J., Sforza E., Champagne V., Ofiara L., Gendron D. Upper airway sensation in snoring and obstructive sleep apnea. Am J Respir Crit Care Med. 2001;164(2):250–255. doi: 10.1164/ajrccm.164.2.2010012. [DOI] [PubMed] [Google Scholar]
- 15.Irwin M.R. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol. 2019;19(11):702–715. doi: 10.1038/s41577-019-0190-z. [DOI] [PubMed] [Google Scholar]
- 16.Besedovsky L., Lange T., Born J. Sleep and immune function. Pflugers Arch. 2012;463(1):121–137. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reutrakul S., Mokhlesi B. Obstructive sleep apnea and diabetes: a state of the art review. Chest. 2017;152(5):1070–1086. doi: 10.1016/j.chest.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nadeem R., Molnar J., Madbouly E.M., et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9(10):1003–1012. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouloukaki I., Mermigkis C., Kallergis E.M., Moniaki V., Mauroudi E., Schiza S.E. Obstructive sleep apnea syndrome and cardiovascular disease: the influence of C-reactive protein. World J Exp Med. 2015;5(2):77–83. doi: 10.5493/wjem.v5.i2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel S.R., Malhotra A., Gao X., Hu F.B., Neuman M.I., Fawzi W.W. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35(1):97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McSharry D., Lam M.T., Malhotra A. OSA as a probable risk factor for severe COVID-19. J Clin Sleep Med. 2020;16(9) doi: 10.5664/jcsm.8708. 1649-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saxena K., Kar A., Goyal A. COVID 19 and OSA: exploring multiple cross-ways. Sleep Med. 2021;79:223. doi: 10.1016/j.sleep.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhat S., Chokroverty S. Sleep disorders and COVID-19. Sleep Med. 2022;91:253–261. doi: 10.1016/j.sleep.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cade B.E., Dashti H.S., Hassan S.M., Redline S., Karlson E.W. Sleep apnea and COVID-19 mortality and hospitalization. Am J Respir Crit Care Med. 2020;202(10):1462–1464. doi: 10.1164/rccm.202006-2252LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lohia P., Sreeram K., Nguyen P., et al. Preexisting respiratory diseases and clinical outcomes in COVID-19: a multihospital cohort study on predominantly African American population. Respir Res. 2021;22(1):37. doi: 10.1186/s12931-021-01647-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strausz S., Kiiskinen T., Broberg M., et al. Sleep apnoea is a risk factor for severe COVID-19. BMJ Open Respir Res. 2021;8(1) doi: 10.1136/bmjresp-2020-000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maas M.B., Kim M., Malkani R.G., Abbott S.M., Zee P.C. Obstructive sleep apnea and risk of COVID-19 infection, hospitalization and respiratory failure. Sleep Breath. 2021;25(2):1155–1157. doi: 10.1007/s11325-020-02203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright J.D., Folsom A.R., Coresh J., et al. The ARIC (Atherosclerosis Risk In Communities) study: JACC Focus Seminar 3/8. J Am Coll Cardiol. 2021;77(23):2939–2959. doi: 10.1016/j.jacc.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan S.F., Howard B.V., Iber C., et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 30.Lutsey P.L., Misialek J.R., Mosley T.H., et al. Sleep characteristics and risk of dementia and Alzheimer's disease: the Atherosclerosis Risk in Communities Study. Alzheimers Dement. 2018;14(2):157–166. doi: 10.1016/j.jalz.2017.06.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagayoshi M., Punjabi N.M., Selvin E., et al. Obstructive sleep apnea and incident type 2 diabetes. Sleep Med. 2016;25:156–161. doi: 10.1016/j.sleep.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowan L.T., Alonso A., Pankow J.S., et al. Hospitalized infection as a trigger for acute ischemic stroke: the Atherosclerosis Risk in Communities Study. Stroke. 2016;47(6):1612–1617. doi: 10.1161/STROKEAHA.116.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folsom A.R., Yatsuya H., Nettleton J.A., Lutsey P.L., Cushman M., Rosamond W.D. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57(16):1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 35.Punjabi N.M. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pizzorni N., Radovanovic D., Pecis M., et al. Dysphagia symptoms in obstructive sleep apnea: prevalence and clinical correlates. Respir Res. 2021;22(1):117. doi: 10.1186/s12931-021-01702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghannouchi I., Speyer R., Doma K., Cordier R., Verin E. Swallowing function and chronic respiratory diseases: systematic review. Respir Med. 2016;117:54–64. doi: 10.1016/j.rmed.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Sato K., Chitose S.I., Sato F., Ono T., Umeno H. Recurrent aspiration pneumonia precipitated by obstructive sleep apnea. Auris Nasus Larynx. 2021;48(4):659–665. doi: 10.1016/j.anl.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Kikuchi R., Watabe N., Konno T., Mishina N., Sekizawa K., Sasaki H. High incidence of silent aspiration in elderly patients with community-acquired pneumonia. Am J Respir Crit Care Med. 1994;150(1):251–253. doi: 10.1164/ajrccm.150.1.8025758. [DOI] [PubMed] [Google Scholar]
- 40.Teramoto S., Fukuchi Y., Sasaki H., et al. High incidence of aspiration pneumonia in community- and hospital-acquired pneumonia in hospitalized patients: a multicenter, prospective study in Japan. J Am Geriatr Soc. 2008;56(3):577–579. doi: 10.1111/j.1532-5415.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 41.Beal M., Chesson A., Garcia T., Caldito G., Stucker F., Nathan C.O. A pilot study of quantitative aspiration in patients with symptoms of obstructive sleep apnea: comparison to a historic control group. The Laryngoscope. 2004;114(6):965–968. doi: 10.1097/00005537-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Shukla S.D., Walters E.H., Simpson J.L., et al. Hypoxia-inducible factor and bacterial infections in chronic obstructive pulmonary disease. Respirology. 2020;25(1):53–63. doi: 10.1111/resp.13722. [DOI] [PubMed] [Google Scholar]
- 43.Moreno-Indias I., Torres M., Montserrat J.M., et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J. 2015;45(4):1055–1065. doi: 10.1183/09031936.00184314. [DOI] [PubMed] [Google Scholar]
- 44.Meerlo P., Sgoifo A., Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Unnikrishnan D., Jun J., Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord. 2015;16(1):25–34. doi: 10.1007/s11154-014-9304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kheirandish-Gozal L., Gozal D. Obstructive sleep apnea and inflammation: proof of concept based on two illustrative cytokines. Int J Mol Sci. 2019;20(3) doi: 10.3390/ijms20030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van der Touw T., Andronicos N.M., Smart N. Is C-reactive protein elevated in obstructive sleep apnea? a systematic review and meta-analysis. Biomarkers. 2019;24(5):429–435. doi: 10.1080/1354750X.2019.1600025. [DOI] [PubMed] [Google Scholar]
- 48.Nácher M., Serrano-Mollar A., Farré R., Panés J., Seguí J., Montserrat J.M. Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol. 2007;155(1):93–96. doi: 10.1016/j.resp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 49.Ross S.D., Sheinhait I.A., Harrison K.J., et al. Systematic review and meta-analysis of the literature regarding the diagnosis of sleep apnea. Sleep. 2000;23(4):519–532. [PubMed] [Google Scholar]
- 50.Redline S., Tosteson T., Boucher M.A., Millman R.P. Measurement of sleep-related breathing disturbances in epidemiologic studies: assessment of the validity and reproducibility of a portable monitoring device. Chest. 1991;100(5):1281–1286. doi: 10.1378/chest.100.5.1281. [DOI] [PubMed] [Google Scholar]
- 51.Miller M.A., Cappuccio F.P. A systematic review of COVID-19 and obstructive sleep apnoea. Sleep Med Rev. 2021;55 doi: 10.1016/j.smrv.2020.101382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.