Abstract
In this article, we present the case of a patient with previously undiagnosed myasthenia gravis who underwent an endoscopic procedure for Zenker’s diverticulum. The patient was readmitted due to ongoing dysphagia and severe respiratory distress caused by myasthenic crisis. This case demonstrates that myasthenia gravis, although rare, can occur in elderly patients and present with other sequelae that may mask the underlying diagnosis.
KEYWORDS: Myasthenia gravis, myasthenic crisis, Zenker’s diverticulum, ZPOEM
CASE SUMMARY
An 81-year-old woman presented with dysphagia that had begun 4 months earlier and had progressed. Her past medical history was significant for anxiety, depression, hypothyroidism, and morbid obesity. To further evaluate her dysphagia, a barium esophagram was obtained and she was found to have a Zenker’s diverticulum (Figure 1). Due to her symptoms and imaging findings, she was scheduled for a per oral endoscopic myotomy for Zenker’s diverticulum (ZPOEM). The ZPOEM procedure was uneventful, and her postoperative esophagram showed no evidence of a leak. Normally, these patients are discharged on postoperative day 1; however, the patient continued to complain of dysphagia despite the success of the procedure, as seen on the postoperative esophagram (Figure 2). She was given dexamethasone to help with nausea and inflammation, which improved her dysphagia. She was discharged on postoperative day 2 when she demonstrated adequate oral intake.
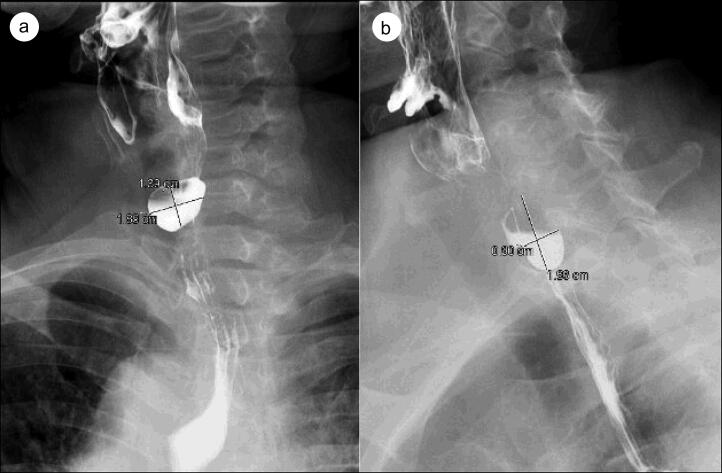
Figure 1.
(a) Frontal and (b) lateral views of preoperative barium esophagram demonstrating a Zenker’s diverticulum in the posterior proximal esophagus.
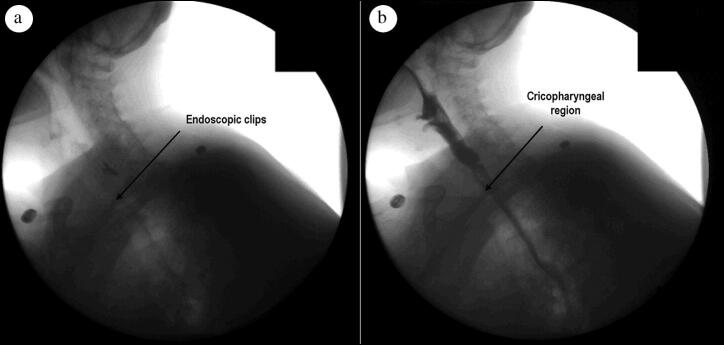
Figure 2.
Postoperative barium esophagram obtained after ZPOEM demonstrating no evidence of a leak, with an arrow identifying (a) the endoscopic clips used to close the mucosal incision in (b) the cricopharyngeal region.
Three days later, she presented to an outside hospital with recurrent dysphagia and severe dehydration. Her electrolytes, specifically magnesium, were repleted and she was given additional steroids. She continued to deteriorate despite these measures and was transferred back to our institution. Upon arrival, the patient was obtunded and her neurologic exam revealed nystagmus, pinpoint pupils, generalized weakness, and dysarthric speech. In addition, she was hypoxic, requiring nasal cannula, and an arterial blood gas was notable for compensated respiratory acidosis. She was placed on bilevel positive airway pressure and admitted to the intensive care unit for close monitoring of her respiratory status. The patient underwent an extensive workup including computed tomography (CT) of the head, CT angiogram, echocardiogram, electrocardiogram, chest x-ray, and full panel laboratory workup that was unremarkable. Over the next few days, she continued to have altered mental status due to hypoxemia, and attempts at weaning her oxygen requirements were unsuccessful. After further discussions with the family, it was discovered that she had issues with intermittent blurry vision and weakness over the previous 4 months in addition to her dysphagia, which the patient did not disclose during her initial preoperative visit.
At this time, a disease of neuromuscular origin, such as myasthenia gravis (MG), became the primary diagnostic concern. Neurology was consulted to evaluate the patient, and on their exam she continued to have nystagmus, fatigue with upward gaze, ptosis, and weakness in all extremities. After careful review, a trial of pyridostigmine and intravenous immunoglobulin was started. The day after therapy initiation, the patient had profound improvement of her altered mental status, ocular symptoms, muscle weakness, and dysphagia. She was able to be transferred out of the intensive care unit and underwent a thorough workup for MG. Her acetylcholine receptor (AChR) analysis was positive, and nerve conduction studies showed evidence of neuromuscular junction compromise and chronic length-dependent sensorimotor axonal polyneuropathy. The results of these studies ultimately confirmed the diagnosis of MG with subsequent myasthenic crisis. The patient improved with appropriate therapy and was discharged home on pyridostigmine in stable condition. At her clinic visit, she was symptom free while taking pyridostigmine.
CLINICAL QUESTIONS
-
An 81-year-old woman presents with fatigue. She reports over the past 4 months that she has been feeling weak, especially by the end of the day. She also reports issues with blurry vision. On exam, she has bilateral ptosis and 4/5 strength in all four extremities. Sensation and underlying reflexes are normal. What is the underlying pathophysiology of the most likely diagnosis?
Demyelination of the peripheral nervous system
Demyelination of the central nervous system
Presynaptic voltage-gated calcium channel antibodies
Postsynaptic acetylcholine receptor antibodies
-
The patient from the previous question presents to the hospital in severe respiratory distress. You suspect myasthenic crisis. What are the primary initial therapies for myasthenic crisis?
Rituximab
Cyclophosphamide
Plasma exchange and intravenous immunoglobulin
Glucocorticoids
DISCUSSION
Background
MG is an autoimmune disorder of the neuromuscular junction that is most commonly mediated by antibodies directed toward the AChR on postsynaptic membranes. Although relative rare, MG is the most common disorder of the neuromuscular junction with a prevalence of 20 per 100,000 population in the United States. It exhibits a female predominance in those <40 years of age and a male predominance in those >50 years of age.1 Both predisposing genetic and environmental factors play crucial roles in the induction of MG. Factors known to uncover myasthenia include physiologic stress, infections, immunizations, and certain drugs like checkpoint inhibitors.2
Presentation and diagnosis
The distinguishing clinical feature of MG is fluctuating weakness that varies in severity, worsens with activity, and improves with rest.2 MG can produce weakness in any musculoskeletal group. Isolated ocular symptoms, such as ptosis or diplopia, are the presenting feature in over 50% of patients.3 Bulbar presentation occurs in approximately 15% of patients, and symptoms may include dysphagia, dysarthria, or chewing difficulties.4 Other presentations, such as proximal limb weakness without additional symptoms, are less common. Patients can also present with life-threatening respiratory depression, known as myasthenic crisis. Interestingly, approximately 20% of patients who experience a myasthenic crisis have it as their initial presentation upon diagnosis.5 The course of MG is varied. The symptoms can be episodic and worsen over time. Alternatively, patients can reach maximum severity within 6 months after the onset of symptoms.6
Physical examination may reveal muscle weakness, ptosis, or impaired extraocular movements. However, physical examination may reveal normal muscle strength due to a fluctuating disease pattern. In these cases, repeated muscle use can demonstrate weakness. In a patient with suspected MG, serum testing for AChR antibodies is the first recommended step in diagnosis.7 Multiple other diagnostic tests can be performed. Electrodiagnostic techniques, such as nerve conduction studies and routine needle electromyography, can be performed to exclude alternative diagnoses or to confirm a concomitant disorder.7 Pharmacologic testing with edrophonium can be used, but is associated with serious adverse reactions.8 The ice pack test is a quick and simple bedside method that can be used. If there is improvement in ptosis after ice is placed on a closed lid for 2 minutes, it is a positive test result.9
Management
The mainstay of treatment for MG in symptomatic patients involves the use of cholinesterase enzyme inhibitors, such as pyridostigmine. If patients remain significantly symptomatic on pyridostigmine, they can be placed on immunosuppressive agents. These typically include glucocorticoids initially, and patients may require immunotherapeutic agents such as azathioprine or mycophenolate.10 Although used for treatment, steroids can lead to exacerbation of MG. Predictors of exacerbation from steroids include older age and bulbar symptoms. Numerous medications, including magnesium, may also exacerbate MG and should be used cautiously in these patients after surgery.5 Lastly, all patients with known MG should have a CT scan of the chest to look for thymomas. Thymectomy has been shown to lead to symptomatic improvement in these patients.11
In patients presenting in myasthenic crisis, airway support is paramount for optimal outcomes. Patients with appropriate mentation and secretion management may be candidates for noninvasive positive pressure ventilation or bilevel positive airway pressure. Intubation can be performed if necessary, and decisions regarding this are similar to those for other critically ill patients. Once a patient with myasthenic crisis is stabilized, further evaluation can be performed to evaluate for triggers. Infectious evaluation may include a chest radiograph, complete blood count, urinalysis, and blood cultures. As metabolic abnormalities can result in crisis, electrolyte levels and thyroid function tests should be checked. Pregnancy can precipitate crisis, and beta-human chorionic gonadotropin levels should be obtained in females of childbearing age. Creatinine kinase levels can be measured to evaluate for other myopathies. Electrocardiography is useful in evaluating a cardiac dysrhythmia or a toxicologic etiology. Arterial or venous blood gases may not be useful for diagnosing crisis, but can help with ventilator management. Plasma exchange and intravenous immunoglobulin are the primary treatments for myasthenic crisis.12 If the trigger is identified, the underlying cause should be treated concurrently.
Evaluation
MG is the most common disorder of the neuromuscular junction and can have differing patient presentations. In our case, the patient had ocular symptoms for months preoperatively, but we suspect her MG was triggered by high-dose steroids given her recent surgery for a Zenker’s diverticulum that was masking the real cause of her profound dysphagia. Patients with MG can present in myasthenic crisis as their initial presentation. For these patients, high suspicion and prompt recognition are imperative for good patient outcomes. After the patient is stabilized, treatment can be initiated with plasma exchange and intravenous immunoglobulin.
Disclosure statement/Funding
The authors report no funding or conflicts of interest. The patient gave consent for this case report to be published.
ANSWERS FOR CLINICAL QUESTIONS
Question 1, D; Question 2, C.
References
- 1.Jayam Trouth A, Dabi A, Solieman N, Kurukumbi M, Kalyanam J.. Myasthenia gravis: a review. Autoimmune Dis. 2012;2012:874680. doi: 10.1155/2012/874680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beloor Suresh A, Asuncion RMD.. Myasthenia gravis. In: StatPearls. Treasure Island, FL: StatPearls Publishing; September 16, 2022. [PubMed] [Google Scholar]
- 3.Nair AG, Patil-Chhablani P, Venkatramani DV, Gandhi RA.. Ocular myasthenia gravis: a review. Indian J Ophthalmol. 2014;62(10):985–991. doi: 10.4103/0301-4738.145987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meriggioli MN, Sanders DB.. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8(5):475–490. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wendell LC, Levine JM.. Myasthenic crisis. Neurohospitalist. 2011;1(1):16–22. doi: 10.1177/1941875210382918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grob D, Brunner N, Namba T, Pagala M.. Lifetime course of myasthenia gravis. Muscle Nerve. 2008;37(2):141–149. doi: 10.1002/mus.20950. [DOI] [PubMed] [Google Scholar]
- 7.Rousseff RT. Diagnosis of myasthenia gravis. J Clin Microbiol. 2021;10(8):1736. doi: 10.3390/jcm10081736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ing EB, Ing SY, Ing T, Ramocki JA.. The complication rate of edrophonium testing for suspected myasthenia gravis. Can J Ophthalmol. 2000;35(3):141–144; discussion 145. doi: 10.1016/s0008-4182(00)80007-1. [DOI] [PubMed] [Google Scholar]
- 9.Larner AJ. The place of the ice pack test in the diagnosis of myasthenia gravis. Int J Clin Pract. 2004;58(9):887–888. doi: 10.1111/j.1742-1241.2004.00053.x. [DOI] [PubMed] [Google Scholar]
- 10.Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ.. Treatment of myasthenia gravis. Neurol Clin. 2018;36(2):311–337. doi: 10.1016/j.ncl.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson SP, Akhondi H.. Thymoma. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2022. [Google Scholar]
- 12.Roper J, Fleming ME, Long B, Koyfman A.. Myasthenia gravis and crisis: evaluation and management in the emergency department. J Emerg Med. 2017;53(6):843–853. doi: 10.1016/j.jemermed.2017.06.009. [DOI] [PubMed] [Google Scholar]