Abstract

The ocean constitutes approximately 70% of Earth’s surface. Its average depth is 3688 m, of which depths beyond 200 m are classified as the deep sea. The deep sea is distinct from the surface of the ocean in terms of pressure, temperature, and sunlight. The unique physicochemical processes under the extreme environment of the deep sea and the specialized biochemical mechanisms developed by organisms to survive in the deep sea can serve as a vast source of inspiration for scientific and technological advancements. In this Perspective, we discuss three examples of deep-sea-inspired chemistry: (1) soft materials that respond to high pressures such as those observed in the deep sea; (2) molecular self-assembly inspired by the chemistry of hot and compressed water in deep-sea hydrothermal vents; and (3) nanobiotechnology and biomimetics inspired by survival strategies of deep-sea organisms. Finally, we provide an outlook on deep-sea-inspired chemistry. This Perspective aims to promote the sustainable utilization of the ocean based on knowledge, as opposed to the conventional utilization of the ocean solely based on resources. We hope that this Perspective will encourage chemists to harness their inspiration gleaned from the deep sea.
1. Introduction
Sustainability is the greatest challenge that humanity is facing today. Harnessing the benefits of the oceans, which constitutes approximately 70% of Earth’s surface, is the key to achieving a sustainable society that coexists in harmony with Earth. Thus far, we have been operating under the traditional paradigm of a linear economy, relying on the food and energy resources provided by the oceans. To realize a sustainable society, however, a paradigm shift from a linear to a circular economy is required. The new paradigm should be based on the sustainable benefits of the ocean.
The average depth of the ocean is 3688 m, of which depths beyond 200 m are classified as the deep sea. The deep sea is significantly different from the surface of the ocean (Figure 1).1 The hydrostatic pressure in the deep sea steadily increases by 0.1 MPa for every 10 m of water depth and reaches 110 MPa at the deepest point (approximately 11 000 m), which is the Challenger Deep at the southern end of the Mariana Trench. The deep sea is also lightless because sunlight does not penetrate below 200 m. Hence, the temperature below 1000 m is approximately 5 °C. However, hydrothermal vents have hot and compressed water, sometimes in the supercritical state (Tc = 374 °C, Pc = 22.1 MPa), which erupts into the cold deep water.2 Despite the harsh conditions, the deep sea contains a rich biodiversity.3
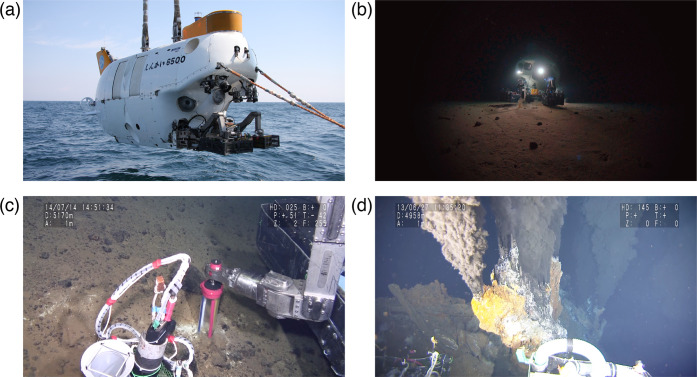
Figure 1.
(a) Manned submersible Shinkai 6500. Shinkai means the “deep sea” in Japanese, and the number 6500 (meters) represents its diving capability. Reproduced with permission from ref (1). Copyright 2012 JAMSTEC. (b) Shinkai 6500 at the bottom of the Izu-Ogasawara Trench at a depth of 6500 m. Reproduced with permission from ref (1). Copyright 2019 JAMSTEC. (c) Sampling operation of deep-sea sediment in the Mariana Trench at a depth of 5170 m. Reproduced with permission from ref (1). Copyright 2014 JAMSTEC. (d) Hydrothermal venting from the deep-sea floor in the Mid-Cayman Rise at a depth of 4958 m. Reproduced with permission from ref (1). Copyright 2013 JAMSTEC.
Nature is a rich source of inspiration for new scientific and technological advancements, and the deep sea is no exception. This Perspective provides an overview of deep-sea-inspired chemistry to inspire interested chemists to pursue innovative chemistry. We believe that deep-sea-inspired chemistry will lead to the sustainable utilization of the ocean based on knowledge, as opposed to the conventional utilization of the ocean based on resources.
2. Pressure-Responsive Soft Materials
Pressure is one of the state quantities that determines the thermodynamic state of a system but is often treated as a constant, implicitly fixed at 0.1 MPa. It becomes a variable between 0.1 and 110 MPa when considering deep-sea conditions. Unlike temperature, pressure propagates instantaneously and uniformly throughout a system, making pressure-responsive systems dynamic, manageable, and scalable. In addition, generating and maintaining high pressures equivalent to hydrostatic pressures in the deep sea does not require much energy (Figure 2a). Thus, materials that respond to high pressures such as those observed in the deep sea could be useful for developing sustainable solutions, such as for material processing with reduced energy consumption and CO2 emissions.
Figure 2.
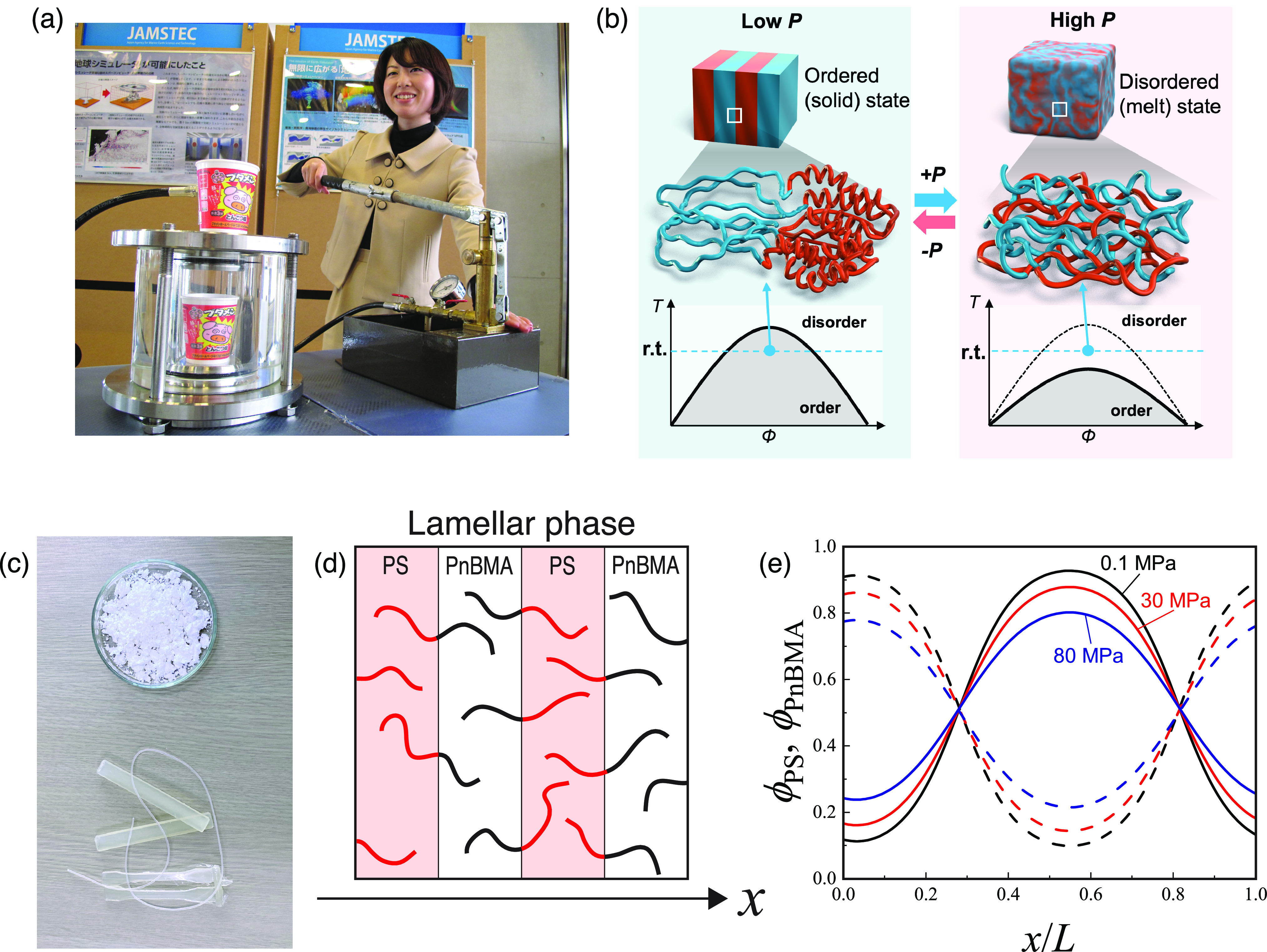
Baroplastic behavior of block copolymers. (a) Experiment demonstrating the isotropic deformation of Styrofoam upon pressurization in water. This hand-operated pump can generate high pressures of up to ∼100 MPa. Reproduced with permission from ref (1). Copyright 2014 JAMSTEC. (b) Schematic of pressure-induced order–disorder transition of block copolymers. (c) Compression melt molding of degradable baroplastic. Polymer powder (top) was compressed at ambient temperature and 50 MPa to obtain a strand (bottom). Other molded products at the bottom were obtained at 100 °C and 1 MPa. Adapted with permission from ref (5). Copyright 2022 Royal Society of Chemistry. (d, e) Changes in the normalized concentration profiles of PS-b-PnBMA upon compression at 443 K in the direction perpendicular to the lamellar interfaces. The length is scaled by the lamellar period L. Adapted with permission from ref (9). Copyright 2022 Society of Polymer Science, Japan.
From the perspective of pressure-responsive soft materials, polymer mixtures that undergo phase transitions between ordered and disordered states upon compression, called baroplastics, are of great interest (Figure 2b).4 Typical baroplastics are block copolymers comprising soft and hard segments. The soft segment exhibits a low glass-transition temperature (Tg) and exists in a rubbery state at ambient temperature, whereas the hard segment shows a high Tg and exists in a glassy state at ambient temperature. Unlike thermoplastic block copolymers, baroplastic block copolymers undergo an order–disorder transition and flow upon compression at ambient temperatures.
Baroplastics have some green advantages over conventional thermoplastics: First, polymers are heated to 200 °C or higher to obtain sufficient fluidity for thermoplastic molding. In contrast, baroplastics require only ambient temperatures (Figure 2c). Low-temperature processing significantly reduces energy consumption and CO2 emissions in polymer processing. Second, low-temperature polymer processing eliminates the thermal degradation of the polymer during processing. A previous study reported that the mechanical properties of the baroplastic remained unchanged even after 10 cycles of repeated processing.5 This degradation-free processability significantly improves the polymer lifetime during materials recycling.
Certain degradable polymers such as aliphatic block polyesters also show baroplasticity.6 Such baroplastics exhibit low-temperature processability and biodegradability and provide unique opportunities for developing biomaterials and sustainable plastics. For example, a baroplastic block copolymer comprising poly(2-isopropoxy-2-oxo-1,3,2-dioxaphospholane) as the soft segment and poly(l-lactide) as the hard segment was successfully composited with a hydrolytic enzyme, proteinase K.7 A composite film was obtained via a simple method of mixing the polymer powders and the enzyme powders and compressing the mixture at 35 MPa for 5 min at ambient temperature. As the enzyme did not lose its catalytic activity during low-temperature processing, the composite film was swiftly degraded when immersed in a buffered solution. In addition, degradable baroplastics prepared from renewable biobased monomers are more environmentally benign.5
Experimental studies on material properties under high pressures require an apparatus equipped with high-pressure cells, limiting available analytical methods. Therefore, theoretical considerations and numerical simulations are more valuable tools for studying material properties under high pressures than under ambient pressures. Compressible regular solution (CRS) theory, which is based on the Flory–Huggins solution theory, has played a major role in the development of baroplastics.8 The CRS theory predicts the miscibility of two incompatible polymers upon compression and has been successfully used to select suitable monomer species for the design of baroplastic block copolymers. However, this theory does not explain the phase behavior of block copolymers upon compression. Nevertheless, a recent study based on the self-consistent field (SCF) theory successfully described the pressure-induced structural transformation of baroplastic block copolymers.9
The SCF theory has been widely used to study microphase-separated structures of block copolymers comprising various chain architectures and block sequences.10 The compressive SCF theory expresses the phase change of block copolymers upon compression using density compressibility via the effective Flory–Huggins interaction parameter, χeff, derived from CRS theory
| 1 |
where β = 1/kBT, z is the coordination number, and ϵij is the monomer interaction energy between the ith and the jth components. The reduced density, ρ̃i (T,P), can be determined from the equation of the state of the ith component.
Figure 2d and 2e shows changes in the concentration profile of the lamellar structure of a typical baroplastic block copolymer, polystyrene-block-poly(n-butyl methacrylate) (PS-b-PnBMA), as a function of pressure. The concentration profile of the lamellar structure flattens with increasing pressure, demonstrating the compatibilization of the two segments upon compression. The density difference, |ρ̃A – ρ̃B|, decreased with increasing pressure owing to the compressibility difference between the two segments. As the second term of eq 1 is proportional to the density difference |ρ̃A – ρ̃B|, a smaller |ρ̃A – ρ̃B| results in a smaller χeff. This suggests that the compressibility difference plays a major role in baroplastic behavior.
As first reported in 1998,11 baroplastics constitute a relatively new category of functional polymers. There is still extensive scope for fundamental research for this category of materials, such as by exploiting molecular designs (e.g., monomer combinations, molecular-weight distributions, and chain architectures) for controlling the pressure response and mechanical properties. On the other hand, developing new applications that take advantage of the pressure responsiveness, such as through adhesion, lubrication, sensors, and reactivity control, is a future challenge.
Pressures above the deep-sea hydrostatic pressure alter the structure of the hydration shell around hydrophobic solutes12 and can sometimes have lethal consequences for biological self-assemblies, such as the disruption of cell membranes13 and protein denaturation.14 Such high pressures (400–600 MPa) have been exploited for the inactivation of bacteria in food engineering since the late 19th century.15 Tissue decellularization is an intriguing application of such high pressures for producing biomaterials.16 A previous study showed that the high-pressure treatment of porcine blood vessels in phosphate-buffered saline (PBS) at 980 MPa disrupted the cells inside the tissue. Washing the cell debris with PBS afforded decellularized blood vessels, which showed promising post-transplantation performance, including reduced inflammation, no clot formation, and cellular infiltration without compromising the mechanical properties of the blood vessels.
3. Molecular Self-Assembly Inspired by the Chemistry of Hydrothermal Vents
Hot and compressed water, especially supercritical water, exhibits properties that are remarkably different from those of ambient water.17 In particular, the dielectric constant of water, which is ∼80 under ambient conditions, decreases to 3 in the supercritical state. This value is comparable to that of typical hydrocarbons. Consequently, oil and water become freely miscible (Figure 3a),18 whereas inorganic salts precipitate out.19 This unique phase behavior inspired us to develop a bottom-up process called monodisperse nanodroplet generation in quenched hydrothermal solution (MAGIQ) for preparing nanoemulsions.20
Figure 3.
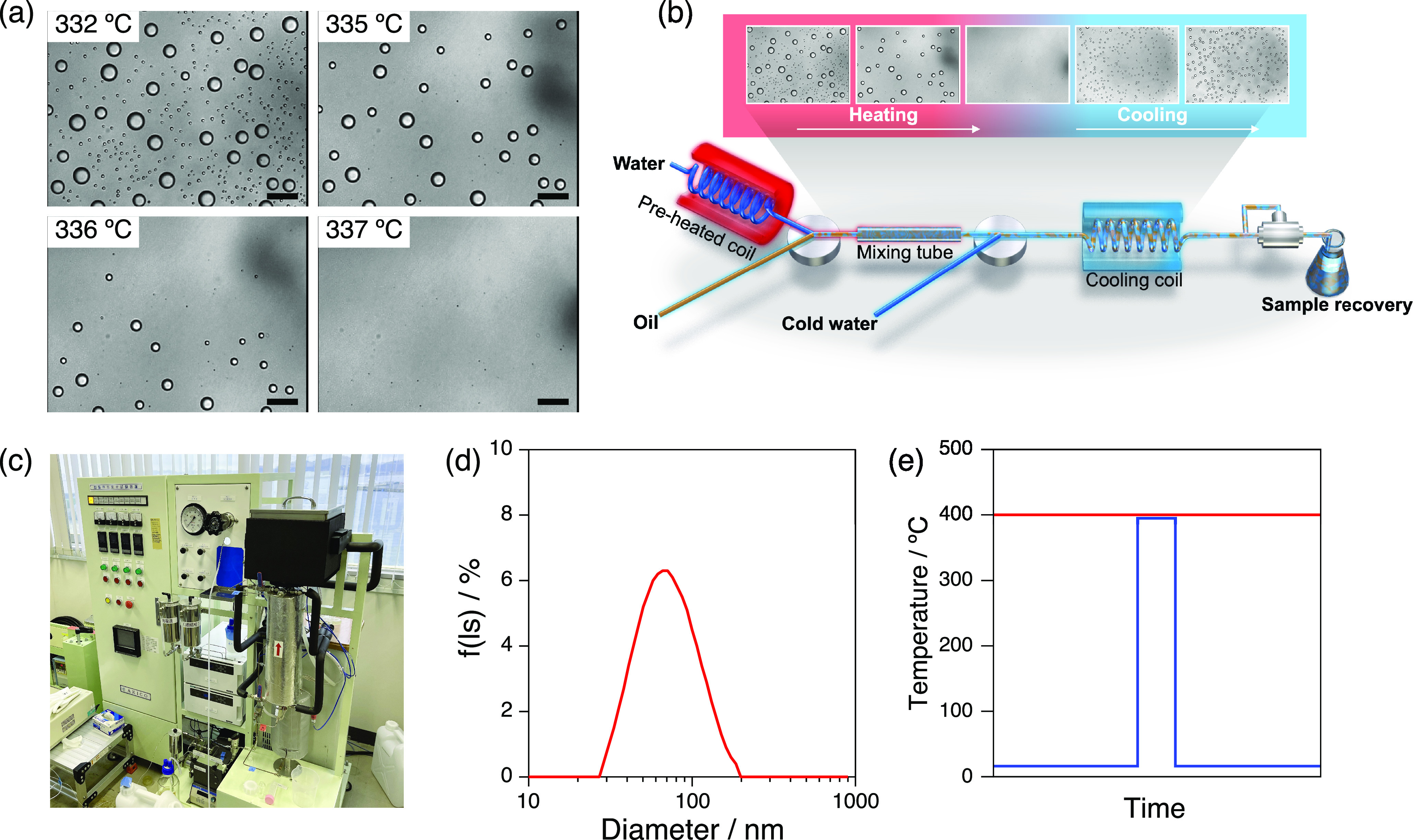
Bottom-up formation of a nanoemulsion via a deep-sea-inspired method called monodisperse nanodroplet generation in a quenched hydrothermal solution (MAGIQ). (a) Optical microscopy image sequence showing the dissolution of dodecane droplets in water at high temperatures and 25 MPa. Scale bars represent 50 μm. Adapted with permission from ref (20). Copyright 2013 Wiley. (b, c) Schematic (b) and photograph (c) of a flow reactor for single-pass continuous production of nanoemulsions via MAGIQ. (d) Size distribution of dodecane droplets obtained from MAGIQ. Adapted with permission from ref (20). Copyright 2013 Wiley. (e) Comparison of the temperature profiles of a conventional supercritical water process (red) and a deep-sea-inspired process (blue).
Conventional emulsification is a typical top-down process in which energy is input into mixtures of oil, water, and an emulsifier to split large droplets into smaller ones. In theory, one can repeat the process as many times as necessary until oil droplets of the desired size are obtained. However, splitting the droplets requires an energy input sufficient to overcome the Laplace pressure, which is the pressure difference between the inside and outside droplets. The Laplace pressure is inversely proportional to the droplet size. Hence, splitting the droplets becomes challenging as the droplet size decreases. Therefore, the preparation of nanoemulsions through a top-down process is not straightforward.
Nanosized solid particles are typically synthesized by bottom-up processes where molecules or atoms are allowed to self-assemble into nanoparticles. The bottom-up approach should also be feasible for obtaining nanosized droplets, but it requires a homogeneous solution of oil in water as a starting point. In the MAGIQ process, a homogeneous solution of oil in supercritical water was prepared. The solution was then quenched by mixing it with cold water containing a surfactant to induce rapid phase separation, during which oil molecules self-assembled into nanosized oil droplets. The interplay of the phase-separation dynamics, coalescence kinetics of the droplets, and adsorption kinetics of the surfactant on the droplet surface determined the final droplet size.21
The MAGIQ process was realized using a flow-type reactor (Figure 3b and 3c). The proof-of-concept experiment was conducted using dodecane and a nonionic surfactant, Brij 97, as the emulsifier and afforded a nanoemulsion containing dodecane droplets with a 61 nm diameter (Figure 3d). The formation of the nanosized droplets via a single-pass continuous process demonstrated the engineering advantage of the deep-sea-inspired bottom-up approach.
Supercritical water has been used as a reaction medium for organic material decomposition, such as the chemical recycling of polymers.22 Thermal decomposition suppression is therefore always a challenge when processing organic materials in supercritical water, such as through MAGIQ. Deep-sea hydrothermal vents inspired a solution for this issue. In addition to the natural occurrence of supercritical water, hydrothermal vents in the deep sea are characterized by a steep thermocline, which is formed when the hot vent water mixes with the surrounding cold, deep water. Molecules in the cold, deep water are instantaneously heated to extremely high temperatures when the vent water engulfs them and are subsequently quenched when they exit the vent stream (Figure 3e). This thermocline had been reproduced in the flow reactor for MAGIQ, where the oil was exposed to high temperatures for less than 5 s to allow its molecular dissolution in water. The brief exposure to high temperatures mitigated the thermal decomposition of dodecane to less than 1%, whereas thermal cracking of up to 20% was reported after the high-temperature treatment of dodecane in supercritical water for 20 min.20
Brief exposure to high temperatures under high pressures also provides unique opportunities for polymer chemistry.23,24 For example, the radical polymerization of acrylic acid with potassium persulfate as an initiator can be completed in 5.2 s in water at 200 °C and 25 MPa, which affords poly(acrylic acid) (Mn = 2.1 × 104, Đ = 2.73) with a monomer conversion of 60.3%.23 Furthermore, chemical reactions in such a steep thermocline from extremely high to near-freezing temperatures may have played a vital role in the chemical origin of life by driving the dehydration condensation of amino acids in aqueous environments to synthesize proteins.25
4. Nanobiotechnology and Biomimetics Inspired by Deep-Sea Organisms
Organisms that inhabit the deep sea are prime examples of biological robustness. For instance, deep-sea extremophilic microorganisms grow at pressures above 100 MPa or temperatures above 100 °C. Although adaptation to physical extremes is one of the key traits characterizing deep-sea organisms, sunlessness has the most profound impact on the deep-sea ecosystem because photosynthesis that supports surface ecosystems cannot occur. Then, the question is, what energy supports the deep-sea ecosystem?
The answer is sunlight. Organic matter that is photosynthetically produced at the ocean surface or that flows into the ocean from the land eventually falls as marine snow26 into the deep sea and nourishes the ecosystem. Readily degradable materials preferentially undergo biodegradation on the way, and residual materials enriched in recalcitrant organic matter reach the deep sea. This indicates that efficient biochemical processes for degrading recalcitrant materials are crucial for survival in the deep sea. As expected, deep-sea organisms have evolved to develop systems that utilize peculiar food sources such as collagen and lipids trapped in whale bones27 and d-amino acids.28 The knowledge of the natural history of deep-sea organisms had inspired us to study the biodegradation of cellulose, which is the most abundant organic polymer on Earth but is highly resistant to chemical and biochemical degradation.29
Cellulose is the primary component of plant cell walls. Although marine organisms such as tunicate also produce cellulose, its distribution in the ocean had remained largely unknown. However, recent studies on plastic pollution in the ocean have revealed a massive discharge of used plastics from land to the deep sea. An estimated 107 tons of plastic is discharged into the oceans annually, and 99% of it reaches the deep sea.30 Considering that the annual production of cellulose (1011 tons)31 far outstrips that of plastic (108 tons),32 an enormous amount of cellulose of terrestrial origin likely settles in the deep sea and nourishes its microbial ecosystems.33
In the enzymatic hydrolysis of water-insoluble polymers such as cellulose, enzymes can access only the surface of the substrate. Therefore, in addition to material properties such as crystallinity, the substrate surface area significantly impacts the hydrolysis rate. Increasing the polymer substrate surface area via processes such as nanofibrillation should accelerate the degradation reaction, where colloids and interface chemistry can play an active role. In this context, we previously developed a nanofiber-based ultrasensitive assay for the enzymatic hydrolysis of cellulose called surface pitting observation technology (SPOT).33,34 In SPOT, a solution of cellulases was inkjet-deposited on the surface of a hydrogel made of nanofibrous cellulose (Figure 4a).35 Then, the enzyme hydrolyzed the nanofibrous cellulose, destroyed the three-dimensional networks of the nanofiber, and pitted the surface of the hydrogel (Figure 4b and 4c). The volume of the pit was analyzed via optical profilometry to quantify the enzymatic hydrolysis, which was less than 1 ng of cellulose (Figure 4d). Surface pitting was also observed when a cellulolytic bacterium was cultured on the surface of the cellulose hydrogel containing nutrients. In this case, the pit grew with increasing incubation time and eventually became visible to the naked eye. By applying SPOT for prospecting deep-sea bioresources, we have discovered novel cellulose degraders in the deep sea (Figure 4e). Bioinformatic analysis and AI-assisted prediction of the protein structure revealed the protein structures specific to deep-sea cellulases (Figure 4f).
Figure 4.
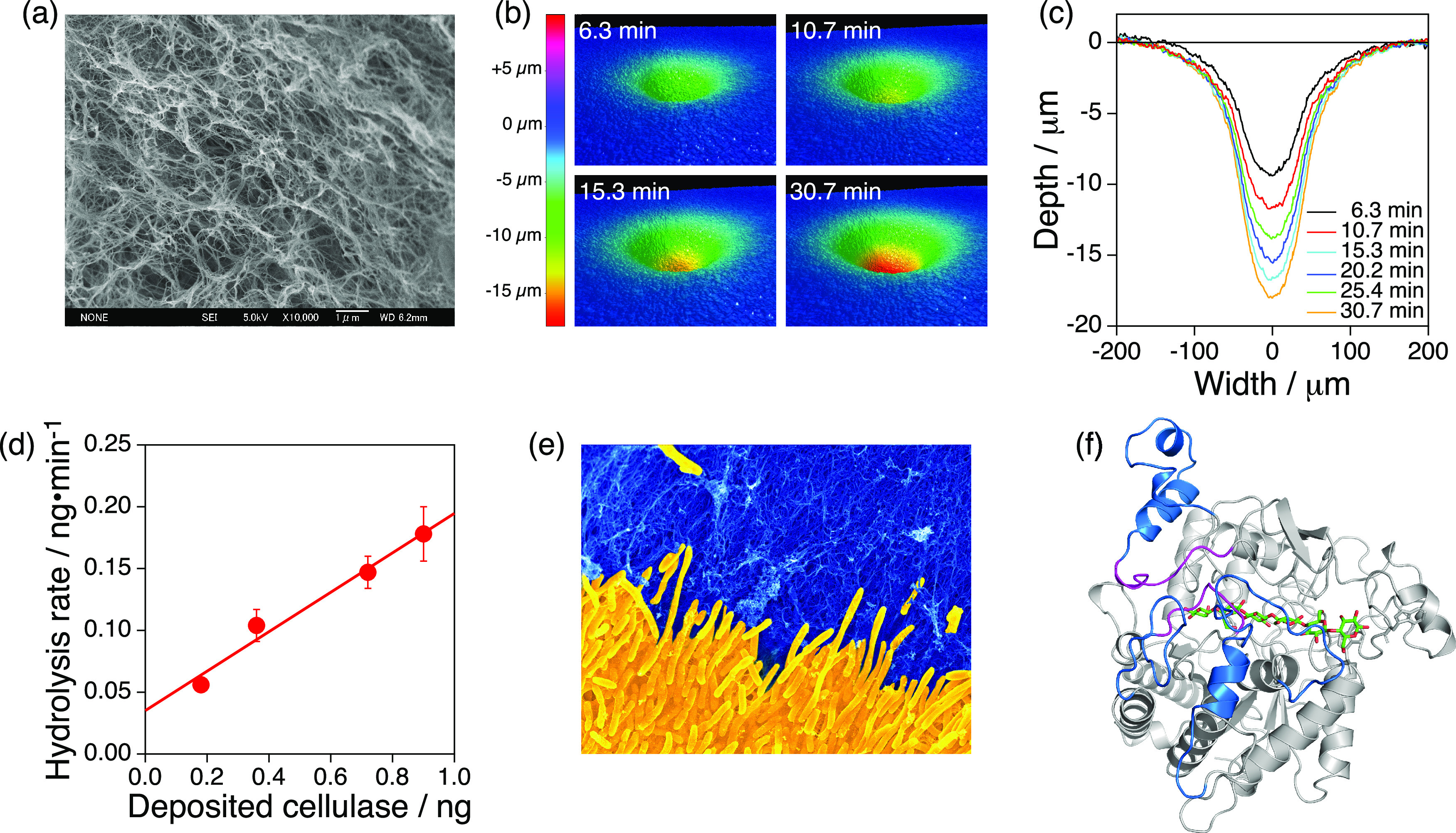
Nanofiber-based ultrasensitive assay called surface pitting observation technology (SPOT) for studying cellulose biodegradation in the deep sea. (a) Scanning electron microscopy (SEM) image showing the surface morphology of a nanofibrous cellulose gel. Adapted with permission from ref (35). Copyright 2007 Royal Society of Chemistry. (b, c) 3D topographic images (b) and cross-sectional profiles (c) showing the time-dependent growth of a pit on the surface of a nanofibrous cellulose gel. Reproduced with permission from ref (33). Copyright 2022 Elsevier. (d) Ultrasensitive quantification of enzymatic hydrolysis of cellulose via SPOT. Reproduced with permission from ref (33). Copyright 2022 Elsevier. (e) Colored SEM image showing a novel deep-sea cellulose-degrader (Marinagarivorans cellulosilyticus sp. nov., yellow) growing in a pit on a nanofibrous cellulose gel (blue). (f) AI-predicted structure of a deep-sea cellulase. Reproduced with permission from ref (33). Copyright 2022 Elsevier.
We expect that SPOT could be applied to study the enzymic hydrolysis of other water-insoluble polymers such as chitin, gelatin, and various synthetic polymers. As the high sensitivity of SPOT is attributable to the large specific surface area and porous structure of the reaction substrate,33 the challenge for such applications is the preparation of nanofibrous substrates from these materials using processes such as microphase separation in solutions and electrospinning.
Harnessing the enzymatic degradation of water-insoluble polymers is crucial to solving key sustainability challenges. Elucidating the structure–function correlation of deep-sea cellulases would help in improving existing cellulases, which are essential to establishing biorefineries to process the inexhaustible cellulosic biomass. Moreover, the genetic diversity in the deep sea is a promising pool for prospecting solutions to other sustainability challenges, such as the remediation of plastic pollution. This also reinforces the fact that solving sustainability challenges will require an interdisciplinary effort that encompasses diverse disciplines from nanotechnology to microbiology.
Deep-sea organisms are also intriguing research subjects for biomimetics. The deep-sea glass sponge, with its multilayer core–cladding structure and fiber-optical properties of the spicules, is a representative example.36 The deep-sea scaly foot gastropod with a shell incorporating iron sulfide37,38 and hydrothermal vent crabs with exceptionally hard exoskeltons39 are other examples of biomineralization. Despite the rich biodiversity in the deep sea, biomimetic research on deep-sea organisms has been minimal thus far.40 Collaboration with marine biologists is crucial to further exploiting the biomimetic potential of deep-sea organisms.41
5. Moving Forward to “42”42
Space and the deep sea are regarded as the final frontiers of human exploration. In the past, space exploration was a monumental scientific endeavor that required a national commitment, but today, the private sector is making significant strides. Similarly, deep-sea exploration is undergoing a transformation. On April 28, 2019, Victor Vescovo became the fourth person to reach the ocean’s deepest point, following Jacques Piccard and Don Walsh in 1960 and James Cameron in 2012. His submersible, the Limiting Factor, is now available for rental, making it possible for anyone who can afford the price of the trip to reach the deepest point. As a result, the number of people who have reached the deepest point has skyrocketed, reaching 27 as of July 2022.
As exemplified by the headline of a BBC article “Mariana Trench: Deepest-ever sub dive finds plastic bag” that reported Vescovo’s first successful dive, reaching the ocean’s deepest point is no longer headline-worthy. Instead, the question now is what will be discovered there. Scientific understanding of the deep sea will continue to grow, and the scientific and technological innovations inspired by these discoveries can transform these natural-history findings into sustainable benefits of the ocean under the circular economy paradigm.
The unique physicochemical processes under the extreme environment of the deep sea and the specialized biochemical mechanisms that organisms have developed to survive in the deep sea are so alien to those of us on the surface that inspiration from them should have the potential to revolutionize even mature and well-established fields of chemistry. We hope that this Perspective convinces readers that there’s plenty of room for chemists at the bottom of the ocean.
Acknowledgments
We thank Tsuyoshi Kimura, Akihiko Nakamura, and Mikako Tachioka for critiquing and improving the manuscript. We also thank the artists from Science Graphics and Ella Maru Studio for their assistance in creating the figures. We are grateful for the financial support from JST CREST (grant number JPMJCR21L4), JST A-STEP (grant number JPMJTR164B), and JSPS KAKENHI (grant number JP20K05633).
Biographies

Shigeru Deguchi is currently the director of the Research Center for Bioscience and Nanoscience, JAMSTEC. He graduated with a Ph.D. in polymer chemistry from Kyoto University, Japan, in 1996. After pursuing a JRDC overseas fellowship in the Department of Physical Chemistry 1, Lund University, Sweden, under Prof. Björn Lindman, he joined JAMSTEC in 1999. His current research interests include soft materials under extreme conditions, nanobiotechnology, and biomimetics from extremophiles. He received a Young Scientist Award from the Division of Colloid and Surface Chemistry, the Chemical Society of Japan; an Osawa Award from the Fullerenes, Nanotubes and Graphene Research Society; and an Ichimura Prize in Technology – Meritorious Achievement Prize from the New Technology Development Foundation. He currently serves as president of the Division of Colloid and Surface Chemistry, the Chemical Society of Japan and is a council member of the International Association for Colloid and Interface Scientists (IACIS).

Hiroki Degaki is currently a graduate student in the Department of Polymer Chemistry at the Graduate School of Engineering, Kyoto University, Japan. He graduated with a bachelor of engineering in polymer chemistry from Kyoto University in 2021, specializing in statistical mechanics of polymers. His current research focuses on the phase behavior of polymer mixtures.

Ikuo Taniguchi is currently a professor in the Graduate School of Fiber Science and Engineering, Kyoto Institute of Technology (KIT), Japan. After receiving his Ph.D. from Kyoto University, Japan, in 1999, he joined KIT as an assistant professor. He then pursued a postdoctoral fellowship at the Massachusetts Institute of Technology, USA (2004–2007), and worked as a senior/researcher at the Research Institute for Innovative Technology for the Earth, Japan (2007–2012) and an associate professor at Kyushu University, Japan (2012–2022) before rejoining KIT in 2022 as a professor. His current research interests include environmentally benign polymer materials and CO2 capture.

Tsuyoshi Koga is currently a professor in the Graduate School of Engineering, Kyoto University, Japan. He received his doctoral degree from Kyushu University, Japan, under the supervision of Prof. Kyozi Kawasaki in 1993. After working as a researcher in the Hashimoto Polymer Phasing Project, Japan Science and Technology (1994–1998), he joined the Department of Polymer Chemistry, Graduate School of Engineering, Kyoto University in 1998. In 2001, he was a visiting scholar at the University of Cambridge under Prof. Sir Sam Edwards. His current research interests include the molecular mechanisms of structure formation and functionalities of various kinds of materials such as polymers, supramolecules, and gels.
The authors declare no competing financial interest.
References
- Japan Agency for Marine-Earth Science and Technology (JAMSTEC). https://www.jamstec.go.jp.
- Koschinsky A.; Garbe-Schönberg D.; Sander S.; Schmidt K.; Gennerich H.-H.; Strauss H. Hydrothermal Venting at Pressure-Temperature Conditions above the Critical Point of Seawater, 5°S on the Mid-Atlantic Ridge. Geology 2008, 36, 615–618. 10.1130/G24726A.1. [DOI] [Google Scholar]
- Paulus E. Shedding Light on Deep-Sea Biodiversity—A Highly Vulnerable Habitat in the Face of Anthropogenic Change. Front. Mar. Sci. 2021, 8, 667048. 10.3389/fmars.2021.667048. [DOI] [Google Scholar]
- Gonzalez-Leon J. A.; Acar M. H.; Ryu S.-W.; Ruzette A.-V. G.; Mayes A. M. Low-Temperature Processing of ‘Baroplastics’ by Pressure-Induced Flow. Nature 2003, 426, 424–428. 10.1038/nature02140. [DOI] [PubMed] [Google Scholar]
- Taniguchi I.; Nguyen T. T. T.; Kinugasa K.; Masutani K. A Strategy to Enhance Recyclability of Degradable Block Copolymers by Introducing Low-Temperature Formability. J. Mater. Chem. A 2022, 10, 25446–25452. 10.1039/D2TA06036A. [DOI] [Google Scholar]
- Taniguchi I.; Lovell N. G. Low-Temperature Processable Degradable Polyesters. Macromolecules 2012, 45, 7420–7428. 10.1021/ma301230y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y.; Takemoto K.; Tanaka S.; Taniguchi I. Low-Temperature Processable Block Copolymers That Preserve the Function of Blended Proteins. Biomacromolecules 2016, 17, 2466–2471. 10.1021/acs.biomac.6b00641. [DOI] [PubMed] [Google Scholar]
- Ruzette A.-V. G.; Banerjee P.; Mayes A. M.; Russell T. P. A Simple Model for Baroplastic Behavior in Block Copolymer Melts. J. Chem. Phys. 2001, 114, 8205–8209. 10.1063/1.1361072. [DOI] [Google Scholar]
- Degaki H.; Taniguchi I.; Deguchi S.; Koga T. Theoretical Study on Baroplastic Behavior of Block Copolymers. Polym. Prepr. Jpn. 2022, 71, 2P2A028. [Google Scholar]
- Matsen M. W.; Schick M. Stable and Unstable Phases of a Diblock Copolymer Melt. Phys. Rev. Lett. 1994, 72, 2660–2663. 10.1103/PhysRevLett.72.2660. [DOI] [PubMed] [Google Scholar]
- Pollard M.; Russell T. P.; Ruzette A. V.; Mayes A. M.; Gallot Y. The Effect of Hydrostatic Pressure on the Lower Critical Ordering Transition in Diblock Copolymers. Macromolecules 1998, 31, 6493–6498. 10.1021/ma980316f. [DOI] [Google Scholar]
- Sawamura S.; Kitamura K.; Taniguchi Y. Effect of Pressure on the Solubilities of Benzene and Alkylbenzenes in Water. J. Phys. Chem. 1989, 93, 4931–4935. 10.1021/j100349a051. [DOI] [Google Scholar]
- Winter R.; Jeworrek C. Effect of Pressure on Membranes. Soft Matter 2009, 5, 3157–3173. 10.1039/b901690b. [DOI] [Google Scholar]
- Dias C. L.; Chan H. S. Pressure-Dependent Properties of Elementary Hydrophobic Interactions: Ramifications for Activation Properties of Protein Folding. J. Phys. Chem. B 2014, 118, 7488–7509. 10.1021/jp501935f. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam V. M.; Martinez-Monteagudo S. I.; Gupta R. Principles and Application of High Pressure–Based Technologies in the Food Industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. 10.1146/annurev-food-022814-015539. [DOI] [PubMed] [Google Scholar]
- Funamoto S.; Nam K.; Kimura T.; Murakoshi A.; Hashimoto Y.; Niwaya K.; Kitamura S.; Fujisato T.; Kishida A. The Use of High-Hydrostatic Pressure Treatment to Decellularize Blood Vessels. Biomaterials 2010, 31, 3590–3595. 10.1016/j.biomaterials.2010.01.073. [DOI] [PubMed] [Google Scholar]
- Deguchi S.; Tsujii K. Supercritical Water: A Fascinating Medium for Soft Matter. Soft Matter 2007, 3, 797–803. 10.1039/b611584e. [DOI] [PubMed] [Google Scholar]
- Weingärtner H.; Franck E. U. Supercritical Water as a Solvent. Angew. Chem., Int. Ed. 2005, 44, 2672–2692. 10.1002/anie.200462468. [DOI] [PubMed] [Google Scholar]
- Odu S. O.; van der Ham A. G. J.; Metz S.; Kersten S. R. A. Design of a Process for Supercritical Water Desalination with Zero Liquid Discharge. Ind. Eng. Chem. Res. 2015, 54, 5527–5535. 10.1021/acs.iecr.5b00826. [DOI] [Google Scholar]
- Deguchi S.; Ifuku N. Bottom-up Formation of Dodecane-in-Water Nanoemulsions from Hydrothermal Homogeneous Solutions. Angew. Chem., Int. Ed. 2013, 52, 6409–6412. 10.1002/anie.201301403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasaki Y. N.; Sagawa N.; Deguchi S. Two-Step Droplet Formation in Monodisperse Nanodroplet Generation in Quenched Hydrothermal Solution as Revealed by Spontaneous Transformation of Nanodroplets to Swollen Micelles in Octane-in-Water Nanoemulsions. J. Colloid Interface Sci. 2021, 604, 221–226. 10.1016/j.jcis.2021.07.005. [DOI] [PubMed] [Google Scholar]
- Queiroz A.; Pedroso G. B.; Kuriyama S. N.; Fidalgo-Neto A. A. Subcritical and Supercritical Water for Chemical Recycling of Plastic Waste. Curr. Opin. Green Sustain. Chem. 2020, 25, 100364. 10.1016/j.cogsc.2020.100364. [DOI] [Google Scholar]
- Kinoshita K.; Takano Y.; Ohkouchi N.; Deguchi S. Free-Radical Polymerization of Acrylic Acid under Extreme Reaction Conditions Mimicking Deep-Sea Hydrothermal Vents. ACS Omega 2017, 2, 2765–2769. 10.1021/acsomega.7b00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S.; Sezai A.; Kohsaka Y.; Deguchi S.; Osada M. High-Speed Synthesis of Thermo-Responsive Polymers by Boosted Polymerization of N,N-Diethyl Acrylamide in High-Temperature Water. Ind. Eng. Chem. Res. 2022, 61, 17012–17016. 10.1021/acs.iecr.2c02831. [DOI] [Google Scholar]
- Alargov D. K.; Deguchi S.; Tsujii K.; Horikoshi K. Reaction Behaviors of Glycine under Super- and Subcritical Water Conditions. Orig. Life Evol. Biosph. 2002, 32, 1–12. 10.1023/A:1013906319253. [DOI] [PubMed] [Google Scholar]
- Silver M. Marine Snow: A Brief Historical Sketch. Limnol. Oceanogr. Bull. 2015, 24, 5–10. 10.1002/lob.10005. [DOI] [Google Scholar]
- Tresguerres M.; Katz S.; Rouse G. W. How to Get into Bones: Proton Pump and Carbonic Anhydrase in Osedax Boneworms. Proc. Royal Soc. B 2013, 280, 20130625. 10.1098/rspb.2013.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T.; Kobayashi T.; Nunoura T.; Maruyama F.; Deguchi S. Enantioselective Utilization of D-Amino Acids by Deep-Sea Microorganisms. Front. Microbiol. 2016, 7, 1135. 10.3389/fmicb.2016.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi S.; Tsujii K.; Horikoshi K. Cooking Cellulose in Hot and Compressed Water. Chem. Commun. 2006, 3293–3295. 10.1039/b605812d. [DOI] [PubMed] [Google Scholar]
- Kane I. A.; Clare M. A.; Miramontes E.; Wogelius R.; Rothwell J. J.; Garreau P.; Pohl F. Seafloor Microplastic Hotspots Controlled by Deep-Sea Circulation. Science 2020, 368, 1140–1145. 10.1126/science.aba5899. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-H. P.; Himmel M. E.; Mielenz J. R. Outlook for Cellulase Improvement: Screening and Selection Strategies. Biotechnol. Adv. 2006, 24, 452–481. 10.1016/j.biotechadv.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Jehanno C.; Alty J. W.; Roosen M.; Meester S. D.; Dove A. P.; Chen E. Y.-X.; Leibfarth F. A.; Sardon H. Critical Advances and Future Opportunities in Upcycling Commodity Polymers. Nature 2022, 603, 803–814. 10.1038/s41586-021-04350-0. [DOI] [PubMed] [Google Scholar]
- Tsudome M.; Tachioka M.; Miyazaki M.; Uchimura K.; Tsuda M.; Takaki Y.; Deguchi S. An Ultrasensitive Nanofiber-Based Assay for Enzymatic Hydrolysis and Deep-Sea Microbial Degradation of Cellulose. iScience 2022, 25, 104732. 10.1016/j.isci.2022.104732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachioka M.; Tsudome M.; Deguchi S. Protocol for Analyzing Enzymatic Hydrolysis of Cellulose Using Surface Pitting Observation Technology. STAR Protoc. 2023, 4, 102066. 10.1016/j.xpro.2023.102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi S.; Tsudome M.; Shen Y.; Konishi S.; Tsujii K.; Ito S.; Horikoshi K. Preparation and Characterisation of Nanofibrous Cellulose Plate as a New Solid Support for Microbial Culture. Soft Matter 2007, 3, 1170–1175. 10.1039/b702504a. [DOI] [PubMed] [Google Scholar]
- Sundar V. C.; Yablon A. D.; Grazul J. L.; Ilan M.; Aizenberg J. Fibre-Optical Features of a Glass Sponge. Nature 2003, 424, 899–900. 10.1038/424899a. [DOI] [PubMed] [Google Scholar]
- Warén A.; Bengtson S.; Goffredi S. K.; Dover C. L. V. A Hot-Vent Gastropod with Iron Sulfide Dermal Sclerites. Science 2003, 302, 1007–1007. 10.1126/science.1087696. [DOI] [PubMed] [Google Scholar]
- Okada S.; Chen C.; Watsuji T.; Nishizawa M.; Suzuki Y.; Sano Y.; Bissessur D.; Deguchi S.; Takai K. The Making of Natural Iron Sulfide Nanoparticles in a Hot Vent Snail. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 20376–20381. 10.1073/pnas.1908533116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B.; Kim D.; Kim T. Exceptional Properties of Hyper-Resistant Armor of a Hydrothermal Vent Crab. Sci. Rep. 2022, 12, 11816. 10.1038/s41598-022-15982-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasiak R.; Jouffray J.-B.; Amon D. J.; Moberg F.; Claudet J.; Søgaard Jørgensen P.; Pranindita A.; Wabnitz C. C. C.; Österblom H. A Forgotten Element of the Blue Economy: Marine Biomimetics and Inspiration from the Deep Sea. PNAS Nexus 2022, 1, pgac196. 10.1093/pnasnexus/pgac196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell-Rood E. Interdisciplinarity: Bring Biologists into Biomimetics. Nature 2016, 529, 277–278. 10.1038/529277a. [DOI] [PubMed] [Google Scholar]
- The number 42 is the “Answer to the Ultimate Question of Life, the Universe, and Everything” according to; Adams D.The Hitchhiker’s Guide to the Galaxy; Pan Books: London, 1979. [Google Scholar]