Abstract
Background
COVID-19 (Coronavirus Disease 2019) vaccinations are a critical control measure for the coronavirus pandemic that began in 2019. Several COVID-19 vaccines have been developed, and their effectiveness will almost certainly vary.
Objective
This study aimed to assess how effective two doses of the Pfizer and Oxford-AstraZeneca vaccines were in preventing SARS‐CoV‐2 infection six months after administration.
Methods
This is a retrospective cohort study of adult individuals from the Jazan Region of Saudi Arabia who received their second dose of the COVID-19 vaccine [Pfizer and Oxford-AstraZeneca (ASZ)] between April and June 2021. The monitoring and follow-up period continued until the end of January 2022. Data were retrieved from the Health Electronic Surveillance Network and National Vaccination Records. Logistic regression was performed to assess the risk of COVID-19 infection among the vaccinated subjects.
Results
This study included randomly enrolled 4458 participants in Jazan who received two doses of the COVID-19 vaccine during the research period. The majority of them received the Pfizer vaccine (3136/4458; 70.3%), while the remaining received the ASZ vaccine (1322/4458; 29.7%). The study participants’ mean age was 59.7 years, with a male-to-female ratio of 1.9:1.0 (2920:1538). The results showed that the Pfizer and ASZ vaccines’ protection against infection decreased from 93.2% and 90.2%, respectively, during the first three months, to 68.5% and 68.1% after a six-month interval. In the current study population, being Saudi Arabian, younger as well as having longer intervals between vaccines or crossing a 6-month period after the second vaccine dose were factors linked to higher rates of breakthrough infections.
Conclusion
Our findings revealed variations in the efficacy of different COVID-19 vaccine types against COVID-19 breakthrough infections. The Pfizer (mRNA-based) vaccine was found to be relatively more effective than the ASZ (DNA-based) vaccine.
Keywords: COVID-19, Vaccine, Effectiveness, Jazan, Saudi Arabia
Introduction
COVID-19 vaccinations are a core control measure for the coronavirus pandemic that began in 2019 [1]. COVID-19 vaccines protect the community by enabling the vaccinated individuals to develop an effective immune response to control the infection. The use of COVID-19 vaccines has thus aided in the return to normalcy and the limited practice of social distancing [2]. After receiving the vaccine, the body recognizes the virus, produces antibodies, and retains a biological memory of the virus [3]. In Saudi Arabia, authorized COVID-19 vaccines [i.e., Pfizer–BioNTech (Pfizer) and Oxford–AstraZeneca (ASZ)] are considered safe owing to their strong immune response, effective testing stages, and consistent antibodies [4]. Pfizer is an mRNA vaccine that uses a lipid nanoparticle carrier to protect the mRNA, which encodes for the spike protein found on the surface of the SARS-CoV-2 virus and aids its entry into cells 56. Several reports have described the safety and efficacy of the Pfizer COVID-19 vaccine. One of the earliest ones underscored the effectiveness of two Pfizer vaccine doses based on the findings of a clinical trial that recruited 43,548 people aged 16 years and older between July and November 2020 from 152 sites in the United States, Argentina, Brazil, South Africa, Germany, and Turkey. The Pfizer vaccine was reported to be 95% effective (95% confidence interval (CI) between 90.3% and 97.6%) [5]. In a follow-up clinical trial in 2021, the Pfizer vaccine’s efficacy was reduced to 91.3% ( 95% CI between 89% and 93.2%) among 44,165 participants from the United States, Argentina, Brazil, South Africa, Germany, and Turkey [6]. Nonetheless, according to a recent review, the Pfizer vaccine’s efficacy in reducing symptomatic infection varied based on the COVID-19 variants, with the vaccine showing higher effectiveness against the Alpha (76–95.3%), Beta (86–100%), and Gamma (90%) variants but lower effectiveness against the Delta (42–93%) and Omicron variants (55.2–70%) [7]. ASZ is a traditional DNA (deoxyribonucleic acid) vaccine [8]. It deploys a replication-deficient chimpanzee viral vector based on a mitigated version of an adenovirus. The vaccine contains the genetic material of the spike protein found on a coronavirus’s outer surface [9]. After injecting the ASZ vaccine into host cells, DNA, including the spike protein, is released, stimulating the immune system to attack the coronavirus [9]. One of the first reports on the efficacy of the ASZ vaccine involved a sample of 23,848 participants from the United Kingdom and Brazil who received two doses of the vaccine between April and November 2020, revealing an overall efficacy of 70.4% ( 95% CI between 54.8% and 80.6%) [10]. According to a review that outlined the ASZ vaccine’s measured effectiveness, its ability to prevent symptomatic infection differed depending on the variants, reaching between 70.4% and 87% against the Alpha variant, between 60% and 82.8% against the Delta variant, and 48.9% against the Omicron variants; however, low effectiveness was indicated at 10.4% against the Beta variant [10]. The Saudi Food and Drug Authority approved the Pfizer and ASZ vaccines for emergency use in early 2021 [11]. The first dose of the COVID-19 vaccine initiates the process of establishing immunity against the coronavirus infection, and the second dose reinforces the protection [12]. Older people may be more likely to develop harmful COVID-19 symptoms [13]. People with significant medical illnesses, such as heart or lung disorders, compromised immune systems, diabetes, or obesity, may also be at risk, with the risk being greater for those with more than one risk factor present.COVID-19 vaccine effectiveness is known to vary according to the type of vaccine used and the sample population. There have been limited studies on the use of COVID-19 vaccines in the Jazan region of Saudi Arabia’s southwest. Only one study assessed vaccine utilization, which measured the incidence of COVID-19 vaccine side effects among healthcare workers in the region and indicated that nearly 75% of the sample of 397 healthcare workers suffered side effects due to the vaccination. The majority of those who experienced side effects reported minor flu-like symptoms or pain at the injection site [14]. Other studies on COVID-19 vaccination in the region focused on vaccine acceptance [15] and vaccine effectiveness perception [16]. However, none of the studies identified assessed the effectiveness of COVID-19 vaccines in the Jazan region. The effectiveness of COVID-19 vaccines authorized for use in Jazan, Saudi Arabia, is currently unknown. Information about vaccination and COVID-19 infections, nevertheless, are recorded in official Saudi Ministry of Health databases: the National Vaccination Records and the Electronic Health Surveillance Network. According to the National Vaccination Records, more than 77.4% of the target population received two doses of the vaccines. In particular, the elderly, those with chronic diseases, and healthcare providers had more than 90% coverage. Despite widespread vaccination, the Jazan region was among the hardest hit by SARS-CoV-2. During the pandemic, the prevalence of COVID-19 cases among vaccinated individuals in Saudi Arabia was assumed to be less than 10% in this region. Moreover, based on the Electronic Health Surveillance Network, after the introduction of the COVID-19 vaccine in February 2021, the Jazan region was hit by two waves of COVID-19 variants: the Delta variant wave from April to September 2021, with approximately 110,024 confirmed positive cases and 1009 deaths; and the Omicron variant wave from November 2021 to February 2022, with a total of 94,269 confirmed positive cases and 105 deaths. This study aimed to determine the effectiveness of two doses of both the Pfizer and the ASZ vaccines in preventing SARS‐CoV‐2 infection six months after administration among vaccinated individuals in Saudi Arabia’s Jazan region.
Materials and methods
Adults from Saudi Arabia’s Jazan Region participated in this retrospective cohort study. The Jazan region is comprised of 13 administrative governorates, wherein health services are operated by eight main health sectors, which are distributed geographically throughout the region. The sectors’ names are detailed in Table 1, and they are operating under the administration of the Directorate of Health in the Jazan region. The region’s 1.1 million population received their second dose of COVID-19 vaccines (Pfizer and ASZ) between April and June 2021. This study involved monitoring and following up on the study participants staring from the month of delivering second dose of COVID-19 vaccines in Jazan region in April 2021 until the end of March 2022. Follow up periods of each participant was variable depending on the date of vaccine receipt and varied between nine and twelve months. The follow up was performed via a national electronic surveillance platform, The Health Electronic Surveillance Network (HESN), and Tawakkalna app. HESN which was established by the Saudi Arabia Ministry of Health (MoH) to monitor the status of communicable diseases testing and notification throughout the country. Additionally, as per the Saudi Public Health Authority COVID-19 guidelines, all health care authorities in Saudi Arabia are mandated to report all suspected COVID-19 cases and their closed contacts to HESN. Furthermore, as part of the COVID-19 pandemic measures Tawakkalna app is the official Covid-19 application in the Kingdom of Saudi Arabia to prevent the spread of the Covid-19 virus, which can verify the status of the disease and possible contact near an infected person [17]. With a minimum sample size of 3457 randomly chosen subjects enrolled in this study, the COVID-19 vaccine’s efficacy was described to have a 95% confidence level and 1% standard error. The SPSS (Statistical Package for the Social Sciences) software for Windows was used to perform the statistical analysis. Data for the statistical analysis, including sociodemographic data as well as vaccine information and clinical data, were captured into case record forms using the Health Electronic Surveillance Network and National Vaccination Records. Administrative access to the data was secured since several investigators of this study were affiliated with the Public Health Administration in Jazan and were authorized to access and analyze the relevant databases. The data were stratified according to the COVID-19 infection status to allow for a comparison of demographic characteristics between those who are and are not infected. The sample was also stratified based on the type of vaccine received, allowing for a comparison of the infection’s clinical characteristics. Moreover, the frequencies and proportions of binary and categorical variables were summarized and compared using Fisher's exact test or Pearson's Χ² test. The continuous variables were expressed as mean ( ± standard deviation (SD) or median (interquartile range [IQR]), while comparisons were made via the Student’s t-test or the Mann–Whitney U test, depending on the data distribution. Finally, multivariate logistic regression was performed to estimate the infection’s odds ratio (OR) among fully immunized individuals according to the measured characteristics. A p-value of less than 0.05 was presumed to be statistically significant for the applied statistical test. This study was approved by the Ministry of Health Ethics Committee, Jazan Region, Saudi Arabia.
Table 1.
Univariate analysis comparing confirmed COVID-19 cases to non-infected individuals.
| Characteristics | All N = 1652 |
Infected N = 523 |
Non-infected N = 1129 |
P-value |
|---|---|---|---|---|
| Age (y): Mean (SD) | 59.7 ( ± 13.4) | 56.1 ( ± 15.3) | 57.6 ( ± 14.9) | 0.057 |
| Age category: N (%) | ||||
| 18–45 | 316 (19.1) | 115 (22.0) | 201 (17.8) | 0.098 |
| 46–59 | 545 (33.0) | 160 (30.6) | 385 (34.1) | |
| > 60 | 791 (47.9) | 248 (47.4) | 543 (48.1) | |
| Gender: N (%) | ||||
| Female | 647 (39.2) | 214 (40.9) | 433 (38.4) | 0.174 |
| Male | 1005 (60.8) | 309 (59.1) | 696 (61.6) | |
| Nationality: N (%) | ||||
| Saudi | 1352 (81.8) | 489 (93.5) | 863 (76.4) | < 0.001 |
| Non-Saudi | 300 (18.2) | 34 (6.5) | 266 (23.6) | |
| Residence according to health sectors: N (%) | ||||
| Central Health Sector | 366 (32.4) | 157 (30.0) | 523 (31.7) | 0.323 |
| Southern Health Sector | 264 (23.4) | 138 (26.4) | 402 (24.3) | |
| Western Health Sector | 137 (12.1) | 45 (8.6) | 182 (11.0) | |
| Middle Health Sector | 158 (14.0) | 80 (15.3) | 238 (14.4) | |
| Northern Health Sector | 105 (9.3) | 55 (10.5) | 160 (9.7) | |
| Al Jabali Health Sector | 54 (4.8) | 26 (5.0) | 80 (4.8) | |
| Banimalik Health Sector | 27 (2.4) | 16 (3.1) | 43 (2.6) | |
| Farasan Health Sector | 18 (1.6) | 6 (1.1) | 24 (1.5) | |
| Type of vaccine received: N (%) | ||||
| Pfizer-BioNTech | 1110 (67.2) | 350 (66.9) | 760 (67.3) | 0.458 |
| Oxford-AstraZeneca | 542 (32.8 | 173 (33.1) | 369 (32.7) | |
| Interval between first and second vaccine doses: | ||||
| Interval (W) Med. (IQR) | 9 (6.0–11.0) | 9 (6.0–12.0) | 9 (6.0–11.0) | 0.156 |
| Vaccine interval category: N (%) | ||||
| = < 3 weeks | 132 (8.0) | 37 (7.1) | 95 (8.4) | 0.159 |
| 4–6 weeks | 331 (20.0) | 99 (18.9) | 232 (20.5) | |
| 7–9 weeks | 472 (28.6) | 139 (26.6) | 333 (29.5) | |
| > 9 weeks | 717 (43.4) | 248 (47.4) | 469 (41.5) | |
| Infection after the second dose of vaccine: Interval (W) Med. (IQR) | ||||
| Infection after the second dose of vaccine: N (%) | 12 (4.0–31.0) | 31 (15.0–34.0) | 8 (4.0–21.0) | < 0.001 |
| < = 3 months | 919 (55.6) | 130 (24.9) | 789 (69.9) | < 0.001 |
| 4–6 months | 136 (8.2) | 23 (4.4) | 113 (10.0) | |
| > 6 months | 597 (36.1) | 370 (70.7) | 227 (20.1) | |
Results
General characteristics of the study participants
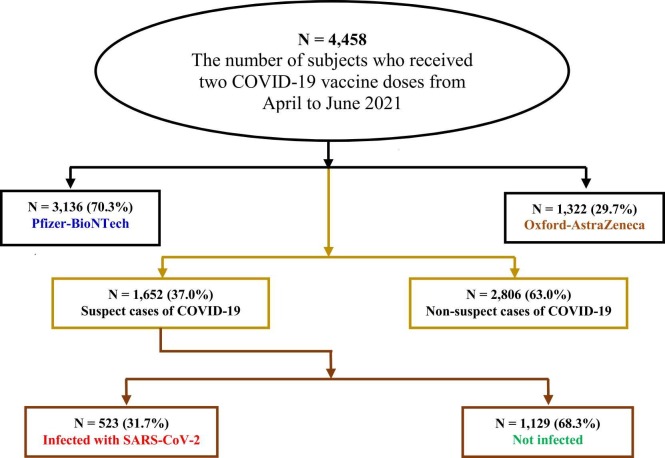
In this study, 4458 randomly chosen participants in Jazan received two doses of the COVID-19 vaccine during the above-mentioned period. Fig. 1 displays a flowchart of the studied sample according to the received vaccine. The majority (3136/4458; 70.3%) received Pfizer, while the rest (1322/4458; 29.7%) received ASZ. The mean age of the study participants was 59.7 years, with a male-to-female ratio of 1.9:1.0 (2920:1538).
Fig. 1.
Study design and results flowchart.
Out of 4458 participants, 37.0% (1652) had contact with positive cases and were tested for COVID-19 between the second dose and the end of February 2022; 31.7% (523) had positive PCR (polymerase chain reaction) tests for SARS-CoV-2, representing 11.7% of the total study population, as shown in Fig. 2.
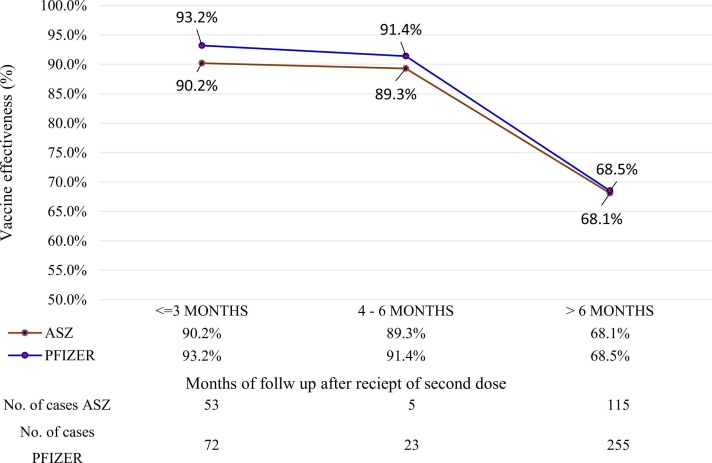
Fig. 2.
Estimated effectiveness of Pfizer-BioNTech and Oxford-AstraZeneca vaccines.
Vaccine effectiveness against COVID-19 infection
For the Pfizer and ASZ vaccines, the results showed that their protection against infection declined from 93.2% and 90.2%, respectively, during the first three months to 91.4% and 89.3% during a six-month interval. The ASZ group was more affected than the Pfizer group during the first three months (30.6% and 20.6%, respectively; P = 0.014), whereas the Pfizer group was more affected than the ASZ group during the remaining study period ( Table 1, Table 2, Table 3; Fig. 2).
Table 2.
Univariate analysis comparing subjects vaccinated with the Pfizer-BioNTech vaccine to those vaccinated with the Oxford-AstraZeneca vaccine.
| Characteristics | All N = 1652 (%) |
Pfizer N = 1110 (%) |
ASZ N = 542 (%) |
P-value |
|---|---|---|---|---|
| PCR results for COVID-19 | ||||
| Positive | 523 (31.7) | 350 (31.6) | 173 (31.9) | 0.468 |
| Negative | 1129 (68.3) | 760 (68.4) | 369 (68.1) | |
| Elapsed time between the second dose of vaccine and PCR testing | ||||
| < = 3 months | 919 (55.6) | 597 (53.8) | 322 (59.4) | 0.082 |
| 4–6 months | 136 (8.2) | 98 (8.8) | 38 (7.0) | |
| > 6 months | 597 (36.1) | 415 (37.4) | 182 (33.6) | |
| Positive COVID-19 test | N = 523 | N = 350 | N = 173 | |
| Elapsed time between the second dose of vaccine and infection with SARS-CoV-2 | ||||
| < = 3 months | 125 (23.9) | 72 (20.6) | 53 (30.6) | 0.014 |
| 4–6 months | 28 (5.4) | 23 (6.6) | 5 (2.9) | |
| > 6 months | 370 (70.7) | 255 (72.9) | 115 (66.0) | |
| Presence of symptoms in PCR-positive cases | ||||
| Asymptomatic | 227 (43.4) | 148 (42.3) | 79 (45.7) | 0.261 |
| Symptomatic | 296 (56.6) | 202 (54.3) | 94 (54.3) | |
| Source of infection in PCR-positive cases | ||||
| Home contact | 482 (92.2) | 321 (91.7) | 161 (93.1) | 0.039 |
| Health care worker | 6 (1.1) | 1 (0.3) | 5 (2.9) | |
| Community contact | 14 (2.7) | 12 (3.4) | 2 (1.2) | |
| Traveling history | 18 (3.4) | 14 (4.0) | 4 (2.3) | |
| Work | 3 (0.6) | 2 (0.6) | 1 (0.6) | |
| ICU admission in PCR-positive cases | ||||
| No | 513 (98.1) | 343 (98.0) | 170 (98.3) | 0.566 |
| Yes | 10 (1.9) | 7 (2.0) | 3 (1.7) | |
| Outcomes in PCR-positive cases | ||||
| Recovered | 521 (99.6) | 349 (99.7) | 172 (99.4) | 0.553 |
| Death | 2 (0.4) | 1 (0.3) | 1 (0.6) | |
| Reinfection in PCR-positive cases | ||||
| No | 356 (99.4) | 241 (99.6) | 115 (99.1) | 0.544 |
| Yes | 2 (0.6) | 1 (0.4) | 1 (0.9) | |
| Comorbidities in PCR-positive cases | ||||
| No | 328 (62.7) | 228 (65.1) | 100 (57.8) | 0.063 |
| Yes | 195 (37.3) | 122 (34.9) | 73 (42.2) | |
Table 3.
Risk factors associated with COVID-19 infection in fully immunized individuals.
| Characteristics | OR (95% CI) | P-value |
|---|---|---|
| Age category, 18–45 | 1.45 (1.04–2.03) | 0.028 |
| Nationality, Saudi | 3.92 (2.59–5.94) | < 0.001 |
| Vaccine interval, 3 weeks | 0.49 (0.36–0.99) | 0.049 |
| Elapsed time between second dose and PCR testing, < = 3 months | 0.10 (0.08–0.13) | < 0.001 |
| Elapsed time between second dose and PCR testing, 4–6 months | 0.16 (0.10–0.26) | < 0.001 |
Comparison between infected and non-infected participants
The mean age of participants in the infected group was lower than that of the non-infected group, having a marginal statistical significance difference of 56.1 and 57.6, respectively (P: 0.057). The infected group also had a higher proportion of females (40.9%) than the non-infected group (38.4%); still, the difference was not statistically significant. Similarly, there was no statistically significant difference in the proportions of those infected versus those not infected based on the health sector, type of vaccine received, and vaccine interval category. Furthermore, the median number of weeks between vaccine doses was similar for the infected group and the non-infected group; thus, there was no statistically significant difference. Additionally, the proportion of participants with a longer time interval between PCR tests after the second dose, particularly after six months of vaccination, was significantly higher in the infected group (70.7%) than in the non-infected group (20.1%) (P < 0.001) (Table 1).
Impact of vaccination
Of the 523 COVID-19-infected patients, 1.9% (n = 10) were admitted to the intensive care unit (ICU) and 0.4% (n = 2) died. However, the impact of different vaccine types on SARS-CoV-2-related morbidity and mortality was not significantly different (Table 2).
Risk factors for developing COVID-19 infection
The protection effect was significantly associated with the vaccine for an interval of ≤ three weeks after the first dose (OR: 0.49, 95%CI: 0.36–0.99, P = 0.049), the first three months after the second dose (OR: 0.10, 95%CI: 0.08–0.13, P < 0.001), and 4–6 months after the second dose (OR: 0.16, 95%CI: 0.10–0.26, P < 0.001). On the other hand, being Saudi Arabian (OR: 3.92, 95%CI: 2.59–5.94, P < 0.001) and being between 18 and 45 years of age (OR: 1.45, 95%CI: 1.04–2.03, P = 0.028) were significantly associated with COVID-19 infection (Table 2).
Discussion
This study investigated the real-world effectiveness of double-dose COVID-19 vaccines in the Jazan Region of Saudi Arabia. This involved a comparison of the Pfizer (BNT162b2) and ASZ (ChAdOx1) vaccines’ efficacy. The results shed light on the variability of these vaccines’ effectiveness as well as the risk factors associated with breakthrough infections in the vaccinated population. Six months after the second dose, the COVID-19 vaccines’ effectiveness (protection rate) against COVID-19 infections was 68.3%. In our study population, being male, Saudi Arabian, younger as well as having a longer interval between vaccine doses or crossing a period of more than six months after the second vaccine dose were all associated with higher rates of breakthrough infections. Understanding the effectiveness of vaccines and the factors influencing breakthrough infections is key for informed decision-making and implementation of plans to control the epidemic and protect public health, especially during the upcoming waves of infection and for similar future events. Consistent with previous studies, our findings revealed variations in the effectiveness of different COVID-19 vaccine types against COVID-19 breakthrough infections, with the Pfizer (mRNA-based) vaccine outperforming the ASZ (DNA-based) vaccine [18]. Because different vaccine development platforms are based on triggering the immune response to produce neutralizing antibodies in different ways, the immune response is assumed to be different for each vaccine [19]. mRNA vaccines superior effectiveness compared to DNA vaccines can be attributed to several factors, including their simplified cellular entry required for expression, the generation of robust immune responses, and enhanced stability and potency [20], [21]. Unlike DNA vaccines, which necessitate nuclear membrane penetration, mRNA vaccines only require crossing the cell membrane, resulting in accelerated protein synthesis and an expedited immune response [20], [22]. Moreover, preclinical and clinical investigations have revealed that mRNA vaccines elicit potent immune reactions, including the production of neutralizing antibodies and the activation of T cells, outperforming DNA vaccines in this regard [23], [24]. Furthermore, mRNA molecules exhibit enhanced stability and potency compared to DNA, reducing the likelihood of degradation when introduced into the body [21]. Similar to previous research, our results indicated that vaccine effectiveness reduced after six months of receiving the second dose [25], [26]. Although multiple factors play a role in the incidence of breakthrough infections [27], this decline is attributed to waning immunity over time [28]. Waning immunity, which may arise from either vaccine-induced protection or natural immunity obtained through previous infection [29], [30], is a plausible explanation for infections among our study population, especially considering that about 56% of our study population is over 60 years old. This waning effect is more robust among older individuals due to age-related factors such as a decline in immune system function, a phenomenon known as immunosenescence, which affects both the adaptive and innate immune responses, leading to a weaker and slower response to infections and vaccinations in older individuals, which in turn results in a more rapid decline in immune memory and protection [31]. The lowest rate of infections among our study participants was reported four to six months after the dose, suggesting that the initial building of immunity takes time, peak at four to six months, and then starts to wane. Infection contracted during the first three months following the second dose could be explained by the time needed for the immune system to produce neutralizing antibodies, non-compliance with basic precautionary measures due to perceived protection, or infection contracted during the first 14 days after vaccination. This study’s follow-up period occurred during the second and third waves in Saudi Arabia when different COVID-19 variants were responsible for circulating infections [32], [33]. BTIs (breakthrough infections) were assessed before and during the presence of the highly transmissible Delta variants and possible vaccine breakthroughs, which demonstrated marked resistance to neutralization via vaccines [34], [35], [36]. Moreover, Liu Y., et al. found that the R0 of the Delta variant was found to be 5.08, compared to the ancestral strain's R0 of 2.79. This suggests that the Delta variant is much more transmissible and has the potential to cause larger outbreaks, posing a significant risk to public health and it is crucial to rapidly increase vaccine coverage rates [37]. In addition, The Delta variant of SARS-CoV-2 may have some ability to evade the immune response and escapes neutralizing monoclonal antibodies and polyclonal antibodies elicited by previous infection or vaccination, which could reduce the effectiveness of certain COVID-19 vaccines. While vaccination still offers significant protection against severe disease, hospitalization, and death, there is evidence that the Delta variant may be associated with higher rates of vaccine breakthrough infections compared to other variants [35]. This might explain the relatively lower effectiveness of vaccines observed in our study compared to their reported effectiveness in the literature [38]. However, no data were collected from our study participants to identify the variant type. Nonetheless, effectiveness was established in our sample when compared to findings from other populations. For example, the Pfizer vaccine’s effectiveness against the Delta variant has been reported to range between 42% and 93%, whereas in our sample, its effectiveness was identified to vary between 93% and 68.5% depending on the interval after the administration. Additionally, the ASZ vaccine’s effectiveness has been reported to be relatively lower against Delta variants ranging between 60% and 82.8%, which is similar to our findings, where its effectiveness ranged between 90.2% and 68.1% based on the measured interval after receipt of the second dose. Our findings indicate that among those who received two vaccine doses, Saudis and those in the lower age groups were at a higher risk of infection. Though our investigation did not measure detailed demographic factors associated with BTI among these categories, several reasons can be postulated to explain the higher infection frequencies. In a study that assessed adherence to personal protective behavior against COVID-19 in the Jazan region among those who are vaccinated, it was concluded that older populations exhibited higher adherence levels than younger ones, which may partially explain the higher infection rates among younger populations [39]. Moreover, the higher incidence of BTI among Saudis can be explained in part by the possibility that expatriates working in Saudi Arabia are single and have lower frequency and intensity levels of social interaction than Saudis, making them less likely to contract the infection. Furthermore, in a study that assessed adherence to COVID-19 preventive precautionary measures among nurses working in Saudi Arabia, it was concluded that non-Saudi nurses reported better adherence levels than Saudis, suggesting, though not conclusive, the presence of an association between nationality and risk of infection [40].
Conclusions
In conclusion, our findings detected variations in the effectiveness of different COVID-19 vaccine types against COVID-19 breakthrough infections, with the Pfizer (mRNA-based) vaccine being more effective than the ASZ (DNA-based) vaccine. The reduced vaccine effectiveness six months after receiving the second dose of the two vaccines suggests that vaccine-induced immunity is waning, especially among the elderly. This study has further practical implications concerning the importance of COVID-19 vaccination and the need for boosting doses after six months to reduce the incidence of SARS-CoV-2 infection and transmission.
Declaration of Competing Interest
We have no conflict of interest to declare.
Acknowledgments
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Tse L.V., Meganck R.M., Graham R.L., Baric R.S. The current and future state of vaccines, antivirals and gene therapies against emerging coronaviruses. Front Microbiol. 2020;11:658. doi: 10.3389/fmicb.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giubilini A., Savulescu J., Wilkinson D. COVID-19 vaccine: vaccinate the young to protect the old. J Law Biosci. 2020:7. doi: 10.1093/jlb/lsaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwasaki A., Omer S.B. Why and how vaccines work. Cell. 2020;183:290–295. doi: 10.1016/j.cell.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soiza R.L., Scicluna C., Thomson E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas S.J., Moreira E.D.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi W.-Y., Li Y.-D., Huang H.-C., Chan T.E.H., Chow S.-Y., Su J.-H., et al. COVID-19 vaccine update: vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection. J Biomed Sci. 2022;29:82. doi: 10.1186/s12929-022-00853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knoll M.D., Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397:72–74. doi: 10.1016/s0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukhari M.H., Syed M., Zain S. The differences between traditional vaccines and RNA vaccines: safety, efficacy, reliability and future of COVID-19 vaccines. Ann King Edw Med Univ. 2021:27. [Google Scholar]
- 10.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet (Lond, Engl) 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Authority P.H. Interim guidelines for the use of SARS-CoV-2 vaccin 2021.
- 12.Padoan A., Dall’Olmo L., Rocca F., della, Barbaro F., Cosma C., Basso D., et al. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin Chim Acta. 2021;519:60–63. doi: 10.1016/j.cca.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y., Ding M., Dong X., Zhang J., Kursat Azkur A., Azkur D., et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 14.Ahsan W., Syed N.K., Alsraeya A.A., Alhazmi H.A., Najmi A., Bratty M.Al, et al. Post-vaccination survey for monitoring the side effects associated with COVID-19 vaccines among healthcare professionals of Jazan province, Saudi Arabia. Saudi Med J. 2021;42:1341–1352. doi: 10.15537/smj.2021.42.12.20210576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalafalla H.E., Tumambeng M.Z., Halawi M.H.A., Masmali E.M.A., Tashari T.B.M., Arishi F.H.A., et al. COVID-19 vaccine hesitancy prevalence and predictors among the students of Jazan University, Saudi Arabia using the health belief model: a cross-sectional study. Vaccines. 2022:10. doi: 10.3390/vaccines10020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alamer E., Hakami F., Hamdi S., Alamer A., Awaf M., Darraj H., et al. Knowledge, attitudes and perception toward COVID-19 vaccines among adults in Jazan Province, Saudi Arabia. Vaccines. 2021:9. doi: 10.3390/vaccines9111259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saudi Public Health Authority COVID-19 GUIDELINES 2023. 〈https://covid19.cdc.gov.sa/wp-content/uploads/2023/04/V3.1COVID-19-Coronavirus-Disease-Guidelinesfinal-editionApr4-en.pdf〉 (Accessed May 31, 2023).
- 18.Yi S., Choe Y.J., Kim J., Kim Y.Y., Kim R.K., Jang E.J., et al. SARS-CoV-2 breakthrough infections after introduction of 4 COVID-19 vaccines, South Korea, 2021. Emerg Infect Dis. 2022;28:753–756. doi: 10.3201/eid2803.212210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UpToDate. COVID-19: Vaccines - UpToDate 2022. 〈https://www.uptodate.com/contents/covid-19-vaccines〉.
- 20.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 23.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 25.Feikin D.R., Higdon M.M., Abu-Raddad L.J., Andrews N., Araos R., Goldberg Y., et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang P., Hasan M.R., Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 delta variant in Qatar. Nat Med. 2021;27:2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 27.Gopinath S., Ishak A., Dhawan N., Poudel S., Shrestha P.S., Singh P., et al. Characteristics of COVID-19 breakthrough infections among vaccinated individuals and associated risk factors: a systematic review. Trop Med Infect Dis. 2022;7:81. doi: 10.3390/tropicalmed7050081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dolgin E. COVID vaccine immunity is waning-how much does that matter. Nature. 2021;597:606–607. doi: 10.1038/d41586-021-02532-4. [DOI] [PubMed] [Google Scholar]
- 29.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet (Lond, Engl) 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavanaugh A.M., Spicer K.B., Thoroughman D., Glick C., Winter K. Reduced Risk of Reinfection with SARS-CoV-2 After COVID-19 Vaccination - Kentucky, May-June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pawelec G. Age and immunity: what is “immunosenescence”? Exp Gerontol. 2018;105:4–9. doi: 10.1016/j.exger.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Agency S.P. Health Ministry Shows Impact of Vaccines, Precautions, Immunization on COVID-19 in Saudi Arabia The official Saudi Press Agency 2022. 〈https://www.spa.gov.sa/viewfullstory.php?lang=en&newsid=2344086〉.
- 33.AlArabiya_Eng. Saudi Arabia detects first case of COVID-19 Omicron variant in Kingdom 2021.
- 34.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Benhassine F., Rajah M.M., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 35.Farinholt T., Doddapaneni H., Qin X., Menon V., Meng Q., Metcalf G., et al. Transmission event of SARS-CoV-2 delta variant reveals multiple vaccine breakthrough infections. BMC Med. 2021;19:255. doi: 10.1186/s12916-021-02103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alhamlan F., Al-Qahtani A., Obeid D., Aljumaah S., Alghamdi S., ALNAFEE K., et al. SARS-CoV-2 Delta Variant Predominant at a Tertiary-Care Hospital in Saudi Arabia 2021. doi:10.21203/rs.3.rs-779549/v1.
- 37.Liu Y., Rocklöv J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. 2021:28. doi: 10.1093/jtm/taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayawi K., Shahriar S., Serhani M.A., Alashwal H., Masud M.M. Vaccine versus variants (3Vs): are the COVID-19 vaccines effective against the variants? A systematic review. Vaccines. 2021;9:1305. doi: 10.3390/vaccines9111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alameer A., Maslamani Y., Gosadi I.M., Elamin M.Y., Muaddi M.A., Alqassim A.Y., et al. Assessing continuity of adherence to precautionary measures for COVID-19 among vaccinated people in Jazan, Saudi Arabia. Microorganisms. 2023:11. doi: 10.3390/microorganisms11030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Dossary R., Alamri M., Albaqawi H., Al Hosis K., Aljeldah M., Aljohan M., et al. Awareness, attitudes, prevention, and perceptions of COVID-19 outbreak among nurses in Saudi Arabia. Int J Environ Res Public Health. 2020:17. doi: 10.3390/ijerph17218269. [DOI] [PMC free article] [PubMed] [Google Scholar]