ABSTRACT
Candida auris is an emerging, multidrug-resistant fungal pathogen that causes refractory colonization and life-threatening, invasive nosocomial infections. The high proportion of C. auris isolates that display antifungal resistance severely limits treatment options. Combination therapies provide a possible strategy by which to enhance antifungal efficacy and prevent the emergence of further resistance. Therefore, we examined drug combinations using antifungals that are already in clinical use or are undergoing clinical trials. Using checkerboard assays, we screened combinations of 5-flucytosine and manogepix (the active form of the novel antifungal drug fosmanogepix) with anidulafungin, amphotericin B, or voriconazole against drug resistant and susceptible C. auris isolates from clades I and III. Fractional inhibitory concentration indices (FICI values) of 0.28 to 0.75 and 0.36 to 1.02 were observed for combinations of anidulafungin with manogepix or 5-flucytosine, respectively, indicating synergistic activity. The high potency of these anidulafungin combinations was confirmed using live-cell microfluidics-assisted imaging of the fungal growth. In summary, combinations of anidulafungin with manogepix or 5-flucytosine show great potential against both resistant and susceptible C. auris isolates.
KEYWORDS: Candida auris, antifungal combination, anidulafungin, flucytosine, manogepix, synergy
INTRODUCTION
Candida auris is an emerging fungal pathogen that causes nosocomial invasive infections and that is difficult to eradicate, following the colonization of hospitalized patients (1). C. auris was first identified in 2009 in Japan, but, since then, outbreaks have been observed on most continents (1, 2). C. auris strains have been subdivided into four genetic clades, namely, the South Asian (I), East Asian (II), South African (III) and South American (IV) clades (3), with a potential fifth Iranian clade having been identified more recently (4). The organism colonizes the skin and can lead to mucosal or bloodstream infections, predominately in immunocompromised hosts (1). Invasive C. auris infections are associated with mortality rates between 28% and 60%, and treatment failure due to antifungal resistance is often observed (1, 3, 5–11).
To date, only four classes of antifungal drugs are available for the treatment of invasive fungal infections: azoles, polyenes, echinocandins and the nucleoside analogue 5-flucytosine. 5-flucytosine has high oral bioavailability with high activity against C. auris, but it is not generally used in monotherapy due to the rapid emergence of resistance (12). Current guidelines recommend echinocandin treatment as a first line therapy for invasive candidiasis and for C. auris infections, in particular (13, 14). However, echinocandin resistance can develop during treatment (15, 16). Resistance to all four existing classes of antifungals has been reported in C. auris, with various drug susceptibilities and resistance mechanisms between clades (17). Around 90% of C. auris isolates show resistance to fluconazole, with various susceptibilities to other azoles (3, 6, 9, 18). Resistance to amphotericin B and the echinocandins appears to be less common, having been reported in 13 to 35% and 2 to 7% of tested isolates, respectively (3, 9, 18). Alarmingly, up to 41% of the isolates exhibit resistance to two or more antifungal classes (3, 18). Consequently, the Centers for Disease Control and Prevention (CDC) recently added C. auris to its list of urgent antibiotic resistance threats (19), and the World Health Organization (WHO) declared it a critical threat in its fungal priority pathogens list (14).
The limited number of antifungal drugs as well as the increased threat of antifungal resistance in C. auris means that novel treatment strategies are urgently needed. Combinations of antifungals with different mechanisms of action provide one proposed therapeutic strategy. Previous in vitro studies investigated combinations of echinocandins with azoles or the polyene amphotericin B (20–24) as well as combinations of 5-flucytosine with the other three antifungal classes in C. auris (25–27). These studies observed either synergy or indifference and no antagonism for all of the tested combinations, with variability between C. auris isolates. The most promising combinations were azoles combined with echinocandins which, in two studies, resulted in synergy against all tested isolates (20, 23).
Combinations with 5-flucytosine are of particular interest, as its combinations with amphotericin B and fluconazole have been shown to be superior to monotherapy in phase III clinical trials against cryptococcal meningitis (28). As a result of these trials, 5-flucytosine is now more widely available globally, including in countries such as South Africa, which suffers a high burden of C. auris candidemia (28, 29). Echinocandin combinations with 5-flucytosine have been reported to be indifferent in most cases, but these combinations have shown 100% growth inhibition and fungicidal activity against multidrug-resistant isolates (25–27).
None of these studies included the new antifungal fosmanogepix, which has recently completed phase 1 and 2 clinical trials and is one of several new antifungals in the pipeline that may also exhibit activity against C. auris (30). Fosmanogepix is a prodrug that is converted into the active compound manogepix by systemic phosphatases (31). Manogepix inhibits a novel antifungal target, namely, Gwt1, which is involved in the GPI-anchor biosynthetic pathway, thereby leading to a decrease in cell wall-anchored mannoproteins (31). In the present study, we examined combinations of manogepix or 5-flucytosine with anidulafungin, amphotericin B, or voriconazole against a range of resistant and susceptible C. auris isolates in vitro.
RESULTS
Antifungal activity against C. auris isolates.
The antifungal susceptibility profiles of 25 C. auris isolates were determined in order to select a subset of isolates with different drug susceptibilities for antifungal combination testing. The ranges of minimal inhibitory concentrations (MICs) for the C. auris isolates against the tested antifungals are summarized in Table 1 and Table S1. The MIC values for amphotericin B clustered around the breakpoint of 2 mg/L, which is a known problem for the broth microdilution susceptibility testing of amphotericin B in RPMI medium, making it difficult to distinguish resistant and susceptible isolates (32). Fluconazole showed a large percentage of resistant C. auris isolates (96%; breakpoint ≥32 mg/L) with high MIC values that ranged from 4 to ≥128 mg/L, whereas the other triazole that was tested (voriconazole) displayed more potent antifungal activity, with the MIC values ranging from 0.06 to 16 mg/L and with 40% resistant isolates (breakpoint ≥ 2 mg/L). Of all of the antifungals tested with an available breakpoint, anidulafungin produced the lowest percentage of resistant isolates (32%; ≥4 mg/L). The most potent antifungal activity against C. auris was observed for manogepix (MIC50/MIC90, 0.008/0.03 mg/L; range, 0.004 to 0.03), and this was followed by 5-flucytosine (MIC50/MIC90, 0.25/0.25 mg/L; range, 0.125 to 0.25).
TABLE 1.
Antifungal MIC distribution for 25 C. auris isolates
| Drug | MIC (mg/L) |
MIC50a | MIC90b | %Rc | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.002 | 0.004 | 0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | ||||
| AMB | 0 | 0 | 0 | 0 | 0 | 1 | 24 d | 0 | 0 | 0 | 2 | 2 | 96.0 | |||||||
| FLC | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 5 | 5 | 14 | ≥128 | ≥128 | 96.0 | |||||||
| VRC | 0 | 1 | 2 | 6 | 1 | 5 | 9 | 0 | 0 | 1 | 1 | 2 | 40.0 | |||||||
| AFG | 0 | 3 | 3 | 5 | 3 | 2 | 0 | 1 | 0 | 8 | 0.25 | ≥8 | 32.0 | |||||||
| 5FC | 0 | 0 | 0 | 0 | 11 | 14 | 0 | 0 | 0 | 0 | 0.25 | 0.25 | No BP | |||||||
| MGX | 0 | 11 | 5 | 1 | 8 | 0 | 0 | 0 | 0 | 0 | 0.008 | 0.03 | No BP | |||||||
MIC at which 50% of isolates were inhibited.
MIC at which 90% of isolates were inhibited.
Percentage of resistant isolates.
Modal MICs are indicated with underlined numbers. A gray background indicates a tentative C. auris breakpoint, according to the CDC. 5FC, 5-flucytosine; AFG, anidulafungin; AMB, amphotericin B; BP, breakpoint; FLC, fluconazole; MGX, manogepix; VRC, voriconazole. Empty cells indicate drug concentrations that were not tested.
Interaction of antifungal drug combinations against C. auris isolates.
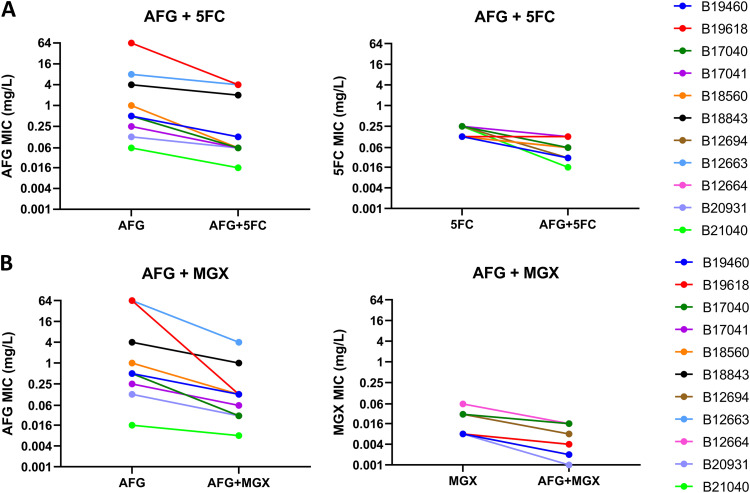
Based on their MIC values, 11 C. auris isolates with different drug susceptibility profiles were selected to investigate the interactions of anidulafungin, amphotericin B, and voriconazole with 5-flucytosine or manogepix. The FICI values for these combinations, as determined by the checkerboard assays, are presented in Table 2 and Fig. 1. The FICI values of separate repeats can be found in Tables S2 and S3). The combination of anidulafungin with 5-flucytosine resulted in synergistic interactions for 10/11 isolates (synergy, 2/11 isolates; partial synergy, 8/11 isolates). Meanwhile the combination of anidulafungin with manogepix led to synergy in all 11 isolates (synergy, 5/11 isolates; partial synergy, 6/11 isolates). These FICI values corresponded to a median (range) decrease in the MIC of 2 log2-fold (1 to 4 log2-fold) for anidulafungin and 2 log2-fold (0 to 4 log2-fold) for 5-flucytosine (Fig. 2A) or 3 log2-fold (1 to 9 log2-fold) for anidulafungin and 2 log2-fold (1 to 3 log2-fold) for manogepix (Fig. 2B). Additionally, both anidulafungin combinations achieved fungistatic activity with log10-fold reductions in the CFU/mL values of 2.2 and 0.8, compared to the starting inoculum for the combination with manogepix and 5-flucytosine, respectively, whereas the corresponding monotherapies only had a negligible antifungal effect (Fig. S7).
TABLE 2.
FICI values for 5 antifungal combinations against 11 C. auris isolatesa
| Isolate | AFG + 5FC | AFG+mGX | AMB + 5FC | VRC + 5FC | MGX + 5FC |
|---|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | Median (range) | Median (range) | |
| B19460 | 0.49 (0.48 to 0.50) | 0.50 (0.50 to 0.52) | 1.01 (1.00 to 1.01) | 4.48 (1.01 to 8.00) | 1.01 (1.00 to 1.01) |
| B19618 | 0.56 (0.49 to 1.01) | 0.52 (0.51 to 1.00) | 1.00 (0.52 to 1.02) | 4.50 (1.02 to 5.00) | 1.00 (1.00 to 1.02) |
| B17040 | 0.56 (0.15 to 0.56) | 0.51 (0.33 to 0.55) | 1.00 (1.00 to 1.01) | 1.01 (1.00 to 1.01) | 1.01 (0.76 to 1.02) |
| B17041 | 0.74 (0.69 to 0.98) | 0.65 (0.37 to 0.77) | 1.00 (0.75 to 1.00) | 1.00 (0.56 to 1.02) | 1.00 (0.63 to 1.01) |
| B18560 | 0.60 (0.30 to 0.61) | 0.28 (0.19 to 0.75) | 1.01 (1.01) | 1.00 (1.00 to 4.48) | 0.56 (0.53 to 1.00) |
| B18843 | 0.98 (0.49 to 1.00) | 0.63 (0.53 to 0.75) | 1.00 (0.63 to 1.01) | 4.50 (1.01 to 4.50) | 1.00 (0.63 to 4.41) |
| B12694 | 0.36 (0.24 to 0.37) | 0.52 (0.20 to 0.62) | 0.63 (0.53 to 1.00) | 1.01 (1.01) | 0.54 (0.50 to 1.05) |
| B12663 | 0.74 (0.38 to 1.00) | 0.33 (0.33 to 1.01) | 0.75 (0.51 to 1.01) | 1.00 (1.00 to 1.01) | 1.01 (0.51 to 1.05) |
| B12664 | 0.75 (0.62 to 0.98) | 0.39 (0.29 to 0.51) | 0.75 (0.62 to 0.75) | 1.01 (0.63 to 1.01) | 0.58 (0.57 to 1.01) |
| B20931 | 0.53 (0.18 to 0.60) | 0.49 (0.30 to 0.56) | 1.00 (0.53 to 1.00) | 1.01 (0.63 to 1.01) | 1.01 (0.56 to 1.01) |
| B21040 | 1.02 (0.56 to 1.03) | 0.75 (0.56 to 1.00) | 0.75 (0.63 to 1.00) | 1.01 (1.01) | 1.00 (0.53 to 1.00) |
Synergy, dark gray; partial synergy, medium gray; indifference/additivity, white; antagonism, light gray. Underlined values indicate resistance to either AFG or VRC. 5FC, 5-flucytosine; AFG, anidulafungin; AMB, amphotericin B; MGX, manogepix; VRC, voriconazole.
FIG 1.
In vitro interactions of AFG, MGX, AMB, VRC, and 5FC, according to the FICI values for 11 C. auris isolates. Minimum FICI values are shown in the absence of antagonism. Otherwise, maximum FICI values are reported. Drug interaction ranges are indicated by background color: Synergy, dark green; partial synergy, light green; indifference, white; antagonism, red. The symbols represent the FICI values of three independent experiments. 5FC, 5-flucytosine; AFG, anidulafungin; AMB, amphotericin B; MGX, manogepix; VRC, voriconazole.
FIG 2.
Changes in MIC values due to antifungal combinations for 11 C. auris isolates. MIC values for 11 C. auris isolates in combinations of anidulafungin with 5-flucytosine (A) and manogepix (B), compared to the antifungals in monotherapy, as determined via checkerboard assays. The symbols represent the median values of three independent experiments. 5FC, 5-flucytosine; AFG, anidulafungin; MGX, manogepix.
The combination of amphotericin B with 5-flucytosine did not show full synergy for any of the tested isolates, though partial synergy was observed in 4/11 isolates (median FICIs, 0.63 to 0.75). The other isolates showed either additive (5/11 isolates) or indifferent (2/11 isolates; median FICIs, 1.01) interactions for amphotericin B with 5-flucytosine. For the combination of manogepix and 5-flucytosine, 3/11 isolates displayed partial synergy (median FICIs, 0.54 to 0.58), and 4/11 isolates showed additive or indifferent interactions (median FICIs, 1.01). The combination of manogepix and 5-flucytosine led to large reductions in the MIC by a median (range) of 7 log2-fold (1 to 8 log2-fold) for 5-flucytosine, whereas the manogepix MIC values were only decreased by a median (range) of 0 log2-fold (0 to 2 log2-fold) (Fig. S1C). The drug combination resulting in the least favorable interactions was voriconazole with 5-flucytosine, with 3/11 isolates displaying antagonistic interactions (median FICIs, 4.48 to 4.50) and the remaining isolates displaying additive (3/11 isolates) or indifferent (5/11 isolates; median FICIs, 1.01) interactions.
Response surface analyses were also used to examine the drug combinations, and an example is shown in Fig. 3 for the multidrug-resistant isolate B12663 (see Fig. S2–S6 for the other isolates). Consistent with the FICI scores, the synergy maps indicate synergy for the combination of anidulafungin and manogepix (median FICI, 0.33) and weak synergy for combinations of 5-flucytosine with anidulafungin (median FICI, 0.74) or amphotericin B (median FICI 0.75). In contrast to the FICI calculation, which only focuses on the drug concentrations that correspond to the MIC values, the response surface analysis permits the examination of drug interactions over a wide range of tested concentrations. This revealed antagonism at the lower end of some concentration ranges that was missed by the FICI approach, highlighting the concentration-dependence of the interactions.
FIG 3.
Synergy maps for 5 antifungal combinations against the multidrug-resistant C. auris isolate B12663. The interactions of 5-flucytosine with anidulafungin (A), amphotericin B (C), or voriconazole (D), as well as the interactions of manogepix with anidulafungin (B) or 5-flucytosine (E) were analyzed with Combenefit (n = 3). The graphs show the growth percentage, relative to the drug-free control, with the color scale representing the drug interaction. 5FC, 5-flucytosine; AFG, anidulafungin; AMB, amphotericin B; MGX, manogepix; VRC, voriconazole.
Real time imaging of anidulafungin combinations against a multidrug-resistant C. auris isolate using microfluidics.
A microfluidics imaging approach was employed to further investigate the effects, at a single-cell level, of the two most promising drug combinations: anidulafungin with manogepix and anidulafungin with 5-flucytosine. This system is less static than the traditional broth microdilution method, as the cells are constantly perfused with fresh medium containing different antifungal drugs. Again, the multidrug-resistant C. auris isolate B12663 was chosen for analysis. Both drug combinations showed dramatic effects upon cell growth, markedly reducing the sizes of colonies, compared to the relevant monotherapies and media-only controls (Fig. 4A; Movies S1 and S2). The doubling times, measured by the two-dimensional colony area changes, increased significantly in the presence of the drug combinations, compared to the individual antifungals. An increase from 3.19 h (5-flucytosine alone) to 4.90 h (P < 0.001) was observed for anidulafungin combined with 5-flucytosine (Fig. 4B). Similarly, an increase from 2.75 h (manogepix alone) to 9.50 h (P < 0.001) was seen for the anidulafungin-manogepix combination (Fig. 4C). These changes in doubling time correspond to 63.5% (anidulafungin-5-flucytosine) and 96.5% (anidulafungin-manogepix) decreases in the colony area after 24 h, compared to 5-flucytosine and manogepix, respectively (data not shown). These findings were again consistent with those of the checkerboard and response surface analysis experiments in that the combination of anidulafungin and manogepix showed the most potent impacts on cell growth and in that this was followed by the combination of anidulafungin plus 5-flucytosine.
FIG 4.
Microfluidics imaging of C. auris under antifungal combination exposure. DIC images from two representative experiments (A) and doubling times (B and C) of C. auris B12663 cells grown in the presence of RPMI 2% G-MOPS for 4 h. These were followed by further RPMI 2% G-MOPS or treatment with anidulafungin, 5-flucytosine, and manogepix, alone or in combination, at their MICs for 16 h. The doubling times were calculated via the two-dimensional colony area changes for several colonies from two independent experiments. Mean ± range. Scale bars: 100 μm. *, P ≤ 0.05; **, P ≤ 0.01; ***, P < 0.001 (one-way analysis of variance (ANOVA) with Bonferroni’s correction). 5FC, 5-flucytosine; AFG, anidulafungin; MGX, manogepix.
The cellular morphology was further examined at higher magnification after exposing the C. auris cells to the antifungals in monotherapy or combination for 24 h (Fig. S8). In drug-free medium, the cells had a well-defined, oval morphology. Under exposure to anidulafungin, manogepix, and both anidulafungin combinations, the cells displayed a rounder morphology with the formation of aggregates, whereas the 5-flucytosine treatment resulted in a more elongated phenotype. Additionally, enlarged, round cells were observed in the presence of manogepix and both combinations.
DISCUSSION
The emergence and global spread of multidrug-resistant C. auris strains poses a serious health threat. The high prevalence of antifungal resistance reported for C. auris isolates (3, 6–9, 11, 18, 24) was also observed in the isolates that were used in this study, with the majority of the isolates being resistant to fluconazole, 40% being resistant to voriconazole, and 32% being resistant to anidulafungin. The ability of C. auris to develop resistance to all of the available classes of antifungal drugs severely limits treatment options.
New antifungal drugs, such as fosmanogepix, are currently under development (reviewed in [30]). C. auris currently appears susceptible to the active version of this new class of drugs (manogepix), but there is a high risk of resistance developing after its introduction into the clinic, unless precautionary measures are taken. Combination therapies provide a proven strategy by which to prevent the emergence of resistance to a single drug, and they have already been employed in the treatment of viral and bacterial infections (33). Additionally, combination therapies have the potential to improve efficacy through additive or synergistic interactions, which allows for lower drug doses to be used, thereby reducing dose-related toxicity.
Thus far, nine studies have examined antifungal drug combinations against C. auris. The majority of these studies focused on combinations of azoles with echinocandins (20, 23, 24, 34), while a smaller number evaluated polyene-echinocandin interactions (21, 22) or combinations with 5-flucytosine (25–27). These studies reported mainly synergistic (including partial synergy) or indifferent interactions, with interstrain variability being observed for some combinations. None of these studies included manogepix. Both manogepix and 5-flucytosine have potent antifungal activity against C. auris, as is shown here and as was observed by others (35–40). Therefore, we examined interactions of the echinocandin anidulafungin, the azole voriconazole, and the polyene amphotericin B with either 5-flucytosine or manogepix via checkerboard assays, response surface analyses, and microfluidics imaging.
According to the FICI values and response-surface analyses, the most potent combination (with respect to the number of C. auris isolates that displayed synergy) was anidulafungin plus manogepix, and this was followed by the combination of anidulafungin with 5-flucytosine. The high efficacy of these combinations was also confirmed by microfluidics imaging, which revealed dramatic reductions in fungal growth, compared to the relevant monotherapies. The interactions between 5-flucytosine with either amphotericin B or manogepix were additive or indifferent for the majority of the isolates, whereas the combination of voriconazole with 5-flucytosine was indifferent or antagonistic.
Applying our FICI thresholds, Bidaud and coworkers also reported mainly partially synergistic or additive interactions for combinations of amphotericin B, voriconazole, or micafungin with 5-flucytosine (25). However, they did not observe the antagonism for the combination of voriconazole with 5-flucytosine that we observed here. Another study reported the 100% growth inhibition of amphotericin B or anidulafungin-resistant C. auris isolates for amphotericin B-5-flucytosine combinations (0.25/1 mg/L) or anidulafungin-5-flucytosine combinations (0.008/1 mg/L) (26). Based on our OD530 measurements, more than 90% growth inhibition was also achieved for the majority of the susceptible and resistant isolates that we analyzed, and this growth inhibition could be reached at lower concentrations for some isolates. To the best of our knowledge, antifungal combinations with fosmanogepix/manogepix have not been studied previously against Candida species. One recent study compared amphotericin B monotherapy with the combination therapy of fosmanogepix and amphotericin B in invasive mouse infection models of Aspergillus fumigatus, Rhizopus arrhizus var. delemar, and Fusarium solani (41). In all three models, mortality and fungal burden were significantly reduced in the mice that were treated with the combination therapy, compared to those that were treated with amphotericin B or fosmanogepix alone (41).
For the majority of the combinations and isolates that we examined, the interactions were partially synergistic or additive. However, even these interactions could be of interest clinically, as the ultimate goal is to reduce the fungal burden with a view to supporting the immune system in clearing the infection. This reduction in fungal growth could be clearly observed in the microfluidics imaging for the combination of anidulafungin with 5-flucytosine, which only displayed a partially synergistic interaction for the imaged isolate in the checkerboard assays. Furthermore, partially synergistic or additive interactions can lead to reductions in the MICs, potentially allowing for a lowering of antifungal doses, thereby reducing toxicity. Reductions in MICs for partially synergistic, additive, and indifferent combinations have also been observed by others (20, 24), and Caballero and colleagues reported that additive combinations of isavuconazole-echinocandin combinations against C. auris can result in fungistatic effects that were absent for single agents in time-kill assays (23). This finding is similar to our results showing negligible antifungal activity for anidulafungin, manogepix, and 5-flucytosine in monotherapy, whereas the combinations of these two antifungals with anidulafungin showed heightened efficacy, with the reductions in CFU/mL approaching the cidality threshold. The lack of fungicidal activity of the echinocandins against C. auris in time-kill assays has also been observed by others who reported either a fungistatic effect or the complete absence of antifungal activity (22, 23, 42, 43). In comparison to anidulafungin monotherapy, the anidulafungin combinations resulted in 2.1 and 3.6 log10-fold reductions in CFU/mL for the 5-flucytosine and manogepix combinations, respectively, highlighting their advantage over monotherapy.
Cost and additional toxicities are potential barriers to the implementation of antifungal combinations, and, to date, the routine use of antifungal combinations has been largely confined to cryptococcal infections. However, affordable generic echinocandins and 5-flucytosine are now available, and short courses of 5-flucytosine are known to be safe, giving feasible current options with which to try to prevent the inevitable increase in C. auris resistance that is a consequence of the continued use of monotherapies. Furthermore, early studies of combination approaches with new agents, such as fosmanogepix, could expand the options for clinical evaluation and prolong their clinical efficacy.
In most cases, the synergistic interactions that we observed for anidulafungin combined with manogepix or 5-flucytosine were within clinically relevant concentrations. Serum anidulafungin concentrations of up to 7 mg/L are achievable in patients (44, 45), which is above the anidulafungin concentrations corresponding to synergistic interactions for most isolates. For 5-flucytosine, all of the concentrations that we tested fell well below the achievable serum concentrations (44). In the case of fosmanogepix, no clinical pharmacokinetics data are publicly available, to our knowledge. Several safety and pharmacokinetics clinical studies for fosmanogepix have been completed, but no results are available yet (NCT02956499, NCT02957929, NCT03333005). However, the manogepix concentrations at which synergy was observed were relatively low, ranging between 0.002 and 0.03 mg/L.
It should be noted that the current study employed a relatively small number of isolates and that there was an unequal representation of C. auris clades. Additionally, the clustering of the amphotericin B MICs around the breakpoint made it difficult to categorize the isolates according to their amphotericin B susceptibility. Hence, other susceptibility testing methods, such as the Etest, are recommended (17).
In summary, combinations of anidulafungin with manogepix or 5-flucytosine show the highest potential against the tested C. auris isolates. Further studies are needed to determine the mechanisms that underlie these drug interactions and to evaluate their efficacy and safety in a murine model as well as whether these combinations also protect against the development of resistance.
MATERIALS AND METHODS
Fungal isolates.
25 clinical C. auris isolates belonging to clades I, III, and IV that were isolated from 6 patients from a range of sites (blood, urine, respiratory tract, skin) were obtained from the CDC (Table 3). The clade designations were based on whole-genome sequencing (Gifford et al., in preparation). The isolates were maintained at −80°C in 25% glycerol broth and subcultured on Sabouraud dextrose agar (SDA) at 37°C for up to 48 h.
TABLE 3.
Candida auris isolates
| Isolate no. | Cladea | Origin | Isolation dayb | Isolated from | Reference |
|---|---|---|---|---|---|
| B12406 | South American | USA | Day 0 | Patient A, urine | 50 |
| B15223 | South American | USA | Day 294 | Patient A, blood | |
| B19460 | South Asian | USA | Day 0 | Patient B, sputum | |
| B19547 | South Asian | USA | Day 16 | Patient B, unknown | |
| B19617 | South Asian | USA | Day 46 | Patient B, urine | |
| B19837 | South Asian | USA | Day 79 | Patient B, urine | |
| B19618 | South Asian | USA | Day 62 | Patient B, urine | |
| B17040 | South Asian | USA | Day 0 | Patient C, urine | |
| B17041 | South Asian | USA | Day 15 | Patient C, sputum | |
| B17073 | South Asian | USA | Day 44 | Patient C, urine | |
| B17201 | South Asian | USA | Day 67 | Patient C, urine | |
| B18560 | South Asian | USA | Day 0 | Patient D, blood | |
| B18845 | South Asian | USA | Day 72 | Patient D, blood | |
| B18841 | South Asian | USA | Day 103 | Patient D, blood | |
| B18843 | South Asian | USA | Day 96 | Patient D, blood | |
| B12692 | South Asian | USA | Day 11 | Patient E, rectal | 51 |
| B12694 | South Asian | USA | Day 0 | Patient E, groin swab | 51 |
| B12663 | South Asian | USA | Day 11 | Patient E, urine | 51 |
| B12664 | South Asian | USA | Day 11 | Patient E, respiratory | 51 |
| B12688 | South Asian | USA | Day 11 | Patient E, groin swab | 51 |
| B20931 | South African | USA | Day 0 | Patient F, blood | |
| B21040 | South African | USA | Day 3 | Patient F, trachea aspirate | |
| B21041 | South African | USA | Day 3 | Patient F, groin swab | |
| B21042 | South African | USA | Day 3 | Patient F, blood | |
| B21043 | South African | USA | Day 3 | Patient F, blood |
Clade designation is based on whole-genome sequencing (Gifford et al., in preparation).
In reference to the isolation date of the first isolate from the respective patient. Empty cells indicate isolates without known references.
Antifungal susceptibility testing.
Antifungal susceptibility testing was performed using the broth microdilution method, according to the EUCAST guidelines (46). Flat-bottom, tissue-treated 96-well plates were used. Anidulafungin (MedChem Express), amphotericin B (Merck), fluconazole (Thermo Scientific), 5-flucytosine (Thermo Scientific), fosmanogepix (MedChem Express), manogepix (MedChem Express), and voriconazole (Sigma-Aldrich) were dissolved in 100% dimethyl sulfoxide (DMSO). The range of antifungal concentrations tested were 0.016 to 8 mg/L for anidulafungin, 0.03 to 16 mg/L for amphotericin B and voriconazole, 0.25 to 128 mg/L for fluconazole, 0.008 to 4 mg/L for 5-flucytosine, 0.004 to 2 mg/L for fosmanogepix, and 0.002 to 1 mg/L for manogepix. Antifungal dilution series were prepared in RPMI supplemented with glucose to 2% and buffered at pH 7 using 3-(N-morpholino) propanesulfonic acid (MOPS) at a final concentration of 0.165 mol/L (RPMI 2% G-MOPS). Spectrophotometer readings at 530 nm were taken after incubation at 37°C for 24 h. The MIC endpoint for amphotericin B was defined as the lowest concentration leading to a 90% reduction in growth, compared to the drug-free control, whereas 50% reductions in growth, compared to the drug-free control, were used for all of the other antifungal agents. The tentative CDC breakpoints for C. auris were used to define resistance to anidulafungin (≥4 mg/L), amphotericin B (≥2 mg/L), fluconazole (≥32 mg/L), and voriconazole (≥2 mg/L) (https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html). A known issue for the broth microdilution susceptibility testing of amphotericin B in RPMI medium is the clustering of the MICs around the breakpoint of 2 mg/L, which makes it difficult to distinguish resistant and susceptible isolates (32). There are no breakpoints available for 5-flucytosine and fosmanogepix. Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality control strains, as recommended by the EUCAST guidelines (46). All experiments were performed in triplicate.
Antifungal combination testing.
The interactions of the antifungal drugs were tested using checkerboard assays that were based on the EUCAST guidelines (46). The range of antifungal concentrations tested was dependent on the MIC of each isolate, with the highest concentration at 4 × MIC. Columns 3 to 12 of a 96-well microtiter plate were filled with 50 μL of drug A, and rows B to H were filled with 50 μL of drug B. Column 1 served as the drug-free growth and sterility control. The inoculum was prepared by suspending five distinct colonies from 40 to 48-hour-old cultures in distilled water, counting the cell number using a haemocytometer, and adjusting the inocula to 5 × 105 cells/mL. The plates were inoculated with 100 μL and incubated at 37°C for 24 h. OD readings were taken after 24 h by using a spectrophotometer at 530 nm. All experiments were performed in triplicate.
Two different approaches were applied in the analysis of the drug interactions. The fractional inhibitory concentration index (FICI) was calculated as follows:
CA and CB are the concentrations of the drugs A and B in combination, and MICA and MICB are the MICs of the drugs alone. The MIC values were rounded to the next highest 2-fold concentration if the endpoint was not reached within the tested concentration range. The interaction was considered synergistic for FICI ≤ 0.5, partially synergistic between >0.5 and <1.0, additive at 1.0, indifferent between >1.0 and <4 and antagonistic >4 (24). In the following, the term “any synergy” refers to FICI values of <1, thereby including complete and partial synergy. In the presence of antagonism, the maximum median FICI values were reported. Otherwise, the minimum median FICI values were given. Additionally, the drug interactions were visualized using a response surface analysis approach with the Combenefit software package (version 2.021) under the application of the Bliss independence model (47).
Microfluidics imaging.
C. auris B12663 cells were grown and prepared as described above. Inocula were adjusted to 2 × 105 cells/mL. Antifungal monotherapy and combination treatments were prepared in RPMI 2% G-MOPS at the MIC. CellASIC ONIX Y04C microfluidic plates (Millipore Merck) were washed with RPMI 2% G-MOPS by applying 5 lb/in2 perfusion for 5 min, using a CellASIC ONIX2 microfluidic system (version 1.0.4, Millipore Merck). Yeasts were loaded into the CellASIC culture chambers by applying 8 lb/in2 for 5 s twice (Thomson et al., in preparation). The adhered cells were then perfused with RPMI 2% G-MOPS for 4 h at 1 lb/in2. After 4 h, the cells were exposed to either the antifungal(s) or RPMI 2% G-MOPS for the drug-free control by applying 5 lb/in2 for 5 min, and following this with perfusion at 1 lb/in2 for 20 h at 37°C, during which the microfluidic plates were subjected to multipoint 4D imaging on an inverted AxioObserver Z1 microscope (Carl Zeiss). Differential interference contrast (DIC) images were captured using a 20×/0.8 NA PlanApochromatic DIC objective and a 16-bit ORCA-Fusion sCMOS camera (Hamamatsu). The area of colonies over time was measured in FIJI 1.53t (48), using an adapted method for migration analysis from Venter and Niesler (49). Briefly, during the time series, the colony edges were found (Process → Find Edges). The image was blurred 15 times (Process → Smooth) and inverted (Edit → Invert) before thresholding (Image → Adjust → Threshold: Default) to quantify the total fungal area (Analyze → Analyze Particles). The increases in the 2-dimensional colony area were used to calculate the doubling times.
ACKNOWLEDGMENTS
We acknowledge funding from the MRC Centre for Medical Mycology at the University of Exeter (MR/N006364/2 and MR/V033417/1), the NIHR Exeter Biomedical Research Centre, and the MRC Doctoral Training Grant MR/P501955/2. Additional work may have been undertaken by the University of Exeter Biological Services Unit. E.M.B. was also funded by a UK Medical Research Council (MRC) project grant [MR/S001824/1] and Biotechnology and Biological Sciences Research Council (BBSRC) project grant [BB/V017004/1]. A.J.P.B. was also funded by an MRC programme grant [MR/M026663/2]. TB was also funded by a Medical Research Foundation Emerging Leaders in Antimicrobial Resistance Award [MRF-160-0009-ELP-BICA-C0802]. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. For the purpose of open access, the author has applied a 'Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising. The research data supporting this publication are provided within this paper or in the supplementary information accompanying this publication.
E.M.B. and D.D.T. were funded by Gilead Sciences. D.D.T. received a consultancy fee from OwlStone Medical within the last 5 years. T.B. has received research funding from Gilead Sciences, MSD, and Pfizer as well as speaker fees from Gilead Sciences and Pfizer and Advisory Board fees from Gilead Sciences and Mundipharma. M.H. reports grants and research funding from Astellas, Gilead, MSD, Pfizer, Euroimmun, F2G, Pulmocide, IMMY, Mundipharma, and Scynexis, outside the submitted work. T.S.H. has received speaker fees from Gilead and Pfizer, has received an investigator award to the institution from Gilead, and has served as an advisor to F2G.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. 2020. Candida auris: epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog 16:e1008921. doi: 10.1371/journal.ppat.1008921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. 2009. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol 53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 3.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow NA, de Groot T, Badali H, Abastabar M, Chiller TM, Meis JF. 2019. Potential fifth clade of Candida auris, Iran, 2018. Emerg Infect Dis 25:1780–1781. doi: 10.3201/eid2509.190686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, Jang H-C. 2011. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol 49:3139–3142. doi: 10.1128/JCM.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chowdhary A, Sharma C, Duggal S, Agarwal K, Prakash A, Singh PK, Jain S, Kathuria S, Randhawa HS, Hagen F, Meis JF. 2013. New clonal strain of Candida auris, Delhi, India. Emerg Infect Dis 19:1670–1673. doi: 10.3201/eid1910.130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhary A, Anil Kumar V, Sharma C, Prakash A, Agarwal K, Babu R, Dinesh KR, Karim S, Singh SK, Hagen F, Meis JF. 2014. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis 33:919–926. doi: 10.1007/s10096-013-2027-1. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Kaur H, Capoor M, Chhina D, Rao R, Eshwara VK, Xess I, Kindo AJ, Umabala P, Savio J, Patel A, Ray U, Mohan S, Iyer R, Chander J, Arora A, Sardana R, Roy I, Appalaraju B, Sharma A, Shetty A, Khanna N, Marak R, Biswas S, Das S, Harish BN, Joshi S, Mendiratta D. 2015. Incidence, characteristics and outcome of ICU-acquired candidemia in India. Intensive Care Med 41:285–295. doi: 10.1007/s00134-014-3603-2. [DOI] [PubMed] [Google Scholar]
- 9.Calvo B, Melo ASA, Perozo-Mena A, Hernandez M, Francisco EC, Hagen F, Meis JF, Colombo AL. 2016. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect 73:369–374. doi: 10.1016/j.jinf.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Chowdhary A, Sharma C, Meis JF. 2017. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13:e1006290. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudramurthy SM, Chakrabarti A, Paul RA, Sood P, Kaur H, Capoor MR, Kindo AJ, Marak RSK, Arora A, Sardana R, Das S, Chhina D, Patel A, Xess I, Tarai B, Singh P, Ghosh A. 2017. Candida auris candidaemia in Indian ICUs: analysis of risk factors. J Antimicrob Chemother 72:1794–1801. doi: 10.1093/jac/dkx034. [DOI] [PubMed] [Google Scholar]
- 12.Robbins N, Wright GD, Cowen LE. 2016. Antifungal drugs: the current armamentarium and development of new agents. Microbiol Spectr 4. doi: 10.1128/microbiolspec.FUNK-0002-2016. [DOI] [PubMed] [Google Scholar]
- 13.PHE. 2017. Guidance for the laboratory investigation, management and infection prevention and control for cases of Candida auris v2.0. Public Health England, London. [Google Scholar]
- 14.WHO. 2022. WHO fungal priority pathogens list to guide research, development and public health action. World Health Organization, Geneva. [Google Scholar]
- 15.Biagi MJ, Wiederhold NP, Gibas C, Wickes BL, Lozano V, Bleasdale SC, Danziger L. 2019. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect Dis 6:ofz262. doi: 10.1093/ofid/ofz262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrowsky B, Greenko J, Adams E, Quinn M, O’Brien B, Chaturvedi V, Berkow E, Vallabhaneni S, Forsberg K, Chaturvedi S, Lutterloh E, Blog D, Bucher C, Denis RJ, Erazo R, Fernandez R, Southwick K, Zhu YC, C. auris Investigation Work Group . 2020. Candida auris isolates resistant to three classes of antifungal medications — New York, 2019. MMWR Morb Mortal Wkly Rep 69:6–9. doi: 10.15585/mmwr.mm6901a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lockhart SR. 2019. Candida auris and multidrug resistance: defining the new normal. Fungal Genet Biol 131:103243. doi: 10.1016/j.fgb.2019.103243. [DOI] [PubMed] [Google Scholar]
- 18.Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, Tarai B, Singh A, Upadhyaya G, Upadhyay S, Yadav P, Singh PK, Khillan V, Sachdeva N, Perlin DS, Meis JF. 2018. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J Antimicrob Chemother 73:891–899. doi: 10.1093/jac/dkx480. [DOI] [PubMed] [Google Scholar]
- 19.CDC. 2019. Antibiotic resistance threats in the United States, 2019. U.S. Department of Health and Human; Services, CDC, Atlanta, GA. [Google Scholar]
- 20.Fakhim H, Chowdhary A, Prakash A, Vaezi A, Dannaoui E, Meis JF, Badali H. 2017. In vitro interactions of echinocandins with triazoles against multidrug-resistant Candida auris. Antimicrob Agents Chemother 61:e01056-17. doi: 10.1128/AAC.01056-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaggavarapu S, Burd EM, Weiss DS. 2020. Micafungin and amphotericin B synergy against Candida auris. Lancet Microbe 1:e314–e315. doi: 10.1016/S2666-5247(20)30194-4. [DOI] [PubMed] [Google Scholar]
- 22.Caballero U, Eraso E, Quindós G, Jauregizar N. 2021. In vitro interaction and killing-kinetics of amphotericin B combined with anidulafungin or caspofungin against Candida auris. Pharmaceutics 13:1333. doi: 10.3390/pharmaceutics13091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caballero U, Kim S, Eraso E, Quindós G, Vozmediano V, Schmidt S, Jauregizar N. 2021. In vitro synergistic interactions of isavuconazole and echinocandins against Candida auris. Antibiotics 10:355. doi: 10.3390/antibiotics10040355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfaller MA, Messer SA, Deshpande LM, Rhomberg PR, Utt EA, Castanheira M. 2021. Evaluation of synergistic activity of isavuconazole or voriconazole plus anidulafungin and the occurrence and genetic characterization of Candida auris detected in a surveillance program. Antimicrob Agents Chemother 65:e02031-20. doi: 10.1128/AAC.02031-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bidaud AL, Botterel F, Chowdhary A, Dannaoui E. 2019. In vitro antifungal combination of flucytosine with amphotericin B, voriconazole, or micafungin against Candida auris shows no antagonism. Antimicrob Agents Chemother 63:e01393-19. doi: 10.1128/AAC.01393-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien B, Chaturvedi S, Chaturvedi V. 2020. In vitro evaluation of antifungal drug combinations against multidrug-resistant Candida auris isolates from New York outbreak. Antimicrob Agents Chemother 64:e02195-19. doi: 10.1128/AAC.02195-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien B, Liang J, Chaturvedi S, Jacobs JL, Chaturvedi V. 2020. Pan-resistant Candida auris: New York subcluster susceptible to antifungal combinations. Lancet Microbe 1:e193–e194. doi: 10.1016/S2666-5247(20)30090-2. [DOI] [PubMed] [Google Scholar]
- 28.Molloy SF, Kanyama C, Heyderman RS, Loyse A, Kouanfack C, Chanda D, Mfinanga S, Temfack E, Lakhi S, Lesikari S, Chan AK, Stone N, Kalata N, Karunaharan N, Gaskell K, Peirse M, Ellis J, Chawinga C, Lontsi S, Ndong J-G, Bright P, Lupiya D, Chen T, Bradley J, Adams J, van der Horst C, Oosterhout JJ, van Sini V, Mapoure YN, Mwaba P, Bicanic T, Lalloo DG, Wang D, Hosseinipour MC, Lortholary O, Jaffar S, Harrison TS. ACTA Trial Study Team. 2018. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 378:1004–1017. doi: 10.1056/NEJMoa1710922. [DOI] [PubMed] [Google Scholar]
- 29.Mashau RC, Meiring ST, Quan VC, Nel J, Greene GS, Garcia A, Menezes C, Reddy DL, Venter M, Stacey S, Madua M, Boretti L, Harrison TS, Meintjes G, Shroufi A, Trivino-Duran L, Black J, Govender NP, Abrahams S, Pearce V, Moncho M, Wadula J, Maloba M, Hoosen A, Verwey C, Menezes C, Moore D, Pombo D, Reubenson G, Ntlemo G, Richards L, Nchabeleng M, Tsitsi M, Moshe M, Said M, Kolojane M, Mothibi L, Plessis ND, Chomba R, Thomas T, Avenant T, Nana T, Chibabhai V, Maharj A, Wilson D, Naby F, Dawood H, Han KSS, Sookan L, Dlamini N, et al. 2022. Outcomes of flucytosine-containing combination treatment for cryptococcal meningitis in a South African national access programme: a cross-sectional observational study. Lancet Infect Dis 22:1365–1373. doi: 10.1016/S1473-3099(22)00234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoenigl M, Sprute R, Egger M, Arastehfar A, Cornely OA, Krause R, Lass-Flörl C, Prattes J, Spec A, Thompson GR, Wiederhold N, Jenks JD. 2021. The antifungal pipeline: fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 81:1703–1729. doi: 10.1007/s40265-021-01611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw KJ, Ibrahim AS. 2020. Fosmanogepix: a review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. JoF 6:239. doi: 10.3390/jof6040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis RE, Diekema DJ, Messer SA, Pfaller MA, Klepser ME. 2002. Comparison of Etest, chequerboard dilution and time–kill studies for the detection of synergy or antagonism between antifungal agents tested against Candida species. J Antimicrob Chemother 49:345–351. doi: 10.1093/jac/49.2.345. [DOI] [PubMed] [Google Scholar]
- 33.Gow NAR, Johnson C, Berman J, Coste AT, Cuomo CA, Perlin DS, Bicanic T, Harrison TS, Wiederhold N, Bromley M, Chiller T, Edgar K. 2022. The importance of antimicrobial resistance in medical mycology. Nat Commun 13:5352. doi: 10.1038/s41467-022-32249-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagy F, Tóth Z, Nyikos F, Forgács L, Jakab Á, Borman AM, Majoros L, Kovács R. 2021. In vitro and in vivo interaction of caspofungin with isavuconazole against Candida auris planktonic cells and biofilms. Med Mycol 59:1015–1023. doi: 10.1093/mmy/myab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berkow EL, Lockhart SR. 2018. Activity of novel antifungal compound APX001A against a large collection of Candida auris. J Antimicrob Chemother 73:3060–3062. doi: 10.1093/jac/dky302. [DOI] [PubMed] [Google Scholar]
- 36.Hager CL, Larkin EL, Long L, Abidi FZ, Shaw KJ, Ghannoum MA. 2018. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 62:e02319-17. doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaller MA, Huband MD, Flamm RK, Bien PA, Castanheira M. 2019. In vitro activity of APX001A (manogepix) and comparator agents against 1,706 fungal isolates collected during an international surveillance program in 2017. Antimicrob Agents Chemother 63. doi: 10.1128/AAC.00840-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arendrup MC, Chowdhary A, Jørgensen KM, Meletiadis J. 2020. Manogepix (APX001A) in vitro activity against Candida auris: head-to-head comparison of EUCAST and CLSI MICs. Antimicrob Agents Chemother 64:e00656-20. doi: 10.1128/AAC.00656-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maphanga TG, Mpembe RS, Naicker SD, Govender NP, Germs-Sa F, for GERMS-SA . 2022. In vitro antifungal activity of manogepix and other antifungal agents against South African Candida auris isolates from bloodstream infections. Microbiol Spectr 10:e01717-21. doi: 10.1128/spectrum.01717-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Y, Kilburn S, Kapoor M, Chaturvedi S, Shaw KJ, Chaturvedi V. 2020. In vitro activity of manogepix against multidrug-resistant and panresistant Candida auris from the New York outbreak. Antimicrob Agents Chemother 64. doi: 10.1128/AAC.01124-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gebremariam T, Gu Y, Alkhazraji S, Youssef E, Shaw KJ, Ibrahim AS. 2022. The combination treatment of fosmanogepix and liposomal amphotericin B is superior to monotherapy in treating experimental invasive mold infections. Antimicrob Agents Chemother 66:e0038022. doi: 10.1128/aac.00380-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dudiuk C, Berrio I, Leonardelli F, Morales-Lopez S, Theill L, Macedo D, Yesid-Rodriguez J, Salcedo S, Marin A, Gamarra S, Garcia-Effron G. 2019. Antifungal activity and killing kinetics of anidulafungin, caspofungin and amphotericin B against Candida auris. J Antimicrob Chemother 74:2295–2302. doi: 10.1093/jac/dkz178. [DOI] [PubMed] [Google Scholar]
- 43.Kovács R, Tóth Z, Locke JB, Forgács L, Kardos G, Nagy F, Borman AM, Majoros L. 2021. Comparison of in vitro killing activity of rezafungin, anidulafungin, caspofungin, and micafungin against four Candida auris clades in RPMI-1640 in the absence and presence of human serum. 4. Microorganisms 9:863. doi: 10.3390/microorganisms9040863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bellmann R, Smuszkiewicz P. 2017. Pharmacokinetics of antifungal drugs: practical implications for optimized treatment of patients. Infection 45:737–779. doi: 10.1007/s15010-017-1042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dupont H, Massias L, Jung B, Ammenouche N, Montravers P. 2017. Pharmacokinetic study of anidulafungin in ICU patients with intra-abdominal candidiasis. J Antimicrob Chemother 72:1429–1432. doi: 10.1093/jac/dkw568. [DOI] [PubMed] [Google Scholar]
- 46.European Committee on Antimicrobial Susceptibility Testing. 2020. EUCAST Definitive Document E.Def 7.3.2 Revision: method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts. ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST; ). [Google Scholar]
- 47.Di Veroli GY, Fornari C, Wang D, Mollard S, Bramhall JL, Richards FM, Jodrell DI. 2016. Combenefit: an interactive platform for the analysis and visualization of drug combinations. Bioinformatics 32:2866–2868. doi: 10.1093/bioinformatics/btw230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venter C, Niesler CU. 2019. Rapid quantification of cellular proliferation and migration using ImageJ. Biotechniques 66:99–102. doi: 10.2144/btn-2018-0132. [DOI] [PubMed] [Google Scholar]
- 50.Chow NA, Gade L, Tsay SV, Forsberg K, Greenko JA, Southwick KL, Barrett PM, Kerins JL, Lockhart SR, Chiller TM, Litvintseva AP, US Candida auris Investigation Team . 2018. Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: a molecular epidemiological survey. Lancet Infect Dis 18:1377–1384. doi: 10.1016/S1473-3099(18)30597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Pilato V, Codda G, Ball L, Giacobbe DR, Willison E, Mikulska M, Magnasco L, Crea F, Vena A, Pelosi P, Bassetti M, Marchese A. 2021. Molecular epidemiological investigation of a nosocomial cluster of C. auris: evidence of recent emergence in Italy and ease of transmission during the COVID-19 pandemic. JoF 7:140. doi: 10.3390/jof7020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aac.01645-22-s0001.pdf, PDF file, 4.1 MB (4.1MB, pdf)
Supplemental material. Download aac.01645-22-s0002.avi, AVI file, 2.4 MB (2.4MB, avi)
Supplemental material. Download aac.01645-22-s0003.avi, AVI file, 2.5 MB (2.5MB, avi)