Abstract
Quorum sensing (QS) is a widespread mechanism of environment sensing and behavioural coordination in bacteria. At its core, QS is based on the production, sensing and response to small signalling molecules. Previous work with Pseudomonas aeruginosa shows that QS can be used to achieve quantitative resolution and deliver a dosed response to the bacteria’s density environment, implying a sophisticated mechanism of control. To shed light on how the mechanistic signal components contribute to graded responses to density, we assess the impact of genetic (AHL signal synthase deletion) and/or signal supplementation (exogenous AHL addition) perturbations on lasB reaction-norms to changes in density. Our approach condenses data from 2000 timeseries (over 74 000 individual observations) into a comprehensive view of QS-controlled gene expression across variation in genetic, environmental and signal determinants of lasB expression. We first confirm that deleting either (∆lasI, ∆rhlI) or both (∆lasIrhlI) AHL signal synthase gene attenuates QS response to density. In the ∆rhlI background we show persistent yet attenuated density-dependent lasB expression due to native 3-oxo-C12-HSL signalling. We then test if density-independent quantities of AHL signal (3-oxo-C12-HSL, C4-HSL) added to the WT either flatten or increase responsiveness to density and find that the WT response is robust to all tested concentrations of signal, alone or in combination. We then move to progressively supplementing the genetic knockouts and find that cognate signal supplementation of a single AHL signal (∆lasI +3-oxo-C12-HSL, ∆rhlI +C4HSL) is sufficient to restore the ability to respond in a density-dependent manner to increasing density. We also find that dual signal supplementation of the double AHL synthase knockout restores the ability to produce a graded response to increasing density, despite adding a density-independent amount of signal. Only the addition of high concentrations of both AHLs and PQS can force maximal lasB expression and ablate responsiveness to density. Our results show that density-dependent control of lasB expression is robust to multiple combinations of QS gene deletion and density-independent signal supplementation. Our work develops a modular approach to query the robustness and mechanistic bases of the central environmental sensing phenotype of quorum sensing.
Keywords: sociomicrobiology, quorum sensing, microbial ecology, reaction norms
Introduction
Many species of bacteria use a form of cell–cell communication known as quorum sensing (QS) to collectively sense and respond to variation in their extracellular environment. QS bacteria secrete and respond to diffusible signal molecules that encode information on aspects of their environment. Canonically, QS is understood as a density-sensing device, as higher-density populations will typically accumulate higher concentrations of QS signals, although other aspects of environmental variation can also impact signal supply (e.g. mass-transfer [1–3], and genetic similarity [4]).
In the context of sensing density, the adoption of a ‘quorum’ analogy [5] leads to a simple threshold, qualitative interpretation of QS behaviour as either ‘quorate’ (high density; QS-controlled genes ‘on’) or ‘sub-quorate’ (low density; QS-controlled genes ‘off’). However, a growing number of studies have revealed substantial heterogeneity in responses to QS signals [1, 6–17] and we recently demonstrated that QS in Pseudomonas aeruginosa does not necessarily function in a threshold manner, on both population and single-cell scales [18]. Specifically, we found that per capita lasB, pqsA and rhlI expression shows a linear, quantitatively graded expression control (or ‘reaction norm’ [19, 20]) on the population scale to variation in density.
The ability of P. aeruginosa to achieve quantitative resolution and deliver a dosed response to its social environment implies a sophisticated mechanism of control. QS in P. aeruginosa represents one of the most intensely studied model systems – revealing a complex intracellular regulatory network driven by multiple signal molecules [21, 22] (Fig. 1). Fig. 1 illustrates the coupling between signal synthesis, signal response and downstream expression. The two primary signal molecules of P. aeruginosa are the N-acyl homoserine lactones (AHLs) N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL, henceforth 3-oxo-C12) and N-butyryl-homoserine lactone (C4-HSL, henceforth C4). The AHL system in PA is conventionally understood as a hierarchical system [23–27], with the lasIR system (producing/responding to 3-oxo-C12) governing the rhlIR system (producing/responding to C4), although alternative las-rhl wirings have been reported [28, 29]. The AHL signalling system is known to interact with multiple additional regulatory components, including the Pseudomonas quinolone signal (PQS) [30, 31].
Fig. 1.

Intracellular mechanics of AHL signalling in Pseudomonas aeruginosa . The P. aeruginosa QS system is dominated by the las (blue) and rhl (red) acyl-homoserone lactone (AHL) signalling systems. Each system codes for a signal synthase gene (lasI, rhlI), which guide the production of a diffusible AHL signal molecule [N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL, henceforth 3-oxo-C12) and N-butyryl-homoserine lactone (C4-HSL, henceforth C4)] at an initially basal level. Binding of each signal to its cognate receptor (LasR-3-oxo-C12, RhlR-C4) results in an active transcriptional factor, which up-regulates cognate synthase activity (signal auto-induction – a positive feedback control of signal production) along with other genes in the QS regulon, e.g. the secreted exoprotease enzyme and virulence factor LasB (purple).
In this study, we focus on lasB, a secreted protease and virulence factor that is under dual AHL signal control (Fig. 1 [24, 32–34]). We focus on lasB because of its widespread use as a model of P. aeruginosa virulence [35, 36], cooperation [37–40] and as a marker of QS-controlled behaviours [41–43]. Previous research has shown that lasB expression is controlled synergistically, with substantially increased expression under dual AHL exposure [24, 32–34]. Expression of lasB is also positively impacted by specific nutrient stress (P or Fe limitation [44]), potentially reflecting direct and indirect interactions of QS with multiple regulatory systems [22]. lasB expression is shaped by a large regulatory region with two distinct functional operator sequences, OP1 and OP2 [45, 46].
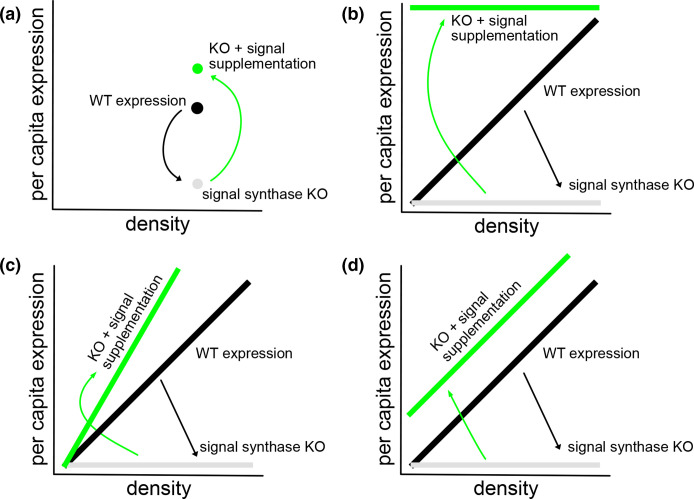
To shed light on how these mechanistic components contribute to graded responses to density, we assess the impact of genetic (AHL signal synthase deletion) and/or chemical (exogenous signal supplementation) perturbations on lasB reaction-norms to changes in density. Fig. 2 illustrates how our approach differs from conventional synthase knockout/signal complementation approaches, which focus on the impact on gene expression in a single environmental condition (typically, growth in complex medium to high density; Fig. 2a). In the context of lasB expression, studies have demonstrated that lasB expression can be attenuated by knockouts of one or both AHL signal synthase gene (lasI and/or rhlI, Fig. 2a grey circle) and can then be restored by addition of missing signal (Fig. 2a green circle) [47].
Fig. 2.
Gene knockout and signal complementation experiments as a function of environmental variation. A reaction-norm approach [18–20, 48, 69, 70] introduces more metrics for the impacts of knockout/complementation experiments, including changes in reaction-norm slope and average expression level across densities. Black=wild-type per-capita gene expression. Grey=attenuated per-capita gene expression in a signal synthase knockout. Green=genetic knockout supplemented with exogenous signal. (a) conventional knockout/complementation experiments are conducted under a single controlled lab condition (for QS experiments, typically high-density environments). (b,c,d) Reaction-norm experiments conducted under a range of environmental conditions (e.g. differing stationary phase densities). The grey lines represent the hypothesis that knocking out one or both signal synthase gene will ‘flatten off’ the wild-type lasB reaction norm shown in the black bars, due to the dependency of lasB expression on both AHL signal inputs (Fig. 1). (b) The ‘flatten up’ hypothesis predicts that sufficient signal supplementation will remove encoding of density information in the signal environment, producing high mean expression and a slope of zero. (c) The ‘acceleration’ hypotheses predicts that signal supplementation can steepen the reaction norm compared to the wild-type due to synergistic lasB expression control, particularly if one or both native synthases are intact. (d) The ‘robust reactivity’ hypothesis predicts that signal supplementation can raise mean expression but retain reactivity (positive slope). In Results we develop more specific hypotheses leading to predictions of patterns (b,c,d) under defined synthase knock-out and signal supplementation manipulations.
In contrast to the conventional complementation approach focused on expression in a single environment (Fig. 2a), we turn to an examination of how synthase knockouts and signal complementation modify reaction norms [48], i.e. the reactivity of QS controlled lasB to changes in the social environment (Fig. 2b, c, d). In light of the synergistic dual signal control model of lasB (Fig. 1 [24, 32, 34, 49]), we predict that genetic knockouts of lasI or rhlI alone or in combination will attenuate the QS response, specifically flattening the reaction norm towards an ‘always off’ phenotype (Fig. 2b–d, grey lines). Turning to supplementation, the hypothesis in analogy to Fig. 2(a) is that complementing the signal synthase knockouts (or indeed the wild-type) with sufficiently high concentrations of both signals will lead to an always-on phenotype, represented by high lasB expression across all density conditions where the slope ‘flattens-up’ (Fig. 2b, green bar).
In light of the synergistic response of lasB expression to a dual input of the two AHL signals, we offer an alternate hypothesis that signal supplementation can in some circumstances lead to enhanced reactivity of the wild-type to density (steeper reaction norms, Fig. 2c green bar), due to the amplifying effect of the synergistic lasB signal response. Specifically, we predict steeper reaction norms under a combination of a single intact signal synthase (providing information on density) with exogenous supplementation of the knocked-out signal synthase (magnifying the response to density due to synergistic lasB expression control). Fig. 2(d) outlines a final potential pattern, outlining that signal supplementation could increase mean expression, while leaving reactivity (positive slope) intact. This ‘robust reactivity’ hypothesis would indicate that while lasB expression is sensitive to exogenous signal, the ability of QS to quantitatively tune responses to changes in density is robust to exogenous signal.
Consistent with the ‘robust reactivity’ hypothesis (Fig. 2d), our results show that a positive reaction norm to density is robust to multiple combinations of gene deletion and density-independent signal supplementation, with little support for the ‘flatten up’ or ‘acceleration’ hypotheses (Fig. 2b, c). We find that density-independent AHL signal supplementation can increase mean expression, but that WT QS still retains the ability to quantitatively respond to density. Finally, a positive reaction norm to density persists even in the absence of both AHL synthase genes, given density-independent AHL signal supplementation. We discuss these results in light of the complex regulatory control of lasB and in the context of evolutionary theory of communication.
Methods
Bacterial strains and growth conditions. The main bacterial strain used in this study is P. aeruginosa NPAO1 (Nottingham-PAO1) containing the PlasB::gfp(ASV) quorum-sensing reporter pMHLAS [50]. Single and double signal synthase knockouts (ΔlasI, ΔrhlI, ΔlasIΔrhlI) were made using double allelic exchange and the quorum-sensing reporter pMHLAS was subsequently electroporated in. Overnight cultures were grown in lysogeny broth (LB), supplemented with 30 ug ml−1 gentamicin to maintain the pMHLAS plasmid, with shaking at 37 °C. Experiments were conducted in lightly buffered (50 mM MOPS) M9 minimal defined media composed of an autoclaved basal salts solution (Na2HPO4, 6.8 gl−1; KH2PO4, 3.0 gl−1; NaCl, 0.5 gl−1), and filter-sterilized 1 mM MgSO4, 100 uM CaCl2, and 1X Hutner’s Trace Elements with casein digest, as the sole carbon source (ThermoFisher Difco Casein Digest CAT 211610).
Controlling culture carrying Capacity. We manipulated density by controlling the limiting resource in the media, carbon, allowing us to tune the carrying capacity of each treatment and verified that carbon was the limiting resource across our range of densities. To cover a variety of densities, we generated a carbon range between 0.05 and 0.25 % via dilutions of a 0.5 % carbon minimal media stock for a total of six different carrying capacities. Quantities of carbon past 0.25 % start exhibiting characteristics of non-logistic growth, so we use 0.25 % to generate our highest densities. This produced a range of densities environments from 1.18×108 cells ml−1 to 2.02×109 cells ml−1. Overnight cultures were grown in LB gentamicin 50 ug ml−1 and centrifuged for 2 min. The cells were then washed twice with carbonless minimal media and then each carbon treatment was adjusted to OD600=0.05. Then, 200 ul of each sample was added to a 96-well microplate with the buffered M9 minimal media described above. Plates were incubated with shaking at 37 °C in a Cytation/BioSpa plate reader with readings of optical density (OD600) and green fluorescence taken at 30 min intervals for 20 h.
Estimating per capita lasB expression. To estimate per capita lasB expression, we first derive steady-state estimates of population density (K OD) and population fluorescence (K RFU), by fitting logistic curves (growthcurver package in R, available at https://cran.r-project.org/) to the OD and RFU timeseries data over 20 h (see Fig. 3a). We then estimate per-capita lasB expression as the ratio of these values, K RFU/K OD . To produce reaction norms, we plot per capita expression (K RFU/K OD) against carrying capacity (K OD) (Fig. S1).
Fig. 3.
A reaction-norm methodology to quantify QS phenotypic responses. In order to dissect broad trends in quorum-sensing behaviour across population densities, we reduced the dimensionality of our data by first summarizing the growth and lasB gene expression of each population (a) across controlled densities (b) and then summarize the data across environmental density (c). (a) Logistic curves were fit to raw expression (RFU) and growth (OD600) data of wild-type NPAO1 containing the short half-life PlasB::gfp(ASV) quorum-sensing reporter (pMHLAS). Cells were grown in triplicate for 20 h (15 h shown above for brevity). We then extract the K, commonly referred to as the carrying capacity, from the logistic equation and use a ratio of K RFU/K od to describe the per capita behaviour of that population. (b) Seven distinct culture carrying capacities were generated by manipulating the concentration of casein digest as the limiting resource, the lowest was removed from analysis due to lack of convergence in the logistic fits. (c) Per capita expression (K RFU/K od) is then plotted against carrying capacity (K od) and a linear regression (grey dotted line) is performed to generate the reaction norm, i.e. the change in behaviour across an environment. This reaction norm can then be further summarized by calculating the slope and mean across density.
Exogenous signal addition. The AHL signalling molecules used in this study are N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL, Sigma-Aldrich CAS# 168982-69-2), N-butyryl-homoserine lactone (C4-HSL, Sigma-Aldrich CAS# 98426-48-3), and 2-Heptyl-3-hydroxy-4(1 h)-quinolone (pqs, Sigma-Aldrich CAS# 108985-27-9) alone and in combination. These signals are suspended in methanol, so methanol was used as a control to account for different amounts of signal being added experimentally (Fig. S2).
Statistical analysis. Statistical analysis was performed using R. Logistic curves were fit to raw expression (RFU) and growth (OD600) data using the growthcurver package. K, commonly called carrying capacity, was extracted from the logistic model. Linear models were then built from the extracted K values using the emmeans package. Both means and slopes were obtained from those linear models using the emmeans() function and emtrends() function, which uses estimated marginal means to construct a reference grid of predicted means or trends. ANOVA was used for pairwise comparisons between all treatments. Dunnett’s test for multiple comparisons was used to compare the manipulations with their respective controls (defined in their respective results sections). Reported P values are adjusted for multiple comparisons using the Šídák method. Statistical tests for hypotheses with specific directionality (i.e. a statistically significant increase or decrease) are denoted in the text as one tailed.
Results
To provide a baseline for subsequent analyses of genetic knockout and signal supplementation manipulations, we begin with a reaction-norm analysis of NPAO1 wildtype lasB expression across a range of population carrying capacities, using an automated analysis approach that extracts growth and expression data from timeseries experiments (Fig. 3). Our results recapitulate earlier findings that lasB obeys a linear, graded reaction norm on the population scale (Fig. 3c [18]) and outlines the high-throughput methodological approach we take throughout the rest of this study (Fig. 3a–c), which allows us to assess reaction norms across a large number of genetic and chemical manipulations.
Deletion of signal synthase genes attenuates quorum-sensing activity and responsiveness
In our first QS manipulation, we assess the impact of signal synthase genetic knockouts on lasB reaction norms. In light of the dual signal control of lasB (Fig. 1 [23–26, 32, 34, 49], we hypothesized that knocking out any combination of lasI and rhlI signal synthases will attenuate QS reaction norms, with greater attenuation for lasI alone compared to rhlI alone due to the hierarchical arrangement of las and rhl systems [27, 28, 32, 51].
Fig. 4 illustrates support for our ‘flattening off’ hypotheses (grey lines, Fig. 2b–d). Specifically, we test for decreases in mean expression and reaction-norm slope compared to the WT and find that knocking out any gene alone or in combination attenuates both mean QS activity and responsiveness to density [ANOVA, F(3,494) = 1910, P<2.2e-16. Post-hoc comparisons using Dunnett’s test (to control for multiple testing), df = (2, 494), one tailed P<0.001 for all comparisons to WT]. Additionally, compared to the double signal synthase knockout, knocking out lasI alone reduces QS response to a similar extent as knocking out lasI and rhlI together [Dunnett’s test, df = (1, 372), one-tail p mean=0.4726, p slope=0.6965]. In the case of the rhlI knockout compared to the double knockout, we still see a residual quorum-sensing response [Dunnett’s test, df = (1, 372), one-tail p mean<0.0001, p slope <0.0001], consistent with partial and density-dependent lasB expression in response to natively produced 3-oxo-C12 alone [41].
Fig. 4.
Deletion of signal synthase genes attenuates quorum-sensing activity. Reaction norms of per capita lasB expression (y-axis) across density (x-axis) in dual whole gene deletion (ΔlasIΔrhlI, 3-oxo-C12 and C4 signal synthase knockout) and single whole gene deletion (ΔlasI, 3-oxo-C12 signal synthase knockout; ΔrhlI, C4 signal synthase knockout) backgrounds. There is a significant reduction in mean expression and slope for all signal synthase knockouts compared to the WT [ANOVA, F(3,494) = 1910, P<2.2e-16]. Post-hoc comparisons using Dunnett’s test (to control for multiple comparisons), df = (2, 494), one tailed P<0.001). ^ Indicates a significant decrease in mean compared to the WT. * Indicates a significant decrease in slope compared to the WT.
Exogenous signal supplementation of the wild-type increases mean response and maintains reactivity to density
We next turn to manipulations to increase signal exposure via exogenous supplementation of 3-oxo-C12 HSL and/or C4 HSL in the wild-type. The AHL signalling molecules used in this study are suspended in methanol, so methanol was used as a control and the amount of methanol used did not significantly impact growth or average expression levels (Figs S2, S3). In Fig. 5, we find that in most cases adding any fixed (density-independent) concentration of either signal, alone or in combination, significantly increases the mean level of expression across the reaction norm compared to the WT with no supplementation [ANOVA, F(15,391) = 106, P<2.2e-16. Dunnett’s test, df = (15, 391), one tail P<0.0001]. The only two exceptions we see are with 3-oxo-C12 (10 uM and 50 uM of 3-oxo-C12), and in the latter case we see a significant decrease of mean expression [Dunnett’s test, df = (15, 391), two tail P<0.0001]. This observed decrease could be a result of a high concentration of 3-oxo-C12 [52], which aggregates into detrimental micelles at high concentrations [53] while C4 does not.
Fig. 5.
Exogenous signal supplementation of the wild-type increases mean response and maintains reactivity to density. Reaction norms of per capita lasB expression (y-axis) across density (x-axis) in the WT quorum-sensing background. WT behaviour with no signal supplementation in grey. Methanol control (green, dotted line) and signal supplemented environments [3-oxo-C12 alone (blue), C4 alone (orange), 3-oxo-C12 and C4 combined (pink)] across five different concentrations of signal (1 uM, 5 uM, 10 uM, 25 uM, 50 uM). Each reaction norm is built using a linear regression on 18 data points (six carrying capacity environments run in triplicate). ^ Indicates a significant increase in mean compared to the non-supplemented methanol control. * Indicates a significant increase in slope compared to the non-supplemented methanol control. We find a significant increase in means for all manipulations compared to the non-supplemented WT [ANOVA, F(15,391) = 106, P<2.2e-16. Dunnett’s Test, df = (15, 391), one tail P<0.0001], but only one significant increase in slope [Dunnett’s test, df = (15, 391), one tail p5uM-3-oxo-C12/C4 = 0.0023]. For legibility, Fig. 5 only shows the fitted linear models and not the underlying 18 datapoints per linear fit. See Figs S1 and S2, available in the online version of this article, for a series of plots of the experimental and control data plus linear model fits.
The increase in mean expression in the WT upon signal supplementation indicates that native signal production is not sufficient to maximize gene expression, averaging across densities. But does the addition of fixed amounts of signal modulate the responsiveness of the WT to different densities? The hypothetical models in Fig. 2 are motivated by gene knockout (grey) plus signal complementation (green) but can be translated to the case of wild-type supplementation. Fig. 2(b) (green line) represents the hypothesis that exogenous supplementation of (sufficient) signal will maximize QS-controlled gene expression (‘flattening up’), regardless of density, while Fig. 2(c, d) (green line) represent the hypotheses that augmenting the availability of either signal past WT levels will enhance (Fig. 2c) or maintain (Fig. 2d) the responsiveness to changes in density. In this paper, we compare our manipulations to the null expression of the ΔlasIΔrhlI (Fig. 4) in order to test for a flattening, and therefore decreased reactivity to density, of the slope. From Fig. 5 it is evident that the ‘flattening up’ hypothesis fails across all supplementation conditions, including high doses of one or both signals. Specifically, we find that in contrast to the Fig. 2(c) hypothesis, all supplemented reaction-norm slopes are significantly greater that of the ΔlasIΔrhlI [Dunnett’s test, df = (15, 391) P<0.05]. We next test the ‘amplified response’ hypothesis by comparing slopes to the non-supplemented WT control and find that only the combined 5 uM 3-oxo-C12 and C4 produces a significant increase in slope compared to the WT [Dunnett’s test, df = (15, 391), one tail P=0.0023].
Overall, from Fig. 5 we can see that in most cases of signal supplementation to the WT we find that the addition of either signal alone or together (at any concentration) increases mean expression (indicated in figure by ^) while retaining a positive slope (consistent with the ‘robust reactivity’ hypothesis, Fig. 2d). In contrast, we find no support for the ‘flatten up’ hypothesis (Fig. 2b) and minimal support for the ‘amplify’ hypothesis (indicated by *; Fig. 2c).
Cognate and dual AHL signal complementation restores QS reactivity to density
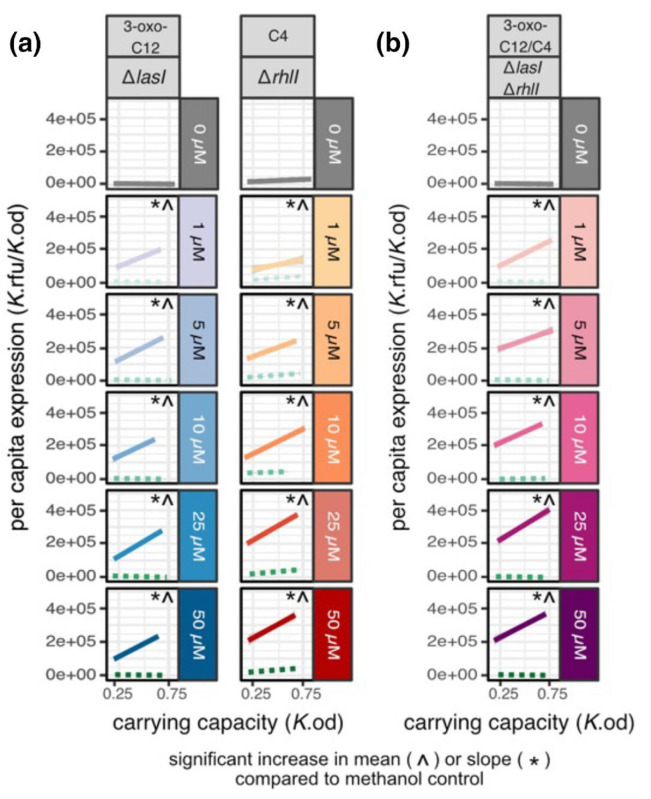
In the next round of supplementation experiments, we now combine signal synthase knockouts with specific signal complementation (Fig. 6) allowing complete experimental control over the level of one or both AHL signals experienced by cells, decoupled from density. In this context, we can now test each step of the synthase knockout and signal complementation hypotheses outlined in Fig. 2. Consistent with the grey lines in Fig. 2(b–d), we can see that the knockouts alone lead to a relative ‘flattening off’ of the reaction norms – lower mean expression and lower slope (Fig. 6 top row in grey; see Fig. 4 and associated text for details on statistics).
Fig. 6.
Cognate and dual AHL signal complementation restores QS reactivity to density. (a) Cognate signal complementation. (b) Dual signal complementation. Behaviour with no signal supplementation in grey. Methanol control (green, dotted line) and signal supplemented environments [3-oxo-C12 alone (blue), C4 alone (orange), 3-oxo-C12 and C4 combined (pink)] across five different concentrations of signal (1 uM, 5 uM, 10 uM, 25 uM, 50 uM). Each reaction norm is built using a linear regression on 18 data points (six carrying capacity environments done in triplicate). ^ Indicates a significant increase in mean compared to the methanol control for that specific strain (Dunnett’s test, adjusted P<0.05) * Indicates a significant increase in slope compared to the methanol control for that specific strain (Dunnett’s test, adjusted P<0.05). For legibility, Fig. 6 only shows the fitted linear models and not the underlying 18 datapoints per linear fit. See Figs S1 and S2 for a series of plots of the experimental and control data plus linear model fits.
Our first prediction is that specifically complementing a single synthase knockout (lasI or rhlI) with cognate signal (3-oxo-C12 or C4) will restore both lasB expression and a positive reaction norm, due to density information being encoded by the remaining intact AHL signal synthase (Fig. 2d). Consistent with this prediction, in Fig. 6(a) we see that cognate signal complementation of lasI and rhlI restores QS response and a positive reaction norm. Specifically, we see that cognate signal complementation restores QS response in lasI (Fig. 6a lasI+C12) by significantly increasing mean expression [Dunnett’s test, df = (5,297), one tail P<0.0001] and slope [Dunnett’s test, df = (5,297), one tail P<0.0001] compared to the lasI methanol-supplemented control (dotted green lines in Fig. 6). We see similar results for cognate signal supplementation in rhlI (Fig. 6a, rhlI +C4), where QS response is also restored through a significant increase in mean [Dunnett’s test, df = (5,274), one tail P<0.0001] and slope [Dunnett’s test, df = (5,274), one tail P<0.0001] for all cases except 1uM C4 where P=0.0168. In these cognate supplement experiments, we again can clearly see that the slope does not ‘flatten-up’ – the slope is significantly higher than the ΔlasIΔrhlI in Fig. 4 [Dunnett’s test, df = (5, 297), one tail, P<0.05]. This is anticipated as each strain still has a functional copy of one signal synthase gene that encodes density-dependent information. In terms of our ‘amplified response’ hypothesis, while cognate signal supplementation can restore a positive slope compared to the non-supplemented knockout, this slope is always lower than the non-supplemented WT [Dunnett’s test, df = (10,353), one tail P<0.001] and therefore not amplified compared to the WT (Fig. S4).
Turning to the double synthase knockout (Fig. 6b), we expect we can restore lasB expression via dual signal complementation, but we do not expect restoration of lasB reactivity, as there is no longer any connection between purely exogenously derived AHL signal supply and bacterial density. While we do see the predicted restoration of mean expression via dual signal supplementation [Dunnett’s test, df = (5, 200), one tail P<0.0001 for all comparisons], in contrast to the ‘flatten up’ prediction (Fig. 2b) we see that this restored expression retains a positive slope on density compared to the ΔlasIΔrhlI [Dunnett’s test, df = (5,200), one tail, P<0.05]. Thus, in contrast to the Fig. 2(b) hypothesis, we see that density-dependent lasB expression (a positive reaction norm) can be restored by adding a density-independent amount of dual signal.
Triple signal supplementation decreases but does not ablate reactivity to density
Given that dual signal supplementation with 3-oxo-C12 and C4 does not support the ‘flatten up’ prediction, we expanded our search to other signals that could be carrying density-encoding information. The non-AHL signal molecule 2-heptyl-3-hydroxy-4(1 h)-quinolone (‘Pseudomonas quinolone signal’, henceforth referred to as PQS) [54–58] is frequently linked to iron limitation and iron acquisition [55], but was first discovered as a QS signal in connection to its impact on the expression of lasB [54]. Knocking out PQS production is more complicated than knocking out AHL production, due to the more complex genetics and pleiotropic effects of the PQS system. Choices for a genetic deletion either lead to ablating production of all 40 quinolones (ΔpqsA) or cell lysis through excess HHQ accumulation (ΔpqsH) [56]. Given these choices have substantial impacts on cell behaviour and fitness outside of cell–cell signalling, and in addition that a ΔpqsA mutant would require supplementing with 40 independent quinolones, we manipulate this non-AHL system solely via supplementation of PQS. The PQS supplementation experiment is sufficient to test whether the addition of exogenous, density-independent quantities of PQS (with or without AHL synthase deletions/AHL supplementation) is sufficient to remove density responsiveness in lasB expression.
First, we supplement the WT with just PQS (Fig. 7a) and find that, similar to the AHLs, density-independent concentrations of PQS significantly increases the mean level of expression across the reaction norm compared to the WT [ANOVA, F(5,78) = 66, P<2.2e-16, Dunnett’s test, df = (5, 78), one tail P<0.0001] and that in contrast to the Fig. 2(b) ‘flatten up’ hypothesis, all supplemented reaction norm slopes are significantly greater than the ΔlasIΔrhlI [Dunnett’s test, df = (5,78), one tail P<0.05]. This lack of a ‘flatten-up’ is likely due to intact production of both AHL signals (3-oxo-C12 and C4).
Fig. 7.
PQS alone or the combination of PQS and dual AHL signal supplementation decrease, but do not always ablate, responsiveness to density. (a) Variable PQS supplementation of the WT. Grey solid line represents no supplementation; green dotted lines, methanol control; teal lines, PQS signal supplemented environments. (b) Fixed supplementation of 3-oxo-C12 and C4 at 25 uM and variable supplementation of PQS of the WT. Grey solid line, dual 25 uM AHL 3-oxo-C12 and C4 supplementation; green dotted lines, 25 uM 3-oxo-C12 and C4 and methanol; brown lines, fixed 25 uM 3-oxo-C12 and C4 supplementation (purple box) and variable PQS supplementation (teal box). Each reaction norm is built using a linear regression on 18 data points (six carrying capacity environments done in triplicate). ^ Indicates a significant increase in mean compared to the methanol control for that specific strain (Dunnett’s test, adjusted P<0.05) # Indicates a significant decrease in slope compared to the methanol control for that specific strain (Dunnett’s test, adjusted P<0.01).
To give the best chance at a ‘flatten-up’ response, we turn to triple signal supplementation in Fig. 7(b) and supplement with a fixed concentration of both AHL signals and variable supplementation of PQS. We chose to supplement with 25 uM of the AHLs instead of 50 uM as 50 uM of 3-oxo-C12 actually lowered expression across the reaction norm in Fig. 5. In agreeance with Fig. 5, we find that adding 25 uM of 3-oxo-C12 and C4 increases expression [Dunnett’s test, df = (2, 36), one tail P<0.05] but not reactivity [Dunnett’s test, df = (2, 36), one tail P<0.05] compared to the non-supplemented WT (Fig. 7(a) 0 uM compared to Fig. 7(b) 0 uM).
When supplementing with all three signals, we find that supplemental PQS significantly increases the mean level of expression across the reaction norm compared to the dual AHL supplemented WT [ANOVA, F(5,72) = 106, P<2.2e-16, Dunnett’s Test, df = (5, 72), one tail P<0.0001]. The increase in mean expression in the dual AHL supplemented WT upon PQS supplementation indicates that dual AHL signal supplementation alone is not sufficient to maximize lasB expression. But does triple supplementation of density-independent amounts of all three signals modulate the responsiveness of the WT to different densities? Across these triple supplementation environments, we find that only the addition of 25 uM of 3-oxo-C12, 25 uM of C4, and 50 uM of PQS is sufficient to ‘flatten’ the reaction norm [no significant difference in slope compared to the QS null ΔlasIΔrhlI; Dunnet’s test, df = (5,72), P=1]. Additionally, we find no case where triple signal supplementation increases reactivity to density [slope does not increase, Dunnett’s test, df = (5, 72), one tail P>0.9]. The results from the triple supplementation imply that only excess quantities of all three signals is sufficient to increase expression past the WT level and ‘flatten up’ the reaction norm.
Discussion
In this study we use a reaction-norm approach (Fig. 2) to assess how signal knockout and supplementation treatments impact the mean QS response and reactivity of QS phenotypes to environmental change. Our approach condenses data from 2000 timeseries (over 74 000 individual observations) into a comprehensive view of QS-controlled gene expression across variation in genetic, environmental and signal determinants of lasB expression. Consistent with canonical understanding of QS regulatory wiring (Fig. 1), we find that knocking out AHL signal synthase genes alone and in combination attenuate the responsiveness of lasB to density (Fig. 4). Overall, we find that both AHL and PQS signal supplementation increases expression past WT levels, indicating that native signal production is not sufficient to maximize gene expression (Figs 4–7). We find that wild-type reactivity to density is robust to AHL and PQS supplementation (Figs 5 and 7a) except for the most extreme case of triple supplementation (Fig. 7b). While AHL supplementation of the WT can increase overall response, it neither enhances reactivity to density nor flattens the response respective to density (Fig. 5). In signal complementation experiments (Fig. 6), we find that a positive lasB reaction norm is dependent on the presence of 3-oxo-C12 and can persist given density-independent AHL supplementation even in the absence of both AHL synthase genes.
A key result across our manipulations is the robustness of QS-mediated density sensing in the face of both genetic and/or chemical manipulations of signal availability (Figs 5–7). In Fig. 5 we outline the robustness of the wild-type in the face of increasing exogenous supplementation with one or both AHL signals. Given we are supplementing the wild-type strain, it remains the case that at higher densities there will likely be larger concentrations of signal, due to greater contributions from the larger density of wild-type cells. While in principle this could explain the robust reactivity (consistent positive slope), we note that the preservation of a positive slope continues given supplementation with concentrations of AHL that greatly exceed observed wild-type levels [52]. To remove the effect of native signal production on signal density we turned to supplementation of signal synthase knock-out strains (Fig. 6), and again see robust density sensing (positive slope) phenotypes under many manipulations.
In Fig. 6(b) we see that a positive lasB reaction norm is robust even in conditions where both AHL signal concentrations are controlled independently of bacterial density. This points to a role for additional density-dependent factors controlling lasB expression, outside of 3-oxo-C12 and C4 HSL signal density. Fig. 7(b) indicates that, in the extreme case, supplementing with PQS can ablate the ability to sense density. We note that our data does not allow us to determine whether the impact of PQS on lasB is due to direct PQS interactions with lasB or due to indirect effects of PQS via other QS regulators. In either case, the results indicate that maximal lasB activation requires PQS, and is consistent with earlier work showing that spent culture supernatant can bring forward QS activation [59] (although the mechanism was not identified in [59]). More recent work points to PqsE, an effector protein of PA QS, as an additional control dial for lasB [57, 58, 60]. While PqsE is not required for RhlR-driven gene expression [58] show that PqsE can modulate the level of expression of RhlR controlled genes, such as lasB. In addition to QS signal molecules, lasB expression is impacted by multiple other environmental factors [44], which may also be modulated by our specific experimental design. For example, by manipulating the availability of limiting carbon (Fig. 3c), we also modify the time and number of generations until arrival at stationary phase.
In a parallel study [61] we examined lasB, lasI and rhlI gene expression in an NPAO1 double-synthase KO, as a function of graded dual AHL signal supplementation at a single density. By fitting this data to a mathematical model of multi-signal dynamics, we were able to conclude that the conventional view of a las – rhl signalling hierarchy (Fig. 1) is incomplete. We found that the rhl system has significant impacts on las signalling behaviour, therefore forming a reciprocal las-rhl signalling network. The current study extends on this work by examining gene expression across a broad range of bacterial densities and timepoints, and therefore provides a valuable dataset for future quantitative modelling analyses.
From an evolutionary perspective, we can view our manipulative experiments in the context of animal communication theory [62, 63]. Communication systems convey reliable information when signals correlate with underlying information of interest [64]. For example, the jumping height of ‘stotting’ gazelles signals to predators reliable information on the athletic ability of potential prey, leading predators to avoid pursuit of the most athletic (high-stotting) individuals [65]. One important challenge to signal reliability is ‘noise’ – environmental forces that degrade or distort the transmitted signal and therefore weaken the correlation with useful information (e.g. factors reducing visibility in the context of stotting signals). Evolutionary theory predicts that if signal reliability is reduced by noise, communication breaks down as receivers are selected to ignore the signal and signalers respond by dropping signal production [64, 66, 67]. Partially consistent with this basic prediction, [68] showed that long-term QS signal supplementation (50 µm of 3-oxo-C12; described as adding uncorrelated noise to QS communication, under the assumption that signal addition will weaken the correlation between bacterial density and signal concentration) over 120 generations in P. aeruginosa selected for an evolved response of reduced signal investment. However, Popat et al. [68] did not examine behaviour across reaction norms so we do not know to what extent the signal manipulation truly functioned to increase ‘noise’, i.e. to what extent it obscured the relationship between density, QS signal and QS response. Our experimental results shed light on this issue and highlight that this supplementation design likely elevated average response, while maintaining responsiveness of the ancestral P. aeruginosa to changes in density, potentially leading to early and excessive production of lasB (required in Popat et al.’s experiment in order to digest the carbon source). Our findings in Fig. 5 indicate that P. aeruginosa QS populations similar to our strain may actually be fairly robust to excess exogenous signal and are still able to sense density when challenged with density-independent signal.
One limitation of our experimental method is that quantifying QS activity from batch culture does not represent true steady-state dynamics. Chemostats present an alternative approach that offer a more controlled steady state environment, although our ability to assess multiple distinct treatments would then be limited by the larger spatial scale and complexity of chemostat approaches. While we cover a 20-fold range of density in this manuscript (the range of cell densities generated from our culture method is roughly 1×108 cells ml−1 to 2×109 cells ml−1), chemostats, or at least larger batch cultures than microtitre plates, would also increase the range of densities that are observable. In an effort to make our results translatable across strains of PAO1, we quantified both AHLs in our highest density environment and find that the levels of signal (0.8 uM 3-oxo-C12 and 2.3uM C4) agree with other high density work done in the field. In addition to signal, it would also be interesting to look at what other factors impact the ability of the WT to sense density even when given a density-independent concentration of signal. This could be done using constructs containing inducible transcription factors like lasR to increase lasR expression, or by using rhlR-specific C4 competitive binders to reduce rhlR-mediated activity. Transcriptomics could provide a good baseline as a discovery step to determine which mechanistic avenues would be promising to pursue via controlled reaction-norm experiments.
Overall, our results show that a positive reaction norm to density is robust to multiple combinations of gene deletion and density-independent signal supplementation. Our work develops a modular approach to query the robustness and mechanistic bases of the central environmental sensing phenotype of quorum sensing.
Supplementary Data
Funding information
This research was supported by the Foundation for the National Institutes of Health grant 1R21AI156817, the Centers for Disease Control and Prevention Foundation grant 75D30120C 09782, the Cystic Fibrosis Foundation grant BROWN21P0, and the Army Research Office grant W911NF1910384.
Acknowledgements
We thank Steve Diggle, Marvin Whitely, and members of the Center for Microbial Dynamics and Infection (CMDI) for valuable comments and discussion on this work.
Conflicts of interest
The authors declare no conflict of interests.
Footnotes
Abbreviations: AHL, acyl homoserine lactone; C4, N-butyryl-homoserine lactone; NPAO1, Nottingham PAO1; OD, optical density; 3-oxo-C12, N-(3-oxododecanoyl)-l-homoserine lactone; PQS, pseudomonas quinolone signal; QS, quorum sensing; RFU, relative fluorescence units; WT, wild type.
Four supplementary figures are available with the online version of this article.
References
- 1.Boedicker JQ, Vincent ME, Ismagilov RF. Microfluidic confinement of single cells of bacteria in small volumes initiates high-density behavior of quorum sensing and growth and reveals its variability. Angew Chem Int Ed Engl. 2009;48:5908–5911. doi: 10.1002/anie.200901550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redfield RJ. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 2002;10:365–370. doi: 10.1016/s0966-842x(02)02400-9. [DOI] [PubMed] [Google Scholar]
- 3.Connell JL, Wessel AK, Parsek MR, Ellington AD, Whiteley M, et al. Probing prokaryotic social behaviors with bacterial “lobster traps.”. mBio. 2010;1:e00202-10. doi: 10.1128/mBio.00202-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen RC, McNally L, Popat R, Brown SP. Quorum sensing protects bacterial co-operation from exploitation by cheats. ISME J. 2016;10:1706–1716. doi: 10.1038/ismej.2015.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez PD, Hagen SJ. Heterogeneous response to a quorum-sensing signal in the luminescence of individual Vibrio fischeri . PLoS One. 2010;5:e15473. doi: 10.1371/journal.pone.0015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plener L, Lorenz N, Reiger M, Ramalho T, Gerland U, et al. The phosphorylation flow of the Vibrio harveyi quorum-sensing cascade determines levels of phenotypic heterogeneity in the population. J Bacteriol. 2015;197:1747–1756. doi: 10.1128/JB.02544-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grote J, Krysciak D, Streit WR. Phenotypic heterogeneity, a phenomenon that may explain why quorum sensing does not always result in truly homogenous cell behavior. Appl Environ Microbiol. 2015;81:5280–5289. doi: 10.1128/AEM.00900-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haseltine EL, Arnold FH. Implications of rewiring bacterial quorum sensing. Appl Environ Microbiol. 2008;74:437–445. doi: 10.1128/AEM.01688-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long T, Tu KC, Wang Y, Mehta P, Ong NP, et al. Quantifying the integration of quorum-sensing signals with single-cell resolution. PLoS Biol. 2009;7:e68. doi: 10.1371/journal.pbio.1000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayakumar P, Thomas SA, Brown SP, Kümmerli R. Pseudomonas aeruginosa reaches collective decisions via transient segregation of quorum sensing activities across cells. bioRxiv . 2021. Mar 22, [ November 1; 2021 ]. https://www.biorxiv.org/content/10.1101/2021.03.22.436499v1 accessed. [DOI] [PubMed]
- 12.Bettenworth V, Steinfeld B, Duin H, Petersen K, Streit WR, et al. Phenotypic heterogeneity in bacterial quorum sensing systems. J Mol Biol. 2019;431:4530–4546. doi: 10.1016/j.jmb.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Pradhan BB, Chatterjee S. Reversible non-genetic phenotypic heterogeneity in bacterial quorum sensing. Mol Microbiol. 2014;92:557–569. doi: 10.1111/mmi.12575. [DOI] [PubMed] [Google Scholar]
- 14.Anetzberger C, Pirch T, Jung K. Heterogeneity in quorum sensing-regulated bioluminescence of Vibrio harveyi . Mol Microbiol. 2009;73:267–277. doi: 10.1111/j.1365-2958.2009.06768.x. [DOI] [PubMed] [Google Scholar]
- 15.Garmyn D, Gal L, Briandet R, Guilbaud M, Lemaître J-P, et al. Evidence of autoinduction heterogeneity via expression of the Agr system of Listeria monocytogenes at the single-cell level. Appl Environ Microbiol. 2011;77:6286–6289. doi: 10.1128/AEM.02891-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darch SE, Simoska O, Fitzpatrick M, Barraza JP, Stevenson KJ, et al. Spatial determinants of quorum signaling in a Pseudomonas aeruginosa infection model. Proc Natl Acad Sci. 2018;115:4779–4784. doi: 10.1073/pnas.1719317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korobkova E, Emonet T, Vilar JMG, Shimizu TS, Cluzel P. From molecular noise to behavioural variability in a single bacterium. Nature. 2004;428:574–578. doi: 10.1038/nature02404. [DOI] [PubMed] [Google Scholar]
- 18.Rattray JB, Thomas SA, Wang Y, Molotkova E, Gurney J, et al. Bacterial quorum sensing allows graded and bimodal cellular responses to variations in population density. mBio. 2022;13:e0074522. doi: 10.1128/mbio.00745-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woltereck R. Weitere experimentelle untersuchungen uber artveranderung, speziel uber das wesen quantitativer artuntershiede bei daphniden. Science. 1979;32:344–345. [Google Scholar]
- 20.Schlichting C, Pigliucci M. Phenotypic Evolution: A Reaction Norm Perspective. 1998. [Google Scholar]
- 21.Guss AM, Roeselers G, Newton ILG, Young CR, Klepac-Ceraj V, et al. Phylogenetic and metabolic diversity of bacteria associated with cystic fibrosis. ISME J. 2011;5:20–29. doi: 10.1038/ismej.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams P, Cámara M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol. 2009;12:182–191. doi: 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 24.Pesci EC, Pearson JP, Seed PC, Iglewski BH. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa . J Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Kievit TR, Kakai Y, Register JK, Pesci EC, Iglewski BH. Role of the Pseudomonas aeruginosa las and rhl quorum-sensing systems in rhlI regulation. FEMS Microbiol Lett. 2002;212:101–106. doi: 10.1016/s0378-1097(02)00735-8. [DOI] [PubMed] [Google Scholar]
- 26.Medina G, Juárez K, Díaz R, Soberón-Chávez G. Transcriptional regulation of Pseudomonas aeruginosa rhlR, encoding a quorum-sensing regulatory protein. Microbiology. 2003;149:3073–3081. doi: 10.1099/mic.0.26282-0. [DOI] [PubMed] [Google Scholar]
- 27.Soto-Aceves MP, Cocotl-Yañez M, Servín-González L, Soberón-Chávez G. The Rhl quorum-sensing system is at the top of the regulatory hierarchy under phosphate-limiting conditions in Pseudomonas aeruginosa PAO1. J Bacteriol. 2021;203:e00475-20. doi: 10.1128/JB.00475-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feltner JB, Wolter DJ, Pope CE, Groleau M-C, Smalley NE, et al. LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa . mBio. 2016;7:e01513-16. doi: 10.1128/mBio.01513-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurney J, Azimi S, Brown SP, Diggle SP. Combinatorial quorum sensing in Pseudomonas aeruginosa allows for novel cheating strategies. Microbiology. 2020;166:777–784. doi: 10.1099/mic.0.000941. [DOI] [PubMed] [Google Scholar]
- 30.Heeb S, Fletcher MP, Chhabra SR, Diggle SP, Williams P, et al. Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev. 2011;35:247–274. doi: 10.1111/j.1574-6976.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster M, Sexton DJ, Diggle SP, Greenberg EP. Acyl-homoserine lactone quorum sensing: from evolution to application. Annu Rev Microbiol. 2013;67:43–63. doi: 10.1146/annurev-micro-092412-155635. [DOI] [PubMed] [Google Scholar]
- 32.Seed PC, Passador L, Iglewski BH. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J Bacteriol. 1995;177:654–659. doi: 10.1128/jb.177.3.654-659.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chugani SA, Whiteley M, Lee KM, D’Argenio D, Manoil C, et al. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa . Proc Natl Acad Sci. 2001;98:2752–2757. doi: 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornforth DM, Popat R, McNally L, Gurney J, Scott-Phillips TC, et al. Combinatorial quorum sensing allows bacteria to resolve their social and physical environment. Proc Natl Acad Sci. 2014;111:4280–4284. doi: 10.1073/pnas.1319175111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casilag F, Lorenz A, Krueger J, Klawonn F, Weiss S, et al. The LasB Elastase of Pseudomonas aeruginosa acts in concert with alkaline protease AprA to prevent flagellin-mediated immune recognition. Infect Immun. 2016;84:162–171. doi: 10.1128/IAI.00939-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cigana C, Castandet J, Sprynski N, Melessike M, Beyria L, et al. Pseudomonas aeruginosa elastase contributes to the establishment of chronic lung colonization and modulates the immune response in a murine model. Front Microbiol. 2020;11:620819. doi: 10.3389/fmicb.2020.620819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diggle SP, Griffin AS, Campbell GS, West SA. Cooperation and conflict in quorum-sensing bacterial populations. Nature. 2007;450:411–414. doi: 10.1038/nature06279. [DOI] [PubMed] [Google Scholar]
- 38.Sexton DJ, Schuster M. Nutrient limitation determines the fitness of cheaters in bacterial siderophore cooperation. Nat Commun. 2017;8:230. doi: 10.1038/s41467-017-00222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruz RL, Asfahl KL, Van den Bossche S, Coenye T, Crabbé A, et al. RhlR-regulated acyl-homoserine lactone quorum sensing in a cystic fibrosis isolate of Pseudomonas aeruginosa . mBio. 2020;11:e00532-20. doi: 10.1128/mBio.00532-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feltner JB, Wolter DJ, Pope CE, Groleau M-C, Smalley NE, et al. LasR variant cystic fibrosis isolates reveal an adaptable quorum-sensing hierarchy in Pseudomonas aeruginosa . mBio. 2016;7:e01513-16. doi: 10.1128/mBio.01513-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gambello MJ, Iglewski BH. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson JP, Passador L, Iglewski BH, Greenberg EP. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa . Proc Natl Acad Sci. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brint JM, Ohman DE. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mellbye B, Schuster M. Physiological framework for the regulation of quorum sensing-dependent public goods in Pseudomonas aeruginosa . J Bacteriol. 2014;196:1155–1164. doi: 10.1128/JB.01223-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson RM, Zimprich CA, Rust L. A second operator is involved in Pseudomonas aeruginosa elastase (lasB) activation. J Bacteriol. 1999;181:6264–6270. doi: 10.1128/JB.181.20.6264-6270.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rust L, Pesci EC, Iglewski BH. Analysis of the Pseudomonas aeruginosa elastase (lasB) regulatory region. J Bacteriol. 1996;178:1134–1140. doi: 10.1128/jb.178.4.1134-1140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith KM, Bu Y, Suga H. Induction and inhibition of Pseudomonas aeruginosa quorum sensing by synthetic autoinducer analogs. Chem Biol. 2003;10:81–89. doi: 10.1016/s1074-5521(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 48.Rattray JB, Brown SP. Beyond thresholds: quorum‐sensing as quantitatively varying reaction norms to multiple environmental dimensions. Isr J Chem. 2023 doi: 10.1002/ijch.202200109. [DOI] [Google Scholar]
- 49.Whiteley M, Greenberg EP. Promoter specificity elements in Pseudomonas aeruginosa quorum-sensing-controlled genes. J Bacteriol. 2001;183:5529–5534. doi: 10.1128/JB.183.19.5529-5534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, et al. Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- 51.Dekimpe V, Déziel E. Revisiting the quorum-sensing hierarchy in Pseudomonas aeruginosa: the transcriptional regulator RhlR regulates LasR-specific factors. Microbiology. 2009;155:712–723. doi: 10.1099/mic.0.022764-0. [DOI] [PubMed] [Google Scholar]
- 52.Pearson JP, Van Delden C, Iglewski BH. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol. 1999;181:1203–1210. doi: 10.1128/JB.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gahan CG, Patel SJ, Boursier ME, Nyffeler KE, Jennings J, et al. Bacterial quorum sensing signals self-assemble in aqueous media to form micelles and vesicles: an integrated experimental and molecular dynamics study. J Phys Chem B. 2020;124:3616–3628. doi: 10.1021/acs.jpcb.0c00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pesci EC, Milbank JB, Pearson JP, McKnight S, Kende AS, et al. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa . Proc Natl Acad Sci. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diggle SP, Matthijs S, Wright VJ, Fletcher MP, Chhabra SR, et al. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol. 2007;14:87–96. doi: 10.1016/j.chembiol.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Dubern JF, Diggle SP. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol Biosyst. 2008;4:882–888. doi: 10.1039/b803796p. [DOI] [PubMed] [Google Scholar]
- 57.Rampioni G, Falcone M, Heeb S, Frangipani E, Fletcher MP, et al. Unravelling the genome-wide contributions of specific 2-Alkyl-4-quinolones and PqsE to quorum sensing in Pseudomonas aeruginosa . PLoS Pathog. 2016;12:e1006029. doi: 10.1371/journal.ppat.1006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Letizia M, Mellini M, Fortuna A, Visca P, Imperi F, et al. PqsE expands and differentially modulates the RhlR quorum sensing regulon in Pseudomonas aeruginosa . Microbiol Spectr. 2022;10:e0096122. doi: 10.1128/spectrum.00961-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yarwood JM, Volper EM, Greenberg EP. Delays in Pseudomonas aeruginosa quorum-controlled gene expression are conditional. Proc Natl Acad Sci. 2005;102:9008–9013. doi: 10.1073/pnas.0503728102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Groleau MC, de Oliveira Pereira T, Dekimpe V, Déziel E. PqsE is essential for RhlR-dependent quorum sensing regulation in Pseudomonas aeruginosa . mSystems. 2020;5:e00194-20. doi: 10.1128/mSystems.00194-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomas S, El-Zayat AS, Gurney J, Rattray J, Brown SP. The las and rhl quorum sensing systems in Pseudomonas aeruginosa form a multi-signal reciprocal network which can tune reactivity to variations in physical and social environments. Microbiology. 2023 doi: 10.1101/2023.02.23.529764. [DOI] [Google Scholar]
- 62.Smith JM. Animal Signals. Oxford University Press, (Oxford series in 705 ecology and evolution); 2003. [Google Scholar]
- 63.Stegmann UE. John Maynard Smith’s notion of animal signals. Biol Philos. 2006;20:1011–1025. doi: 10.1007/s10539-005-9020-8. [DOI] [Google Scholar]
- 64.Smith MJ, Harper DGC. Animal signals: models and terminology. J Theor Biol. 1995;177:305–311. doi: 10.1006/jtbi.1995.0248. [DOI] [Google Scholar]
- 65.FitzGibbon CD, Fanshawe JH. Stotting in Thomson’s gazelles: an honest signal of condition. Behav Ecol Sociobiol. 1988;23:69–74. doi: 10.1007/BF00299889. [DOI] [Google Scholar]
- 66.Searcy WA, Nowicki S. The Evolution of Animal Communication: Reliability and Deception in Signaling Systems. (Monographs in Behavior and Ecology) US: Princeton, NJ: Princeton University Press; 2005. p. xii, 270–xii, 270. p. [Google Scholar]
- 67.Bradbury JW, Vehrencamp SL. Principles of Animal Communication. 2nd ed. US: Sunderland, MA: Sinauer Associates; 2011. xiv, 697–xiv, 697 p. [Google Scholar]
- 68.Popat R, Pollitt EJG, Harrison F, Naghra H, Hong K-W, et al. Conflict of interest and signal interference lead to the breakdown of honest signaling. Evolution. 2015;69:2371–2383. doi: 10.1111/evo.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waddington CH. Canalization of development and the inheritance of acquired characters. Nature. 1942;150:563–565. doi: 10.1038/150563a0. [DOI] [PubMed] [Google Scholar]
- 70.Paaby AB, Testa ND. Evolutionary Developmental Biology. Springer International Publishing; 2018. Developmental plasticity and evolution; pp. 1–14. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.