Abstract
RNA–RNA recognition is a critical process in controlling many key biological events, such as translation and ribozyme functions. The recognition process governing RNA–RNA interactions can involve complementary Watson–Crick (WC) base pair binding, or can involve binding through tertiary structural interaction. Hence, it is of interest to determine which of the RNA–RNA binding events might emerge through an in vitro selection process. The A-site of the 16S rRNA decoding region was chosen as the target, both because it possesses several different RNA structural motifs, and because it is the rRNA site where codon/anticodon recognition occurs requiring recognition of both mRNA and tRNA. It is shown here that a single family of RNA molecules can be readily selected from two different sizes of RNA library. The tightest binding aptamer to the A-site 16S rRNA construct, 109.2-3, has its consensus sequences confined to a stem–loop region, which contains three nucleotides complementary to three of the four nucleotides in the stem–loop region of the A-site 16S rRNA. Point mutations on each of the three nucleotides on the stem–loop of the aptamer abolish its binding capacity. These studies suggest that the RNA aptamer 109.2-3 interacts with the simple 27 nt A-site decoding region of 16S rRNA through their respective stem–loops. The most probable mode of interaction is through complementary WC base pairing, commonly referred to as a loop–loop ‘kissing’ motif. High affinity binding to the other structural motifs in the decoding region were not observed.
INTRODUCTION
Ever since in vitro selection was introduced in the early nineties (1–3), the power of this combinatorial approach has been utilized to generate aptamers that bind targets ranging from organic molecules to proteins, and even DNA (4,5). There also have been recent reports on the selection of RNA motifs that bind RNA tetraloops (6) and on the selection of an RNA substrate for the P RNA of Bacillus subtilis (7). Studies on RNA–RNA binding and recognition are not at the stage where it is possible to predict the nature of the RNA–RNA binding interactions that would prevail in a particular instance. Therefore, the fundamental question of understanding the principles that govern intermolecular RNA–RNA recognition still remains in the exploratory stage.
RNA–RNA interactions are known to control many key biological events, such as translation and ribozyme function. Most of the RNA–RNA recognition processes occur through complementary Watson–Crick (WC) base pair binding. Specifically, loop–loop interactions generally occur from the single stranded region of the RNA, e.g. stem–loop, internal bulge, followed by formation of an extended intermolecular helix. These types of interactions have been observed in the dimerization of the HIV-1 genome (8,9), the formation of ribonucleoprotein particles for transport and localization in Drosophila (10), and in the self-splicing of subgroups in Tetrahymena thermophilia ribozymes (11). Alternatively, recent reports demonstrate that the catalytic P RNA ribozyme is able to recognize its substrate (RNA) through its three-dimensional structural or tertiary interactions (12,13). Hence, it is of interest to investigate which of the RNA–RNA binding events will emerge through an in vitro selection protocol.
The decoding region of prokaryotic 16S rRNA is a region of RNA which is thought to interact with mRNA and tRNA (14–16). It has long been known that the interaction of the codon and anticodon occurs on the A-site of the decoding region of prokaryotic 16S rRNA, which is itself part of the 30S ribosomal subunit (17,18). This interaction is disrupted by aminoglycoside antibiotics, which cause misreading of the mRNA, as well as inhibition of translocation (18). The aminoglycoside antibiotics are thought to primarily bind to 16S rRNA (19,20). While the entire 16S rRNA is too large for molecular dissection, recent experiments have shown that the 16S rRNA can be treated in a modular fashion (21). The binding-site of the aminoglycoside antibiotics in 16S rRNA appears to be confined to a discrete region (decoding region) as revealed by a series of mutational and chemical protection experiments (20,21). A simple decoding analog of 49 nt has been shown to be able to bind to aminoglycosides, tRNA and mRNA (21). The decoding region contains the A and P sites for tRNA binding, mRNA binding, as well as an important aminoglycoside binding region (21). The aminoglycoside binding region has been studied in some detail. An NMR spectroscopic study on the interaction between the aminoglycosides paramomycin and gentamycin with the internal bulged A-site of a 27-nt 16S rRNA construct has been reported (22–24). A more extensive 16S rRNA construct has been shown to stoichiometrically bind aminoglycosides known to interact with the A-site, while not binding to antibiotics known not to bind to the A-site (25,26). In contrast to the relatively advanced state of knowledge concerning A-site decoding region–aminoglycoside interactions, little is known about the nature of the interactions between tRNA and mRNA with the A-site. Thus the work described should allow for a further understanding of the RNA–RNA recognition process. In addition, it could also reveal novel molecules able to prevent the interaction of aminoglycosides with the 27-nt A-site decoding region construct of 16S rRNA first described by Fourmy et al. (22).
We have recently reported the development of a sensitive and quantitative binding fluorescence method that allows the efficient and accurate measurements of the dissociation constant (Kd) for aminoglycoside–RNA interactions (25–28). The method entails the use of fluorescent dye tagged aminoglycosides to measure binding affinities to RNA molecules selected to bind to particular aminoglycosides. Here, we report a modification of this technique that enabled us to study RNA–RNA interactions efficiently and quantitatively. In this instance, the attachment of a fluorescein fluorophore to the target RNA, instead of to the aminoglycoside, allows us to monitor and measure the Kd of RNA species through fluorescence anisotropy measurements. In addition, previously published aminoglycoside binding measurements were performed on a substantially more complex decoding region construct (157 nt) than the one under investigation here (25). Therefore, it was of interest to determine the specificity and affinity of aminoglycoside binding to the simplified 27-nt 16S rRNA construct which comprises only the A-site.
MATERIALS AND METHODS
Materials
Neomycin B sulfate (>90%), and paramomycin sulfate were purchased from Sigma (St Louis, MO). The primers and the DNA template (69mer and 109mer) used for the library were purchased from Integrated DNA Technologies (Coralville, IA). The two primers used for the PCR reactions have the following sequences. Primer 1: 5′-AGTAATACGACTCACTATAGGGAGAATTCCGACCAGAAG-3′; and primer 2: 5′-TGAGGATCCATGTAGACGCACATA-3′ where the underlined sequence is the promoter for T7 polymerase reactions (shown in Fig. 1B).
Figure 1.
(A) The structure of the simplified 27-nt A-site 16S ribosomal RNA used by Fourmy et al. (22). This structure is employed as the target for the in vitro selection protocol. (B) The nucleotide sequences of the 109mer and 69mer DNA template have embedded within them a 60 random nt and a 20 random nt cassette, respectively. The DNA templates are then amplified through seven cycles of PCR using primers #1 and #2. Primer #2 has a promoter sequence for T7 polymerase reactions (sequence in italic). The dsDNA was then reverse transcribed to the corresponding RNA using the T7 transcription kit from Promega.
The Wizard Miniprep DNA purification system and the Ribomax Large scale RNA T7 production kit were purchased from Promega (Madison, WI). The GeneAmp PCR and the GeneAmp Thermostable rTth Reverse Transcriptase RNA PCR kits were purchased from Perkin Elmer (Foster City, CA). The Original TA cloning kit was purchased from Invitrogen (Carlsbad, CA). Sephadex G-50 gel in NICK column and thiopropyl Sepharose 6B solid support from Pharmacia Biotech (Piscataway, NJ). Purification of dsDNA was performed through electrophoresis with agarose gels and subsequently using the QIAEX II gel extraction kit or through PAGE gels and purification by the ‘crush-and-soak method’ using 0.3 M Na2SO4. Both [α-32P]ATP and [γ-32P]ATP were purchased from New England Nuclear (NEN) (Boston, MA). All the DNA oligomers, A-site 16S rRNA stem–loop RNA oligomer mimic constructs, and both the 5′-thiol C6 linked and 5′-fluorescein-labeled 27mer A-site RNA construct were purchased from Oligos Etc. Inc. (Wilsonville, OR). 5-Carboxy-tetramethylrhodamine labeled paramomycin (CRP) was synthesized according to the method used by Wang et al. (25).
Affinity column preparation
Thiopropyl sepharose 6B solid support (containing 20 µmol of 2-thiopyridone per ml of gel) was derivatized with either 3.0 or 1.3 nmol of 5′-thiol C6 linked A-site 16S RNA according to the procedure described by Pei et al. (5). The thiopropyl sepharose 6B gel was activated by incubation with 10 mol dithiothreitol (DTT) in 50 mM Tris–HCl pH 8.1 for 3 h with constant shaking. The thiol modified A-site RNA construct was heated to 80°C for 3 min and allowed to cool back to room temperature over a 10 min period before being added to the activated thiopropyl sepharose 6B gel. The mixture was then vortexed vigorously for 1 min and incubated for 15 h at room temperature. After removal of the unreacted RNA with 30 vol incubation buffer, the efficiency and amount of RNA was determined by reducing an aliquot of the sepharose gel with DTT.
Preparation of the nucleic acid library and selections
The ssDNA template library was amplified through seven cycles of PCR, and the dsDNA was purified by agarose gel using the QIAEX II gel extraction kit. Ten milligrams of the dsDNA template was then subjected to the T7 Transcription process according to the instructions provided by the manufacturer (Promega). The RNA library was then purified with a 12% denaturing gel according to the method described by Sambrook et al. (29). An aliquot of 400 µg of the purified RNA library was heated to 80°C for 3 min, and allowed to cool to room temperature over a 10 min period prior to being loaded onto the column containing the derivatized target A-site RNA in selection buffer (1 M NaCl, 50 mM Tris–HCl, 3 mM MgCl2, pH 7.4). The column was incubated for 30 min at room temperature, and then rinsed with 3 column vol of the selection buffer with 10 mM DTT. For the first three rounds, 400 µg RNA was applied to the column. In succeeding rounds, 40 µg RNA was used. The RNA eluted was precipitated with ethanol, with glycogen as a carrier. RNA reverse transcription and PCR were performed in a single tube, using GeneAmp Thermostable rTth Reverse Transcriptase RNA PCR kit. About 50 ng of the RNA template was used for the 20 µl scale reverse transcription reactions. The succeeding PCR reaction was done for 10 thermocycles to ensure high quality PCR products. Cloning of the PCR DNA products was conducted using an Original TA cloning kit that contains INVαF′ Escherichia coli as the host bacteria.
Fluorescence measurements
The affinities of RNA for the fluorescein-labeled A-site RNA were determined by fluorescence anisotropy. Fluorescence measurements were performed on a LS-50B spectrofluorimeter (Perkin-Elmer) at 20.0 ± 0.1°C in the selection buffer (1 M NaCl, 50 mM Tris–HCl, 3 mM MgCl2, pH 7.4). The samples were excited at 490 nm, and fluorescence was monitored at 516 nm. Slits on both excitation and emission sides were 10 nm for aptamer–16S rRNA interaction. Equation 1 is used for the calculation of Kd by curve fitting the fluorescence anisotropy measurements between the RNA aptamers and fluorescein-labeled RNA (assuming a 1:1 complex).
I = I0 + 0.5Δɛ{[F·RNA]0 – ([F·RNA]0 + [RNA]0 Kd)2 –
4([F·RNA]0[RNA]0)0.5} 1
where I0 and I are the fluorescence anisotropy of fluorescein-labeled RNA in the absence and presence of aptamers, respectively; Δɛ is the difference between the fluorescence anisotropy of 1 µM fluorescein-labeled RNA in the presence of an infinite concentration of RNA and in its absence; [RNA]0 is the total concentration of RNA added; and [F·RNA]0 is the total concentration of fluorescein-labeled RNA.
The competition binding measurements between aminoglycosides and RNA–RNA complex were performed at constant concentration of the CRP and the 27-nt A-site 16S RNA by monitoring fluorescence anisotropy changes as a function of the changing concentrations of the selected aptamers. The Kd values for the selected aptamers were subsequently calculated by curve fitting the fluorescence anisotropy measurements of CRP as a function of the aptamers.
[aminoglycosides]0 = {Kd(A∞ – A)/Kd(A – A0) + 1} ×
{[RNA]0 – Kd(A – A0)/(A∞ – A) – [CRP]0(A – A0)/(A∞ – A0)} 2
where Kd is the dissociation constant between the aptamers and the aminoglycosides; [aminoglycosides]0 is the initial concentration of the aminoglycosides; and A, A∞ and A0 are the fluorescence anisotropy values of sample, totally-bound tracer and totally-free tracer, respectively.
RESULTS
Selection of RNA aptamers that bind to the immobilized target A-site 16S rRNA
In order to determine if the size of the oligomers in the library is important for the in vitro selection process to work, we chose to apply two pools of random RNA library to the target 27-nt A-site 16S rRNA construct previously described by Fourmy et al. (22). Each of the pools comprised a stretch of 20 and 60 random nucleotides, respectively, which were flanked by the appropriate primers with a promoter site for T7 polymerase (Fig. 1B). The sequences of the RNA primers used are described in Materials and Methods. The RNA library generated from the two templates was incubated with the derivatized thiopropyl sepharose 6B column containing the target A-site RNA. After elution of the bound RNA, the RNA was reverse transcribed to the cDNA, amplified though 10 cycles of PCR, and transcribed using T7 transcription polymerase. The RNA pool was then incubated with underivatized Sepharose 6B (pre-column) to eliminate any possible unwanted binding of the RNA molecules to the solid support. The eluted RNA molecules were then subjected to the same selection cycle as described above for enrichment of the RNA ligands.
After undergoing 10 rounds of selection cycles for the two libraries (69mer and 109mer) with the target A-site 16S rRNA at ‘low stringency’, the percentage of the RNA that eluted increased from ∼0.1 to ∼64% for both RNA pools (Table 1). At this point, the concentration of the target RNA was decreased 10-fold, and the selection process was repeated. Initially, the percentage of the eluted RNA was drastically decreased to ∼4%, but after five rounds of similar selection at ‘high stringency’ for the two pools, the number again rose and levelled off at 66%. It was of interest to note that further attempts to decrease the concentration of the target RNA to increase the stringency did not result in an increase in percentage of the eluted RNA, suggesting that there were no higher affinity aptamers in the pool.
Table 1. Stepwise enrichment of the eluted RNA from the 69mer and 109mer library pools.
| Cycle | % Input eluted for 69mer | % Input eluted for 109mer |
|---|---|---|
| (20 nt random cassette) | (60 nt random cassette) | |
| Low stringency (3.02 mmol) | ||
| 1 | 0.87 | 2.85 |
| 2 | 2.66 | 6.79 |
| 3 | 2.98 | 11.33 |
| 4 | 6.81 | 14.43 |
| 5 | 9.64 | 40.90 |
| 6 | 18.78 | 36.44 |
| 7 | 37.12 | 47.74 |
| 8 | 53.72 | 55.41 |
| 9 | 59.88 | 60.63 |
| 10 | 63.23 | 64.02 |
| High stringency (1.30 nmol) | ||
| 1 | 4.68 | 5.89 |
| 2 | 17.34 | 15.88 |
| 3 | 53.43 | 48.67 |
| 4 | 66.00 | 64.32 |
| 5 | 65.65 | 66.06 |
Progression of each cycle was monitored by determining the percentage (%) of the input RNA that was eluted after incubation with the target A-site 16S rRNA construct immobilized on a thiopropyl sepharose 6B column. A pre-column containing the underivatized sepharose 6B beads was utilized on cycle 2 and 5 for the low stringency selection process, and at cycle 2 during the high stringency selection process.
Cloning and sequencing of the selected pool
The RNA eluted after the fifth cycle after the ‘high stringency’ round was reversed transcribed, amplified and cloned. Ten out of the 25 clones sequenced for the 109mer library showed a high degree of homology, and seven out of the 22 clones sequenced for the 69mer library are closely related (Fig. 2). The consensus sequences for the selected aptamers from the two different libraries also proved to be >80% homologous when the sequences were read from the opposite direction. These results clearly indicate that the selected RNA ligands belong to a single family, indicating that a single species of ligands can be selected from pools of RNA molecules with different sizes.
Figure 2.
Randomized nucleotide sequences of the selected RNA aptamer constructs from the (A) 109mer and (B) 69mer libraries that exhibit high specificity and affinity towards the A-site 16S rRNA construct. The consensus sequences within each library are highlighted in bold lettering.
The consensus sequences of the aptamers obtained from the two libraries were then examined for WC complementary base pairing. It turned out that >60% of the consensus sequences of the aptamers have nucleotides that are complementary to the stem–loop and the duplex region around the target A-site RNA construct.
Dissociation constant (Kd) measurements of selected aptamers
Three sequences from each pool of selected aptamers which exhibit high homology were chosen for quantitative studies to determine the Kd to the target A-site 16S rRNA. To study the binding behavior of the various aptamers to the target A-site 16S rRNA through fluorescence spectroscopy, we first obtain the target A-site 16S rRNA with its 5′ end adducted to a fluorescein fluorophore through a six-carbon linker. Fluorescein was chosen since it is a neutral functional group, and thus is not expected to interact with the phosphate backbone of the RNA.
Upon binding of the selected aptamer to the fluorescein modified A-site 16S rRNA, the anisotropy of the fluorescein fluorophore was observed to be markedly increased in a concentration dependent manner (Fig. 3). This allows the binding constant to be calculated by curve-fitting using equation 1 as described in Materials and Methods. The Kds for the three selected aptamers from the 109mer pool were all observed to be in the low µM range with the target A-site 16S rRNA, with aptamer 109.2-3 exhibiting the tightest binding of all with a Kd of 1.24 µM (Table 2). The binding studies of the three selected aptamers from the 69mer pool all generally showed dissociation constants 2–3-fold higher (∼2.7–4 µM) than the 109mer library (Table 2).
Figure 3.
(A) The structure of the fluorescein-labeled 27-nt A-site 16S ribosomal RNA utilized in the fluorescence measurements. (B) Fluorescence anisotropy of fluorescein-labeled A-site 16S rRNA as a function of the 109.2-3 aptamer concentrations. Curve fitting (solid line) using equation 1 gave Kd = 1.236 µM.
Table 2. Summary of the Kds of the selected aptamers, from both the 69mer and 109mer libraries, with the target A-site 16S rRNA construct.
| Aptamers | Kd/µM |
|---|---|
| 109.1-7 | 1.446 ± 0.088 |
| 109.2-3 | 1.236 ± 0.051 |
| 109.2-15 | 1.655 ± 0.101 |
| 69.1-4 | 2.946 ± 0.149 |
| 69.1-11 | 2.786 ± 0.188 |
| 69.2-4 | non-specific binding |
A proposed two-dimensional structure of aptamer 109.2-3 from the 109mer library could be generated using the Mfold program (30). The putative structure of the 109.2-3 aptamer revealed that its consensus sequence was primarily found in either a stem–loop structure or in an internal bulge structure (Fig. 4). Since most of the consensus sequences are proposed to exist as a single stranded RNA, it is likely that this stem–loop, or the internal bulge, are regions that interact with the stem–loop of the A-site 16S RNA. A reasonable mode of interaction is through the loop–loop ‘kissing’ motif (31–35).
Figure 4.
The secondary structure of the 109mer aptamer 109.2-3 as predicted by the Mfold program (30). The circled nucleotides indicate the consensus sequences of the aptamer.
Binding of aminoglycosides to a simplified decoding construct
The A-site bulge of the 16S rRNA is shown to comprise part of the aminoglycoside binding site (21,22). It was our intention to determine whether the RNA aptamer that recognizes the A-site decoding region construct does so by binding to this region. We have previously reported that fluorescent dye tagged aminoglycosides can be used to accurately measure stoichiometry and binding affinities to RNA molecules (25,26). Specifically, CRP, a rhodamine substituted analog of paramomycin, has been shown to specifically bind to the internal bulge of the A-site. Subsequent competition experiments between CRP and other aminoglycosides allowed for a direct determination of affinities of the latter molecules. Previously published measurements on the aminoglycosides–16S rRNA were performed on a more complex (129 nt) decoding region construct (25). Therefore, it was of interest to determine the specificity and affinity of aminoglycoside binding to the simplified construct which comprises only the A-site of the decoding region. As shown in Figure 5, aminoglycosides CRP and neomycin B bind to the simplified construct stoichiometrically and with affinities of 0.207 and 0.248 µM, respectively. These dissociation constants obtained for the aminoglycosides against the simplified decoding region construct are similar to the more complex construct previously described which contained 129 nt (25). Thus, the 27-nt A-site fragment appears to behave in a similar fashion to the more complex decoding region construct as described previously (25). Given these results, it was of interest to determine whether the selected RNA aptamers would compete with aminoglycoside for binding to the A-site RNA construct.
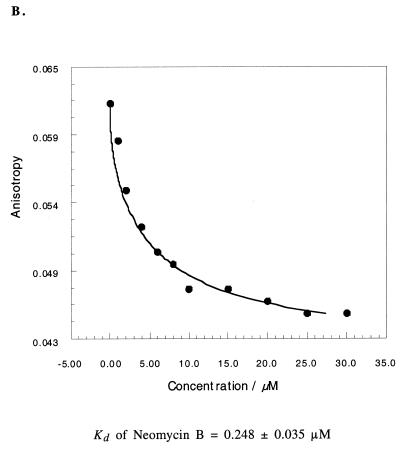
Figure 5.
(A) Fluorescence anisotropy of CRP (10 nM) as a function of the simplified 16S rRNA analogue concentrations. Curve fitting (solid line) using equation 1 gave Kd = 0.207 µM. (B) Fluorescence anisotropy of CRP (10 nM) containing the 16S rRNA analogue as a function of neomycin B concentrations. Curve fitting (solid line) using equation 2 gave Kd = 0.248 µM.
Competition of aminoglycosides with the aptamer–RNA complex
Since CRP is able to bind to the simplified construct under investigation here as well as to the more complex construct, competition experiments were carried out to determine if the selected RNA aptamers could displace CRP from the CRP–A-site construct complex. In the competition binding measurements between aminoglycosides and RNA–RNA complex, the affinity of neomycin and paramomycin for the aptamer–RNA complex is determined in two ways. First, the competition is conducted by titrating the particular aminoglycoside with the selected aptamer–fluorescein-labeled-RNA complex. Second, we also investigated the titration of the selected aptamer with the CRP–RNA complex. In both cases, equation 2 (see Materials and Methods) was used to curve fit the obtained data for the quenching of anisotropy displayed by CRP.
As shown in Figure 6, upon titrating the CRP–16S RNA complex with increasing concentrations of aptamer 109.2-3, no displacement of CRP was observed up to an aptamer concentration of 3 µM. This observation shows that the binding of the 109.2-3 aptamer to the 16S rRNA does not occur at the paramomycin binding site (the A-site). More importantly, this observation lends support to the hypothesis that the interaction of the selected RNA aptamers occurs at the stem–loop of the A-site 16S rRNA, and not at the internal bulge region.
Figure 6.
(A) Fluorescence anisotropy of CRP (10 nM) containing the A-site 16S rRNA construct as a function of the RNA aptamer 109.2-3 concentrations. (B) Fluorescence anisotropy of fluorescein-labeled A-site 16S rRNA construct (10 nM) containing the RNA aptamer 109.2-3 as a function of the neomycin aminoglycoside concentrations.
Competition of antisense DNA oligomer and A-site 16S rRNA stem–loop RNA oligomer mimic constructs with the aptamer–RNA complex
To determine if the stem–loop of the aptamer 109.2-3 is critical in binding the A-site 16S rRNA, a short antisense DNA oligomer predicted to bind the stem–loop of the aptamer 109.2-3 through WC base pairing (Fig. 7A), and RNA mimic constructs of the A-site 16S rRNA stem–loop (Fig. 7B), were synthesized and tested for their binding activities to the 16S A-site construct. The RNA and DNA oligomers were subsequently examined by fluorescence binding assay for competition against the binding of the aptamer 109.2-3 to the A-site 16S rRNA construct.
Figure 7.
(A) Sequence of the antisense DNA oligomer to the consensus stem–loop of aptamer 109.2-3. (B) Minimized structure of the A-site 16S rRNA decoding region stem–loop mimic constructs as determined by the Mfold program (30).
The aptamer 109.2-3 was incubated with one equivalent of either the DNA or RNA oligomers, and fluorescence binding studies were carried out by titrating increasing concentrations of each aptamer–oligomer complex with the fluorescein-labeled A-site 16S rRNA and monitoring the changes in anisotropy values. It was observed that the incubation of one equivalent of antisense DNA oligomer with the aptamer 109.2-3 had no disruptive effect on the binding interaction of the DNA oligomer–aptamer complex and the A-site 16S rRNA construct (Fig. 8A). However, when one equivalent each of the A-site 16S rRNA stem–loop RNA mimic construct A was incubated with the aptamer, the binding specificity between the resulting complex with the fluorescein-labeled A-site 16S rRNA was drastically diminished. Upon the addition of two equivalents of construct A with aptamer 109.2-3, the binding efficiency of the resulting complex with the A-site 16S rRNA construct is decreased even further as measured by fluorescence anisotropy (Fig. 8B).
Figure 8.
(A) Fluorescence anisotropy of fluorescein-labeled A-site 16S rRNA as a function of the antisense DNA oligomer–109.2-3 aptamer complex concentrations. (B) Fluorescence anisotropy of fluorescein-labeled A-site 16S rRNA as a function of the RNA oligomer–109.2-3 aptamer concentrations. Open squares, incubation of aptamer 109.2-3 with 1 equivalent of construct A; open diamonds, incubation of aptamer 109.2-3 with 2 equivalents of construct A; closed circles, incubation of aptamer 109.2-3 with 1 equivalent of construct B.
To further investigate the importance of the stem–loop of A-site 16S rRNA in binding the aptamer 109.2-3, the stem–loop of the wild-type mimic construct A was point mutated to afford construct B. We reasoned that the mutated construct B should not bind to the aptamer since its stem–loop lacks the appropriate nucleotide sequence for WC binding to the aptamer’s stem–loop. As expected, the binding of the resulting aptamer–oligomer complex (1:1) to the fluorescein-labeled A-site 16S rRNA was restored. These results suggest that the stem–loop of the aptamer 109.2-3 is critical for binding to the A-site 16S rRNA, and that the 4-nt stem–loop of the A-site 16S rRNA construct confers binding capacity to the aptamer 109.2-3.
Binding of mutant aptamer and the A-site 16S rRNA
To further confirm that the stem–loop of the aptamer is indeed the likely site of interaction between the aptamer and the stem–loop of the A-site 16S rRNA, point mutations were sequentially introduced at each of the three nucleotides of the aptamer 109.2-3 suspected to be involved in the WC base pairing with the stem–loop of the A-site 16S rRNA construct (Fig. 9A). The secondary structure of the three resulting mutant aptamer 109.2-3 constructs, 1–3, were predicted using the Mfold program (30). Fluorescence binding studies of the three mutant constructs with the fluorescein-labeled A-site 16S rRNA construct were carried out. It was observed that the constructs 1–3 (containing point mutations at each of the three nucleotides) were unable to specifically bind the fluorescein-labeled A-site 16S rRNA construct (Fig. 9B). These observations suggest that the three nucleotides on the aptamer’s stem–loop are critical for specific binding between the aptamer 109.2-3 and the A-site 16S rRNA.
Figure 9.
(A) Point mutations were performed on each of the three nucleotides in the consensus sequence confined to the stem–loop region. The structural integrity of the resulting constructs 1, 2 and 3 remained unchanged as predicted by the Mfold program (29). (B) Fluorescence anisotropy of fluorescein-labeled A-site 16S rRNA as a function of the three mutant 109.2-3 aptamer construct concentrations. Open diamonds, construct 1; crossed square boxes, construct 2; closed triangles, construct 3; closed circles, wild type aptamer 109.2-3 construct.
Taken together, these observations strongly support the conclusion that the interactions between the aptamer 109.2-3 and A-site 16S rRNA occurs through simple WC base pairing through their stem–loops.
DISCUSSION
The 16S rRNA decoding region in prokaryotes is part of a complex RNA molecule which is itself an essential component of the 30S ribosomal subunit (17,18). The decoding region is of substantial interest pharmacologically and medically because it constitutes the binding-site of the aminoglycoside antibiotics (19,20). While the A and P sites of the decoding region constitute only a small fraction of 16S rRNA, mini-constructs of the decoding region are biochemically active (21), suggesting that the structure of the decoding region is preserved in the absence of the remainder of the RNA molecule. In fact, a 27-nt A-site construct has been prepared which binds aminoglycosides (22,23), and the structure of this construct bound to the aminoglycoside paramomycin has been elegantly determined by NMR studies (22,23).
This small construct is quantitatively well-behaved with respect to aminoglycoside binding. CRP and neomycin B were bound to a significantly larger (129 nt) 16S rRNA construct with dissociation constants in the range of 0.165 and 0.132 µM, respectively (25). Specific aminoglycoside binding was observed with dissociation constants for CRP and neomycin B aminoglycosides of 0.175 and 0.248 µM, respectively, with the simplified 16S rRNA construct containing only the A-site. These and other data (21,22) strongly suggest that complicated RNA molecules can be broken down into relatively simple fragments without losing biochemical activity.
While aminoglycosides specifically bind to the A-site decoding region, they bind with only moderate affinities (23). Clearly, higher affinity ligands would be desirable given the inherent toxicities (nephrotoxicity and ototoxicity) (29). One approach to this problem is to select RNA molecules against an A-site construct. The 27-nt A-site construct described by Fourmy et al. was chosen for this study (22,23). This construct contains three different structural motifs: a double helical region, a bulged region and a stem–loop region (22,23). It was therefore of interest to select for high affinity aptamers against this construct to determine which region of the construct they would be directed. High affinity aptamer binding mediated via base-pairing (including triple helix formation) or shape recognition would define two distinct modes of RNA–RNA recognition.
It is shown here that a single family of RNA molecules could be readily selected from two different sizes of RNA libraries. The affinities of 109mer aptamers generally showed an ~2-fold higher binding propensity as compared to their 69mer counterpart. This small difference suggests that there are no important differences in the interactions between the two different size aptamers and that base pairing between the aptamers and the A-site construct dominate the binding interactions.
The aptamer 109.2-3 that binds the A-site 16S rRNA is predicted to have its consensus sequence confined to a stem–loop region by modeling using the Mfold program (30). The consensus sequence has three WC nucleotides complementary to the stem–loop region of the A-site 16S rRNA. Point mutations of each of the three nucleotides abolishes specific binding between the aptamer and the A-site 16S rRNA construct, suggesting that the likely mode of recognition motif between the two RNA constructs is between their stem–loops. This type of interaction, characterized as a loop-loop ‘kissing’ motif, has already been shown to exist in many biological systems (8–11,32–34). The binding between the loops observed here was significantly lower in affinity than those previously reported (32,34). We attribute this difference to the smaller, 4 nt, stem–loop for the A-site 16S rRNA. It has previously been observed that loops of >6 nt are generally more stable in the formation of such loop–loop interactions (32,33). Moreover only triplet–triplet interloops occur between the 4 nt A-site loop and the 7 nt aptamer loop. It is possible that base pairing at the four nucleotides in the A-site loop is sterically prohibited.
The studies described here have also led to the development of a fluorescence technique that enables the efficient and quantitative study of RNA–RNA interactions. Fluorescence methods are extremely sensitive, convenient to use and readily adaptable for the screening of libraries. In this case, we have utilized a fluorescein fluorophore attached to the target RNA, and this system allows for the measurement of the binding dissociation between two RNA species through fluorescence spectroscopy.
In conclusion, these studies clearly illustrate that the favored RNA–RNA recognition in aptamer–A-site construct binding involves complementary WC base pairing. It is interesting that the A-site bulge was not recognized, since this bulge is normally thought to interact with mRNA and tRNA, as revealed by chemical protection experiments (21). It is possible that RNA–RNA interactions involving the A-site bulge generate very weak-binding aptamers which would have been lost during the higher stringency selection protocols. The RNA aptamers that were generated against the A-site 16S rRNA construct recognize the loop region of the construct with an affinity in the low µM range. These observations imply that the general interaction of RNA with the 16S rRNA A-site is not favorable. From a teleological standpoint, it might be anticipated that the A-site region would not be prone to making high affinity inter-complementary contacts with RNA molecules. Although the A-site may bind to mRNAs and tRNAs, the underlying interactions must be weak and relatively non-specific. High affinity interactions would tend to inhibit the translation process. The fact that a site contiguous to the aminoglycoside binding bulge is targeted suggests the possibility of the design of hybrid aminoglycoside–RNA species which could have enhanced affinities for the decoding region.
Acknowledgments
ACKNOWLEDGEMENTS
We gratefully acknowledge the input and stimulating discussion from the members of the laboratory. This work was partially supported by US Public Health Service National Institutes of Health Grant EY-12375.
REFERENCES
- 1.Ellington A.D. and Szostak,J.W. (1990) Nature, 346, 818–822. [DOI] [PubMed] [Google Scholar]
- 2.Tuerk C. and Gold,L. (1990) Science, 249, 505–510. [DOI] [PubMed] [Google Scholar]
- 3.Beaudry A. and Joyce,G.F. (1992) Science, 257, 635–641. [DOI] [PubMed] [Google Scholar]
- 4.Gold L., Polisky,B., Uhlenbeck,O. and Yarus,M. (1995) Annu. Rev. Biochem., 64, 763–797. [DOI] [PubMed] [Google Scholar]
- 5.Pei D., Ulrich,H.D. and Schultz,P.G. (1991) Science, 253, 1408–1411. [DOI] [PubMed] [Google Scholar]
- 6.Costa M. and Michel,F. (1997) EMBO J., 16, 3289–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan T. (1995) Biochemistry, 34, 8458–8464. [DOI] [PubMed] [Google Scholar]
- 8.Laughrea M. and Jette,L. (1996) Biochemistry, 35, 9366–9374. [DOI] [PubMed] [Google Scholar]
- 9.Laughrea M. and Jette,L. (1997) Biochemistry, 36, 9501–9508. [DOI] [PubMed] [Google Scholar]
- 10.Ferrandon D., Koch,I., Westhof,E. and Nusslein-Volhard,C. (1997) EMBO J., 16, 1751–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehnert V., Jaeger,L. Michel,F. and Westhof,E. (1996) Chem. Biol., 3, 993–1009. [DOI] [PubMed] [Google Scholar]
- 12.Westhof E. and Altman,S. (1994) Proc. Natl Acad. Sci. USA, 91, 5133–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris M.E., Nolan,J.M., Malhotra,A., Brown,J.W., Harvery,S.C. and Pace,N.R. (1994) EMBO J., 13, 4870–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moazed D. and Noller,H.F. (1986) Cell, 47, 985–994. [DOI] [PubMed] [Google Scholar]
- 15.Moazed D. and Noller,H.F. (1990) J. Mol. Biol., 211, 135–145. [DOI] [PubMed] [Google Scholar]
- 16.Moazed D. and Noller,H.F. (1987) Nature, 327, 389–394. [DOI] [PubMed] [Google Scholar]
- 17.Cundliffe E. (1989) Annu. Rev. Microbiol., 43, 207–233. [DOI] [PubMed] [Google Scholar]
- 18.Cundliffe E. (1990) In Hill,W.E., Dahlberg,A.E., Garrett,R.A., Moore,P.B., Schlessinger,D. and Warner,J.R. (eds), The Ribosome: Structure, Function and Evolution. American Society for Microbiology, Washington, DC, pp. 479–490.
- 19.Gale E.F., Cundliffe,E., Reynolds,P.E., Richmond,M.H. and Waring,M.J. (1981) The Molecular Basis of Antibiotic Action, 2nd edition. John Wiley & Sons, London, UK, pp. 419–439.
- 20.Woodcook J., Mozaed,D., Cannon,M., Davies,J. and Noller,H.F. (1991) EMBO J., 10, 3099–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purohit P. and Stern,S. (1994) Nature, 370, 659–662. [DOI] [PubMed] [Google Scholar]
- 22.Fourmy D., Recht,M.I., Blanchard,S.C. and Puglisi,J.D. (1996) Science, 274, 1367–1371. [DOI] [PubMed] [Google Scholar]
- 23.Recht M.I., Fourmy,D., Blanchard,S.C., Dahlquist,K.D. and Puglisi,J.D. (1996) J. Mol. Biol., 262, 421–426. [DOI] [PubMed] [Google Scholar]
- 24.Ulenbeck O.C., Pardi,A. and Feigon,J. (1997) Cell, 90, 833–840. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Hamasaki,K. and Rando,R.R. (1997) Biochemistry, 36, 768–779. [DOI] [PubMed] [Google Scholar]
- 26.Hamasaki K. and Rando,R.R. (1997) Biochemistry, 36, 12323–12328. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y. and Rando,R.R. (1996) Chem. Biol., 3, 129–131.8807838 [Google Scholar]
- 28.Wang Y., Killian,J., Hamasaki,K. and Rando,R.R. (1996) Biochemistry, 35, 12338–12346. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, Section 6.46.
- 30.Jaeger J.A., Turner,D.H. and Zuker,M. (1989) Methods Enzymol., 183, 281–306. [DOI] [PubMed] [Google Scholar]
- 31.Marino J.P., Gregorian,R.S.,Jr, Csankovszki,G. and Crothers,D.M. (1995) Science, 268, 1448–1454. [DOI] [PubMed] [Google Scholar]
- 32.Chang K.Y. and Tinoco,I.,Jr (1997) J. Mol. Biol., 269, 52–66. [DOI] [PubMed] [Google Scholar]
- 33.Chang K.Y. and Tinoco,I.,Jr (1994) Proc. Natl Acad. Sci. USA, 91, 8705–8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henderson B.S. and Schimmel,P. (1997) Biorg. Med. Chem., 5, 1071–1079. [DOI] [PubMed] [Google Scholar]
- 35.Chambers H.F. and Sande,M.A. (1996) In Hardman,J.G., Limbird,L.E., Molinoff,P.B., Ruddon,R.W. and Goodman Gilman,A. (eds), The Aminoglycosides in Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 9th edition. McGraw-Hill, New York, Chapter 46, pp. 1103–1121.