Abstract
Background
To improve patient tolerability and satisfaction as well as minimize complications, procedural sedation has been widely used. Propofol is the most widely used agent for induction of anesthesia and sedation by anesthesiologists. With a different mechanism compared to propofol, remimazolam is a new short-acting GABA-A receptor agonist. It is an ester-based benzodiazepine. This meta-analysis aims to clarify the efficacy and safety of remimazolam versus propofol for procedure sedation.
Methods
Electronic databases were searched for randomized controlled trials (RCTs) comparing efficacy or safety of remimazolam versus propofol. Meta-analysis were conducted using RStudio with “metafor” package with random-effects model.
Results
A total of twelve RCTs were included in the meta-analysis. The pooled results demonstrated that patients with remimazolam for procedural sedation had lower risk of bradycardia (OR 0.28, 95% CI [0.14–0.57]), hypotension (OR 0.26, 95% CI [0.22–0.32]), and respiratory depression (OR 0.22, 95% CI [0.14–0.36]). There was no difference in the risk of developing postoperative nausea and vomiting (PONV) (OR 0.65, 95% CI [0.15–2.79]) and dizziness (OR 0.93, 95% CI [0.53–1.61]) between the remimazolam and propofol groups. Using remimazolam for procedural sedation is significantly associated with less injection pain compared to propofol (OR 0.06, 95% CI [0.03–0.13]). Regarding the sedation efficacy, there was no difference in sedation success rate or time to loss of consciousness, recover and discharge between the remimazolam and the propofol groups.
Conclusions
Based on our meta-analysis, patients receiving procedural sedation with remimazolam had lower risk of bradycardia, hypotension, respiratory depression and injection pain compared with propofol. On the other hand, there was no difference in sedation success rate, risk of PONV, dizziness, time to LOC, recovery and discharge between these two sedatives.
PROSPERO registration number
CRD42022362950
Keywords: Remimazolam, Propofol, Procedural sedation, Meta-analysis
Introduction
Advancements in the medical care system in the 21st century have helped to increase accessibility to health care services, including medical procedures (e.g., colonoscopy Stock, Haug & Brenner, 2010, gastrointestinal endoscopy Zagari et al., 2016, and hysteroscopy Orlando & Bradley, 2022) that are now more often performed worldwide. During procedures, patients may feel anxiety, fear, and physical or emotional stress due to discomfort or pain, and such distress can lead to complications or unfavorable outcomes (Morrison et al., 1998; Zubarik et al., 2002). Procedural sedation has been widely used to improve patient tolerability and satisfaction and subsequently minimize these complications (Tobias & Leder, 2011).
Propofol is the most widely used agent for inducing anesthesia and sedation by anesthesiologists (Fulton & Sorkin, 1995; Trapani et al., 2000). Propofol is a potent intravenous sedative/hypnotic agent with a rapid onset of action and an extremely short half-life, indicating rapid awakening and quick recovery of cognitive functions after anesthesia or sedation (Fulton & Sorkin, 1995; Trapani et al., 2000). However, several propofol-associated adverse events have been reported in the literature, such as hypotension, bradycardia, respiratory depression, and injection pain (Marik, 2004; Qadeer et al., 2005; Jalota et al., 2011; Newstead et al., 2013).
Remimazolam is a new short-acting GABA-A receptor agonist that can address the distinct mechanisms associated with propofol; it is an ester-based benzodiazepine (BZD) and can be rapidly hydrolyzed into inactive metabolites by tissue esterases (Lee & Shirley, 2021). The onset of remimazolam also takes approximately 1–3 min and has a short metabolic half-life, thereby providing adequate moderate sedation but faster recovery after intervention (Lee & Shirley, 2021). A recent published meta-analysis reported that remimazolam had a lower success rate for sedation or anesthesia and a lower incidence of adverse events than propofol (Zhang et al., 2022a). This review by Zhang et al. (2022a) included not only patients who underwent procedural sedation but also general surgery and heart surgery, but conceptual heterogeneity was encountered because of the great variations in the included studies. Other emerging studies have reported the better suitability of remimazolam for procedural sedation compared with propofol (Guo et al., 2022a; Guo et al., 2022b; Qiu et al., 2022; Yao et al., 2022). In the present study, our aim was to conduct a systematic review and meta-analysis to update and clarify the efficacy and safety of remimazolam against propofol for procedural sedation.
Method
We performed a systematic review and meta-analysis following the Cochrane Handbook for Systematic Reviews of Interventions (Higgins et al., 2021) and presented our findings according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Our study was registered as CRD42022362950 on PROSPERO.
Study selection
Yun-Ting Huang and Yu Chang independently searched electronic databases, including PubMed, Embase, and Cochrane Library, from January 1900 to December 2022 to identify relevant studies. In cases of discrepancies, a consensus was reached with senior reviewers, specifically Yen-Ta Huang Additional details about the search are shown in Method S1.
Eligibility criteria
The three predefined criteria for evidence selection were as follows: (1) randomized controlled trials (RCTs); (2) studies that involved adult patients older than 18 years who underwent medical procedures with procedural sedation using propofol or remimazolam; and (3) studies that reported at least one comparative outcome of remimazolam and propofol.
Exclusion criteria
The studies were considered ineligible when any of the following criteria were met: (1) they took the form of review articles, case reports, case series, retrospective data analyses, or nonrandomized prospective studies; (2) data were unavailable or irrelevant for the meta-analysis; (3) the trials compared other sedatives in the control group instead of propofol; and (4) they were duplicate publications.
Data extraction
Two investigators (Yun-Ting Huang and Yu Chang) independently extracted relevant information from eligible articles, including (1) first author’s name; (2) publication year; (3) country; (4) study period; (5) American Society of Anesthesiology (ASA) score; (6) sample size; (7) procedure type; and (8) protocol of procedural sedation. Any discrepancy was addressed by reaching a consensus with senior reviewers (Yen-Ta Huang).
Quality assessment
Two investigators (Yun-Ting Huang and Yu Chang) independently completed a critical appraisal of the included trials using Cochrane Risk of Bias 2.0 (ROB 2.0) (Sterne et al., 2019). Any discrepancy was addressed by consulting a third investigator (Yen-Ta Huang).
Primary and secondary outcomes
Our main objective was to compare the safety of remimazolam and propofol by analyzing the incidence of adverse events following their administration. Additionally, we assessed the efficacy of these two sedatives by measuring their success rate, time to sedation, and postprocedural recovery as secondary outcomes.
Statistical analysis
Meta-analyses were conducted using RStudio with the “metafor” package (Viechtbauer, 2010) (Method S2). For binary outcomes, we extracted the odds ratios (ORs) from the included studies and pooled them by using a random-effects model with the Mantel–Haenszel method. The pooled OR was presented with 95% confidence intervals (CIs) and p values.
For the continuous outcomes, we extracted the mean and standard deviation (SD) from the included studies and pooled the results by using a random-effects model with the inverse variance method. The pooled results of the continuous outcomes were summarized with weighted mean difference (WMD) and presented with 95% CIs and p values.
Heterogeneity was assessed using I-square, with values of I2 < 25%, 25% < I2 < 50%, and I2 > 50%, indicating low, moderate, and high heterogeneity, respectively (Higgins, Deeks & Altman, 2003).
Results
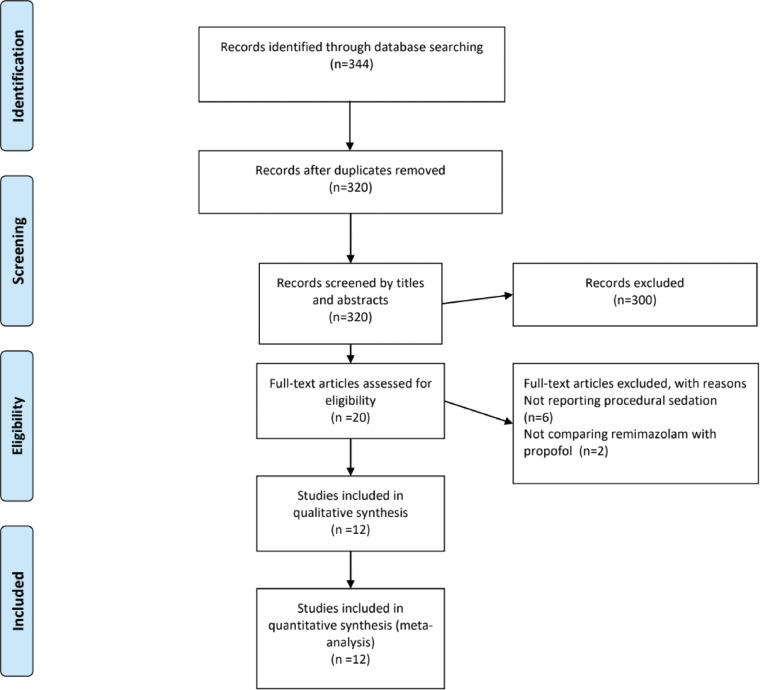
Our search strategy identified 344 references from the electronic databases, with 20 studies for full-text inspection. Ultimately, we included 12 studies (Guo et al., 2022a; Guo et al., 2022b; Qiu et al., 2022; Yao et al., 2022; Cao et al., 2022; Chen et al., 2020; Chen et al., 2021; Shi et al., 2022; Tan et al., 2022; Zhang et al., 2022b; Zhang, Li & Liu, 2021; Lu et al., 2022) for qualitative and quantitative syntheses (Fig. 1).
Figure 1. PRISMA flowchart diagram.
We initially extracted 344 potential references. Screening the titles and abstracts yielded 20 full-text articles, the eligibility of which was assessed. Eventually, 12 studies were included for qualitative and quantitative syntheses. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Characteristics of the included studies
Table 1 shows that 12 RCTs involving 2,170 patients who underwent medical procedures with procedural sedation (remimazolam = 1,119; propofol = 1,051) were enrolled between 1987 and 2019. Among the 12 RCTs, five studies included patients who underwent colonoscopy, three included patients who underwent upper gastrointestinal (UGI) endoscopy, two included patients who underwent hysteroscopy, one included patients who underwent endoscopic submucosal dissection, and one included patients who underwent endoscopic variceal ligation.
Table 1. Characteristics of included studies.
| Study | Procedures | ASA | Patient number | Intervention |
|---|---|---|---|---|
| Zhang et al. (2022b) | Hysteroscopy | I–II | 90 | Propofol 5 mg/kg/h (n = 30) |
| Remit 0.48 mg/kg/h (n = 30) | ||||
| Remit 0.6 mg/kg/h (n = 30) | ||||
| Guo et al. (2022a) | Gastrointestinal endoscopy | I–II | 77 | Propofol 1.5 mg/kg (n = 38) |
| Remit 0.15 mg/kg (n = 39) | ||||
| Guo et al. (2022b) | Colonoscopy | I–II | 248 | Propofol 1.5 mg/kg (n = 124) |
| Remin 5 mg/kg (n = 124) | ||||
| Shi et al. (2022) | Endoscopic Variceal Ligation | II–III | 76 | Propofol 4–10 mg/kg/h (n = 38) |
| Remit 1–2 mg/kg/h (n = 38) | ||||
| Zhang, Li & Liu (2021) | Hysteroscopy | I–II | 82 | Propofol 3–6 mg/kg/h (n = 41) |
| Remib 1 mg/kg/h (n = 41) | ||||
| Chen et al. (2020) | Colonoscopy | I–II | 384 | Propofol 1.5 mg/kg (n = 190) |
| Remit 5.0 mg (n = 194) | ||||
| Chen et al. (2020) | Upper gastrointestinal endoscopy | I–II | 378 | Propofol 1.5 mg/kg (n = 189) |
| Remit 5.0 mg (n = 189) | ||||
| Cao et al. (2022) | Gastroscopy: | II–III | 148 | Propofol: 2 mg/kg (n = 74) |
| Remit: 0.107 mg/kg (n = 74) | ||||
| (Sufentanil 0.15 µg/kg) | ||||
| Qiu et al. (2022) | Endoscopic submucosal dissection | I–III | 56 | Propofol: 2.0 mg/kg (n = 28) |
| Remin: 0.3 mg/kg (n = 28) | ||||
| Tan et al. (2022) | Gastrointestinal endoscopy | I–II | 99 | Propofol 1–1.5 mg/kg (n = 33) |
| Remit 0.1 mg/kg (n = 33) | ||||
| Remit 0.2 mg/kg (n = 33) | ||||
| Yao et al. (2022) | Colonoscopy | I–II | 132 | Propofol 1 mg/kg (n = 66) |
| Remin 0.2 mg/kg (n = 66) | ||||
| Lu et al. (2022) | Upper gastrointestinal endoscopy | I–III | 400 | Propofol 3 g/h (n = 200) |
| Remit: 300 mg/h (n = 200) |
Notes.
- ASA
- American Society of Anesthesiologists classification
- remib
- remimazolam besilate
- remit
- remimazolam tosilate
- remin
- substituent not reported
Risk of bias assessments
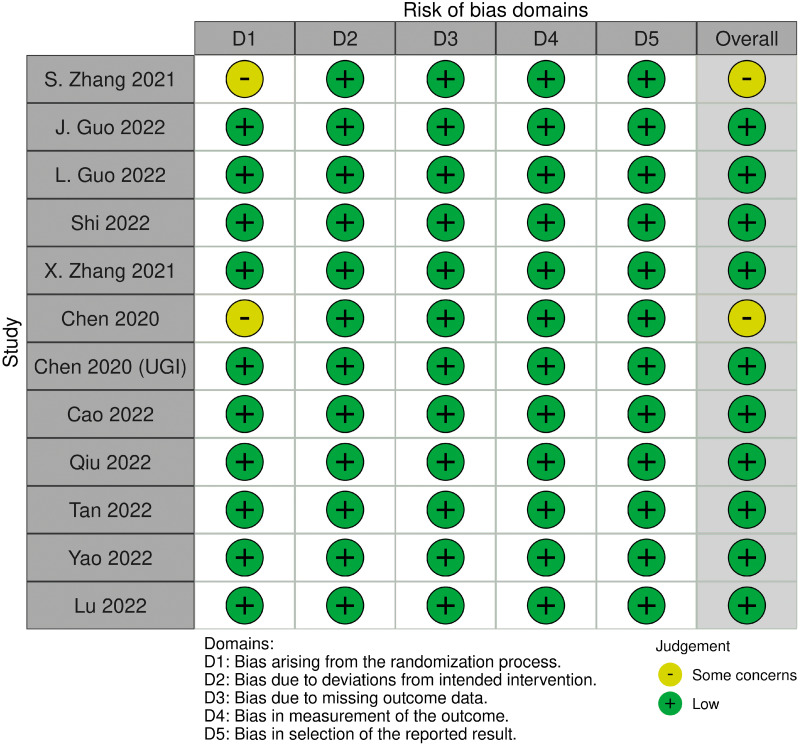
Using ROB 2.0 for quality of assessment of the included studies, we identified 10 of the included trials as having a low risk of bias. We encountered limitations in evaluating the risks of bias in the other two studies because of bias in randomization (Fig. 2).
Figure 2. Visual summary of risk of bias using ROB 2.0 evaluation tool.
Ten included trials are evaluated as low overall risk of bias and two as some concerns for risk of bias.
Safety and efficacy of remimazolam versus propofol
The pooled results demonstrated that patients given remimazolam for procedural sedation had a lower risk of bradycardia (OR 0.28; 95% CI [0.14–0.57]; I2 = 0%; Fig. S1), hypotension (OR 0.26; 95% CI [0.22–0.32]; I2 = 0%; Fig. S2), and respiratory depression (OR 0.22; 95% CI [0.14–0.36]; I2 = 27%; Fig. S3). No differences were observed in the risk of developing postoperative nausea and vomiting (PONV) (OR 0.65; 95% CI [0.15–2.79]; I2 = 70%; Fig. S4) and dizziness (OR 0.93; 95% CI [0.53–1.61]; I2 = 41%; Fig. S5) between the remimazolam and propofol groups. The use of remimazolam for procedural sedation was significantly associated with less injection pain than the use of propofol (OR 0.06; 95% CI [0.03–0.13]; I2 = 37%; Fig. S6). Regarding sedation efficacy, no differences were found in the sedation success rate between the remimazolam and propofol groups (OR 1.40; 95% CI [0.41–4.76]; I2 = 41%; Fig. S7). The pooled results did not demonstrate significant differences in time to loss of consciousness (LOC) (WMD 12.68; 95% CI [−5.55 to 30.9 s]; I2 = 99%; Fig. S8), recovery (WMD −84.55; 95% CI [−258.34 to 89.23 s]; I2 = 1.0%; Fig. S9), and discharge (WMD −2.52; 95% CI [−6.82 to 1.78 min]; I2 = 99%; Fig. S10) with high statistical heterogeneity. The results of our meta-analysis are summarized in Table 2.
Table 2. Summary of meta-analyses.
| Outcome | OR | WMD | Number of included studies | I2 |
|---|---|---|---|---|
| Bradycardia | 0.28 (0.14–0.57) | 6 | 0% | |
| Hypotension | 0.26 (0.22–0.32) | 11 | 0% | |
| Respiratory depression | 0.22 (0.14–0.36) | 11 | 27% | |
| PONV | 0.65 (0.15–2.79) | 8 | 70% | |
| Dizziness | 0.93 (0.53–1.61) | 7 | 41% | |
| Injection pain | 0.06 (0.03–0.13) | 9 | 37% | |
| Successful sedation | 1.40 (0.41–4.76) | 8 | 41% | |
| Time to LOC | 12.68 (−5.55–30.9) | 7 | 99% | |
| Time to recovery | −84.55 (−258.34–89.23) | 9 | 100% | |
| Time to discharge | −2.52 (−6.82–1.78) | 7 | 99% |
Notes.
- LOC
- loss of consciousness
- OR
- odds ratio
- WMD
- weighted mean difference
Discussion
This meta-analysis focused on patients who received procedural sedation. Our results demonstrated a significantly lower risk of adverse events, including bradycardia, hypotension, respiratory depression, and injection pain, in the remimazolam group than in the propofol group. Regarding sedation efficacy, no differences were observed in sedation success, time to LOC, recovery, and discharge between the two groups.
Procedural sedation may be administered to patients to improve their comfort during diagnostic or therapeutic procedures (Benzoni & Cascella, 2022). Remimazolam is a rapidly metabolized and intravenously administered BZD sedative that induces sedation by binding to specific neurotransmitter receptors in the brain. Regarding drug safety, hemodynamic events, including hypotension and bradycardia, are the most common adverse events (Kampo et al., 2019). From a drug safety perspective, we compared the adverse events of remimazolam and propofol. Our analysis found that propofol, despite being a traditional and widely used sedative, entails a higher risk of varying adverse events. In contrast to the findings of a previous review, our results were reinforced by minimal statistical heterogeneity and a greater sample size with more included studies.
Although pain during injection of sedatives is not considered a major complication, it causes patient discomfort and anxiety. Explanations for pain on propofol injection include phenol-associated irritation of the skin and mucous membrane, vein endothelium, and delayed pain because of the release of mediators such as kininogen from the kinin cascade (Desousa, 2016). Remimazolam is an ester-based BZD with different components, and it theoretically causes less injection pain. Our results support the notion that remimazolam is significantly associated with a much lower risk of injection compared with propofol.
Previous evidence has suggested the antiemetic properties of propofol; in our study, we found a similar incidence of PONV between the remimazolam and propofol groups (Kampo et al., 2019). We believe that the development of PONV or dizziness is multifactorial and associated not only with sedatives themselves but also with the procedure type. For example, Cao et al. (2022) studied patients who underwent UGI endoscopy examination and found the incidence of dizziness to be approximately 1.4% in the remimazolam group and 4.1% in the propofol group. Chen et al. (2020) studied patients who underwent colonoscopy and determined the incidence of dizziness to be approximately 25% in both the remimazolam and propofol groups.
Zhang et al. (2022a) and Jalota et al. (2011) reported the lower performance of remimazolam compared with propofol in terms of sedation success rate. However, our meta-analysis did not find significant differences in sedation success rates between the two sedative groups. The discrepancy in results may be explained by the inclusion of various patient populations by the authors, such as those who underwent general anesthesia and procedural sedation, whereas we only included patients who underwent procedural sedation. The comparable time to LOC, recovery, and discharge between remimazolam and propofol should be interpreted with caution. First, the pooled results presented extremely high statistical heterogeneity, and the reported mean time to LOC, recovery, or discharge across the studies presented wide variations. Moreover, different procedural durations and complexities likely affected the recovery course and time, a potential bias that could not be justified.
Limitations
One of the main limitations of our meta-analysis was related to the variability in study design among the included studies. In particular, the protocols for sedative administration and assessment of adverse events varied across studies. Second, although we included 12 studies, some of them did not report relevant comparative safety or efficacy outcomes, which caused the numbers of studies pooled in the meta-analysis of several outcomes to be less than expected. Third, the high heterogeneity in the overall estimation of time to LOC, recovery, and discharge could not be adequately explained. Future well-designed studies should aim to clarify the reasons for the aforementioned heterogeneity. Fourth, although flumazenil can be used to reverse the effects of remimazolam after a procedure, only two of the studies included in our analysis reported the use of flumazenil. This finding might have affected our comparison of recovery outcomes following procedural sedation between remimazolam and propofol. Finally, all of the included studies were conducted in East Asia; therefore, the races represented in these studies may be primarily Asian. This geographic and racial homogeneity limits the generalizability of our findings to other populations. We hope that in the future, more relevant research will be conducted in other parts of the world to ensure higher applicability of the results to different populations.
Conclusions
Our meta-analysis did not show differences in sedation success rate, time to loss of consciousness, recovery, and discharge between remimazolam and propofol. However, in terms of adverse events, our findings suggest that patients who received procedural sedation with remimazolam had a lower risk of bradycardia, hypotension, respiratory depression, and injection pain than those who received propofol.
Supplemental Information
Acknowledgments
We are grateful to the English editors of Knowledge Growth Support for helping with language editing.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yu Chang conceived and designed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Yun-Ting Huang conceived and designed the experiments, performed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Kuan-Yu Chi performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Yen-Ta Huang conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary File.
References
- Benzoni & Cascella (2022).Benzoni T, Cascella M. StatPearls. Treasure Island StatPearls Publishing LLC: 2022. Procedural sedation. [PubMed] [Google Scholar]
- Cao et al. (2022).Cao Y, Chi P, Zhou C, Lv W, Quan Z, Xue FS. Remimazolam tosilate sedation with adjuvant sufentanil in Chinese patients with liver cirrhosis undergoing gastroscopy: a randomized controlled study. Medical Science Monitor. 2022;28:e936580. doi: 10.12659/MSM.936580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2020).Chen S, Wang J, Xu X, Huang Y, Xue S, Wu A, Jin X, Wang Q, Lyu J, Wang S, Li Y, Yu Y, Ai D, Luo A, Min S, Li L, Zou X, Liu J, Lv P, Chai X, Sun X, Zhao Z, Zhang J. The efficacy and safety of remimazolam tosylate versus propofol in patients undergoing colonoscopy: a multicentered, randomized, positive-controlled, phase III clinical trial. American Journal of Translational Research. 2020;12(8):4594–4603. [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2021).Chen SH, Yuan TM, Zhang J, Bai H, Tian M, Pan CX, Bao HG, Jin XJ, Ji FH, Zhong TD, Wang Q, Lv JR, Wang S, Li YJ, Yu YH, Luo AL, Li XK, Min S, Li L, Zou XH, Huang YG. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, phase III trial. Journal of Gastroenterology and Hepatology. 2021;36(2):474–481. doi: 10.1111/jgh.15188. [DOI] [PubMed] [Google Scholar]
- Desousa (2016).Desousa KA. Pain on propofol injection: causes and remedies. Indian Journal of Pharmacology. 2016;48(6):617–623. doi: 10.4103/0253-7613.194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton & Sorkin (1995).Fulton B, Sorkin EM. Propofol. An overview of its pharmacology and a review of its clinical efficacy in intensive care sedation. Drugs. 1995;50(4):636–657. doi: 10.2165/00003495-199550040-00006. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2022b).Guo L, Liu T, Zhang Y, Qi D. Effect of remimazolam versus propofol sedation on the quality of recovery after colonoscopy: a randomised, controlled, noninferiority trial. European Journal of Anaesthesiology. 2022b;39(12):953–955. doi: 10.1097/EJA.0000000000001701. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2022a).Guo J, Qian Y, Zhang X, Han S, Shi Q, Xu J. Remimazolam tosilate compared with propofol for gastrointestinal endoscopy in elderly patients: a prospective, randomized and controlled study. BMC Anesthesiology. 2022a;22(1):180. doi: 10.1186/s12871-022-01713-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, Deeks & Altman (2003).Higgins JPTS, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins et al. (2021).Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. http://www.training.cochrane.org/handbook Cochrane handbook for systematic reviews of interventions version 6.2 (updated 2021) 2021
- Jalota et al. (2011).Jalota L, Kalira V, George E, Shi YY, Hornuss C, Radke O, Pace NL, Apfel CC. Perioperative clinical research core. Prevention of pain on injection of propofol: systematic review and meta-analysis. BMJ. 2011;342:d1110. doi: 10.1136/bmj.d1110. [DOI] [PubMed] [Google Scholar]
- Kampo et al. (2019).Kampo S, Afful AP, Mohammed S, Ntim M, Buunaaim ADB, Anabah TW. Sub-hypnotic dose of propofol as antiemetic prophylaxis attenuates intrathecal morphine-induced postoperative nausea and vomiting, and pruritus in parturient undergoing cesarean section—a randomized control trial. BMC Anesthesiology. 2019;19(1):177. doi: 10.1186/s12871-019-0847-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee & Shirley (2021).Lee A, Shirley M. Remimazolam: a review in procedural sedation. Drugs. 2021;81(10):1193–1201. doi: 10.1007/s40265-021-01544-8. [DOI] [PubMed] [Google Scholar]
- Lu et al. (2022).Lu K, Wei S, Ling W, Wei Y, Ran X, Huang H, Wang M, Wei N, Liao Y, Qin Z, Pan M, Wei Q, Fu L, Xiong B, Ma C, Jiang J, Huang Y. Remimazolam versus propofol for deep sedation/anaesthesia in upper gastrointestinal endoscopy in elderly patients: a multicenter, randomized controlled trial. Journal of Clinical Pharmacy and Therapeutics. 2022;47(12):2230–2236. doi: 10.1111/jcpt.13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik (2004).Marik PE. Propofol: therapeutic indications and side-effects. Current Pharmaceutical Design. 2004;10(29):3639–3649. doi: 10.2174/1381612043382846. [DOI] [PubMed] [Google Scholar]
- Morrison et al. (1998).Morrison RS, Ahronheim JC, Morrison GR, Darling E, Baskin SA, Morris J, Choi C, Meier DE. Pain and discomfort associated with common hospital procedures and experiences. Journal of Pain and Symptom Management. 1998;15(2):91–101. doi: 10.1016/S0885-3924(97)00261-3. [DOI] [PubMed] [Google Scholar]
- Newstead et al. (2013).Newstead B, Bradburn S, Appelboam A, Reuben A, Harris A, Hudson A, Jones L, McLauchlan C, Riou P, Jadav M, Lloyd G. Propofol for adult procedural sedation in a UK emergency department: safety profile in 1008 cases. British Journal of Anaesthesia. 2013;111(4):651–655. doi: 10.1093/bja/aet168. [DOI] [PubMed] [Google Scholar]
- Orlando & Bradley (2022).Orlando MS, Bradley LD. Implementation of office hysteroscopy for the evaluation and treatment of intrauterine pathology. Obstetrics & Gynecology. 2022;140(3):499–513. doi: 10.1097/AOG.0000000000004898. [DOI] [PubMed] [Google Scholar]
- Qadeer et al. (2005).Qadeer MA, Vargo JJ, Khandwala F, Lopez R, Zuccaro G. Propofol versus traditional sedative agents for gastrointestinal endoscopy: a meta-analysis. Clinical Gastroenterology and Hepatology. 2005;3(11):1049–1056. doi: 10.1016/S1542-3565(05)00742-1. [DOI] [PubMed] [Google Scholar]
- Qiu et al. (2022).Qiu Y, Gu W, Zhao M, Zhang Y, Wu J. The hemodynamic stability of remimazolam compared with propofol in patients undergoing endoscopic submucosal dissection: a randomized trial. Frontiers in Medicine. 2022;9:938940. doi: 10.3389/fmed.2022.938940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi et al. (2022).Shi F, Chen Y, Li H, Zhang Y, Zhao T. Efficacy and safety of remimazolam tosilate versus propofol for general anesthesia in cirrhotic patients undergoing endoscopic variceal ligation. International Journal of General Medicine. 2022;15:583–591. doi: 10.2147/IJGM.S345390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne et al. (2019).Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- Stock, Haug & Brenner (2010).Stock C, Haug U, Brenner H. Population-based prevalence estimates of history of colonoscopy or sigmoidoscopy: review and analysis of recent trends. Gastrointestinal Endoscopy. 2010;71(2):366–381.e362. doi: 10.1016/j.gie.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Tan et al. (2022).Tan Y, Ouyang W, Tang Y, Fang N, Fang C, Quan C. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. Journal of Gastroenterology and Hepatology. 2022;37(3):576–583. doi: 10.1111/jgh.15761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias & Leder (2011).Tobias JD, Leder M. Procedural sedation: a review of sedative agents, monitoring, and management of complications. Saudi Journal of Anaesthesia. 2011;5(4):395–410. doi: 10.4103/1658-354X.87270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapani et al. (2000).Trapani G, Altomare C, Liso G, Sanna E, Biggio G. Propofol in anesthesia. Mechanism of action, structure–activity relationships, and drug delivery. Current Medicinal Chemistry. 2000;7(2):249–271. doi: 10.2174/0929867003375335. [DOI] [PubMed] [Google Scholar]
- Viechtbauer (2010).Viechtbauer W. Conducting meta-analyses in R with the metafor Package. Journal of Statistical Software. 2010;36(3):1–48. [Google Scholar]
- Yao et al. (2022).Yao Y, Guan J, Liu L, Fu B, Chen L, Zheng X. Discharge readiness after remimazolam versus propofol for colonoscopy: a randomised, double-blind trial. European Journal of Anaesthesiology. 2022;39(12):911–917. doi: 10.1097/EJA.0000000000001715. [DOI] [PubMed] [Google Scholar]
- Zagari et al. (2016).Zagari RM, Eusebi LH, Rabitti S, Cristoferi L, Vestito A, Pagano N, Bazzoli F. Prevalence of upper gastrointestinal endoscopic findings in the community: a systematic review of studies in unselected samples of subjects. Journal of Gastroenterology and Hepatology. 2016;31(9):1527–1538. doi: 10.1111/jgh.13308. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2022a).Zhang J, Cairen Z, Shi L, Pang S, Shao Y, Wang Y, Lu Z. Remimazolam versus propofol for procedural sedation and anesthesia: a systemic review and meta-analysis. Minerva Anestesiologica. 2022a;88(12):1035–1042. doi: 10.23736/S0375-9393.22.16817-3. [DOI] [PubMed] [Google Scholar]
- Zhang, Li & Liu (2021).Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-centre randomized controlled trial. BMC Anesthesiology. 2021;21(1):156. doi: 10.1186/s12871-021-01373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2022b).Zhang S, Wang J, Ran R, Peng Y, Xiao Y. Efficacy and safety of remimazolam tosylate in hysteroscopy: a randomized, single-blind, parallel controlled trial. Journal of Clinical Pharmacy and Therapeutics. 2022b;47(1):55–60. doi: 10.1111/jcpt.13525. [DOI] [PubMed] [Google Scholar]
- Zubarik et al. (2002).Zubarik R, Ganguly E, Benway D, Ferrentino N, Moses P, Vecchio J. Procedure-related abdominal discomfort in patients undergoing colorectal cancer screening: a comparison of colonoscopy and flexible sigmoidoscopy. The American Journal of Gastroenterology. 2002;97(12):3056–3061. doi: 10.1111/j.1572-0241.2002.07101.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplementary File.