ABSTRACT
Methicillin-resistant Staphylococcus aureus (MRSA) infections are an increasing concern due to their intrinsic resistance to most standard-of-care β-lactam antibiotics. Recent studies of clinical isolates have documented a novel phenotype, termed NaHCO3 responsiveness, in which a substantial proportion of MRSA strains exhibit enhanced susceptibility to β-lactams such as cefazolin and oxacillin in the presence of NaHCO3. A bicarbonate transporter, MpsAB (membrane potential-generating system), was recently found in S. aureus, where it plays a role in concentrating NaHCO3 for anaplerotic pathways. Here, we investigated the role of MpsAB in mediating the NaHCO3 responsiveness phenotype. Radiolabeled NaH14CO3 uptake profiling revealed significantly higher accumulation in NaHCO3-responsive vs nonresponsive MRSA strains when grown in ambient air. In contrast, under 5% CO2 conditions, NaHCO3-responsive (but not nonresponsive) strains exhibited repressed uptake. Oxacillin MICs were measured in four prototype strains and their mpsABC deletion mutants in the presence of NaHCO3 supplementation under 5% CO2 conditions. NaHCO3-mediated reductions in oxacillin MICs were observed in the responsive parental strains but not in mpsABC deletion mutants. No significant impact on oxacillin MICs was observed in the nonresponsive strains under the same conditions. Transcriptional and translational studies were carried out using both quantitative reverse transcription-PCR (qRT-PCR) and mpsA-green fluorescent protein (GFP) fusion constructs; these investigations showed that mpsA expression and translation were significantly upregulated during mid-exponential-phase growth in oxacillin-NaHCO3-supplemented medium in responsive versus nonresponsive strains. Taken together, these data show that the NaHCO3 transporter MpsABC is a key contributor to the NaHCO3–β-lactam responsiveness phenotype in MRSA.
IMPORTANCE MRSA infections are increasingly difficult to treat, due in part to their resistance to most β-lactam antibiotics. A novel and relatively common phenotype, termed NaHCO3 responsiveness, has been identified in which MRSA strains show increased susceptibility in vitro and in vivo to β-lactams in the presence of NaHCO3. A recently described S. aureus NaHCO3 transporter, MpsAB, is involved in intracellular NaHCO3 concentration for anaplerotic pathways. We investigated the role of MpsAB in mediating the NaHCO3 responsiveness phenotype in four prototype MRSA strains (two responsive and two nonresponsive). We demonstrated that MpsABC is an important contributor to the NaHCO3–β-lactam responsiveness phenotype. Our study adds to the growing body of well-defined characteristics of this novel phenotype, which could potentially translate to alternative targets for MRSA treatment using β-lactams.
KEYWORDS: methicillin-resistant Staphylococcus aureus, MRSA, sodium bicarbonate, CO2, β-lactams, bicarbonate transporter, membrane potential-generating system ABC, MpsABC
INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) infections are a growing public health threat worldwide and the cause of a number of serious infections, including bacteremia, endocarditis, osteomyelitis, and invasive skin and soft tissue infections (1, 2). A major concern with MRSA therapies is the in vitro resistance of these organisms to most β-lactam agents (except ceftaroline and ceftobiprole) (3) as well as other antibiotics (e.g., aminoglycosides and quinolones).
One recent goal in clinical microbiology has been to improve standardized antimicrobial susceptibility testing (AST) for resistant bacteria, such as MRSA, by modifying in vitro growth conditions to better reflect the host physiologic microenvironment (4). In MRSA, such AST modifications have identified a novel phenotype in a relatively large proportion of clinical MRSA isolates, known as NaHCO3–β-lactam responsiveness. Such isolates exhibit enhanced susceptibility (i.e., ≥4-fold reductions in MICs) to early-generation β-lactams, such as cefazolin and oxacillin, when grown in vitro in the presence of NaHCO3 supplementation (4–7). According to the Clinical and Laboratory Standards Institute (CLSI) guidelines, MIC breakpoints for oxacillin are interpreted as susceptible (S) at ≤2 μg/mL and resistant (R) at ≥4 μg/mL for S. aureus (8).
Recent investigations from our laboratory have defined a number of key phenotypic and genotypic characteristics of MRSA strains that distinguish NaHCO3-responsive from NaHCO3-nonresponsive isolates when exposed to NaHCO3, including (i) reduced production of PBP2a (a major determinant of β-lactam resistance); (ii) reduced expression of mecA (a gene responsible for PBP2a production), blaZ (coregulator of PBP2a production), and sarA (required for maintenance of the MRSA phenotype); (iii) reduced expression of pbp4, vraS, and prsA (required for intramembrane maturation and final folding of PBP2a); (iv) a synergistic impact on killing when NaHCO3 is combined with host defense cationic peptides; (v) increased β-lactam binding to the MRSA surface and to membrane-localized PBP2a; (vi) the presence of specific genotypes within the ribosome binding site (RBS) and coding regions of mecA (e.g., 246G versus 246E); (vii) an association with the combination of in vitro susceptibility to amoxicillin-clavulanate, specific mecA susceptible genotypes (246G), and specific spa types (t002 or t008); (viii) reductions in wall teichoic acid (WTA) synthesis via posttranslational mechanisms (e.g., gene products involved in peptidoglycan synthesis and functionality); and (ix) enhanced clearance from simulated cardiac vegetations ex vivo, as well as from vegetations and other target tissues in experimental endocarditis in vivo (4, 6, 7, 9–14).
Importantly, a NaHCO3 transporter named MpsAB was recently identified in S. aureus, the first example of such transporters in the phylum Firmicutes and other nonautotrophic bacteria (15). It is encoded by the mpsABC operon, which was initially characterized for its functionality as a key membrane potential-generating system (16). Although the small mpsC gene is part of the operon, it apparently does not contribute to NaHCO3 transport. MpsAB has now been confirmed as the principal determinant of NaHCO3 transport in S. aureus (15). Of note, such NaHCO3 cotransporters are widespread in both autotrophic and nonautotrophic bacteria. As the only dissolved inorganic carbon supply system in S. aureus, MpsAB plays an important role in concentrating bicarbonate for key anaplerotic pathways (15, 17, 18). Deletion of mpsAB genes in S. aureus leads to severe growth delay under ambient-air conditions, which is reversible only by NaHCO3 or CO2 supplementation (15).
Many investigations have been done independently on NaHCO3 responsiveness (4–7) and on the NaHCO3 transport systems (15, 17–19). However, the interplay between these two factors remains unknown. We postulated that these phenotypic differences in NaHCO3 responsiveness among MRSA strains might be impacted, at least in part, by either structural or functional characteristics of the MpsAB transporter.
The aims of the current study were to compare the role of the NaHCO3 transporter MpsAB in mediating the NaHCO3–β-lactam responsiveness phenotype using four prototype MRSA strains (two NaHCO3-responsive and two NaHCO3-nonresponsive strains). We compared strains with these two distinct MRSA phenotypes for (i) the amino acid sequence of their MpsAB transporters; (ii) NaHCO3 uptake in ambient air versus in CO2, using radiolabeled NaH14CO3; (iii) the effect of mpsABC deletions on oxacillin MICs; and (iv) the effects of NaHCO3-oxacillin exposure on mpsA transcription and translation analyzed by quantitative reverse transcription-PCR (qRT-PCR) and flow cytometry.
RESULTS
NaH14CO3 uptake is significantly higher in NaHCO3-responsive strains vs NaHCO3-nonresponsive MRSA.
Our previous studies demonstrated that a substantial proportion of clinical MRSA strains exhibit enhanced susceptibility in vitro to early-generation β-lactams (e.g., oxacillin and cefazolin) when grown in the presence of NaHCO3 (4–6). To evaluate if the known NaHCO3 transporter MpsAB plays a role in NaHCO3 responsiveness, we compared the NaHCO3 uptake activity in two NaHCO3-responsive strains (JE2 and MRSA 11/11) and two NaHCO3-nonresponsive strains (BMC1001 and COL) using radiolabeled NaH14CO3. Prior to the addition of NaH14CO3, fluorocitrate and glucose were added to the cell suspensions. Fluorocitrate, an aconitase inhibitor, was added to prevent the rapid expiration of CO2 by the decarboxylation reactions of the tricarboxylic acid (TCA) cycle (15). The NaH14CO3 uptake occurred almost immediately, peaking within 2 min, before decreasing to a steady-state level at the end of 15 min (Fig. 1A). The total NaH14CO3 uptake under ambient-air conditions for each strain was calculated from the area under the time-accumulation curve (AUC). These studies showed that the NaH14CO3 uptake was highest in the two NaHCO3-responsive strains, MRSA 11/11 and JE2, and lowest in the two NaHCO3-nonresponsive strains, BMC1001 and COL (Fig. 1A). Statistical analyses revealed that the responsive strains, JE2 and MRSA 11/11, had significantly higher H14CO3 uptake than the nonresponsive strains BMC1001 and COL (Fig. 1B).
FIG 1.
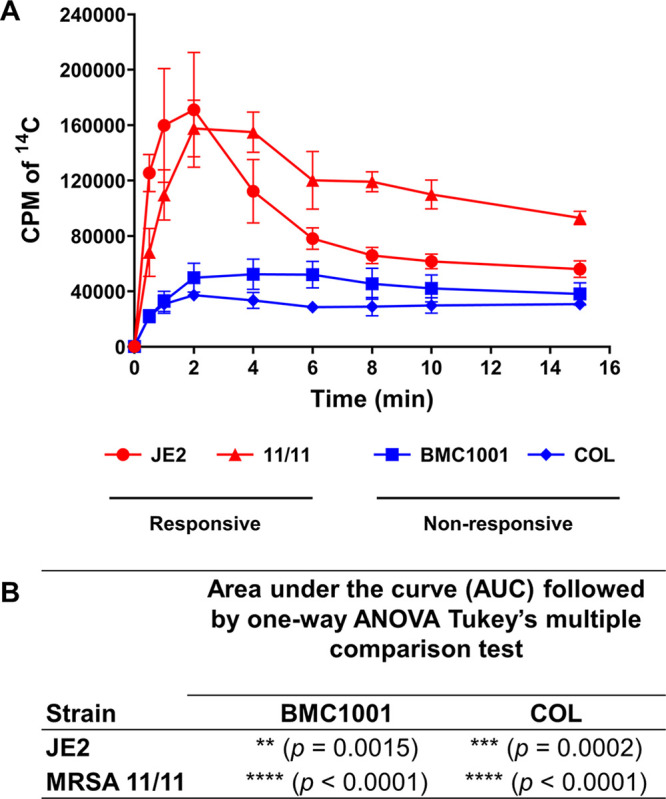
(A) Uptake of NaH14CO3 by bicarbonate-responsive strains (S. aureus JE2 and MRSA 11/11; red lines and symbols) and nonresponsive strains (S. aureus BMC1001 and COL; blue lines and symbols). Bacterial cultures grown in ambient air until mid-exponential phase were washed and adjusted to the same OD. Fluorocitrate was added to the cells and incubated for 30 min before the addition of NaH14CO3 (50 μCi). Aliquots of cell suspensions were collected at the indicated time points, and the H14CO3 uptake was determined by the 14C accumulation in cells, as measured by liquid scintillation counting. Each value is the mean and SD from three independent biological replicates. (B) The P values represent the significant differences in the AUC for the uptake of each strain, analyzed using one-way ANOVA followed by Tukey’s multiple-comparison test. The H14CO3 uptake in the responsive strains JE2 and MRSA 11/11 was significantly higher than that in the nonresponsive strains BMC1001 and COL.
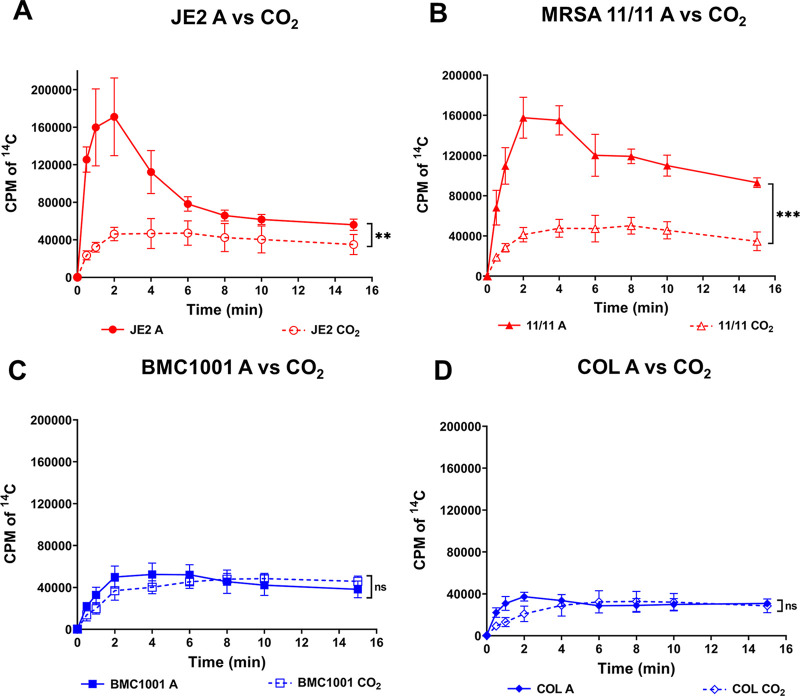
NaHCO3-responsive strains show lower NaH14CO3 uptake when grown in 5% CO2 than in ambient air.
To assess the influence of CO2 on NaH14CO3 uptake, the above studies were carried out in parallel in the presence of 5% CO2. As shown in Fig. 2, both responsive strains showed significantly lower NaH14CO3 uptake when grown with CO2 (2.2 and 2.8 times lower for JE2 and MRSA 11/11, respectively) (Fig. 2A and B). In contrast, the two nonresponsive strains (BMC1001 and COL) showed no significant differences in the NaH14CO3 uptake when grown in ambient air versus CO2 (Fig. 2C and D). This suggests the possibility that the mpsAB NaHCO3 uptake system in responsive strains is partially repressed by an exogenous supply of CO2 (as it is not required in the latter scenario); on the other hand, this uptake system is more constitutive in nonresponsive strains and is not repressible by exogenous CO2.
FIG 2.
Uptake of NaH14CO3 by strain sets grown in ambient air (A; solid lines and symbols) and 5% CO2 conditions (CO2; dashed lines and symbols). The bicarbonate-responsive strains (A) JE2 and (B) MRSA 11/11 showed significantly higher H14CO3 uptake when grown in ambient air than in CO2. **, P = 0.0092; ***, P = 0.0003. The H14CO3 uptake for cells grown in ambient air and 5% CO2 was not significantly different in the nonresponsive strains (C) BMC1001 (ns, not significant [P = 0.7847]) and (D) COL (P = 0.6652). Each point represents the mean and SD from three independent biological replicates. Statistical significance between the uptake in ambient air and CO2 was calculated using Student’s t test from the AUC values for each strain set.
mpsABC sequences are highly similar in all four NaHCO3-responsive and NaHCO3-nonresponsive MRSA strains.
Since differences were seen in the NaHCO3 uptake under ambient-air and CO2 growth conditions, we examined whether the mpsABC operon exhibited substantial differences in the four study strains. Multiple-sequence alignment of the mpsABC locus of JE2, MRSA 11/11, BMC1001, and COL showed that there are only two mismatches out of 4,727 bp, resulting in 99.4% similarity among the four prototype strains. In both BMC1001 and COL, there is a nucleotide change from T to C in the coding region of mpsB, causing a change in the 264th amino acid position of MpsB from tyrosine to histidine. Additionally, COL has another nucleotide change, from C to A, resulting in the change in the 466th amino acid of MpsA from alanine to glutamic acid (see Fig. S1A in the supplemental material). The promoter sequences of mpsA were 100% identical among the four prototype strains. To verify whether the two single nucleotide polymorphisms (SNPs) found in the mpsABC locus of the nonresponsive strains are determinative of the latter phenotype, we introduced these SNPs into the responsive strain backgrounds by “swapping” the mpsABC region from COL into JE2 and MRSA 11/11 (Fig. S1B); this generated JE2 and MRSA 11/11 swap variants which harbor both SNPs. The oxacillin MICs were then checked using these swap variants to see whether the phenotype switched from oxacillin-NaHCO3 responsiveness to nonresponsiveness. The oxacillin MIC data showed that both the JE2 and MRSA 11/11 swap variants harboring the SNPs from nonresponsive strain COL remained responsive to NaHCO3 (Table S1).
Growth studies of prototype strains.
In our previous studies, NaHCO3 supplementation had a minimal impact on the 24-h growth kinetics of our four prototype strains used in the current investigation. Thus, the 24-h growth yields were not significantly different for growth in cation-adjusted Mueller-Hinton broth (CA-MHB)–Tris medium with NaHCO3 versus growth in the same medium without NaHCO3 for any strain tested except COL, which grew somewhat more slowly than the other three strains (4). The long doubling time of COL was documented previously (20). Growth yields of these four prototype parental strains at 24 h in ambient air versus 5% CO2 did not show any major differences (Fig. S2).
Deletion of mpsABC reversed the NaHCO3-responsive phenotype in responsive strains, determined by oxacillin MIC testing.
To determine whether a functional NaHCO3 transporter affected the NaHCO3-responsive phenotype, mpsABC deletion mutants and their respective plasmid complementation constructs were constructed in the four prototype MRSA strains; these strain sets were then assessed for oxacillin MICs in the presence versus the absence of NaHCO3 under either ambient-air or 5% CO2 growth conditions. CA-MHB–Tris alone was used as the control (4).
In ambient air, the two responsive parental strains showed the expected NaHCO3 responsiveness phenotype, with 64-fold reductions in oxacillin MICs in ambient air in the presence of NaHCO3 (Table 1). The ambient-air oxacillin MICs for the deletion mutants and complemented variants could not be obtained, because these constructs grew very poorly under such conditions.
TABLE 1.
MICs of oxacillin in NaHCO3-responsive and -nonresponsive MRSA strainsa
| Strain | Oxacillin MIC (μg/mL) |
|||
|---|---|---|---|---|
| Ambient air |
5% CO2 |
|||
| CA-MHB–Tris | CA-MHB–Tris + 44 mM NaHCO3 | CA-MHB–Tris | CA-MHB–Tris + 44 mM NaHCO3 | |
| Responsive | ||||
| JE2 | 64 | 1 | 8 | 1 |
| JE2 ΔmpsABC | — | — | 16 | 16 |
| JE2 ΔmpsABC compl. | — | — | 16 | 1 |
| MRSA 11/11 | 32 | 0.5 | 2 | 1 |
| MRSA 11/11 ΔmpsABC | — | — | 4 | 8 |
| MRSA 11/11 ΔmpsABC compl. | — | — | 1 | 0.5 |
| Nonresponsive | ||||
| BMC1001 | 256 | 512 | 64 | 128 |
| BMC1001 ΔmpsABC | — | — | 32 | 64 |
| BMC1001 ΔmpsABC compl. | — | — | 32 | 64 |
| COL | 256 | 512 | 64 | 128 |
| COL ΔmpsABC | — | — | 64 | 128 |
| COL ΔmpsABC compl. | — | — | 64 | 128 |
CA-MHB–Tris, cation-adjusted Mueller-Hinton broth supplemented with 100 mM Tris maintained at pH ~7.2; —, the MIC was not obtained because the growth was severely affected in ambient air; compl., complemented. All MHB used was supplemented with 2% NaCl for testing with oxacillin. MICs were obtained from at least three independent biological replicates.
In the presence of 5% CO2, the baseline MICs of both responsive parental strains were lower, but each displayed a 2- to 8-fold reduction in oxacillin MICs in the presence of NaHCO3 supplementation. In contrast, in the mpsABC deletion mutants of these two responsive strains, the NaHCO3-responsive phenotype was not observed. NaHCO3 responsiveness was restored in both complementation constructs in the presence of NaHCO3 supplementation (Table 1).
For the nonresponsive strains, oxacillin MICs were unaffected when the two parental strains were compared with their respective deletion or complementation mutants, in the presence versus the absence of NaHCO3 supplementation, whether in ambient air or 5% CO2 (Table 1).
Transcription and translation of mpsA are increased by NaHCO3 exposure in responsive versus nonresponsive strains.
During mid-exponential phase, by qRT-PCR, mpsA expression, as the first gene of the mpsABC operon, was significantly upregulated (~2-fold) in both responsive strains (JE2 and MRSA 11/11) by NaHCO3 supplementation in the presence of oxacillin. In contrast, NaHCO3 had no impact on mpsA expression in the two nonresponsive strains at this growth phase (Fig. 3A). In early stationary phase, in the two responsive strains, NaHCO3 supplementation had no impact on mpsA expression, while mpsA expression was reduced 2-fold in nonresponsive strains (Fig. 3B). As a control, the addition of oxacillin alone (i.e., in the absence of NaHCO3) did not have any effect on the expression of mpsA at either growth phase (Fig. S3).
FIG 3.
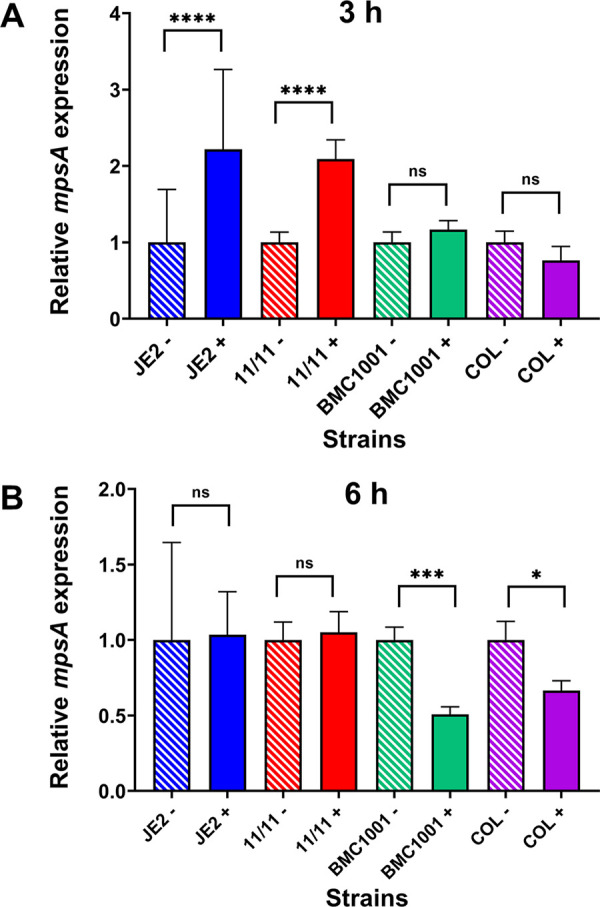
Expression of mpsA in NaHCO3-responsive (JE2 and MRSA 11/11) and NaHCO3-nonresponsive (BMC1001 and COL) strains. Gene expression data were obtained by qRT-PCR of RNA from (A) mid-exponential-phase (3 h) and (B) early-stationary-phase (6 h) strains grown in CA-MHB–Tris with (+) or without (−) NaHCO3 and 1/2 MIC of oxacillin. NaCl (2%) was included in growth media in which oxacillin was also included. For each strain, mpsA expression was normalized to the value obtained in CA-MHB–Tris (−), with this value set to 1.0. Data are means and SD from three independent biological replicates. Statistical comparisons were determined by Student’s t test. ns, not significant; *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
For confirmation of the impact of NaHCO3-oxacillin exposure on mpsA transcription, as well as on translation, strategic mpsA-green fluorescent protein (GFP) fusion constructs were assessed by flow cytometry. Transcriptional (txn) constructs carry the promoter region of the mpsA with the heterologous sarA RBS fused to the gfp gene; translational (tln) constructs harbor the promoter region of the mpsA with its intrinsic mpsA RBS fused to the gfp gene (Fig. S4). As noted in Materials and Methods, the two flow-cytometric readouts which were assessed and quantitatively compared were (i) percent GFP fluorescence, indicating the proportion of the 10,000-cell population that expressed GFP above the control baselines for the strain set, and (ii) the mean fluorescence intensity (MFI), measuring the mean per-cell GFP expression.
Consistent with the qRT-PCR data shown in Fig. 3, MFI data also demonstrated that the two responsive strains had significantly higher GFP expression at both txn and tln levels after exposure to NaHCO3-oxacillin than the two nonresponsive strains (Fig. 4). This difference was even more impressive when the changes in MFI metrics (ΔMFI) in the responsive and nonresponsive strains in the absence (baseline with oxacillin alone) versus the presence of NaHCO3-oxacillin exposure were compared (Fig. 5A).
FIG 4.
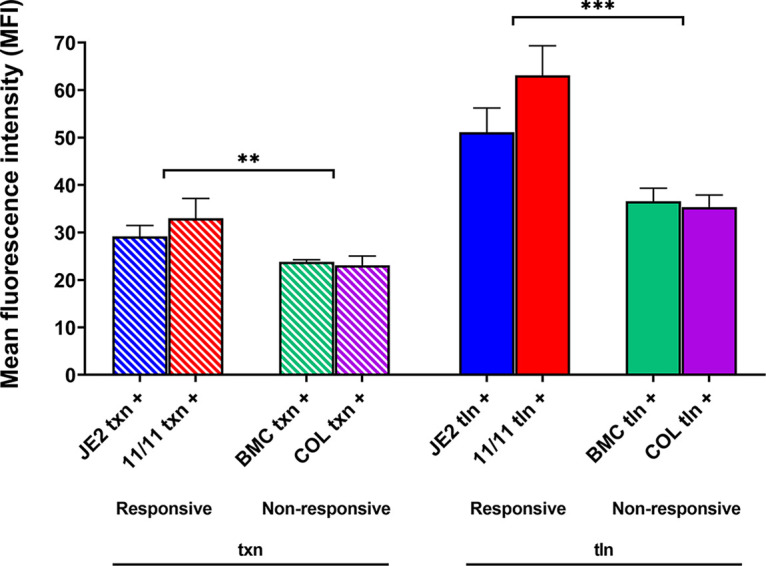
Translational efficiency of mpsA promoter sequences for all the prototype strains in the presence of NaHCO3 and oxacillin. Translational efficiency was assessed by flow cytometry using strains harboring the promoter-GFP fusions (Fig. S4) for JE2, MRSA 11/11, BMC1001, and COL promoter regions. Transcriptional (txn) constructs harbor the promoter region of mpsA with the sarA RBS fused to gfp (left), while translational (tln) constructs harbor the promoter region of mpsA with the mpsA RBS fused to gfp (right). Cells were grown in CA-MHB–Tris, 1/2 MIC of oxacillin (16 μg/mL for JE2 and MRSA 11/11, 64 μg/mL for BMC1001, and 128 μg/mL for COL), and 2% NaCl for 3 h before being assessed for MFI via flow cytometry. NaHCO3 (44 mM) was added to all the media (+). Statistical significance of the MFI between the responsive and nonresponsive strains was determined by Student’s t test. **, P = 0.0044; ***, P = 0.0003. Data are the results of four independent biological replicates for each strain/condition.
FIG 5.
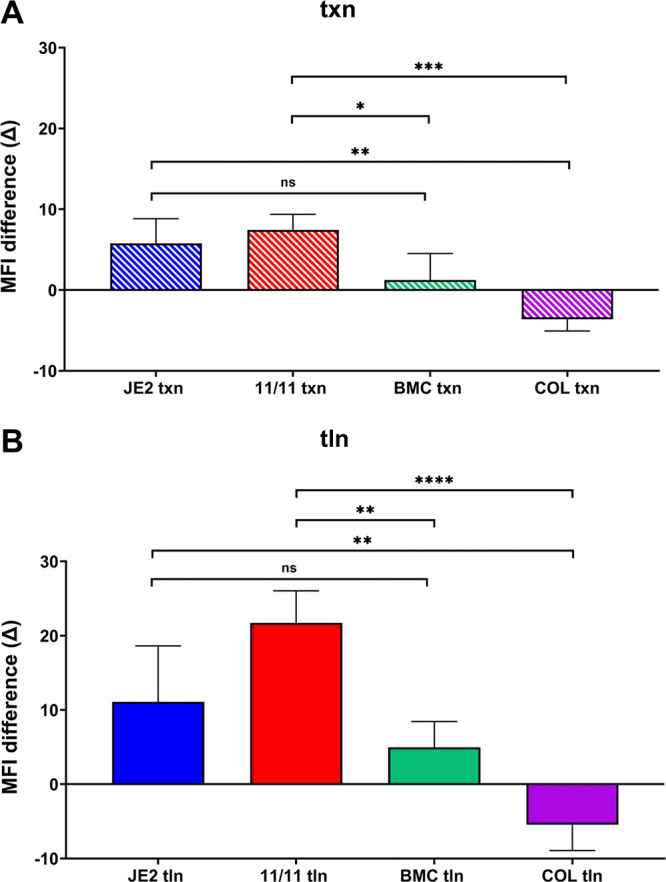
Differences in MFI of strains JE2, MRSA 11/11, BMC1001, and COL grown with and without NaHCO3 in the presence of 1/2 MIC of oxacillin for (A) transcriptional (txn) and (B) translational (tln) constructs. The constructs and conditions used are indicated in the legend to Fig. 4. Each bar shows the difference (increase/decrease) between the MFIs of the strain grown with and without NaHCO3 (baseline levels), and the statistical significance between them was calculated using one-way ANOVA followed by Tukey’s multiple-comparison test. ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. Data are the results from four independent biological replicates for each strain/condition.
As expected, the tln metrics (utilizing MFI readouts) closely paralleled those observed in the txn assays mentioned above. Thus, in both responsive strains, the MFI txn metrics were significantly higher than those in the nonresponsive strains (Fig. 4). These tln differences were further magnified when baseline expression in the absence of NaHCO3 was compared with expression in the presence of NaHCO3-oxacillin exposure for the two phenotype groups (Fig. 5B).
In parallel, we assessed the percent GFP expression for both the txn and tln constructs, in the presence and the absence of NaHCO3-oxacillin exposure. As noted above, this metric indicates the proportion of the overall 10,000 cell population tested by flow cytometry expressing GFP above the control, background gated level. In contrast to the MFI data detailed above, there were essentially no differences in the percent GFP data sets comparing the responsive versus nonresponsive strain groups (Fig. S5 and S6). This indicated that the same proportion of the cell populations for each phenotype group transcribed and translated the mpsA message; the differences between the phenotype groups appear to depend upon the extent to which txn and tln proceed (Fig. S6). As a control, the addition of oxacillin alone (i.e., in the absence of NaHCO3) did not have any influence on the txn and tln efficiency of each corresponding strains (Fig. S7 and S8).
DISCUSSION
The overall goal of the current study was to investigate the effect of the mpsABC NaHCO3 uptake system on the NaHCO3-responsive and NaHCO3-nonresponsive MRSA phenotypes in prototype strains. A number of interesting outcomes were observed when MRSA strains which are NaHCO3 responsive were compared with those that are not, as defined in vitro. First, among our prototype MRSA strain set, the mpsABC coding sequences were essentially identical, with only two amino acid substitutions. The promoter sequences of mpsA also shared 100% identity among the prototype strain set. The oxacillin MIC data for the JE2 and MRSA 11/11 swap variants confirmed that the two SNPs contained within the mpsABC coding region do not dictate NaHCO3 responsiveness phenotype. The mpsAB SNPs found in nonresponsive strains seem to yield this phenotype only in selected genetic backgrounds, implying that regulatory factors beyond the mpsABC operon are in play. In addition to the above-mentioned reasoning, the high degree of identity in the mpsABC sequence and its promoter in all four prototype strains suggests that the phenotypic differences in NaHCO3-responsive versus NaHCO3-nonresponsive MRSA strains were likely due to genes or pathways outside mpsABC that impacted its functionality and/or posttranslational modifications of the MpsABC transporter.
Second, deletions of mpsABC caused loss of the NaHCO3-responsive, oxacillin-susceptible phenotype in both intrinsically responsive MRSA strains (a defect which was plasmid complementable) but had no impact in the intrinsically nonresponsive strains. Of importance, the deletion mutants of both responsive and nonresponsive strains could not grow in ambient air; in contrast, all deletion mutants grew well in the presence of 5% CO2. The latter phenomenon is expected, since CO2 can diffuse freely into the MRSA cell, although the rate and extent of this diffusibility can be substantially impacted by the degree of biofilm formation in a given strain (17). One notable observation was that CO2 supplementation was able to suppress NaHCO3 uptake in both responsive strains but not in either nonresponsive strain. There are two possibilities here. (i) It may be that in NaHCO3-responsive strains, a feedback inhibition system recognizes the availability of exogenous CO2 and is able to repress the mpsABC NaHCO3 uptake system; in contrast, this uptake system is relatively constitutive in nonresponsive strains but at a much lower setpoint. (ii) The intracellular CO2 fixation systems may be quantitatively or functionally different in NaHCO3-responsive versus -nonresponsive strains.
This concept of fixation of CO2 is that this molecule, which is small and uncharged, moves freely into and out of the cell, based only on concentration differences. On the other hand, HCO3− is a charged molecule which remains within the organism once taken up. While CO2 can spontaneously be converted to HCO3− in an aqueous environment, the reverse reaction occurs as well. In many organisms, an enzyme with an extremely high turnover number can rapidly convert CO2 to HCO3− intracellularly, thereby capturing it inside the cell. Carbonic anhydrase is not present in S. aureus; thus, for S. aureus to retain CO2 (i.e., fix it), the spontaneously produced HCO3− must be rapidly integrated into an intracellular molecule. As examples, two enzymes, PcyA and PurK, fix HCO3− intracellularly by carboxylating pyruvate and 5-amino-1-(5-phospho-d-ribosyl)imidazole, respectively. This prevents CO2 from diffusing out of the cell, thereby enhancing growth. Pyruvate carboxylase adds HCO3− to pyruvate to form oxaloacetate, which then feeds the TCA cycle; importantly, the TCA cycle is linked to the cytochrome system, which can also help produce a membrane potential. Thus, even when mpsABC is deleted, intracellular CO2 can help generate a membrane potential, albeit at a much lower level (15).
Third, NaHCO3-responsive MRSA took up NaHCO3 more quickly and to a greater extent than NaHCO3-nonresponsive strains (over a 15-min period); these early uptake differences roughly correlate with higher early growth rates in NaHCO3-responsive than NaHCO3-nonresponsive MRSA strains. The β-lactam antibiotics are more active during accelerated growth, which correlates with upregulated ATP production to compensate for the large amounts being consumed during cell wall biosynthesis.
Fourth, our data show differences in NaHCO3 uptake profiles of NaHCO3-responsive and -nonresponsive strains; responsive strains exhibited enhanced NaHCO3 uptake under ambient-air conditions. In this study, we found that CO2 blocks the uptake of HCO3 in responsive versus nonresponsive strains. There are many metabolic pathways in S. aureus that require CO2, so defining which specific reaction is pivotal is not possible at this time. However, from our data, we can make some predictions about glutamine metabolism impacting maintenance of the methicillin resistance (MRSA) phenotype (i.e., HCO3 nonresponsiveness). Several lines of evidence suggest that responsive strains are not able to generate sufficient endogenous CO2 under ambient-air conditions (versus nonresponsive strains). (i) Endogenous production of CO2 is dependent upon intracellular glutamate levels and, to some extent, aspartate levels (21). Other amino acids and carbohydrates do not contribute to endogenous CO2 production. This paradigm was studied by labeling staphylococcal cells with 14C. Moreover, the addition of glutamate or glucose to the growth medium does not impact the release of 14CO2. (ii) In S. aureus, glutamate is shuttled to glutamine by glutamine synthase, GlnA, and then onto carbamoyl phosphate, which is metabolized by carbamate kinase (ArcC). This process ultimately produces HCO3, ATP, and NH3, which are the principal metabolic products reported by Ramsey (21). (iii) Others have also noted a connection between glutamine and expression/maintenance of the MRSA phenotype (22). Of course, the regulator of the glutamine synthase operon, GlnR (also called FemC), is an accessory factor, which itself is required for expression of the MRSA phenotype (23). (iv) Finally, there are differences between the arc genes (important determinants within the glutamine biosynthetic pathway) of the USA300 (MRSA 11/11) and USA400 (MW2) clonal lineages (designated arcC) versus strains of the USA500 lineage (BMC1001) (designated arc2) (24, 25). Of note, the USA300 and USA400 lineages represent the two HCO3-responsive strains in our study, while the USA500 lineage represents one of our HCO3-nonresponsive strains. Thus, there are several lines of evidence to link glutamate metabolism, CO2, and the maintenance of expression of MRSA (i.e., a HCO3-nonresponsive state). We recognize (i) the complexity of the endogenous CO2-generating systems as outlined above and (ii) the somewhat speculative nature of our hypothesis (i.e., that HCO3-responsive strains need to take up more exogenous HCO3). We plan to investigate these CO2-generating systems further in future experiments.
Fifth, two additional factors that undoubtedly impact the differences in NaHCO3 uptake profiles seen in NaHCO3-responsive versus NaHCO3-nonresponsive strains are the expression (transcription) and translation metrics of the mpsA gene itself in the presence of exogenous NaHCO3. In the current study, when baseline expression was compared with NaHCO3-stimulated expression, the transcription of this gene during log phase was substantially increased by exogenous NaHCO3 exposure in NaHCO3-responsive strains while remaining at near-baseline levels in NaHCO3-nonresponsive strains. As anticipated, at stationary phase of growth, when mpsA expression is minimal, exogenous NaHCO3 exposure exerted no enhancement of mpsA expression. Similar to the transcription outcomes above, the profiles of exogenous NaHCO3-stimulated translation of the mpsA message were significantly higher in NaHCO3-responsive than NaHCO3-nonresponsive strains.
Last, the transcriptional data above, showing increased expression of mpsA in NaHCO3-responsive versus NaHCO3-nonresponsive strains in the presence of exogenous NaHCO3, were confirmed by both qRT-PCR and flow cytometry. This technique provided an additional key piece of information to this metric. These flow cytometry data demonstrated that the overall population of cells expressing this gene was not significantly different in the two phenotype groups; however, the quantitative per-cell expression (MFI) of this gene was significantly amplified by exogenous NaHCO3 exposure in NaHCO3-responsive versus NaHCO3-nonresponsive MRSA cells.
These investigations have several limitations. Our study strain set, although consisting of well-characterized and previously published strains, was relatively small. Future studies will be required to assess the mpsABC uptake system in a larger MRSA strain cohort. Also, the identification of genes/pathways which can significantly impact the mpsABC system, especially to explain the differential phenotypic and genotypic outcomes in NaHCO3-responsive versus -nonresponsive strains, will be required. Transcriptome sequencing (RNA-seq) analyses comparing parental JE2 versus JE2 mpsABC deletion constructs are in progress to help answer this question. Preliminary expression profiles have revealed the upregulation of genes responsible for cell wall hydrolase/modification, transporters, and phage-related genes in the JE2 mpsABC mutant, with the most highly upregulated gene being sceD, a transglycosylase gene (unpublished data). SceD is a divisome-specific autolysin postulated to participate in peptidoglycan turnover, cell wall hydrolysis, and cell division; its inactivation results in impaired cell separation and clumping of bacterial cultures (26). This result is consistent with our previous and independently derived RNA-seq data, where it was demonstrated that NaHCO3 strongly represses sceD expression (11). Thus, high levels of intracellular NaHCO3 appear to repress sceD expression, whereas low levels of intracellular NaHCO3 (such as those seen in mpsABC mutants) result in upregulation of sceD. Finally, RNA-seq analyses in the JE2 mpsABC mutant showed a downregulation of genes related to cell wall-bound and secreted enzymes and protein and ion transporters, with the most downregulated genes encoding toxins such as phenol-soluble modulins. Given that many of these differentially expressed genes are linked to cell wall homeostasis, it is tempting to speculate that the absence of the NaHCO3 transporter MpsAB exerts most of its effects on the cell wall and its adaptations in distinct microenvironments.
Clearly, additional investigations will be required to assess the impacts of endogenous CO2 generation systems, inhibitors of individual components of the proton motive force (ΔΨ and ΔpH), as well as the proton transport systems (e.g., FoF1 ATPase). While MpsAB can establish a ΔΨ, growing S. aureus in the presence of 5% CO2 allows the organism to maintain its normal ΔΨ (15), probably because the presence of CO2 enhances pyruvate carboxylase activity, thereby increasing TCA activity, leading to FoF1 ATPase upregulation.
Importantly, the translatability of this uptake system to β-lactam hypersusceptibility in vivo is currently being examined in the experimental endocarditis model, using strategic deletion and complementation mpsABC variants of our prototype NaHCO3-responsive and -nonresponsive strains treated with oxacillin.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All the strains used in this study are listed in Table S2. The four prototype strains which form the basis for this investigation (JE2, MRSA 11/11, BMC1001, and COL) have been well characterized by our laboratory and others (27–30).
For cloning procedures, all staphylococcal and Escherichia coli strains were grown with aeration in basic medium (BM) (1% soy peptone, 0.5% yeast extract, 0.5% NaCl, 0.1% glucose, and 0.1% K2HPO4 [pH 7.2]), unless specified otherwise. All cultures were grown in baffled shake flasks (flask-to-medium ratio, 1:10). When applicable, the medium was supplemented with 10 μg/mL chloramphenicol for selected staphylococcal strains and 100 μg/mL ampicillin for E. coli strains.
Bicarbonate uptake analysis.
Bicarbonate uptake analysis was performed as previously described (15). Briefly, overnight cultures cultivated in tryptic soy broth (TSB) in ambient air and 5% CO2 were inoculated to an optical density at 578 nm (OD578) of 0.1 and grown until mid-exponential phase for 3 h under their respective conditions. Cells were then washed with 10 mM Tris buffer (pH 7) before resuspension in the same buffer and adjusted to an OD578 of 1 in a final volume of 10 mL. Fluorocitrate (1 mM) and glucose (5 mM) were added to the cell suspensions prior to incubation for 30 min at room temperature with magnetic stirring. Next, 50 μCi of NaH14CO3 (specific activity, 40 to 60 mCi/mmol; PerkinElmer) was added. One-milliliter aliquots of each sample were collected before (time zero) and after the addition of NaH14CO3 and at 0.5, 1, 2, 4, 6, 8, 10, and 15 min. After the collection of each sample, cells were immediately filtered by vacuum filtration onto membrane filters (Pall GN-6 Metricel membrane disc filter; 0.45-μm pore size, 25-mm diameter) and washed with 10 mL of Tris NaCl buffer (10 mM Tris with 100 mM NaCl). Membrane filters were subsequently placed in a vial containing 10 mL of liquid scintillation cocktail (Ultima Gold; PerkinElmer). Radioactivity retained on the membrane filters was measured in a PerkinElmer liquid scintillation analyzer (Tri-Carb 2800TR). The H14CO3 uptake was determined by the accumulation of 14C in the cells and recorded as counts per minute.
Multiple-sequence alignment.
mpsABC sequences for JE2 are found at locus tags B7H15_02370, B7H15_02375, and B7H15_02380 of the complete genome of S. aureus JE2 retrieved from the NCBI database (31) under the accession number CP020619 (https://www.ncbi.nlm.nih.gov/nuccore/CP020619.1/). For COL, the mpsABC sequences are found at locus tags SACOL0494, SACOL0495, and SACOL0496 of the complete genome of an early methicillin-resistant isolate, S. aureus COL, which was isolated in the 1960s (30) and sequenced in 2005 (32) under the accession number CP000046, also from the NCBI database. MRSA 11/11 (28) and BMC1001 (29) were sequenced by our group with the BioSample IDs SAMN17703515 and SAMN17703518, respectively. Both can be found in the NCBI database with the accession number PRJNA697971 (https://www.ncbi.nlm.nih.gov/bioproject/697971). mpsABC and its promoter sequences from all the four prototype strains were aligned using Clustal Omega (33).
Construction of mpsABC deletion mutants and respective complementation plasmid construct variants in the prototype MRSA strains.
The oligonucleotides used in this can be found in Table S3. The mpsABC deletion mutants in the background of S. aureus MRSA 11/11, BMC1001, and COL were constructed as markerless deletions using allelic replacements as previously described (17). The upstream and downstream flanking regions of mpsABC are approximately 2 kb each, and the sequences are identical for all three strains. Briefly, the up- and downstream regions were amplified from the chromosomal DNA of MRSA 11/11. The amplified fragments were assembled using SmaI-linearized plasmid pBASE6 (34) via Gibson assembly (35) with Hi-Fi DNA assembly master mix (New England Biolabs). The resulting plasmid was transformed into chemically competent E. coli DC10B (36). The authenticated constructs were first introduced into S. aureus RN4220 via electroporation and then into MRSA 11/11, BMC1001, and COL. Mutagenesis was performed as previously described (37). Deletion of mpsABC was confirmed by chromosomal PCR and DNA sequencing of the PCR products.
Complementation of the mpsABC deletion mutants in all strains was performed using the plasmid pRB473 harboring mpsABC along with its native promoter from a previous study (16). The recombinant plasmid was transformed into competent ΔmpsABC mutants of MRSA 11/11, BMC1001, and COL via electroporation and confirmed with PCR.
Construction of GFP reporters for mpsA transcription and translation.
To quantify transcriptional and translational activity of the mpsA genes under the control of a reporter gene, gfpuvr, the upstream promoter region was cloned with or without the RBS of mpsA before the translational start codon (ATG) of the gfp reporter gene (Fig. S4) in the shuttle plasmid pALC1484 (38). First, the pALC1484 vector was modified by removing the RBS along with the spacing region between the RBS and start codon (ATG) of the gfp gene and replaced with the RBS and spacing region of the mpsA gene using pairwise primers with flanking EcoRI and XbaI sites, and template DNA as pALC1484 by PCR. A 394-bp promoter fragment with flanking EcoRI and XbaI sites of the mpsA gene without the RBS was amplified by PCR and cloned into the modified pALC1484 and pALC1484 plasmids in E. coli IM08B (39). Final constructs were verified by enzymatic digestion and DNA sequencing, mobilized into strains JE2, MBC1001, MRSA 11/11, and COL by electroporation, and selected on tryptic soy agar (TSA) with chloramphenicol (10 μg/mL).
Construction of the mpsAB region SNP swap variant.
To construct strains with interchange (swap) of the mpsAB region, a 5.5-kb DNA fragment was amplified that contained the intact mpsAB genes from COL by PCR using primers with flanking BamHI sites at both ends (Table S3). The DNA fragment was cloned into a temperature-sensitive shuttle vector, pMAD-X (13), and then selected in E. coli IM08B (39) for the correct construct. After verification by restriction digestion and DNA sequencing, the interchanged construct was introduced into JE2 and MRSA 11/11 by electroporation and selected on chloramphenicol (10 μg/mL)- and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-containing plates for blue colonies at 30°C. Plasmid DNA was isolated and digested with BamHI for the verification of the presence of DNA fragment in the respective construct in the strains. The construction of chromosomal mutations in the respective strain by recombination or two-point crossover was performed by a routine procedure as described previously (40). Briefly, two-point crossover of the mpsAB region was performed by temperature shift by growing the strains at 43°C with chloramphenicol, followed by subculturing at 30°C without any antibiotic. Cells were plated with and without chloramphenicol in the presence of X-Gal (40 μg/mL) for selection and incubated at 37°C. White/nonblue colonies were cross-streaked to select chloramphenicol-susceptible colonies for the potential two-point crossover clones or mutants. The mutants were verified by chromosomal PCR and DNA sequencing of the PCR product.
MIC determinations.
The MICs of oxacillin were determined by broth microdilution according to the CLSI guidelines as previously described (4, 41, 42). Briefly, cells were grown overnight in the indicated medium (CA-MHB, CA-MHB–Tris, or CA-MHB–Tris plus 44 mM NaHCO3) and then diluted into the same medium containing 2-fold serial dilutions of oxacillin with 2% NaCl. The final concentration of the cells used were 5 × 105 CFU/mL. Microtiter plates were incubated overnight at 37°C in ambient air or 5% CO2. The MIC was read as the first well in which visual turbidity was reduced compared to the no-drug control well. All MICs are the modes from at least six independent determinations.
Assessment of mpsA promoter-GFP fusions by flow cytometry.
For flow cytometry, strains containing the plasmid pALC1484 with various promoter-GFP constructs (Table S2) were grown overnight in the indicated medium (CA-MHB–Tris with or without 44 mM NaHCO3) at 37°C with aeration and then diluted 1:10 into the same medium with or without 1/2 MIC of oxacillin (16 μg/mL for JE2 and 11/11, 64 μg/mL for BMC1001, and 128 μg/mL for COL) and 2% NaCl. Cells were incubated at 37°C with aeration and grown for 3 h to mid-exponential phase before being diluted 1:10 into phosphate-buffered saline (PBS), and 10,000 cells were analyzed for GFP production by flow cytometry with FACSCalibur (Becton Dickinson). The MFI and percentage of cells expressing GFP in each sample were determined with FlowJo software (version 10.8) using data obtained from the FL1-H channel and expressed as relative fluorescent units per cell population or percentage of cells expressing GFP per cell population, respectively. All samples were performed in four independent biological replicates.
RNA isolation and qRT-PCR analysis of mpsA expression.
To quantify the combined effect of NaHCO3 exposure and oxacillin stimulation on the expression of mpsA, overnight cultures of the strains were grown in CA-MHB Tris with or without 44 mM NaHCO3 and 1/2 MIC of oxacillin, inoculated to an OD578 of 0.1, and grown for 3 h and 6 h, respectively. As a control to check if oxacillin alone affects the expression of mpsA, cultures were also grown in CA-MHB–Tris with or without 1/2 MIC of oxacillin for 3 h (Fig. S3). NaCl (2%) was included in growth media in which oxacillin was also included. The same amount of cells at a given OD578 was collected for all the strains and conditions and RNA was extracted from the cell pellets using FastPrep (FP120; Thermo Savant) with lysing matrix B tubes (MP Biomedicals). Total RNA was isolated by column purification (Qiagen), followed by DNase treatment (Turbo DNA-free; Invitrogen, Thermo Fisher Scientific) and then reverse transcribed to generate a cDNA library (Superscript IV; Invitrogen, Thermo Fisher Scientific). qRT-PCR was performed using a StepOne real-time PCR system (Applied Biosystems) with the primers listed in Table S3. gyrB was used as a housekeeping gene to normalize transcript quantifications. Relative quantification was carried out using the cycle threshold (ΔΔCT) method. The data are presented as the fold change in mpsA expression in the presence of oxacillin and NaHCO3 compared to CA-MHB–Tris for each strain, with the latter being normalized to 1.0. All qRT-PCR gene expression data were determined from at least two independent biological replicates for each condition and tested in duplicate.
Statistical analyses.
All data are presented as the sample means and standard deviations (SD), unless otherwise indicated. The bicarbonate uptake data were also quantified as the AUC for comparison among the strains under different conditions. All statistical analyses were carried out using Student’s t test for comparison between two groups, while one-way analysis of variance (ANOVA) followed by Tukey’s multiple-comparison test to compare the means among three or more groups. P values of <0.05 were considered statistically significant. All statistical analyses were performed using Microsoft Excel or GraphPad Prism 9 software.
Data availability.
The main data supporting the findings of this work are available within the article and its supplemental material or from the corresponding author upon request.
ACKNOWLEDGMENTS
This work was supported by the following grant from the National Institutes of Health: 1RO1-AI146078 (to A.S.B.). F.G. acknowledges support from the Deutsche Forschungsgemeinschaft, Germany’s Excellence Strategy—EXC 2124—390838134, Controlling Microbes to Fight Infections.
We thank David Applebaum and Mike Lopiccolo for their excellent assistance with the 14C measurement.
A.S.B. conceived the idea and designed the study with help from S.-H.F. and S.C.E. S.-H.F. and S.C.E. performed the experiments. A.C.M. and A.L.C. constructed the GFP reporter strains. A.S.B., S.-H.F., and R.A.P. analyzed the data and wrote the manuscript. F.G. and H.F.C. critically reviewed and edited the manuscript. All authors read and approved the final manuscript.
We all declare that no conflict of interest exists.
Footnotes
Supplemental material is available online only.
Contributor Information
Sook-Ha Fan, Email: sook-ha.fan@lundquist.org.
Arnold S. Bayer, Email: abayer@lundquist.org.
Matthew J. Culyba, University of Pittsburgh
REFERENCES
- 1.Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, Carugati M, Holland TL, Fowler VG, Jr. 2019. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol 17:203–218. doi: 10.1038/s41579-018-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. 2010. Community-associated meticillin-resistant Staphylococcus aureus. Lancet 375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakhundi S, Zhang K. 2018. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev 31:e00020-18. doi: 10.1128/CMR.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ersoy SC, Abdelhady W, Li L, Chambers HF, Xiong YQ, Bayer AS. 2019. Bicarbonate resensitization of methicillin-resistant Staphylococcus aureus to beta-lactam antibiotics. Antimicrob Agents Chemother 63:e00496-19. doi: 10.1128/AAC.00496-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ersoy SC, Heithoff DM, Barnes L, Tripp GK, House JK, Marth JD, Smith JW, Mahan MJ. 2017. Correcting a fundamental flaw in the paradigm for antimicrobial susceptibility testing. EBioMedicine 20:173–181. doi: 10.1016/j.ebiom.2017.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ersoy SC, Otmishi M, Milan VT, Li L, Pak Y, Mediavilla J, Chen L, Kreiswirth B, Chambers HF, Proctor RA, Xiong YQ, Fowler VG, Bayer AS. 2020. Scope and predictive genetic/phenotypic signatures of bicarbonate (NaHCO3) responsiveness and beta-lactam sensitization in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 64:e02445-19. doi: 10.1128/AAC.02445-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ersoy SC, Rose WE, Patel R, Proctor RA, Chambers HF, Harrison EM, Pak Y, Bayer AS. 2021. A combined phenotypic-genotypic predictive algorithm for in vitro detection of bicarbonate: beta-lactam sensitization among methicillin-resistant Staphylococcus aureus (MRSA). Antibiotics (Basel) 10:1089. doi: 10.3390/antibiotics10091089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel JB. 2017. Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Rose WE, Bienvenida AM, Xiong YQ, Chambers HF, Bayer AS, Ersoy SC. 2020. Ability of bicarbonate supplementation to sensitize selected methicillin-resistant Staphylococcus aureus strains to beta-lactam antibiotics in an ex vivo simulated endocardial vegetation model. Antimicrob Agents Chemother 64:e02072-19. doi: 10.1128/AAC.02072-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ersoy SC, Chambers HF, Proctor RA, Rosato AE, Mishra NN, Xiong YQ, Bayer AS. 2021. Impact of bicarbonate on PBP2a production, maturation, and functionality in methicillin-resistant Staphylococcus aureus (MRSA). Antimicrob Agents Chemother 65:e02621-20. doi: 10.1128/AAC.02621-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ersoy SC, Hanson BM, Proctor RA, Arias CA, Tran TT, Chambers HF, Bayer AS. 2021. Impact of bicarbonate-beta-lactam exposures on methicillin-resistant Staphylococcus aureus (MRSA) gene expression in bicarbonate-beta-lactam-responsive vs. non-responsive strains. Genes (Basel) 12:1650. doi: 10.3390/genes12111650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ersoy SC, Chan LC, Yeaman MR, Chambers HF, Proctor RA, Ludwig KC, Schneider T, Manna AC, Cheung A, Bayer AS. 2022. Impacts of NaHCO(3) on beta-lactam binding to PBP2a protein variants associated with the NaHCO(3)-responsive versus NaHCO(3)-non-responsive phenotypes. Antibiotics (Basel) 11:462. doi: 10.3390/antibiotics11040462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ersoy SC, Manna AC, Proctor RA, Chambers HF, Harrison EM, Bayer AS, Cheung A. 2022. The NaHCO(3)-responsive phenotype in methicillin-resistant Staphylococcus aureus (MRSA) is influenced by mecA genotype. Antimicrob Agents Chemother 66:e00252-22. doi: 10.1128/aac.00252-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ersoy SC, Goncalves B, Cavaco G, Manna AC, Sobral RG, Nast CC, Proctor RA, Chambers HF, Cheung A, Bayer AS. 2022. Influence of sodium bicarbonate on wall teichoic acid synthesis and beta-lactam sensitization in NaHCO(3)-responsive and nonresponsive methicillin-resistant Staphylococcus aureus. Microbiol Spectr 10:e03422-22. doi: 10.1128/spectrum.03422-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan SH, Ebner P, Reichert S, Hertlein T, Zabel S, Lankapalli AK, Nieselt K, Ohlsen K, Gotz F. 2019. MpsAB is important for Staphylococcus aureus virulence and growth at atmospheric CO(2) levels. Nat Commun 10:3627. doi: 10.1038/s41467-019-11547-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer S, Steffen W, Steuber J, Gotz F. 2015. The Staphylococcus aureus NuoL-like protein MpsA contributes to the generation of membrane potential. J Bacteriol 197:794–806. doi: 10.1128/JB.02127-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan SH, Matsuo M, Huang L, Tribelli PM, Gotz F. 2021. The MpsAB bicarbonate transporter is superior to carbonic anhydrase in biofilm-forming bacteria with limited CO(2) diffusion. Microbiol Spectr 9:e00305-21. doi: 10.1128/Spectrum.00305-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan SH, Liberini E, Gotz F. 2021. Staphylococcus aureus genomes harbor only MpsAB-like bicarbonate transporter but not carbonic anhydrase as dissolved inorganic carbon supply system. Microbiol Spectr 9:e00970-21. doi: 10.1128/Spectrum.00970-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangiapia M, Usf M, Brown TW, Chaput D, Haller E, Harmer TL, Hashemy Z, Keeley R, Leonard J, Mancera P, Nicholson D, Stevens S, Wanjugi P, Zabinski T, Pan C, Scott KM, USF MCB4404L . 2017. Proteomic and mutant analysis of the CO(2) concentrating mechanism of hydrothermal vent chemolithoautotroph Thiomicrospira crunogena. J Bacteriol 199:e00871-16. doi: 10.1128/JB.00871-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbert S, Ziebandt AK, Ohlsen K, Schafer T, Hecker M, Albrecht D, Novick R, Gotz F. 2010. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect Immun 78:2877–2889. doi: 10.1128/IAI.00088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramsey HH. 1962. Endogenous respiration of Staphylococcus aureus. J Bacteriol 83:507–514. doi: 10.1128/jb.83.3.507-514.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munch D, Roemer T, Lee SH, Engeser M, Sahl HG, Schneider T. 2012. Identification and in vitro analysis of the GatD/MurT enzyme-complex catalyzing lipid II amidation in Staphylococcus aureus. PLoS Pathog 8:e1002509. doi: 10.1371/journal.ppat.1002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafson J, Strassle A, Hachler H, Kayser FH, Berger-Bachi B. 1994. The femC locus of Staphylococcus aureus required for methicillin resistance includes the glutamine synthetase operon. J Bacteriol 176:1460–1467. doi: 10.1128/jb.176.5.1460-1467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Portillo BC, Moreno JE, Yomayusa N, Alvarez CA, Cardozo BE, Perez JA, Diaz PL, Ibanez M, Mendez-Alvarez S, Leal AL, Gomez NV. 2013. Molecular epidemiology and characterization of virulence genes of community-acquired and hospital-acquired methicillin-resistant Staphylococcus aureus isolates in Colombia. Int J Infect Dis 17:e744–e749. doi: 10.1016/j.ijid.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Strauß L, Stegger M, Akpaka PE, Alabi A, Breurec S, Coombs G, Egyir B, Larsen AR, Laurent F, Monecke S, Peters G, Skov R, Strommenger B, Vandenesch F, Schaumburg F, Mellmann A. 2017. Origin, evolution, and global transmission of community-acquired Staphylococcus aureus ST8. Proc Natl Acad Sci USA 114:E10596–E10604. doi: 10.1073/pnas.1702472114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stapleton MR, Horsburgh MJ, Hayhurst EJ, Wright L, Jonsson IM, Tarkowski A, Kokai-Kun JF, Mond JJ, Foster SJ. 2007. Characterization of IsaA and SceD, two putative lytic transglycosylases of Staphylococcus aureus. J Bacteriol 189:7316–7325. doi: 10.1128/JB.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murthy MH, Olson ME, Wickert RW, Fey PD, Jalali Z. 2008. Daptomycin non-susceptible meticillin-resistant Staphylococcus aureus USA 300 isolate. J Med Microbiol 57:1036–1038. doi: 10.1099/jmm.0.2008/000588-0. [DOI] [PubMed] [Google Scholar]
- 29.Yang SJ, Xiong YQ, Boyle-Vavra S, Daum R, Jones T, Bayer AS. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob Agents Chemother 54:3161–3169. doi: 10.1128/AAC.00487-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyke KG. 1969. Penicillinase production and intrinsic resistance to penicillins in methicillin-resistant cultures of Staphylococcus aureus. J Med Microbiol 2:261–278. doi: 10.1099/00222615-2-3-261. [DOI] [PubMed] [Google Scholar]
- 31.Sayers EW, Bolton EE, Brister JR, Canese K, Chan J, Comeau DC, Connor R, Funk K, Kelly C, Kim S, Madej T, Marchler-Bauer A, Lanczycki C, Lathrop S, Lu Z, Thibaud-Nissen F, Murphy T, Phan L, Skripchenko Y, Tse T, Wang J, Williams R, Trawick BW, Pruitt KD, Sherry ST. 2022. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res 50:D20–D26. doi: 10.1093/nar/gkab1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM. 2005. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol 187:2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madeira F, Pearce M, Tivey ARN, Basutkar P, Lee J, Edbali O, Madhusoodanan N, Kolesnikov A, Lopez R. 2022. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res 50:W276–W279. doi: 10.1093/nar/gkac240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, Schrenzel J, Lalk M, Wolz C. 2012. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog 8:e1003016. doi: 10.1371/journal.ppat.1003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 36.Monk IR, Shah IM, Xu M, Tan MW, Foster TJ. 2012. Transforming the untransformable: application of direct transformation to manipulate genetically Staphylococcus aureus and Staphylococcus epidermidis. mBio 3:e00277-11. doi: 10.1128/mBio.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bae T, Schneewind O. 2006. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid 55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Kahl BC, Goulian M, van Wamel W, Herrmann M, Simon SM, Kaplan G, Peters G, Cheung AL. 2000. Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect Immun 68:5385–5392. doi: 10.1128/IAI.68.9.5385-5392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monk IR, Tree JJ, Howden BP, Stinear TP, Foster TJ. 2015. Complete bypass of restriction systems for major Staphylococcus aureus lineages. mBio 6:e00308-15. doi: 10.1128/mBio.00308-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim S, Reyes D, Beaume M, Francois P, Cheung A. 2014. Contribution of teg49 small RNA in the 5’ upstream transcriptional region of sarA to virulence in Staphylococcus aureus. Infect Immun 82:4369–4379. doi: 10.1128/IAI.02002-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cockerill FR, Wikler MA, Alder J, Dudley MN, Eliopoulos GM, Ferraro MJ, Hardy DJ, Hecht DW, Hindler JA, Patel JB, Powell M. 2012. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M07-A9. Clinical and Laboratory Standareds Institute, Wayne, PA. [Google Scholar]
- 42.Weinstein MP, Lewis JS, II.. 2020. The Clinical and Laboratory Standards Institute Subcommittee on Antimicrobial Susceptibility Testing: background, organization, functions, and processes. J Clin Microbiol 58:e01864-19. doi: 10.1128/JCM.01864-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.00141-23-s0001.pdf, PDF file, 0.9 MB (958.6KB, pdf)
Data Availability Statement
The main data supporting the findings of this work are available within the article and its supplemental material or from the corresponding author upon request.