Abstract
INTRODUCTION:
Epidemiological studies report an association between traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) and clinically diagnosed Alzheimer’s disease (AD). We examined the association between TBI/PTSD and biomarker-defined AD.
METHODS:
We identified 289 non-demented veterans with TBI and/or PTSD and controls who underwent clinical evaluation, cerebrospinal fluid (CSF) collection, magnetic resonance imaging (MRI), β-amyloid (Aβ) and tau positron emission tomography, and APOE testing. Participants were followed for up to 5.2 years.
RESULTS:
Exposure groups (TBI, PTSD, and TBI + PTSD) had higher prevalences of mild cognitive impairment (MCI: p<0.0001) and worse Mini-Mental State Examination scores (PTSD: p=0.008; TBI & PTSD: p=0.009) than controls. There were no significant differences in other cognitive scores, MRI volumes, Aβ or tau accumulation, or in most longitudinal measures.
DISCUSSION:
TBI and/or PTSD were not associated with elevated AD biomarkers. The poorer cognitive status of exposed veterans may be due to other comorbid pathologies.
Keywords: traumatic brain injury, post-traumatic stress disorder, Alzheimer’s disease, cognitive decline, head injury, amyloid, tau, neurodegeneration, cerebrovascular disease, veterans
1. INTRODUCTION
Traumatic brain injury (TBI) is associated with long term consequences including cognitive impairment and neurological disease[1]. A history of moderate to severe TBI has been associated with an approximate doubling of the risk of all-cause dementia[2-4]. Post-traumatic stress disorder (PTSD) is a psychiatric disorder that may follow exposure to traumatic stress including military combat[5]. Like TBI, PTSD has been associated with the development of dementia in later life[6, 7] although evidence for this association is mixed [8, 9]. TBI and PTSD are commonly comorbid[10].
Many epidemiological studies[2] investigating cognitive sequelae of TBI or PTSD have reported associations with all cause dementia and with Alzheimer’s disease (AD). AD, a progressive cognitive disorder initially causing mild cognitive impairment (MCI)[11] and ultimately leading to dementia, is among the pathologies for up to 80% of dementia cases[12] and is therefore an important candidate for the pathological outcome of both TBI and PTSD. A meta-analysis[4] of 32 studies reported head injury was associated with a relative risk (RR) of 1.63 [95% CI: 1.34-1.99] for all-cause dementia, and 1.49 [95% CI: 0.91 – 2.43] for AD, and recent large cohort studies[3, 13, 14] reported similar associations. There is variable evidence for PTSD as a risk factor for MCI/dementia. A meta-analysis of seven studies calculated a pooled hazard ratio (HR) of 1.55 (1.41-1.81) for dementia following PTSD [15] and a recent large study reported that individuals with PTSD exposure had a HR of 1.36 (1.12-1.67) for AD, and 1.80 (1.40--2.31) for vascular neurodegenerative diseases[16]. Conversely, a smaller meta-analysis[8] and a recent study[9] found no associations between PTSD and subsequent dementia. Almost all previous studies suggesting that TBI and PTSD are risk factors for dementia/AD have been hampered by methodological issues including limited sample size, variability in clinical diagnosis of dementia/AD, and poorly defined, self-reported TBI[17, 18] and PTSD[19]. While self-reporting of TBIs occurring decades earlier can capture exposures that did not result in clinical evaluation and subsequent medical records, it may also be inaccurate due to impairment of recollection from the injury [18] and difficulty encoding events around the injury particularly during military combat. A combination of self-report TBI and medical records therefore has the highest likelihood of capturing all TBIs.
As the majority of research on PTSD, TBI, and risk of dementia has relied on clinical diagnosis, the biological mechanism underlying this increased risk is not understood. AD is biologically defined based on the presence of abnormal Aβ plaques and tau tangles and disease severity may be additionally characterized by neurodegeneration [20]. However, clinical and neuropathological diagnoses are not always concordant[21], and a variety of in vivo biomarkers including Aβ positron emission tomography (PET), tau PET, measurements of abnormal Aβ and tau in cerebrospinal fluid (CSF), and brain magnetic resonance imaging (MRI) have now been shown to be closely associated with AD pathology[22]. Cerebrovascular disease markers such as white matter hyperintensities (WMHs) are also associated with cognitive impairment and play a complex role in the clinical expression of AD and related dementias [23].
We tested the hypothesis that non-demented veteran elders with TBI and/or PTSD have elevated AD and cerebrovascular pathology compared to their veteran counterparts without lifetime TBI and/or PTSD, using the framework of Alzheimer’s Disease Neuroimaging Initiative (ADNI)[24]. We measured AD pathology using in vivo measurements of Aβ (18F-florbetapir) and tau (18F-flortaucipir) PET, CSF Aβ42, total tau (t-tau), and ptau181, and MRI. As the high frequency of abnormal AD biomarkers in participants with dementia would have required an impractically large sample size to detect an effect of TBI or PTSD on these biomarkers, we elected to exclude these veterans. We also measured cerebrovascular disease with WMHs measured on MRI. This study was conducted in 289 veterans with a history of TBI before, during or after the Vietnam war and/or subsequent ongoing PTSD, and controls, and updates previously reported preliminary results[25]. Because the accumulation of Aβ precedes clinical AD diagnosis by 10-20 years [26], we examined non-demented veterans to determine whether there was any evidence for AD-specific pathological change linked to subtle cognitive deficits that precede clinical dementia diagnosis. The relatively young age of Vietnam veterans (Table 1) and corresponding low frequency of clinically diagnosed dementia in this group made it a useful sample to determine whether TBI or PTSD increases the appearance of abnormal AD pathophysiology prior to the clinical diagnosis of dementia.
Table 1.
Participant characteristics by group
| Variable | Control (n=71) |
PTSD (n=81) |
TBI (n=43) |
TBI & PTSD (n=94) |
|---|---|---|---|---|
| Age, mean (SD), y | 71.4 (5.8) | 68.2 (3.3) | 70.4 (5.4) | 69.8 (3.1) |
| Education, mean (SD), y | 16.1 (2.1) | 14.5 (2.3) | 16.0 (2.3) | 14.8 (2.5) |
| Male, n (%) | 71 (100) | 80 (99) | 43 (100) | 93 (99) |
| Hispanic, n (%) | 4 (6) | 10 (12) | 2 (5) | 6 (6) |
| Race, n (%) | ||||
| Black | 4 (6) | 6 (7) | 4 (9) | 8 (8) |
| Caucasian | 61 (86) | 69 (85) | 37 (86) | 77 (82) |
| Other | 6 (8) | 6 (7) | 2 (5) | 9 (10) |
| American Indian/Alaska native | 1 (1.4) | 1 (1.2) | 0 (0.0) | 2 (2.1) |
| Asian | 3 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| More than 1 race | 1 (1.4) | 4 (4.9) | 2 (4.6) | 6 (6.4) |
| Unknown | 1 (1.4) | 1 (1.1) | 0 (0.0) | 1 (1.1) |
|
APOE ε4+, n (%)a |
19 (27) | 19 (24) | 13 (32) | 21 (25) |
| MCI, n (%) | 2 (3) | 16 (20) | 9 (21) | 24 (25) |
| Progress to MCIb, n (%) | 4 (6) | 5 (8) | 1 (3) | 5 (6) |
APOE results are missing for 1 Control, 1 PTSD, 3 TBI, and 10 TBI & PTSD participants.
percentage calculated out of the cognitively unimpaired participants in each group.
Abbreviations: MCI, mild cognitive impairment; PTSD, post-traumatic stress disorder; TBI, traumatic brain injury.
2. METHODS
2.1. Identification, screening, enrollment, and assessment of study subjects
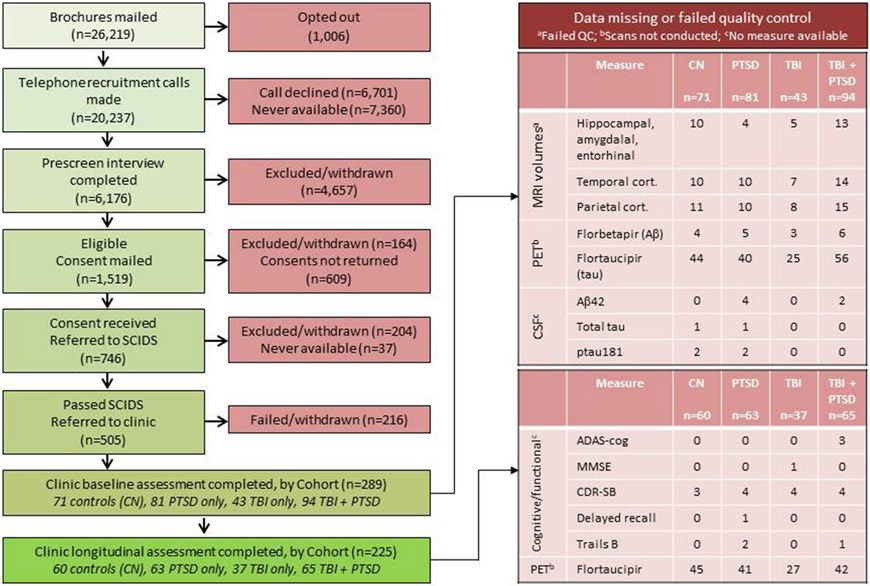
Study recruitment is illustrated in Figure 1. Between 2013 and 2020 we identified non-demented United States Vietnam veterans with a service-connected traumatic head injury and/or ≥1 moderate/severe TBI or TBI-related diagnostic code and/or ongoing PTSD, and controls from military and Veterans Benefits Administration records and in response to advertisements. Details of recruitment are provided in the Section S1 of Supplementary Material. Our goal was to cast a wide net for capturing TBIs in Vietnam veterans. The Glasgow Coma Scale was not available during the Vietnam Conflict and the military medical personnel categorization of closed TBIs not requiring neurosurgery was variable. Thus we used search terms, definitions and diagnostic codes of that period that might appear in the accessible medical records. We used an operational definition of TBI consisting of non-penetrating head injury with amnesia, and/or loss of consciousness for 5 minutes, and/or being dazed and confused for longer than one day and additionally searched for diagnostic codes potentially related to TBI such as post-trauma headache, brain hemorrhage, and traumatic brain disease (Table S1). Controls were demographically comparable and service-connected for conditions other than TBI-related injuries or PTSD (Table S2), and had no record of TBI or PTSD before, during or after the Vietnam war.
Figure 1. Flow of DOD ADNI recruitment and enrollment.
Abbreviations: ADAS-cog; Alzheimer’s Disease Assessment Scale – cognitive; CDR-SB, Clinical Dementia Rating – Sum of Boxes; CN, control; cort., cortical; CSF, cerebrospinal fluid; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; PET, positron emission tomography; PTSD, posttraumatic stress disorder; SCIDS, Structured Clinical Interview for DSM; TBI, traumatic brain injury.
We sent study invitation packets to veterans meeting initial criteria and living within 150 miles of 19 ADNI clinics and prescreened them by telephone after obtaining verbal consent to ensure that the TBI-related diagnostic codes were head injury related (Sections S2 and S3 in Supplementary Material), to diagnose MCI, and to identify exclusions (Section S4 in Supplementary Material). As enrollment of veterans with TBI or TBI + PTSD was challenging and as these individuals often had MRI contraindications, we allowed their MRI requirement to be waived. Head injuries before, during, and after the Vietnam War were identified using a version of the Ohio State University TBI identification method – interview form which captured TBI exposure before, during and after Vietnam. MCI was diagnosed using the Telephone Interview for Cognitive Status 11 item questionnaire and an adapted version of the AD8. Final MCI diagnosis was made by the study clinician during the in-person baseline and follow-up visits.
Veterans meeting inclusion criteria were mailed self-report questionnaires and a written consent form for telephone psychiatric clinical interview. After reviewing self-report questionnaires, we conducted a telephone clinical evaluation to assess current and lifetime PTSD and to rule out drug and alcohol issues and/or psychosis. Remaining veterans, either cognitively unimpaired (CU) or with MCI, were placed into one of four groups: TBI only, PTSD only, TBI and PTSD, and controls (no TBI/PTSD). Types of TBI and age at TBI, military and combat experience, and overall combat experience by group are detailed in Tables S3, S4, and S5, respectively.
Clinical evaluation
Study veterans were referred to ADNI sites for clinical consent and evaluation, final diagnosis of MCI, lumbar puncture for CSF collection, neuropsychological testing, and APOE ε4 and genetic testing using ADNI procedures as described previously (Section S5 in Supplementary Material). A subset (Figure 1) was followed for up to 5.2 years.
MRI [27], florbetapir-PET (10 mCi with 4X5min frames acquired at 50-70 min post-injection) and flortaucipir-PET acquisition (10 mCi with 6X5min frames acquired at 75-105min post-injection) imaging were carried out as previously described (adni.loni.usc.edu/methods/pet-analysis-method/pet-analysis). Briefly, hippocampal, amygdala, entorhinal and parietal cortex volumes were determined using FreeSurfer (v.5.1) T1-weighted images by a single processing site to minimize measurement bias and these volumes were normalized by intracranial volume[28]. WMHs were determined from T1 and FLAIR images using a semi-automated Bayesian approach and normalized by intracranial volume[29].
PET quantification differed from the usual ADNI MRI-dependent pipeline and enabled MRI-free calculation of regions of interest allowing all available PET scans to be analyzed, even those of participants with contraindications to MRI[30]. Full details are available in the “UC Berkeley- MRI-free processing methods” file in the DOD-ADNI section of: https://ida.loni.usc.edu/. Briefly, florbetapir and flortaucipir images were spatially normalized to MNI-152 space tracer-specific templates. Florbetapir cortical summary standardized uptake volume ratios (SUVrs) were calculated using the mean uptake from within frontal, temporal, parietal, precuneus, anterior striatum, and insula regions relative to whole cerebellum[31]. Flortaucipir entorhinal and temporal composite SUVrs [32] relative to inferior cerebellar cortex [33] were calculated in MNI152-space and FreeSurfer-defined regions from 200 ADNI participants that were spatially normalized to MNI-152 space and averaged together.
Baseline assessments of DOD participants with MCI (n=49; all male) with TBI, PTSD and TBI+PTSD were compared with those of MCI male participants (n=317) from the NIH funded ADNI study. Follow-up assessments for clinical evaluation, neuropsychological testing, MRI, and tau (flortaucipir) PET scans were made for 77.2%, 77.8%, 43%, and 24.2% of participants, respectively. The low number of follow-up assessments for flortaucipir is due to the availability of tau PET for which funding was approved in September 2014.
2.2. Statistical analysis
Our study was designed to have 80% power to detect a minimum difference in means of 0.55 standard deviations (SD), assuming 65 participants in each group, an α of 0.025, and a two-sided test. All groups but the TBI group exceeded this target sample size. For comparison of the TBI group with the control group, we have 80% power to detect a difference as small as 0.78 SD. Minimum detectable differences are larger for the imaging and CSF outcomes, where sample sizes are smaller. We calculated means and SD for all continuous variables (age, education, MRI volumetrics, florbetapir and flortaucipir SUVr, neuropsychological tests, CSF biomarkers) and percentages for all categorical variables of interest (sex, race/ethnicity, diagnosis, APOE ε4 status). Analyses focused on all available data for each measure of interest. Primary comparisons of interest for all analyses were between each exposure group and the control group. In cross-sectional analyses, we compared demographic and clinical characteristics of groups using analysis of variance or Fisher’s exact test, and MRI volumetrics and most neuropsychological scores using linear regression. Mini-mental state examination (MMSE) was operationalized as the number of errors (30-MMSE). As Clinical dementia rating sum of boxes (CDR-SoB) scores and MMSE errors did not meet the assumptions of linear regression, we compared them using Poisson and negative binomial regression, respectively. WMH volumes were log-transformed to meet model assumptions. Aβ positivity determined using a threshold of 1.17 for florbetapir cortical SUVr was compared across groups using logistic regression. For comparison of change between groups, mixed effects models with a random intercept and slope, assuming an unstructured variance-covariance structure, were used. These methods assume missing assessments are missing completely at random. The interaction between exposure group and years since study start was of primary interest, since it captured differences between the groups in annual rates of change. All models were adjusted for age, education, APOE ε4 status and baseline diagnosis of TBI/PTSD. Finally, to compare individuals with MCI exposed to TBI or PTSD (all males) with individuals known to be on the AD spectrum, age and APOE ε4 status adjusted comparisons between were made with ADNI MCI male participants on key Alzheimer’s disease markers and clinical indicators, using similar methods described above. We conducted all analyses using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) with a two-sided p-value <0.05 considered statistically significant.
3. RESULTS
3.1. Demographic characteristics
Of 26,219 potential participants, 6176 completed the prescreening interview, 746 gave written consent and were referred to the structured telephone interview, 505 were referred to a clinic, and 289 were ultimately enrolled (Figures 1, S1-S3; Tables S6 and S7). These veterans were placed in four groups: PTSD (n=81), TBI (n=43), TBI & PTSD (n=94) and controls (no TBI or PTSD; n=71) (Table 1). The PTSD group was younger, on average, than the control and TBI groups (p<.05). The PTSD and TBI & PTSD groups had fewer years of education than the other two groups (p<.05). There was a difference in frequency of MCI among the groups, with each of the exposure groups associated with higher frequency of MCI than the control group (p<.001). There was no significant difference between groups in participant sex (p>.99), Hispanic ethnicity (p=.48), race (p=.96), or APOE ε4 positivity (p=.76). Fourteen individuals progressed from CU to MCI during follow-up and none progressed from MCI to dementia; the percentage who progressed did not differ across groups (p=.88).
3.2. Baseline assessments
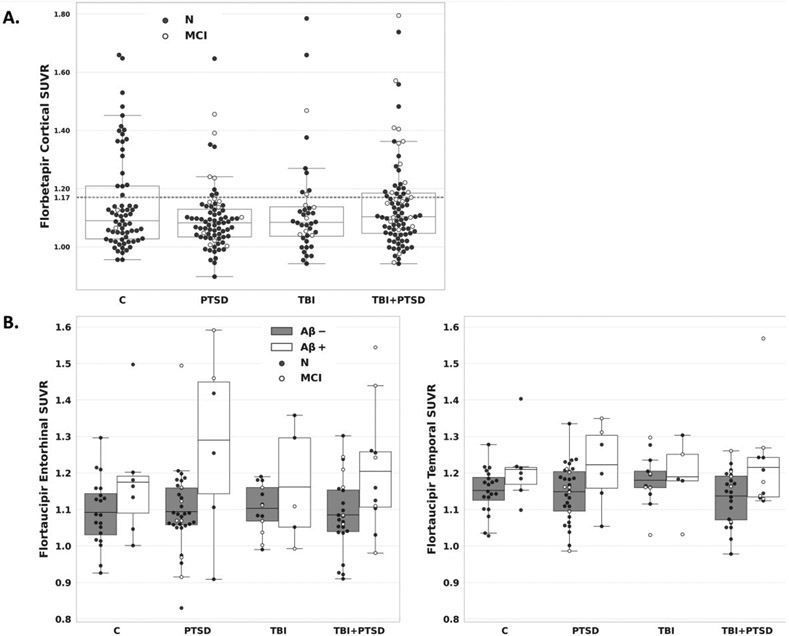
At initial assessment (Table 2) no groups differed in AD assessment scale-13 item subscale (ADAS-Cog13) score after Tukey-Kramer adjustment for multiple comparisons, despite an overall group difference (overall difference: p=.04; pairwise comparisons: PTSD vs controls: adjusted p-value =.08; PTSD vs TBI: adjusted p-value =.11; all other comparisons had p>.34). The number of errors on MMSE differed between the groups (p=.03), with PTSD (p=.008) and TBI & PTSD (p=.009) groups having more errors than control group, both of which remained significant after the Tukey-Kramer adjustment for multiple comparisons. No other cognitive scores differed significantly between the groups. There were no significant differences between any groups in florbetapir or flortaucipir SUVr (Figure 2) nor in regional MRI volumes, WMH volume and CSF biomarkers available for a subset of participants (Figure 1).
Table 2.
Cognitive outcomes and biomarker levels at the initial assessment by group
| Variable | Control (n=71) |
PTSD (n=81) |
TBI (n=43) |
TBI & PTSD (n=94) |
p-valuea |
|---|---|---|---|---|---|
| ADAS-Cog Total 13, mean (SD) | 10.7 (4.6) | 13.0 (4.1) | 11.0 (4.2) | 12.6 (5.4) | .04 |
| MMSE, mean (SD) | 28.8 (1.3) | 28.0 (1.5) | 28.5 (1.4) | 27.9 (1.9) | .03 |
| CDR SoB, mean (SD) | 0.2 (0.4) | 0.6 (0.8) | 0.4 (0.6) | 0.5 (0.8) | .11 |
| Delayed Recall, mean (SD) | 11.2 (3.8) | 9.8 (3.8) | 10.6 (3.5) | 10.3 (4.1) | .64 |
| RAVLT Sum of 5 trials, mean (SD) | 41.5 (9.3) | 39.0 (7.5) | 40.5 (8.9) | 39.1 (9.3) | .72 |
| Trails B, mean (SD) | 88.2 (42.0) | 98.9 (45.1) | 88.6 (37.6) | 107.7 (55.1) | .18 |
| Hippocampal volume, mean (SD), % of intracranial volume | 0.51 (0.06) | 0.53 (0.06) | 0.52 (0.05) | 0.51 (0.06) | .59 |
| Amygdala volume, mean (SD), % of intracranial volume | 0.21 (0.03) | 0.22 (0.03) | 0.21 (0.03) | 0.21 (0.03) | .58 |
| Entorhinal Cortex volume, mean (SD), % of intracranial volume | 0.26 (0.04) | 0.28 (0.04) | 0.27 (0.03) | 0.27 (0.04) | .14 |
| Temporal Cortex volume, mean (SD), % of intracranial volume | 5.8 (0.4) | 5.9 (0.5) | 5.9 (0.4) | 5.9 (0.5) | .99 |
| Parietal Cortex volume, mean (SD), % of intracranial volume | 6.3 (0.4) | 6.3 (0.6) | 6.3 (0.5) | 6.3 (0.4) | .98 |
| White matter Hyperintensities volume, mean (SD) |
5.3 (5.3) | 4.6 (4.2) | 5.4 (7.9) | 4.9 (8.3) | .68 |
| CSF Aβ42, mean (SD), pg/ml | 1102.4 (558.7) | 1325.6 (501.3) | 1183.8 (562.4) | 1240.7 (569.0) | .37 |
| CSF t-tau, mean (SD), pg/ml | 226.0 (95.9) | 231.3 (107.5) | 224.2 (87.4) | 210.0 (79.7) | .49 |
| CSF p-tau181, mean (SD), pg/ml | 20.4 (10.1) | 20.4 (12.2) | 19.6 (8.6) | 18.4 (7.1) | .31 |
| Florbetapir cortical SUVR, mean (SD) | 1.15(0.17 | 1.10 (0.12) | 1.13 (0.18) | 1.14 (0.16) | .37 |
| Florbetapir positive, % | 28.4 | 13.2 | 22.5 | 29.5 | .10 |
| Flortaucipir entorhinal SUVR, mean (SD) | 1.11 (0.11) | 1.12 (0.15) | 1.12 (0.10) | 1.12 (0.14) | .96 |
| Flortaucipir temporal SUVR, mean (SD) | 1.17 (0.07) | 1.16 (0.08) | 1.18 (0.08) | 1.16 (0.10) | .91 |
p-value for an overall group comparison obtained from a model that adjusts for age, education, APOE E4 status (E4+ or E4-), and diagnosis (Normal or MCI).
Missing data:
CDR SoB: 2 Control, 4 PTSD, 3 TBI, 2 TBI & PTSD
RAVLT: 1 PTSD
Trails B: 3 PTSD, 1 TBI & PTSD
Failed QC or no measurement provided from available sample:
Hippocampal volume: 10 Control, 4 PTSD, 5 TBI, 13 TBI & PTSD (Failed QC)
Amygdala volume: 10 Control, 4 PTSD, 5 TBI, 13 TBI & PTSD (Failed QC)
Entorhinal cortex volume: 10 Control, 4 PTSD, 5 TBI, 13 TBI & PTSD (Failed QC)
Temporal cortex volume: 10 Control, 10 PTSD, 7 TBI, 14 TBI & PTSD (Failed QC)
Parietal cortex volume: 11 Control, 10 PTSD, 8 TBI, 15 TBI & PTSD (Failed QC)
Florbetapir PET: 4 Control, 5 PTSD, 3 TBI, 6 TBI & PTSD (scans not conducted)
Flortaucipir PET: 44 Control, 40 PTSD, 25 TBI, 56 TBI & PTSD (scans not conducted; scanning began in 2015)
CSF Aβ: 4 PTSD, 2 TBI & PTSD (no extrapolated value >1700available)
CSF tau: 1 Control, 1 PTSD (no measure from sample available)
CSF p-tau: 2 Control, 2 PTSD, 2 TBI & PTSD (no measure from sample available)
Abbreviations: ADAS-cog, Alzheimer’s disease assessment scale-cognitive subscale; CDR-SoB, Clinical dementia rating, sum of boxes; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; MMSE, Mini-mental state examination; PTSD, post-traumatic stress disorder; RAVLT, Rey auditory verbal learning test; SUVR, standardized uptake volume ratio; TBI, traumatic brain injury.
Figure 2. Florbetapir and flortaucipir binding by participant group.
Florbetapir cortical SUVRs are shown for each participant group, with a dotted line reflecting the positivity threshold at 1.17 and separate points for cognitively normal and MCI individuals (A). Flortaucipir entorhinal cortex and temporal metaROI SUVRs are shown for each participant group by amyloid status, with separate points for cognitively normal and MCI individuals (B). Abbreviations: C, controls; MCI, mild cognitive impairment; CN, cognitively unimpaired; PTSD, posttraumatic stress disorder; TBI, traumatic brain injury.
3.3. Longitudinal assessments
A subset of veterans underwent longitudinal testing. We obtained an average of 2.3 longitudinal cognitive/functional assessments (SD=0.6; range=2-4) over an average of 1.8 years (SD=1.1 years; range=0.8-5.2 years) for estimation of annual rates of change (Table 3). These did not differ significantly between groups except for CDR-SoB, which worsened more in the TBI group than in the control group. We obtained longitudinal MRI for a subset of veterans over an average of 1.8 years (SD=1.2 years; range=0.9-5.2 years) with an average of 2.3 images per person (SD=0.6; range=2-5). Among 65-year old CU veterans with 12 years of education and no APOE ε4 alleles, hippocampal volume (% total intracranial volume) significantly declined only in the TBI group by 0.01 (SE=0.004; p=.01). The difference in annual rates of change across groups did not reach significance (p=.07). There were no significant differences between the groups in rate of change in flortaucipir PET SUVR (p>.30), but due to the small sample size obtained (15 control, 22 PTSD, 10 TBI, and 23 TBI+PTSD) and variability in the measure of change, there was limited power to detect effects.
Table 3.
Annual change by group in cognitive/functional measures
| Variable | Control (n=60) |
PTSD (n=63) |
TBI (n=37) |
TBI & PTSD (n=65) |
p-value a |
|---|---|---|---|---|---|
| ADAS-Cog, mean (SD) | −0.02 (0.32) | −0.28 (0.26) | 0.09 (0.42) | 0.37 (0.33) | .29 |
| MMSE, mean (SD) | −0.14 (0.14) | −0.22 (0.11) | −0.13 (0.19) | −0.13 (0.15) | .91 |
| CDR SoB, mean (SD) | −0.05 (0.05) | −0.09 (0.04) | 0.10 (0.07) | −0.07 (0.05) | .03 |
| Delayed Recall, mean (SD) | 0.71 (0.30) | 0.33 (0.24) | 0.51 (0.39) | 0.55 (0.31) | .58 |
| RAVLT Sum of 5 trials, mean (SD) | 0.007 (0.80) | −0.59 (0.64) | −1.24 (1.01) | −0.34 (0.80) | .61 |
| Trails B, mean (SD) | 4.63 (3.39) | 3.23 (2.75) | 6.00 (4.48) | 8.75 (3.55) | .49 |
p-value for an overall group difference in rate of change
Annual change is estimated for a 65 year old cognitively unimpaired individual with 12 years of education and no APOE ε4 alleles. Annual change and the corresponding standard error are presented for each group.
Missing Data:
ADAS-Cog: 3 TBI & PTSD
MMSE: 1 TBI
CDR SoB: 3 Control, 4 PTSD, 4 TBI, 4 TBI & PTSD
Delayed Recall: 1 PTSD
Trails B: 2 PTSD, 1 TBI & PTSD
Abbreviations: ADAS-cog, Alzheimer’s disease assessment scale-cognitive subscale; CDR-SoB, Clinical dementia rating, sum of boxes; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; MMSE, Mini-mental state examination; PTSD, post-traumatic stress disorder; RAVLT, Rey auditory verbal learning test; TBI, traumatic brain injury.
3.4. Comparison of DOD MCI participants with ADNI MCI participants
We compared the 49 DOD MCI participants (all male) with TBI, PTSD and TBI+PTSD with those of 317 MCI male participants from the NIH funded ADNI study to understand differences between MCI veterans selected for history of TBI and/or PTSD and ADNI MCI participants on the AD continuum (Table 4). DOD MCI participants were slightly younger with a lower frequency of at least one APOE ε4 allele than MCI in the NIH funded study. They also had slightly lower MMSE scores and higher hippocampal volumes compared with ADNI MCI participants.
Table 4.
Comparison of MCI male participants in the DOD and NIH funded studies.
| Variable | NIH Male MCI | DOD Male MCI | p-valuea |
|---|---|---|---|
| Age, mean (SD), y | 72.5 (7.1) (n=315) |
69.8 (3.7) (n=49) |
<.001 |
| APOE ε4+, % | 47.5 (n=301) |
20.4 (n=44) |
<.001 |
| ADAS-Cog Total 13, mean (SD) | 16.1 (6.5) (n=316) |
15.1 (5.4) (n=49) |
.75 |
| MMSE, mean (SD) | 28.0 (1.7) (n=317) |
27.4 (2.1) (n=49) |
<.001 |
| Florbetapir cortical SUVR, mean (SD) |
1.22 (0.23) (n=286) |
1.19 (0.17) (n=42) |
.23 |
| Florbetapir positive, % | 55.2 (n=286) |
38.1 (n=42) |
.98 |
| Hippocampus, mean (SD), % of intracranial volume | 0.46 (0.08) (n=248) |
0.50 (0.07) (n=33) |
.03 |
| White matter Hyperintensities volume, mean (SD) |
6.8 (9.1) (n=316) |
4.9 (5.2) (n=38) |
.85 |
| CSF Aβ42, mean (SD), pg/ml | 1034.0 (554.9) (n=291) |
1196.5 (550.5) (n=25) |
.67 |
| CSF t-tau, mean (SD), pg/ml | 260.3 (111.2) (n=291) |
263.0 (151.2) (n=25) |
.10 |
| CSF p-tau181, mean (SD), pg/ml | 25.1 (12.5) (n=291) |
25.2 (17.1) (n=24) |
.10 |
Age and APOE ε4+ prevalence was compared between groups using the two-sample t-test and chi-square test, respectively. All other variables were tested using a linear or generalized linear regression model adjusted for age and APOE ε4 status.
Abbreviations: ADAS-cog, Alzheimer’s disease assessment scale-cognitive subscale; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; MMSE, mini-mental state examination; PTSD, post-traumatic stress disorder; SUVR, standardized uptake volume ratio; TBI, traumatic brain injury.
4. DISCUSSION
In this study of Vietnam veterans exposed to TBI and/or PTSD, there was no evidence for increased AD or cerebrovascular pathology in any exposure group compared to unexposed controls despite the greater frequency of MCI in all exposure groups (p<.001), and the lower MMSE scores in the PTSD (p=.008) and TBI & PTSD (p=.009) groups. We did not detect any group differences in AD biomarkers including PET and CSF measurements of Aβ and tau, nor in neurodegeneration measured by hippocampal volume on MRI, or cerebrovascular disease measured with WMH. Longitudinally, there were no group differences in CSF or PET measures of Aβ or tau change over time and the sole change in cognition detected was a slightly a greater decline in CDR-SoB in the TBI only group compared to controls. Our results suggest that TBI or PTSD or TBI/PTSD together do not contribute to an increase or acceleration in AD biomarkers.
Our data showing comparable degree of abnormality in biomarkers of Aβ, tau, and neurodegeneration between exposure and control groups do not support the hypothesis that TBI and PTSD are associated with biomarker-defined AD. This is consistent with reports using biomarker- or neuropathologically-defined AD. Veterans with PTSD did not differ in Aβ and tau PET SUVr, or MRI volumes from those with no PTSD, despite having a lower Montréal Cognitive Assessment score [34]. Self-reported TBI with LOC was not associated with neuritic plaques or neurofibrillary tangles at autopsy in a pooled dataset from the Religious Orders Study (ROS), The Memory and Aging Project (MAP), and the Adult Changes in Thought (ACT) cohorts or in the National Alzheimer’s Coordinating Center (NACC) cohort [35]. A history of self-reported mild TBI (mTBI) in 134 CU elders was not significantly associated with cortical Aβ burden or accumulation [36]. Conversely, a study reported a slight elevation of cortical tau but not Aβ in unimpaired and impaired participants with a self-reported history of head injury from ADNI and Indiana University ADRC [37]. Differences in TBI definitions and severity, and the use of unverified self-report data [17] together with a small sample size of more severely impaired participants may contribute to these inconsistencies. Nevertheless, the overall consistency of these studies with our results strengthens our conclusions.
Our major finding that neither TBI nor PTSD was associated with evidence of increased Aβ, tau or neurodegeneration biomarkers must be reconciled with the well-documented association of TBI and PTSD with increased frequency of dementia, often diagnosed clinically as AD. This discrepancy may be attributable to the fundamental differences between the biological definition of AD used in this study [20] that identifies underlying neuropathology, and the broader clinical and behavioral definition of AD dementia used in most epidemiological studies. The clinical diagnosis of AD dementia is based on clinical evaluations of patients drawn from both community samples and specialty clinics over time with evolving diagnostic criteria related to cognitive symptoms and functional decline that interferes with daily activities, and may include a range of progressive neurodegenerative diseases[38]. Indeed, co-pathologies such as α-synuclein and TDP-43 are commonly found to accompany Aβ and tau at autopsy in patients with clinically diagnosed probable AD and MCI. TDP-43 has also been observed in chronic traumatic encephalopathy[39] which is associated with repetitive mild TBI[40]. The prevalence of frontotemporal dementia (FTD) or Parkinson’s disease (PD) dementia was also increased following TBI[38]. These studies support polypathology but not AD-specific pathology as a long-term outcome following TBI[1]. Similarly, various studies reported that PTSD was associated with an increased risk of FTD, PD/Lewy body dementia and vascular dementia as well as AD[6], and a large cohort study reported a greater increase in risk for vascular neurodegenerative diseases than for primary neurodegenerative diseases[16]. Therefore, reports of increased clinically diagnosed dementia as a result of TBI and/or PTSD in epidemiological studies likely reflect a broad range of pathologies.
Beyond the presence of other pathologies, other mechanisms may account for the discrepancies between our results and previous epidemiological studies. TBI and PTSD may also lead to cognitive decline/dementia via reduced cognitive reserve[41] and/or non-Aβ/tau mediated synaptic dysfunction and neuroinflammation [42]. Synaptic dysfunction is implicated as an early pathological event in AD and may be the final common biological mechanism most proximal to neurodegeneration linking protein pathologies to disease symptoms [42]. Future studies may therefore examine changes in markers of synaptic function such as SNAP-25, neurogranin, PSD-95 [43] and NMDAR2A [44] or neuroimaging markers such as synaptic vesicle glycoprotein 2A (SV2) PET imaging [45], as changes in this protein have been observed with TBI in mice[46].
In contrast, MCI is a clinical rather than biological construct, and is considered the earliest clinical stage of AD and other dementias[11], although some MCI patients do not progress to dementia. The higher frequency of MCI in veterans with TBI and PTSD in the absence of elevated Aβ and tau is consistent with the association of self-reported TBI with slightly increased odds of MCI after accounting for other factors such as depression and sex[47]. It could also be attributed to exacerbation of age-related decline [48], the influence of cognitive reserve[49], and non-Aβ and tau copathologies and/or synaptic dysfunction as discussed above.
Although exposure groups had a higher frequency of MCI and lower MMSE scores, they did not differ in auditory verbal learning, typically impaired early in AD progression (Table 3). This suggests that these participants may have non-amnestic MCI (naMCI) characterized by deficits in other cognitive domains[11]. TBI was reported to impair processing speed and executive function[50, 51], but not memory[50-53] consistent with naMCI, although mTBI was additionally associated with impaired working memory and visual learning[54]. Veterans with PTSD had primarily impaired processing speed, executive function, and learning[55], also consistent naMCI, which has a number of neurological sequelae distinct from AD including frontotemporal dementia, dementia with Lewy bodies, and vascular dementia[11], and which may also be due to psychiatric problems.
Our lack of detection of Aβ was likely not attributable to the younger age of non-demented veterans compared to most studies of dementia, as Aβ begins to accumulate 10-20 years prior to symptom onset and approximately 30% of people in this age group have abnormal levels. Our study suggests that PTSD and/or TBI do not contribute to this accumulation. However, acute moderate or severe TBI has been associated with elevated levels of amyloid precursor protein, Aβ and associated enzymes, as well as hyperphosphorylated microtubule associated tau and associated enzymes, but it is unknown whether these changes are transient or ultimately lead to accumulation of AD neuropathology [56]. Such short-term changes would not captured by our study methodology. It is also possible that the tracers used in this study were not sensitive to Aβ and tau accumulation that is specific to TBI and PTSD, as recent work has suggested that the currently available tracers are only minimally sensitive to detecting pathology in TBI and chronic traumatic encephalopathy [57].
The use of biomarkers to define AD in this study circumvents the limitations of previous epidemiological reports of both TBI and PTSD which have been hampered by clinical AD and dementia phenotypes that do not align with biomarker and neuropathological evidence in a substantial proportion of patients[21]. Our use of medical records to determine TBI history overcomes the documented unreliability of self-report for TBI [18]. However, self-report of TBI was also necessary as many veterans who experienced TBI during Vietnam service were not admitted to hospitals, and no contemporary records of TBI while deployed to Vietnam are available. Although we are confident that our methodologies capture the vast majority of TBIs, it is possible that controls had unreported exposures to blasts or head impacts during training. It is also possible that alternative definitions of TBI may have changed our results. This study was not epidemiologically sampled. Written consent was received from only 3.7% of veterans who were initially contacted and up to 75% of veterans were excluded at different points. Some did not return for follow-up assessments due to health issues, travel challenges or lack of interest (Figures S1 and S2). Despite our efforts, the number of black and Hispanic veterans who enrolled was low relative to their proportions in the veteran population. Additionally, up to 20% of participants MRI scans failed quality control, and tau PET data were available for only 46% (N=124) participants due to later availability of this modality. Therefore, the number of participants and scans limited the statistical power to test the underlying hypothesis. Due to the difficulty in enrolling veterans, we were unable to analyze impacts of TBI severity and number, or of the influence of medical comorbidities known to affect cognition such as vascular risk factors and depression, although there were no group differences in measures of vascular risk factors (Table S8). Difficulties in recruitment also resulted in the enrollment of only two women therefore we cannot extend our results to female veterans.
Consistent with epidemiological studies of TBI and PTSD, we observed increased cognitive impairment and prevalence of MCI in exposure groups. However, in contrast to studies based on clinically diagnosed AD dementia, neither TBI nor PTSD were associated with AD biomarkers of Aβ, tau, and neurodegeneration, or with WMH, consistent with other biomarker or neuropathological studies[34, 36, 58]. This information raises questions about alternative mechanisms underlying cognitive symptoms and AD diagnosis in these patients.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank the clinic site Principal Investigators and Site Study Coordinators of the DOD-ADNI study. A full list is given in Table S9 in the Supplementary Material.
This study was funded by Department of Defense (grant numbers W81XWH-12-2-0012, W81XWH-13-1-0259, and W81XWH-14-1-0462), and by the NIH (grant number U19-AG024904). SML declares additional NIH funding and honoraria for presentations at the Hillblom Symposium and IMPACT AD workshop, and travel support from the Alzheimer’s Association. TCN is a member of the US Department of Veterans Affairs PTSD Psychopharmacology Initiative Executive Committee. AJS receives additional NIH funding and participates in the Scientific Advisory Boards of Bayer Oncology and Siemens Medical Solutions USA, Inc., and is Editor-in-Chief for Brain Imaging and Behaviour, Springer Nature Publishing. He receives in kind support (tau PET radiotracer precursor) from Avid Radiopharmaceuticals, a subsidiary of Eli Lilly. PSA declares additional funding from the Foundation for NIH, Alzheimer’s Association, Eisai and Eli Lilly. He has received consulting fees from Biogen, Abbvie, Shionogi, Merck, ImmunoBrain Checkpoint, and Rainbow Medical. DH has participated on the Statistical Advisory Board for PLOS One. MWW receives additional support from the NIH and the California Department of Health. He has directly received consulting fees from Cerecin, Bioclinica, Nestle, Roche/Genentech, The Buck Institute on Aging, NIH, Fujifilm – Toyama Chemical, Garfield-Weston, Baird Equity Capital, University of Southern California, Cytox, the Japanese Organization for Medical Device Development, and T3D Pharmaceuticals. He has received lecturing fees from the Buck Institute for Research on Aging, and the China Association for Alzheimer’s Disease, and travel expenses from University of Southern California, NervGen and CTAD Congress. He is an unpaid committee member of the University of California San Francisco Internal Review Board, and personally owns stock options in Anven, Alzecai and Alzheon. CRJ was an unpaid member of the Roche Advisory Board. All other authors have reported that they have no relationships relevant to the content of this paper to disclose.
REFERENCES
- [1].Wilson L, Stewart W, Dams-O'Connor K, Diaz-Arrastia R, Horton L, Menon DK, et al. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017;16:813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Peterson K, Veazie S, Bourne D, Anderson J. Association Between Traumatic Brain Injury and Dementia in Veterans: A Rapid Systematic Review. J Head Trauma Rehabil. 2020;35:198–208. [DOI] [PubMed] [Google Scholar]
- [3].Nordström A, Nordström P. Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS medicine. 2018;15:e1002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li Y, Li Y, Li X, Zhang S, Zhao J, Zhu X, et al. Head Injury as a Risk Factor for Dementia and Alzheimer's Disease: A Systematic Review and Meta-Analysis of 32 Observational Studies. PloS one. 2017;12:e0169650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science (New York, NY. 2006;313:979–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Desmarais P, Weidman D, Wassef A, Bruneau MA, Friedland J, Bajsarowicz P, et al. The Interplay Between Post-traumatic Stress Disorder and Dementia: A Systematic Review. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2020;28:48–60. [DOI] [PubMed] [Google Scholar]
- [7].Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Archives of general psychiatry. 2010;67:608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kuring JK, Mathias JL, Ward L. Risk of Dementia in persons who have previously experienced clinically-significant Depression, Anxiety, or PTSD: A Systematic Review and Meta-Analysis. Journal of affective disorders. 2020;274:247–61. [DOI] [PubMed] [Google Scholar]
- [9].Islamoska S, Hansen AM, Ishtiak-Ahmed K, Garde AH, Andersen PK, Garde E, et al. Stress diagnoses in midlife and risk of dementia: a register-based follow-up study. Aging & mental health. 2021;25:1151–60. [DOI] [PubMed] [Google Scholar]
- [10].Stein MB, McAllister TW. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. The American journal of psychiatry. 2009;166:768–76. [DOI] [PubMed] [Google Scholar]
- [11].Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Crous-Bou M, Minguillón C, Gramunt N, Molinuevo JL. Alzheimer's disease prevention: from risk factors to early intervention. Alzheimer's research & therapy. 2017;9:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fann JR, Ribe AR, Pedersen HS, Fenger-Grøn M, Christensen J, Benros ME, et al. Long-term risk of dementia among people with traumatic brain injury in Denmark: a population-based observational cohort study. The lancet Psychiatry. 2018;5:424–31. [DOI] [PubMed] [Google Scholar]
- [14].Schneider ALC, Selvin E, Latour L, Turtzo LC, Coresh J, Mosley T, et al. Head injury and 25-year risk of dementia. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2021;17:1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Günak MM, Billings J, Carratu E, Marchant NL, Favarato G, Orgeta V. Post-traumatic stress disorder as a risk factor for dementia: systematic review and meta-analysis. The British Journal of Psychiatry. 2020;217:600–8. [DOI] [PubMed] [Google Scholar]
- [16].Song H, Sieurin J, Wirdefeldt K, Pedersen NL, Almqvist C, Larsson H, et al. Association of Stress-Related Disorders With Subsequent Neurodegenerative Diseases. JAMA neurology. 2020;77:700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hicks AJ, James AC, Spitz G, Ponsford JL. Traumatic Brain Injury as a Risk Factor for Dementia and Alzheimer Disease: Critical Review of Study Methodologies. Journal of neurotrauma. 2019;36:3191–219. [DOI] [PubMed] [Google Scholar]
- [18].McKinlay A, Horwood LJ, Fergusson DM. Accuracy of Self-report as a Method of Screening for Lifetime Occurrence of Traumatic Brain Injury Events that Resulted in Hospitalization. Journal of the International Neuropsychological Society : JINS. 2016;22:717–23. [DOI] [PubMed] [Google Scholar]
- [19].McCarron KK, Reinhard MJ, Bloeser KJ, Mahan CM, Kang HK. PTSD diagnoses among Iraq and Afghanistan veterans: comparison of administrative data to chart review. Journal of traumatic stress. 2014;27:626–9. [DOI] [PubMed] [Google Scholar]
- [20].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2018;14:535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. Journal of neuropathology and experimental neurology. 2012;71:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB, Salloway S, et al. Alzheimer's disease. Lancet (London, England). 2016;388:505–17. [DOI] [PubMed] [Google Scholar]
- [23].Ye BS, Seo SW, Kim GH, Noh Y, Cho H, Yoon CW, et al. Amyloid burden, cerebrovascular disease, brain atrophy, and cognition in cognitively impaired patients. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2015;11:494–503.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Veitch DP, Weiner MW, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. Understanding disease progression and improving Alzheimer's disease clinical trials: Recent highlights from the Alzheimer's Disease Neuroimaging Initiative. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2019;15:106–52. [DOI] [PubMed] [Google Scholar]
- [25].Weiner MW, Harvey D, Hayes J, Landau SM, Aisen PS, Petersen RC, et al. Effects of traumatic brain injury and posttraumatic stress disorder on development of Alzheimer's disease in Vietnam veterans using the Alzheimer's Disease Neuroimaging Initiative: preliminary report. Alzheimer's & Dementia: Translational Research & Clinical Interventions. 2017;3:177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. The Lancet Neurology. 2010;9:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jack CR Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. [DOI] [PubMed] [Google Scholar]
- [29].Schwarz C, Fletcher E, DeCarli C, Carmichael O. Fully-automated white matter hyperintensity detection with anatomical prior knowledge and without FLAIR. Information processing in medical imaging : proceedings of the conference. 2009;21:239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Landau S, Ward T, Murphy A, Harrison T, Baker S, Iaccarino L, et al. Quantification of β-amyloid and tau PET without a structural MRI. Alzheimer’s Dement. 2022;in press. [DOI] [PubMed] [Google Scholar]
- [31].Klunk WE, Koeppe RA, Price JC, Benzinger TL, Devous MD Sr., Jagust WJ, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11:1–15 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jack CR Jr., Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017;13:205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Maass A, Landau S, Baker SL, Horng A, Lockhart SN, La Joie R, et al. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer's disease. Neuroimage. 2017;157:448–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Elias A, Cummins T, Lamb F, Tyrrell R, Dore V, Williams R, et al. Amyloid-β, Tau, and 18F-Fluorodeoxyglucose Positron Emission Tomography in Posttraumatic Stress Disorder. Journal of Alzheimer's disease : JAD. 2020;73:163–73. [DOI] [PubMed] [Google Scholar]
- [35].Sugarman MA, McKee AC, Stein TD, Tripodis Y, Besser LM, Martin B, et al. Failure to detect an association between self-reported traumatic brain injury and Alzheimer's disease neuropathology and dementia. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2019;15:686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Asken BM, Mantyh WG, La Joie R, Strom A, Casaletto KB, Staffaroni AM, et al. Association of remote mild traumatic brain injury with cortical amyloid burden in clinically normal older adults. Brain Imaging Behav. 2021;15:2417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Risacher SL, West JD, Deardorff R, Gao S, Farlow MR, Brosch JR, et al. Head injury is associated with tau deposition on PET in MCI and AD patients. Alzheimer's & dementia (Amsterdam, Netherlands). 2021;13:e12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].LoBue C, Munro C, Schaffert J, Didehbani N, Hart J, Batjer H, et al. Traumatic Brain Injury and Risk of Long-Term Brain Changes, Accumulation of Pathological Markers, and Developing Dementia: A Review. Journal of Alzheimer's disease : JAD. 2019;70:629–54. [DOI] [PubMed] [Google Scholar]
- [39].McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, et al. TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. Journal of neuropathology and experimental neurology. 2010;69:918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Smith DH, Johnson VE, Trojanowski JQ, Stewart W. Chronic traumatic encephalopathy - confusion and controversies. Nature reviews Neurology. 2019;15:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bigler ED, Stern Y. Traumatic brain injury and reserve. Handbook of clinical neurology. 2015;128:691–710. [DOI] [PubMed] [Google Scholar]
- [42].Jackson J, Jambrina E, Li J, Marston H, Menzies F, Phillips K, et al. Targeting the Synapse in Alzheimer's Disease. Frontiers in neuroscience. 2019;13:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kivisäkk P, Carlyle BC, Sweeney T, Quinn JP, Ramirez CE, Trombetta BA, et al. Increased levels of the synaptic proteins PSD-95, SNAP-25, and neurogranin in the cerebrospinal fluid of patients with Alzheimer's disease. Alzheimer's research & therapy. 2022;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tian C, Stewart T, Hong Z, Guo C, Aro M, Soltys D, et al. Blood extracellular vesicles carrying synaptic function and brain related proteins as potential biomarkers for Alzheimer’s disease. Alzheimer's & Dementia. 2022;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cai Z, Li S, Matuskey D, Nabulsi N, Huang Y. PET imaging of synaptic density: A new tool for investigation of neuropsychiatric diseases. Neuroscience letters. 2019;691:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Fronczak KM, Li Y, Henchir J, Dixon CE, Carlson SW. Reductions in Synaptic Vesicle Glycoprotein 2 Isoforms in the Cortex and Hippocampus in a Rat Model of Traumatic Brain Injury. Molecular neurobiology. 2021;58:6006–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].LoBue C, Denney D, Hynan LS, Rossetti HC, Lacritz LH, Hart J Jr, et al. Self-reported traumatic brain injury and mild cognitive impairment: increased risk and earlier age of diagnosis. Journal of Alzheimer's disease. 2016;51:727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. Cognitive decline in older adults with a history of traumatic brain injury. The Lancet Neurology. 2012;11:1103–12. [DOI] [PubMed] [Google Scholar]
- [49].Fraser EE, Downing MG, Biernacki K, McKenzie DP, Ponsford JL. Cognitive Reserve and Age Predict Cognitive Recovery after Mild to Severe Traumatic Brain Injury. Journal of neurotrauma. 2019;36:2753–61. [DOI] [PubMed] [Google Scholar]
- [50].Peltz CB, Gardner RC, Kenney K, Diaz-Arrastia R, Kramer JH, Yaffe K. Neurobehavioral Characteristics of Older Veterans With Remote Traumatic Brain Injury. J Head Trauma Rehabil. 2017;32:E8–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kaup AR, Peltz C, Kenney K, Kramer JH, Diaz-Arrastia R, Yaffe K. Neuropsychological Profile of Lifetime Traumatic Brain Injury in Older Veterans. Journal of the International Neuropsychological Society : JINS. 2017;23:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Grasset L, Glymour MM, Yaffe K, Swift SL, Gianattasio KZ, Power MC, et al. Association of traumatic brain injury with dementia and memory decline in older adults in the United States. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2020;16:853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tripodis Y, Alosco ML, Zirogiannis N, Gavett BE, Chaisson C, Martin B, et al. The Effect of Traumatic Brain Injury History with Loss of Consciousness on Rate of Cognitive Decline Among Older Adults with Normal Cognition and Alzheimer's Disease Dementia. Journal of Alzheimer's disease : JAD. 2017;59:251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Alosco ML, Tripodis Y, Baucom ZH, Mez J, Stein TD, Martin B, et al. Late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology. 2020;95:e793–e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cohen BE, Neylan TC, Yaffe K, Samuelson KW, Li Y, Barnes DE. Posttraumatic stress disorder and cognitive function: findings from the mind your heart study. The Journal of clinical psychiatry. 2013;74:1063–70. [DOI] [PubMed] [Google Scholar]
- [56].Brett BL, Gardner RC, Godbout J, Dams-O'Connor K, Keene CD. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biological psychiatry. 2022;91:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Asken BM, Rabinovici GD. Identifying degenerative effects of repetitive head trauma with neuroimaging: a clinically-oriented review. Acta neuropathologica communications. 2021;9:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Crane PK, Gibbons LE, Dams-O'Connor K, Trittschuh E, Leverenz JB, Keene CD, et al. Association of Traumatic Brain Injury With Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA neurology. 2016;73:1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.