ABSTRACT
The mycobacterial cytochrome bcc:aa3 complex deserves the name “supercomplex” since it combines three cytochrome oxidases—cytochrome bc, cytochrome c, and cytochrome aa3—into one supramolecular machine and performs electron transfer for the reduction of oxygen to water and proton transport to generate the proton motive force for ATP synthesis. Thus, the bcc:aa3 complex represents a valid drug target for Mycobacterium tuberculosis infections. The production and purification of an entire M. tuberculosis cytochrome bcc:aa3 are fundamental for biochemical and structural characterization of this supercomplex, paving the way for new inhibitor targets and molecules. Here, we produced and purified the entire and active M. tuberculosis cyt-bcc:aa3 oxidase, as demonstrated by the different heme spectra and an oxygen consumption assay. The resolved M. tuberculosis cyt-bcc:aa3 cryo-electron microscopy structure reveals a dimer with its functional domains involved in electron, proton, oxygen transfer, and oxygen reduction. The structure shows the two cytochrome cIcII head domains of the dimer, the counterpart of the soluble mitochondrial cytochrome c, in a so-called “closed state,” in which electrons are translocated from the bcc to the aa3 domain. The structural and mechanistic insights provided the basis for a virtual screening campaign that identified a potent M. tuberculosis cyt-bcc:aa3 inhibitor, cytMycc1. cytMycc1 targets the mycobacterium-specific α3-helix of cytochrome cI and interferes with oxygen consumption by interrupting electron translocation via the cIcII head. The successful identification of a new cyt-bcc:aa3 inhibitor demonstrates the potential of a structure-mechanism-based approach for novel compound development.
KEYWORDS: cytochrome bcc:aa3 oxidase, bioenergetics, OXPHOS, tuberculosis, Mycobacterium tuberculosis, inhibitor
INTRODUCTION
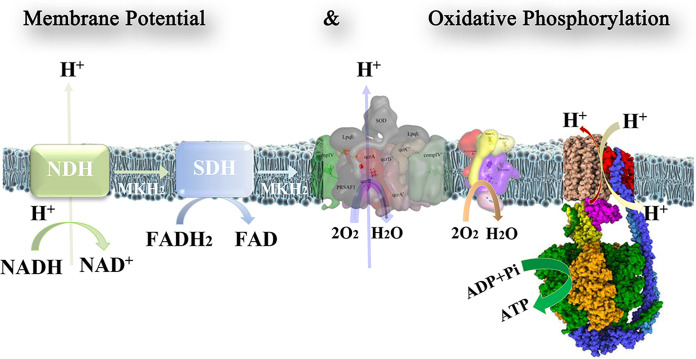
The tuberculosis (TB) causing pathogen Mycobacterium tuberculosis is an obligate aerobe that is strictly dependent on oxygen to meet its energetics demand during growth. Without any effective fermentative process, the oxidative phosphorylation (OXPHOS) pathway is crucial to maintain redox homeostasis and synthesis of sufficient amount of ATP (Fig. 1) (1, 2). Most mycobacteria contain a proton-pumping cytochrome bcc:aa3 oxidase supercomplex (cyt-bcc:aa3) and a non-proton-pumping, less energetically efficient, cytochrome bd oxidase (cyt-bd) (3, 4). The discovery and visualization of the phase 2 candidate Telacebec (Q203), which targets the substrate-binding site of menaquinol of mycobacterial cyt-bcc:aa3, validated this complex as a target for anti-TB treatment (5–8).
FIG 1.
Mycobacterial respiratory chain complexes generate the electrochemical gradient, providing the energy for the formation of ATP by the F1Fo-ATP synthase. Complexes I (NDH) and II (SDH) oxidize NADH and succinate, respectively. These electrons are transferred to the mycobacterial cyt-bcc:aa3 and cyt-bd oxidases to reduce oxygen to water. While complex I and the cyt-bcc:aa3 pump protons into the intermembrane lumen to generate the proton gradient, the Fo domain of the F1Fo ATP synthase uses these protons to drive ATP formation in the F1 domain. The maintenance of a proton gradient and the regulation of ATP production are crucial parameters governing the survival of the bacterium.
From an evolutionary perspective, the mycobacterial cyt-bcc:aa3 supercomplex is a fusion of the mitochondrial cytochrome bc (cyt-bc), cytochrome c, and cytochrome aa3 oxidases (cyt-aa3), with cytochrome c connecting the electron flow from the substrate quinol in cyt-bc oxidase to the cyt-aa3 oxidase in which reduction of O2 to water occurs (9, 10).
The mycobacterial cyt-bcc domain consists of subunits QcrC, QcrA, and QcrB, while the cyt-aa3 part includes the subunits CtaC and CtaD, relevant for electron transfer and O2 reduction, as well as the assembly subunits CtaE, CtaF, CtaI, and CtaJ. The mycobacterial subunit QcrC is the counterpart of the mitochondrial cyt-c, consisting of a transmembrane helical segment, followed by the two cytochrome cIcII (cyt-cIcII) domains, including heme cI and cII, as demonstrated by the recent cryo-electron microscopy (cryo-EM) structures of the Mycobacterium smegmatis cyt-bcc:aa3 oxidase (9, 10). The cyt-cIcII domains are located at the intermembrane space and couple the electron flow from the cyt-bcc:aa3 to subunit CtaC of cyt-aa3 (1). Mutants of the related M. smegmatis lacking subunit CtaC have profoundly impaired growth, indicating the importance of this subunit for bacterial survival and coupling of electrons between cyt-cIcII and CtaC for the final reduction of oxygen (11).
Understanding the structure, mechanisms, regulation, and M. tuberculosis-specific modifications of the cyt-bcc:aa3 oxidase requires the isolation of a pure, complete, and enzymatically active M. tuberculosis cyt-bcc:aa3 oxidase, which is presented here. The first cryo-EM structure of this entire M. tuberculosis supercomplex sheds light into the pathways involved in O2 reduction and the generation of the proton motive force and revealed a mycobacterium-specific epitope essential for electron transfer that we exploited to discover a specific inhibitor.
RESULTS AND DISCUSSION
Expression, production, and purification of the recombinant M. tuberculosis cyt-bcc:aa3.
The expression and production of the membrane-embedded, multisubunit complexes of M. tuberculosis represents a challenge (12, 13). Here, we used M. bovis BCG as an expression system, a nonpathogenic species in which the cyt-bcc:aa3 is 100% homolog to the M. tuberculosis counterpart. cyt-bcc:aa3 was tagged at the C terminus of subunit QcrB using the ORBIT (oligonucleotide-mediated recombineering, followed by Bxb1 integrase targeting) technology, as described in Materials and Methods (14).
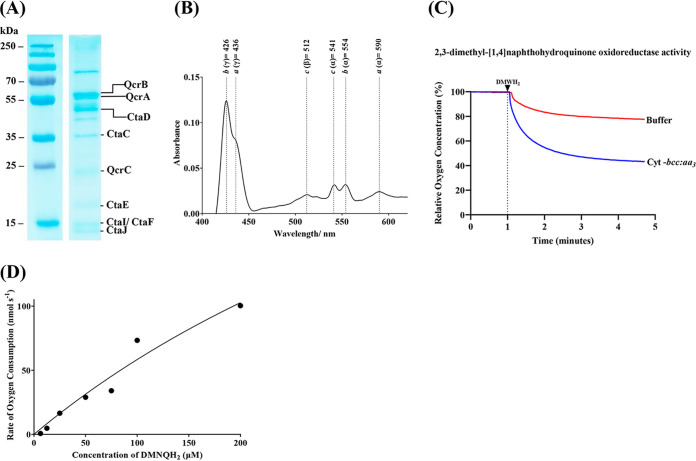
The M. tuberculosis cyt-bcc:aa3 was solubilized in a buffer containing 1% (wt/vol) n-dodecyl-β-d-maltoside (DDM; 20 mM morpholinepropanesulfonic acid [MOPS; pH 7.4], 100 mM NaCl, 2 mM Pefabloc, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1% [wt/vol] DDM, 10% glycerol) by stirring for 1 h at 4°C. Afterward, the mixture was ultracentrifuged (39,500 × g for 30 min at 4°C), and the supernatant was incubated with 1 mL of FLAG beads at 4°C for 1 h. To reduce the detergent content and prevent subunit dissociation, the resin was washed with a buffer of lower DDM content (20 mM MOPS [pH 7.4], 100 mM NaCl, 2 mM Pefabloc, 1 mM PMSF, 0.1% [wt/vol] DDM, 5% glycerol). The recombinant M. tuberculosis cyt-bcc:aa3 was eluted by a buffer including FLAG peptide (20 mM MOPS [pH 7.4], 100 mM NaCl, 2 mM Pefabloc, 1 mM PMSF, 0.1% [wt/vol] DDM, 100 μg/mL of 3× FLAG peptide). SDS-PAGE (Fig. 2A) and MALDI analysis (see Table S1A to C in the supplemental material) revealed that the eluted complex contained the subunits QcrA-C, CtaC, CtaD, CtaE, CtaF, CtaI, and CtaJ.
FIG 2.
Characterization of purified M. tuberculosis cyt-bcc:aa3. (A) 12% SDS gel profile of purified supercomplex. The bands for QcrB, QcrA, and QcrC were further confirmed through matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (MS) (see (Table S1A to C in the supplemental material). (B) Difference spectrum of purified M. tuberculosis cyt-bcc:aa3 after reduction with Na2S2O4. The spectrum registered peaks for heme a (437 nm, 592 nm), heme b (424 nm, 555 nm), and heme c (512 nm, 543 nm). (C) Characterization of the purified enzyme in an oxygen consumption assay. In the presence of 2,3-dimethyl[1,4]naphthohydroquinone, the purified supercomplex reduced oxygen levels significantly, as shown by the profile after subtracting background activity. (D) Enzyme kinetics of M. tuberculosis cyt-bcc:aa3. A dose response of 2,3-dimethyl[1,4]naphthohydroquinone was used to determine the Km, kcat, and kcat/Km values of the enzyme.
To prove the electron transfer, M. tuberculosis cyt-bcc:aa3 was first oxidized by 100 mM potassium ferricyanide (K3[Fe(CN)6]), followed by the chemical reduction using sodium dithionite (Na2S2O4). The respective difference spectrum confirmed that all the hemes—a, b, and c—are within the purified complex and contribute to electron translocation (Fig. 2B). To further test whether the recombinant M. tuberculosis cyt-bcc:aa3 was also capable to reduce oxygen to water, a 2,3-dimethyl-[1,4]naphthohydroquinone (DMNQ) oxidoreductase activity assay was carried out. The data shown in Fig. 2C demonstrate that the enzyme complex was able to reduce oxygen significantly. Titration of cyt-bcc:aa3 with increasing concentrations of the substrate (2,3-dimethyl-[1,4]naphthohydroquinol [DMNQH2]) gave a graded increase in Oxygen Consumption Rate (OCR) measurements with a calculated Michaelis constant (Km) of about 75 μM and a catalytic rate constant (kcat) of about 154 s−1 (Fig. 2D). These data enabled the catalytic efficiency (kcat/Km) to be estimated at 2 μM−1 s−1. Based on this and to allow comparison of data published on other mycobacterial cyt-bcc:aa3 oxygen consumption assays (7, 8), we used 100 μM DMNQH2 for our downstream experiments.
Cryo-EM studies of recombinant M. tuberculosis cyt-bcc:aa3.
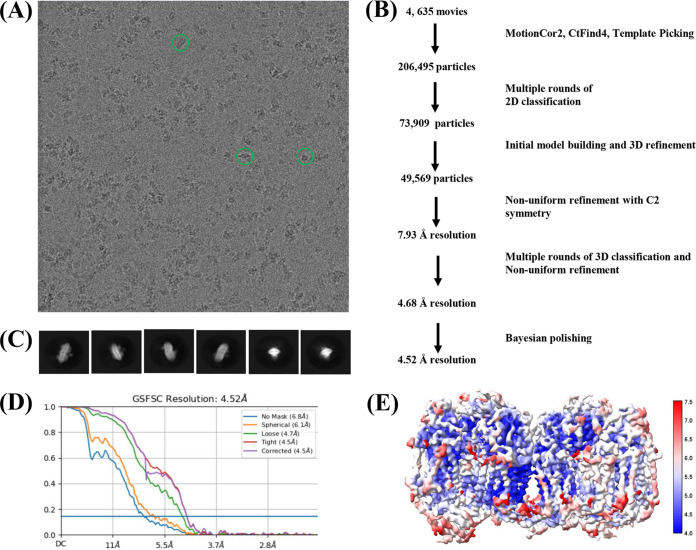
The recombinant M. tuberculosis cyt-bcc:aa3 was monodispersed as visualized in cryo-EM images (Fig. 3A). In order to resolve a three-dimensional (3D) structure of the supercomplex, 4,635 movies were collected. Data processing was performed using cryoSPARC, as well as RELION v3.1 (Fig. 3A and B). Autopicking yielded 206,495 particles, which were classified into 100 2D classes, which provided 21 good classes (73,909 particles), that were selected for ab initio model building (Fig. 3C). The model was subjected to nonuniform refinement, yielding a resolution of 4.63 Å, and further transferred to RELION v3.1 for Bayesian polishing. The resulting structure from RELION improved the resolution to 4.52 Å. Although the superoxide dismutase (sodC) was found to be associated with the M. tuberculosis cyt-bcc:aa3 (see Fig. S1), the sodC resolution was low, which could be attributed to preferred orientation, leading to fewer particles displaying visible sodC. The final local resolution of the M. tuberculosis cyt-bcc:aa3 density map (Fig. 3D and E) revealed an inner core of the cyt-bcc domain to have a higher resolution of closer to 4 Å compared to the exterior of the entire M. tuberculosis cyt-bcc:aa3 complex. The model of the M. tuberculosis cyt-bcc:aa3 was built into the map, using the structural model of the recently described mycobacterial cyt-bcc:aa3 (PDB 7E1V) (7). The final structure, which was refined using Phenix and Coot, yielded a MolProbity score of 1.96. The cryo-EM map statistics and model statistics are presented in Tables 1 to 3. While the densities of most of the M. tuberculosis cyt-bcc:aa3 subunits can be visualized clearly (see Fig. S2), the densities of subunits CtaI and CtaJ cannot be resolved properly in view of the low resolution (Table 3 and Fig. 3E).
FIG 3.
Cryo-EM data collection and processing. (A) Grid screening revealed the desired protein particles that resembled either a rod or eclipse shape. (B) Strategy of image analysis. (C) The acceptable 2D classes revealed rod- and eclipse-like structures. (D) A 4.6-Å structure was determined and further transferred to RELION v3.1 for Bayesian polishing, resulting in a final structure with a global 4.52-Å resolution. (E) The local resolution of the various regions was highlighted in a red-white-blue scale. The lowest resolution of 4 Å is represented by blue, and the highest resolution of 7 Å is represented by red.
TABLE 1.
Cryo-EM data acquisition and image processing of M. tuberculosis cyt-bcc:aa3
| Data acquisition and image processing | M. tuberculosis cyt-bcc:aa3 |
|---|---|
| Data collection | |
| Electron microscope | Titan Krios |
| Camera | Gatan K2 |
| Mode | Super-resolution counting |
| Voltage (kV) | 300 |
| Nominal magnification | 130,000 × |
| Calibrated physical pixel size (Å) | 1.06 |
| Exposure time (s) | 9 |
| Total exposure (e–/Å2) | 40 |
| No. of frames | 40 |
| Defocus range (μM) | 1.0–2.0 |
| Image processing | |
| Motion correction software | MotionCor2 |
| CTF estimation software | CTFFind4 |
| Particle selection software | cryoSPARC v3.3.1 |
| 2D classifications | cryoSPARC v3.3.1 |
| 3D classification and refinement software | RELION v3.1 |
| Particle motion correction software | RELION v3.1 |
TABLE 2.
Cryo-EM map statistics of resolved M. tuberculosis cyt-bcc:aa3
| Cryo-EM map parameter | M. tuberculosis cyt-bcc:aa3 |
|---|---|
| No. of micrographs | 4,635 |
| No. of particle images selected | 206,495 |
| No. of particle images after clean-up | 73,909 |
| Particle images contributing to maps | 49,569 |
| Applies symmetry | C2 |
| Applied box size (pixels) | 500 |
| Applied B-factor (Å2) | 142.2 |
| Global resolution (FSC = 0.143, Å) | 4.5 |
TABLE 3.
Model building, model refinement, and model statistics for M. tuberculosis cyt-bcc:aa3
| EM model | M. tuberculosis cyt-bcc:aa3 |
|---|---|
| Modeling software | Coot |
| Refinement software | Phenix |
| MolProbity score | 1.96 (78th percentile) |
| EMRinger score | 1.12 |
| No. of residues | 5,060 |
| Clash score | 11.03 |
| CC (mask) | 0.78 |
| RMS bond | |
| Length (Å) | 0.003 |
| Angle (°) | 0.742 |
| Ramachandran | |
| Favored (%) | 94.07 |
| Outliers (%) | 0.06 |
| Rama-Z | 0.98 ± 0.13 |
| Ligands | Heme a, heme b, heme c, FeS |
Structure of the dimeric M. tuberculosis cyt-bcc:aa3 supercomplex.
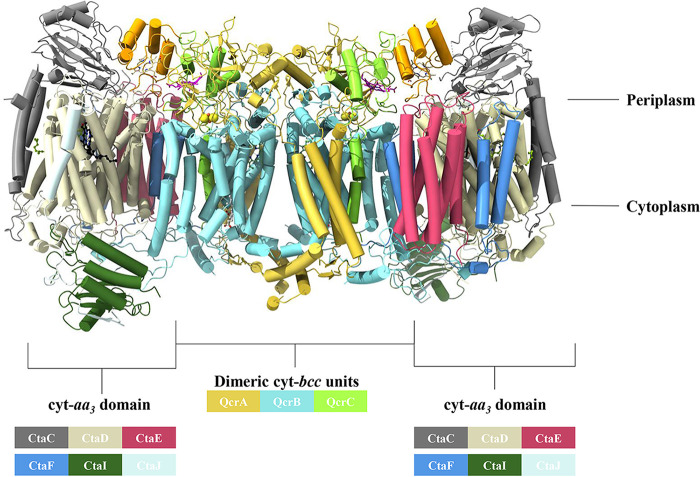
The resolved M. tuberculosis cyt-bcc:aa3 structure represents a dimer, with a C2 symmetry, consisting of the cyt-bcc and cyt-aa3 domains (Fig. 4; see also Movie S1 in the supplemental material), with the core formed by the dimeric cyt-bcc units and the two cyt-aa3 domains flanked at the outsides of the supercomplex. The structures of the three individual M. tuberculosis cyt-bcc subunits QcrC, QcrA, and QcrB are presented in Fig. S2A and include the cofactors heme bH and bL (QcrB), the iron-sulfur cluster (QcrA), and the hemes cI and cII of subunit QcrC. Figure S2B and C shows the determined structures of the M. tuberculosis cyt-bcc:aa3 unit with the six subunits CtaC, CtaD, CtaE, CtaF, CtaI, and CtaJ. The cofactors of the catalytically active subunit CtaD (heme a, heme a3) could be clearly assigned (see Fig. S3), while the density for CuB and CuC in CtaD and CuA in CtaC could not be determined.
FIG 4.
Dimeric cryo-EM structure of the resolved M. tuberculosis cyt-bcc:aa3 oxidase. The cyt-bcc subunits in the structure consist of QcrC (green and orange), QcrA (gold), and QcrB (cyan). The cyt-aa3 subunits constitute CtaC (gray), CtaJ (light blue), CtaD (cream), CtaE (magenta), CtaF (blue), and CtaI (dark green).
The recent deposition of the analogous respiratory complex from Corynebacterium glutamicum (PDB 7QHO) (15) allowed for the comparison of the respiratory complexes between C. glutamicum and M. tuberculosis, which are both classified under the Actinomycetota phylum. The overall architectures between the two supercomplexes are similar, with both complexes sharing QcrA (root mean square deviation [RMSD] = 1.185 Å), QcrB (RMSD = 0.689 Å), QcrC (RMSD = 1.159 Å), CtaC (RMSD = 0.489 Å), CtaD (RMSD = 0.512 Å), CtaE (RMSD = 0.476 Å), and CtaF (RMSD = 0.669 Å) subunits with their respective RMSD values annotated. Notably, the superoxide dismutase observed in mycobacteria is absent and instead replaced with a soluble ThiX domain. The C. glutamicum supercomplex consists of additional subunits such as P29, P20, P12, P8, and P6 (15).
Architecture of and electron transfer within the M. tuberculosis cyt-bcc domain.
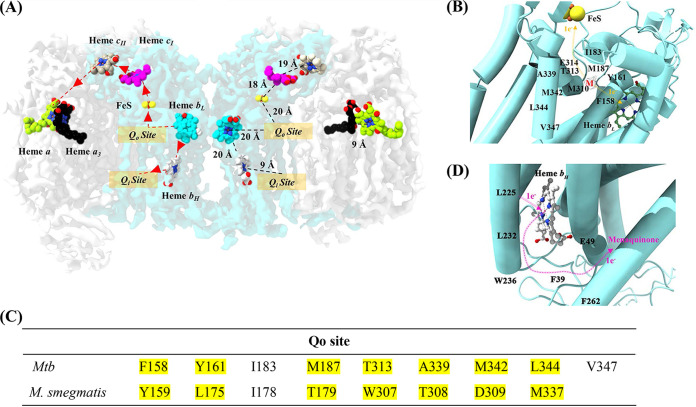
The oxidoreductase enzyme begins its cascade of events by binding of the electron donor menaquinol in the QcrB subunit of the M. tuberculosis cyt-bcc unit. The electron transfer pathway is initiated with the binding of menaquinol to the Qo site in QcrB (Fig. 5A and B). Although the density obtained for the bound menaquinone tail was weak, the density for the head of the molecule was sufficient to highlight its binding within the Qo site. The first electron from menaquinol can be transferred to the FeS cluster, which is ~20 Å away from the Qo site of the presented M. tuberculosis QcrB structure (Fig. 5A and B). As shown in Fig. 5C, the M. tuberculosis residues F158, Y161, I183, M187, T313, A339, M342, L344, and V347 interacting with menaquinol differ from the ones of the M. smegmatis enzyme (Y158, L175, I178, T179, W307, T308, D309, and M337). Interestingly, substitution of the M. tuberculosis residue T313 to an alanine like in its M. smegmatis counterpart (T313A mutation) was associated with a high level of resistance to Q203 (6). The binding pocket of Q203 was recently revealed by Zhou et al. (7) with residues H375 of subunit QcrA and T313, as well as E314 of subunit QcrB mainly interacting with Q203. The strong anchoring interactions between the inhibitor at this site prevent menaquinol from binding, thus preventing electron transfer to both heme bL and Fe-S, leading to the arrest of electron transfer within the complex.
FIG 5.
Electron transfer in resolved M. tuberculosis cyt-bcc:aa3. (A) Electron transfer pathway. The left side of the dimer describes the electron transfer pathway, while the right side depicts the distances between the reaction centers. The binding of menaquinol to the Qo site in QcrB initiates the electron transfer process. The first electron from menaquinol is transferred to FeS, which is ~20 Å from the Qo site. The second electron from menaquinol is transferred to heme bL, which is ~20 Å apart from the Qo site and subsequently transferred to heme bH. The electron from heme bH is used to reduce a menaquinone bound at the Qo site. FeS transfers its electron to the cytochrome c domain present in QcrC, which transfers the electron further to the cyt-aa3 domain. The electrons are carried in the following order: from FeS to cyt-cI, cyt-cII, and CuA. The final electron transfer pathway occurs from CuA to heme a followed by CuB and heme a3. The electrons at heme a3 are used to reduce O2 to H2O. (B) Electron transfer in the Qo site. The Qo binding site with side chains interacting with menaquinol (M). The electron transfer pathway is illustrated with orange arrows. The binding of menaquinol results in the transfer of one electron to heme bL and the other to FeS. (C) Amino acid residues that interact with menaquinol at the Qo site revealed in the M. tuberculosis cyt-bcc:aa3 and the M. smegmatis cyt-bcc:aa3 structure. (D) Electron transfer in the QN site. The Qi binding site with side chains is predicted to interact with menaquinone. The electron transfer pathway is illustrated by pink arrows. The electron, which originated from heme bL, is transferred to heme bH before being transferred to menaquinone in the Qi site. The [2Fe-2S] and heme groups are shown as balls and sticks and are labeled in the figures. Mtb, M. tuberculosis.
The second electron from menaquinol can be translocated to the low spin heme bL of the M. tuberculosis QcrB, which is ~20 Å apart from the Qo site (Fig. 5D). The electron from heme bL is then transferred to the high spin heme bH to reduce a menaquinone bound at the Qi site (Fig. 5D). The heme bL–heme bH distance (~20 Å) is shorter compared to the heme bL–FeS distance (~26 Å). The amino acids F39, E49, L225, L232 W236, and F262 are involved in the interaction with menaquinone, which becomes reduced to menaquinol in the Qi site. While residues F34 and E44 of the M. smegmatis menaquinone binding site are similar, the latter differs by the two additional residues L47 and Y48, as well as the different amino acids L227, W231, F257, and S261.
Switching the cyt-cIcII head transfers electrons between the bcc and aa3 domains.
The cyt-cIcII head domains of both QcrCs, the counterpart of the soluble mitochondrial subunit c, are located between the cyt-bcc and cyt-aa3 domains in the presented M. tuberculosis cyt-bcc:aa3 dimer structure (Fig. 3 and 6), reflecting the so-called “closed state.” In this closed state, the FeS cluster of QcrC is in ~18 Å proximity to heme cI, while the second heme in cyt-cII is ~19 Å distant from cyt-cI (Fig. 6). Helix α3 of the cyt-cII with its residues 70DTS72 comes in close proximity (~10 Å) to the loop regions 205GTS207 from CtaC, as well as the cyt-cII loop 111MA117 and the CtaC loop 278GTY280 (~3.8 Å), providing structural bridges for the smooth electron transfer to CuA inside subunit CtaC (Fig. 6). In comparison, the recently described M. smegmatis cyt-bcc:aa3 dimer structure revealed a closed state, whereby the second cyt-cIcII head domain was in a so-called open conformation, in which the cyt-cIcII head domain interacts only with the cyt-aa3 part (10). As a consequence, the FeS and cyt-cI distance increases to about 48 Å and prevents electron transfer between the cyt-bcc and cyt-aa3 domains (10). Switching from an open to a closed state is archived by the linker region of the QcrC transmembrane helix and the cyt-cIcII head domain. The M. tuberculosis linker 237VATEARQPGGYLLGGF252 includes four glycine residues and one proline residue (see Fig. S2A), proposed to provide the flexibility for this region. Figure 6B shows the differences in amino acid composition of the M. tuberculosis linker compared to the M. smegmatis one.
FIG 6.
In silico strategy to identify M. tuberculosis QcrC inhibitors. (A) Identification of region of interest in the QcrC functional domain. (B) Analysis of linker residue differences in QcrC between M. tuberculosis and M. smegmatis. Mtb, M. tuberculosis.
Structural, mechanistic and assembly elements of the M. tuberculosis cyt-aa3 units.
The final electron transfer pathway occurs from CuA to heme a, CuB, and heme a3 of the CtaC and CtaD subunits of the M. tuberculosis cyt-aa3 domains (Fig. 5A). The electrons at heme a3 are used for the reduction of O2 to H2O. The M. tuberculosis CtaD structure superimposes well with the M. smegmatis structure with an RMSD of 0.56 Å.
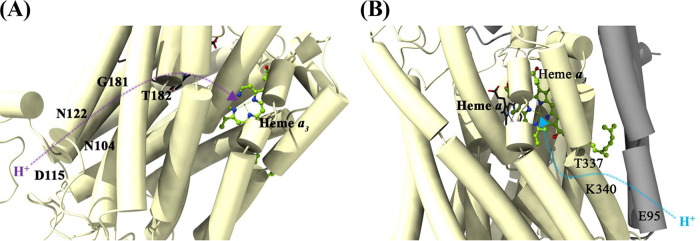
The reduction of O2 inside the M. tuberculosis CtaD requires an uptake of eight protons from the negative side of the membrane. The K-proton pathway aids in the transfer of “substrate” protons to the catalytic site before O2 binds to the catalytic Fea3/CuB site. The D-proton pathway aids in both the pumping of protons across the membrane and in transferring “substrate” protons to O2. The amino acids N104, D115, N122, G181, T182, and E266 of subunit CtaD constitute the D pathway within the M. tuberculosis cyt-aa3 domain (Fig. 7A), whereas the amino acids E95, T337, and K340 of subunit CtaC and CtaD, respectively, constitute the K pathway (Fig. 7B). The importance of this pathway is highlighted in the study, wherein the disruption of this pathway leads to growth impairment in M. tuberculosis (16).
FIG 7.
D- and K-proton pathways in M. tuberculosis cyt-bcc:aa3. (A) The residues N104, D115, N122, G181, T182, and E266 of subunit CtaD constitute the D pathway. (B) Amino acids E95 (CtaC), T337 (CtaD), and K340 (CtaD) are assigned to the K pathway. The proton transfer direction and channels are shown in purple (D pathway) and blue (K pathway).
The oxygen diffusion pathway in any mycobacterial cytochrome aa3 has not been described thus far. Here, a prediction of the oxygen diffusion pathway within the M. tuberculosis CtaD is made based on the observations seen for the O2 pathway in the Thermus thermophilus cytochrome c oxidase (17). The proposed oxygen diffusion pathway to M. tuberculosis heme a3 has a Y-shaped channel with two entry points in subunit CtaD (see Fig. S4). The first entrance is flanked by amino acids A223 and L219, while the second entrance is flanked by residues F543 and L554. The two channels converge at F275 to continue to I270 and finally reach M. tuberculosis heme a3.
Finally, M. tuberculosis CtaE, CtaF, CtaI, and CtaJ are described to function as assembly factors (see Fig. S2C). They are structurally similar to the M. smegmatis ones with an RMSD of 0.99 Å. CtaE consists of five transmembrane spanning α-helices (see Fig. S2C), while the 14.9-kDa M. tuberculosis CtaF is composed of four transmembrane spanning α-helices (α1, α2, α4, and α5) and the short helix α3, connected by random coiled elements (see Fig. S2C). The M. tuberculosiss CtaI subunit is located at the cytoplasmic site (Fig. 4), composed of an N-terminal tail, four α-helices, and three β-sheets (see Fig. S2C), while subunit CtaJ shows a transmembrane spanning α-helix, being linked to a terminal β-sheet via a long disordered region (Fig. 4; see also Fig. S2C).
Identification of a novel cyt-cI target and inhibitor.
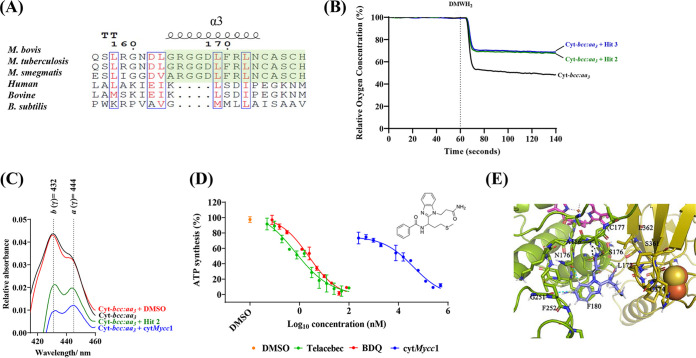
The CtaC and cyt-cIcII junction serves as an important interface orchestrating the transfer of electrons from the cyt-bcc to cyt-aa3. The importance of subunit CtaC for growth (11) and the critical ability of cyt-cIcII head to switch between open / closed states, as observed in M. smegmatis cytbcc:aa3 (10), attracted attention to this site in M. tuberculosis. These distinct mycobacterial traits motivated us to pursue this cyt-cIcII interface for structure based ligand discovery in M. tuberculosis. Ligands that target this domain could potentially disturb interactions between the cyt-bcc and cyt-aa3, disrupt the process of electron transfer to the catalytic center and thereby inhibit the oxidase functioning required for growth (11). While targeting the cyt-cII and CtaC interacting regions 70DTS72-205GTS207 and the loops 111MA117, as well a 278GTY280 (Fig. 6A), may cause steric clashes with any compound during the switching mode of an open to a closed state, we focused on helix α3 of the subunit cyt-cI (Fig. 6A), since compound binding to that site would interrupt electron transfer between the FeS of QcrB and heme cI of the cyt-cyt-cIcII head and, thereby, the flow of electrons to the catalytic center in the M. tuberculosis cyt-aa3 domain. Importantly, the sequence of helix α3 is unique to mycobacteria and does not exist in human or bovine mitochondrial or other bacterial cytochrome oxidase counterparts (Fig. 8A). Targeting this region may avoid undesirable interactions with potential enzymatic counterparts in the human host and evades off-target toxicity.
FIG 8.
(A) Epitope sequence alignment between M. bovis, M. tuberculosis, M. smegmatis, humans, bovines, and B. subtilis. The region of interest is highlighted in green and found to be unique to mycobacteria. (B) Characterization of in silico screen hits 2 (green line) and 3 (blue line) in oxygen consumption assay with purified recombinant M. tuberculosis cyt-bcc:aa3 (black line). In the presence of 2,3-dimethyl-[1,4]naphthohydroquinone, the supercomplex reduced oxygen levels by 50%, while hits 2 and 3 decreased respiration of the recombinant M. tuberculosis cyt-bcc:aa3. (C) Effect of hits on electron transfer in the recombinant M. tuberculosis cyt-bcc:aa3. cytMycc1 was incubated with recombinant M. tuberculosis cyt-bcc:aa3 prior to oxidation with potassium ferricyanide. The conditions tested were compound free (black), DMSO (red), cytMycc1 (blue), or hit 2 (green). The heme a (444 nm) and heme b (432 nm) wavelengths showed drastic differences. All experiments were repeated at least once. (D) Effect of cytMycc1 on ATP synthesis of M. smegmatis IMVs. cytMycc1 (blue circles) had a starting concentration of 500 μM and was serially diluted by 2-fold. The starting concentration of Telacebec (red circles) and bedaquiline (BDQ; green circles) was 100 and 80 nM, respectively. The data points are expressed as means ± the standard deviations of triplicates from a representative experiment. The experiment was performed in triplicates and repeated more than once. (E) Binding pose of cytMycc1: the amide (NH2) group on N-propionamide form H-bonding interactions (black dotted lines) with main chain carbonyl (CO) atoms of residues S176 and C177 and main chain NH atoms of A186 of QcrC. Benzimidazole has strong aromatic interactions with amino acids F180 and close contacts with N176 and G251. The propyl-thio-methyl fragment was in vicinity of QcrA (L362, S361, and C374) residues adjacent to the Fe-S cluster.
A virtual screening campaign (see Fig. S5) to identify novel chemical entities that could bind to the helix α3 residues was carried out. Our primary glide high-throughput virtual screening with 2 million compounds from the virtual enamine library (18) enabled us to segregate nonbinders from putative binders. Next, the ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties of binder(s) subset poses were evaluated using the Qikprop tool to further exclude ligands with unfavorable solubility, clogP, and Human Ether-a-go-go-Related Gene (HERG) binding predictions. Only ligands (4,120) with a docking score below −5 kcal/mol and predicted to be non-hERG K+ inhibitors (>−5 kcal/mol) were progressed to the next standard precision (SP) docking run. Ligand poses (n = 402) with an SP glide score of <−5 kcal/mol were taken further into the extra precision (XP) docking run (see the supplemental material). Finally, four hits that had favorable interactions with the amino acid residues surrounding the target region were selected for validation.
First, the effect of the four hits was tested by O2 comsumption of the enzyme using DMNQ oxidoreductase activity. Figure 8B demonstrates a clear drop in O2 comsumption of M. tuberculosis cyt-bcc:aa3 in the presence of hits 2 and 3 after the addition of the electron donor DMNQH2. Interestingly, the reduction profile of both hits is comparable to the one observed for the enzyme in the absence (addition of buffer) of DMNQH2 (Fig. 2C), indicating their potency as an enzyme inhibitor.
To further test whether the drop in O2 reduction within the heme a catalytic center is in line with the interruption of electron transfer to the O2 reduction center, difference spectra of the supercomplex were measured with or without inhibitors (Fig. 8C). The difference spectrum (415 to 460 nm) of M. tuberculosis cyt-bcc:aa3 energized with using 100 mM sodium dithionite (Na2S2O6) demonstrated electron transfer, followed by the reduction of the cytochrome bcc:aa3 hemes (b at 432 nm and a at 444). In comparison, the difference spectrum of M. tuberculosis cyt-bcc:aa3 in the presence of sodium dithionite and hit 2 or hit 3 (N-(1-(1-(3-amino-3-oxopropyl)-1H-benzo[d]imidazol-2-yl)-3-(methylthio)propyl)benzamide, here called cytMycc1; Fig. 8C) displayed a drastic reduction of the peaks corresponding to the two hemes, confirming compound binding and inhibition of the electron transfer within the cytochrome M. tuberculosis cytochrome supercomplex. As a control, addition of dimethyl sulfoxide (DMSO; solvent) revealed no major change in the heme difference spectrum (Fig. 8C). The reduction of the heme a intensity supports the computational docking model where the disruption of the electron flow at the FeS and cyt-cIcII domain junction would inhibit electron flow at the O2 reduction center. The reduction in intensity rather than the absence of a peak for heme a could imply that the compound has low binding affinity at the predicted epitope. The drop of heme b which was not initially predicted, could possibly be due to a feedback mechanism, by which the FeS cannot be regenerated to an electron acceptor for the next electron coming from the sodium dithionite/menaquinol, as the electron flow to the heme cI is interrupted. This may affect the electron transfer from menaquinol to the Qi site, including heme bL and bH. At the moment, these scenarios can only be speculated and remain to be determined by mutational and/or cryo-EM efforts of an cytMycc1-M. tuberculosis cyt-bcc:aa3 complex in future studies.
The ability of cytMycc1 to disrupt oxygen respiration in the complex was investigated further by adopting a dose-response strategy (see Fig. S7 in the supplemental material). In this experiment, the compound was serially diluted 2-fold from a starting concentration of 250 μM and subsequently incubated with the enzyme complex for 1 h before determining the effect of the compound on the rate of respiration. A graded dose-response was observed (see Fig. S7) with a 50% inhibitory concentration (IC50) of 59 ± 3.7 μM. This further lends support to the ability of the hit compound cytMycc1 to target the M. tuberculosis cyt-bcc:aa3.
Since switching of the opened and closed states of the cyt-cIcII head domain has only been demonstrated in the M. smegmatis cyt-bcc:aa3 (10), we investigated whether inhibition of cyt-bcc-aa3 by cytMycc1 may also reduce the process of oxidative phosphorylation within M. smegmatis inside outside vesicles (IMVs). As shown in Fig. 8D, cytMycc1 inhibited NADH-driven ATP synthesis of IMVs of parental M. smegmatis with an IC50 of 39.5 ± 0.2 μM, which is in the range of the IC50 value of the recombinant M. tuberculosis cyt-bcc:aa3 described above. In comparison, cytMycc1 did not show a major effect on ATP formation of Escherichia coli IMVs, underlying its specificity, while the E. coli cytochrome bO3 inhibitor potassium cyanide (KCN) (19) significantly reduced ATP synthesis levels by ~70% (see Fig. S8). Since E. coli IMVs contain also the NADH-dehydrogenase, succinate-dehydrogenases, cyt-bd oxidase, and the F-ATP synthase, the data underline also that cytMycc1 does not nonspecifically inhibit the complexes of the entire E. coli oxidative phosphorylation pathway.
While cytMycc1 inhibited ATP formation on IMVs, no effect was observed in mycobacterial growth using M. smegmatis mc2 155 or M. bovis BCG, or in a whole-cell ATP assay (see Fig. S6). This may indicate that the compound does not reach the required concentration at the plasma membrane to exert antimicrobial activity. This is a well-known limitation of target-based approaches, as described before in the context of pantothenate kinase inhibitors, including problems such as limited cell envelope penetration, the presence of efflux pumps, and intrabacterial metabolism (20).
cytMycc1 interactions with mycobacterial subunits cyt-cI and QcrA.
cytMyccI showed good docking XP score of −6.025 kcal/mol to subunit cyt-cI. The amide (NH2) group on N-propionamide form H-bonding interactions with main chain carbonyl (CO) atoms of residues S176 and C177 and the main-chain NH atoms of A186 of cyt-cI (Fig. 8E). The 1H-benzo[d]imidazol-2-yl fragment was involved in strong aromatic π-π interactions with F180, as well as van der Waals contacts with N176 and G251 (Fig. 8E, blue hashed lines). The benzamide fragment was seated toward L172, whereas the methyl-thio-propyl fragment was in close vicinity to residues L362, S361, P373, and C374-C358 residues lining the Fe-S cluster of subunit QcrA (Fig. 8E). Taken together, cytMycc1 with its interactions to key residues such as S176 and C177 being in close proximity to residue H178, which is ligated to heme cI, could potentially hinder the electron transfer between the cyt-bcc and cyt-aa3 domains and reduce cytochrome oxidase activity.
Conclusions.
By establishing an expression system, the enzymatically active M. tuberculosis cyt-bcc:aa3 could be produced and purified as a monodispersed enzyme complex, a prerequisite for the determination of the first entire M. tuberculosis cyt-bcc:aa3 supercomplex structure. The resolved dimeric complex provides insights into the electron cascade from the substrate menaquinol via FeS, the cyt-cIcII head to the catalytic center within subunit CtaD, as well as the electron transfer of the second electron of the substrate for the regeneration of menaquinone in the Qi site. The proton pathways responsible for O2 reduction and proton motive force, as well as oxygen entrance to the catalytic site, were discussed. So far, inhibitors targeting the mycobacterial cyt-bcc:aa3 focused on the inhibition of the menaquinol binding site (5, 6). Insights into the closed confirmation of the M. tuberculosis cyt-cIcII head relative to subunit CtaC and the unique mycobacterial cyt-cI helix α3 paved the way for the identification of a new inhibitor target within the supercomplex and the novel inhibitor cytMycc1, which targets the mycobacterial cyt-cI and QcrA interface of cyt-bcc:aa3 and affects electron transfer within the enzyme complex and finally, reduces ATP formation of the oxidative phosphorylation pathway within mycobacterial IMVs. The data presented underline that interrupting electron transfer within the mycobacterial supercomplex may open the door for novel mycobacterial cyt-bcc:aa3 inhibitors.
MATERIALS AND METHODS
Generation of M. bovis BCG with genomic cyt-bcc:aa3-FLAG/6×HIS tag.
The mycobacterial shuttle vector pKM444 was first electroporated into wild-type (WT) M. bovis BCG. The resulting M. bovis BCG::pKM444 strain was selected on 7H10 complemented with oleic-acid-dextrose-catalase (OADC) plates, supplemented with 50 μg/mL kanamycin. Colonies were expended and grown to an optical density at 600 nm (OD600) of 0.5 before induction with 500 ng/mL anhydrotetracycline for 24 h to express the Che9c phage RecT annealase and the Bxb1 phage integrase. The induced strain was washed in 0.05% Tween 80 at room temperature three times and finally resuspended to an OD600 of 100, before being coelectroporated with 200 ng of pKM 491 and 1 μg of targeting oligonucleotide 5′-CGA ACA ACG TGC CCT TGC CGC ACT GCG CGA ACA CCA GGA CAG CAT CAT GGG TTC GCC AGA CGG CGA GCA CGG TTT GTC TGG TCA ACC ACC GCG GTC TCA GTG GTG TAC GGT ACA AAC CTG ACC CGG CGA CGA CCC GGG TCG GCA CGA CCC GGG AAG GAA CCG GGC AAA TCA AGC ACA GCC CGG CGA CGA CC-3′. The electroporated cells were recovered overnight in 7H9-albumin-dextrose (ADS) culture broth medium. The recombinant strain was selected on 7H10-OADC plates, supplemented with 50 μg/mL hygromycin. The strain was validated by PCR with the forward primer 5′-CAC CAT TGA TGA CTC GAG TCT AGA GCA TG-3′ and the reverse primer 5′-ATG ATG GTG GTG GTG GTG GTG GTG-3′. The expression of the FLAG tag was validated by Western blotting.
Western blot validation of genomic FLAG tag.
First, 1 mL of the culture strain (OD600 = 1) strain, containing the FLAG genomic tag, was sonicated for 1 min at 30% power using Bandelin Sonopuls (Bandelin, Berlin, Germany) for five cycles. The sonicated sample was centrifuged at 12,000 rpm. The supernatant was applied to a 12% SDS gel and transferred to a nitrocellulose membrane using a semidry transfer apparatus according to the manufacturer’s protocols (Carl Roth, Karlsruhe, Germany). After blocking with 3% gelatin in a TBSN-Tween buffer (20 mM Tris-HCl [pH 7.5], 500 mM NaCl, 0.02% NaN3, and 0.05% Tween 20) for 1 h at room temperature, the membrane was washed with 1% gelatin in TBSN-Tween for 3 × 10 min. Afterward, the membranes were incubated with a rabbit anti-FLAG HRP-conjugated antibodies against the FLAG tag (Abcam, Cambridge, United Kingdom) diluted (1:2,000) with 1% gelatin TBSN-Tween 20 for 1 h at room temperature, followed by three washing steps (15 min) with TBSN-Tween 20 buffer. The antibodies were detected using SuperSignal West Pico Plus chemiluminescent substrate according to the manufacturer’s protocol (Thermo Fisher Scientific, Waltham, MA).
Purification of M. tuberculosis cyt-bcc:aa3.
The strain containing the genomic tag was grown in 7H9-ADS-glycerol medium until reaching an OD600 of 2.0 to 2.5. Cell pellets were harvested and stored in −80°C. Then, 10 g of cell pellets was dissolved in 100 mL of buffer A (20 mM MOPS [pH 7.4], 100 mM NaCl, 2 mM Pefabloc, 1 mM PMSF, 10% glycerol) and briefly sonicated in ice to ensure complete resuspension. Cell lysis was achieved by three passes through a microfluidizer (Microfluidics, Newton, MA) at 4°C and 1.2E+8 Pa. The lysate was centrifuged at 12,000 rpm for 20 min to remove cell debris and unlysed cells. The resulting supernatant was centrifuged at 36,900 rpm for 1 h in a P50AT2 rotor (Hitachi Himac Ultracentrifuge; Eppendorf Himac Technologies Co., Ltd., Japan). The membrane pellets from the previous step were resuspended in buffer B (20 mM MOPS [pH 7.4], 100 mM NaCl, 2 mM Pefabloc, 1 mM PMSF, 1% [wt/vol] DDM, 5% glycerol). The mixture was stirred for 1 h at 4°C on a rotating wheel. After solubilization with the detergent, the mixture was centrifuged at 18,000 rpm for 30 min at 4°C. The supernatant was allowed to equilibrate with 1 mL of FLAG beads for 1 h; the resin was then washed with buffer C (20 mM MOPS [pH 7.4], 100 mM NaCl, 2 mM Pefabloc, 1 mM PMSF, 0.1% [wt/vol] DDM). The protein was eluted with buffer D (20 mM MOPS [pH 7.4], 100 mM NaCl, 2 mM Pefabloc, 1 mM PMSF, 0.1% [vol/wt] DDM, 100 μg/mL). Protein samples that were collected during the course of the experiment were analyzed using 12% SDS-PAGE.
Heme absorbance spectra of purified M. tuberculosis cyt-bcc:aa3.
The spectra were analyzed with an Amersham Biosciences Ultrospec 2100 Pro-UV-Visible absorption spectroscopy (Amersham, Piscataway, NJ). The spectra were recorded from wavelengths of 400 to 700 nm. The purified M. tuberculosis cyt bcc:aa3 at a concentration of 1 mg/mL was oxidized with 100 μM potassium ferricyanide. The oxidized UV spectra were subsequently recorded. The sample was reduced using 100 mM sodium dithionite. The reduced UV spectrum was recorded promptly. The difference spectra were then obtained by subtracting the absorbance value of the reduced state from that of the oxidized state.
2,3-Dimethyl-1,4-naphthoquinol oxygen consumption assay.
DMNQ was purchased from Enamine, Cincinnati, OH. Next, 20 mM DMNQ was prepared in 1 mL of ethanol containing 6 mM HCl. The DMNQ solution was reduced with a few grains of sodium borohydride (NaBH4) in an ice bath. Then, 10 μL of 12 N HCl was used to quench the reaction. This reaction resulted in the formation of DMNQH2.
The oxygen consumption assay was performed based on published protocols (7, 8). Briefly, the purified M. tuberculosis cyt-bcc:aa3 was resuspended in 500 μL of reaction buffer (20 mM MOPS [pH 7.4], 100 mM NaCl, 0.01% DDM) to a final concentration of 65 nM. Next, 5 μL of DMNQH2 was added to the mixture to yield a final concentration of 25 μM. This initiated respiration within the supercomplex, which was monitored by a Clark-type oxygen electrode (Oxytherm+, Hansatech, Pentney, United Kingdom). The oxygen consumption curve was plotted using GraphPad Prime 8.0 software (21).
Cryo-EM.
The purified M. tuberculosis cyt-bcc:aa3 was concentrated to 9 mg/mL. Portions (4 μL) of the sample were applied to glow-discharged Quantifoil R1.2/1.3 holey carbon grids. The grids were blotted for 2 s at 100% humidity and 4°C and plunge-frozen using a FEI Vitrobot Mark IV (Thermo Fisher Scientific). Images were taken using an FEI Titan Krios electron microscope operating at 300 kV with a K2 Summit detector (Gatan, Pleasanton, CA) at a magnification of ×130,000. Images were recorded in super-resolution mode. Automated single-particle data acquisition was performed using EPU software. A defocus range of 1.0 to 2.0 μm was implemented. Movies were collected at 40 frames per stack with an exposure time of 9 s. The total dose was set at 40 e–/Å2. Motion correction was performed using MotionCor2 and CTF refinement was performed using CTFFind4 (22, 23). The initial data processing was performed on CryoSPARC and RELION v3.1 (24, 25). Structure refinement was done using COOT and Phenix software (26, 27).
Data availability.
All relevant data are available from the authors. Structural data that supports the findings of this study are openly available from the Protein Data Bank (https://www.rcsb.org; PDB ID 8HCR) and the EM Data Bank (https://www.ebi.ac.uk/emdb/; EMDB ID EMD-34664).
ACKNOWLEDGMENTS
This study was supported by National Research Foundation (NRF) Singapore, NRF Competitive Research Program (CRP), grants NRF-CRP18-2017-01 and NRF-CRP27-2021-0002. V.M. acknowledges a NTU Research Scholarship. C.-F.W.’s Ph.D. scholarship was funded by an NRF CRP grant (award NRF-CRP18-2017-01). We also acknowledge the use of the EM facility at the NTU Institute of Structural Biology.
V.M., C.-F.W., A.H., K.P., and G.G. designed the experiments. V.M., C.-F.W., A.H., K.P., and G.G. conducted the formal analysis. V.M., C.-F.W., and A.H. performed investigations. V.M., A.H., K.P., and G.G. wrote the original draft. All authors contributed to the writing (both review and editing). K.P. and G.G. obtained funding.
We declare no competing interests.
Footnotes
Supplemental material is available online only.
Contributor Information
Kevin Pethe, Email: kevin.pethe@ntu.edu.sg.
Gerhard Grüber, Email: ggrueber@ntu.edu.sg.
REFERENCES
- 1.Rao SPS, Alonso S, Rand L, Dick T, Pethe K. 2008. The proton motive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci USA 105:11945–11950. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koul A, Vranckx L, Dendouga N, Balemans W, Van den Wyngaert I, Vergauwen K, Göhlmann HWH, Willebrords R, Poncelet A, Guillemont J, Bald D, Andries K. 2008. Diarylquinolines are bactericidal for dormant mycobacteria as a result of disturbed ATP homeostasis. J Biol Chem 283:25273–25280. doi: 10.1074/jbc.M803899200. [DOI] [PubMed] [Google Scholar]
- 3.Cook GM, Hards K, Dunn E, Heikal A, Nakatani Y, Greening C, Crick DC, Fontes FL, Pethe K, Hasenoehrl E, Berney M. 2017. Oxidative phosphorylation as a target space for tuberculosis: success, caution, and future directions. Microbiol Spectr 5:5.3.14. doi: 10.1128/microbiolspec.TBTB2-0014-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalia NP, Hasenoehrl EJ, Ab Rahman NB, Koh VH, Ang MLT, Sajorda DR, Hards K, Grüber G, Alonso S, Cook GM, Berney M, Pethe K. 2017. Exploiting the synthetic lethality between terminal respiratory oxidases to kill Mycobacterium tuberculosis and clear host infection. Proc Natl Acad Sci USA 114:7426–7431. doi: 10.1073/pnas.1706139114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang J, Wang B, Wu T, Wan J, Tu Z, Njire M, Wan B, Franzblauc SG, Zhang T, Lu X, Ding K. 2015. Design, synthesis, and biological evaluation of pyrazolo[1,5-a]pyridine-3-carboxamides as novel antitubercular agents. ACS Med Chem Lett 6:814–818. doi: 10.1021/acsmedchemlett.5b00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pethe K, Bifani P, Jang J, Kang S, Park S, Ahn S, Jiricek J, Jung J, Jeon HK, Cechetto J, Christophe T, Lee H, Kempf M, Jackson M, Lenaerts AJ, Pham H, Jones V, Seo MJ, Kim YM, Seo M, Seo JJ, Park D, Ko Y, Choi I, Kim R, Kim SY, Lim S, Yim S-A, Nam J, Kang H, Kwon H, et al. 2013. Discovery of Q203, a potent clinical candidate for the treatment of tuberculosis. Nat Med 19:1157–1160. doi: 10.1038/nm.3262. [DOI] [PubMed] [Google Scholar]
- 7.Zhou S, Wang W, Zhou X, Zhang Y, Lai Y, Tang Y, Xu J, Li D, Lin J, Yang X, Ran T, Chen H, Guddat LW, Wang Q, Gao Y, Rao Z, Gong H. 2021. Structure of Mycobacterium tuberculosis cytochrome bcc in complex with Q203 and TB47, two anti-TB drug candidates. Elife 10:e69418. doi: 10.7554/eLife.69418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanofsky DJ, Di Trani JM, Król S, Abdelaziz R, Bueler SA, Imming P, Brzezinski P, Rubinstein JL. 2021. Structure of mycobacterial CIII2CIV2 respiratory supercomplex bound to the tuberculosis drug candidate Telacebec (Q203). Elife 10:e71959. doi: 10.7554/eLife.71959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong H, Li J, Xu A, Tang Y, Ji W, Gao R, Wang S, Yu L, Tian C, Li J, Yen H-Y, Lam SM, Shui G, Yang X, Sun Y, Li X, Jia M, Yang C, Jiang B, Lou Z, Robinson CV, Wong L-L, Guddat LW, Sun F, Wang Q, Rao Z. 2018. An electron transfer path connects subunits of a mycobacterial respiratory supercomplex. Science 362:eaat8923. doi: 10.1126/science.aat8923. [DOI] [PubMed] [Google Scholar]
- 10.Wiseman B, Nitharwal RG, Fedotovskaya O, Schäfer J, Guo H, Kuang Q, Benlekbir S, Sjöstrand D, Ädelroth P, Rubinstein JL, Brzezinski P, Högbom M. 2018. Structure of a functional obligate complex III2IV2 respiratory supercomplex from Mycobacterium smegmatis. Nat Struct Mol Biol 25:1128–1136. doi: 10.1038/s41594-018-0160-3. [DOI] [PubMed] [Google Scholar]
- 11.Matsoso LG, Kana BD, Crellin PK, Lea-Smith DJ, Pelosi A, Powell D, Dawes SS, Rubin H, Coppel RL, Mizrahi V. 2005. Function of the cytochrome bc1:aa3 branch of the respiratory network in mycobacteria and network adaptation occurring in response to its disruption. J Bacteriol 187:6300–6308. doi: 10.1128/JB.187.18.6300-6308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saw WG, Wong CF, Dick T, Grüber G. 2020. Overexpression, purification, and enzymatic and microscopic characterization of recombinant mycobacterial F-ATP synthase. Biochem Biophys Res Commun 522:374–380. doi: 10.1016/j.bbrc.2019.11.098. [DOI] [PubMed] [Google Scholar]
- 13.Zhang AT, Montgomery MG, Leslie AGW, Cook GM, Walker JE. 2019. The structure of the catalytic domain of the ATP synthase from Mycobacterium smegmatis is a target for developing antitubercular drugs. Proc Natl Acad Sci USA 116:4206–4211. doi: 10.1073/pnas.1817615116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy KC, Nelson SJ, Nambi S, Papavinasasundaram K, Baer CE, Sassetti CM. 2018. ORBIT: a new paradigm for genetic engineering of mycobacterial chromosomes. mBio 9:e01467-18. doi: 10.1128/mBio.01467-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kao WC, Ortmann de Percin Northumberland C, Cheng TC, Ortiz J, Durand A, von Loeffelholz O, Schilling O, Biniossek ML, Klaholz BP, Hunte C. 2022. Structural basis for safe and efficient energy conversion in a respiratory supercomplex. Nat Commun 13:545. doi: 10.1038/s41467-022-28179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beites T, O’Brien K, Tiwari D, Engelhart CA, Walters S, Andrews J, Yang H-J, Sutphen ML, Weiner DM, Dayao EK, Zimmerman M, Prideaux B, Desai PV, Masquelin T, Via LE, Dartois V, Boshoff HI, Barry CE, Ehrt S, Schnappinger D. 2019. Plasticity of the Mycobacterium tuberculosis respiratory chain and its impact on tuberculosis drug development. Nat Commun 10:4970. doi: 10.1038/s41467-019-12956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu G, Zeng H, Zhang S, Juli J, Tai L, Zhang D, Pang X, Zhang Y, Lam SM, Zhu Y, Peng G, Michel H, Sun F. 2021. The unusual homodimer of a heme-copper terminal oxidase allows itself to utilize two electron donors. Angew Chem Int Ed Engl 60:13323–13330. doi: 10.1002/anie.202016785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin JJ, Shoichet BK. 2005. ZINC: a free database of commercially available compounds for virtual screening. J Chem Inf Model 45:177–182. doi: 10.1021/ci049714+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asseri AH, Godoy-Hernandez A, Goojani HG, Lill H, Sakamoto J, McMillan DGG, Bald D. 2021. Cardiolipin enhances the enzymatic activity of cytochrome bd and cytochrome bo3 solubilized in dodecyl-maltoside. Sci Rep 11:8006. doi: 10.1038/s41598-021-87354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barry CE, III, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. 2009. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 7:845–855. doi: 10.1038/nrmicro2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motulsky H, Christopoulos A. 2003. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. GraphPad Software, Inc, San Diego, CA. [Google Scholar]
- 22.Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, Agard DA. 2017. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohou A, Grigorieff N. 2015. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J Struct Biol 192:216–221. doi: 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. 2017. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods 14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 25.Zivanov J, Nakane T, Forsberg BO, Kimanius D, Hagen WJH, Lindahl E, Scheres SHW. 2018. New tools for automated high-resolution cryo-EM structure determination in RELION-3. Elife 7:e42166. doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noble M, Perrakis A. 2004. Model building and refinement. Acta Crystallographica Section D 60. [Google Scholar]
- 27.Liebschner D, Afonine PV, Baker ML, Bunkoczi G, Chen VB, Croll TI, Hintze B, Hung L-W, Jain S, McCoy AJ, Moriarty NW, Oeffner RD, Poon BK, Prisant MG, Read RJ, Richardson JS, Richardson DC, Sammito MD, Sobolev OV, Stockwell DH, Terwilliger TC, Urzhumtsev AG, Videau LL, Williams CJ, Adams PD. 2019. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr D Struct Biol 75:861–877. doi: 10.1107/S2059798319011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.01531-22-s0001.pdf, PDF file, 1.3 MB (1.3MB, pdf)
Supplemental material. Download aac.01531-22-s0002.mov, MOV file, 16.0 MB (16MB, mov)
Data Availability Statement
All relevant data are available from the authors. Structural data that supports the findings of this study are openly available from the Protein Data Bank (https://www.rcsb.org; PDB ID 8HCR) and the EM Data Bank (https://www.ebi.ac.uk/emdb/; EMDB ID EMD-34664).