ABSTRACT
Cefiderocol and aztreonam-avibactam (ATM-AVI) both had activity against carbapenem-resistant Gram-negative bacilli, including those that produce metallo-β-lactamases (MBLs). We compared the in vitro activities and inoculum effects of these antibiotics against carbapenemase-producing Enterobacteriaceae (CPE), especially MBL-producing isolates. The MICs of cefiderocol and ATM-AVI were determined using broth microdilution method for a 2016 to 2021 collection of Enterobacteriaceae isolates which produced MBL, KPC, or OXA-48-like carbapenemases. MICs with high bacteria inoculum were also evaluated for susceptible isolates. A total of 195 CPE were tested, including 143 MBL- (74 NDM, 42 IMP, and 27 VIM), 38 KPC-, and 14 OXA-48-like-producing isolates. The susceptible rates of MBL-, KPC-, and OXA-48-like producers to cefiderocol were 86.0%, 92.1%, and 92.9%, respectively, and that to ATM-AVI were 95.8%, 100%, and 100%, respectively. NDM producers displayed lower susceptibility and higher MIC50s/MIC90s of cefiderocol (78.4%, 2/16 mg/L) than IMP (92.9%, 0.375/4 mg/L) and VIM (96.3%, 1/4 mg/L) producers. NDM- and VIM-producing Escherichia coli showed lower susceptibility to ATM-AVI (77.3% and 75.0%, respectively) compared to MBL-CPE of other species (100% susceptible). Inoculum effects for cefiderocol and ATM-AVI were observed among 95.9% and 95.2% of susceptible CPE, respectively. A switch from susceptible to resistant category was observed in 83.6% (143/171) of isolates for cefiderocol and 94.7% (179/189) for ATM-AVI. Our results revealed that NDM-producing Enterobacteriaceae had lower susceptibility to cefiderocol and ATM-AVI. Prominent inoculum effects on both antibiotics were observed for CPE, which suggested a risk of microbiological failure when they were used for CPE infections with high bacteria burden.
IMPORTANCE The prevalence of infections caused by carbapenem-resistant Enterobacteriaceae is increasing worldwide. Currently, therapeutic options for metallo-β-lactamase (MBL)-producing Enterobacteriaceae remain limited. We demonstrated that clinical metallo-β-lactamase (MBL)-producing Enterobacteriaceae isolates were highly susceptible to cefiderocol (86.0%) and aztreonam-avibactam (ATM-AVI) (95.8%). However, inoculum effects on cefiderocol and ATM-AVI were observed for over 90% of susceptible carbapenemase-producing Enterobacteriaceae (CPE) isolates. Our findings highlight a potential risk of microbiological failure when using monotherapy with cefiderocol or ATM-AVI to treat severe CPE infection.
KEYWORDS: carbapenem-resistant Enterobacteriaceae, metallo-β-lactamase, cefiderocol, β-lactam and β-lactamase inhibitor, inoculum effect
INTRODUCTION
The prevalence of carbapenem resistance has increased among clinical isolates of Enterobacteriaceae in the past decade. Enterobacteriaceae carrying carbapenemase-encoding plasmids raised special concern because the antimicrobial resistance could be transferred between different strains and species (1). The predominant carbapenemase types in Enterobacteriaceae varied across geographic regions. In Southeast Asia, metallo-β-lactamases (MBLs) and oxacillinase-48 (OXA-48) were the main carbapenemases in Enterobacteriaceae, whereas Klebsiella pneumoniae carbapenemase (KPC) was the most common carbapenemase in North America (2). In Europe, OXA-48 and KPC were more frequently detected than MBLs, but an increase in NDM-1 producers was noted since 2022, which was associated with the migration of people (3, 4).
Newer β-lactam with β-lactamase inhibitor combinations that were actively against carbapenem-resistant Enterobacteriaceae (CRE) had been developed in recent years, but MBLs could hydrolyze most of these antibiotics, such as ceftazidime-avibactam, ceftolozane-tazobactam, and meropenem-vaborbactam (5). Therefore, treatment options for MBL carbapenemase-producing Enterobacteriaceae (CPE) remained limited, and 14-day mortality-following infections with these pathogens could be up to 60% (6). Among newer antibiotics, a siderophore cephalosporin cefiderocol and aztreonam combined with avibactam (ATM-AVI) were proposed for treating infections with MBL CPE based on the in vitro susceptibility data and clinical reports (7). Nonetheless, MBL CPE were not universally susceptible to cefiderocol or ATM-AVI. Published studies reported higher cefiderocol minimum inhibitory concentrations (MICs) for New Delhi metallo-β-lactamase (NDM)-producing Enterobacteriaceae than those carrying other MBLs (8, 9). For ATM-AVI, Vasoo et al. found that two (1.2%) of 172 CPE had elevated MICs of ATM-AVI (8/4 and 16/4 mg/L), and both produced NDM carbapenemase (10). Data on the susceptibilities of multidrug-resistant Enterobacteriaceae to cefideocol or ATM-AVI have been increasingly reported (11, 12). However, many did not compare the results across bacterial species or types of MBL.
The MIC of many beta-lactam antibiotics can be influenced by the inoculum effect. Inoculum effect refers to a laboratory phenomenon in which the MIC of an antibiotic increases significantly when the number of inoculated organisms is increased. In vitro inoculum effect of cefazolin has been associated with worse outcomes when used to treat methicillin-susceptible Staphylococcus aureus bacteremia (13). In the literature, cephalosporins displayed inoculum effect against various bacterial pathogens, including Escherichia coli and Klebsiella pneumoniae (14). For extended-spectrum β-lactamases-producing Enterobacteriaceae, both cephalosporins and aztreonam tended to show inoculum effect (14, 15). At present, only three studies investigated the inoculum effect of cefiderocol or ATM-AVI against CRE (16–18), and the numbers of enrolled MBL CPE isolates were small. In addition, the impact of carbapenemase types or species of Enterobacteriaceae on inoculum effect had rarely been evaluated.
In this study, we aimed to compare the in vitro activities of cefiderocol and ATM-AVI against different types of CPE, especially the MBL-producing isolates, and determine the MICs at standard and high inoculum.
RESULTS
A total of 195 CPE were tested, including 143 MBL- (74 NDM, 42 imipenemase [IMP], and 27 verona integron-encoded [VIM] type carbapenemase), 38 KPC-, and 14 OXA-48-like-producing Enterobacteriaceae isolates. Of the 74 NDM producers, 49 (66.2%) were NDM-1, 24 (32.4%) were NDM-5, and one (1.4%) was NDM-4. All IMP producers were IMP-8, and all VIM producers were VIM-1. The subtypes of KPC carbapenemase included KPC-2 (89.5%, 34/38), KPC-17 (5.3%, 2/38), KPC-3 (2.6%, 1/38), and KPC-65 (2.6%, 1/38). Of the 14 OXA-48-like CPE, 13 were OXA-48 and one was OXA-181.
Among the 195 CPE isolates, K. pneumoniae (n = 79, 40.5%) was the most common species, followed by E. coli (n = 56, 28.7%) and E. cloacae complex (n = 44, 22.6%). Other species included nine Citrobacter freundii, six Klebsiella oxytoca, and one Klebsiella aerogenes. The majority of CPE were clinical isolates from urine (n = 68, 34.9%), blood (n = 50, 25.6%), sputum (n = 37, 19.0%), skin pus or wound (n = 14, 7.2%), body fluid (n = 5, 2.6%), and catheter tip (n = 1, 0.5%). Twenty (10.3%) of the 195 CPE were from anal screening cultures.
Susceptibilities and MICs of CPE to antimicrobial agents.
Table 1 presents the MIC values and antimicrobial susceptibilities of the CPE isolates. The susceptibility rates of all CPE to amikacin, aztreonam, ciprofloxacin, and tigecycline were 92.3% (180/195), 21.5% (42/195), 24.1% (47/195), and 99.0% (193/195), respectively. A total of 87.7% (171/195) of CPE isolates had a colistin MIC ≤2 mg/L. The susceptible rates of MBL-, KPC-, and OXA-48-like CPE to cefiderocol were 86.0% (123/143), 92.1% (35/38) and 92.9% (13/14), respectively. Among MBL CPE, NDM producers showed lower susceptibility and higher MIC50/MIC90 values of cefiderocol (78.4% [58/74], 2/16 mg/L) than IMP (92.9% [39/42], 0.375/4 mg/L) and VIM (96.3% [26/27], 1/4 mg/L) producers. Fig. 1A shows the cumulative percentages of different CPE inhibited at various cefiderocol concentrations.
TABLE 1.
In vitro susceptibilities and MICs (mg/L) of cefiderocol and aztreonam-avibactam against carbapenemase-producing Enterobacteriaceae
| Carbapenemase type and bacteria species | AMKc | ATM | CIP | COL | TGC | Cefiderocol |
Aztreonam-avibactama |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S (%) | S (%) | S (%) | I (%) | S (%) | MIC range | MIC50 | MIC90 | S (%) | MIC range | MIC50 | MIC90 | S (%) | |
| MBL (n = 143) | 92.3 | 26.8 | 30.8 | 92.3 | 98.6 | ≤0.06–>32 | 2 | 8 | 86.0 | ≤0.06–16 | 0.25 | 1 | 95.8 |
| NDM (n = 74) | 90.5 | 18.9 | 12.2 | 93.2 | 100 | 0.25–>32 | 2 | 16 | 78.4 | ≤0.06–16 | 0.25 | 4 | 93.2 |
| E. coli (n = 22) | 95.5 | 18.2 | 4.5 | 100 | 100 | 0.5–16 | 4 | 16 | 72.7 | ≤0.06–16 | 2 | 8 | 77.3 |
| ECC (n = 31) | 100 | 3.2 | 6.5 | 100 | 100 | 1–>32 | 2 | 16 | 77.4 | ≤0.06–1 | 0.25 | 0.5 | 100 |
| K. pneumoniae (n = 14) | 78.6 | 42.9 | 35.7 | 85.7 | 100 | 0.5–8 | 1.5 | 4 | 92.9 | ≤0.06–1 | 0.188 | 0.5 | 100 |
| Other speciesb (n = 7) | 57.1 | 42.9 | 14.3 | 57.1 | 100 | 0.25–32 | 4 | 32 | 71.4 | ≤0.06–0.5 | 0.25 | 0.5 | 100 |
| IMP (n = 42) | 95.2 | 51.2 | 52.4 | 92.9 | 95.2 | ≤0.06–32 | 0.375 | 4 | 92.9 | ≤0.06–0.5 | 0.125 | 0.5 | 100 |
| E. coli (n = 17) | 94.1 | 62.5 | 52.9 | 100 | 100 | ≤0.06–4 | 0.25 | 4 | 100 | ≤0.06–0.5 | 0.125 | 0.5 | 100 |
| ECC (n = 13) | 92.3 | 30.8 | 53.8 | 76.9 | 92.3 | ≤0.06–32 | 1 | 16 | 76.9 | ≤0.06–0.5 | 0.125 | 0.5 | 100 |
| K. pneumoniae (n = 5) | 100 | 100 | 100 | 100 | 80.0 | ≤0.06–0.25 | 0.06 | 0.25 | 100 | ≤0.06–0.25 | 0.125 | 0.25 | 100 |
| Other speciesb (n = 7) | 100 | 28.6 | 42.9 | 100 | 100 | ≤0.06–4 | 0.5 | 4 | 100 | ≤0.06–0.5 | 0.125 | 0.5 | 100 |
| VIM (n = 27) | 92.6 | 11.1 | 48.1 | 88.9 | 100 | ≤0.06–8 | 1 | 4 | 96.3 | ≤0.06–16 | 0.25 | 0.25 | 96.3 |
| E. coli (n = 4) | 100 | 25.0 | 50.0 | 100 | 100 | ≤0.06–2 | 0.313 | 2 | 100 | ≤0.06–16 | 0.188 | 16 | 75.0 |
| K. pneumoniae (n = 21) | 90.5 | 9.5 | 47.6 | 85.7 | 100 | ≤0.06–8 | 1 | 4 | 95.2 | ≤0.06–0.5 | 0.25 | 0.25 | 100 |
| Other speciesb (n = 2) | 100 | 0.0 | 50.0 | 100 | 100 | 0.5–2 | 1.25 | 2 | 100 | ≤0.06–0.25 | 0.155 | 0.25 | 100 |
| KPC (n = 38) | 94.7 | 2.6 | 5.3 | 71.1 | 100 | ≤0.06–16 | 0.75 | 4 | 92.1 | ≤0.06–4 | 0.5 | 1 | 100 |
| OXA-48-like (n = 14) | 85.7 | 21.4 | 7.1 | 85.7 | 100 | ≤0.06–16 | 0.75 | 4 | 92.9 | ≤0.06–2 | 0.25 | 1 | 100 |
| Total (n = 195) | 92.3 | 21.6 | 24.1 | 87.7 | 99.0 | ≤0.06–>32 | 1 | 8 | 87.7 | ≤0.06–16 | 0.25 | 1 | 96.9 |
Avibactam was tested at a fixed concentration of 4 mg/L.
Other species included nine Citrobacter freundii, six Klebsiella oxytoca, and one Klebsiella aerogenes.
AMK, amikacin; ATM, aztreonam; CIP, ciprofloxacin; COL, colistin; ECC, Enterobacter cloacae complex; I, intermediate; MBL, metallo-β-lactamase; MIC, minimum inhibitory concentration; S, susceptibility; TGC, tigecycline.
FIG 1.

In vitro activity of (A) cefiderocol and (B) aztreonam-avibactam against various carbapenemase-producing Enterobacteriaceae. Avibactam was tested at a fixed concentration of 4 mg/L.
Among 24 cefiderocol nonsusceptible CPE, 16 (66.7%) carried NDM, of which 13 carried NDM-1 (seven E. cloacae complex, three E. coli, one K. pneumoniae, one Klebsiella oxytoca, and one Citrobacter freundii) and three carried NDM-5 (all were E. coli). The rest of eight cefiderocol nonsusceptible CPE carried IMP (n = 3), KPC (n = 3), VIM (n = 1), and OXA-181 (n = 1). Fig. S1A demonstrates cefiderocol MIC distributions for different species of MBL CPE. Overall, MBL-producing E. cloacae complex were less susceptible to cefiderocol than MBL-producing E. coli or K. pneumoniae isolates (77.3% [34/44] versus 86.0% [37/43] and 95.0% [38/40]).
ATM-AVI inhibited 95.8% (137/143) of MBL CPE and 100% of KPC CPE and OXA-48-like CPE at the 4/4 mg/L breakpoint. Although KPC CPE was 100% susceptible to ATM-AVI, it demonstrated higher MIC50 of ATM-AVI (0.5 mg/L) than MBL CPE (0.25 mg/L) and OXA-48-like CPE (0.25 mg/L) (Table 1; Fig. 1B). The MIC distributions of ATM-AVI by species of MBL CPE are presented in Fig. S1B. NDM- and VIM-positive E. coli showed elevated MIC90s (8 mg/L and 16 mg/L, respectively) and lower susceptibility to ATM-AVI (77.3% [17/22] and 75.0% [3/4], respectively) compared to MBL CPE of other species. All six ATM-AVI nonsusceptible CPE were E. coli (four NDM-5, one NDM-1, and one VIM-1). Among 24 cefiderocol nonsusceptible CPE isolates, 22 (91.7%) were inhibited by ATM-AVI at the 4/4 mg/L breakpoint. Two E. coli isolates demonstrated high MICs for cefiderocol and ATM-AVI, and both carried NDM carbapenemase (1 NDM-1 and 1 NDM-5).
Clonal relationship of cefiderocol or ATM-AVI nonsusceptible isolates.
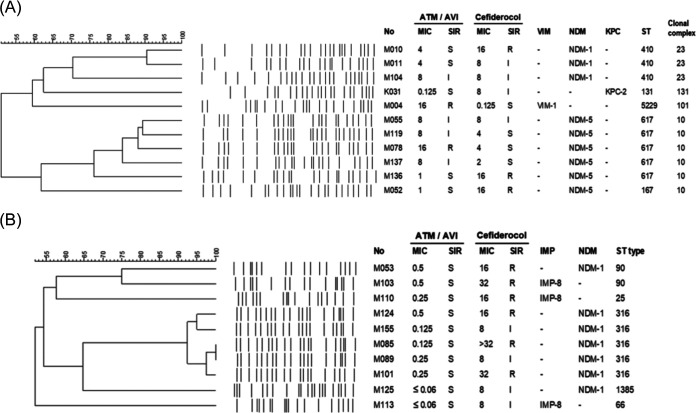
E. coli and E. cloacae complex accounted for the majority of cefiderocol or ATM-AVI nonsusceptible isolates, and NDM was the dominant carbapenemase type. Therefore, we investigated the clonal and epidemiological relatedness of 11 E. coli which were nonsusceptible to cefiderocol (n = 5), ATM-AVI (n = 4), or both (n = 2), as well as the 10 cefiderocol nonsusceptible E. cloacae complex. Among cefiderocol nonsusceptible E. coli, only two NDM-1-positive E. coli belonging to ST410 displayed the same pulsotype. As for ATM-AVI nonsusceptible E. coli, a cluster of four NDM-5-positive E. coli were found, and all belonged to ST617 (Fig. 2A). Two of the four ST617 E. coli isolates (M119 and M137) were from patients who had recently been discharged from two adjacent hospital wards within a 2-month period (Table S1). Among 10 cefiderocol nonsusceptible E. cloacae complex (seven NDM-1 and three IMP-8 producers), five NDM-1-positive isolates with the same pulsotype were assigned ST316, while others showed distinct pulsotype and diverse ST (25, 66, 90, 1385) (Fig. 2B). Analysis of the epidemiologic data showed that three ST316 E. cloacae complex isolates (M89, M101, M124) were from patients who had been admitted to the hematology ward within the same year, but their admission periods did not overlap (Table S1).
FIG 2.
Comparisons of (A) aztreonam-avibactam or cefedirocol nonsusceptible E. coli and (B) cefedirocol nonsusceptible Enterobacter cloacae complex isolates by PFGE dendrogram and MLST. Avibactam was tested at a fixed concentration of 4 mg/L.
Inoculum effect.
Overall, inoculum effects for cefiderocol and ATM-AVI were detected in 95.9% (164/171) and 95.2% (180/189) of susceptible CPE isolates, respectively (Table 2). A switch from susceptible to resistant category was observed in 83.6% (143/171) of isolates for cefiderocol and 94.7% (179/189) for ATM-AVI. The inoculum effect of cefiderocol and ATM-AVI were observed in 98.4% (121/123) and 94.2% (129/137) of MBL CPE isolates, respectively. Compared to MBL CPE, KPC CPE showed a significantly lower rate of inoculum effect for cefiderocol (98.4% [121/123] versus 88.6% [31/35], P = 0.022). The frequency of inoculum effect for cefiderocol or ATM-AVI were comparable among NDM, IMP, and VIM CPE. Over 90% of carbapenemase-producing E. coli, E. cloacae complex, and K. pneumoniae isolates displayed an inoculum effect for cefiderocol, whereas for ATM-AVI, inoculum effect was less commonly detected in carbapenemase-producing E. coli (82.0%, 41/50) than in carbapenemase-producing E. cloacae complex (100%, 44/44) or K. pneumoniae (100%, 79/79) (Table 2).
TABLE 2.
The frequency of inoculum effect among susceptible carbapenemase-producing Enterobacteriaceae isolatesa,b
| Antimicrobial Agent | Total % (n/n) | Metallo-β-Lactamase, % (n/n) |
KPC, % (n/n) | OXA-48-like, % (n/n) |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| All MBL | NDM | IMP | VIM | MBL vs. KPC vs. OXA-48-like | MBL vs. KPC | MBL vs. OXA-48-like | ||||
| Cefiderocol | 95.9 (164/171) | 98.4 (121/123) | 100 (58/58) | 94.5 (37/39) | 100 (26/26) | 88.6 (31/35) | 92.3 (12/13) | 0.027 | 0.022 | 0.262 |
| E. coli (n = 49) | 93.9 (46/49) | 94.6 (35/37) | 100 (16/16) | 88.2 (15/17) | 100 (4/4) | 100 (7/7) | 80.0 (4/5) | |||
| ECC (n = 34) | 100 (34/34) | 100 (34/34) | 100 (24/24) | 100 (10/10) | NA | NA | NA | |||
| K. pneumoniae (n = 74) | 94.6 (70/74) | 100 (38/38) | 100 (13/13) | 100 (5/5) | 100 (20/20) | 85.7 (24/28) | 100 (8/8) | |||
| Aztreonam-avibactam | 95.2 (180/189) | 94.2 (129/137) | 94.2 (65/69) | 92.9 (39/42) | 96.2 (25/26) | 97.4 (37/38) | 100 (14/14) | 0.845 | 0.686 | >0.999 |
| E. coli (n = 50) | 82.0 (41/50) | 78.4 (29/37) | 76.5 (13/17) | 82.4 (14/17) | 66.7 (2/3) | 87.5 (7/8) | 100 (5/5) | |||
| ECC (n = 44) | 100 (44/44) | 100 (44/44) | 100 (31/31) | 100 (13/13) | NA | NA | NA | |||
| K. pneumoniae (n = 79) | 100 (79/79) | 100 (40/40) | 100 (14/14) | 100 (5/5) | 100 (21/21) | 100 (30/30) | 100 (9/9) | |||
The inoculum effect was investigated on CPE isolates with a cefiderocol MIC ≤ 4 mg/L or aztreonam-avibactam MIC ≤ 4/4 mg/L at standard inoculum.
ECC, Enterobacter cloacae complex; MBL, metallo-β-Lactamase; NA, not applicable.
DISCUSSION
This in vitro study demonstrated high susceptibility of MBL CPE to cefiderocol (86.0%) and ATM-AVI (95.8%). CPE with NDM carbapenemase showed higher MICs of cefiderocol and ATM-AVI than those with VIM or IMP. By species, MBL-producing E. cloacae complex were less susceptible to cefiderocol than E. coli or K. pneumoniae, whereas MBL-producing E. coli were more frequently associated with elevated ATM-AVI MICs compared to other species. Over 90% of CPE isolates that were susceptible to cefiderocol or ATM-AVI at standard inoculum exhibited 8-fold or greater increase in MICs at high inoculum.
MBL-producing Enterobacteriaceae were more susceptible to ATM-AVI than cefiderocol in our study. Likewise, a higher susceptibility to ATM-AVI (70.3%) than cefiderocol (45.3%, using the CLSI breakpoint) among MBL CPE was reported by Terrier et al. (19). The detailed β-lactamases profile of MBL producers in Terrier’s work was not provided, but in another study published by the same group, presence of extended-spectrum β-lactamases such as BEL and SHV conferred a greater impact on cefiderocol MICs than ATM-AVI MICs among E. coli (20). Furthermore, Kocer et al. showed that the acquisition of extrachromosomal fec operon in conjunction with VIM-type MBL could lead to a significant elevation of the cefiderocol MIC (21). These results indicated that the presence of transferable resistance determinant or other β-lactamases among MBL CPE might affect their susceptibilities to ATM-AVI or cefiderocol.
Isolates harboring NDM carbapenemases demonstrated higher cefiderocol MICs than other CPE. This finding was in line with previous reports (22, 23). Mechanisms responsible for cefiderocol resistance among NDM CPE included increased expression of blaNDM gene (11) and mutation in cirA siderophore receptor or its regulators (24, 25). Several previous studies reported that NDM-5 was the NDM variant correlated with cefiderocol resistance (11, 22, 26). NDM-5 showed increased carbapenemase activity than NDM-1 due to the V88L substitution (27). In our study, NDM-1 but not NDM-5 was the main NDM variant of cefiderocol nonsusceptible NDM CPE, including a cluster of five E. cloacae complex strain ST316. In addition, the E. coli strain ST167, ST410, and ST617 identified in our cefiderocol nonsusceptible NDM producers were the widely distributed sequence types across countries (28). Among them, ST410 had been identified as the predominant strain of NDM-positive E. coli isolates in our institute (29). Although our investigation of the epidemiologic relationship among the cefiderocol nonsusceptible E. coli or E. cloacae complex isolates did not support a hospital outbreak, the increase in prevalence of cefiderocol nonsusceptibility among Enterobacteriaceae via the dissemination of certain bacterial clones is worthy of potential concern.
MBL-producing E. cloacae complex showed lower susceptibility to cefiderocol compared to MBL-producing E. coli or K. pneumoniae in our study. These cefiderocol nonsusceptible E. cloacae complex carried IMP-8 or NDM-1, and six different ST were identified among them. To date, no specific sequence type of E. cloacae complex was associated with cefiderocol resistance. E. cloacae complex could harbor a variety of antibiotic resistance gene, and epidemic carbapenem-resistant clones of E. cloacae complex such as ST78, ST171, and ST114 had emerged (30, 31). Therefore, the trend of cefiderocol nonsusceptibility in E. cloacae complex should be monitored further after the wide use of this novel agent.
ATM-AVI demonstrated potent in vitro activity against MBL CPE or cefiderocol nonsusceptible CPE. In one systemic review, 20.4% of MBL-producing Enterobacterales had MIC >4 mg/L for ATM when combined with AVI or CAZ-AVI, and 31 (93.9%) of them carried NDM carbapenemase (32). The susceptibility to ATM-AVI has not been compared between different MBLs. Our study showed that NDM-positive E. coli had the highest MIC50 of ATM-AVI compared to VIM CPE, IMP CPE, or other species of NDM CPE. In addition, a cluster of four ATM-AVI nonsusceptible NDM-5-positive E. coli ST617 was found. NDM-5-positive E. coli with reduced susceptibility to ATM-AVI had been discovered worldwide, and molecular typing showed diverse clonal background (33). More than 10 sequence types were identified, including ST617 (33). The spread of these ATM-AVI-resistant clones could be a concern in clinical practice. Although ATM-AVI showed promising in vitro activity against MBL CPE and may serve as an option for cefiderocol nonsusceptible Enterobacteriaceae, cautions should be taken while prescribing ATM-AVI in area with high prevalence of NDM producers, especially when antimicrobial susceptibility test for ATM-AVI was not available.
The inoculum size of CPE substantially impacted the MICs of cefiderocol. In studies by Hobson et al. and Danjean et al., the inoculum effect of cefiderocol against CPE occurred in 37/37 of KPC-producing Enterobacteriaceae and 39/40 of CPE (including 11 NDM- and eight VIM-producing isolates), respectively (16, 17). Our results were consistent with theirs that 95.9% of CPE exhibited inoculum effect on cefiderocol. Moreover, we found inoculum effect on cefiderocol more commonly occurred among MBL CPE isolates than KPC CPE. For ATM-AVI, 95.2% susceptible CPE exhibited inoculum effect in our study, which was much higher than the report by Kim et al. (15/35, 42.9%) (18). The reason leading to this discrepancy was unclear. However, both of ours and Kim’s studies revealed that carbapenemase-producing K. pneumoniae isolates exhibited a higher rate of ATM-AVI inoculum effect than E. coli. In the literature, variations in PBP3 enzymes and carbapenemase activity, or the production of other β-lactamases among different species of Enterobacteriaceae might all affect the magnitude of inoculum effect (14, 34, 35). The presence of inoculum effects suggested potential benefits of higher doses or combination therapy to combat CPE infections with high bacterial burden. In vivo or clinical studies are warrants to confirm the correlation between high inoculum and failure of cefiderocol or ATM-AVI monotherapy. In most cases, amikacin, tigecycline, or colistin would be the options of companion drug for CPE infections if based on in vitro susceptibilities. Further investigations are also needed to identify the superior combination regimen for MBL CPE infections.
This study has several limitations. First, the CPE isolates were from one single center, thus the distribution of bacteria species and MBL types may not be representative. Second, we did not investigate other molecular mechanisms which were associated with elevated cefiderocol or ATM-AVI MICs, or the β-lactamases that might impact the inoculum effect. Third, not all CPE isolates were tested for their MICs at high inoculum. The inoculum effect of CPE against cefiderocol or ATM-AVI might be more significant if all isolates were included.
In conclusion, cefiderocol and ATM-AVI showed potent in vitro activity against MBL-producing Enterobacteriaceae. NDM producer had lower susceptibility to cefiderocol and ATM-AVI compared to VIM or IMP producers. For most CPE isolates, inoculum effects on cefiderocol and ATM-AVI were observed. Our results suggested a potential risk of microbiological failure when using monotherapy with these newer antibiotics to treat CPE infections with high bacteria burden.
MATERIALS AND METHODS
Hospital setting and bacterial isolates.
This study was conducted at National Taiwan University Hospital (NTUH), a 2,200-bed tertiary care center in Northern Taiwan. At NTUH, nonduplicate meropenem-resistant (MIC ≥4 mg/L, according to Clinical and Laboratory Standards Institute [CLSI] 2021 criteria [36]) Enterobacteriaceae isolates were prospectively screened for the carriage of carbapenemase-encoding genes by PCR since 2013 based on NTUH infection control policy, and isolates positive for carbapenemase-encoding genes were stored. The susceptibility to meropenem was determined with Vitek 2 automated system (BioMé rieux, Marcy l’Etoile, France).
In the present study, all Enterobacteriaceae carrying MBL genes or OXA-48-like genes, and randomly selected KPC-producing Enterobacteriaceae (with a one in 10 ratio) collected during 2016 to 2021 at NTUH were included and subjected to in vitro susceptibility studies. Isolates carrying dual-carbapenemase genes were excluded from the analysis. These Enterobacteriaceae isolates were isolated from various types of clinical specimens and anal screening cultures. We also recorded the specimen collection date, the type and location of patient ward, and the comorbidities of the source patient. The species identification were performed with Bruker Biotyper matrix assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) system (Bruker Corporation, Billerica, MA, USA). The study was approved by the NTUH Research Ethics Committee (registration number 201912161RINC) and written or oral informed consent was waived.
Detection of carbapenemase-encoding genes.
Bacterial DNA was extracted using Viogene DNA extraction kit (Blood Genomic DNA Extraction Midiprep System; Viogene, Taipei, Taiwan) following the manufacturer’s instruction. Genes encoding for carbapenemases, including class A (blaKPC), class B (blaIMP, blaVIM, blaNDM), and class D (blaOXA-48-like) were detected by PCR using the primers described previously (37, 38). All PCR amplicons were sequenced by Sanger sequencing. The sequencing results were compared to the National Center for Biotechnology Information (NCBI) database at https://blast.ncbi.nlm.nih.gov/Blast.cgi to determine the subtypes of carbapenemase. In this study, Enterobacteriaceae isolates positive for the carbapenemase-encoding genes by PCR tests were considered CPE.
Molecular typing.
Pulsed-field gel electrophoresis (PFGE) was performed as previously described method (29). Genomic DNA of bacterial isolates were prepared in agarose plugs and digested with the restriction enzyme Spel (New England Biolabs, NEB, Beverly, MA, USA). The PFGE banding patterns were analyzed by BioNumerics software version 8.0 (Applied Maths, Sint-Martens-Latem, Belgium). Isolates that exhibited similarity of ≥80% of their banding patterns were considered as closely related strains. Multilocus sequence typing (MLST) with housekeeping genes of E. coli (including adk, fumC, gyrB, icd, mdh, purA, and recA) and Enterobacter cloacae (including dnaA, fusA, gyrB, leuS, pyrG, rplB, and rpoB) were performed according to previously described protocol (39–41). Information from the PubMLST website https://pubmlst.org/organisms were used to determine the sequence type (ST) of E. coli and E. cloacae isolates.
Antimicrobial susceptibility testing.
The MICs of each CPE isolate to amikacin, aztreonam, ciprofloxacin, colistin, and tigecycline were determined by commercialized broth microdilution using Sensititre 96-well plates (GNX2F; Trek Diagnostic Systems, East Grinstead, United Kingdom). The MICs of each CPE isolate to cefiderocol and ATM-AVI were determined by broth microdilution method and the results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) 2021 criteria (36). Cefiderocol was tested using iron-depleted cation-adjusted Mueller-Hinton broth prepared following the CLSI guidance (36). Cefiderocol and avibactam were obtained from Shionogi & Co., Ltd. (Osaka, Japan). Currently, ATM-AVI does not have approved susceptibility breakpoints for Enterobacteriaceae, thus the CLSI susceptible breakpoint for aztreonam (MIC ≤ 4 mg/L) for Enterobacteriaceae was taken as reference to interpret the results at with a fixed concentration of avibactam at 4 mg/L in this study. For tigecycline, the U.S. Food and Drug Administration susceptibility breakpoint (MIC ≤ 2 mg/L) for Enterobacteriaceae was used. For isolates that were susceptible to cefiderocol or having an ATM-AVI MIC ≤ 4/4 mg/L at standard inoculum (105 CFU [CFU]/mL), the MICs at high inoculum (107 CFU/mL) would be determined. The in vitro inoculum effect was defined as a ≥8-fold MIC increase at the high inoculum (14).
Statistical analysis.
The difference in antimicrobial susceptibility or inoculum effect between groups were compared using the chi-squared test or Fisher’s exact test. All P values were two-sided, and a P value <0.05 was considered statistically significant. Statistical analyses were performed using Stata software (version 11; StataCorp, College Station, TX, USA).
Ethical approval statement.
The study was approved by the NTUH Research Ethics Committee (registration number 201912161RINC) and written or oral informed consent was waived.
Data availability.
Deidentified participant-level data will be available on publication of the study. Requests for data should be sent to the corresponding author by email and, on review of the proposed protocol and signing of a data sharing agreement, the data will be made available.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by the National Taiwan University Hospital, Taipei, Taiwan (grant number NTUH110-35) and Ministry of Science and Technology, Taiwan (grant number MOST110-2314-B-002-251). The sponsors had no role in the study design, data collection and analysis, manuscript preparation, or the decision to submit for publication.
The authors have no conflicts of interest to declare.
Conceived and designed the experiments: Y.-S.H.; acquisition of data: Y.-S.H., P.-C.C.; analysis of data: Y.-S.H., P.-C.C., P.-Y. C.; drafting and revision of the manuscript: Y.-S.H., J.-T. W.
Footnotes
Supplemental material is available online only.
Contributor Information
Jann-Tay Wang, Email: wang.jt1968@gmail.com.
Pablo Power, Universidad de Buenos Aires.
Dennis Nurjadi, Universitat zu Lubeck.
REFERENCES
- 1.van Duin D, Doi Y. 2017. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 8:460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nordmann P, Poirel L. 2019. Epidemiology and diagnostics of carbapenem resistance in Gram-negative bacteria. Clin Infect Dis 69:S521–s528. doi: 10.1093/cid/ciz824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević AT, Cantón R, Carmeli Y, Friedrich AW, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Nordmann P, Poirel L, Rossolini GM, Seifert H, Vatopoulos A, Walsh T, Woodford N, Monnet DL, European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group . 2017. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis 17:153–163. doi: 10.1016/S1473-3099(16)30257-2. [DOI] [PubMed] [Google Scholar]
- 4.Sandfort M, Hans JB, Fischer MA, Reichert F, Cremanns M, Eisfeld J, Pfeifer Y, Heck A, Eckmanns T, Werner G, Gatermann S, Haller S, Pfennigwerth N. 2022. Increase in NDM-1 and NDM-1/OXA-48-producing Klebsiella pneumoniae in Germany associated with the war in Ukraine, 2022. Euro Surveill 27. doi: 10.2807/1560-7917.ES.2022.27.50.2200926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doi Y. 2019. Treatment options for carbapenem-resistant Gram-negative bacterial infections. Clin Infect Dis 69:S565–s575. doi: 10.1093/cid/ciz830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guducuoglu H, Gursoy NC, Yakupogullari Y, Parlak M, Karasin G, Sunnetcioglu M, Otlu B. 2018. Hospital outbreak of a colistin-resistant, NDM-1- and OXA-48-producing Klebsiella pneumoniae: high mortality from pandrug resistance. Microb Drug Resist 24:966–972. doi: 10.1089/mdr.2017.0173. [DOI] [PubMed] [Google Scholar]
- 7.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. 2022. Infectious Diseases Society of America 2022 Guidance on the treatment of extended-spectrum β-lactamase producing enterobacterales (ESBL-E), carbapenem-resistant enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P aeruginosa). Clin Infect Dis 75:187–212. doi: 10.1093/cid/ciac268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamano Y. 2019. In vitro activity of cefiderocol against a broad range of clinically important Gram-negative bacteria. Clin Infect Dis 69:S544–s551. doi: 10.1093/cid/ciz827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mushtaq S, Sadouki Z, Vickers A, Livermore DM, Woodford N. 2020. In vitro activity of cefiderocol, a siderophore cephalosporin, against multidrug-resistant Gram-negative bacteria. Antimicrob Agents Chemother 64. doi: 10.1128/AAC.01582-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasoo S, Cunningham SA, Cole NC, Kohner PC, Menon SR, Krause KM, Harris KA, De PP, Koh TH, Patel R. 2015. In vitro activities of ceftazidime-avibactam, aztreonam-avibactam, and a panel of older and contemporary antimicrobial agents against carbapenemase-producing Gram-negative bacilli. Antimicrob Agents Chemother 59:7842–7846. doi: 10.1128/AAC.02019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simner PJ, Mostafa HH, Bergman Y, Ante M, Tekle T, Adebayo A, Beisken S, Dzintars K, Tamma PD. 2021. Progressive development of cefiderocol resistance in Escherichia coli during therapy is associated with increased blaNDM-5 copy number and gene expression. Clin Infect Dis 75:47–54. doi: 10.1093/cid/ciab888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alm RA, Johnstone MR, Lahiri SD. 2015. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: role of a novel insertion in PBP3. J Antimicrob Chemother 70:1420–1428. doi: 10.1093/jac/dku568. [DOI] [PubMed] [Google Scholar]
- 13.Miller WR, Seas C, Carvajal LP, Diaz L, Echeverri AM, Ferro C, Rios R, Porras P, Luna C, Gotuzzo E, Munita JM, Nannini E, Carcamo C, Reyes J, Arias CA. 2018. The cefazolin inoculum effect is associated with increased mortality in methicillin-susceptible Staphylococcus aureus bacteremia. Open Forum Infect Dis 5:ofy123. doi: 10.1093/ofid/ofy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenhard JR, Bulman ZP. 2019. Inoculum effect of β-lactam antibiotics. J Antimicrob Chemother 74:2825–2843. doi: 10.1093/jac/dkz226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang CI, Cha MK, Kim SH, Wi YM, Chung DR, Peck KR, Lee NY, Song JH. 2014. Extended-spectrum cephalosporins and the inoculum effect in tests with CTX-M-type extended-spectrum β-lactamase-producing Escherichia coli: potential clinical implications of the revised CLSI interpretive criteria. Int J Antimicrob Agents 43:456–459. doi: 10.1016/j.ijantimicag.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Danjean M, Hobson CA, Gits-Muselli M, Courroux C, Monjault A, Bonacorsi S, Birgy A. 2022. Evaluation of the inoculum effect of new antibiotics against carbapenem-resistant enterobacterales. Clin Microbiol Infect 28:1503.e1–1503.e3. doi: 10.1016/j.cmi.2022.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Hobson CA, Cointe A, Jacquier H, Choudhury A, Magnan M, Courroux C, Tenaillon O, Bonacorsi S, Birgy A. 2021. Cross-resistance to cefiderocol and ceftazidime-avibactam in KPC β-lactamase mutants and the inoculum effect. Clin Microbiol Infect 27:1172.e7–1172.e10. doi: 10.1016/j.cmi.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Kim T, Lee SC, Bae M, Sung H, Kim MN, Jung J, Kim MJ, Kim SH, Lee SO, Choi SH, Kim YS, Chong YP. 2020. In vitro activities and inoculum effects of ceftazidime-avibactam and aztreonam-avibactam against carbapenem-resistant Enterobacterales isolates from South Korea. Antibiotics (Basel) 9:912. doi: 10.3390/antibiotics9120912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Terrier C, Nordmann P, Poirel L. 2022. In vitro activity of aztreonam in combination with newly developed β-lactamase inhibitors against MDR Enterobacterales and Pseudomonas aeruginosa producing metallo-β-lactamases. J Antimicrob Chemother 78:101–107. doi: 10.1093/jac/dkac360. [DOI] [PubMed] [Google Scholar]
- 20.Poirel L, Ortiz de la Rosa JM, Sadek M, Nordmann P. 2022. Impact of acquired broad-spectrum β-lactamases on susceptibility to cefiderocol and newly developed β-lactam/β-lactamase inhibitor combinations in Escherichia coli and Pseudomonas aeruginosa. Antimicrob Agents Chemother 66:e0003922. doi: 10.1128/aac.00039-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocer K, Boutin S, Heeg K, Nurjadi D. 2022. The acquisition of transferable extrachromosomal fec operon is associated with a cefiderocol MIC increase in Enterobacterales. J Antimicrob Chemother 77:3487–3495. doi: 10.1093/jac/dkac347. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Jin L, Sun S, Yin Y, Wang R, Chen F, Wang X, Zhang Y, Hou J, Zhang Y, Zhang Z, Luo L, Guo Z, Li Z, Lin X, Bi L, Wang H. 2022. Occurrence of high levels of cefiderocol resistance in carbapenem-resistant Escherichia coli before its approval in China: a report from China CRE-Network. Microbiol Spectr 10:e0267021. doi: 10.1128/spectrum.02670-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang C, Yang D, Wang Y, Ni W. 2022. Cefiderocol for the treatment of multidrug-resistant Gram-negative bacteria: a systematic review of currently available evidence. Front Pharmacol 13:896971. doi: 10.3389/fphar.2022.896971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nurjadi D, Kocer K, Chanthalangsy Q, Klein S, Heeg K, Boutin S. 2022. New Delhi metallo-beta-lactamase facilitates the emergence of cefiderocol resistance in Enterobacter cloacae. Antimicrob Agents Chemother 66:e0201121. doi: 10.1128/AAC.02011-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElheny CL, Fowler EL, Iovleva A, Shields RK, Doi Y. 2021. In vitro evolution of cefiderocol resistance in an NDM-producing Klebsiella pneumoniae due to functional loss of CirA. Microbiol Spectr 9:e0177921. doi: 10.1128/Spectrum.01779-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein S, Boutin S, Kocer K, Fiedler MO, Störzinger D, Weigand MA, Tan B, Richter D, Rupp C, Mieth M, Mehrabi A, Hackert T, Zimmermann S, Heeg K, Nurjadi D. 2022. Rapid development of cefiderocol resistance in carbapenem-resistant enterobacter cloacae during therapy is associated with heterogeneous mutations in the catecholate siderophore receptor cirA. Clin Infect Dis 74:905–908. doi: 10.1093/cid/ciab511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hornsey M, Phee L, Wareham DW. 2011. A novel variant, NDM-5, of the New Delhi metallo-β-lactamase in a multidrug-resistant Escherichia coli ST648 isolate recovered from a patient in the United Kingdom. Antimicrob Agents Chemother 55:5952–5954. doi: 10.1128/AAC.05108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poirel L, Ortiz de la Rosa JM, Sakaoglu Z, Kusaksizoglu A, Sadek M, Nordmann P. 2022. NDM-35-producing ST167 Escherichia coli highly resistant to β-lactams including cefiderocol. Antimicrob Agents Chemother 66:e0031122. doi: 10.1128/aac.00311-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang YS, Tsai WC, Li JJ, Chen PY, Wang JT, Chen YT, Chen FJ, Lauderdale TL, Chang SC. 2021. Increasing New Delhi metallo-β-lactamase-positive Escherichia coli among carbapenem non-susceptible Enterobacteriaceae in Taiwan during 2016 to 2018. Sci Rep 11:2609. doi: 10.1038/s41598-021-82166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Simmonds A, Annavajhala MK, Wang Z, Macesic N, Hu Y, Giddins MJ, O’Malley A, Toussaint NC, Whittier S, Torres VJ, Uhlemann A-C. 2018. Genomic and geographic context for the evolution of high-risk carbapenem-resistant enterobacter cloacae complex clones ST171 and ST78. mBio 9. doi: 10.1128/mBio.00542-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Annavajhala MK, Gomez-Simmonds A, Uhlemann AC. 2019. Multidrug-resistant enterobacter cloacae complex emerging as a global, diversifying threat. Front Microbiol 10:44. doi: 10.3389/fmicb.2019.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mauri C, Maraolo AE, Di Bella S, Luzzaro F, Principe L. 2021. The revival of aztreonam in combination with avibactam against metallo-β-lactamase-producing Gram-negatives: a systematic review of in vitro studies and clinical cases. Antibiotics (Basel) 10:1012. doi: 10.3390/antibiotics10081012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadek M, Ruppé E, Habib A, Zahra R, Poirel L, Nordmann P. 2021. International circulation of aztreonam/avibactam-resistant NDM-5-producing Escherichia coli isolates: successful epidemic clones. J Glob Antimicrob Resist 27:326–328. doi: 10.1016/j.jgar.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Adler A, Ben-Dalak M, Chmelnitsky I, Carmeli Y. 2015. Effect of resistance mechanisms on the inoculum effect of carbapenem in Klebsiella pneumoniae isolates with borderline carbapenem resistance. Antimicrob Agents Chemother 59:5014–5017. doi: 10.1128/AAC.00533-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyazaki H, Horii T, Nagura O, Suda T, Chida K, Nakamura H. 2009. Effect of the inoculum size on carbapenem susceptibilities of beta-lactamase-negative, ampicillin-resistant Haemophilus influenzae. Curr Microbiol 58:18–24. doi: 10.1007/s00284-008-9259-9. [DOI] [PubMed] [Google Scholar]
- 36.CLSI. 2021. Performance standards for antimicrobial susceptibility testing. 30th ed CLSI supplement M100. CLSI. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 37.Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis 70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Paul D, Dhar Chanda D, Maurya AP, Mishra S, Chakravarty A, Sharma GD, Bhattacharjee A. 2015. Co-Carriage of blaKPC-2 and blaNDM-1 in clinical isolates of Pseudomonas aeruginosa associated with hospital infections from India. PLoS One 10:e0145823. doi: 10.1371/journal.pone.0145823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirth T, Falush D, Lan R, Colles F, Mensa P, Wieler LH, Karch H, Reeves PR, Maiden MC, Ochman H, Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol Microbiol 60:1136–1151. doi: 10.1111/j.1365-2958.2006.05172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyoshi-Akiyama T, Hayakawa K, Ohmagari N, Shimojima M, Kirikae T. 2013. Multilocus sequence typing (MLST) for characterization of Enterobacter cloacae. PLoS One 8:e66358. doi: 10.1371/journal.pone.0066358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.García-González T, Sáenz-Hidalgo HK, Silva-Rojas HV, Morales-Nieto C, Vancheva T, Koebnik R, Ávila-Quezada GD. 2018. Enterobacter cloacae, an emerging plant-pathogenic bacterium affecting chili pepper seedlings. Plant Pathol J 34:1–10. doi: 10.5423/PPJ.OA.06.2017.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.00569-23-s0001.docx, DOCX file, 0.6 MB (652.6KB, docx)
Data Availability Statement
Deidentified participant-level data will be available on publication of the study. Requests for data should be sent to the corresponding author by email and, on review of the proposed protocol and signing of a data sharing agreement, the data will be made available.