ABSTRACT
Beta-hemolytic streptococci are common causes of bloodstream infection (BSI). There is emerging data regarding oral antibiotics for BSI but limited for beta-hemolytic streptococcal BSI. We conducted a retrospective study of adults with beta-hemolytic streptococcal BSI from a primary skin/soft tissue source from 2015 to 2020. Patients transitioned to oral antibiotics within 7 days of treatment initiation were compared to those who continued intravenous therapy, after propensity score matching. The primary outcome was 30-day treatment failure (composite of mortality, infection relapse, and hospital readmission). A prespecified 10% noninferiority margin was used for the primary outcome. We identified 66 matched pairs of patients treated with oral and intravenous antibiotics as definitive therapy. Based on an absolute difference in 30-day treatment failure of 13.6% (95% confidence interval 2.4 to 24.8%), the noninferiority of oral therapy was not confirmed (P = 0.741); on the contrary, the superiority of intravenous antibiotics is suggested by this difference. Acute kidney injury occurred in two patients who received intravenous treatment and zero who received oral therapy. No patients experienced deep vein thrombosis or other vascular complications related to treatment. In patients treated for beta-hemolytic streptococcal BSI, those who transitioned to oral antibiotics by day 7 showed higher rates of 30-day treatment failure than propensity-matched patients. This difference may have been driven by underdosing of oral therapy. Further investigation into optimal antibiotic choice, route, and dosing for definitive therapy of BSI is needed.
KEYWORDS: Beta-hemolytic Streptococcus, cellulitis, oral therapy, propensity score
INTRODUCTION
Bloodstream infection (BSI) is a common complication of skin and soft tissue infection (1). Beta-hemolytic streptococci are among the most common pathogens causing BSI, particularly among those with primary soft tissue infections (2, 3). Cellulitis complicated by BSI has been associated with higher rates of infection recurrence (4). Partially on account of this difference in outcomes, streptococcal BSI has traditionally been treated with intravenous (i.v.) antibiotic therapy. However, i.v. therapy has been associated with adverse events, including i.v. catheter-related complications (5, 6).
There are emerging data suggesting equivalence of clinical outcomes with the transition of i.v. to definitive oral antibiotic therapy in infectious syndromes traditionally treated with fully i.v. regimens, such as infective endocarditis and orthopedic infections (7, 8). Preliminary data have suggested similar outcomes between i.v. and oral therapy for patients with BSI with Enterobacterales (9–11). More limited data have shown similar findings for low-risk Staphylococcus aureus BSI (12, 13). While there is a paucity of data regarding the use of definitive oral antibiotics with streptococcal BSI, a recent survey showed a majority of surveyed infectious diseases physicians are completing treatment of beta-hemolytic streptococcal BSIs with oral agents (14).
In this study, we aimed to compare outcomes between i.v. and oral definitive therapy in patients with beta-hemolytic Streptococcus BSI from a skin or soft tissue source. We also aimed to assess factors in those with treatment failure, as well as analyze differences in complications from antibiotic therapy.
RESULTS
Patient characteristics.
Overall, 609 with beta-hemolytic streptococcal bloodstream infection were identified in the study period, 315 of which were eligible for inclusion. Among these, 96 were transitioned to oral antibiotic therapy within 7 days of treatment initiation. After propensity score estimation, 66 patient pairs from the i.v. and oral treatment groups were successfully matched. Baseline characteristics of the treatment groups before and after matching are presented in Table 1.
TABLE 1.
Baseline characteristics before and after propensity score matchinga
| i.v. (n = 219) | Oral (n = 96) | SMD | i.v. (n = 66) | Oral (n = 66) | SMD | |
|---|---|---|---|---|---|---|
| Characteristic | Before propensity score matching | After propensity score matching | ||||
| Age, yr | 70.6 (60.0–81.1) | 72.7 (61.8–84.7) | 0.127 | 74.5 (64.1–82.9) | 71.8 (63.3–84.6) | 0.088 |
| Mayo site | 0.413 | 0.266 | ||||
| Arizona/Florida | 23 (10.5%) | 23 (24.0%) | 11 (16.7%) | 17 (25.8%) | ||
| Rochester, MN | 79 (36.1%) | 22 (22.9%) | 15 (22.7%) | 17 (25.8%) | ||
| Midwest health system sites | 117 (53.4%) | 51 (53.1%) | 40 (60.6%) | 32 (48.5%) | ||
| Pitt bacteremia score | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.138 | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.147 |
| Charlson Comorbidity index | 4.0 (2.0–8.0) | 5.0 (2.0–9.0) | 0.092 | 5.0 (2.0–8.0) | 5.0 (2.0–9.8) | 0.083 |
| Infectious syndrome | 0.419 | 0.036 | ||||
| Cellulitis | 143 (65.3%) | 72 (75.0%) | 49 (74.2%) | 48 (72.7%) | ||
| Unknown source | 48 (21.9%) | 22 (22.9%) | 15 (22.7%) | 16 (24.2%) | ||
| Otherb | 28 (12.8%) | 2 (2.1%) | 2 (3.0%) | 2 (3.0%) | ||
| Preexisting long-term i.v. accessc | 12 (5.5%) | 0 (0.0%) | 0.341 | 0 (0.0%) | 0 (0.0%) | |
| Body mass index, kg/mc | 33.8 (27.9–43.9) | 32.4 (26.4–38.6) | 0.284 | 32.6 (28.9–37.2) | 32.1 (26.4–38.1) | 0.058 |
| ICU admission | 54 (24.7%) | 9 (9.4%) | 0.415 | 4 (6.1%) | 5 (7.6%) | 0.060 |
| Hospital length of stay ≥7 days | 124 (56.6%) | 17 (17.7%) | 0.880 | 18 (27.3%) | 16 (24.2%) | 0.069 |
| Infectious diseases consult | 176 (80.4%) | 42 (43.8%) | 0.815 | 39 (59.1%) | 33 (50.0%) | 0.183 |
| Malabsorptive disorderc | 6 (2.7%) | 0 (0.0%) | 0.237 | 0 (0.0%) | 0 (0.0%) | |
| Recent chemotherapy | 13 (5.9%) | 7 (7.3%) | 0.055 | 3 (4.5%) | 4 (6.1%) | 0.068 |
| Immunosuppression | 21 (9.6%) | 9 (9.4%) | 0.007 | 7 (10.6%) | 7 (10.6%) | 0.000 |
| Beta-lactam allergy | 34 (15.5%) | 23 (24.0%) | 0.213 | 10 (15.2%) | 10 (15.2%) | 0.000 |
| Streptococcus species | 0.253 | 0.158 | ||||
| S. pyogenes | 45 (20.5%) | 19 (19.8%) | 15 (22.7%) | 11 (16.7%) | ||
| S. agalactiae | 101 (46.1%) | 55 (57.3%) | 37 (56.1%) | 41 (62.1%) | ||
| S. dysgalactiae | 73 (33.3%) | 22 (22.9%) | 14 (21.2%) | 14 (21.2%) | ||
| Prosthetic device | 50 (22.8%) | 21 (21.9%) | 0.023 | 14 (21.2%) | 16 (24.2%) | 0.072 |
| Delayed admission | 13 (5.9%) | 4 (4.2%) | 0.081 | 2 (3.0%) | 2 (3.0%) | 0.000 |
| Propensity score | 0.13 (0.06–0.31) | 0.57 (0.34–0.72) | 1.429 | 0.38 (0.30–0.62) | 0.44 (0.30–0.71) | 0.171 |
Data are median (IQR) for continuous variables and N (%) for categorical variables. Delayed admission was defined as hospitalization on a different day than initial culture acquisition. ICU, intensive care unit; i.v., intravenous; SMD, standardized mean difference.
Other infectious syndromes included diabetic foot infection with osteomyelitis (17), necrotizing fasciitis (6), abscess (4), and pyomyositis (3). After propensity matching, all 4 other syndromes were diabetic foot infections with osteomyelitis.
As no patients in the oral therapy group had a preexisting long-term i.v. access or a malabsorptive disorder, these patients were excluded from data set before propensity score matching.
Among the propensity-matched patients, the most common Streptococcus species was S. agalactiae and the most common infectious syndrome was cellulitis. Slightly more than half (54.5%) received an infectious diseases consult. Most factors incorporated in the propensity score were balanced between the matched groups (Fig. S1 in the supplemental material). Only the treatment site showed residual imbalance based on a standardized difference greater than 0.25. When comparing the matched groups on baseline variables not incorporated in the propensity score, the i.v. group had slightly higher proportions with a history of cellulitis (19.7% versus 12.1%) but comparable rates of diabetes mellitus (51.5% versus 45.5%), chronic kidney disease (34.8% versus 31.8%), chronic extremity edema (39.4% versus 37.9%), chronic soft tissue ulceration (18.2% versus 21.2%), and sex (female 33.3% versus 31.8%). There were no reported cases of i.v. drug use in either group. Four patients received secondary cellulitis prophylaxis with penicillin following primary therapy: three in the i.v. group and one in the oral group. From 120 patients in the propensity-matched patients with follow-up negative blood cultures, 20 34.5% had a duration of bacteremia >2 days. The median duration of bacteremia was 2.0 (interquartile range [IQR], 2.0 to 3.0) days overall and for both treatment groups.
Antibiotic therapy.
Details of antibiotic therapy for the matched groups are presented in Table 2. The median total duration of antibiotic therapy was 16 days in the i.v. group and 15 days in the oral group from the first initiation of an active antibiotic. Ceftriaxone was the most common i.v. antibiotic while amoxicillin and amoxicillin-clavulanate were the most common oral antibiotics. Twelve patients in the i.v. group were transitioned to oral antibiotic therapy after more than 7 days of i.v. therapy. In the oral group, most patients received the usual dosing (n = 49, 74.2%), nine (13.6%) received low dosing and eight (12.1%) received higher than usual dosing after considering renal function at the time of transition to an oral antibiotic. Further details about antibiotic dosing are included in Table S2.
TABLE 2.
Antibiotic treatment details after propensity score matchinga
| Characteristic | i.v. (n = 66) | Oral (n = 66) |
|---|---|---|
| i.v. Antibiotic | ||
| Ampicillin | 1 (1.5%) | 5 (7.6%) |
| Ampicillin-sulbactam | 2 (3.0%) | 3 (4.5%) |
| Cefazolin | 4 (6.1%) | 5 (7.6%) |
| Cefepime | 1 (1.5%) | 5 (7.6%) |
| Ceftriaxone | 52 (78.8%) | 41 (62.1%) |
| Daptomycin | 1 (1.5%) | 0 (0.0%) |
| Penicillin | 1 (1.5%) | 0 (0.0%) |
| Piperacillin-tazobactam | 1 (1.5%) | 4 (6.1%) |
| Vancomycin | 3 (4.5%) | 1 (1.5%) |
| Vancomycin + cefepime | 0 (0.0%) | 2 (3.0%) |
| Duration of i.v. antibiotics, days | 16.0 (15.0–17.0) | 4.5 (4.0–5.8) |
| Oral antibiotic transition | 12 (18.2%) | 66 (100.0%) |
| Oral antibiotic agent | ||
| Amoxicillin | 1 (8.3%) | 17 (25.8%) |
| Amoxicillin-clavulanate | 4 (33.3%) | 17 (25.8%) |
| Amoxicillin-clavulanate + trimethoprim-sulfamethoxazole | 1 (8.3%) | 0 (0.0%) |
| Cefadroxil | 1 (8.3%) | 12 (18.2%) |
| Cefdinir | 1 (8.3%) | 10 (15.2%) |
| Cephalexin | 1 (8.3%) | 5 (7.6%) |
| Clindamycin | 1 (8.3%) | 2 (3.0%) |
| Doxycycline | 0 (0.0%) | 1 (1.5%) |
| Levofloxacin | 1 (8.3%) | 0 (0.0%) |
| Linezolid | 1 (8.3%) | 0 (0.0%) |
| Penicillin | 0 (0.0%) | 2 (3.0%) |
| Duration of oral antibiotics, days | 11.5 (6.5–15.0) | 10.0 (8.0–12.0) |
| Total duration of antibiotics, days | 16.0 (15.0–17.0) | 15.0 (12.0–16.0) |
Data are median (IQR) for continuous variables and n (%) for categorical variables; i.v., intravenous.
Outcomes.
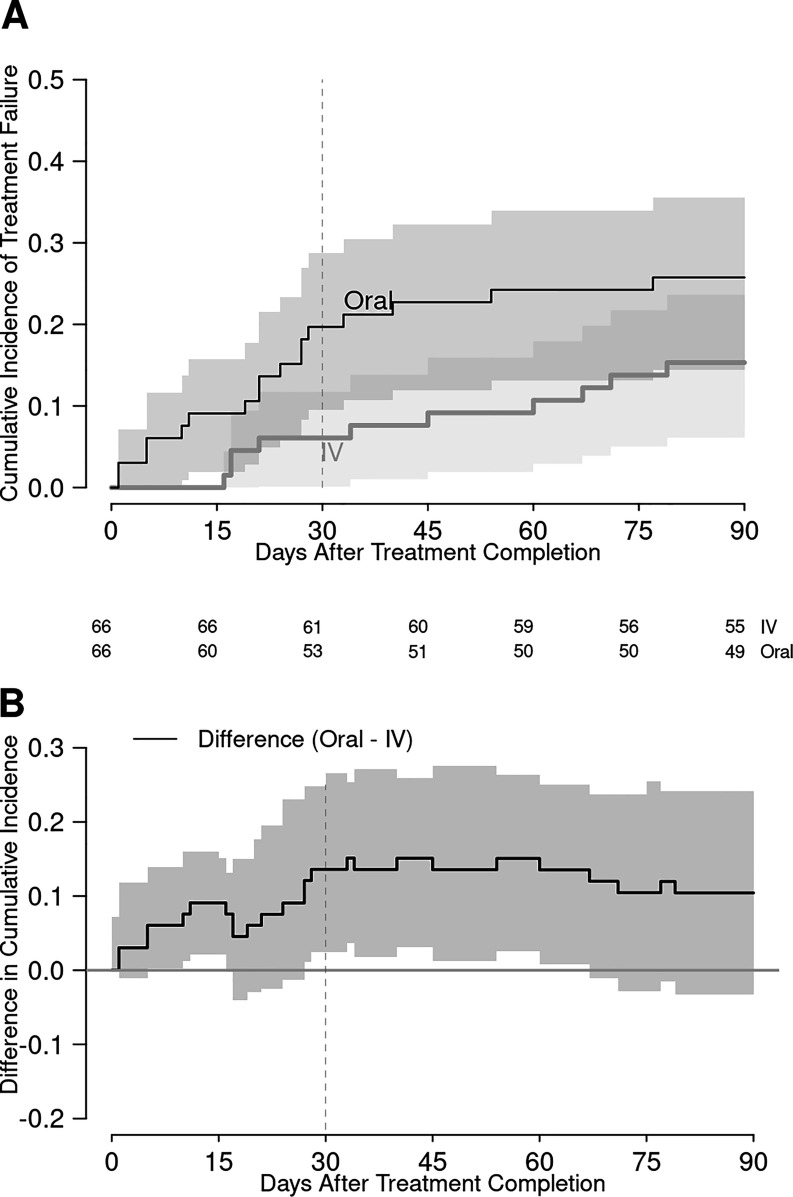
Seventeen of 132 (12.9%) patients experienced treatment failure within 30 days of completing therapy, and 27 (20.5%) failed within 90 days. This included 10 patients with infection relapse, 2 of whom had recurrent BSI. None of these relapses resulted in complications such as osteomyelitis or infective endocarditis. The oral group had a higher incidence of treatment failure at both time points, with absolute differences of 13.6% and 10.6%, respectively (Table 3). Formal testing of the primary outcome, 30-day treatment failure, did not meet the criteria for noninferiority (P = 0.741) and the confidence interval for the treatment difference suggested superiority of i.v. therapy (95% confidence interval, 2.4% to 24.8%). Kaplan-Meier curves of treatment failure are shown over the 90 days after therapy completion in Fig. 1.
TABLE 3.
Summary of study outcomesa
| Outcome | i.v. (n = 66) | Oral (n = 66) | Treatment difference (95% confidence interval) |
|---|---|---|---|
| 30-day treatment failure | 4 (6.1%) | 13 (19.7%) | 13.6% (2.4%, 24.8%)b |
| 30-day mortality | 0 (0.0%) | 3 (4.5%) | 4.5% (−0.5%, 9.6%) |
| 30-day relapse | 2 (3.0%) | 5 (7.6%) | 4.5% (−3.1%, 12.2%) |
| 30-day readmission | 3 (4.5%) | 6 (9.1%) | 4.5% (−4.0%, 13.1%) |
| 90-day treatment failure | 10 (15.2%) | 17 (25.8%) | 10.6% (−3.0%, 24.2%) |
| 90-day mortality | 1 (1.5%) | 4 (6.1%) | 4.5% (−1.9%, 11.0%) |
| 90-day relapse | 4 (6.1%) | 6 (9.1%) | 3.0% (−6.0%, 12.0%) |
| 90-day readmission | 7 (10.6%) | 9 (13.6%) | 3.0% (−8.1%, 14.2%) |
| Acute kidney injury during treatment | 2 (3.0%) | 0 (0.0%) | −3.0% (−7.2%, 1.1%) |
Treatment difference is the outcome frequency in the oral group minus the outcome frequency in the i.v. group (in percentage points), with a corresponding 95% confidence interval.
Test for noninferiority of oral therapy based on a preestablished margin of 10 percentage points: P = 0.741.
FIG 1.
Kaplan-Meier curves showing cumulative incidence of treatment failure (A) and difference in cumulative incidence of treatment failure (B) between the intravenous and oral antibiotic groups. The vertical dashed line indicates day 30 of posttreatment follow-up. The numbers under the x axis are number of patients at risk at the respective time points.
Details of the 17 patients who developed 30-day treatment failure are shown in Table S3. The four failures in the i.v. antibiotic group had received either cefazolin (2) or ceftriaxone (2). The 13 failures in the oral group received cefdinir (4), amoxicillin-clavulanate (4), cefadroxil (2), penicillin, clindamycin, and cephalexin. Patients that received low dosing had the highest rate of 30-day treatment failure (n = 4/9, 44.4%). Those that received the usual dosing had a rate of 30-day treatment failure (n = 8/49, 16.3%) comparable to the high-dose group (n = 1/8, 12.5%). Further details about antibiotic dosing are included in Table 4.
TABLE 4.
Outcomes of patients in the oral antibiotic group, stratified by relative antibiotic dosing
| Characteristic | Usual (n = 49) | Low (n = 9) | High (n = 8) | Total (n = 66) |
|---|---|---|---|---|
| Oral antibiotic agent | ||||
| Amoxicillin | 12 (24.5%) | 0 | 5 (62.5%) | 17 (25.8%) |
| Amoxicillin-clavulanate | 16 (32.7%) | 1 (11.1%) | 0 | 17 (25.8%) |
| Cefadroxil | 7 (14.3%) | 3 (33.3%) | 2 (25.0%) | 12 (18.2%) |
| Cefdinir | 9 (18.4%) | 0 | 1 (12.5%) | 10 (15.2%) |
| Cephalexin | 1 (2.0%) | 4 (44.4%) | 0 | 5 (7.6%) |
| Clindamycin | 1 (2.0%) | 1 (11.1%) | 0 | 2 (3.0%) |
| Doxycycline | 1 (2.0%) | 0 | 0 | 1 (1.5%) |
| Penicillin | 2 (4.1%) | 0 | 0 | 2 (3.0%) |
| 30-day treatment failure | 8 (16.3%) | 4 (44.4%) | 1 (12.5%) | 13 (19.7%) |
| 30-day mortality | 1 (2.0%) | 2 (22.2%) | 0 | 3 (4.5%) |
| 30-day readmission | 5 (10.2%) | 1 (11.1%) | 0 | 6 (9.1%) |
| 30-day relapse | 3 (6.1%) | 1 (11.1%) | 1 (12.5%) | 5 (7.6%) |
| 90-day treatment failure | 11 (22.4%) | 4 (44.4%) | 2 (25.0%) | 17 (25.8%) |
| 90-day mortality | 2 (4.1%) | 2 (22.2%) | 0 | 4 (6.1%) |
| 90-day readmission | 7 (14.3%) | 1 (11.1%) | 1 (12.5%) | 9 (13.6%) |
| 90-day relapse | 3 (6.1%) | 1 (11.1%) | 2 (25.0%) | 6 (9.1%) |
Two patients in the i.v. group developed acute kidney injury during the treatment period, compared to zero in the oral antibiotic group. No patients in either group developed a deep vein thrombosis or other vascular complications during therapy.
DISCUSSION
This study found that transitioning to oral antibiotic therapy within 7 days of treatment initiation for beta-hemolytic streptococcal BSI from the skin and soft tissue sources was not noninferior to continued i.v. therapy. On the contrary, our data suggest that the transition to oral therapy was associated with a higher risk of 30-day treatment failure. Rates for the other complications studied were low in both treatment groups.
There are multiple potential factors that could explain the higher rate of treatment failure in the oral antibiotic group. One is the bioavailability of the commonly used oral agents. This has been evaluated in the treatment of BSI from Enterobacterales, where treatment with an oral antibiotic with low or moderate bioavailability has been associated with higher rates of treatment failure (15). Agents with high bioavailability in these studies were primarily fluoroquinolones, which were scarcely used in the present study. However, a recent study of streptococcal BSI that compared oral therapy with fluoroquinolone to oral beta-lactam agents found similar rates of treatment success, with oral beta-lactams being found to be noninferior (16). Additionally, existing data pertaining to oral therapy in other Gram-positive BSIs have commonly employed highly bioavailable non-beta-lactam agents, such as linezolid, clindamycin, levofloxacin, or trimethoprim-sulfamethoxazole (13, 17–19). Our study cohort primarily received oral beta-lactam agents. Of the commonly used oral agents, cefdinir had a disproportionate rate of treatment failure (40%). Cefdinir has a low bioavailability of 21% (20), and it is possible this is a suboptimal choice for treatment of BSI. Amoxicillin-clavulanate also represented a large proportion of treatment failures. Interestingly, patients who received amoxicillin without clavulanate did not have any episodes of treatment failure, although the number of those patients was small.
It has also been noted that transition to oral therapy before day 3 and utilization of low-dose oral therapy may be associated with higher odds of treatment failure (16). It should be noted that the prior study analyzed all Streptococcus species from multiple sources, and it is possible that species or source-specific differences could have influenced outcomes. For example, that study included 34% with S. pneumoniae bloodstream infection, which likely has differences from beta-hemolytic streptococci in terms of source of infection and potential for infection relapse. However, this finding regarding dosing is similar to our study, where patients that received low-dose oral therapy had the highest rate of treatment failure (44.4%). Thus, the difference in outcomes may be driven by suboptimal dosing of primarily oral beta-lactam therapy.
Other recent studies evaluating the transition from i.v. to oral therapy in Gram-positive BSIs have suggested factors unrelated to therapy may impact rates of treatment failure. A cohort study of uncomplicated streptococcal BSI did not find a difference in 30-day outcomes in univariable analysis (21). This study found diabetes mellitus and hospitalization within 3 months before the index BSI episode to be associated with readmission. However, it should be noted that definitive therapy with i.v. or oral antibiotics was not included in the multivariable analysis and the described associations with readmission were not balanced between groups.
This study did show a very low rate of therapy-related complications regardless of the route of antibiotic administration. This may have been influenced by the specific antibiotics used in the outpatient setting and the relatively short duration of therapy. A recent study examining catheter-related complications of outpatient parenteral antibiotic therapy for a median of 29 days found the receipt of vancomycin or daptomycin to be associated with catheter complications (22), which very few patients received in our study. The rate of acute kidney injury was also similar to a study including patients receiving outpatient parenteral antibiotic therapy for a variety of infections (23). It should be noted that patients who received oral antibiotic therapy likely underwent less frequent toxicity monitoring, which may have influenced the rate of detected complications.
Our study was not able to evaluate social and economic factors that could have influenced outcomes. There are data that socioeconomic status is associated with a higher rate of infectious complications (24). Certain social factors such as occupation and less social support may be associated with receiving oral antibiotics for definitive treatment after hospital discharge given the greater burden continued i.v. therapy places on individuals. As such, lower socioeconomic status may be a source of confounding in the present study, which requires further evaluation. Additionally, we were unable to evaluate oral drug adherence. It is possible that oral regimens may have poorer adherence rates compared to structured outpatient parenteral therapy.
This study has multiple limitations of note. It was performed retrospectively and is susceptible to multiple sources of bias, including treatment selection bias. For this purpose, we used propensity score matching to minimize baseline differences between groups and facilitate fair comparisons of study outcomes. However, there are likely other possible sources of confounding that were not completely accounted for, such as socioeconomic status. Also, the reduced sample size after propensity score matching was relatively small, and event rates were generally too low to pursue statistical testing. Our outcome definitions, such as readmission, may not necessarily reflect direct effects from the route of antibiotic therapy. However, these were chosen to avoid subjectivity surrounding allocating an outcome as infection-related or unrelated. The epidemiology and risk factors for different beta-hemolytic streptococci are also not completely overlapping (25), and these differences should be considered. Superficial soft tissue infections were largely documented as cellulitis; however, it is possible that some may have been other forms of soft tissue infection such as erysipelas. Finally, most relapsed infections were not confirmed through microbiologic testing, and some may have been due to different bacteria than their primary episode.
In conclusion, these data could not establish the transition of i.v. to definitive oral therapy as noninferior to continued i.v. treatment in patients with beta-hemolytic streptococcal BSI from a skin or soft tissue source rather suggested a worse 30-day outcome. This difference in outcomes may be related to the choice of oral antibiotics, specifically the use of primarily oral beta-lactam agents with limited bioavailability. Patients who received low-dose oral therapy had the highest rate of treatment failure, and dose optimization of oral beta-lactam therapy may improve outcomes. Further study analyzing more optimal oral antibiotic agents and dosing strategies is needed.
MATERIALS AND METHODS
Study design.
We performed a retrospective, multicenter, propensity score-matched case-control study of adults with beta-hemolytic Streptococcus species bloodstream infections from a skin or soft tissue source at our centers in Arizona, Florida, Minnesota, and Wisconsin from January 2015 through December 2020. Inclusion criteria were age ≥18 years at the time of diagnosis, isolation of a beta-hemolytic Streptococcus species from a blood culture, and the bloodstream infection originating from a skin or soft tissue source. Patients were excluded if they had a polymicrobial bloodstream infection, a nonskin or soft tissue primary source of infection, a metastatic focus of infection such as infective endocarditis or hematogenous osteomyelitis, hospice enrollment before completing antibiotic therapy, or if they lacked research authorization per state statutes. Patients with diabetic foot osteomyelitis who underwent an appropriate source-controlling procedure within 7 days of antibiotic initiation, such as amputation, were eligible for inclusion as their remaining antibiotic course would be primarily aimed at resolving their bloodstream infection rather than osteomyelitis. Those without a defined primary focus of infection were eligible for inclusion if they underwent thorough investigation for occult sources of infection, as these were presumed to be related to unrecognized soft tissue infection.
Potentially eligible patients were identified from our internal microbiologic lab blood culture database. These patients were then manually screened and data from included patients were manually extracted into a prespecified data collection form. Abstracted data included demographics, preexisting comorbidities, infection-related characteristics, treatment characteristics, and outcomes. Study data were collected and managed using REDCap electronic data capture tools hosted at Mayo Clinic (26, 27).
Treatment characteristics included choice and duration of an initial i.v. antibiotic agent. For applicable patients, this also included the choice and duration of a subsequent oral antibiotic agent. Patients were included in the oral group if they received 7 or less days of initial i.v. therapy. The i.v. group received more than 7 days of i.v. therapy. These groups were compared for the outcomes of interest. The primary outcome was 30-day treatment failure, defined as a composite of mortality, infection, and/or bacteremic relapse, and all-cause hospital readmission. Infection relapse was defined as the recurrence of clinical signs or symptoms of bacteremia or soft tissue infection. Bacteremic relapse required repeat isolation of the same streptococcal species, while relapsed soft tissue infection did not require microbiologic confirmation. Secondary outcomes were 90-day treatment failure and individual components of treatment failure at 30 and 90 days. Antibiotic agents were defined as those received for at least 50% of i.v. or oral therapy, respectively. After considering creatinine clearance (incorporating adjusted body weight for patients whose weight was >120% of ideal body weight), oral antibiotic doses were classified as usual or high- or low-dose relative to the usual reference doses listed in Table S1. Patients whose adjusted doses were above the usual dose were classified as “high” and those below were classified as “low.” Delayed admission was defined as admission to the hospital on a later date than when the initial positive blood culture was drawn. This was defined to capture patients who may have presented to the emergency department and were discharged but were called back for admission once blood cultures returned positive. The Pitt bacteremia score and Charlson comorbidity index were calculated as previously described (28, 29).
Propensity scoring.
Propensity score matching was used to facilitate comparisons between the two treatment groups. For propensity score estimation, a multivariable logistic regression model was constructed to predict the actual treatment received using 15 of 17 prespecified baseline variables thought to be relevant to treatment selection (Table 1). Because the other two variables were too sparse for statistical modeling (zero counts in the oral group), we removed those individuals with either exposure from entry into the matching procedure to avoid potential imbalance. Next, we used a nearest neighbor matching algorithm to sample the oral antibiotic-treated patients and an equal number of i.v.-treated patients, matching them on propensity score within a tolerance of 0.2 standard deviations of the logit-transformed scores (i.e., predicted log odds for receiving oral treatment). Covariate balance before and after matching was evaluated with standardized mean differences.
Statistical analysis.
Continuous and categorical variables are summarized using median (IQR) and frequency (percentage), respectively. Treatment differences in all 30-day and 90-day outcomes between the matched groups were estimated along with 95% confidence intervals. The primary outcome of 30-day treatment failure was formally tested for noninferiority in the oral versus i.v. therapy group, based on a prespecified noninferiority margin set at 10%. This noninferiority margin was chosen as a clinically relevant difference in effectiveness between oral and i.v. therapy based on previously published studies (12, 15, 16). Kaplan-Meier curves were also constructed to illustrate group differences in the cumulative incidence of treatment failure as a function of time since treatment completion. All analyses were performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
Data availability.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ACKNOWLEDGMENTS
This project was supported by Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This work was also supported by internal funding from Mayo Clinic.
A.V. reports being an inventor for Mayo Clinic Travel App interaction with Smart Medical Kit and Medical Kit for Pilgrims and receiving funding from Moderna Inc. for Advisory Board participation. All other authors have no potential conflicts of interest to report.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Peralta G, Padrón E, Roiz MP, De Benito I, Garrido JC, Talledo F, Rodríguez-Lera MJ, Ansorena L, Sánchez MB. 2006. Risk factors for bacteremia in patients with limb cellulitis. Eur J Clin Microbiol Infect Dis 25:619–626. doi: 10.1007/s10096-006-0186-z. [DOI] [PubMed] [Google Scholar]
- 2.Uslan DZ, Crane SJ, Steckelberg JM, Cockerill FR, St Sauver JL, Wilson WR, Baddour LM. 2007. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med 167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson CG, Martinello RA. 2012. A systematic review of bacteremias in cellulitis and erysipelas. J Infect 64:148–155. doi: 10.1016/j.jinf.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Tay E-Y, Thirumoorthy T, Pang S-M, Lee H-Y. 2014. Clinical outcomes of bacteraemia in cellulitis of the leg. Clin Exp Dermatol 39:683–688. doi: 10.1111/ced.12366. [DOI] [PubMed] [Google Scholar]
- 5.Seaton RA, Sharp E, Bezlyak V, Weir CJ. 2011. Factors associated with outcome and duration of therapy in outpatient parenteral antibiotic therapy (OPAT) patients with skin and soft-tissue infections. Int J Antimicrob Agents 38:243–248. doi: 10.1016/j.ijantimicag.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Quintens C, Steffens E, Jacobs K, Schuermans A, Van Eldere J, Lagrou K, De Munter P, Derdelinckx I, Peetermans WE, Spriet I. 2020. Efficacy and safety of a Belgian tertiary care outpatient parenteral antimicrobial therapy (OPAT) program. Infection 48:357–366. doi: 10.1007/s15010-020-01398-4. [DOI] [PubMed] [Google Scholar]
- 7.Iversen K, Ihlemann N, Gill SU, Madsen T, Elming H, Jensen KT, Bruun NE, Høfsten DE, Fursted K, Christensen JJ, Schultz M, Klein CF, Fosbøll EL, Rosenvinge F, Schønheyder HC, Køber L, Torp-Pedersen C, Helweg-Larsen J, Tønder N, Moser C, Bundgaard H. 2019. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med 380:415–424. doi: 10.1056/NEJMoa1808312. [DOI] [PubMed] [Google Scholar]
- 8.Li H-K, Rombach I, Zambellas R, Walker AS, McNally MA, Atkins BL, Lipsky BA, Hughes HC, Bose D, Kümin M, Scarborough C, Matthews PC, Brent AJ, Lomas J, Gundle R, Rogers M, Taylor A, Angus B, Byren I, Berendt AR, Warren S, Fitzgerald FE, Mack DJF, Hopkins S, Folb J, Reynolds HE, Moore E, Marshall J, Jenkins N, Moran CE, Woodhouse AF, Stafford S, Seaton RA, Vallance C, Hemsley CJ, Bisnauthsing K, Sandoe JAT, Aggarwal I, Ellis SC, Bunn DJ, Sutherland RK, Barlow G, Cooper C, Geue C, McMeekin N, Briggs AH, Sendi P, Khatamzas E, Wangrangsimakul T, Wong THN, OVIVA Trial Collaborators , et al. 2019. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 380:425–436. doi: 10.1056/NEJMoa1710926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamma PD, Conley AT, Cosgrove SE, Harris AD, Lautenbach E, Amoah J, Avdic E, Tolomeo P, Wise J, Subudhi S, Han JH, Antibacterial Resistance Leadership Group . 2019. Association of 30-day mortality with oral step-down vs continued intravenous therapy in patients hospitalized with Enterobacteriaceae bacteremia. JAMA Intern Med 179:316–323. doi: 10.1001/jamainternmed.2018.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurber KM, Arnold JR, Narayanan PP, Dierkhising RA, Sampathkumar P. 2019. Comparison of intravenous and oral definitive antibiotic regimens in hospitalised patients with Gram-negative bacteraemia from a urinary tract infection. J Glob Antimicrob Resist 18:243–248. doi: 10.1016/j.jgar.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Saad S, Mina N, Lee C, Afra K. 2020. Oral beta-lactam step down in bacteremic E. coli urinary tract infections. BMC Infect Dis 20:785. doi: 10.1186/s12879-020-05498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bupha-Intr O, Blackmore T, Bloomfield M. 2020. Efficacy of early oral switch with β-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 64:e02345-19. doi: 10.1128/AAC.02345-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorgensen SCJ, Lagnf AM, Bhatia S, Shamim M-D, Rybak MJ. 2019. Sequential intravenous-to-oral outpatient antibiotic therapy for MRSA bacteraemia: one step closer. J Antimicrob Chemother 74:489–498. doi: 10.1093/jac/dky452. [DOI] [PubMed] [Google Scholar]
- 14.Hospenthal DR, Waters CD, Beekmann SE, Polgreen PM. 2020. Practice patterns of infectious diseases physicians in transitioning from intravenous to oral therapy in patients with bacteremia. Open Forum Infect Dis 7:ofz386. doi: 10.1093/ofid/ofz386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kutob LF, Justo JA, Bookstaver PB, Kohn J, Albrecht H, Al-Hasan MN. 2016. Effectiveness of oral antibiotics for definitive therapy of Gram-negative bloodstream infections. Int J Antimicrob Agents 48:498–503. doi: 10.1016/j.ijantimicag.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Arensman K, Shields M, Beganovic M, Miller JL, LaChance E, Anderson M, Dela-Pena J. 2020. Fluoroquinolone versus beta-lactam oral step-down therapy for uncomplicated streptococcal bloodstream infections. Antimicrob Agents Chemother 64:e01515-20. doi: 10.1128/AAC.01515-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willekens R, Puig-Asensio M, Ruiz-Camps I, Larrosa MN, González-López JJ, Rodríguez-Pardo D, Fernández-Hidalgo N, Pigrau C, Almirante B. 2019. Early oral switch to linezolid for low-risk patients with staphylococcus aureus bloodstream infections: a propensity-matched cohort study. Clin Infect Dis 69:381–387. doi: 10.1093/cid/ciy916. [DOI] [PubMed] [Google Scholar]
- 18.Kouijzer IJE, van Leerdam EJ, Gompelman M, Tuinte RAM, Aarntzen EHJG, Berrevoets MAH, Maat I, Bleeker-Rovers CP, van Crevel R, Ten Oever J. 2021. Intravenous to oral switch in complicated staphylococcus aureus bacteremia without endovascular infection: a retrospective single-center cohort study. Clin Infect Dis 73:895–898. doi: 10.1093/cid/ciab156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dagher M, Fowler VG, Wright PW, Staub MB. 2020. A narrative review of early oral stepdown therapy for the treatment of uncomplicated staphylococcus aureus bacteremia: yay or nay? Open Forum Infect Dis 7:ofaa151. doi: 10.1093/ofid/ofaa151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Food & Drug Administration. Omnicef (cefdinir) sNDA 50-739. https://www.accessdata.fda.gov/drugsatfda_docs/label/1999/50739S2LBL.PDF. Accessed 3 November 2022.
- 21.Kang A, Beuttler R, Minejima E. 2022. Evaluation of step-down oral antibiotic therapy for uncomplicated streptococcal bloodstream infections on clinical outcomes. Ther Adv Infect 9:204993612110732. doi: 10.1177/20499361211073248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller SC, Dzintars K, Gorski LA, Williams D, Cosgrove SE. 2018. Antimicrobial agents and catheter complications in outpatient parenteral antimicrobial therapy. Pharmacotherapy 38:476–481. doi: 10.1002/phar.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrestha NK, Blaskewicz C, Gordon SM, Everett A, Rehm SJ. 2020. Safety of outpatient parenteral antimicrobial therapy in nonagenarians. Open Forum Infect Dis 7:ofaa398. doi: 10.1093/ofid/ofaa398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnelly JP, Lakkur S, Judd SE, Levitan EB, Griffin R, Howard G, Safford MM, Wang HE. 2018. Association of neighborhood socioeconomic status with risk of infection and sepsis. Clin Infect Dis 66:1940–1947. doi: 10.1093/cid/cix1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi T, Sunaoshi K, Sunakawa K, Fujishima S, Watanabe H, Ubukata K, Invasive Streptococcal Disease Working Group . 2010. Clinical aspects of invasive infections with Streptococcus dysgalactiae ssp. equisimilis in Japan: differences with respect to Streptococcus pyogenes and Streptococcus agalactiae infections. Clin Microbiol Infect 16:1097–1103. doi: 10.1111/j.1469-0691.2009.03047.x. [DOI] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. 2009. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, REDCap Consortium . 2019. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow JW, Yu VL. 1999. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 11:7–12. doi: 10.1016/s0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.00120-23-s0001.docx, DOCX file, 0.07 MB (67.6KB, docx)
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.