ABSTRACT
To investigate community synergy of lactic acid bacteria (LAB) and cleaner fermentation of oat silage, oat silages were prepared with or without (control) commercial LAB inoculants LI1 (containing Lactiplantibacillus plantarum, Lentilactobacillus buchneri, Lacticaseibacillus paracasei, and Pediococcus acidilactici) and LI2 (containing Lactiplantibacillus plantarum and Lentilactobacillus buchneri). The microbial community, LAB synergy, and cleaner fermentation were analyzed at 1, 3, 6, 15, 35, and 90 days of ensiling. The LAB inoculant improved fermentation quality, with significantly (P < 0.05) lower pH, ammonia nitrogen content, and gas production and higher lactic acid and acetic acid contents than those of the control. Enterobacteriaceae was the main bacterial community in early stage of fermentation, which utilizes sugar to produce CO2 gas, causing dry matter (DM) and energy loss. As fermentation progressed, the microbial diversity decreased, and the microbial community shifted from Gram-negative to Gram-positive bacteria. The inoculation of multispecies LAB displayed community synergy; Pediococcus acidilactici formed a dominant community in the early stage of fermentation, which produced an acid and anaerobic environment for the subsequent growth of Lentilactobacillus and Lacticaseibacillus species, thus forming a LAB-dominated microbial community. The predicted functional profile indicated that the silage inoculated with LI1 enhanced the carbohydrate metabolism pathway but inhibited the amino acid metabolism pathway, which played a role in promoting faster lactic acid production, reducing the decomposition of protein to ammonia nitrogen, and improving the fermentation quality of silage. Therefore, oat silage can be processed to high-quality and cleaner fermented feed by using an LAB inoculant, and LI1 showed better efficiency than LI2.
IMPORTANCE Oat natural silage is rich in Enterobacteriaceae, increasing gas production and fermentation loss. Lactic acid bacteria interact synergistically to form a dominant community during ensiling. Pediococci grow vigorously in the early stage of fermentation and create an anaerobic environment. Lactobacilli inhibit the harmful microorganisms and result in cleaner fermentation of oat silage.
KEYWORDS: clean fermentation, community synergy, gas production, lactic acid bacteria, oat
INTRODUCTION
Oat (Avena sativa) is a dual-purpose crop, widely planted in Europe, North America, and northern China for food and feed production (1). Livestock production in many countries around the world is plagued by feed shortages during cold winter and early spring, increasing the demand for good-quality oat production (2). However, high temperatures and rain during the harvest period often delay the harvest, resulting in high levels of crop dry matter (DM) and fiber contents and losses of nutrients during the processing and storing of oat hay (3). Therefore, oat needs to be appropriately stored to ensure provision throughout the year of good-quality forage for herbivores. Silage preservation is crucial for maintaining forage quality and alleviating seasonal and regional imbalances in quality forage supply. Recently, the study of oat silage has attracted global attention, because of its palatability, high digestibility, nutritional value, and production benefits for herbivores in most countries (4, 5).
Ensiling, a simple and low-cost technique, has been traditionally and widely used for better nutrient preservation of fresh forages for year-round forage supply, particularly under unfavorable climatic conditions (6). The investigation by Wang et al. (7) revealed that ensiling fermentation may happen spontaneously as a result of microorganisms attached to the surface of the plants. In addition, adding microbial inoculants can speed up the fermentation progress, thus resulting in better silage quality and lower DM and nutrient losses during ensiling and the feed-out period. Commercial microbial inoculants are in common use in silage production, in which lactic acid bacteria (LAB) are the most promising microbial inoculants (8). In general, LAB inoculants increase the speed of fermentation and minimize nutrition losses during the initial phase of ensiling and prolong the ensiling time of ensilage in well-sealed silos (9). To date, oat silage studies on specific LAB inoculants and microbial community have been performed systematically (7, 10). Oliveira et al. (11) reported that only 5.7% of studies compared the effects of various types of Lactobacillus on oat silage quality, and 67% of studies tested the effect of Lactobacillus plantarum. In recent years, however, individual inoculants such as Lactiplantibacillus plantarum, Lentilactobacillus buchneri, Lacticaseibacillus paracasei, or Pediococcus acidilactici have been tested for oat silage fermentation and nutritional quality (7, 12, 13). Clearly, specific LAB inoculants have their unique functions. For instance, as a homofermentative LAB, Pediococcus acidilactici occupies a dominant position during the initial period of fermentation, resulting in the production of lactic acid to inhibit spoilage organisms (14), and as a functional genus of LAB in silage, Lacticaseibacillus can also directly restrain the growth of undesirable microorganisms (15). In addition, Lactiplantibacillus, one of the most commonly used inoculants, can rapidly produce lactic acid and lower the pH in forage fermentation, and Lentilactobacillus can enhance the aerobic stability of various forages (16). In the ensiling process, particularly in the initial phase of ensiling, the metabolisms of plant cells and microbes contribute to gas production (carbon dioxide [CO2] greater than 60%), resulting in greenhouse gas emissions and losses of DM and energy from the ensiled material (17). Chen et al. (18) found that Lactobacillus plantarum can decrease CO2 production by the control microbial community during silage fermentation.
Recently, detailed results regarding specific individual microbial inoculants have provided sufficient information to develop compound LAB in a more precise way (19). Therefore, studies on the performance of different LAB species combinations in terms of microbial community dynamics, silage fermentation characterization, feed value, and aerobic stability in the silo and feed-out periods are required.
Compound LAB inoculants have shown beneficial effects on silage quality for different whole crops and forage biomass silages (19). Several studies have shown that proper compound LAB inoculants can improve silage fermentation and nutritional quality (20). In fact, numerous commercial inoculants use combinations of LAB, which usually contain Lentilactobacillus, Lactiplantibacillus, Pediococcus, Lacticaseibacillus, etc., ensuring rapid fermentation and increasing silage stability in the silo and feed-out periods. However, studies on the application of different commercial inoculants on fermentation and nutritional quality of oat silage are scarce. Therefore, the community synergy of LAB and cleaner fermentation of oat silage prepared with microbial inoculant were studied.
RESULTS
Changes in chemical and microbial compositions of oat during ensiling.
Data on the nutrient composition, microbial community, and diversity index of pre-ensiled oat are summarized in Table 1, and data on the changes in chemical and microbial composition during ensiling (0 to 90 days), as affected by the LAB inoculants, are shown in Table 2. The DM content was not affected (P > 0.05) by the inoculants, the ensiling duration, or their interaction. DM loss increased significantly (P < 0.05) with ensiling duration. Higher DM loss was detected in LI2-treated silage on average in comparison with the control silage, whereas DM loss in LI1- and LI2-treated silages had no significant difference. The inoculant, ensiling duration, and their interaction altered (P < 0.05) lactate buffer capacity (LBC), water-soluble-carbohydrate (WSC) content, and LAB and aerobic-bacterium counts in silages. The LBC of fresh oat was 250.84 meq/kg DM. Overall, a lower level (P < 0.05) of LBC was observed for LI2-inoculated silage than for LI1- and control-treated silages. The level of LBC markedly varied during the ensiling process. The LI1-treated silage had higher (P < 0.05) contents of WSC, while the control and LI2-inoculated silages showed no difference in WSC contents. The WSC content was significantly (P < 0.05) influenced by the ensiling duration. However, at 1, 3, 6, and 90 days of fermentation, the WSC content of LI1-inoculated silage was significantly (P < 0.05) greater than that of the control and LI2 silages. The inoculant and ensiling duration did not alter the content of crude protein (CP) in silages. However, the contents of neutral detergent fiber (NDF) and acid detergent fiber (ADF) altered due to ensiling duration. In particular, the NDF content in the LI1-inoculated silage significantly increased at 90 days compared to 1, 3, 6, and 15 days of ensiling. The numbers of yeast and aerobic bacteria were lower (P < 0.05) in LI1-inoculated silage than control and LI2-inoculated silage. Moreover, the numbers of yeast and aerobic bacteria decreased (P < 0.05) with increase in ensiling duration. Coliform bacteria in fresh oats exceeded 7 log10 CFU/g FM, declined to <5 log10 CFU/g FM in inoculated silage on the 1st day of ensiling, and disappeared after the 3rd day of fermentation, while coliform bacteria of control silage were detected continuously on the 3rd day of fermentation.
TABLE 1.
Chemical composition, microbial community, and diversity index of fresh oata
| Parameter | Value |
|---|---|
| Dry matter (g/kg) | 300.90 ± 5.46 |
| Lactate buffering capacity (meq/kg DM) | 250.84 ± 5.04 |
| Water-soluble carbohydrates (g/kg DM) | 73.06 ± 2.18 |
| Crude protein (g/kg DM) | 96.85 ± 4.67 |
| Neutral detergent fiber (g/kg DM) | 364.17 ± 63.49 |
| Acid detergent fiber (g/kg DM) | 179.00 ± 6.78 |
| pH | 6.20 ± 0.04 |
| Lactic acid bacteria (log10 CFU/g FM) | 5.92 ± 0.05 |
| Aerobic bacteria (log10 CFU/g FM) | 7.63 ± 0.10 |
| Yeasts (log10 CFU/g FM) | 6.35 ± 0.35 |
| Coliform bacteria (log10 CFU/g FM) | 7.10 ± 0.14 |
| OTUs | 228.25 ± 55.52 |
| Shannon index | 1.46 ± 0.40 |
| Simpson index | 0.46 ± 0.15 |
| Chao1 index | 340.59 ± 97.93 |
| Coverage | 0.9972 ± 0.0011 |
The values are means and standard deviations for four replicates. FM, fresh matter; DM, dry matter; OTUs, operational taxonomic units.
TABLE 2.
Chemical composition and microbial population of oat silagea
| Item | Treatment | Value on ensiling day |
Mean | SEM |
P value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 15 | 35 | 90 | I | D | I × D | ||||
| DM (g/kg) | Control | 306.58A | 294.99bB | 298.88AB | 300.49AB | 299.97AB | 292.49B | 298.90b | 4.14 | 0.0570 | 0.0783 | 0.1098 |
| LI1 | 305.79AB | 300.25abAB | 304.74AB | 315.06A | 293.20B | 304.94AB | 304.00a | |||||
| LI2 | 300.24AB | 304.23aA | 301.10AB | 298.07AB | 298.41AB | 291.44B | 298.92b | |||||
| Mean | 304.21A | 299.82AB | 301.57AB | 304.54A | 297.20AB | 296.29B | ||||||
| DM loss (g/kg DM) | Control | 4.51F | 8.03aE | 13.20D | 21.20C | 60.49B | 86.90A | 32.39b | 1.92 | 0.0703 | <0.0001 | 0.2545 |
| LI1 | 4.26E | 7.59abDE | 13.30D | 23.01C | 63.04B | 96.22A | 34.57ab | |||||
| LI2 | 4.81E | 7.10bE | 13.24D | 22.98C | 63.77B | 96.49A | 34.73a | |||||
| Mean | 4.53E | 7.57E | 13.25D | 22.40C | 62.43B | 93.20A | ||||||
| Lactate buffering capacity (meq/kg DM) | Control | 298.66bA | 217.84aD | 227.14aD | 238.25aC | 217.72aD | 257.51bB | 242.85a | 3.22 | <0.0001 | <0.0001 | <0.0001 |
| LI1 | 326.86aA | 208.44bD | 207.17cD | 220.77bC | 198.37bD | 277.43aB | 239.84a | |||||
| LI2 | 193.49cE | 201.58bD | 218.64bB | 218.94bB | 209.68abC | 257.68bA | 216.67b | |||||
| Mean | 273.00A | 209.29E | 217.65D | 225.99C | 208.59E | 264.21B | ||||||
| Water-soluble carbohydrate (g/kg DM) | Control | 61.65bAB | 66.52bA | 58.08bB | 55.07B | 35.21bC | 41.19bC | 52.95b | 2.43 | <0.0001 | <0.0001 | 0.0074 |
| LI1 | 73.83aA | 74.23aA | 71.50aAB | 63.10B | 43.31aC | 64.44aB | 65.07a | |||||
| LI2 | 66.30bA | 62.04bA | 60.73bA | 52.69B | 44.08aC | 46.48bBC | 55.39b | |||||
| Mean | 67.26A | 67.60A | 63.44A | 56.96B | 40.87D | 50.70C | ||||||
| Crude protein (g/kg DM) | Control | 100.60 | 95.94b | 98.69 | 100.29 | 101.99a | 100.83 | 99.72 | 1.74 | 0.4369 | 0.9562 | 0.0044 |
| LI1 | 98.96 | 98.29b | 99.47 | 98.18 | 100.68a | 99.69 | 99.21 | |||||
| LI2 | 97.10BC | 105.37aA | 97.37BC | 99.30B | 95.17bC | 96.27BC | 98.43 | |||||
| Mean | 98.89 | 99.87 | 98.51 | 99.26 | 99.28 | 98.93 | ||||||
| Neutral detergent fiber (g/kg DM) | Control | 267.71AB | 277.43A | 273.65A | 240.47bB | 264.72AB | 285.50A | 268.25b | 17.62 | 0.0085 | 0.0014 | 0.1132 |
| LI1 | 283.78BC | 261.06C | 271.90C | 256.54abC | 348.45AB | 358.90A | 296.77a | |||||
| LI2 | 290.19 | 290.60 | 283.79 | 288.05a | 319.59 | 307.65 | 296.64a | |||||
| Mean | 280.56B | 276.36B | 276.45B | 261.69B | 310.92A | 317.35A | ||||||
| Acid detergent fiber (g/kg DM) | Control | 149.78AB | 159.16A | 154.48A | 133.06bB | 151.46bAB | 166.71A | 152.44 | 7.60 | 0.1352 | 0.0287 | 0.4508 |
| LI1 | 158.55AB | 146.22B | 153.54AB | 148.28abB | 176.32aA | 163.20AB | 157.68 | |||||
| LI2 | 157.87 | 164.17 | 161.56 | 154.27a | 161.86ab | 168.34 | 161.35 | |||||
| Mean | 155.40AB | 156.52AB | 156.53AB | 145.20B | 163.21A | 166.08A | ||||||
| Lactic acid bacteria (log10 CFU/g FM) | Control | 9.21bAB | 9.24aA | 8.71abC | 8.96aABC | 8.87aBC | 7.91aD | 8.82a | 0.09 | <0.0001 | <0.0001 | 0.0017 |
| LI1 | 9.38aA | 8.98bB | 8.40bC | 8.47bC | 8.45bC | 7.32bD | 8.50b | |||||
| LI2 | 9.40aA | 9.31aA | 9.01aB | 8.87aB | 9.03aB | 7.72aC | 8.89a | |||||
| Mean | 9.33A | 9.18B | 8.71C | 8.77C | 8.78C | 7.65D | ||||||
| Aerobic bacteria (log10 CFU/g FM) | Control | 9.36A | 9.1175aB | 8.86C | 8.34aD | 8.38aD | 7.73aE | 8.63a | 0.07 | <0.0001 | <0.0001 | <0.0001 |
| LI1 | 9.39A | 8.81bB | 8.71B | 7.66cC | 7.83bC | 7.31bD | 8.28b | |||||
| LI2 | 9.41A | 9.27aA | 8.77B | 7.95bD | 8.41aC | 7.79aD | 8.60a | |||||
| Mean | 9.39A | 9.07B | 8.78C | 7.98E | 8.20D | 7.61F | ||||||
| Yeasts (log10 CFU/g FM) | Control | 9.33A | 9.24aAB | 9.01B | 8.50abC | 9.03B | 7.64D | 8.79a | 0.11 | 0.0003 | <0.0001 | 0.1017 |
| LI1 | 9.21A | 8.83bA | 8.86A | 8.17bB | 9.08A | 7.29C | 8.57b | |||||
| LI2 | 9.39A | 9.37aA | 8.98BC | 8.71aC | 9.13AB | 7.34D | 8.82a | |||||
| Mean | 9.31A | 9.15AB | 8.95C | 8.46D | 9.08BC | 7.42E | ||||||
| Coliform bacteria (log10 CFU/g FM) | Control | 7.87 | 3.87 | N | N | N | N | 0.12 | ||||
| LI1 | 4.83 | N | N | N | N | N | ||||||
| LI2 | 4.43 | N | N | N | N | N | ||||||
| Mean | 5.71 | N | N | N | N | N | ||||||
DM, dry matter; FM, fresh matter; I, inoculant; D, ensiling days; I × D, interaction between additive and ensiling days; N, not detected. Means with different letters in the same row (A to F) or column (a to c) differ significantly (P < 0.05).
Fermentation characteristics of oat silages during ensiling.
The inoculants, ensiling duration, and their interaction significantly (P < 0.05) influenced pH and acetic acid, ammonia N, and gas production of oat silages (Table 3). The LI1-inoculated silages had lower (P < 0.05) pH than control and LI2-inoculated silages in this investigation. The pH values in control and LI2-inoculated silages gradually decreased, reaching their lowest levels at 15 days of ensiling, while they significantly increased from 15 to 90 days. In contrast, the pH of LI1 silages rapidly decreased to below 4.0 at 3 days of ensiling and remained at their lowest level (<4.0) until 15 days. Moreover, the pH values of LI1 silages at 35 and 90 days were lower (P < 0.05) than those of LI2 silages. Ensiling duration and interaction of ensiling duration and inoculant significantly (P < 0.05) affected lactic acid concentration. Unexpectedly, lactic acid and acetic acid contents did not vary between LI1- and LI2-inoculated silages. However, the lactic acid and acetic acid contents were significantly (P < 0.05) higher in the inoculated silages than in the control. The lactic acid content in LI1-inoculated silages remained high (>51 g/kg DM) at 1 to 6 days of fermentation and then significantly (P < 0.05) declined, reaching its lowest level at 90 days of ensiling. Consistently, on 1 and 3 days of ensiling, lactic acid content in the LI2 treatment was significantly (P < 0.05) lower than that in LI1 treatment; nevertheless, lactic acid content between LI1and LI2 treatments had no significant difference (P > 0.05) on 6 and 15 days of ensiling. The acetic acid contents in all treatments consistently increased during ensiling. Propionic acid content ranging from 2 to 9 g/kg DM was detected in LI1-inoculated silage at 35 and 90 days of ensiling. Inoculations of the LAB decreased the ammonia N concentrations of oat silages. On average, the ammonia N concentration gradually increased during ensiling. At 1, 3, 15, and 35 days of ensiling, the LI1- and LI2-inoculated silages had significantly lower (P < 0.05) ammonia N concentrations than the control. However, at 6 and 90 days of ensiling, the LI1-inoculated silage had significantly lower (P < 0.05) ammonia N concentrations than control and LI2-inoculated silages. The gas production was lower (P < 0.05) in LI1-inoculated silage than in control and LI2-inoculated silages. The gas production of each treatment increased rapidly in the initial phase of fermentation but gradually decreased with the prolongation of fermentation.
TABLE 3.
Fermentation characteristics of oat silagea
| Item | Treatment | Value on ensiling day |
Means | SEM |
P value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 15 | 35 | 90 | I | D | I × D | ||||
| pH | Control | 4.61aB | 4.15aC | 4.09aD | 4.12aCD | 4.69bA | 4.68bA | 4.39a | 0.01 | <0.0001 | <0.0001 | <0.0001 |
| LI1 | 4.11bC | 3.98bD | 3.98cD | 3.97bD | 4.48cB | 4.68bA | 4.20c | |||||
| LI2 | 4.10bC | 4.09aCD | 4.05bD | 4.12aC | 4.78aA | 4.72aB | 4.31b | |||||
| Mean | 4.27C | 4.07D | 4.04E | 4.07D | 4.65B | 4.69A | ||||||
| Lactic acid (g/kg DM) | Control | 33.12cBC | 36.50cB | 46.72bA | 32.43C | 18.80bD | 15.96aD | 30.59b | 0.81 | 0.1925 | <0.0001 | <0.0001 |
| LI1 | 51.28aB | 57.25aA | 57.72aA | 31.27C | 23.05aD | 7.66bE | 38.04a | |||||
| LI2 | 46.17bC | 52.39bB | 57.59aA | 32.71D | 20.08bE | 15.63 aF | 37.43a | |||||
| Mean | 43.52C | 48.71B | 54.01A | 32.13D | 20.64E | 13.08F | ||||||
| Acetic acid (g/kg DM) | Control | 5.42bD | 6.54bC | 7.12bC | 11.86cB | 26.76aA | 25.92bA | 13.93b | 0.41 | <0.0001 | <0.0001 | <0.0001 |
| LI1 | 5.55bE | 7.78abD | 8.35aD | 17.22aC | 25.11bB | 30.29aA | 15.72a | |||||
| LI2 | 10.19aD | 8.07aE | 7.87abE | 16.08bC | 28.05aA | 24.91bB | 15.86a | |||||
| Mean | 7.05D | 7.46CD | 7.78C | 15.05B | 26.64A | 27.04A | ||||||
| Propionic acid (g/kg DM) | Control | N | N | N | N | N | N | N | ||||
| LI1 | N | N | N | N | 2.13 | 8.51 | ||||||
| LI2 | N | N | N | N | N | N | N | |||||
| Mean | N | N | N | N | N | N | N | |||||
| Ammonia nitrogen (g/kg TN) | Control | 19.19aD | 23.99aBC | 22.88aC | 27.49aB | 37.54aA | 36.20aA | 27.88a | 0.89 | <0.0001 | <0.0001 | 0.0340 |
| LI1 | 15.82bD | 17.85bCD | 19.91bBC | 21.44bB | 32.72bA | 32.09bA | 23.30c | |||||
| LI2 | 15.64bE | 20.53bD | 24.65aB | 22.60bC | 33.80bA | 34.55abA | 25.29b | |||||
| Mean | 16.88D | 20.79C | 22.48B | 23.84B | 34.69A | 34.28A | ||||||
| Gas production (mL) | Control | 281.25aA | 307.50aA | 292.50aA | 190.00aB | 85.00C | 55.00bD | 201.88a | 9.72 | <0.0001 | <0.0001 | <0.0001 |
| LI1 | 237.50bB | 275.00bA | 182.50bC | 90.00bD | 50.00E | 75.00aD | 151.67c | |||||
| LI2 | 262.50aA | 265.00bA | 207.50bB | 130.00bC | 67.50D | 45.00bD | 162.92b | |||||
| Mean | 260.42B | 282.50A | 282.50C | 136.667D | 136.67E | 58.33E | ||||||
DM, dry matter; TN, total nitrogen; I, inoculant; D, ensiling days; I × D, the interaction between additive and ensiling days; N, not detected. Means with different letters in the same row (A to F) or column (a to c) differ significantly (P < 0.05).
Bacterial community diversity, compositions, and successions in oat silages during ensiling.
Tables 1 and 4 show richness and diversity indicators of bacterial communities in fresh oat and silage. The ensiling duration, inoculant, and their interaction significantly (P < 0.05) influenced numbers of operational taxonomic units (OTUs) and Chao1, Shannon, and Simpson indexes of silages. In general, lower numbers of OTUs and lower Chao1 and Shannon indexes were detected in the LI2 silages than control and LI1 silages. The average numbers of OTUs and Chao1 indexes gradually decreased until the 35th day but increased significantly after 90 days of ensiling. Higher Shannon indexes were observed in LI1 silages than in control and LI2 silages at 15 days of fermentation. After 90 days of ensiling, control silages had the highest Shannon index compared to LAB-inoculated silages. At 6 and 15 days of fermentation, lower Simpson indexes were observed in the LI1 silages. High Good’s coverage (>99.70%) was detected in all samples.
TABLE 4.
Richness and diversity indexes of microbial community in oat silagea
| Index | Treatment | Value on ensiling day |
Mean | SEM |
P value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 15 | 35 | 90 | I | D | I × D | ||||
| OTUs | Control | 307.50AB | 344.75aA | 259.50abC | 292.75aBC | 192.00D | 275.50aBC | 278.67a | 12.47 | <0.0001 | <0.0001 | 0.0025 |
| LI1 | 331.50A | 301.75abAB | 296.00aAB | 280.50aBC | 180.50D | 239.25aC | 271.58a | |||||
| LI2 | 324.25A | 271.50bB | 237.50bC | 248.25bBC | 164.00D | 181.00bD | 237.75b | |||||
| Means | 321.08A | 306.00A | 264.33B | 273.83B | 178.83D | 231.92C | ||||||
| Shannon | Control | 1.90B | 2.18aA | 1.91aB | 1.95bB | 1.46C | 1.53aC | 1.82a | 0.07 | <0.0001 | <0.0001 | <0.0001 |
| LI1 | 1.95A | 1.66bB | 2.16aA | 2.17aA | 1.53B | 1.30bC | 1.80a | |||||
| LI2 | 2.02A | 1.63bB | 0.90bD | 1.72cB | 1.39C | 1.28bC | 1.49b | |||||
| Means | 1.96A | 1.82B | 1.65C | 1.95A | 1.46D | 1.37D | ||||||
| Simpson | Control | 0.30ABC | 0.22bC | 0.28bBC | 0.26aC | 0.37abA | 0.36AB | 0.30b | 0.03 | <0.0001 | <0.0001 | <0.0001 |
| LI1 | 0.25C | 0.37aB | 0.18cC | 0.17bC | 0.34bB | 0.46A | 0.29b | |||||
| LI2 | 0.27D | 0.36aC | 0.68aA | 0.28aD | 0.42aBC | 0.45B | 0.41a | |||||
| Means | 0.27D | 0.32C | 0.38B | 0.24D | 0.37B | 0.42A | ||||||
| Chao1 | Control | 450.14AB | 521.13aA | 430.53abB | 455.39aAB | 328.58C | 433.04aB | 436.47a | 22.36 | <0.0001 | <0.0001 | 0.0026 |
| LI1 | 485.81A | 436.22bAB | 447.70aAB | 433.63aAB | 319.57C | 400.38aB | 420.55a | |||||
| LI2 | 511.68A | 433.58bB | 377.65bB | 400.58bB | 261.11C | 275.62bC | 376.70b | |||||
| Means | 482.54A | 463.64AB | 418.63C | 429.87BC | 303.09E | 369.68D | ||||||
| Coverage | Control | 0.9981AB | 0.9978bAB | 0.9982bAB | 0.9970B | 0.9987A | 0.9983bAB | 0.9980b | 0.0003 | 0.0376 | 0.0013 | 0.2682 |
| LI1 | 0.9979C | 0.9981aBC | 0.9980bC | 0.9983BC | 0.9987A | 0.9985bAB | 0.9983ab | |||||
| LI2 | 0.9978E | 0.9982aD | 0.9986aBC | 0.9983CD | 0.9990A | 0.9989aAB | 0.9985a | |||||
| Means | 0.9980C | 0.9980C | 0.9983BC | 0.9979C | 0.9988A | 0.9985AB | ||||||
OTUs, operational taxonomic units; I, inoculant; D, ensiling days; I × D, the interaction between additive and ensiling days. Means with different letters in the same row (A to D) or column (a to c) differ significantly (P < 0.05).
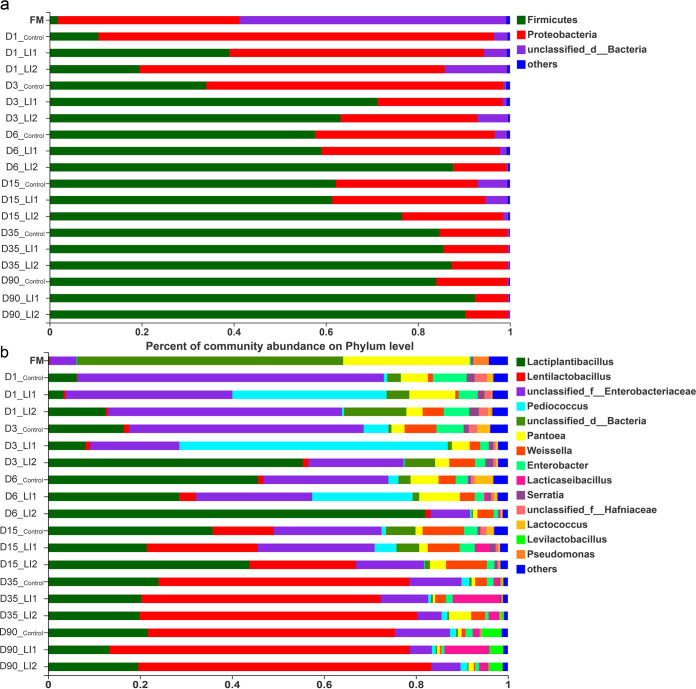
Figure 1a shows the bacterial community composition at the phylum level in oat silage. Notably, Proteobacteria and unclassified bacteria were both dominant in the FM group, whereas Proteobacteria and Firmicutes played a predominant role in silages. The relative abundance of Firmicutes increased and Proteobacteria declined during the ensiling process. Moreover, the LI1 inoculant decreased the relative abundance of Proteobacteria and increased that of Firmicutes after 1 and 3 days of ensiling. However, the LI2-inoculated silage showed a low relative abundance of Proteobacteria and a high relative abundance of Firmicutes at 6 and 15 days of ensiling compared to control and LI1 silages. At the later stages of ensiling (35 and 90 days), the relative abundances of Firmicutes in LI1 and LI2 treatments reached more than 85%, and there was no significant difference between them.
FIG 1.
Relative abundance of bacteria community in oat and silage at the phylum (a) and genus (b) levels across different groups and fermentation times. LI1, silage inoculated with Lactiplantibacillus plantarum, Lacticaseibacillus casei, Lentilactobacillus buchneri, and Pediococcus acidilactici; LI2, silage inoculated with Lactiplantibacillus plantarum and Lentilactobacillus buchneri; D1, D3, D6, D15, D35, and D90, days 1, 3, 6, 15, 35, and 90 of silage, respectively.
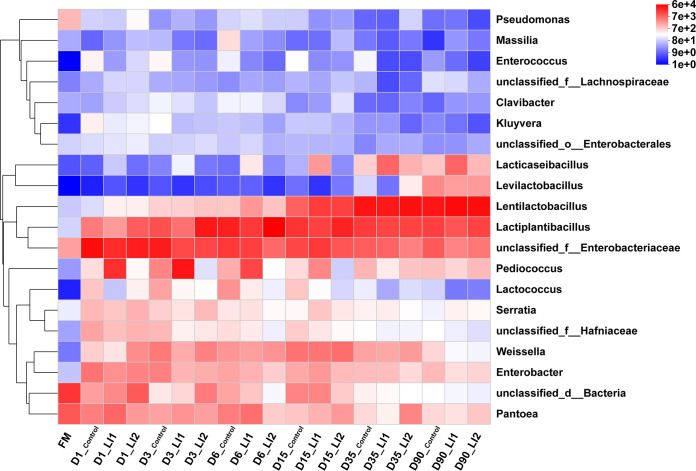
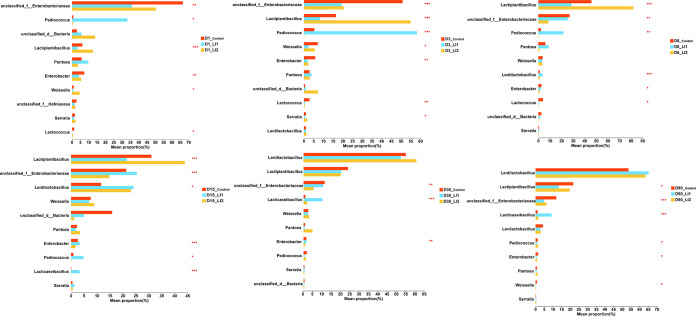
Figures 1b, 2, and 3 display intuitional and statistical comparison of the bacterial compositions at genus level across different treatments and ensiling days. The unclassified bacteria and Pantoea dominated the fresh oat, with a relative abundance of more than 57% and 27%, respectively. The relative abundance of Lactobacillus in the fresh oat was less than 1%. Control silage had a higher (P < 0.05) percentage of members of the family Enterobacteriaceae than the LAB-inoculated silages at 1 and 3 days of ensiling. Enterobacteriaceae were gradually replaced by Lactiplantibacillus and Lentilactobacillus in the control group after 6 days of ensiling. Lentilactobacillus and Lactiplantibacillus play a predominant role at 90 days of ensiling in control group. Relative abundances of Enterobacteriaceae also decreased during ensiling in inoculated silages. The relative abundance of Enterobacteriaceae in control treatment at 1, 3, and 90 days of ensiling was significantly (P < 0.01) greater than that in the LAB-inoculated treatments (Fig. 3). Nevertheless, the relative abundance of Enterobacteriaceae in the LI1 treatment was the same as in the control but higher (P < 0.01) than in the LI2 treatment at 6, 15, and 35 days of ensiling. At the 1st day of fermentation, Enterobacteriaceae and Pediococcus were dominant in the LI1 group, while only Pediococcus dominated at 3 days of fermentation and inhibited the growth of Enterobacteriaceae. Much more interestingly, Lactiplantibacillus was just starting to become the predominant genus, followed by Enterobacteriaceae and Pediococcus, at 6 days of ensiling in the LI1 group, and during the end period of ensiling, Lentilactobacillus was the most predominant, followed by Lactiplantibacillus. Lacticaseibacillus showed significantly higher (P < 0.001) relative abundance at only the late stage of ensiling (15, 35, and 90 days) in the LI1 group than in the LI2 and control groups. Interestingly, for the LI2 group, the silage was dominated by Enterobacteriaceae at 1 day of ensiling. However, Lactiplantibacillus became the most predominant in LI2 at 3, 6, and 15 days of ensiling and then was replaced by Lentilactobacillus at 35 and 90 days of ensiling.
FIG 2.
Heat map of prominent bacterial genera (20 most abundant genera) for oat silage before and after ensiling for 1, 3, 6, 15, 35, and 90 days with or without LI1 and LI2.
FIG 3.
One-way analysis of variance bar plots of the 10 most abundant genera among different oat silage treatments. *, 0.01 < P ≤ 0.05; **, 0.001 < P ≤ 0.01; ***, P ≤ 0.001.
Relationship between silage bacterial taxonomic profile and quality variables.
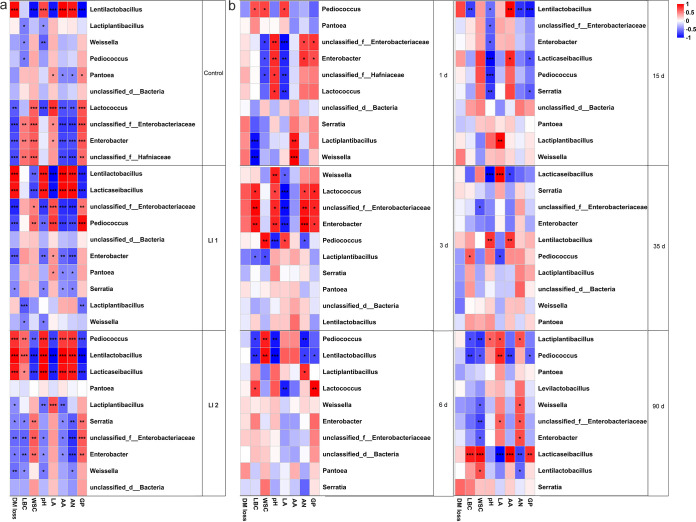
Figure 4a shows the results of association analysis between the major quality variables and the top10 bacterial genera. Among the three treatments, Enterobacteriaceae had a positive correlation with gas production, and Enterobacter had negative correlation with DM loss; Lentilactobacillus had a positive correlation (P < 0.05) with pH value, acetic acid, ammonia N, and DM loss and a negative correlation (P < 0.05) with lactic acid, WSC, and gas production in all groups. This result was the same as that for Lacticaseibacillus from LI1 and LI2 treatments and Pediococcus only from the LI2 treatment and the opposite of that for Pediococcus from the LI1 treatment. Lactiplantibacillus was negatively correlated with LBC and gas production of the LI1 treatment and pH and acetic acid of the LI2 treatment. In addition, Fig. 4b shows the effect of the top 10 bacterial genera on the main quality variables at different ensiling days. The correlations between bacterial genera and quality variables were more complex when the silage fermentation started (1 and 3 days) and finished (35 and 90 days) and simpler in the intermediate stage (6 and 15 days) of ensiling.
FIG 4.
Association analysis between bacterial genera and quality variables. Quality variables are displayed horizontally, and the bacterial genera are displayed vertically. The corresponding value of the middle heat map is the Pearson correlation coefficient r, which ranges between −1 and 1; an r value of <0 indicates a negative correlation (blue), and a value of >0 indicates a positive correlation (red). *, P < 0.05; **, P < 0.01; ***, P < 0.001. (a) Correlations between bacterial genera and quality variables in different silage groups. (b) Correlations between bacterial genera and quality variables at different ensiling time points (1, 3, 6, 15, 35 and 90 days of ensiling). LBC, lactate buffer capacity; WSC, water-soluble carbohydrate; LA, lactic acid; AA, acetic acid; AN, ammonia nitrogen; GP, gas production.
Predicted functions of bacterial communities during oat ensiling.
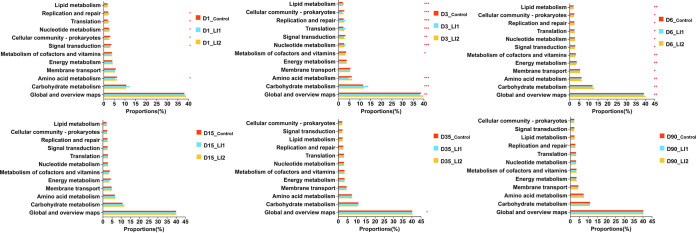
Figure 5 displays the bacterial metabolic functions determined with PICRUSt2 and based on KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways. We obtained 46 metabolic categories, which belong to the metabolism pathway, environmental information processing pathway, genetic information processing pathway, and cellular process pathway, among the three treatments. Figure 5 shows the differences of the first 12 metabolic functions with high abundance in three kinds of silages after 1, 3, 6, 15, 35, and 90 days of ensiling. The global and overview maps pathway showed the most significant role in metabolic categories, followed by carbohydrate and amino acid metabolic pathways in all silages after ensiling. The global and overview maps predominated (P < 0.05) in the LI2-inoculated silage at 3, 6, and 35 days of ensiling. In the LI1 treatment, the amino acid metabolism was significantly lower (P < 0.05) than that in control and LI2 treatments at 1, 3, and 6 days of ensiling. The carbohydrate metabolism pathway was more (P < 0.05) dominant in the LI1-treated silages at 3 days than in LI2 silages, while the opposite result was found for LI1 at 6 days of ensiling. In general, at the early stage of ensiling (before 6 days), there were at least 6 metabolic pathways with significant differences at each ensiling day, but there were few metabolic pathways with significant differences in the late period of ensiling.
FIG 5.
Level 2 KEGG orthologous sequences of ensiled oat as influenced by additives and ensiling time. *, 0.01 < P ≤ 0.05; **, 0.001 < P ≤ 0.01; ***, P ≤ 0.001. Functional prediction of bacterial changes in oat after fermentation was made using PICRUSt2.
DISCUSSION
The DM content at ensiling is a crucial index for fermentation characteristics and preservation of nutritional value of forages (21). The oat was ensiled at a high DM content (301 g/kg), and there were few changes in DM content due to ensiling duration and LAB inoculants. As reported for barley silages, there are few changes in DM content in a well-preserved silage process (22). The higher DM content of LI1-inoculated silage, compared with the control and LI2-treated silages in our study could be related to the effectiveness of LAB inoculation, which restricts extensive fermentation (11). Producing preferred-quality silage while minimizing DM loss is a challenge (19). In general, the DM loss ranging from 2% to 6% caused by lactic acid fermentation is a bit low (23). In our study, the DM losses increased from 0.4% to 6% during 1 to 35 days of ensiling in all treatments, showing a restricted fermentation, which might be due to the predominant lactic acid fermentation pathway in the silages. Unusually, the average DM loss of oat silages in the three treatments was up to about 9% after 90 days of ensiling. This might be related to the higher DM content (≥300 g/kg) of the oat in the silage and to the relative abundance of Lentilactobacillus buchneri, resulting in higher fermentative losses (4). In addition, the higher levels of DM loss in LI2-treated silage compared to control silage might be attributed to the significantly positive correlation between DM loss and Lentilactobacillus, Lacticaseibacillus, and Pediococcus in LI2 treatment (Fig. 4). The WSC was used mainly by microorganisms for energy metabolism during the ensiling process (13). The content of WSC (73.06 g/kg) of fresh oat in the present study was enough for fermentation (24). Moreover, inoculation with LI1 supported efficient fermentation during ensiling, resulting in higher WSC levels in this silage than in control and LI2 silages. The lower WSC content in LI2 and control silages reflects more extensive fermentation of WSC than in LI1 silage, which might be related to the relative abundance of Lentilactobacillus (25). The decreases in WSC contents in all silage could be related to the conversion of WSC into organic acid by LAB during ensiling (26). The LAB-treated silages had higher ADF contents than the control silage. The reason for this is not entirely clear. It may be due to the addition of LAB, which consumes a large amount of fermentation substrates such as WSC, resulting in an increase in the relative content of ADF. The LI1-inoculated silage had higher NDF content at 90 days than at 1, 3, 6 and 15 days of ensiling. Generally, as silage fermentation progresses, it is difficult for microorganisms to decompose the fiber components, but they can decompose part of the soluble carbohydrates into carbon dioxide, water, ethanol, etc. (19), which can easily cause the loss of dry matter and thus lead to the relatively high content of fiber components in the silage.
Table 1 shows that the epiphytic LAB count in fresh oat exceeded the lowest requirement (5 log10 CFU/g FM) for preferred quality of silage preparations (27). However, the yeast count in fresh forage was above 6 log10 CFU/g FM, which increases the possibility of aerobic spoilage, dry matter loss, and CO2 production during ensiling. Cheng et al. (12) reported lower counts of LAB and yeasts in fresh oats grown in different regions than in this experiment. This may be related to the difference in growing conditions, such as differences in geographical location and ambient temperature.
The pH value of silage is a key indicator of silage quality, and the benchmark for high-quality silage fermentation is a pH below 4.2 (28). In the present study, a rapid acidification, with lower pH and higher lactic acid was observed in LAB-treated groups during initial stages of fermentation (before 15 days of ensiling), especially in the LI1 group. This was probably because the LI1 inoculant contains two homofermentative LAB (Lactiplantibacillus plantarum and Pediococcus acidilactici), which have been reported to speed up the fermentation during initial periods of fermentation by more efficiently shifting WSC into lactic acid, resulting in a rapid drop in pH (19). Conversely, silages with higher relative abundances of heterofermentative Lentilactobacillus have a high acetic acid content because of their ability to change some lactic acid into acetic acid (29). The acetic acid content increased progressively with the ensiling duration, which might be because of the obvious enhancement of the relative abundance of Lentilactobacillus at the late period of ensiling (15, 35, and 90 days). Chen et al. (30) suggested that a better alternative LAB inoculant for ensiling is mainly marked by fast increase, resistance to low pH, and rapid production of preferred metabolites. The present study showed that a rapid drop in pH and quick production of lactic acid in oat silage were promoted by LI1 inoculation. Ammonia N is an important index for reflecting the degree of crude protein degradation based on fermentation of Clostridium in the silage (31, 32). The LAB inoculant reduced the ammonia N content of silages, which indicated that LAB restricted the reproduction of undesirable microbes in silage, resulting in a decrease in the degradation of protein (33). As reflected by the negative correlation between ammonia N and Pediococcus, Lacticaseibacillus, and Lentilactobacillus during fermentation (Fig. 4). Overall, the ammonia N concentration gradually increased during ensiling until 35 days of ensiling, indicating that stable conditions were achieved after 35 days of ensiling, stopping further proteolysis.
Gas production (GP) during ensiling not only is bad for the environment but also causes dry matter loss in silage. A previous study found that approximately 6.0 L/kg FM of gas was produced after 60 days of ensiling (34) and mainly emitted at the early stages of fermentation (35). The present study indicated that the average gas production in all silages was greater during the initial stage (1 and 3 days) and then sharply declined at later ensiling days. Another study stated that the relative abundance of Lactococcus had a positive correlation with gas production (18). The higher gas production during the initial stage in our study was also associated with a higher relative abundance of Lactococcus. Moreover, the decrease of the relative abundance of Enterobacteriaceae and increase of the relative abundances of Lentilactobacillus and Lacticaseibacillus during ensiling were observed at all silages. Association analysis showed that the relative abundance of Enterobacteriaceae was positively correlated with GP, whereas the relative abundance of Lentilactobacillus and Lacticaseibacillus was negatively correlated with GP (Fig. 4). It could further explain the reduction in gas production at the later stage of fermentation.
All the results above verify that LI1 improved the fermentation quality of oat silage. Similar findings were showed by Li et al. (36) and Na et al. (33), who suggested that the inoculation of exogenous LAB (Lacticaseibacillus paracasei, Pediococcus acidilactici, Lentilactobacillus buchneri and Lactiplantibacillus plantarum) improved the fermentation quality of silages. Generally, silages with good fermentation quality have low alpha diversity (37). In the present study, the higher Shannon index in LI1-treated silage at 6 and 15 days of ensiling compared with that in LI2 silage was inconsistent with the lower pH and relatively high fermentation quality, which can be explained by the higher relative abundance of Enterobacteriaceae in LI1-inoculated silages. A previous study reported that a combination of homofermentative Lactiplantibacillus plantarum and heterofermentative Lentilactobacillus buchneri was advantageous to control the early fermentation stage by reducing the activity of enterobacteria and other aerobic bacteria (19). This result was confirmed in our bacterial community diversity and composition analysis data (Fig. 1 to 3). Romero et al. (38) reported that the application of inoculant enhanced oat silage quality partially by a change in the microbial community composition during ensiling. During the growing season, Proteobacteria are the main bacterial group in plant leaves, followed by Firmicutes in some cases (39). In the present study, the same phenomenon was found in fresh oat. In addition, Yuan et al. (40) suggested that lactic acid fermentation happens naturally in an anaerobic environment. Likewise, Eikmeyer et al. (41) and Ogunade et al. (42) also found that the epiphytic microorganisms ferment the available nutrients in forage, so as to achieve the production of organic acids, reduction of pH, and prevention of harmful microorganisms’ propagation. In this investigation, the relative abundance of Lactobacillus in the fresh oat was less than 1%, while the total relative abundance of Lentilactobacillus and Lactiplantibacillus in the control increased sharply to 46.97% at 6 days of ensiling and reached 75% at 90 days of ensiling. All oat silages, regardless of the addition of the inoculant, had greater relative abundance of Enterobacteriaceae at the 1st day of ensiling, indicating that the LAB started the fermentation of oat silage after 1 day of ensiling. During the first 6 days of ensiling, the relative abundance of Pediococcus, possessing high acidification potential, was greater in the LI1-inoculated silages. As a result, there was a rapid decrease in pH of LI1-inoculated silages, which might have restricted plant and microbial proteolytic activity, thereby leading to a lower ammonia N concentration. Cai (43) reported that during the ensiling process, lactic acid-producing cocci, e.g., Enterococcus, Lactococcus, and Pediococcus, grow actively during the initial period of the ensiling process, while Lactobacillus is the major LAB during the later stages of ensiling. This might explain the higher relative abundance of Pediococcus, Lactococcus, and Enterobacteriaceae (including Enterococcus) in early stage of ensiling. Our study indicated that Pediococcus had a negative correlation with DM loss but a positive correlation with LA in LI1. Inoculation of Pediococcus acidilactici enhanced lactate production and DM recovery. Therefore, LAB exert microbial synergy to form a dominant community, effectively inhibiting the growth of undesirable microbes, gas production, and fermentation loss.
The silage fermentation process is regulated by microbial activities via complex metabolic pathways to decompose substrates or shift metabolites (37). The predicted function profiles of bacterial communities in silage enable us to obtain more information about the metabolic pathways of the bacterial community affecting silage quality. Generally, most metabolic categories strengthened with the fermentation progress, indicating that the metabolic capacity of the microbial community was augmented with increasing microbial abundance and diversity (44). Therefore, it is meaningful and necessary to determine metabolic pathways of the bacteria in relation to the oat silage. Figure 5 demonstrates the predicted functional profiles of the bacterial community of control and LAB inoculated silages at different ensiling durations in level 2, determined by analysis of the KEGG databases by PICRUSt2. In this study, among 12 metabolic functions with high abundance, three pathways—global and overview maps, carbohydrate metabolism, and amino acid metabolism—play the predominant role in all silages at different days of ensiling. This suggested that three types of metabolism were essential for microbial metabolism. The pathway of amino acid metabolism represents an ability to convert the macromolecular proteins in forage into peptide substances or amino acids that can easily be taken in by the animals (45). In this study, the lowest amino acid metabolism in the LI1-treated silage at the initial stage of fermentation (1, 3 and 6 days) was consistent with lower pH and ammonia N. Therefore, this result further illustrated that the lowest pH restrained the amino acid metabolism related to Enterobacter in the LI1-inoculated silage (46). In general, the metabolic pathways of glycolysis, pyruvate, and butanoate, which are included in carbohydrate metabolism, may have a substantial impact on palatability of silages for herbivores (45). A higher relative abundance of carbohydrate metabolism was found in LI1-treated silages at 3 days of ensiling. Accordingly, a higher relative abundance of all LAB in the bacterial communities was also found in the LI1-treated silage than in the LI2 silage. Conversely, a higher relative abundance of carbohydrate metabolism was found in LI2 silage at 6 days of ensiling; meanwhile, the relative abundance of total LAB was higher in its bacterial community. This strongly demonstrates that the relative abundance of the LAB community was related to the expression of the carbohydrate metabolism pathway. Few metabolic pathways with significant differences were observed at the end period of fermentation relative to the early stage, which may reflect stable and undifferentiated metabolism by the dominant bacterial community. These findings show that LI1-treated silage improved silage fermentation and reduced protein decomposition compared to LI2-treated silage.
Conclusions.
The community synergy of LAB and cleaner fermentation of oat silage prepared with a multispecies microbial inoculant were studied. LAB exert microbial synergy to form a dominant community during ensiling, effectively inhibiting the growth of harmful microorganisms, gas production, and fermentation loss. The combined inoculation of Lactiplantibacillus, Lentilactobacillus, and Pediococcus acidilactici species effectively enhanced the carbohydrate metabolism pathway but inhibited the amino acid metabolic pathway, resulting in improved fermentation quality and reduced protein decomposition of oat silage. The results indicate that oat silage with the L11 inoculant can be used to prepare high-quality, cleaner fermented feed and is a potential feed resource for ruminants.
MATERIALS AND METHODS
Microbial inoculants.
Two commercial multispecies inoculants, LI1 (Bonsilage containing Lentilactobacillus buchneri, Lactiplantibacillus plantarum, Lacticaseibacillus paracasei, and Pediococcus acidilactici; Schaumann Agricultural Trading Co., Ltd., Shanghai, China) and LI2 (Zhuanglemei IV containing Lentilactobacillus buchneri and Lactiplantibacillus plantarum; Sichuan Gaofu Ji Biotechnology Co., Ltd., Chengdu, China), were utilized for the silage preparation of oat. The viable counts of LAB in inoculants were ≥1.0 × 1011 CFU/g for LI1 and ≥1.3 × 1010 CFU/g for LI2.
Silage preparation.
Oat (Avena sativa L. cultivar Qinghai444) was cultivated in an experimental field of the Inner Mongolia Academy of Agricultural and Animal Husbandry Sciences (40°46.265 N, 111°39.851 E; altitude, 1,056 m), located at the foot of Yinshan Mountain on the Tumochuan plain in Inner Mongolia, China. The crop was reaped at the milk stage with approximately 300 g/kg DM on 24 July 2021 and immediately chopped into 1- to 2-cm lengths. Silages were prepared with a laboratory fermentation system (47), and the treatments were designated control, LI1, and LI2. After homogenization of the material, LAB inoculants LI1 and LI2 were processed to 2 g/t and 10 g/t based on FM (fresh matter), respectively. The inoculant was dissolved in sterile distilled water according to the recommended dosage and sprayed with a sprayer. The control was added with an equal amount of sterilized water. After the materials were mixed evenly, 500 g of fresh material was packed in plastic bags (food grade; 300 mm by 400 mm; Anxi County Chengxiang Fengdu Machine Processing Co., Ltd., Quanzhou, China), and the bags were sealed quickly with a sealer (Xiawei ZK-420; Anxi County Chengxiang Fengdu Machine Processing Co., Ltd., Quanzhou, China). For each treatment, 24 bags of silage were prepared and stored at 22°C to 28°C. After 1, 3, 6, 15, 35, and 90 days of ensiling, four bags of silage were randomly selected from each treatment for analysis of microbial community and fermentation parameters.
Fermentation characteristics and chemical composition analyses.
For analysis of silage fermentation, 180 mL of distilled water was added to 20 g of silage sample, and the mixture was immediately homogenized in a homogenizer (LW-09; Shanghai Jingxin Industrial Development Co., Ltd., Shanghai, China) for 10 min and then filtered through 4 layers of cheesecloth. Extracts of sample were used for pH measurement with a pH meter (PB-10; Sartorius, Gottingen, Germany). Then, the extract sample was filtered through a 0.22-mm filter for analysis of organic acids and ammonium nitrogen by high-pressure liquid chromatography (HPLC) and the Kjeltech autodistillation system (2200; Foss Tecator, Hoganas, Sweden) as described by Cai (48). For microbial population analysis, 20 g of silage sample and 180 mL of 0.85% sterile saline were placed in a sterile beaker and mixed well; then, the mixture was diluted 101 to 109 with a sterile test tube. An MRS agar plate, nutrient agar plate, violet red bile agar plate, and potato dextrose agar plate were used to incubate the LAB, aerobic bacteria, coliform bacteria, and yeasts, respectively, as described by Cai et al. (47). The counts of microorganism were expressed as CFU per gram of FM. For chemical analysis, the silage samples were dried in a ventilated heating dryer at 65°C for 48 h to detect the dry matter. The DM loss of silage was calculated using the difference in DM (by weight) before and after fermentation. The WSC content was determined based on the anthrone-sulfuric acid colorimetry (49). LBC was measured following the method of Playne and McDonald (50). The total N content was determined using a Kjeltech 8400 autoanalyzer (Foss Co., Ltd., Hillerød, Denmark), and CP content was computed by multiplying total N by 6.25. The contents of ADF and NDF were quantified as recommended by Van Soest et al. (51).
Gas production analyses.
The gas volume in silage bags was measured in a 5,000-mL measuring cup kept in a constant-temperature water bath (25°C) as described by Chen et al. (18). The difference in expansion volume before and after ensiling was calculated as the gas production.
DNA extraction and 16S rRNA gene sequencing.
DNA extraction and 16S rRNA gene sequencing data processing were performed by LC-Bio Technology Co., Ltd. (Hang Zhou, Zhejiang Province, China). Briefly, the library quantification kit for Illumina (Kapa Biosciences, Woburn, MA, USA) was used to assess the size and quantity of the amplicon. Then, the NovaSeq PE250 platform was used to sequence the library. Alpha diversity was analyzed by QIIME (version 1.9.1), and the relative abundances of microbial communities were measured at the phylum and genus levels using the NT-16S with a confidence threshold of 70%. Bacterial function prediction was proof checked from the KEGG database using phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt2 v2.3.0_b).
Statistical analysis.
All the reported results are the means from four replicates. Chemical composition, fermentation quality, alpha diversity, and microbial count data were analyzed using two-way analysis of variance (ANOVA) with Duncan’s multiple-range test and with silage inoculant and ensiling time as the main variables by SAS version 9.1 (SAS Institute, Cary, NC, USA). Differences between results were considered statistically significant when the P value was lower than 0.05. One-way ANOVA bar plots were made to demonstrate which genera contributed to the changes in the bacterial community. The relationships between the bacterial taxonomic profile and silage quality variables were determined by calculating the Pearson correlation coefficients and were plotted by using the online Majorbio Cloud platform (www.majorbio.com).
Data availability.
Raw sequencing files and associated metadata have been deposited in NCBI’s Sequence Read Archive (SRP410438).
ACKNOWLEDGMENTS
This work was funded by the central government guides local funds for science and technology development–basic research projects with free exploration (2022ZY0152), the major science and technology program of Inner Mongolia (2021SZD0017), and Inner Mongolia Agriculture and Animal Husbandry Innovation Fund (2022CYZX04).
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yanlin Xue, Email: xueyanlin_1979@163.com.
Yimin Cai, Email: cai@affrc.go.jp.
Luca Cocolin, University of Torino.
REFERENCES
- 1.Li X, Chen F, Wang X, Sun L, Guo L, Xiong Y, Wang Y, Zhou H, Jia S, Yang F, Ni K. 2021. Impacts of low temperature and ensiling period on the bacterial community of oat silage by SMRT. Microorganisms 9:274. doi: 10.3390/microorganisms9020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Islam MR, Alam AMS, Eneji AE, Ren C, Song W, Hu Y. 2011. Evaluation of a water-saving superabsorbent polymer for forage oat (Avena saliva L.) production in arid regions of northern China. J Food Agric Environ 9:514–518. [Google Scholar]
- 3.Chen L, Guo G, Yuan XJ, Zhang J, Wen AY, Sun XH, Shao T. 2017. Effect of ensiling whole crop oat with Lucerne in different ratios on fermentation quality, aerobic stability and in vitro digestibility on the Tibetan plateau. J Anim Physiol Anim Nutr (Berl) 101:e144–e153. doi: 10.1111/jpn.12577. [DOI] [PubMed] [Google Scholar]
- 4.Gomes ALM, Jacovaci FA, Bolson DC, Nussio LG, Jobim CC, Daniel JLP. 2019. Effects of light wilting and heterolactic inoculant on the formation of volatile organic compounds, fermentative losses and aerobic stability of oat silage. Anim Feed Sci Technol 247:194–198. doi: 10.1016/j.anifeedsci.2018.11.016. [DOI] [Google Scholar]
- 5.Xie Y, Sun H, Zhang C, Cheng Q, Zheng Y, Wang C, Xiao B, Li P, Chen C. 2022. Ambient ultraviolet radiation: a new factor affecting anaerobic fermentation of oat and subsequent methane emissions. Bioresour Technol 355:127243. doi: 10.1016/j.biortech.2022.127243. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Guo G, Chen L, Li J, Yuan X, Yu C, Shimojo M, Shao T. 2015. Effect of applying lactic acid bacteria and propionic acid on fermentation quality and aerobic stability of oats-common vetch mixed silage on the Tibetan plateau. Anim Sci J 86:595–602. doi: 10.1111/asj.12340. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Dong Z, Li J, Chen L, Shao T. 2019. Pediococcus acidilactici strains as silage inoculants for improving the fermentation quality, nutritive value and in vitro ruminal digestibility in different forages. J Appl Microbiol 126:424–434. doi: 10.1111/jam.14146. [DOI] [PubMed] [Google Scholar]
- 8.Filya I. 2003. The effect of Lactobacillus buchneri, with or without homofermentative lactic acid bacteria, on the fermentation, aerobic stability and ruminal degradability of wheat, sorghum and maize silages. J Appl Microbiol 95:1080–1086. doi: 10.1046/j.1365-2672.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- 9.Sun L, Bai C, Xu H, Na N, Jiang Y, Yin G, Liu S, Xue Y. 2021. Succession of bacterial community during the initial aerobic, intense fermentation, and stable phases of whole-plant corn silages treated with lactic acid bacteria suspensions prepared from other silages. Front Microbiol 12:655095. doi: 10.3389/fmicb.2021.655095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu X, Jiang D, Yuan B, Ni K. 2022. Effect of low-temperature-tolerant lactic acid bacteria on the fermentation quality and bacterial community of oat silage at 5°C vs. 15°C. Fermentation (Basel) 8:158. doi: 10.3390/fermentation8040158. [DOI] [Google Scholar]
- 11.Oliveira AS, Weinberg ZG, Ogunade IM, Cervantes A, Arriola KG, Jiang Y, Kim D, Li X, Goncalves M, Vyas D, Adesogan AT. 2017. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J Dairy Sci 100:4587–4603. doi: 10.3168/jds.2016-11815. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Q, Chen L, Chen Y, Li P, Chen C. 2022. Effects of LAB inoculants on the fermentation quality, chemical composition, and bacterial community of oat silage on the Qinghai-Tibetan plateau. Microorganisms 10:787. doi: 10.3390/microorganisms10040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong Y, Xu J, Guo L, Chen F, Jiang D, Lin Y, Guo C, Li X, Chen Y, Ni K, Yang F. 2022. Exploring the effects of different bacteria additives on fermentation quality, microbial community and in vitro gas production of forage oat silage. Animals (Basel) 12:1122. doi: 10.3390/ani12091122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wang M, Usman S, Li F, Bai J, Zhang J, Guo X. 2022b. Lignocellulose conversion of ensiled Caragana korshinskii Kom. facilitated by Pediococcus acidilactici and cellulases. Microb Biotechnol 16:432–447. doi: 10.1111/1751-7915.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du G, Zhang G, Shi J, Zhang J, Ma Z, Liu X, Yuan C, Li X, Zhang B. 2021. Keystone taxa Lactiplantibacillus and Lacticaseibacillus directly improve the ensiling performance and microflora profile in co-ensiling cabbage byproduct and rice straw. Microorganisms 9:1099. doi: 10.3390/microorganisms9051099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia T, Wang B, Yu Z, Wu Z. 2021. The effects of stage of maturity and lactic acid bacteria inoculants on the ensiling characteristics, aerobic stability and in vitro digestibility of whole‐crop oat silages. Grassl Sci 67:55–62. doi: 10.1111/grs.12285. [DOI] [Google Scholar]
- 17.Zhang Q, Zou X, Wu S, Wu N, Chen X, Zhou W. 2022. Effects of pyroligneous acid on diversity and dynamics of antibiotic resistance genes in alfalfa silage. Microbiol Spectr 10:e01554-22. doi: 10.1128/spectrum.01554-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen D, Zheng M, Guo X, Chen X, Zhang Q. 2021. Altering bacterial community: a possible way of lactic acid bacteria inoculants reducing CO2 production and nutrient loss during fermentation. Bioresour Technol 329:124915. doi: 10.1016/j.biortech.2021.124915. [DOI] [PubMed] [Google Scholar]
- 19.Okoye CO, Wang Y, Gao L, Wu Y, Li X, Sun J, Jiang J. 2023. The performance of lactic acid bacteria in silage production: a review of modern biotechnology for silage improvement. Microbiol Res 266:127212. doi: 10.1016/j.micres.2022.127212. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Lee KD, Choi KC. 2021. Role of LAB in silage fermentation: effect on nutritional quality and organic acid production—an overview. Aims Agric Food 6:216–234. doi: 10.3934/agrfood.2021014. [DOI] [Google Scholar]
- 21.Hu W, Schmidt RJ, McDonell EE, Klingerman CM, Kung LJ. 2009. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J Dairy Sci 92:3907–3914. doi: 10.3168/jds.2008-1788. [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Huan H, Gu H, Xu N, Shen Q, Ding C. 2019. Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour Technol 273:212–219. doi: 10.1016/j.biortech.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 23.Villa R, Ortega Rodriguez L, Fenech C, Anika OC. 2020. Ensiling for anaerobic digestion: a review of key considerations to maximise methane yields. Renew Sustain Energy Rev 134:110401. doi: 10.1016/j.rser.2020.110401. [DOI] [Google Scholar]
- 24.Leibeinsperger RYA, Pitt REA. 1988. Modeling the effects of formic acid and molasses on ensilage. J Dairy Sci 71:1220–1231. doi: 10.3168/jds.S0022-0302(88)79677-0. [DOI] [Google Scholar]
- 25.Kleinschmit DH, Kung LJ. 2006. A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain silages. J Dairy Sci 89:4005–4013. doi: 10.3168/jds.S0022-0302(06)72444-4. [DOI] [PubMed] [Google Scholar]
- 26.Bai J, Ding Z, Su R, Wang M, Cheng M, Xie D, Guo X. 2022. Storage temperature is more effective than lactic acid bacteria inoculations in manipulating fermentation and bacterial community diversity, co-occurrence and functionality of the whole-plant corn silage. Microbiol Spectr 10:e00101-22. doi: 10.1128/spectrum.00101-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Zhao J, Dong Z, Li J, Kaka NA, Shao T. 2020. Sequencing and microbiota transplantation to determine the role of microbiota on the fermentation type of oat silage. Bioresour Technol 309:123371. doi: 10.1016/j.biortech.2020.123371. [DOI] [PubMed] [Google Scholar]
- 28.Muck RE, Nadeau E, McAllister TA, Contreras-Govea FE, Santos MC, Kung LJ. 2018. Silage review: Recent advances and future uses of silage additives. J Dairy Sci 101:3980–4000. doi: 10.3168/jds.2017-13839. [DOI] [PubMed] [Google Scholar]
- 29.Reich LJ, Jr, Kung L. 2010. Effects of combining Lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Anim Feed Sci Technol 159:105–109. doi: 10.1016/j.anifeedsci.2010.06.002. [DOI] [Google Scholar]
- 30.Chen L, Bai S, You M, Xiao B, Li P, Cai Y. 2020. Effect of a low temperature tolerant lactic acid bacteria inoculant on the fermentation quality and bacterial community of oat round bale silage. Anim Feed Sci Technol 269:114669. doi: 10.1016/j.anifeedsci.2020.114669. [DOI] [Google Scholar]
- 31.Wang Y, He L, Xing Y, Zhou W, Pian R, Yang F, Chen X, Zhang Q. 2019. Bacterial diversity and fermentation quality of Moringa oleifera leaves silage prepared with lactic acid bacteria inoculants and stored at different temperatures. Bioresour Technol 284:349–358. doi: 10.1016/j.biortech.2019.03.139. [DOI] [PubMed] [Google Scholar]
- 32.Queiroz O, Ogunade IM, Weinberg Z, Adesogan AT. 2018. Silage review: Foodborne pathogens in silage and their mitigation by silage additives. J Dairy Sci 101:4132–4142. doi: 10.3168/jds.2017-13901. [DOI] [PubMed] [Google Scholar]
- 33.Na N, Qili M, Wu N, Sun L, Xu H, Zhao Y, Wei X, Xue Y, Tao Y. 2022. Bacterial community and fermentation quality of ensiling alfalfa with commercial lactic acid bacterial additives. Front Microbiol 13:836899. doi: 10.3389/fmicb.2022.836899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai Y, Ohmomo S, Ogawa M, Kumai S. 1997. Effect of NaCl-tolerant lactic acid bacteria and NaCl on the fermentation characteristics and aerobic stability of silage. J Appl Microbiol 83:307–313. doi: 10.1046/j.1365-2672.1997.00229.x. [DOI] [PubMed] [Google Scholar]
- 35.McEniry J, Forristal PD, Kiely PO. 2011. Gas composition of baled grass silage as influenced by the amount, stretch, colour and type of plastic stretch-film used to wrap the bales, and by the frequency of bale handling. Grass Forage Sci 66:277–289. doi: 10.1111/j.1365-2494.2011.00788.x. [DOI] [Google Scholar]
- 36.Li P, Zhang Y, Gou W, Cheng Q, Bai S, Cai Y. 2019. Silage fermentation and bacterial community of bur clover, annual ryegrass and their mixtures prepared with microbial inoculant and chemical additive. Anim Feed Sci Technol 247:285–293. doi: 10.1016/j.anifeedsci.2018.11.009. [DOI] [Google Scholar]
- 37.Bai J, Ding Z, Ke W, Xu D, Wang M, Huang W, Zhang Y, Liu F, Guo X. 2021. Different lactic acid bacteria and their combinations regulated the fermentation process of ensiled alfalfa: ensiling characteristics, dynamics of bacterial community and their functional shifts. Microb Biotechnol 14:1171–1182. doi: 10.1111/1751-7915.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero JJ, Zhao Y, Balseca-Paredes MA, Tiezzi F, Gutierrez-Rodriguez E, Castillo MS. 2017. Laboratory silo type and inoculation effects on nutritional composition, fermentation, and bacterial and fungal communities of oat silage. J Dairy Sci 100:1812–1828. doi: 10.3168/jds.2016-11642. [DOI] [PubMed] [Google Scholar]
- 39.Yin X, Zhao J, Wang S, Dong Z, Li J, Shao T. 2022. Separating the chemical and microbial factors of oat harvested at two growth stages to determine the main factor on silage fermentation. J Appl Microbiol 132:4266–4276. doi: 10.1111/jam.15566. [DOI] [PubMed] [Google Scholar]
- 40.Yuan X, Dong Z, Li J, Shao T. 2020. Microbial community dynamics and their contributions to organic acid production during the early stage of the ensiling of Napier grass (Pennisetum purpureum). Grass Forage Sci 75:37–44. doi: 10.1111/gfs.12455. [DOI] [Google Scholar]
- 41.Eikmeyer FG, Kofinger P, Poschenel A, Junemann S, Zakrzewski M, Heinl S, Mayrhuber E, Grabherr R, Puhler A, Schwab H, Schluter A. 2013. Metagenome analyses reveal the influence of the inoculant Lactobacillus buchneri CD034 on the microbial community involved in grass ensiling. J Biotechnol 167:334–343. doi: 10.1016/j.jbiotec.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 42.Ogunade IM, Martinez-Tuppia C, Queiroz OCM, Jiang Y, Drouin P, Wu F, Vyas D, Adesogan AT. 2018. Silage review: Mycotoxins in silage: occurrence, effects, prevention, and mitigation. J Dairy Sci 101:4034–4059. doi: 10.3168/jds.2017-13788. [DOI] [PubMed] [Google Scholar]
- 43.Cai Y. 2001. The role of lactic acid bacteria in the preparation of high quality silage. Grassl Sci 47:527–533. [Google Scholar]
- 44.Qiu Z, Li N, Lu X, Zheng Z, Zhang M, Qiao X. 2018. Characterization of microbial community structure and metabolic potential using Illumina MiSeq platform during the black garlic processing. Food Res Int 106:428–438. doi: 10.1016/j.foodres.2017.12.081. [DOI] [PubMed] [Google Scholar]
- 45.Du Z, Sun L, Chen C, Lin J, Yang F, Cai Y. 2021. Exploring microbial community structure and metabolic gene clusters during silage fermentation of paper mulberry, a high-protein woody plant. Anim Feed Sci Technol 275:114766. doi: 10.1016/j.anifeedsci.2020.114766. [DOI] [Google Scholar]
- 46.Yuan X, Li J, Dong Z, Shao T. 2020. The reconstitution mechanism of Napier grass microbiota during the ensiling of alfalfa and their contributions to fermentation quality of silage. Bioresour Technol 297:122391. doi: 10.1016/j.biortech.2019.122391. [DOI] [PubMed] [Google Scholar]
- 47.Cai Y, Benno Y, Ogawa M, Kumai S. 1999. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J Dairy Sci 82:520–526. doi: 10.3168/jds.S0022-0302(99)75263-X. [DOI] [PubMed] [Google Scholar]
- 48.Cai Y. 2004. Analysis method for silage, p 279–282. In Japanese society of grassland science (ed), Field and laboratory methods for grassland science. Tosho Printing Co. Ltd., Tokyo, Japan. [Google Scholar]
- 49.McDonald P, Henderson AR. 1964. Determination of water-soluble carbohydrates in grass. J Sci Food Agric 15:395–398. doi: 10.1002/jsfa.2740150609. [DOI] [Google Scholar]
- 50.Playne MJ, McDonald P. 1966. The buffering constituents of herbage and of silage. J Sci Food Agric 17:264–268. doi: 10.1002/jsfa.2740170609. [DOI] [Google Scholar]
- 51.Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw sequencing files and associated metadata have been deposited in NCBI’s Sequence Read Archive (SRP410438).