ABSTRACT
Hypermucoviscosity is a hallmark of hypervirulent Klebsiella pneumoniae (hvKP). However, the molecular basis of its regulation is largely unknown. We hypothesize that hypermucoviscosity is modulated via two-component signal transduction systems (TCSs). In-frame deletion mutants of all 33 response regulators of hvKP ATCC43816 were generated using CRISPR/CAS and evaluated for their impacts on hypermucoviscosity. The response regulator OmpR is required for hypermucoviscosity in vitro and virulence in vivo in a mouse pneumonia model. The ΔompR mutant lost its mucoidy but retained its capsule level and comparable rmpADC expression, so transcriptomic analysis by RNA-Seq was performed to identify differentially expressed genes (DEGs) in ΔompR mutant. The top 20 Gene Ontology terms of 273 DEGs belong to purine ribonucleotide triphosphate biosynthetic and metabolic process, transmembrane transport, and amino acid metabolism. Among the overexpressed genes in the ΔompR mutant, the atp operon encoding F-type ATP synthase and the gcvTHP encoding glycine cleavage system were characterized further as overexpression of either operon reduced the mucoviscosity and increased the production of ATP. Furthermore, OmpR directly bound the promoter region of the atp operon, not the gcvTHP, suggesting that OmpR regulates the expression of the atp operon directly and gcvTHP indirectly. Hence, the loss of OmpR led to the overexpression of F-type ATP synthase and glycine cleavage system, which altered the energetic status of ΔompR cells and contributed to the subsequent reduction in the mucoviscosity. Our study has uncovered a previously unknown regulation of bacterial metabolism by OmpR and its influence on hypermucoviscosity.
IMPORTANCE Hypermucoviscosity is a critical virulent factor for Klebsiella pneumoniae infections, and its regulation remains poorly understood at the molecular level. This study aims to address this knowledge gap by investigating the role of response regulators in mediating hypermucoviscosity in K. pneumoniae. We screened 33 response regulators and found that OmpR is essential for hypermucoviscosity and virulence of K. pneumoniae in a mouse pneumonia model. Transcriptomic analysis uncovered that genes involved in energy production and metabolism are highly upregulated in the ΔompR mutant, suggesting a potential link between bacterial energy status and hypermucoviscosity. Overexpression of those genes increased production of ATP and reduced mucoviscosity, recapitulating the ΔompR mutant phenotype. Our findings provide new insights into the regulation of K. pneumoniae hypermucoviscosity by a two-component signal transduction system, highlighting the previously unknown role of OmpR in regulating bacterial energy status and its influence on hypermucoviscosity.
KEYWORDS: Klebsiella pneumoniae, hypermucoviscosity, OmpR, F-type ATP synthase, glycine cleavage system
INTRODUCTION
Hypervirulent Klebsiella pneumoniae (hvKP) has become a dominant pathotype of K. pneumoniae worldwide since it was first reported in the 1980s in Taiwan (1, 2). Epidemiologic studies have revealed that hvKP strains are capable of metastatic to distant sites, including lung, liver, kidney, spleen, fascia, eye, and center nervous system (3). The identification of hvKP is often based on its clinical presentations in the infected host and its ability to infect healthy individuals systematically. However, many cases of hvKP infection are highly variable and difficult to identify and treat promptly, underscoring the urgent need for reliable biomarkers.
Several genotypic and phenotypic virulence biomarkers of hvKP have been identified, including K1/K2 serotype capsule polysaccharides, putative metabolite transporter gene peg-344, aerobactin synthesis gene iucA, salmochelin synthesis gene iroB, mucoid phenotype regulators rmpADC, and hypermucoviscosity (4–6). However, each of these biomarkers has limitations when it comes to highly varied clinical hvKP isolates that are distinct from classic KP (cKP). Hypermucoviscosity, in particular, has been regarded as a unique visual feature of hvKP, with high accuracy in predicting its presence (4, 7). Patients infected with hypermucoviscous strains tend to have a distinct invasive syndrome compared to those infected with nonhypermucoviscosity variants (8, 9). Thus, hypermucoviscosity is a promising and reliable biomarker of hvKP.
Hypermucoviscosity is a unique phenotype that distinguishes hvKP from other encapsulated strains, such as cKP, Escherichia coli, Acinetobacter baumannii, and Pseudomonas aeruginosa. Unlike the coalesce and watery mucoid colonies of these other strains, hypermucoviscous colonies form a viscous string of more than 5 mm in length when stretched with a loop from a blood agar plate (7). Abundant capsular polysaccharides and the regulator of mucoid phenotype (RmpA) are well-known determinants of hypermucoviscosity (10, 11). However, recent studies have identified additional factors that are required for hypermucoviscosity, including RmpD and central metabolism (12, 13). Thus, a further in-depth research is needed to uncover the underlying mechanisms involved in the formation of hypermucoviscosity.
Under favorable environmental conditions, non mucoid parent strains of various bacteria, including Klebsiella spp., Salmonella spp., E. coli, Vibrio parahaemolyticus, and P. aeruginosa, can evolve into mucoid variants (14, 15). Two putative determinants in hypermucoviscosity, capsule biosynthesis and RmpA regulation in hvKP, are related to RcsBCD, a two-component signal transduction system (TCS) (16, 17). Based on this, we hypothesize that hvKP develops the hypermucoviscous phenotype in response to environmental cues via TCS, which is a common regulation mechanism used by many bacteria (18). TCS can regulate the expression of capsules or lipopolysaccharide (LPS) modification to modulate bacterial virulence. For instance, AlgZ/AlgR in P. aeruginosa is required for alginate production under osmolarity and nitrate signals (19, 20). BfmR/BfmS in A. baumannii enhances capsule genes expression in response to antibiotics to confer virulence (21); EnvZ/OmpR regulates Vi polysaccharide synthesis in S. Typhi (22), and PmrA/PmrB regulates LPS modification to acquire polymyxin B resistance in K. pneumoniae (23). Therefore, the systematical study of TCSs can help uncover the molecular regulation mechanisms regulating hypermucoviscosity in hvKP.
In this study, we investigated the effects of 33 response regulators on hypermucoviscosity in hvKP ATCC43816 and discovered that OmpR is essential for both hypermucoviscosity and virulence. The OmpR governs hypermucoid characteristic independently of the capsule and RmpD. Transcriptomic analysis revealed that the absence of OmpR resulted in the overexpression of F-type ATP synthase and the glycine cleavage system, which in turn affects the cellular energy status and contributes to the subsequent inhibition of mucoviscosity. Our findings present a previously unknown regulatory role of OmpR in bacterial energy and its impact on hypermucoviscosity.
RESULTS
Response regulator OmpR is required for hypermucoviscosity.
To systematically investigate the impacts of the TCSs on mucoviscosity, we first predicted TCS homologs using the Prokaryotic 2-Component Systems (P2CS) (http://www.p2cs.org/) (24), an integrated and comprehensive database of TCS proteins. We identified 33 genes encoding TCS response regulators in the K. pneumoniae ATCC43816 genome. Subsequently, we constructed markerless in-frame deletion mutants for all 33 response regulator genes by the CRISPR-Cas9 system, as described (25). The morphology characteristics of all the isogenic mutants are listed in Table 1. Moreover, we determined the hypermucoviscosity of each isogenic strain using the string test.
Table 1.
The characteristics of in-frame deletion mutants of 33 response regulators of hvKP ATCC43816
| Strain | Genotype | Name of deleted gene | String test | Growth curve | Morphology |
|---|---|---|---|---|---|
| TH14397 | ΔVK055_0032 | uvrY | Positive | Normal | Typical |
| TH14423 | ΔVK055_0233 | narL | Positive | Normal | Typical |
| TH14429 | ΔVK055_0249 | rssB | Positive | Normal | Typical |
| TH14411 | ΔVK055_0682 | 0682 | Positive | Normal | Typical |
| TH14412 | ΔVK055_0953 | dcuR | Positive | Normal | Typical |
| TH14413 | ΔVK055_0985 | rstA | Positive | Normal | Typical |
| TH14415 | ΔVK055_1326 | phoP | Positive | Normal | Typical |
| TH14416 | ΔVK055_1598 | 1598 | Positive | Normal | Typical |
| TH14428 | ΔVK055_1724 | pmrA | Positive | Normal | Typical |
| TH14417 | ΔVK055_1819 | kdpE | Positive | Normal | Typical |
| TH14424 | ΔVK055_2216 | phoB | Positive | Normal | Typical |
| TH14398 | ΔVK055_2521 | 2521 | Positive | Normal | Typical |
| TH16060 | ΔVK055_2547 | 2547 | Positive | Normal | Typical |
| TH14426 | ΔVK055_2575 | arcA | Positive | Defect | Elongation |
| TH14418 | ΔVK055_2578 | creB | Positive | Normal | Typical |
| TH14419 | ΔVK055_2704 | cusR | Positive | Normal | Typical |
| TH14402 | ΔVK055_2984 | 2984 | Positive | Normal | Typical |
| TH14401 | ΔVK055_3087 | zraR | Positive | Normal | Typical |
| TH14420 | ΔVK055_3257 | cpxR | Positive | Normal | Typical |
| TH14406 | ΔVK055_3297 | ntrC | Positive | Normal | Typical |
| TH14421 | ΔVK055_3393 | uhpA | Positive | Normal | Typical |
| TH14427 | ΔVK055_3696 | ompR | Negative | Normal | Typical |
| TH14410 | ΔVK055_3990 | evgA | Positive | Normal | Typical |
| TH14400 | ΔVK055_4034 | qseB | Positive | Normal | Typical |
| TH16062 | ΔVK055_4175 | 4175 | Positive | Normal | Typical |
| TH14425 | ΔVK055_4178 | 4178 | Positive | Normal | Typical |
| TH16064 | ΔVK055_4202 | evgA | Positive | Normal | Typical |
| TH14409 | ΔVK055_4622 | yfhA | Positive | Normal | Typical |
| TH14422 | ΔVK055_4775 | mrkE | Positive | Normal | Typical |
| TH14399 | ΔVK055_4883 | rcsB | Negative | Normal | Typical |
| TH14408 | ΔVK055_4961 | btsR | Positive | Normal | Typical |
| TH14404 | ΔVK055_4995 | baeR | Positive | Normal | Typical |
| TH14403 | ΔVK055_5004 | 5004 | Positive | Normal | Typical |
Among the 33 response regulator deletion mutants, only ΔompR and ΔrcsB mutants lost the hypermucoviscous phenotype, as indicated by a negative string test (Table 1). These findings suggest that response regulators RcsB and OmpR are required for hypermucoviscosity. RcsB (regulator of capsule synthesis) has been documented as a mucoviscosity determinant via the activation of capsule biosynthesis (10, 26). However, the mechanism by which OmpR mediates mucoviscosity remains to be elucidated.
To assess the role of OmpR in hypermucoviscosity formation, we investigated its effects in three additional hypermucoviscous clinical strains, including two serotype K2 strains (TH12887 and TH13044) and a serotype K23 strain (TH12896), by deleting the ompR gene using the CRISPR-Cas9 system. Similarly, the mucoviscosity of all ΔompR mutants (TH16268, TH16270, and TH16070) from these strains was significantly reduced compared to the corresponding parent strains (Fig. 1A).
FIG 1.
OmpR is required for hypermucoviscosity of K. pneumoniae. (A) OmpR deficiency reduced hypermucoviscosity in three K2 serotype strains (ATCC43816, TH12887, and TH13044) and one serotype K23 strain (TH12896). The ompRcomp is the complementation of the ΔompR mutant, and Δwzab capsule-null mutant is a negative control. (B) Effects of phosphorylation of OmpR on K. pneumoniae mucoviscosity. The ompRD55A encodes the unphosphorylated form of OmpR, and ΔenvZ is a histidine kinase EnvZ deletion mutant. Data are presented as mean ± SD from six biological replicates. Two-tailed t test and one-way ANOVA with Dunnett’s multiple-comparison test were performed to determine the statistical significance of two group comparisons. ***, P < 0.001; ****, P < 0.0001; ns, no significance.
EnvZ/OmpR constitutes a two-component signal transduction system that controls a wide range of gene expression in response to osmotic signals or acid stress in many species of Enterobacteriaceae (27, 28). OmpR can not only act canonically (requiring phosphorylation) to regulate the porin genes but also noncanonically (without phosphorylation) to activate the acid stress response (29). Given that a single amino acid substitution of Aspirate to Alanine (D55A) mimics the unphosphorylated form of OmpR (30), we constructed a site-directed mutant ompRD55A (TH14495) and a markerless deletion mutant of envZ (TH14889) to evaluate whether OmpR phosphorylation and EnvZ are required for hypermucoviscosity.
The mucoviscosity of ΔenvZ and unphosphorylated form ompRD55A decreased compared to that of the wild-type strain (Fig. 1B). Notably, ΔompR completely lost mucoviscosity (sedimentation value < 0.2), while ompRD55A and EnvZ deficiency reduced mucoviscosity to a lesser extent (sedimentation value 0.2 to 0.4). These data suggest that OmpR phosphorylation and EnvZ/OmpR phosphoryl relay partially account for hypermucoviscosity.
OmpR contributes to virulence in a mouse model of pneumonia.
To determine whether mucoviscosity alterations by OmpR influences virulence, we assessed the virulence of isogenic strains in a pneumonia model with an intranasal inoculation of 2,000 CFU log-phase grown bacteria. We first evaluated virulence by examining the mortality rate postinfection. The mortality of the ΔompR mutant significantly decreased compared with that of the wild-type strain (P < 0.01) (Fig. 2A).
FIG 2.
OmpR contributes to virulence in vivo in a mouse model of pneumonia. (A) Survival of mice infected with K. pneumoniae variants observed for 7 days postinfection (n = 5). Gehan-Breslow-Wilcoxon test was performed. After 72 h postinoculation, the lungs (B), livers (C), and spleens (D) were harvested for bacterial enumeration. Each circle represents one mouse, and dotted lines represent the detection limit. One-way ANOVA with Dunnett’s multiple-comparison test was performed to determine the statistical significance of two group comparisons. **, P < 0.01; ****, P < 0.0001; ns, no significance.
As the clinical definition of hvKP involves the ability to disseminate to multiple organs, we further assessed the bacteria burden in various organs, including the lung, liver, and spleen, at 72 h postinfection. The wild-type bacteria colonized as high as 1 × 109 CFU/g in the primary lung lesion and up to 1 × 107 CFU/g in metastatic sites (liver and spleen). In contrast, the ΔompR mutant was barely or not at all detectable in these organs (Fig. 2B to D). These data suggest that the ΔompR mutant, with reduced mucoviscosity, attenuates the virulence of K. pneumoniae in vivo.
Effects of OmpR on capsule production and rmpADC expression.
Capsule biosynthesis and RmpADC expression contribute to mucoviscosity (10, 13). Accordingly, we assessed whether OmpR regulates either capsule production or rmpADC expression to determine the underlying regulatory mechanisms.
We evaluated capsule production in three aspects in K. pneumoniae ATCC43816: capsular thickness, gene expression, and uronic acid content (10). The ΔompR mutant exhibited the same capsule appearance and thickness as the wild-type strain using the Anthony direct-dry staining method (Fig. 3A and B). We further determined the expression of three representative capsule synthesis genes (galF, wzi, and manC) (31). Expression of galF and wzi remained unchanged, while the expression of manC increased about 5-fold in the ΔompR mutant compared to the wild-type strain (Fig. 3C). Additionally, we detected the same level of uronic acid content in both wild-type and the ΔompR mutant (Fig. 3D). Thus, alternation in capsule production is not responsible for OmpR-mediated hypermucoviscosity.
FIG 3.
Effects of OmpR on capsule and rmpADC expression. Capsules were visualized by Anthony direct-dry staining (A), and the capsule thickness was quantified by measuring the minor axis of the pale blue zone surrounding 20 random cells using Image J (B). Expression of representative capsule genes was determined by quantitative RT-PCR analysis (C) (expression of each gene in the isogenic strains was normalized to that of wild-type strain), and capsule production was assessed by measuring uronic acid content (D). The rmpADC expression levels were monitored using a luciferase transcriptional reporter (E). Relative luminescence units (RLU) were normalized to the OD600nm of culture. The ompRcomp is the complementation of the ΔompR mutant, and Δwzab capsule-null mutant is a negative control. Data are presented as mean ± SD from six biological replicates. Two-tailed t-tests were performed to determine the statistical significance of two group comparisons. ***, P < 0.001; ****, P < 0.0001; ns, no significance.
Furthermore, we investigated rmpADC expression using luciferase reporter assays. Both wild-type and ΔompR strains displayed similar reporter activities, suggesting that OmpR had no significant impact on rmpADC expression (Fig. 3E). Collectively, these findings suggest that the OmpR-mediated mucoid phenotype is not regulated through changes in capsule production or rmpADC expression, two known determinants, indicating of an unknown mechanism involved.
Effects of OmpR on metabolic pathways of K. pneumoniae.
The response regulator OmpR has been extensively studied as a global regulatory factor, directly controlling the transcription of numerous genes in E. coli and Salmonella typhimurium (32). Although OmpR is identical in both species, only 15 target genes of OmpR overlap between them (33). The OmpR regulon in K. pneumoniae has not been fully explored. To investigate the OmpR regulon involved in mucoviscosity, we determined the transcriptional profiles of wild-type and ΔompR cells using RNA sequencing.
We identified a total of 273 differentially expressed genes (DEGs) between wild-type and ΔompR strains, comprising 136 upregulated and 137 repressed genes in ΔompR (Fig. 4A). We performed Gene Ontology (GO) enrichment analysis and identified the top 20 GO terms for DEGs, which include purine ribonucleotide triphosphate biosynthesis, metabolic processes, transmembrane transport, and amino acid metabolism. (Fig. 4B). These results suggest that cellular metabolism plays a pivotal role in mucoviscosity. Consequently, we integrated all DEGs into metabolic pathways to identify underlying molecular mechanisms using the Pathway Tools software v26.0 (34, 35). The DEGs are primarily associated with metabolic pathways such as ATP de novo biosynthesis, l-serine, glycine, and methionine metabolism (Fig. 4C). Our observations align with the results from a previous systematic analysis of hypermucoviscosity, which revealed the role of genes related to purine metabolism in the regulation of mucoviscosity (12).
FIG 4.
Transcriptomic analysis of OmpR-mediated gene expression. Differential gene expression between wild-type K. pneumoniae and its ΔompR mutant was determined by RNA-sequencing. (A) Volcano plot analysis of differentially expressed genes. A total of 273 differentially expressed genes (DEGs), including 136 upregulated and 137 downregulated genes in ΔompR, are highlighted. Representative genes are depicted in Table 2 (name or ID). The red and green dots denote significantly upregulated and downregulated genes, respectively, and the gray dots represent not differentially expressed genes. (B) The top 20 GO terms were identified by Gene Ontology analysis. (C) Metabolic pathways associated with DEGs were determined by an integrated Pathway Tools software V26.0.
In addition to genes related to metabolic pathways, other DEGs are involved in various cellular functions, such as transport and RNA binding. By combining GO enrichment analysis and DEG fold changes, we identified 24 potent hits (Table 2). These hits include 16 upregulated and eight downregulated genes, which were further investigated to evaluate their contributions to mucoviscosity.
TABLE 2.
Genes differentially expressed between wild type (WT) and ΔompR mutant
| Description | Gene_id | Gene name | Description | TPMa |
log2FCb | P value | |
|---|---|---|---|---|---|---|---|
| WT | ΔompR | ||||||
| Up-regulated | VK055_5116 | fyuA | siderophore yersiniabactin receptor FyuA | 2.27 | 75.1 | 4.89 | 6.55E-47 |
| VK055_3329 | atpI | F0F1 ATP synthase subunit I | 48.11 | 176.64 | 1.71 | 0.001 | |
| VK055_3331 | atpE | F0F1 ATP synthase subunit C | 166.48 | 687.31 | 1.92 | 0.038 | |
| VK055_3332 | atpF | F0F1 ATP synthase subunit B | 121.57 | 557.59 | 2.02 | 9.40E-18 | |
| VK055_3333 | atpH | F0F1 ATP synthase subunit delta | 87.74 | 296.23 | 1.59 | 3.05E-09 | |
| VK055_3334 | atpA | F0F1 ATP synthase subunit alpha | 101.13 | 434.34 | 1.93 | 2.14E-42 | |
| VK055_3335 | atpG | F0F1 ATP synthase subunit gamma | 92.27 | 359.93 | 1.79 | 1.67E-24 | |
| VK055_3336 | atpD | F0F1 ATP synthase subunit beta | 69.98 | 338.65 | 2.11 | 1.03E-39 | |
| VK055_3337 | atpC | F0F1 ATP synthase subunit epsilon | 102.25 | 558.91 | 2.28 | 4.11E-16 | |
| VK055_4142 | gcvT | glycine cleavage system aminomethyltransferase GcvT | 38.07 | 150.16 | 1.81 | 2.39E-17 | |
| VK055_4143 | gcvH | glycine cleavage system protein GcvH | 140.99 | 643.30 | 2.03 | 2.31E-13 | |
| VK055_4144 | gcvP | aminomethyl-transferring glycine dehydrogenase | 52.96 | 208.12 | 1.81 | 5.76E-41 | |
| VK055_0304 | 0304 | DUF1852 domain-containing protein | 20.92 | 82.08 | 1.81 | 4.84E-10 | |
| VK055_0305 | 0305 | methionine synthase | 41.13 | 153.21 | 1.73 | 9.42E-16 | |
| VK055_3379 | 3379 | PTS transporter subunit EIIC | 4.93 | 26.94 | 2.28 | 4.35E-09 | |
| VK055_3380 | 3380 | 6-phospho-alpha-glucosidase | 11.15 | 99.92 | 2.99 | 3.15E-27 | |
| Down-regulated | VK055_4983 | 4983 | mannitol dehydrogenase family protein | 93.2 | 20.01 | −2.39 | 4.94E-28 |
| VK055_4481 | 4481 | ATP-binding cassette domain-containing protein | 201.03 | 69.63 | −1.70 | 2.62E-14 | |
| VK055_4482 | 4482 | metal ABC transporter permease | 82.72 | 21.67 | −2.1 | 1.49E-11 | |
| VK055_4483 | 4483 | metal ABC transporter substrate-binding protein | 67.12 | 18.80 | −2.00 | 4.13E-10 | |
| VK055_1148 | pspC | envelope stress response membrane protein PspC | 221.79 | 73.53 | −1.76 | 7.81E-05 | |
| VK055_1149 | pspB | envelope stress response membrane protein PspB | 1233.65 | 320.17 | −2.01 | 0.015 | |
| VK055_1150 | pspA | phage shock protein PspA | 825.94 | 320.45 | −1.53 | 7.92E-24 | |
| VK055_3555 | 3555 | MFS transporter | 75.77 | 23.22 | −1.87 | 2.95E-15 | |
TPM, transcripts per million reads.
Fold change.
OmpR regulons related to hypermucoviscosity.
The transcriptomic results of significantly regulated genes (Table 2) were validated by qRT-PCR. The qRT-PCR data demonstrated the transcriptional levels of the atp operon, gcvT, gcvH, gcvP, VK055_0304-0305, VK055_3379, and fyuA were significantly upregulated (Fig. 5A). Similarly, the transcriptional levels of pspABC and VK055_3555 were downregulated 2-fold, while those of VK055_4481-4483 and VK055_4983 were reduced approximately 5-fold (Fig. 5B), which is consistent with the transcriptomic results. Subsequently, we constructed corresponding isogenic mutants with markerless deletion or overexpression strains containing low-copy-number plasmid (Fig. S1) to evaluate whether they exhibited consistent viscosity phenotypes.
FIG 5.
Overexpression of either F-type atp operon or gcvTHP reduces mucoviscosity. The relative expression of each upregulated gene (A) and downregulated gene (B) was validated by qRT-PCR. A dotted line denotes the mean value of wild type. Mucoviscosity was determined by sedimentation assays (C to E). Mutants for representative downregulated genes (C, E) and overexpression strains for representative upregulated genes (D) were constructed and evaluated for their mucoviscosity. Wild-type or wild-type strain carrying an empty vector (WT vector) was used as a control. (F) Uronic acid contents of wild-type and isogenic variants. Data are presented as mean ± SD from six biological replicates. A two-tailed t test was performed to determine the statistical significance of two group comparison. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, no significance.
In the ΔompR mutant, the expression of the following genes was repressed: pspABCD, VK055_3555, VK055_4481-4483, and VK055_4983-4986. However, none of the deficient mutants altered the mucoviscosity compared to the wild-type strain (Fig. 5C). Nevertheless, overexpressing either F-type atp operon (pF-type, TH16249) or gcvTHP (pgcvTHP, TH16248) in trans in the wild-type strain led to reduced mucoidy (Fig. 5D). Although the expression of VK055_0305, a gene involved in the methionine metabolism supplying methyl group pool for DNA synthesis (Fig. 4C), increased approximately 8-fold in ΔompR mutant (Fig. 5B), overexpressing of this gene in wild-type (p0304-0305, TH16246) did not affect mucoidy (Fig. 5D). Furthermore, we deleted the F-type atp operon and gcvTHP in the wild-type strain, respectively, to determine whether their deficiency would alter the mucoviscosity. The GcvTHP deficiency mutant ΔgcvTHP (TH16266) exhibited increased mucoviscosity (P < 0.01). Intriguingly, the F-type ATP synthase-deficiency mutant ΔF-type (TH16066) exhibited low mucoviscosity (Fig. 5E), likely due to a growth defect observed (Fig. S2). Moreover, complementation of the ΔF-type in trans (F-typecomp, TH16941) produced a phenotype similar to overexpressing it in the wild-type strain background. Likewise, the complementation of ΔgcvTHP in trans (gcvcomp, TH16942) resulted in a similar phenotype. Multiple copy numbers of the complementation plasmid used in the study are likely responsible for the observed overexpression phenotype (Fig. 5E).
Additionally, neither inactivation nor overexpression of F-type atp operon and gcvTHP genes altered the uronic acid content (Fig. 5F). These results suggest that F-type ATP synthase and GcvTHP are involved in the mucoviscosity formation, independent of the capsule level.
Binding of recombinant OmpR to the promoter region of the F-type atp operon.
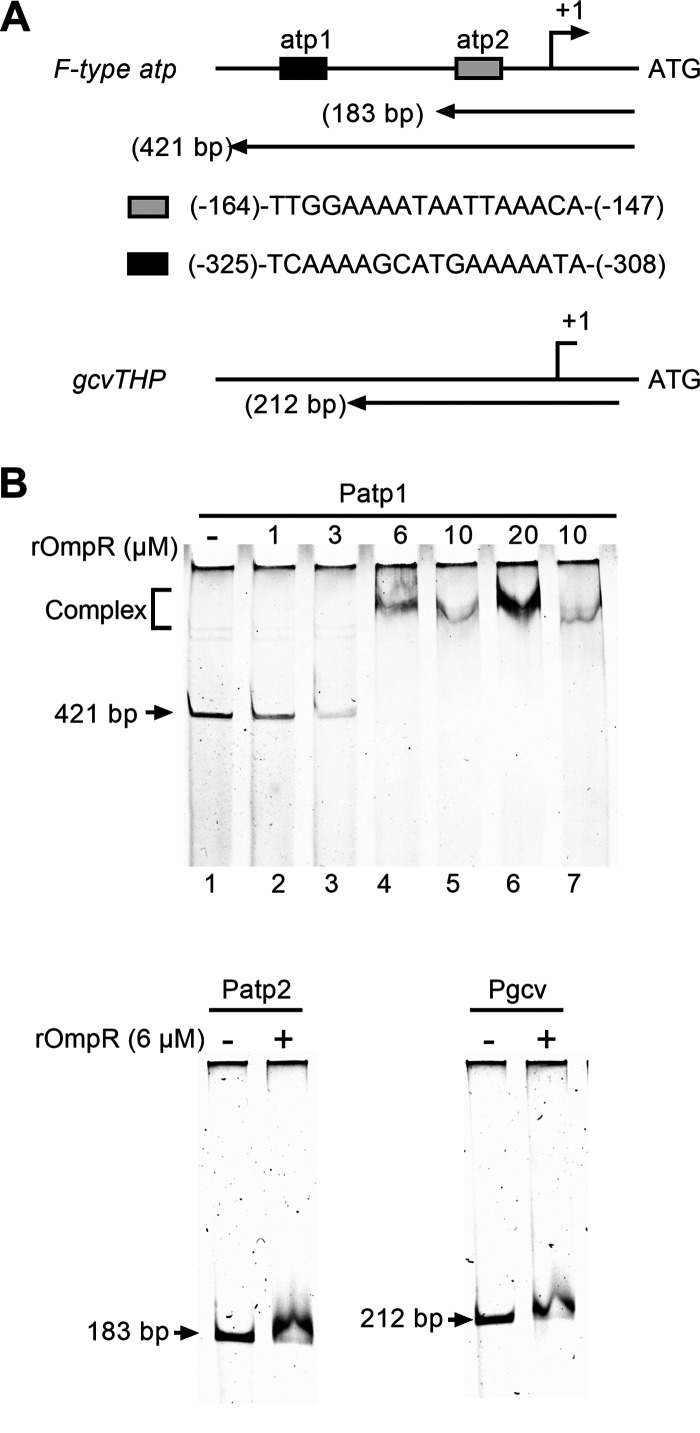
As the expression of genes encoding F-type ATP synthase and GcvTHP was dramatically increased in the ΔompR mutant, we hypothesized that OmpR might directly modulate these genes. To determine whether OmpR directly binds to their promoter regions, we conducted an electrophoretic mobility shift assay (EMSA). We searched the ATCC43816 genomic sequence (ID: CP009208) on an online gene regulation and gene expression database (https://www.prodoric.de/) using the OmpR matrix (MX000141 or MX000338) to identify putative binding sites (36). Two potential OmpR binding sites, (-164)-TTGGAAAATAATTAAACA-(-147) and (-325)-TCAAAAGCATGAAAAATA-(-308), were identified upstream of the atpI ATG codon, while no typical OmpR binding site was found upstream of the gcvT gene (Fig. 6A). In line with this, EMSA data suggest that recombinant OmpR (rOmpR) binds to the distant sequence upstream of atpI (Patp1) not the near sequence (Patp2) (Fig. 6B). Furthermore, unphosphorylated rOmpR directly bound to the promoter region of Patp1, which may explain why ompRD55A and ΔenvZ partially maintain mucoviscosity (Fig. 6B, lane 7). No binding activity was observed for the phosphorylated rOmpR with the promoter of gene gcvT. These results indicate that OmpR directly represses the expression of the F-type atp operon but indirectly represses the expression of the gcvTHP genes.
FIG 6.
EMSA analysis of binding of recombinant OmpR to the F-type atp operon and gcvTHP promoter regions. (A) Diagrams of promoter regions of F-type atp operon and gcvTHP (not drawn to scale). Gray and black boxes represent two putative OmpR binding sequences, atp1 and atp2. Nucleotide numberings are relative to the atpI and gcvT ATG codons, respectively. The relative positions and lengths of the three DNA fragments used in EMSA are shown. (B) EMSA results of rOmpR. EMSA of Patp1 (Upper panel): the Patp1 DNA fragment was mixed with increasing concentrations of rOmpR-P (in the presence of acetyl phosphate) at 0, 1, 3, 6, 10, and 20 μM (Lanes 1 to 6) or 10 μM non-P rOmpR (in the absence of acetyl phosphate) (lane 7). EMSA of Patp2 and Pgcv (Lower panels) DNA fragments specified in the absence and presence of 6 μM rOmpR-P (in the presence of acetyl phosphate), respectively (−/+), were analyzed. DNA bands were detected by Gelred staining. The positions of DNA fragments not shifted were labeled.
High intracellular energy in low mucoviscosity isogenic strains.
It is known that the central metabolism genes related to pyruvate metabolism and the TCA cycle influence hypermucoviscosity (12). We found, in our study, that the regulons of OmpR are associated with ATP or nucleic acid biosynthesis. Consequently, we hypothesized that intracellular energy levels play a role in reducing mucoviscosity. To test this hypothesis, we employed an ATP-dependent luciferase assay to measure the intracellular ATP levels as a proxy for cellular energy status. Our findings showed that the ATP level of log-phase ΔompR cells was approximately twice as high as that of wild-type cells (4.25 versus 2.23 pmol/108 CFU). Similarly, stationary-phase ΔompR cells exhibited elevated ATP levels compared to wild-type cells (Fig. 7A). In accordance with these observations, we also found increased intracellular ATP levels in overexpression of either F-type atp operon or gcvTHP during both log phase and stationary phase (Fig. 7B). These results suggest that cellular energy status plays a role in modulating mucoviscosity. However, the precise mechanism by which elevated ATP production influences mucoviscosity remains unclear and warrants further investigation.
FIG 7.
Intracellular ATP levels of cells at log phase and stationary phase. Intracellular ATP amounts determined in ΔompR mutant (A) and overexpression of F-type atp operon or gcvTHP isogenic strains (B) were compared to the levels in wild-type cells. Data are presented as mean ± SD from six biological replicates. An unpaired t test was performed to determine the statistical significance of two-group comparisons and one-way ANOVA for multiple-group comparisons. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
DISCUSSION
Hypervirulent K. pneumoniae is known to cause severe infections (37). A key characteristic of HvKP is its highly stretchable colonies (38, 39). Capsule abundance (10) and elevated RmpD level (13) have been recognized as two major factors contributing to hypermucoviscosity. Although capsule production is required for hypermucoviscosity of K. pneumoniae, the mechanisms of RmpD-mediated hypermucoviscosity remain unknown. Furthermore, RmpD-negative hypermucoviscosity has been identified in numerous clinical strains (40–43). Therefore, the molecular event precisely modulating hypermucoviscosity formation is still unclear. Our current study systematically assessed the contributions of TCS response regulators to hypermucoviscosity and uncovered a metabolic association with OmpR-mediated hypermucoviscosity in K. pneumoniae.
Environmental factors are known to affect the hypermucoviscosity phenotype of K. pneumoniae (44, 45). As a result, K. pneumoniae may sense the environmental stimuli to regulate its mucoidy characteristic. Bacterial TCSs serve as critical sensors of these environmental cues. By systematic inactivation of genes coding for all 33 TCS response regulators in K. pneumoniae, we found that OmpR is essential for the hypermucoviscous trait in multiple clinical strains. Additionally, an ΔompR mutant of K1 serotype strain NTUH-K2044 exhibited reduced mucoviscosity (46). Interestingly, only two response regulators, OmpR and RcsB, were required for hypermucoviscosity. While RcsB has been implicated in capsule regulation, affecting hypermucoviscosity, we investigated the potential cross-regulation between OmpR and RcsB due to the well-known interplay of TCSs in prokaryotes (47). Our results showed that OmpR deficiency did not affect the expression of all genes in the rcsB locus. Additionally, the expression of rmpD was also not altered in the ΔompR mutant. These findings suggest that the OmpR response regulator independently regulates mucoviscosity. Although OmpR modulates mucoviscosity in various serotypes of K. pneumoniae strains, the molecular mechanisms underlying these regulatory processes have not been elucidated.
In this study, we determined that OmpR regulates mucoviscosity independently of the two well-documented determinants of hypermucoviscosity of K. pneumoniae, capsule and RmpD, indicating the involvement of a previously unknown mechanism in OmpR-mediated hypermucoviscosity. Transcriptomic analysis revealed that the absence of OmpR led to changes in the expression of a large number of genes in K. pneumoniae. Genes responsible for ATP biosynthesis and serine metabolism were the most differentially upregulated genes among 273 DEGs identified by enrichment analysis. The downregulation of genes encoding membrane proteins was also evident in the ΔompR mutant. Only the atp operon, encoding F-type ATP synthase, and the gcvTHP encoding glycine cleavage system affected the mucoviscosity. Furthermore, OmpR directly bound the promoter region of the atp operon, not the gcvTHP, suggesting that OmpR directly regulates the expression of the atp operon while likely indirectly regulating gcvTHP, which requires further investigation.
F-type ATP synthase is a nanomotor in bacterial plasma membrane involved in ATP synthesis and hydrolysis. The direction of catalysis depends on the cellular bioenergetic conditions (48). ATP is a critical regulator of central metabolism, playing a crucial role in RNA, DNA, and protein synthesis (49). Similarly, the glycine cleavage system is a crucial enzyme complex of one-carbon metabolism, which catalyzes glycine into CO2, NH4(+), and a methylene group. The methylene group is accepted by tetrahydrofolate (THF) to generate 5,10-methylenetetrahydrofolate, a precursor of amino acid and DNA synthesis. The glycine cleavage system is responsible for serine biosynthesis in Francisella tularensis and is required for its pathogenesis in vivo (50). Thus, both the F-type ATP synthase and the glycine cleavage system are closely related to cellular metabolism. In K. pneumoniae, cellular carbon metabolism has been shown to affect capsule biosynthesis via either glucose through the cAMP-dependent carbon catabolite repression (CCR) (51) or fucose-mediated expression of the rmpD operon (52). This study demonstrated that capsule levels remain unchanged in ΔompR and variants of overexpression of either F-type atp operon or gcvTHP. Therefore, the precise link between the F-type ATP synthase and glycine cleavage system to mucoviscosity remains unknown.
As F-type ATP synthase is directly related to cellular energy conditions, the regulatory mechanism appears to be connected to energy metabolism. The ΔompR mutant significantly increases ATP production. The overexpression of either F-type ATP synthase or GcvTHP enhanced ATP production, which may increase the ATP/ADP ratio. A high cytosolic ATP/ADP ratio inhibits glycolysis, while a lower cytosolic ATP/ADP ratio enhances glycolysis (53). Consequently, high ATP levels in ΔompR cells could inhibit glycolysis, leading to the high production of secondary lactic acid or carboxylic acid. These acids influence the intracellular pH, which drives the switch from a proton-driven ATP synthase to a proton-pumping ATPase (54). Alongside the proton-pumping activity, relatively low extracellular pH appears to promote the breakdown of the glycosidic bonds of polysaccharides to reduce mucoviscosity. We also noted that the ΔF-type mutant also exhibited reduced mucoviscosity, indicating the important role of energy homeostasis in mucoviscosity. Notably, overexpression of either F-type ATP synthase or GcvTHP modestly inhibited the mucoviscosity compared with the ΔompR mutant, suggesting genes or gene clusters other than atp operon and gcvTHP also contribute to hypermucoviscosity formation. The precise modulation of mucoviscosity by energy metabolism alteration due to the OmpR deficiency awaits further investigation.
In conclusion, OmpR is required for hypermucoviscosity and virulence of K. pneumoniae. OmpR regulates energy metabolism, thereby reducing mucoviscosity. This new insight into the link between OmpR-mediated bacterial energy metabolism and hypermucoviscosity enhances our understanding of the complex relationship between bacterial metabolism and mucoviscosity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All bacterial isolates and derivatives of K. pneumoniae ATCC43816 are listed in Table S1. K. pneumoniae strains were grown in Luria-Bertani (LB) (Miller) broth or on Columbia blood plate (BAP) (Oxoid). The following antibiotics or chemicals were added as specified: apramycin (30 μg/mL), spectinomycin (300 μg/mL), L-arabinose (0.2%), kanamycin (50 μg/mL), and sucrose (5%).
String test and sedimentation assay used to determine mucoviscosity.
The hypermucoviscosity was assessed by a positive string test and a semiquantitative sedimentation assay. The formation of >5-mm viscous string indicates a positive string test when a loop was used to lift a portion of a colony from a fresh colony of K. pneumoniae on BAP (55). In addition, the sedimentation assay was conducted as previously described (46). Briefly, K. pneumoniae was grown overnight in a 6-mL LB medium at 37°C with shaking at 180 rpm. The culture's optical density at 600 nm (OD600nm) was measured by spectrophotometry. The culture was sedimented at 1,000 g for 10 min unless otherwise mentioned. The OD600nm ratio of the supernatant to the culture before centrifugation indicated the mucoviscosity semiquantitatively. Sedimentation values were used to classify mucoviscosity: hypermucoviscosity, >0.4; low viscosity, 0.2 to 0.4; and no viscosity, <0.2.
Anthony’s capsule stain and capsule quantification.
Capsules of K. pneumoniae grown on BAP were stained following Anthony’s staining protocol (56) and imaged at ×1,000 magnification. The minor axis of the pale blue zone surrounding 20 random cells was measured by Image J to quantify capsule thickness.
Quantification of the capsule was performed as previously described (57). In brief, bacterial culture grown overnight in LB was mixed with 1% Zwittergent 3-14 detergent (Sigma-Aldrich) in 100 mM citric acid (pH 2.0) and incubated at 50°C for 20 min. The supernatant was precipitated with absolute ethanol. The resulting pellet was dried and resuspended in distilled water. Subsequently, 12.5 mM borax (Sigma-Aldrich) in H2SO4 was added to samples and boiled followed by addition of 0.15% 3-hydroxydiphenol (Sigma-Aldrich), and the absorbance at 520 nm was measured. The uronic acid content was determined from a standard curve of glucuronic acid (Sigma-Aldrich) and presented as micrograms per OD600nm.
Construction of in-frame deletion mutants and site-directed mutagenesis.
All isogenic strains in K. pneumoniae, including markerless deletion mutants, point mutations, and complementation, were generated by a CRISPR-Cas9-mediated genome-editing method (25) and listed in Table S2. Briefly, designed sgRNAs were cloned onto the pSGKP plasmid, and dsDNA homologous arms of genes of interest were amplified by fusion PCR using PrimeSTAR DNA polymerase (TaKaRa). Plasmid pSGKP-sgRNA and its matched dsDNA homologous arms were electro-transformed into pCasKP-harboring K. pneumoniae competent cells cultured in LB supplemented with 0.2% L-arabinose. Transformants were selected with apramycin and spectinomycin at 30°C and verified by DNA sequencing. Finally, the plasmids were cured with 5% sucrose at 37°C. The plasmids and primers used in mutagenesis are listed in Table S3 and S4, respectively.
Overexpression of genes.
Plasmid pACYC184-spe or pIB166-spe was constructed by replacing the chloramphenicol resistance gene of the pACYC184 with the spectinomycin resistance gene (spe). The promoter region of rpsL in K. pneumoniae ATCC43816 was cloned into pACYC184-spe and designated pTH16235. Strains that overexpressed genes of choice were generated by cloning the genes of interest into the pTH16235 vector by NEBuilder HiFi DNA Assembly Cloning Kit (New England BioLabs, USA). Briefly, the open reading frame and the plasmid backbone were amplified with primers listed in Table S4. The gel-purified PCR products were then assembled at 50°C for 30 min according to the manufacturer’s instructions. Next, the assembled products were directly transferred into E. coli DH5α to amplify plasmids overexpressing the genes of interest. Finally, the overexpression plasmids were electro-transformed into K. pneumoniae competent cells to generate corresponding overexpression strains.
RNA-seq analysis.
Total RNA was extracted from a culture grown on BAP overnight using RNAprep pure Cell/Bacteria Kit (Tiangen BioTech, China) according to the manufacturer’s instructions and further purified with an RNeasy minikit (Qiagen, Germany). RNA-seq was performed at the Novogene Bioinformatics Technology (Beijing, China). Trimmed reads were mapped to the genome of K. pneumoniae ATCC43816 (CP009208) using Bowtie 2.3.1 and Tophat 2.1.1. The result of each sample represents the means of two biological replicates. All the bioinformatics analyses were performed using the free online platform of Majorbio Cloud Platform (www.majorbio.com) from Shanghai Majorbio Bio-pharm Technology Co., Ltd. In brief, the RSEM software was used to quantify the gene, and its isoform abundances from paired-end RNA-Seq data, the calculated TPMs (transcripts per million reads) were then directly used for comparing the differences in gene expression among samples using the DESeq2 software, and the corrected P-values (false discovery rate, FDR) were calculated with Benjamini/Hochberg’s method. Subsequently, the DEGs were identified based on |log2FC| ≥1, FDR < 0.05, and TPM > 20. Finally, GO enrichment of the DEGs was carried out to identify the statistically significant differences in functional and biological metabolic pathways levels using the Goatools (https://github.com/tanghaibao/GOatools) and Pathway Tools 26.0 (34), respectively.
Quantitative RT-PCR.
Total RNA was extracted from bacterial cultures grown on BAP overnight using RNAprep pure Cell/Bacteria Kit (Tiangen Biotech, China) according to the manufacturer’s instructions. cDNA was produced using ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO Biotech, China). qRT-PCR was conducted using UltraSYBR Mixture (CWBIO, China) with the Bio-Rad CFX96 system. The gene expression was normalized to 16S rRNA transcript abundance. The relative difference in mRNA levels was calculated using the 2-ΔΔCt method (58). Two independent experiments with three technical repeats were performed for each qRT-PCR analysis.
Quantitation of intracellular ATP.
BacTiter-Glo Microbial Cell Viability Assay (Promega, USA) was utilized to determine the intracellular ATP level. Bacteria were grown in LB Broth (Miller) at 37°C overnight. The overnight culture was diluted in fresh LB Broth and incubated at 37°C with shaking at 180 rpm. Samples were taken at log-phase grown cultures (OD600nm = 0.6) and measured by the BacTiter-Glo Assay. Luminescence was recorded on a luminometer (BioTek Synergy H1). The CFU were determined in parallel and used to calculate the ATP amount per 108 CFU.
Luciferase reporter assay.
The luciferase reporter of rmpADC was constructed as follows: the promoter region of rmpADC was cloned into the upstream of the promoterless luciferase gene in pTH15802 using NEBuilder HiFi DNA Assembly Cloning Kit (New England BioLabs). The resultant plasmid was transformed into competent cells of K. pneumoniae. The reporter strain was confirmed by PCR and DNA sequencing. Relative luminescence units (RLU) were measured by a luminometer (BioTek Synergy H1) and normalized to bacterial OD600nm.
Purification of recombinant OmpR.
The C-terminally His-tagged recombinant OmpR (rOmpR) protein was constructed and purified. In brief, the full-length OmpR coding open reading frame was amplified with a primer pair Pr16788/Pr16789. The PCR product was ligated to the pET28a vector (Novagen) and transformed into E. coli BL21(DE3). E. coli strain BL21(DE3) carrying rOmpR (TH14685) grown to OD600nm = 0.6 at 37°C was induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 18°C for 16 h in LB. Protein rOmpR was purified from the harvested cell pellets using nickel-nitrilotriacetic acid (Ni-NTA) affinity resin (Sangon Biotech, Shanghai, China) according to the manufacturer’s instructions. The purity of rOmpR was >95%, as determined by Coomassie blue-stained SDS polyacrylamide gel. Protein rOmpR was dialyzed overnight with a buffer with 20 mM Tris, 500 mM NaCl, 5% glycerol, pH 8.0.
Electrophoretic mobility shift assay.
Electrophorectic mobility shift assays (EMSAs) were performed as previously described (59) using an EMSA kit (Viagene Biotech Inc., USA) and modified. Promoter regions of atpI and gcvT in K. pneumoniae ATCC43816 were amplified using respective primer sets. Purified OmpR-6×His from 0 to 20 μM mixed with 40 ng of amplified DNA fragments, along with 25 mM acetyl phosphate, 1 mM MgCl2, and 1 μL of poly(dI-dC), were incubated for 20 min at room temperature, and the reaction mixtures were stopped by adding loading buffer. The mixtures were separated by electrophoresis on 6% nondenaturing acrylamide gel running in 0.25× Tris Borate EDTA (TBE) buffer and stained by Gelred staining for bound DNAs.
Murine pneumonia model.
The murine pneumonia model was used to evaluate virulence. Female CD1 mice (6 to 8 weeks old) were intranasally inoculated with approximately 2,000 CFU of log-phase grown K. pneumoniae per mouse. The virulence of strains was evaluated using a survival curve (n = 5). Because hvKP can disseminate to multiple organs, bacterial colonization was assessed by numerating bacteria numbers in multiple organs, including the lung, liver, and spleen, at 72 h postinfection. The data are presented as CFU/gram tissue (n = 3). The experiments were repeated twice. The study was approved by the Institutional Animal Care and Use Committee at Tsinghua University in Beijing.
Statistical analysis.
The statistical analyses were conducted with the GraphPad Prism software 9.3.1, and the relevant data are presented as mean ± SD unless stated otherwise. Significant differences are defined by P values of < 0.05 (*), <0.01 (**), <0.001 (***), and < 0.0001 (****). Statistical analysis for each figure is denoted in the figure legends.
Data availability.
The raw RNA-seq data presented in this study are available in NCBI’s Gene Expression Omnibus (GEO) database (accession GSE197039).
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (31820103001, 31530082, 81671972, and 31728002) and the Beijing Municipal Administration of Hospitals Incubating Program (PX2020043). H.W. was supported by Weight Endowed Professorship from Oregon Health & Science University School of Dentistry.
Footnotes
Supplemental material is available online only.
Contributor Information
Jing-Ren Zhang, Email: zhanglab@tsinghua.edu.cn.
Hui Wu, Email: wuhu@ohsu.edu.
Justin R. Kaspar, The Ohio State University Division of Biosciences
REFERENCES
- 1.Fierer J, Walls L, Chu P. 2011. Recurring Klebsiella pneumoniae pyogenic liver abscesses in a resident of San Diego, California, due to a K1 strain carrying the virulence plasmid. J Clin Microbiol 49:4371–4373. doi: 10.1128/JCM.05658-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CR, Lee JH, Park KS, Jeon JH, Kim YB, Cha CJ, Jeong BC, Lee SH. 2017. Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Front Cell Infect Microbiol 7:483. doi: 10.3389/fcimb.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shon AS, Russo TA. 2012. Hypervirulent Klebsiella pneumoniae: the next superbug? Future Microbiol 7:669–671. doi: 10.2217/fmb.12.43. [DOI] [PubMed] [Google Scholar]
- 4.Russo TA, Olson R, Fang C-T, Stoesser N, Miller M, MacDonald U, Hutson A, Barker JH, La Hoz RM, Johnson JR, Backer M, Bajwa R, Catanzaro AT, Crook D, de Almeda K, Fierer J, Greenberg DE, Klevay M, Patel P, Ratner A, Wang J-T, Zola J. 2018. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol 56:e00776-18. doi: 10.1128/JCM.00776-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li B, Zhao Y, Liu C, Chen Z, Zhou D. 2014. Molecular pathogenesis of Klebsiella pneumoniae. Future Microbiol 9:1071–1081. doi: 10.2217/fmb.14.48. [DOI] [PubMed] [Google Scholar]
- 6.Choby JE, Howard-Anderson J, Weiss DS. 2020. Hypervirulent Klebsiella pneumoniae - clinical and molecular perspectives. J Intern Med 287:283–300. doi: 10.1111/joim.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalan-Najera JC, Garza-Ramos U, Barrios-Camacho H. 2017. Hypervirulence and hypermucoviscosity: two different but complementary Klebsiella spp. phenotypes? Virulence 8:1111–1123. doi: 10.1080/21505594.2017.1317412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HC, Chuang YC, Yu WL, Lee NY, Chang CM, Ko NY, Wang LR, Ko WC. 2006. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. J Intern Med 259:606–614. doi: 10.1111/j.1365-2796.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 9.Wiskur BJ, Hunt JJ, Callegan MC. 2008. Hypermucoviscosity as a virulence factor in experimental Klebsiella pneumoniae endophthalmitis. Invest Ophthalmol Vis Sci 49:4931–4938. doi: 10.1167/iovs.08-2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker KA, Miller VL. 2020. The intersection of capsule gene expression, hypermucoviscosity and hypervirulence in Klebsiella pneumoniae. Curr Opin Microbiol 54:95–102. doi: 10.1016/j.mib.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker KA, Miner TA, Palacios M, Trzilova D, Frederick DR, Broberg CA, Sepúlveda VE, Quinn JD, Miller VL. 2019. A Klebsiella pneumoniae regulatory mutant has reduced capsule expression but retains hypermucoviscosity. mBio 10:e00089-19. doi: 10.1128/mBio.00089-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mike LA, Stark AJ, Forsyth VS, Vornhagen J, Smith SN, Bachman MA, Mobley HLT. 2021. A systematic analysis of hypermucoviscosity and capsule reveals distinct and overlapping genes that impact Klebsiella pneumoniae fitness. PLoS Pathog 17:e1009376. doi: 10.1371/journal.ppat.1009376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker KA, Treat LP, Sepúlveda VE, Miller VL. 2020. The small protein RmpD drives hypermucoviscosity in Klebsiella pneumoniae. mBio 11:e01750-20. doi: 10.1128/mBio.01750-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam S, Monteiro E. 1984. Isolation of mucoid Vibrio parahaemolyticus strains. J Clin Microbiol 19:87–88. doi: 10.1128/jcm.19.1.87-88.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H, Shin J, Chung YJ, Baek JY, Chung DR, Peck KR, Song JH, Ko KS. 2019. Evolution of Klebsiella pneumoniae with mucoid and non-mucoid type colonies within a single patient. Int J Med Microbiol 309:194–198. doi: 10.1016/j.ijmm.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Wall E, Majdalani N, Gottesman S. 2018. The complex Rcs regulatory cascade. Annu Rev Microbiol 72:111–139. doi: 10.1146/annurev-micro-090817-062640. [DOI] [PubMed] [Google Scholar]
- 17.Cheng HY, Chen YS, Wu CY, Chang HY, Lai YC, Peng HL. 2010. RmpA regulation of capsular polysaccharide biosynthesis in Klebsiella pneumoniae CG43. J Bacteriol 192:3144–3158. doi: 10.1128/JB.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capra EJ, Laub MT. 2012. Evolution of two-component signal transduction systems. Annu Rev Microbiol 66:325–347. doi: 10.1146/annurev-micro-092611-150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimbara K, Chakrabarty AM. 1989. Control of alginate synthesis in Pseudomonas aeruginosa: regulation of the algR1 gene. Biochem Biophys Res Commun 164:601–608. doi: 10.1016/0006-291x(89)91502-7. [DOI] [PubMed] [Google Scholar]
- 20.Okkotsu Y, Tieku P, Fitzsimmons LF, Churchill ME, Schurr MJ. 2013. Pseudomonas aeruginosa AlgR phosphorylation modulates rhamnolipid production and motility. J Bacteriol 195:5499–5515. doi: 10.1128/JB.00726-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geisinger E, Isberg RR. 2015. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11:e1004691. doi: 10.1371/journal.ppat.1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santander J, Roland KL, Curtiss R. 2008. Regulation of Vi capsular polysaccharide synthesis in Salmonella enterica serotype Typhi. J Infect Dev Ctries 2:412–420. doi: 10.3855/jidc.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng HY, Chen YF, Peng HL. 2010. Molecular characterization of the PhoPQ-PmrD-PmrAB mediated pathway regulating polymyxin B resistance in Klebsiella pneumoniae CG43. J Biomed Sci 17:60. doi: 10.1186/1423-0127-17-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortet P, Whitworth DE, Santaella C, Achouak W, Barakat M. 2015. P2CS: updates of the prokaryotic two-component systems database. Nucleic Acids Res 43:D536–41. doi: 10.1093/nar/gku968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wang S, Chen W, Song L, Zhang Y, Shen Z, Yu F, Li M, Ji Q. 2018. CRISPR-Cas9 and CRISPR-assisted cytidine deaminase enable precise and efficient genome editing in Klebsiella pneumoniae. Appl Environ Microbiol 84:e01834-18. doi: 10.1128/AEM.01834-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su K, Zhou X, Luo M, Xu X, Liu P, Li X, Xue J, Chen S, Xu W, Li Y, Qiu J. 2018. Genome-wide identification of genes regulated by RcsA, RcsB, and RcsAB phosphorelay regulators in Klebsiella pneumoniae NTUH-K2044. Microb Pathog 123:36–41. doi: 10.1016/j.micpath.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty S, Winardhi RS, Morgan LK, Yan J, Kenney LJ. 2017. Non-canonical activation of OmpR drives acid and osmotic stress responses in single bacterial cells. Nat Commun 8:1587. doi: 10.1038/s41467-017-02030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakraborty S, Kenney LJ. 2018. A new role of OmpR in acid and osmotic stress in Salmonella and E. coli. Front Microbiol 9:2656. doi: 10.3389/fmicb.2018.02656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenney LJ, Anand GS. 2020. EnvZ/OmpR two-component signaling: an archetype system that can function noncanonically. EcoSal Plus 9. doi: 10.1128/ecosalplus.ESP-0001-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenney LJ, Bauer MD, Silhavy TJ. 1995. Phosphorylation-dependent conformational changes in OmpR, an osmoregulatory DNA-binding protein of Escherichia coli. Proc Natl Acad Sci USA 92:8866–8870. doi: 10.1073/pnas.92.19.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. 1995. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol 177:1788–1796. doi: 10.1128/jb.177.7.1788-1796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oshima T, Aiba H, Masuda Y, Kanaya S, Sugiura M, Wanner BL, Mori H, Mizuno T. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol Microbiol 46:281–291. doi: 10.1046/j.1365-2958.2002.03170.x. [DOI] [PubMed] [Google Scholar]
- 33.Quinn HJ, Cameron AD, Dorman CJ. 2014. Bacterial regulon evolution: distinct responses and roles for the identical OmpR proteins of Salmonella Typhimurium and Escherichia coli in the acid stress response. PLoS Genet 10:e1004215. doi: 10.1371/journal.pgen.1004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paley SM, Karp PD. 2006. The Pathway Tools cellular overview diagram and Omics Viewer. Nucleic Acids Res 34:3771–3778. doi: 10.1093/nar/gkl334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caspi R, Billington R, Keseler IM, Kothari A, Krummenacker M, Midford PE, Ong WK, Paley S, Subhraveti P, Karp PD. 2020. The MetaCyc database of metabolic pathways and enzymes - a 2019 update. Nucleic Acids Res 48:D445–d453. doi: 10.1093/nar/gkz862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudek CA, Jahn D. 2022. PRODORIC: state-of-the-art database of prokaryotic gene regulation. Nucleic Acids Res 50:D295–D302. doi: 10.1093/nar/gkab1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marr CM, Russo TA. 2019. Hypervirulent Klebsiella pneumoniae: a new public health threat. Expert Rev Anti Infect Ther 17:71–73. doi: 10.1080/14787210.2019.1555470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Q, Yang X, Chan EWC, Chen S. 2021. The hypermucoviscosity of hypervirulent K. pneumoniae confers the ability to evade neutrophil-mediated phagocytosis. Virulence 12:2050–2059. doi: 10.1080/21505594.2021.1960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SH, Jeon CH, Kim HT, Wi YM. 2022. Clinical characteristics and manifestations in patients with hypermucoviscous Klebsiella pneumoniae bacteremia from extra-hepatobiliary tract infection. Infection doi: 10.1007/s15010-022-01940-6. [DOI] [PubMed] [Google Scholar]
- 40.Li W, Sun G, Yu Y, Li N, Chen M, Jin R, Jiao Y, Wu H. 2014. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis 58:225–232. doi: 10.1093/cid/cit675. [DOI] [PubMed] [Google Scholar]
- 41.Tan TY, Ong M, Cheng Y, Ng LSY. 2019. Hypermucoviscosity, rmpA, and aerobactin are associated with community-acquired Klebsiella pneumoniae bacteremic isolates causing liver abscess in Singapore. J Microbiol Immunol Infect 52:30–34. doi: 10.1016/j.jmii.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Morales-León F, Opazo-Capurro A, Caro C, Lincopan N, Cardenas-Arias A, Esposito F, Illesca V, Rioseco ML, Domínguez-Yévenes M, Lima CA, Bello-Toledo H, González-Rocha G. 2021. Hypervirulent and hypermucoviscous extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Klebsiella variicola in Chile. Virulence 12:35–44. doi: 10.1080/21505594.2020.1859274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dey T, Chakrabortty A, Kapoor A, Warrier A, Nag VL, Sivashanmugam K, Shankar M, Garcia OR. 2022. Unusual hypermucoviscous clinical isolate of Klebsiella pneumoniae with no known determinants of hypermucoviscosity. Microbiol Spectr 10:e00393-22. doi: 10.1128/spectrum.00393-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Le MN, Kayama S, Wyres KL, Yu L, Hisatsune J, Suzuki M, Yahara K, Terachi T, Sawa K, Takahashi S, Okuhara T, Kohama K, Holt KE, Mizutani T, Ohge H, Sugai M. 2022. Genomic epidemiology and temperature dependency of hypermucoviscous Klebsiella pneumoniae in Japan. Microb Genom 8:mgen000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khadka S, Ring B, Krzeminski LR, Hathaway M, Walker RS, Mobley HLT, Mike LA. 2022. Regulation of Klebsiella pneumoniae mucoidy by the bacterial tyrosine kinase Wzc. bioRxiv. doi: 10.1101/2022.06.05.494587. [DOI]
- 46.Dorman MJ, Feltwell T, Goulding DA, Parkhill J, Short FL. 2018. The capsule regulatory network of Klebsiella pneumoniae defined by density-TraDISort. mBio 9:e01863-18. doi: 10.1128/mBio.01863-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noriega CE, Lin HY, Chen LL, Williams SB, Stewart V. 2010. Asymmetric cross-regulation between the nitrate-responsive NarX-NarL and NarQ-NarP two-component regulatory systems from Escherichia coli K-12. Mol Microbiol 75:394–412. doi: 10.1111/j.1365-2958.2009.06987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sielaff H, Duncan TM, Börsch M. 2018. The regulatory subunit ε in Escherichia coli F(O)F(1)-ATP synthase. Biochim Biophys Acta Bioenerg 1859:775–788. doi: 10.1016/j.bbabio.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neupane P, Bhuju S, Thapa N, Bhattarai HK. 2019. ATP Synthase: structure, function and inhibition. Biomol Concepts 10:1–10. doi: 10.1515/bmc-2019-0001. [DOI] [PubMed] [Google Scholar]
- 50.Brown MJ, Russo BC, O'Dee DM, Schmitt DM, Nau GJ. 2014. The contribution of the glycine cleavage system to the pathogenesis of Francisella tularensis. Microbes Infect 16:300–309. doi: 10.1016/j.micinf.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin CT, Chen YC, Jinn TR, Wu CC, Hong YM, Wu WH. 2013. Role of the cAMP-dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae. PLoS One 8:e54430. doi: 10.1371/journal.pone.0054430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hudson AW, Barnes AJ, Bray AS, Ornelles DA, Zafar MA. 2022. Klebsiella pneumoniae l-fucose metabolism promotes gastrointestinal colonization and modulates its virulence determinants. Infect Immun 90:e0020622. doi: 10.1128/iai.00206-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maldonado EN, Lemasters JJ. 2014. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 19 Pt A:78–84. doi: 10.1016/j.mito.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Ballmoos C, Wiedenmann A, Dimroth P. 2009. Essentials for ATP synthesis by F1F0 ATP synthases. Annu Rev Biochem 78:649–672. doi: 10.1146/annurev.biochem.78.081307.104803. [DOI] [PubMed] [Google Scholar]
- 55.Fang C-T, Chuang Y-P, Shun C-T, Chang S-C, Wang J-T. 2004. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med 199:697–705. doi: 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anthony EE, Jr. 1931. A note on capsule staining. Science 73:319–320. doi: 10.1126/science.73.1890.319. [DOI] [PubMed] [Google Scholar]
- 57.Palacios M, Miner TA, Frederick DR, Sepulveda VE, Quinn JD, Walker KA, Miller VL. 2018. Identification of two regulators of virulence that are conserved in Klebsiella pneumoniae classical and hypervirulent strains. mBio 9:e01443-18. doi: 10.1128/mBio.01443-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 59.Gerken H, Vuong P, Soparkar K, Misra R. 2020. Roles of the EnvZ/OmpR two-component system and porins in iron acquisition in Escherichia coli. mBio 11:e01192-20. doi: 10.1128/mBio.01192-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 and S2 and Tables S1 to S4. Download spectrum.00544-23-s0001.pdf, PDF file, 1.2 MB (1.2MB, pdf)
Data Availability Statement
The raw RNA-seq data presented in this study are available in NCBI’s Gene Expression Omnibus (GEO) database (accession GSE197039).