Abstract
Administration of individual chemicals and mixtures during sexual differentiation that disrupt the androgen signaling pathway can induce reproductive abnormalities in male rats. In the current study, we co-administered the heptafluoroisopropyl pesticide pyrifluquinazon (PFQ), and dibutyl phthalate (DBP) to pregnant rats during sexual differentiation of the reproductive tract. Both chemicals have been shown to disrupt reproductive tract differentiation in a dose-related manner reducing male anogenital distance (AGD), permanently reducing androgen-dependent tissue weights and sperm counts, and inducing reproductive malformations in male offspring, albeit by different mechanisms of action that converge downstream in the androgen signaling pathway on a common key event. Rats were orally dosed from gestation days 14–18 with dilutions of PFQ and DBP at 0, 12.5, 25, 50, 75 and 100% of the top dose (100 mg/kg PFQ and 750 mg/kg DBP). The mixture ratio was selected such that each chemical would contribute equally to multiple effects on the male offspring reproductive tract and the dose range was designed to determine if the mixture produced additive effects predicted by dose addition or response addition models, or whether significant interactions occurred. Observed data were compared to dose and response addition model predictions. As hypothesized, the mixture reduced F1 male AGD, reproductive organ weights and sperm counts and induced hypospadias with dose addition consistently providing a better prediction of the observed effects than response addition. These results support our hypothesis that chemicals that disrupt the androgen signaling pathway induce dose-additive male reproductive abnormalities regardless of the specific mechanism of action.
Keywords: mixtures toxicology, male reproductive toxicity, pesticides, phthalates, perfluorinated chemicals
Introduction
Per- and polyfluoroalkyl substances (PFAS) vary greatly in structure, and depending on the definition (e.g., OECD, US EPA, etc.), the term “PFAS” may refer to as few as a couple hundred individual chemicals to more than 9,000. The Organization of Economic Cooperation and development (OECD) and the EPA are currently reviewing their definition of PFAS (Wang et al. 2021), but currently pyrifluquinazon (PFQ) has been identified as a PFAS by the OECD as well as the USEPA CompTox PFAS Master List (https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster) and is one of several registered pesticides with a heptafluoroisopropyl group (Qacemi et al. 2019). The structure of this subclass of PFAS is quite different from legacy/straight-chain structures like perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS), showing the diversity in structure and bioactivity among the PFAS. PFQ is currently registered under the Federal Insecticide, Fungicide and Rodenticide Act (FIFRA) for use on a variety of food crops and ornamental plants and aerial application in the United States (US EPA 2018, 2020).
PFQ and dibutyl phthalate (DBP) have been shown individually to disrupt male reproductive tract differentiation of many of the same tissues in a dose-dependent manner, including reduction of anogenital distance (AGD), androgen-dependent tissue weights and sperm counts (Gray et al. 2019a), (Hotchkiss et al. 2010; Mylchreest et al. 1998; Mylchreest et al. 1999; Mylchreest et al. 2000). In EPA’s human health assessment of PFQ, the Health Effects Division of the Office of Pesticide Programs selected reduced male rat AGD as the endpoint of concern for females of childbearing age and fetuses. An Acute Population Adjusted Dose (aPAD) of 0.05 mg/kg/day was derived from a developmental NOAEL of 5mg/kg day and a 100-fold UF (10X for inter-species extrapolation, and 10X for intra-species variation).
The current study is similar in design to our fixed ratio binary mixture dilution study with DBP and the androgen receptor (AR) antagonist procymidone (Hotchkiss et al. 2010) (Table 1). Herein, we conducted a fixed ratio binary mixture study with PFQ, a perfluoroalkyl-isopropyl pesticide (PFAP), and DBP. To our knowledge, this is the first study showing that a mixture of a PFAS with another chemical produces cumulative, dose additive adverse effects on reproductive development in male rats. There are few, if any, published studies on the effects of PFAS mixed with other chemicals on reproductive development after in utero exposure. A few in vivo mixture studies with PFAS have been published, but these did not compare dose- and response addition models so it is not possible to determine if the mixture effects were additive, synergistic or antagonistic (Marques et al. 2021; Roth et al. 2021).
Table 1.
Binary Mixture Studies Summary
| Administered by Oral Gavage Gestational Days 14 to 18 | A+B=? | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chemical A | Chemical B | Chemical A | Chemical B | Chemical A+B | Effect | ||||
| Name | Dose mg/kg/d | MOA/MIE | Name | Dose mg/kg/d | MOA/MIE | % Malformed | % Malformed | % Malformed | |
|
| |||||||||
| Vinclozolin | 50 | AR antagonist | Procymidone | 50 | AR antagonist | 0 | 0 | 54 | Vaginal Pouch |
| 10.2 | 0 | 96 | Hypospadias | ||||||
|
| |||||||||
| DBP | 500 | Fetal Testis Insl3 and Androgen Levels | Procymidone | 50 | AR antagonist | 0 | 0 | 26.7 | Vaginal Pouch |
| 0 | 1.5 | 48.8 | Hypospadias | ||||||
| 0 | 0 | 29 | Gubernaculum Testis Agenesis | ||||||
|
| |||||||||
| DBP | 500 | Fetal Testis Insl3 and Androgen Levels | BBP | 500 | Fetal Testis Insl3 and Androgen Levels | 0 | 0 | 40 | Vaginal Pouch |
| 0 | 0 | 50 | Hypospadias | ||||||
| 0 | 8 | 100 | Gubernaculum Testis Agenesis | ||||||
| 9 | 23 | 100 | Epididymal Agenesis | ||||||
|
| |||||||||
| DBP | 500 | Fetal Testis Insl3 and Androgen Levels | DEHP | 500 | Fetal Testis Insl3 and Androgen Levels | 3 | 0 | 25 | Hypospadias |
| 3 | 27 | 75 | Gubernaculum Testis Agenesis | ||||||
| 26 | 5 | 65 | Epididymal Agenesis | ||||||
|
| |||||||||
| BBP | 500 | Fetal Testis Function | Linuron | 75 | Fetal Testis Androgen Levels AR antagonist | 0 | 0 | 41 | Vaginal Pouch |
| 0 | 0 | 56 | Hypospadias | ||||||
| 12 | 62 | 97 | Epididymal Agenesis | ||||||
| 12 | 13 | 62 | Seminal Vesicle Agenesis | ||||||
|
| |||||||||
| DBP | 500 | Fetal Testis Insl3 and Androgen Levels | 2, 3, 7, 8 TCDD | 0.0013 to 0.002 | AhR Agonist | 24 | 0 | 45 | Epididymal Agenesis or Atrophy |
| 7 | 21 | 37 | Epididymal Sperm % Reduced | ||||||
| 9 | 0 | 21 | Epididymal aspermia | ||||||
| 5 | 0 | 28 | Seminal Vesicle | ||||||
| 14 | 0 | 26 | Vas Deferns Agenesis | ||||||
|
| |||||||||
| DBP | Fixed ratio dilution study 94 to 750 | Fetal Testis Insl3 and Androgen Levels | Pyrifluquinazon | Fixed ratio diultion study 12.5 to 100 | AR degradation | See Current Study Results | |||
One advantage of binary mixture studies, like the current study, is that interpretation of the nature of the mixture effects is clear. In contrast, it is possible, albeit unlikely, that the effects in a more complex mixture study could appear dose additive, when in reality, some interactions in the mixture were antagonistic while others were synergistic. An emerging body of information supports the hypothesis that when chemicals that disrupt androgen signaling via different mechanisms of action are administered as a mixture to pregnant female rats during the critical window of sexual differentiation, permanent adverse effects occur in the offspring at doses lower than those at which the individual chemicals produce effects, and the mixture effects are best predicted by dose addition modeling.
Over the last 20 years, we have conducted several component-based mixture studies with chemicals that disrupt the androgen signaling pathway in utero via a variety of mechanisms (reviewed by (Howdeshell et al. 2017). These studies demonstrate that chemicals with diverse mechanisms of toxicity and modes of action, with different structures, from different chemical classes produce cumulative effects that are more accurately predicted by dose addition than response addition. Studies have included binary mixture designs including mixtures of benzyl butyl phthalate and the pesticide linuron (Hotchkiss et al. 2004), DBP and the pesticide procymidone (Hotchkiss et al. 2010), and dipentyl phthalate and the drug simvastatin (Beverly et al. 2014) (Table 1), and multichemical mixture studies with 7 (Rider et al. 2008), 10 (Rider et al. 2010), 15 (Conley et al. 2021a) and 18 chemicals (Conley et al. 2018). Two of the multichemical studies (Conley et al. 2018; Conley et al. 2021b) reported effects at mixture dose levels well below the individual chemical no-observed effect level on the developing reproductive system. Taken together, these studies, along with similar studies from other researchers (Christiansen et al. 2008; Hass et al. 2007; Kortenkamp 2007; Metzdorff et al. 2007), all indicate that when considering cumulative assessment chemicals should be grouped based upon their ability to disrupt common toxicological targets as opposed to grouping them by chemical structure or class, molecular mechanism, or mode of action.
In the current study, pregnant rats were exposed by oral gavage to a mixture of PFQ and DBP from gestational days (GD) 14–18, the stage of pregnancy in rat during the critical window of rat sexual differentiation (Wolf et al. 2000) (Gray et al. 1999) (Carruthers and Foster 2005). The study was a fixed-ratio dilution design and included doses of 0, 12.5, 25, 50, 75 and 100% of the top dose which contained 100 mg/kg for PFQ and 750 mg/kg for DBP. The dose range was designed to determine if the mixture acted in a dose additive, response additive, synergistic (at low doses) or antagonistic (at high doses) manner. Based upon the results of our previous studies, we hypothesized that these two chemicals would act in a dose additive manner because they both disrupt the androgen signaling pathway during sexual differentiation and induce common effects even though they act via different mechanisms of action.
Methods
The study consisted of 6 treatment groups administered at 0, 12.5, 25, 50, 75 and 100% of the top dose corresponding to 0, 12.5mg PFQ + 93.75mg DBP, 25mg PFQ + 187.5 mg DBP, 50mg PFQ + 375mg DBP, 75mg PFQ + 562mg DBP, and a top dose of 100mg PFQ + 750mg DBP) with 5 pregnant dams in each group. The top dose of each chemical has been shown to produce a high rate of reproductive tract malformations when administered from GD 14–18 (Gray et al. 2019a) (Hotchkiss et al. 2010). An examination of the ED50s for each chemical indicates that the relative potency factors for PFQ to DBP varies from androgen-dependent endpoint to endpoint. For this reason, PFQ and DBP contribute equally to the development of some abnormalities in the current study, whereas one chemical or the other is primarily responsible for the induction of other effects.
Animals and administration of chemicals
Timed-pregnant Sprague Dawley (SD) rats, approximately 90 days old, were purchased from Charles River Laboratories (Raleigh, NC, USA) and shipped to EPA on GD 2 (date of sperm plug positive = GD 1). Animals were housed individually in clear, polycarbonate cages (20 × 25 × 47 cm) lined with laboratory-grade heat treated pine shavings (Northeastern Products, Warrensburg, NY), with a 12:12 light: dark photoperiod (lights off at 18:00) at 20–22 °C and 45–55% humidity. Dams and offspring were provided with NIH07 rat chow during gestation and lactation and offspring were provided NTP 2000 rodent diet after weaning. Rats had access to filtered (5 micron) municipal tap water ad libitum which is tested every 4 months for a subset of heavy metals, pesticides, and other chemical contaminants and tested monthly for Pseudomonas. These studies were conducted under protocols approved by the National Health and Environmental Effects Research Laboratory Institutional Animal Care and Use Committee at a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Pregnant rat dams were randomly assigned to treatment groups on GD 14 in a manner that provided each group with similar means and variances in body weight (randomized complete block design). Dams were weighed and dosed daily by oral gavage at approximately 0730 hr. (EST) from GD 14–18 with the vehicle (laboratory-grade corn oil [CAS #8001-30-7] or the test substance mixture at 0.25 ml corn oil kg bodyweight. PFQ was purchased from Chem Services, Inc. (CAS 337458-27-2, Lot # 2110000, Cat # RPN-13158-5G, purity = 99%) and DBP was obtained from Research Triangle -Chemical Services for the National Toxicology Program (Sample Date 12/15/09, RTI Log #: 031609-A-17, Chem ID I16, CAS 84-74-2, Lot # 91997PJ).
Endpoints modeled in the F1 offspring include:
Anogenital distance (AGD) was measured in 2 day old offspring using a dissecting microscope with an ocular micrometer with the observer blind to treatment (as previously described (Ostby and Gray Jr 2004). AGD represents the distance between the base of the genital papilla and the rostral end of the anal opening. Female-like nipple/areola retention was evaluated when the pups were 13 days old and the position of the areolae on the milk lines and the number of areole/nipples were recorded. The age at puberty (as indicated by full preputial separation- PPS) was examined from 40 days of age, when it was first detected in this study, until 47 days of age and the age and weight at puberty were recorded. PPS is considered complete when the glans penis is completely separated from the prepuce (Korenbrot et al. 1977).
Male offspring (total n=120 males) were necropsied as adults and androgen dependent organ weight changes were measured (glans penis, ventral prostate, seminal vesicles, testes, epididymis, levator ani/bulbocavernosus muscle, Cowper’s glands, kidneys, and liver) and males were examined for the presence of hypospadias. Other malformations were noted in the higher dose levels of the mixture, but these were not modeled because we could not determine ED50 and slope values with any confidence due to the low effect range (0 to 15%) observed. For example, a sensitivity analysis indicated that adding or removing a single affected male from one of the two higher dose groups in the PFQ dose response study changed the actual ED50 value of 532 mg/kg to 262 mg/kg or 1360 mg/kg, all of which were well above the PFQ content of 100 mg/kg/d in the top dose of the mixture. In addition, these malformations often display very steep slopes with ambiguous logistic regression parameter estimates.
Epididymal sperm counts were also measured. When present, the right epididymis was used to determine caput/corpus and caudal sperm counts as previously described (Gray et al. 1989; Gray et al. 1995). The right epididymis was cut 13 mm from the tip of the cauda, and the cauda and caput and corpus were placed in separate 20 mL glass scintillation vials containing 2 mL modified M199 media (pre-warmed to 37 °C in an incubator) and minced uniformly with iris scissors. An additional 3 mL of modified M199 were added to each vial after mincing, and the vial was then incubated for approximately 1 hour at 37 °C. At the end of 1 hour, 15 mL of 10% buffered formalin acetate was added to each vial, bringing the total volume to 20 mL (considered to be a 1:20 dilution). The vials are then vortexed for approximately 30 seconds. An aliquot of the diluted sample was then brought to a final dilution of 1:8000 by pipetting 50 μl of the dilution containing minced epididymal tissue and adding 20 mL of 10% buffered formalin acetate to form a second dilution. A homogenous sample of 0.5 mL of the final dilution was counted three independent times using a Beckmann-Coulter cell counter and the average of the three independent counts was used for statistical analyses. The total number of sperm per sample was determined as [(the average of 3 counts) × (16,000)]. This method yields accurate total sperm counts within the normal range of numbers for adult and pubertal male rats (Robb et al. 1978) and a low intra-sample coefficient of variation (less than 5%) and low coefficients of variation among control adult male rats (CV control males; PFQ study =19%, DBP study=17%, Mix study = 21%).
Statistical Methods
Maternal and litter data were analyzed using PROC GLM in SAS 9.4 and each dose group was compared to control using the LSMEANS option. Data collected on multiple F1 offspring per litter were analyzed using litter means values with PROC GLM. Males displaying complete agenesis of an organ were assigned a weight of 0 mg. In addition, males with complete agenesis of the epididymis were assigned a sperm count value of zero.
Dose response data were analyzed using a four-parameter logistic regression (4PL) model in GraphPad Prism 8.4.3 to identify the ED50 and slope parameters for each variable.
| Equation 1 |
where is the response, is the chemical dose, is the power or Hill slope of the curve, and is the exposure dose eliciting a 50 % response. The and slopes generated in the individual chemical analyses were used in equations to predict the overall mixture response.
Data were normalized to percent of control in Prism and logistic regression models for most variables were run with the top constrained to 100% and 0% at the bottom, except for AGD at PND2 and testis weights collected at necropsy. The logistic regression model for AGD was constrained to 100% at the top using the mean F1 control male value and the female rat AGD value at the bottom (representing the maximum biologically plausible degree of demasculinization). Since the effect on testis weight is due to the absence or reduction in spermatogenesis due to fluid pressure atrophy that results from epididymal lesions rather than agenesis of the testis, the bottom of the model for paired testes weight was set to the value for paired testis weight typically seen with bilateral testis hypospermatogenesis which was 2.0 g in the PFQ and mixture studies and 1.47 g in the DBP dose response study.
Data from published in utero DBP and PFQ studies in our laboratory with GD 14–18 dosing were used to calculate ED50 and slope parameters for use in dose- (DA) and response- (RA) addition mixture models to predict the effects of the mixture of DBP and PFQ observed in the current study (Hotchkiss et al. 2010) (Gray et al. 2019a). DA was calculated using two different statistical models. The first model, our laboratory has traditionally used (Rider and LeBlanc 2005) (Olmstead and LeBlanc 2005) (Rider et al. 2008), uses an average slope value to calculate DA and assumes that the slopes for each chemical in the mixture are similar to one another for a specific endpoint (referred to herein as DA1). The joint toxicity of these binary mixtures of like-acting chemicals was computed using the following equation (Olmstead and LeBlanc, 2005):
| Equation 2 |
where is the response to the mixture, is the concentration of chemical in the mixture, is the concentration of chemical that causes a 50% response, and is the power (Hillslope) associated with the chemicals.
Since this assumption is not always met, we also calculated DA using the model described by several authors [for example (Altenburger et al. 2000) (Kortenkamp et al. 2007) (Metzdorff et al. 2007)] and by the NAS (NAS 2008) which does not require parallel slopes for the chemicals in the mixture (referred to as DA2). For DA2, the dose-additivity predictions were estimated using Equation 3. These are defined by the effect dose of the mixture of DBP plus PFQ for the effect doses of the two chemicals where is the proportion of chemical in the mixture, denotes the fractional effect (in %) of the chemical at
| Equation 3 |
Response addition (RA) was estimated using Equation 4 where is the predicted effect of the mixture and and are the individual effects of DBP and PFQ at a given dose levels.
| Equation 4 |
The objective of using two DA models was to determine if they provided similar predictions and, if not, to what degree differences in slopes of the dose response curves resulted in differences in DA mixture predictions. We also predicted mixture effects using independent action with a “response addition” (RA) model as described in (Rider et al. 2008).
Following these analyses, the “better” mixture model among the two DA models and the RA model was determined by fitting the data with the each 4PL model, constrained to 100% at the top, 0% at the bottom, and to the ED50 and Hillslope value of each model. Following this the Akaike information criterion (AIC) values among the DA1, DA2 and RA models were compared by calculating the probability that one model was more or less informative than another using the equation which is known as the relative likelihood of model (Burnham and Anderson 2004).
RESULTS
Maternal and F1 Effects
Maternal body weight on GD 18 was not significantly reduced at any dose level, being reduced by about 5% in the top dose group, but maternal weight gain was significantly reduced by about 20 g as compared to control dams in the high dose group (Table 2). Postnatal litter sizes were reduced in the top dose group on PND13 and PND23 and the percent of pup mortality was significantly increased in the top dose group by PND23 (Table 2). In addition, pup body weight was significantly reduced on PND 2 in the top dose group by about 1.25 g, approximately 15% of control, but there was no significant effect on male body weight on PND 13 or at weaning.
Table 2.
Litter and neonatal effects of in utero administration of a Dibutyl Phthalate and the PFAS pesticide Pyrifluquqinazon
| Percent of Top Dose | 0 | 12.5 | 25 | 50 | 75 | 100 |
|
|
||||||
| Pyrifluquinazon Dose (mg/kg/d) | 0 | 12.5 | 25 | 50 | 75 | 100 |
|
|
||||||
| Dibutyl Phthalate Dose (mg/kg/d) | 0 | 93.75 | 187.5 | 375 | 562 | 750 |
|
|
||||||
| Number of Dams | 5 | 5 | 5 | 5 | 5 | 5 |
|
| ||||||
| Implantations | 13.6 ±1.3 | 12.8 ±0.2 | 13.4 ±0.6 | 14.2 ±0.6 | 13.6 ±0.2 | 12.4 ±1.2 |
| GD 18 Maternal Body Weight (g) | 354.7 ±15.5 | 354.2 ±4.9 | 358.3 ±6.4 | 361.8 ±4.0 | 364.2 ±8.7 | 337.7 ±10.1 |
| GD 14 to 18 Maternal Weight Gain (g) | 42.2 ±3.4 | 30.2 ±1.1 * | 33.8 ±0.7 | 35.0 ±1.9 | 30.5 ±3.2 * | 20.1 ±7.1 ** |
|
| ||||||
| Litter Size PND 2 | 13.4 ±1.3 | 12.0 ± 0.5 | 13.0 ±0.7 | 13.4 ±0.9 | 11.2 ±1.0 | 10.8 ±1.3 |
| Female Pup weight (g) PND 2 | 7.82 ±0.10 (35) | 7.93 ±0.27 (29) | 7.45 ±0.12 (39) | 7.51 ±0.09 (39) | 7.52 ±0.14 (26) | 6.72 ±0.13(29) ** |
| Male Pup weight (g) PND 2 | 8.20 ±0.12 (32) | 8.23 ±0.14 (30) | 7.94 ±0.09 (36) | 7.79 ±0.11 (28) | 7.60 ±.12 (30) | 6.93 ±0.13 (22) ** |
| Female AGD (mm) | 1.54 ±0.03 (35) | 1.65 ±0.04 (29) | 1.51 ±0.3 (30) | 1.42 ±0.03 | 1.47 ±.03 (26) | 1.59 ±0.03 (29) |
| Male AGD (mm) | 3.71 ±0.06 (32) | 3.46 ±0.07 (30) | 2.78 ±0.04 (36) ** | 2.51 ±0.08 (28) ** | 2.15 ±0.04(30) ** | 2.18 ±0.07 (22) ** |
| Male AGD (% of Androgen Effect) | 100 ±2.82 | 88.1 ±3.3 | 55.6 ±1.98 | 42.9 ±3.6 | 26.1 ±1.9 | 27.4 ±3.2 |
| % Pup Loss (Implants - PND23 litter size) | 18.3 ±8.2 | 15.6 ±2.4 | 15.8 ±10.4 | 19.2 ±3.6 | 26.7 ±5.7 | 45.2 ±8.9 * |
| Retained Nipples-Areolae/Male PND 13 | 0 ± (29) | 0.6 ± (29) | 4.7 ±0.4 (34) ** | 6.6 ±0.4 (22) ** | 11.7 ±0.2 (27) ** | 12 ±0 (17) ** |
| % Males with any Retained Nipples-Areolae PND 13 | 0 ±0 | 30.0 ±0.09 ** | 91.2 ±0.05 ** | 100 ±0 ** | 100 ±0 ** | 100 ±0 ** |
F1 data were analyzed using litter means including body weight, AGD, nippes-aerolae, survival and preputial separation
F1 means ± standard error (sample size) are based upon individual pup values, not litter means
p <0.01
p <0.05
AGD was not affected in female pups on PND2, whereas AGD in male rat pups was reduced in all dose groups being significant at 25% of the top dose level and above (p < 0.0001). The magnitude of the reduction in AGD in males ranged from 10 to 75% of control, being reduced by 45% of control in the 25% of top dose group (control female AGD = 0% of control) (Table 2).
At 13 days of age, control males did not display female-like retained nipples/areolae but treated males displayed nipples/areolae in all dose groups ranging from 0.65 to 12 nipples-areolae/per male, being significant at dose levels of 25% of the top dose and above. The percentage of males displaying any nipples-areolae was significant at all dose levels ranging from 30% affected at 12.5% to 100% in the top two dose groups (Table 2).
The age at puberty could not be evaluated in F1 males in the top two dose groups due to genital malformations and was not significantly affected in males with normal genital morphology (data not shown).
When adult F1 male rat offspring were necropsied, all reproductive organ weights were significantly reduced in the 50, 75% and the top dose group (100%) (Table 3). In the 25% mixture group, males displayed a significant increase in permanently retained female-like nipples, and reduced epididymal sperm. Testicular histopathological results confirmed the observations of gross testicular abnormalities noted at necropsy (Table 4).
Table 3.
Adult F1 male rat organ weights, gubernaclum testis length (paired), number of retained female-like nipples/areolae and epididymal sperm counts.
| Percent of Top Dose | 0 | 12.5 | 25 | 50 | 75 | 100 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| F1 Male Offspring | Mean | SE | N | Mean | SE | N | Mean | SE | N | Mean | SE | N | Mean | SE | N | Mean | SE | N |
|
| ||||||||||||||||||
| Body Weight at Necropsy (g) | 745.5 | 17.79 | 21 | 718.3 | 15.01 | 21 | 702.5 | 15.49 | 21 | 685.6 | 16.54 | 20 | 696.3 | 32.99 | 15 | 667.7 ** | 22.97 | 20 |
| Glans Penis (mg) | 141.9 | 1.82 | 21 | 134.8 | 1.94 | 21 | 128.9 * | 2.80 | 19 | 126.7 ** | 4.04 | 12 | 97.8 * | 1 | 82.9 ** | 1 | ||
| Levator Ani-bulbocavernosus (g) | 1.76 | 0.05 | 21 | 1.61 | 0.03 | 21 | 1.51 | 0.05 | 21 | 1.06 ** | 0.05 | 20 | 0.71 ** | 0.04 | 15 | 0.49 ** | 0.06 | 20 |
| Ventral Prostate (mg) | 673.8 | 42.47 | 21 | 556.1 | 30.10 | 21 | 531.8 | 34.11 | 21 | 386.0 ** | 48.52 | 20 | 56.4 ** | 40.61 | 15 | 0 ** | 0.00 | 20 |
| Seminal Vesicle (g) | 2.07 | 0.06 | 21 | 1.91 | 0.05 | 21 | 2.01 | 0.05 | 21 | 1.73 * | 0.11 | 18 | 0.16 ** | 0.09 | 15 | 0.02 ** | 0.02 | 20 |
| Paired Testes (g) | 4.24 | 0.07 | 21 | 4.47 | 0.06 | 21 | 3.86 | 0.08 | 21 | 3.77 ** | 0.11 | 20 | 2.20 ** | 0.19 | 15 | 2.46 ** | 0.22 | 20 |
| Gubernaculum Testis (mm) | 19.2 | 0.46 | 21 | 18.1 | 0.61 | 21 | 15.6 | 0.48 | 21 | 19.4 | 0.97 | 20 | 110.0 ** | 23.30 | 15 | 161.8 ** | 14.26 | 20 |
| Paired Epididymides (mg) | 1460.7 | 26.24 | 21 | 1495.1 | 20.26 | 21 | 1305.3 | 39.36 | 21 | 1287.3 ** | 53.24 | 20 | 121.8 ** | 51.28 | 15 | 1.3 ** | 1.29 | 19 |
| Cauda Epididymis (mg) | 309.9 | 7.10 | 21 | 307.2 | 9.10 | 21 | 281.4 * | 7.50 | 21 | 274.9 ** | 11.30 | 20 | 23.5 ** | 20.50 | 15 | 1.23 ** | 0.00 | 19 |
| Epididymal sperm count ×106 | 177.1 | 9.65 | 15 | 179.3 | 6.92 | 16 | 135.2 * | 13 | 16 | 142.6 * | 14.5 | `6 | 7.83 ** | 2.3 | 3 | 1.55 ** | 1 | |
N is the number of individual males, analyses for dose effects used Litter Means, organ weights were not taken on all males but they were all examined for testis and epiddymal histopathology Organ weights were not taken on all males but they were all examined for testis and epididymal histopathology
p<0.01
p<0.05
Table 4.
Dose related Reproductive alterations in F1 Male Rat Offspring after in utero exposure to a mixture of dibutly phthalate and pyrifluquinazon
| Percent of Top Dose of the Mixture | 0 | 12.5 | 25 | 50 | 75 | 100 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abnormalities | Mean | SE | N males | Mean | SE | N males | Mean | SE | N males | Mean | SE | N males | Mean | SE | N males | Mean | SE | N males |
|
| ||||||||||||||||||
| Agenesis of the Coagulating Glands | 0 | 0 | 28 | 0 | 0 | 27 | 0 | 0 | 31 | 0 | 0 | 19 | 52.4 ** | 11.2 | 21 | 50 ** | 11.5 | 20 |
| Epididymal Agenesis* | 0 | 0 | 28 | 0 | 0 | 27 | 3.2 | 3.2 | 31 | 15.8 * | 8.6 | 19 | 100 ** | 0 | 21 | 85 ** | 8.2 | 20 |
| Epididymal Histological Hypospermia | 0 | 28 | 0 | 27 | 3.2 | 31 | 5.3 | 75.0 | 3 | no sample | 0 | |||||||
| Gubernaculum Testis Agenesis | 0 | 0 | 28 | 0 | 0 | 27 | 0.0 | 0.0 | 31 | 0 | 0 | 19 | 47.6 ** | 11.2 | 21 | 90 ** | 6.9 | 20 |
| Hypospadias | 0 | 0 | 28 | 0 | 0 | 27 | 6.5 | 4.5 | 31 | 47.4 ** | 11.8 | 19 | 100 ** | 0 | 21 | 100 ** | 0 | 20 |
| Any Major Abnormality | 3.6 | 3.6 | 28 | 3.70 | 3.70 | 27 | 16.1 | 6.7 | 31 | 57.9 ** | 11.6 | 19 | 100 ** | 0 | 21 | 100 ** | 0 | 20 |
| % of Males with and Nipples | 0 | 0 | 28 | 3.70 | 3.70 | 27 | 64.5 ** | 8.7 | 31 | 68.4 ** | 11.0 | 19 | 100 ** | 0 | 21 | 100 ** | 0 | 20 |
| Seminal Vesicle Agenesis | 0 | 0 | 28 | 0 | 0 | 27 | 0 | 0 | 31 | 10.5 | 7.2 | 19 | 61.9 ** | 10.9 | 21 | 90 ** | 6.9 | 20 |
| Testis Abnormalities | 3.6 | 3.6 | 28 | 0 | 0 | 27 | 9.7 | 5.4 | 31 | 15.8 | 8.6 | 19 | 100 ** | 0 | 21 | 100 ** | 0 | 20 |
| Undescended Testis | 0 | 0 | 28 | 0 | 0 | 27 | 0 | 0 | 31 | 0 | 0 | 19 | 23.8 ** | 9.5 | 21 | 75 ** | 9.9 | 20 |
| Unilateral or Bilateral Testis Tubular Atrophy | 3.6 | 28 | 0 | 27 | 9.7 | 31 | 11.3 | 19 | 100 | 21 | 100 | 20 | ||||||
| Vaginal Pouch | 0 | 0 | 28 | 0 | 0 | 27 | 0 | 0 | 31 | 10.5 | 7.2 | 19 | 90.5 ** | 6.6 | 21 | 90 ** | 6.9 | 20 |
| Vas Deferens Agenesis | 0 | 0 | 28 | 0 | 0 | 27 | 0 | 0 | 31 | 5.3 | 5.3 | 19 | 52.4 ** | 11.2 | 21 | 70 ** | 10.5 | 20 |
| Ventral Prostate Agenesis | 0 | 0 | 28 | 0 | 0 | 27 | 0 | 0 | 31 | 5.3 | 5.3 | 19 | 66.7 ** | 10.5 | 21 | 90 ** | 6.9 | 20 |
N is the number of individual males, analyses for dose effects used Litter Means
p<0.01
p<0.05
Agenesis refers to organs that display gross partial or complete agenesis or hypoplasia at necropsy
Mixture model analyses
We determined the ED50 and slope values for 12 endpoints for each chemical and used these values to calculate the DA1, DA2 and RA model predictions of joint action of PFQ and DBP binary mixtures (Table 5). These endpoints included AGD, percent of males with any retained female-like nipples/areolae at 13 days of age, number of nipples per males, six androgen-dependent organ weights (ventral prostate, seminal vesicle plus coagulating glands and fluid, levator ani-bulbocavernsus muscles (LABC), testis, Cowper’s glands and epididymal weights), epididymal sperm counts and percent incidence of hypospadias (Figures 1a,b).
Table 5.
Results of four parameter logistic (4PL) regression modeling of the individual dibutyl phthalate (DBP) and pyrifluquinazon (PFQ) (two columns on the right) and the two dose addition (DA) and response addition models fit to the observed mixture data. DA1 and DA2 4PL models were calculated using Equations 2 and 3, respectively, in the described in the methods.
| Observed | DA2 | DA1 | RA | DBP | PFQ | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Anogenital distance | ED50 CI | 30.36 24.48 to 37.15 |
43.68 | 47.31 | 48.18 | 829.6 | 70.95 |
| HillSlope | 1.634 | 1.497 | 1.859 | 1.67 | 3.408 | 1.451 | |
|
| |||||||
| Ventral Prostate Weight | ED50 CI | 40.92 | 42.88 | 44.78 | 67 | 987 | 67.15 |
| HillSlope | 2.697 | 3.361 | 2.478 | 2.752 | 13.5 | 2.734 | |
|
| |||||||
| Seminal Vesicle Weight | ED50 CI | 56.76 50.87 to 63.32 |
52.1 | 48.29 | 95.99 | 737.9 | 111.4 |
| HillSlope | 8.948 | 9.746 | 52.32 | 12.02 | 39.55 | 5.247 | |
|
| |||||||
| Testis Weight | ED50 CI | 54.98 50.87 to 63.32 |
53.59 | 50.96 | 98.32 | 755.0 | 115.0 |
| HillSlope | 3.758 | 10.07 | 60.89 | 8.099 | 26.30 | 5.89 | |
|
| |||||||
| Testis Tubular Semiferous Hypospermatogenesis | ED50 CI | 52.28 ambiguous |
46.56 | 46.54 | 68.89 | 550 | 127.70 |
| HillSlope | 29.96 | 3.407 | 3.339 | 3.945 | 3.791 | 2.89 | |
|
| |||||||
| Epididymal Weight | ED50 CI | 56.93 52.41 to 61.83 |
51.43 | 46.53 | 76.91 | 579.4 | 153.9 |
| HillSlope | 8.513 | 6.615 | 8.011 | 6.815 | 6.725 | 6.431 | |
|
| |||||||
| LABC Weight | ED50 CI | 60.17 51.10 to 70.81 |
62.6 | 62.92 | 85.79 | 967 | 122.8 |
| HillSlope | 1.878 | 2.136 | 2.363 | 1.993 | 2.966 | 1.707 | |
|
| |||||||
| % OF 12 Nipples/Areolae per male | ED50 CI | 34.45 28.34 to 41.48 |
30.52 | 31.34 | 41.33 | 954 | 41.33 |
| HillSlope | 2.36 | 2.308 | 2.295 | 1.734 | 25 | 1.734 | |
|
| |||||||
| % males with any Nipples/Areolae | ED50 CI | 15.09 13.41 to 16.99 |
15.08 | 14.41 | 18.52 | 584.7 | 19.05 |
| HillSlope | 4.498 | 4.839 | 3.142 | 8.114 | 1.638 | 9.203 | |
|
| |||||||
| HYPOSPADIAS | ED50 CI | 50.3 47.81 to 52.80 |
38.27 | 38.73 | 54.54 | 1000 | 54.54 |
| HillSlope | 17.64 | 3.668 | 34.07 | 2.607 | 64 | 2.607 | |
|
| |||||||
| Epididymal Sperm Count | ED50 CI | 57.07 46.51 to 70.02 |
52.63 1.721 |
52.64 1.721 |
75.74 4.41 |
587 | 160.9 |
| HillSlope | 10.71 | 4.067 | 3.96 | 4.4 | 4.27 | 3.710 | |
|
| |||||||
| Cowper’s Gland Weight | ED50 CI | 67.84 48.50 to 94.89 |
54.07 | 56.26 | 90.71 | 1084 | 93.81 |
| HillSlope | 15.44 | 1.299 | 3.146 | 0.929 | 8.2 | 0.909 | |
CI - 95% confidence interval
DA1 - Dose Addition Prediction model 1
DA2 - Dose Addition Prediction model 2
RA - Response Addition
DBP - Dibutyl Phthalate
PFQ - Pyrifluquinazon
Figure 1.
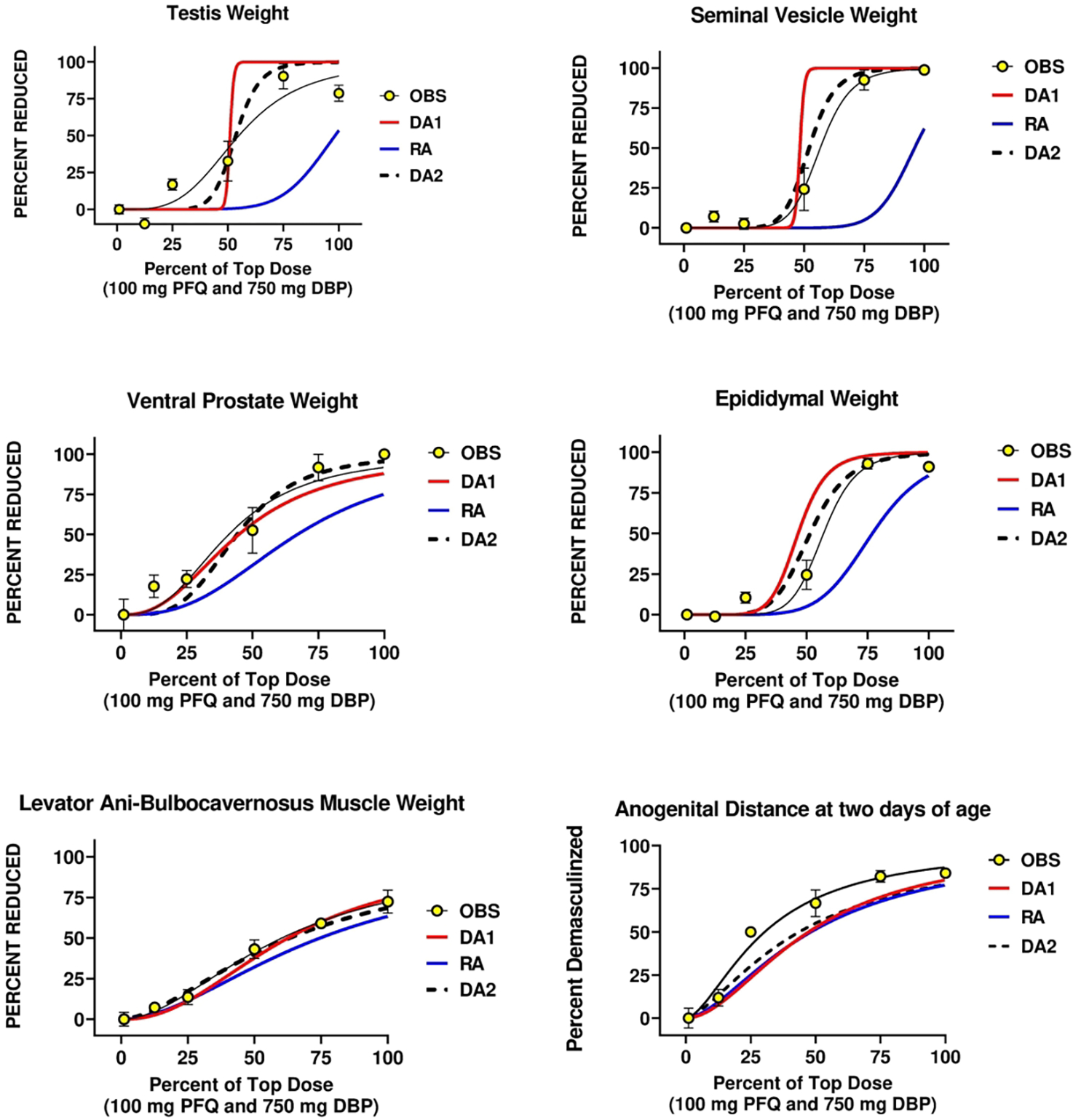
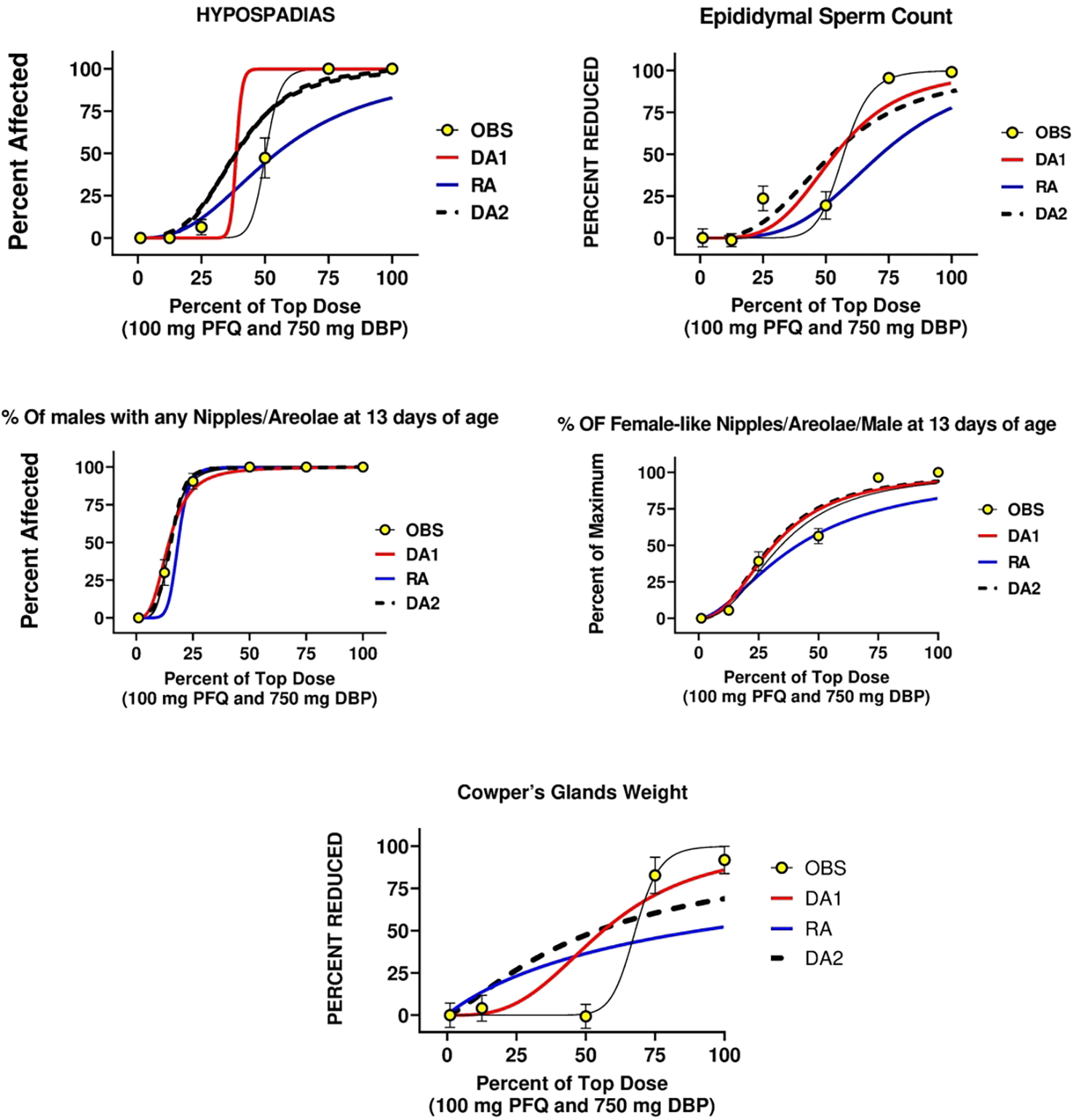
a,b. A comparison of the two dose addition (DA1 and DA2) and the response addition (RA) models with the observed effects of in utero administration of the mixture of dibutyl phthalate (DBP) and the PFAS pesticide pyrifluquinazon (PFQ) on androgen-dependent tissues in the male rat offspring. The major difference between DA1 and DA2 is that DA1 assumes that the two chemicals have similar dose response slope parameters whereas DA2 does not. DA2 was generally the better model among the three models.
When we compared DA and RA models to the observed “best-fit” models using AIC values, we found that DA2 models produced the most accurate predictions and RA models were the least accurate model for all 11 endpoints (Figure 1 a, b). DA2 model AIC values also were significantly better than RA predictions for 10 of 11 endpoints (Table 6). DA2 models were as good as the best fit model for 3 of 11 endpoints. A secondary objective was to compare the fit of two different DA models to the observed mixture data. We found that DA1 models were equivalent to DA2 models for 7 of 11 endpoints (Table 6). The ED50 values with 95% confidence intervals of the observed effects (Table 5) show that the RA models underpredicted the toxicity of the mixture.
Table 6.
AIC values ranked from the “best” (lowest AIC) to the worst fit. Observed data were fit to two dose addition models (AIC_DA1 AND AI_DA2) and the response addition model (AIC_RA) and compared to the “best fit” model (AIC_OBS). A summary of the preferred models is shown in the lower right panel (based upon p<0.05 of the AIC values)
| AIC Table | ||||||||
| Anogenital Distance | % Of Males With Any Nipples | % OF 12 NipplesS | ||||||
|
|
|
|
||||||
| AIC DA OBS | 143.4 | a | AIC DA OBS | 998.6 | a | AIC DA OBS | 142.6 | a |
|
|
|
|
||||||
| AIC DA2 | 162.8 | b | AIC DA2 | 1OO5 | b | AIC_DA1 | 144.6 | a |
|
|
|
|
||||||
| AIC_RA | 17O | c | AIC_DA1 | 1OO5 | b | AIC DA2 | 145.7 | a |
|
|
|
|
||||||
| AIC_DA1 | 170.7 | c | AIC_RA | 1031 | c | AIC_RA | 157.2 | b |
|
|
|
|
||||||
| Testis Weight | Ventral Prostate Weight | LABC Weight | ||||||
|
|
|
|
||||||
| AIC DA OBS | 159.6 | a | AIC DA OBS | 179.5 | a | AIC DA OBS | 139.3 | a |
|
|
|
|
||||||
| AIC DA2 | 188.3 | b | AIC DA2 | 179.7 | a | AIC DA2 | 140.6 | a |
|
|
|
|
||||||
| AIC_DA1 | 188.4 | b | AIC_DA1 | 180.8 | a | AIC_DA1 | 141.7 | a |
|
|
|
|
||||||
| AIC_RA | 202.1 | c | AIC_RA | 196.5 | b | AIC_RA | 151.2 | b |
|
|
|
|
||||||
| Epididymal Weight | Seminal Vesicle Weight | Cowper's Gland Weight | ||||||
|
|
|
|
||||||
| AIC DA OBS | 141.5 | a | AIC DA OBS | 157.3 | a | AIC DA OBS | 176.3 | a |
|
|
|
|
||||||
| AIC DA2 | 156.8 | b | AIC DA2 | 164.1 | b | AIC_DA1 | 199.7 | b |
|
|
|
|
||||||
| AIC_DA1 | 179.3 | c | AIC_DA1 | 203.3 | c | AIC DA2 | 212.4 | b |
|
|
|
|
||||||
| AIC_RA | 190.5 | d | AIC_RA | 223.4 | d | AIC_RA | 215.6 | b |
|
|
|
|
||||||
| Epididymal Sperm Count | Hypospadias | DA2=OBS | 3 of 11 | |||||
|
|
|
|
||||||
| AIC DA OBS | 442.6 | a | AIC DA OBS | 897.6 | a | DA2 > RA | 10 of 11 | |
|
|
|
|
||||||
| AIC DA2 | 452.6 | b | AIC_DA1 | 924.5 | b | DA1>RA | 9 OF 11 | |
|
|
|
|
||||||
| AIC_DA1 | 452.6 | b | AIC DA2 | 928.9 | b | DA2=DA1 | 7 of 11 | |
|
|
|
|
||||||
| AIC_RA | 477.9 | c | AIC_RA | 943.8 | c | DA2>DA1 | 3 OF 11 | |
|
|
|
|
||||||
| DA1>DA2 | 0 OF 11 | |||||||
models with different letters differ from one another
Several effects could not be modeled with DA equations due to a lack of significant response from one or both individual chemicals, but the mixture effect exceeded the value predicted by the RA model. For example, the 27% increase in pup mortality versus control (45% versus 18% in the control group) up to weaning in the high dose mixture group exceed the RA prediction of no increase in pup mortality (0% above control) so it is unclear if this represents an additive or a synergistic outcome.
DISCUSSION
Results of the current mixture study demonstrate that in utero exposure to a PFQ (a perfluoroalkyl pesticide) and DBP (a diortho-phthalate ester) mixture, induces reproductive tract abnormalities in male rat offspring in a dose- rather than response additive manner, even though they act via different molecular mechanisms. These results are consistent with numerous mixture studies our group has conducted over the last 20 years with combined exposure to chemicals that disrupt the androgen signaling pathway during sexual differentiation via different mechanisms of action (reviewed by (Howdeshell et al. 2017)) as well as the work of others (Kortenkamp 2020).
The validity of using DA models to predict the in utero effects of diverse mixtures of chemicals, including PFQ and DBP, that disrupt androgen signaling and male rat development is supported by an examination of the bioactivity of these chemicals at the pathway level. These chemicals act via adverse outcome pathways (AOPs) with diverse molecular initiating events (MIEs) that converge on a common key event (KE) in an AOP network that regulates development of androgen-dependent tissues (Figure 2). The common KE is dysregulation and reduction of androgen-dependent gene expression in male reproductive tissues. AR antagonists, like vinclozolin or procymidone, accomplish this by blocking androgens from binding to ARs, whereas PFQ has been hypothesized to act as a selective androgen receptor degrader (SARD) (Yasunaga et al. 2013), which enhances AR degradation. The phthalates (e.g., di-n-butyl phthalate, di(2-ethylhexyl) phthalate, dipentyl phthalate, butyl benzyl phthalate, di-isobutyl phthalate) reduce fetal testis testosterone production reducing the levels of androgens available to the cell/receptor. Chemicals like finasteride inhibit type II and III 5α reductase, the enzyme in tissues that converts testosterone to dihydrotestosterone (a more active androgen that has higher affinity for the AR). All these molecular perturbations reduce AR/AR homodimer levels, androgen response element (ARE) DNA activation, androgen-dependent mRNA and protein synthesis levels and growth and differentiation of androgen-dependent tissues in the fetus. As a result, male offspring display agenesis or hypoplasia or malformations of androgen-dependent tissues. In addition, we have found that a mixture of DBP and 2,3,7,8-TCDD (dioxin), an AhR agonist, disrupted differentiation of several reproductive tissues in a manner that exceeded response addition (Rider et al. 2010). In summary, an examination of the events disrupted in the androgen signaling pathway by DBP and PFQ at the cellular-molecular level explains why they behave in a dose additive manner. Overall, it is not important to an androgen-dependent cell what MIE reduced the androgen signal to the receptor, it is only important that the signal is reduced.
Figure 2.
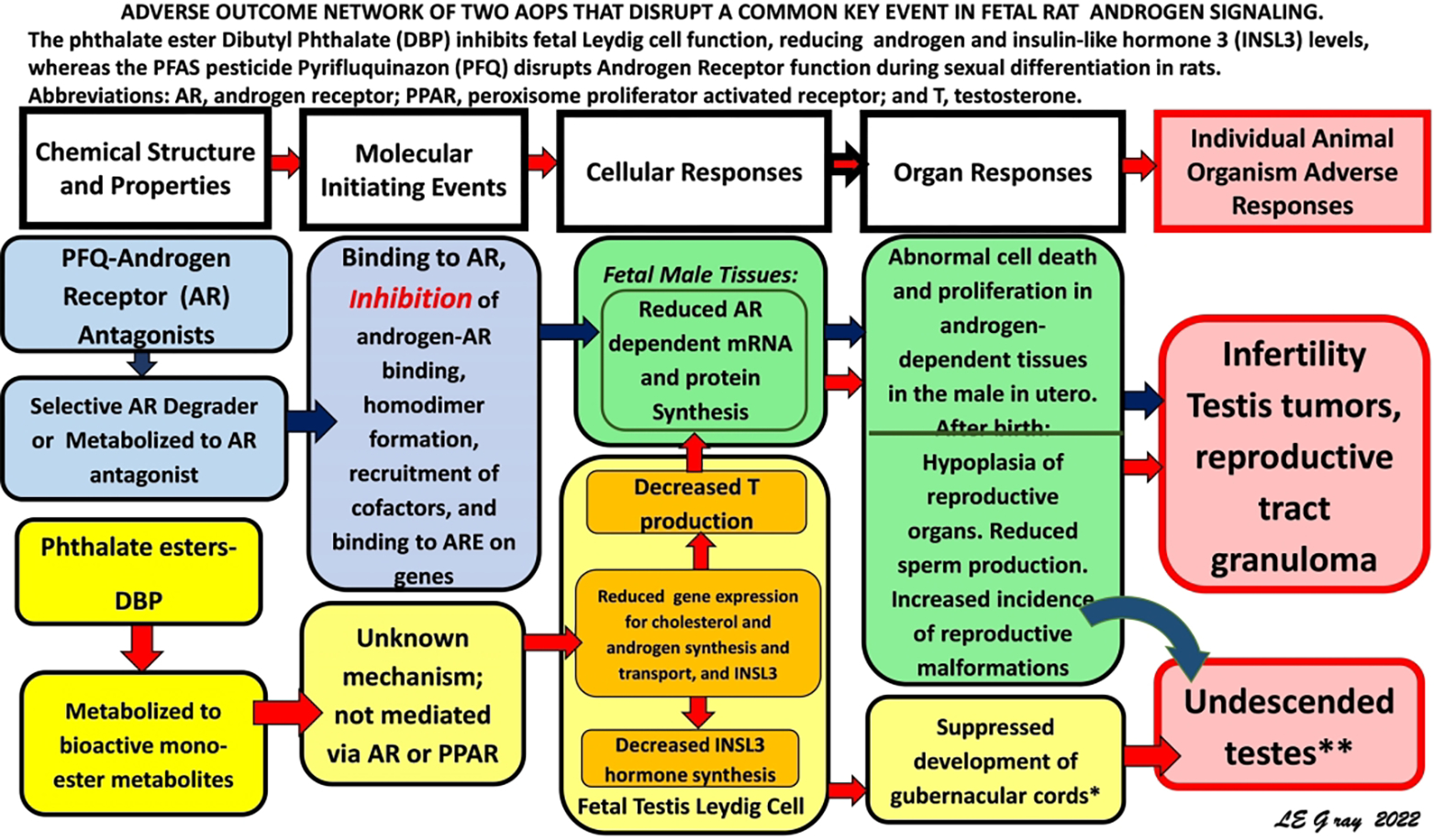
An adverse outcome pathway (AOP) network showing the two AOPs disrupted via different molecular initiating events and different AOPs that converge on a common key event in the AOP network.
As stated above, PFQ has been hypothesized to disrupt AR function by enhancing receptor degradation (Yasunaga et al. 2013) as opposed to acting as a competitive inhibitor of androgen binding to AR. Although the focus on the development of drugs to treat prostate cancer has been on competitive antagonists like flutamide that compete with androgens for binding to the AR ligand binding domain (LBD), many efforts have shifted to developing drugs that target the N-terminal domain (NTD) rather than the LBD. These include chemicals that function as SARDs or inhibit nuclear AR localization by interacting with specific sites on the NTD (Mohler et al. 2021; Narayanan et al. 2008). While PFQ may function as a SARD, PFQ also does antagonize the effects of dihydrotestosterone in vitro, albeit at high concentrations (Gray et al. 2019a). Furthermore, PFQ is extensively metabolized, so it is possible that one of the more than a dozen metabolites acts as a competitive AR antagonist rather than PFQ acting as a SARD.
In contrast to PFQ, DBP and MBP, the active metabolite of DBP, do not interact with the AR in vitro (Parks et al. 2000) or in vivo (Lee and Koo 2007). DBP is one of several diortho-phthalate esters that act as reproductive toxicants in utero in the rat by disrupting fetal testis gene expression (Gray et al. 2021; Hannas et al. 2011) and hormone production (Furr et al. 2014) sufficiently to induce permanent reproductive tract abnormalities in F1 male offspring known as the “Phthalate Syndrome” (Foster 2006). Since most people including pregnant women, are exposed to one or more phthalates like DBP (Adibi et al. 2008) (Zota et al. 2014), any human exposure to PFQ would also very likely be concurrent with exposure to phthalates and other ubiquitous contaminants.
PFQ is classified by several agencies, including the OECD and USEPA, as a PFAS because PFQ is one of a number of perfluoroalkyl pesticides that contain a heptafluoroisopropyl group. However, PFQ has a notably different structure from legacy/straight-chain PFAS like PFOS and PFOA or perfluoroalkyl ether PFAS like HFPO-DA (GenX) (Figure 3). Similar to other pesticides, and unlike many PFAS, there is an extensive data base on PFQ toxicity, fate, and transport. In the last few years, the numbers of PFQ-like hepta- and hexa-fluoroisopropyl PFAPs registered or under development have been increasing because addition of this functional group has been reported to provide many traits considered to be beneficial for pesticides, including increased uptake, enhanced insecticidal toxicity and persistence (Qacemi et al. 2019). Examples include other heptafluoroisopropyl PFAPs include brofranilide [(registered by EPA early in 2021) (https://www.regulations.gov/docket/EPA-HQ-OPP-2018-0053/document)], and pyflubumide [(residue levels established by EPA in late 2021) (https://www.regulations.gov/search?filter=pyflubumide%20)]. In 2016 EPA stated that it intended to cancel the registration of four pesticides containing flubendiamide, concluding that the continued use would result in unreasonable adverse effects on the environment, particularly benthic invertebrates (https://www.epa.gov/ingredients-used-pesticide-products/flubendiamide-notice-intent-cancel-and-other-supporting, accessed 12/30/2021). In addition, the fungicide ZJ5337 (used to control rice blast in China (Hu et al. 2014) also contains a heptafluoroisopropyl group. Taken together, the literature indicates that there are a number of PFAP pesticides in use or under development indicating that PFQ is not unique in this regard. However, for those PFAP pesticides with available summary assessments (regulations.gov), the data suggest that the presence of a hepta- or hexa-fluoroisopropyl side chain does not necessarily confer PFQ-like antiandrogenic bioactivity in vivo.
Figure 3.
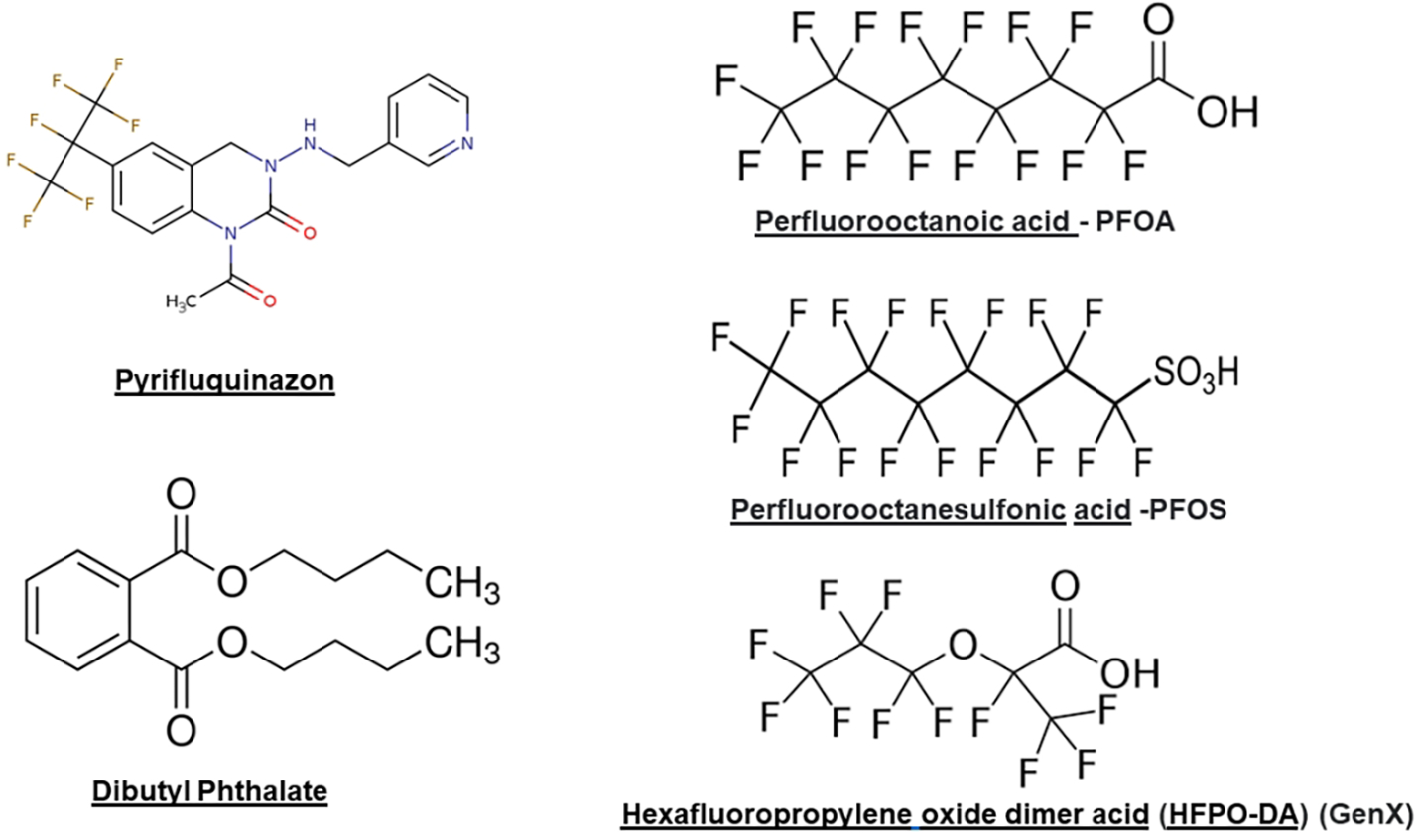
Two dimensional structures of dibutyl phthalate (DBP and pyrifluquinazon (PFQ) (on th left) compared to linear PFAS found in some drinking and surface waters.
In addition to the above PFAPs, there are PFAPs and inert ingredients in pesticides with structures that are similar to PFOA and PFOS that have been used in the US and in other countries including sulfuramid, flusulamid, LPOS, and SIOC-I-013. Examination of the risk assessment summaries provided in EPA documents (in Regulations.gov) for the four aforementioned PFAPs does not indicate that any of these have reproductivity toxicity profiles like PFQ.
Although PFQ is the only PFAS that we are aware of that target development of the male reproductive tract via AR disruption in vivo, a number of in silico and in vitro studies provide a different perspective. These studies have reported that a considerable number of PFAS interact with the ligand binding domain of AR (Di Nisio et al. 2019; Yu et al. 2022). Interestingly, a number of these PFAS also contain perfluoroisopropyl side chains, like PFQ (Azhagiya Singam et al. 2020; Tachachartvanich et al. 2022). Furthermore, Kjeldsen et al. (Kjeldsen and Bonefeld-Jorgensen 2013) reported that a mixture of five PFAS displayed antiandrogenic activity in vitro that was additive at the 95% and 90 % levels but appeared synergistic at the 85% of control level. Because in silico and in vitro assays of AR bioactivity have a high false positive rates (Gray et al. 2020) (Charles et al. 2005; Fabian et al. 2018; Sonneveld et al. 2006) these observations would need to be confirmed in vivo in (anti)androgen screening assays (EPA 2009; Gray et al. 2005) and/or in utero (Ostby and Gray Jr 2004) to determine if any of these PFAS have the potential to cause adverse reproductive effects via disruption of the androgen signaling pathway.
As discussed above, the validity of using DA models rather than RA models to predict the in utero effects of diverse mixtures of chemicals that disrupt androgen signaling and male rat development is supported by an examination of the bioactivity of these chemicals at the cellular-molecular level. All these chemicals act via adverse outcome pathways (AOPs) that converge on a common key event (KE) in an AOP network that regulates the sequence of molecular events in cells that participate in the development of the androgen-dependent tissues.
In summary, a DBP and PFQ mixture behaved in a dose additive manner because these two chemicals target common reproductive tissues via disruption of overlapping AOPs that converge on a common KE in a fetal androgen signaling AOP network (Figure 2). They act via different MIEs and MOAs but they both reduce the hormone signal at the AR, thus reducing androgen-dependent mRNA, and protein synthesis during fetal life, which in turn induces permanent reproductive abnormalities. Overall, an examination of our mixture studies and similar studies in the literature on the effects of antiandrogens on male reproductive tract development consistently indicate: 1) Dose addition should be considered as the default model for assessing the effects of mixture composed of chemicals with different mechanisms that disrupt common effects regardless of whether or not they share a common MIE, 2) RA models can grossly underestimate the hazard of a mixtures of chemicals, and 3) Failure to account for the effects of mixtures during development and other life stages is not fully protective and could potentially lead to adverse consequences for human and ecosystem health.
Footnotes
Disclaimer: The research described in this article has been reviewed by U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, Nelson H, Bhat HK, Perera FP, Silva MJ, and Hauser R (2008). Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect 116, 467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenburger R, Backhaus T, Boedeker W, Faust M, Scholze M, and Grimme LH (2000). Predictability of the toxicity of multiple chemical mixtures to Vibrio fischeri: Mixtures composed of similarly acting chemicals. Environmental Toxicology and Chemistry 19, 2341–2347. [Google Scholar]
- Azhagiya Singam ER, Tachachartvanich P, Fourches D, Soshilov A, Hsieh JCY, La Merrill MA, Smith MT, and Durkin KA (2020). Structure-based virtual screening of perfluoroalkyl and polyfluoroalkyl substances (PFASs) as endocrine disruptors of androgen receptor activity using molecular docking and machine learning. Environmental research 190, 109920. [DOI] [PubMed] [Google Scholar]
- Beverly BE, Lambright CS, Furr JR, Sampson H, Wilson VS, McIntyre BS, Foster PM, Travlos G, and Gray LE Jr. (2014). Simvastatin and dipentyl phthalate lower ex vivo testicular testosterone production and exhibit additive effects on testicular testosterone and gene expression via distinct mechanistic pathways in the fetal rat. Toxicol Sci 141, 524–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, and Anderson DR (2004). Multimodel Inference: Understanding AIC and BIC Model Selection. SOCIOLOGICAL METHODS & RESEARCH 33, 261–304. [Google Scholar]
- Carruthers CM, and Foster PM (2005). Critical window of male reproductive tract development in rats following gestational exposure to di-n-butyl phthalate. Birth Defects Res B Dev Reprod Toxicol 74, 277–85. [DOI] [PubMed] [Google Scholar]
- Charles GD, Kan HL, Schisler MR, Bhaskar Gollapudi B, and Sue Marty M (2005). A comparison of in vitro and in vivo EDSTAC test battery results for detecting antiandrogenic activity. Toxicol Appl Pharmacol 202, 108–20. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Scholze M, Axelstad M, Boberg J, Kortenkamp A, and Hass U (2008). Combined exposure to anti-androgens causes markedly increased frequencies of hypospadias in the rat. Int J Androl 31, 241–8. [DOI] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, Cardon M, Furr J, Wilson VS, and Gray LE Jr. (2018). Mixed “Antiandrogenic” Chemicals at Low Individual Doses Produce Reproductive Tract Malformations in the Male Rat. Toxicol Sci 164, 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, Cardon M, Medlock-Kakaley E, Wilson VS, and Gray LE Jr. (2021a). A mixture of 15 phthalates and pesticides below individual chemical no observed adverse effect levels (NOAELs) produces reproductive tract malformations in the male rat. Environment international 156, 106615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley JM, Lambright CS, Evans N, McCord J, Strynar MJ, Hill D, Medlock-Kakaley E, Wilson VS, and Gray LE Jr. (2021b). Hexafluoropropylene oxide-dimer acid (HFPO-DA or GenX) alters maternal and fetal glucose and lipid metabolism and produces neonatal mortality, low birthweight, and hepatomegaly in the Sprague-Dawley rat. Environ Int 146, 106204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nisio A, Sabovic I, Valente U, Tescari S, Rocca MS, Guidolin D, Dall’Acqua S, Acquasaliente L, Pozzi N, Plebani M, Garolla A, and Foresta C (2019). Endocrine Disruption of Androgenic Activity by Perfluoroalkyl Substances: Clinical and Experimental Evidence. J Clin Endocrinol Metab 104, 1259–1271. [DOI] [PubMed] [Google Scholar]
- EPA (2009). Hershberger Bioassay (OPPTS E, ed., Vol. OPPTS 890.1400.
- Fabian E, Gomes C, Birk B, Williford T, Hernandez TR, Haase C, Zbranek R, van Ravenzwaay B, and Landsiedel R (2018). In vitro-to-in vivo extrapolation (IVIVE) by PBTK modeling for animal-free risk assessment approaches of potential endocrine-disrupting compounds. Arch Toxicol. [DOI] [PubMed] [Google Scholar]
- Foster PMD (2006). Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International Journal of Andrology 29, 140–147. [DOI] [PubMed] [Google Scholar]
- Furr JR, Lambright CS, Wilson VS, Foster PM, and Gray LE Jr. (2014). A short-term in vivo screen using fetal testosterone production, a key event in the phthalate adverse outcome pathway, to predict disruption of sexual differentiation. Toxicol Sci 140, 403–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Furr JR, Conley JM, Lambright CS, Evans N, Cardon MC, Wilson VS, Foster PM, and Hartig PC (2019a). A Conflicted Tale of Two Novel AR Antagonists In Vitro and In Vivo: Pyrifluquinazon Versus Bisphenol C. Toxicol Sci 168, 632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE, Furr JR, Lambright CS, Evans N, Hartig PC, Cardon MC, Wilson VS, Hotchkiss AK, and Conley JM (2020). Quantification of the Uncertainties in Extrapolating From In Vitro Androgen Receptor Antagonism to In Vivo Hershberger Assay Endpoints and Adverse Reproductive Development in Male Rats. Toxicol Sci 176, 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Furr J, and Ostby JS (2005). Hershberger assay to investigate the effects of endocrine-disrupting compounds with androgenic or antiandrogenic activity in castrate-immature male rats. Curr Protoc Toxicol Chapter 16, Unit16 9. [DOI] [PubMed] [Google Scholar]
- Gray LE Jr., Furr JR, Conley JM, Lambright CS, Evans N, Cardon MC, Wilson VS, Foster PM, and Hartig PC (2019b). A Conflicted Tale of Two Novel AR Antagonists In vitro and In vivo: Pyrifluquinazon versus Bisphenol C. Toxicol Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE Jr., Ostby J, Ferrell J, Rehnberg G, Linder R, Cooper R, Goldman J, Slott V, and Laskey J (1989). A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam Appl Toxicol 12, 92–108. [DOI] [PubMed] [Google Scholar]
- Gray LE Jr., Wolf C, Lambright C, Mann P, Price M, Cooper RL, and Ostby J (1999). Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p’-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicology and industrial health 15, 94–118. [DOI] [PubMed] [Google Scholar]
- Gray LE, Kelce WR, Monosson E, Ostby JS, and Birnbaum LS (1995). Exposure to TCDD during development permanently alters reproductive function in male Long-Evans rats and hamsters: reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicology and applied pharmacology 131, 108–118. [DOI] [PubMed] [Google Scholar]
- Gray LE, Lambright CS, Conley JM, Evans N, Furr JR, Hannas BR, Wilson VS, Sampson H, and Foster PMD (2021). Genomic and Hormonal Biomarkers of Phthalate-Induced Male Rat Reproductive Developmental Toxicity Part II: A Targeted RT-qPCR Array Approach That Defines a Unique Adverse Outcome Pathway. Toxicol Sci 182, 195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannas BR, Lambright CS, Furr J, Howdeshell KL, Wilson VS, and Gray LE Jr. (2011). Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate, and diisononyl phthalate. Toxicol Sci 123, 206–16. [DOI] [PubMed] [Google Scholar]
- Hass U, Scholze M, Christiansen S, Dalgaard M, Vinggaard AM, Axelstad M, Metzdorff SB, and Kortenkamp A (2007). Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ Health Perspect 115 Suppl 1, 122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AK, Parks-Saldutti LG, Ostby JS, Lambright C, Furr J, Vandenbergh JG, and Gray LE Jr. (2004). A mixture of the “antiandrogens” linuron and butyl benzyl phthalate alters sexual differentiation of the male rat in a cumulative fashion. Biol Reprod 71, 1852–61. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Furr J, Howdeshell KL, Blystone CR, Wilson VS, and Gray LE Jr. (2010). In utero exposure to an AR antagonist plus an inhibitor of fetal testosterone synthesis induces cumulative effects on F1 male rats. Reprod Toxicol 30, 261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, and Gray LE Jr. (2017). Cumulative effects of antiandrogenic chemical mixtures and their relevance to human health risk assessment. International journal of hygiene and environmental health 220, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Zhu W, Zhang RH, Chen J, Xu T, and Zheng J (2014). Biological activity of a novel quinoline compound ZJ5337. Chinese Journal of Pesticide Science 14, 414–419. [Google Scholar]
- Kjeldsen LS, and Bonefeld-Jorgensen EC (2013). Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int 20, 8031–44. [DOI] [PubMed] [Google Scholar]
- Korenbrot CC, Huhtaniemi IT, and Weiner RI (1977). Preputial separation as an external sign of pubertal development in the male rat. Biology of reproduction 17, 298–303. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A (2007). Ten years of mixing cocktails: a review of combination effects of endocrine-disrupting chemicals. Environ Health Perspect 115 Suppl 1, 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A (2020). Which chemicals should be grouped together for mixture risk assessments of male reproductive disorders? Mol Cell Endocrinol 499, 110581. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Faust M, Scholze M, and Backhaus T (2007). Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect 115 Suppl 1, 106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BM, and Koo HJ (2007). Hershberger assay for antiandrogenic effects of phthalates. J Toxicol Environ Health A 70, 1365–70. [DOI] [PubMed] [Google Scholar]
- Marques ES, Agudelo J, Kaye EM, Modaresi SMS, Pfohl M, Becanova J, Wei W, Polunas M, Goedken M, and Slitt AL (2021). The role of maternal high fat diet on mouse pup metabolic endpoints following perinatal PFAS and PFAS mixture exposure. Toxicology 462, 152921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzdorff SB, Dalgaard M, Christiansen S, Axelstad M, Hass U, Kiersgaard MK, Scholze M, Kortenkamp A, and Vinggaard AM (2007). Dysgenesis and histological changes of genitals and perturbations of gene expression in male rats after in utero exposure to antiandrogen mixtures. Toxicol Sci 98, 87–98. [DOI] [PubMed] [Google Scholar]
- Mohler ML, Sikdar A, Ponnusamy S, Hwang DJ, He Y, Miller DD, and Narayanan R (2021). An Overview of Next-Generation Androgen Receptor-Targeted Therapeutics in Development for the Treatment of Prostate Cancer. International journal of molecular sciences 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylchreest E, Cattley RC, and Foster PMD (1998). Male reproductive tract malformations in rats following gestational and lactational exposure to di(n-butyl) phthalate: An antiandrogenic mechanism? Toxicological Sciences 43, 47–60. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Sar M, Cattley RC, and Foster PMD (1999). Disruption of androgen-regulated male reproductive development by di(n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicology and Applied Pharmacology 156, 81–95. [DOI] [PubMed] [Google Scholar]
- Mylchreest E, Wallace DG, Cattley RC, and Foster PM (2000). Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol Sci 55, 143–51. [DOI] [PubMed] [Google Scholar]
- Narayanan R, Mohler ML, Bohl CE, Miller DD, and Dalton JT (2008). Selective androgen receptor modulators in preclinical and clinical development. Nucl Recept Signal 6, e010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAS (2008). Phthalates and Cumulative Risk Assessment The Task Ahead. National Academy of Sciences, National Academies Press. [PubMed] [Google Scholar]
- Olmstead AW, and LeBlanc GA (2005). Toxicity Assessment of Envrionmentally Relevant Pollutant Mixtures Using a Heuristic Model Integrated Environmental Assessment and Management 1, 114–122. [DOI] [PubMed] [Google Scholar]
- Ostby J, and Gray E Jr (2004). Transgenerational (in utero/lactational) exposure to investigate the effects of endocrine disrupting compounds (EDCs) in rats. John Wiley and Sons, Inc. [DOI] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, and Gray LE (2000). The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicological Sciences 58, 339–349. [DOI] [PubMed] [Google Scholar]
- Qacemi EM, Rendine S, and Maienfisch P (2019). Recent applications of fluorine in crop protectiondnew discoveries originating from the unique heptafluoroisopropyl group. 2019 Elsevier Inc. [Google Scholar]
- Rider CV, Furr J, Wilson VS, and Gray LE Jr. (2008). A mixture of seven antiandrogens induces reproductive malformations in rats. International journal of andrology 31, 249–62. [DOI] [PubMed] [Google Scholar]
- Rider CV, Furr JR, Wilson VS, and Gray LE Jr. (2010). Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl 33, 443–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CV, and LeBlanc GA (2005). An integrated addition and interaction model for assessing toxicity of chemical mixtures. Toxicological Sciences 87, 520–528. [DOI] [PubMed] [Google Scholar]
- Robb GW, Amann RP, and Killian GJ (1978). Daily sperm production and epididymal sperm reserves of pubertal and adult rats. Journal of reproduction and fertility 54, 103–7. [DOI] [PubMed] [Google Scholar]
- Roth K, Yang Z, Agarwal M, Liu W, Peng Z, Long Z, Birbeck J, Westrick J, Liu W, and Petriello MC (2021). Exposure to a mixture of legacy, alternative, and replacement per- and polyfluoroalkyl substances (PFAS) results in sex-dependent modulation of cholesterol metabolism and liver injury. Environment international 157, 106843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld E, Riteco JA, Jansen HJ, Pieterse B, Brouwer A, Schoonen WG, and van der Burg B (2006). Comparison of in vitro and in vivo screening models for androgenic and estrogenic activities. Toxicol Sci 89, 173–87. [DOI] [PubMed] [Google Scholar]
- Tachachartvanich P, Singam ERA, Durkin KA, Furlow JD, Smith MT, and La Merrill MA (2022). In Vitro characterization of the endocrine disrupting effects of per- and poly-fluoroalkyl substances (PFASs) on the human androgen receptor. J Hazard Mater 429, 128243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Buser AM, Cousins IT, Demattio S, Drost W, Johansson O, Ohno K, Patlewicz G, Richard AM, Walker GW, White GS, and Leinala E (2021). A New OECD Definition for Per- and Polyfluoroalkyl Substances. Environ Sci Technol 55, 15575–15578. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, LeBlanc GA, Ostby JS, and Gray LE Jr. (2000). Characterization of the period of sensitivity of fetal male sexual development to vinclozolin. Toxicol Sci 55, 152–61. [DOI] [PubMed] [Google Scholar]
- Yasunaga R, Ikuta J, Murata Y, Inoue K, Koga H, Masaki T, and Tamura H (2013). Ligand-independent androgen receptor antagonism caused by the newly developed pesticide pyrifluquinazon (PFQ). Reprod Toxicol 35, 1–6. [DOI] [PubMed] [Google Scholar]
- Yu S, Ren J, Lv Z, Li R, Zhong Y, Yao W, and Yuan J (2022). Prediction of the endocrine-disrupting ability of 49 per- and polyfluoroalkyl substances: In silico and epidemiological evidence. Chemosphere 290, 133366. [DOI] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, and Woodruff TJ (2014). Temporal Trends in Phthalate Exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Perspect. [DOI] [PMC free article] [PubMed] [Google Scholar]


