ABSTRACT
Population-based HIV Impact Assessments (PHIAs) are national household (HH) surveys that provide HIV diagnosis and CD4 testing with an immediate return of results. Accurate CD4 results improve HIV-positive participants’ clinical care and inform the effectiveness of HIV programs. Here, we present CD4 results from the PHIA surveys that were conducted in 11 countries in sub-Saharan Africa between 2015 and 2018. All of the HIV-positive participants and 2 to 5% of the HIV-negative participants were offered Pima CD4 (Abbott, IL, USA) point-of-care (POC) tests. The quality of the CD4 test was ensured by conducting instrument verification, comprehensive training, quality control, a review of testing errors and an analysis of unweighted CD4 data by HIV status, age, gender, and antiretroviral (ARV) treatment status. Overall, CD4 testing was completed for 23,085 (99.5%) of the 23,209 HIV-positive and 7,329 (2.7%) of the 270,741 negative participants in 11 surveys. The instrument error rate was 11.3% (range, 4.4% to 15.7%). The median CD4 values among HIV-positive and HIV-negative participants (aged 15+) were 468 cells/mm3 (interquartile range [IQR], 307 to 654) and 811 cells/mm3 (IQR, 647 to 1,013), respectively. Among the HIV-positive participants (aged 15+), those with detectable ARVs had higher CD4 values (508 cells/mm3) than those with undetectable ARVs (385.5 cells/mm3). Among the HIV-positive participants (aged 15+), 11.4% (2,528/22,253) had a CD4 value of less than 200 cells/mm3, and approximately half of them (1,225/2,528 = 48.5%) had detectable ARVs, whereas 51.5% (1,303/2,528) had no detectable ARVs (P < 0.0001). We successfully implemented high quality POC CD4 testing using Pima instruments. Our data come from nationally representative surveys in 11 countries and provide unique insights regarding the CD4 distribution among HIV-positive individuals as well as the baseline CD4 values among HIV-negative individuals.
IMPORTANCE The manuscript describes CD4 levels among HIV-positive individuals and baseline CD4 levels among HIV-negative individuals from 11 sub-Saharan countries, thereby highlighting the importance of CD4 markers in the context of the HIV epidemic. Despite increased ARV access in each country, advanced HIV disease (CD4 < 200 cells/mm3) persists among approximately 11% of HIV-positive individuals. Therefore, it is important that our findings are shared with the scientific community to assist with similar implementations of point-of-care testing and to conduct a review of HIV programmatic gaps.
KEYWORDS: point-of-care CD4, Pima, HIV surveys, PHIA, household testing
INTRODUCTION
CD4 T-cell lymphocytes are the most common indicators of immunologic health and AIDS-related mortality among people living with HIV (PLHIV) (1, 2). CD4 cell counts of <200 cells/mm3 in PLHIV are associated with an increased risk of advanced HIV disease (AHD), other opportunistic infections, and mortality (3–6), and CD4 levels at the time of a new diagnosis can be used to assess HIV program success. Prior to widely available antiretroviral (ARV) therapy and HIV viral load (VL) testing, CD4 cell counts had routinely been used to determine eligibility for ARV treatment and to monitor clinical improvement. Baseline CD4 testing is no longer the standard of care in most sub-Saharan African (SSA) countries and has not been since 2016, when the World Health Organization (WHO) recommended that all confirmed HIV-positive individuals start ARV therapy, regardless of CD4 cell count, and continued to recommend HIV VL for the monitoring of patients who were undergoing an ARV treatment (1). While the utility of CD4 is questioned in the test and treat era, it is still an important component of HIV care (1, 7), particularly for identifying late diagnoses and those with AHD (8).
Historically, CD4 testing required a laboratory setting, expensive and complex technologies, skilled laboratory professionals, and an adherence to a robust specimen transportation protocol to ensure quality of testing. These requirements were not always readily available in resource-limited settings. Moreover, delays in the availability of test results for immediate patient care continued to be a major challenge (9). However, CD4 point-of-care (POC) platforms bring testing capacity to communities outside traditional laboratory settings. Given the discontinuation of routine CD4 testing in SSA, we lost an important data source with which to understand the progress of HIV programs in diagnosing HIV infections earlier or later during AHD, based on CD4 levels (10).
POC instruments facilitate the implementation of CD4 testing in household (HH) surveys with appropriate staff training and quality assurance (QA) practices. The Pima CD4 POC assay (Abbott, IL, USA) has shown a good correlation, compared to standard laboratory methods, and it has indicated feasibility for use in multiple countries and HIV programs (11–13). An added benefit of the Pima CD4 is its Internet connectivity solution, which enables data to be transferred and results to be reviewed in real-time.
Nationally representative Population-based HIV Impact Assessment (PHIA) surveys provide behavioral and laboratory data that are used to evaluate the impact of HIV programs and progress toward the Joint United Nations Programme on HIV and AIDS (UNAIDS) 90-90-90 goals. In this study, we present the results of CD4 testing, including quality indicators, from 11 PHIA surveys that were conducted in SSA, using Pima CD4 instruments in HH settings.
RESULTS
More than 98% of the Pima CD4 analyzers (528/538) met the initial verification criteria, prior to the testing of survey participants. The low-level external quality control (QC) had a mean value of 204 (coefficient of variation [%CV], of 2.63%), and the normal level QC had a mean of 1,020 (CV of 2.15%) across all surveys (data not shown), indicating high reproducibility.
Collectively, 30,221 CD4 tests were conducted (range, 897 to 4,988 tests per country) for survey participants (Table 1). Out of the total tests performed, 3,416 (11.3%) errors were identified by the instrument (range, 4.4% to 15.7% per country). The error rates for the first three surveys (Zimbabwe, Malawi, and Zambia), which were conducted from 2015 to 2016, were higher (1,710/12,155; 14.1%) than those that were subsequently conducted (1,706/18,066; 9.4%). The most common errors were a deteriorated reagent, an air bubble on the reagent control position, or misaligned instrument optics, as defined by the manufacturer. 79 (0.26%) runs were manually aborted by the operators at the time of testing and were excluded from the error analysis. After resolving the errors, more than 99% of the targeted HIV-positive participants received valid CD4 results across the 11 PHIA countries. This analysis excludes 193 HIV-positive individuals who are missing ARV measurements.
TABLE 1.
Total number of Pima instruments and CD4 tests performed in households as well as error frequencies by country, listed in order of population-based HIV impact assessment survey implementation start datea
| Country | Survey start (yr) | Number of PIMAs | Number of CD4 tests performed | Number of CD4 tests performed with an errorb | % error |
|---|---|---|---|---|---|
| Zimbabwe | 2015 | 31 | 4,988 | 781 | 15.7 |
| Zambia | 2015 | 54 | 3,828 | 493 | 12.9 |
| Malawi | 2016 | 27 | 3,339 | 436 | 13.1 |
| Eswatini | 2016 | 52 | 3,377 | 150 | 4.4 |
| Uganda | 2016 | 58 | 1,761 | 258 | 14.7 |
| Tanzania | 2016 | 38 | 2,734 | 238 | 8.7 |
| Lesotho | 2016 | 64 | 3,538 | 381 | 10.8 |
| Namibia | 2017 | 83 | 3,005 | 293 | 9.8 |
| Cameroon | 2017 | 41 | 1,661 | 147 | 8.9 |
| Cote D'Ivoire | 2017 | 30 | 897 | 95 | 10.6 |
| Ethiopia | 2017 | 50 | 1,093 | 144 | 13.2 |
| All countries | 528 | 30,221 | 3,416 | 11.3 | |
Note: A CD4 test with any error is considered invalid, and there is no CD4 result.
Excludes manually aborted CD4 tests (n = 79).
Overall, among the HIV-negative and HIV-positive participants who were aged 15 years and older, the median CD4 cell counts were 811 cells/mm3 and 468 cells/mm3, respectively (Table 2). Furthermore, Table 2 highlights the breakdown between the 7,329 HIV negative participants and the 23,085 HIV-positive survey participants by age group. Among the HIV-negative participants, the median CD4 value ranged from 1,470 cells/mm3 for those aged 0 to 5 years to 776.5 cells/mm3 for those aged 45+ years. A similar decline in the median CD4 value was observed with an increase in age among HIV-positive participants. These values ranged from 995 cells/mm3 to 451 cells/mm3 from those aged 0 to 5 years to those aged 45+ years, respectively. Among the HIV-positive participants, the CD4 cell counts were higher in those with detectable ARVs, compared to those with undetectable ARVs, in each age category (e.g., for all participants aged 15 years and older [508 cells/mm3 versus 385.5 cells/mm3; P < 0.0001]).
TABLE 2.
Overall median CD4 among HIV-positive and HIV-negative individuals (excluding Uganda), reported by age group from aggregate data in 11 population-based HIV impact assessment (PHIA) countries as well as by detectable antiretrovirals (ARV) and undetectable ARVsa
| Ageb (yrs) | HIV negativec (n = 7,329) |
HIV positived |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median (Q1, Q3) | Total (n = 22,892) |
Detectable ARVs (n = 15,439) |
Non-detectable ARVs (n = 7,453) |
|||||
| n | Median (Q1, Q3) | n | Median (Q1, Q3) | n | Median (Q1, Q3) | P valuee | |||
| 0 to 5 | 983 | 1,470 (1,104, 1,938) | 163 | 995 (665, 1,418) | 83 | 1,071 (689, 1,513) | 80 | 857 (640, 1,271) | 0.0229 |
| 6 to 14 | 1,140 | 999 (797, 1,267) | 476 | 772.5 (527, 1,108) | 323 | 884 (587, 1,192) | 153 | 596 (413, 824) | <0.0001 |
| 15 to 29 | 2,659 | 830 (662, 1,025) | 4,919 | 508 (344, 706) | 2,538 | 580 (406, 771) | 2,381 | 441 (295, 618) | <0.0001 |
| 30 to 44 | 1,455 | 807 (656, 1,006) | 10,737 | 461 (299, 642) | 7,394 | 505 (347, 678) | 3,343 | 359 (223, 529) | <0.0001 |
| 45+ | 1,092 | 776.5 (596, 988) | 6,597 | 451 (295, 634) | 5,101 | 478 (326, 654) | 1,496 | 352 (211, 531.5) | <0.0001 |
| 0 to 14 | 2,123 | 1,171 (885, 1,580) | 639 | 823 (561, 1,171) | 406 | 914 (615, 1,244) | 233 | 675 (465, 951) | <0.0001 |
| 15+ | 5,206 | 811 (647, 1,013) | 22,253 | 468 (307, 654) | 15,033 | 508 (349, 686) | 7,220 | 385.5 (242, 563.5) | <0.0001 |
All of the CD4 data are unweighted. Abbreviations: Q1, first quartile; Q3, third quartile.
The eligible age-range to participate in the PHIA study was 0 to 64 years in Cameroon, Côte d’Ivoire, Ethiopia, Malawi, Namibia, Zimbabwe and Uganda; 0 to 59 years in Zambia and Lesotho; and 15+ years in Eswatini and Tanzania.
Excludes Uganda. CD4 cell count measurements for participants who tested HIV-negative were not part of the protocol.
The HIV-positive participants with missing ARV measurements (n = 193) were excluded from analysis.
The Wilcoxon P values are among the HIV-positive groups, comparing detectable and undetectable ARVs.
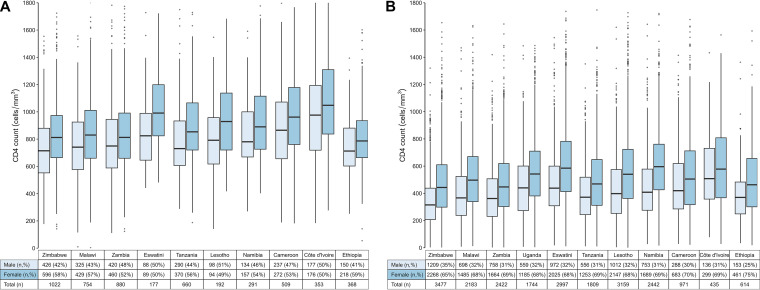
Fig. 1A and B illustrates a box plot comparison of the median CD4 cell counts among males and females (aged 15+ years) and display the interquartile range by country for the HIV-negative (n = 5,206/7,329; 71%) and HIV-positive (n = 22,253/22,892; 97%) participants, respectively. The median CD4 cell count was generally higher for females than for males in both groups. Fig. 1A shows that the median CD4 value among the HIV-negative individuals was 854 cells/mm3 in females versus 759 cells/mm3 in males, whereas for the HIV-positive individuals (Fig. 1B), the median CD4 value was 511 cells/mm3 in females versus 385 cells/mm3 in males. These data also demonstrated country-specific differences among participants. The median CD4 ranged from 755 cells/mm3 in Ethiopia to 1,014 cells/mm3 in Côte d’Ivoire among HIV-negative participants. In comparison, the median CD4 among HIV-positive participants ranged from 394 cells/mm3 in Zimbabwe to 552 cells/mm3 in Côte d’Ivoire.
FIG 1.
Summary of the unweighted median CD4 results from 11 population-based HIV impact assessment surveys, reported by gender (male, female, total) among HIV-negative (A) and HIV-positive (B) individuals (ages 15+ years). Panel A shows the median CD4 for HIV-negative adults (n = 5,206), reported by country and gender. The median CD4 among HIV-negative individuals was 854 cells/mm3 in females versus 759 cells/mm3 in males. 40 of the 5,206 individuals had CD4 values that were greater than 1,800/mm3, and these are not shown. Panel B shows the median CD4 for HIV-positive adults (n = 22,253), reported by country and gender. The median CD4 among HIV-positive individuals was 511 cells/mm3 in females versus 385 cells/mm3 in males.16 of the 5,206 individuals had CD4 values that were greater than 1,800/mm3, and these are not shown. Note that the median measurements exclude Uganda and that the CD4 cell count measurements for participants who tested HIV-negative were not part of the protocol. Also, the Ethiopia survey was conducted only in urban and semiurban settings. Further, note that the horizontal black lines in the vertical center of each box represent the medians. The ends of the box are the Q1 (first quartile) and Q3 (third quartile) values. The vertical box length represents the interquartile range. The vertical lines above and below the boxes extend to the most extreme values that are no further than 1.5 times the interquartile range from each of the upper and lower quartiles. The dots represent outlying CD4 values beyond this range.
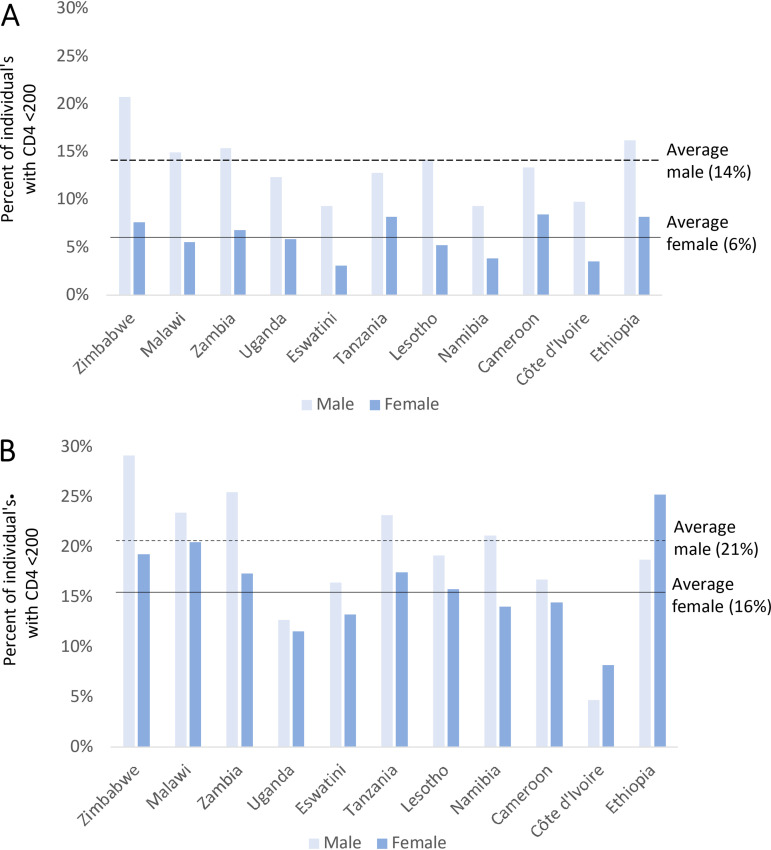
Of the 22,253 CD4 tests conducted among HIV-positive participants (aged 15+), 2,528 (11.4%) individuals had a CD4 value of <200 cells/mm3. Of those individuals with a CD4 value of <200 cells/mm3, 1,225 of the 2,528 (48.5%) and 1,303 of the 2,528 (51.5%) were participants with detectable and undetectable ARVs, respectively. Overall, among those with detectable ARVs, 13.9% of males and 5.7% of females had a CD4 value of <200 cells/mm3, and this varied by country (Fig. 2A). Among those with undetectable ARVs, 21.1% of males and 16.3% of females had a CD4 value of <200 cells/mm3 (Fig. 2B). Only 26/639 (4.1%) participants aged less than 15 years had a CD4 value of <200 cells/mm3 (data not shown). Among the HIV-negative participants, only 20 (0.38%) cases had CD4 counts below 200 cells/mm3 (data not shown).
FIG 2.
Proportion of HIV-positive individuals (aged 15+ years) with CD4 values of less than 200 cells/mm3, reported by gender in 11 population-based HIV impact assessment surveys. The data are unweighted. (A) Detectable antiretrovirals (ARV; n = 1,225). (B) No detectable ARVs (n = 1,303). The overall proportions for males and females are shown by horizontal lines. HIV-positive participants with missing ARV measurements (n = 193) were excluded from the analysis. Header for panel A: Proportion of individuals with CD4 < 200 cells/mm3, among HIV-positive adults (male and female) with detectable ARVs, reported by country. Header for panel B: Proportion of individuals with CD4 < 200 cells/mm3, among HIV-positive adults (male and female) with no detectable ARVs, reported by country.
DISCUSSION
To our knowledge, our assessment is the first to comprehensively present the largest CD4 data set from testing conducted across multiple countries in SSA, using Pima POC instruments in household settings from national population-based surveys. The data set includes a CD4 analysis of 30,221 individuals that includes not only 22,892 HIV-positive individuals but also 7,328 HIV-negative individuals, providing baseline CD4 values for several countries. Since the implementation of test and treat, routine CD4 measurements in HIV programs have decreased substantially (1, 2). However, CD4 testing continues to be an important immunological marker by which to identify patients with AHD, indicating late diagnosis, poor ARV adherence, or patients who are failing treatment so that AHD care is adequately provided (14). Quality CD4 data enables decision makers and program implementers to identify gaps, focus on early diagnosis, and improve prevention interventions.
Prior to 2016, the requirements of CD4 testing before ARV initiation had major limitations regarding the availability of testing. Some of the limitations included requirements for complex laboratory equipment (e.g., FACSCalibur) that required maintenance, trained laboratory personnel, robust specimen transport networks, data management, QA/QC plans, and other resources (15). In 2012, the Kenya AIDS Indicator Survey (KAIS) was the first to use Pima POC instruments in HH settings and, therefore, include a comparison to laboratory based CD4 (16). However, about 54% of the HIV-positive blood specimens were heavily hemolyzed during transport to the central laboratory, limiting the ability to assess the quality of the POC CD4 (17). These challenges and associated poor outcomes further highlighted the importance of ensuring both specimen quality and a robust specimen transport system. Our study demonstrated that CD4 POC testing can be conducted at a HH level with various QA measures and appropriate training. The ongoing monitoring of CD4 among newly diagnosed HIV-positive individuals can provide critical information about the status of HIV diagnoses and about the effectiveness of HIV care and treatment programs.
A thorough review of each equipment verification process, proper hands-on-training for all survey staff regarding specimen collection, the real-time monitoring of QC data, and appropriate troubleshooting have provided valuable data (18). For our surveys, testing was completed for 99.5% of targeted HIV-positive individuals with high quality results. The utilization of Pima CD4 POC instruments has provided added benefits for participants: results are received within 20 min, and data are transmitted to a secure cloud server via an Internet modem. This allowed for the real-time monitoring of all survey data. On average, the CD4 test error rate was 11%, and lessons learned from the first three surveys were implemented in subsequent countries, which likely reduced error rates (Table 1). Additional strategies, such as limiting the number of CD4 testers, increasing practical training time, providing refresher trainings, and reinforcing the use of job aides and standard operating procedures, greatly reduced error rates.
This study has two main limitations. The first limitation is that our data analysis was unweighted to describe both process and outcome results and is therefore not generalizable to national populations. The second limitation is that the Kenya and Rwanda surveys did not include CD4 as part of the survey protocol. These are more recent surveys (2018), so we missed an opportunity to see whether the QA improved as time went on (as well as the data for these countries).
Our results are consistent with those reported by Malaza et al., in which the median CD4 values in both populations (HIV-positive and HIV-negative) were consistently higher in females versus males (19). In addition to the CD4 levels among HIV-positive individuals, we report the median baseline CD4 cell counts among HIV-negative individuals from several countries. Although they are in the expected range, the CD4 levels in Eswatini and Côte d’Ivoire consistently trended higher in both HIV-positive and HIV-negative populations. Our results also demonstrate overall higher median CD4 cell counts in individuals with detectable ARVs, compared to those with undetectable ARVs, which indicates better outcomes for those undergoing treatment. Some of those with undetectable ARVs may be newly diagnosed, and additional analysis is needed for this group to better understand the impact of treatment. Additionally, a decrease in median CD4 cell values was observed with age among HIV-positive and HIV-negative individuals, as expected. The median CD4 cell values in different groups (female versus male, detectable ARVs versus undetectable ARVs) are in the expected range (Table 2; Fig. 1 and 2), further affirming the plausibility of these measurements and the high quality of testing. Successful testing of this magnitude in a HH-based survey has been conducted effectively due to access to POC instrumentation and a strict adherence to quality assurance measures, including training.
Our findings highlight the importance of comprehensive, population based CD4 cell data for national HIV program planning. Despite increased ARV access, based on PHIA CD4 results, AHD persists, on average, in 11% (range, 6% in Côte d’Ivoire to 15% in Zimbabwe) of HIV-positive individuals. More than half in this category were attributed to individuals with undetectable ARVs, perhaps indicating PLHIV who were undiagnosed, untreated, or experienced ART interruption. Balachandra et al. noted AHD persistence in Zimbabwe among those who were virally suppressed (VL < 1000 copies/mL), highlighting a need for further studies on the implementation of AHD care packages to guide the programmatic implementation of CD4 cell testing (8). In addition, population-based CD4 data, including PHIA data, have been used to revise global HIV mortality estimation models (20).
In conclusion, implementing HH POC CD4 testing was complex, in terms of field logistics that included rough terrain, harsh environmental conditions, the recharging of equipment, and unreliable network connectivity. Further, these challenges increased the overall cost of the survey. Despite these challenges, we successfully implemented POC CD4 testing with multilevel QA processes in PHIA surveys, thereby highlighting its importance in the surveys and in future assessments. Our data confirm the feasibility of using POC CD4 instruments in HH settings and provide unique insights regarding CD4 levels and their distribution among HIV-positive and negative individuals from nationally representative HH surveys in 11 countries.
MATERIALS AND METHODS
Survey design and related testing.
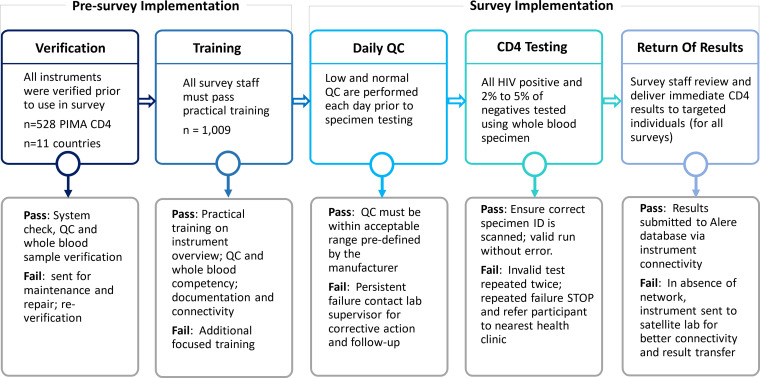
We conducted cross-sectional, household-based surveys between 2015 and 2018, across 11 countries: Zimbabwe, Malawi, Zambia, Uganda, Eswatini, Tanzania, Lesotho, Namibia, Cameroon, Côte d'Ivoire, and Ethiopia. The Ethiopia survey was limited to urban and semiurban settings. The methods that were used to conduct these surveys have been described elsewhere (18, 21). Eligible and consenting (both assent and parental permission for the younger age group) participants, generally aged 0 to 64 years, were offered home-based HIV testing and counseling, using the respective national HIV rapid testing algorithm. Regardless of age, all HIV-positive participants, as well as a random sample (2 to 5%) of HIV-negative participants (excluding Uganda), were offered immediate CD4 testing via the Pima CD4 POC assay in a HH setting. We collected data across 11 countries, using a total of 528 Pima CD4 analyzers (range, 27 to 83 per country) with 1,009 certified survey testers (Fig. 3). Testing was conducted according to the manufacturer’s instructions, using whole blood (venous or capillary) specimens that were collected in an ethylenediaminetetraacetic acid (EDTA) tube. Rapid HIV test and CD4 results were returned to the participants immediately in the HH.
FIG 3.
The standard quality assurance approach for implementing point of care CD4 tests in multiple household, population-based HIV impact assessment (PHIA) surveys. Abbreviations: QC, quality control.
Select first-line and second-line ARV drugs were detected in dried blood spot (DBS) specimens at the University of Cape Town, South Africa, using qualitative high-performance liquid chromatography and a tandem mass spectrometry assay (22).
Instrument verification.
Prior to their deployment in the survey, all of the Pima CD4 analyzers were assessed for mechanical function, accuracy, and precision (Fig. 3). Similarly, all ancillary items, such as printers, barcode scanners, and power adapters were identified by team numbers and were made available to the HH testers. Analyzer precision was determined by calculating the %CV from low and normal level QC values and whole blood specimens’ values to assess analyzer function both for verification and throughout the survey. Both QC materials and whole blood were tested for CD4 over a period of 5 days. Instruments passed verification if they met the preestablished limits for both QC and whole blood specimen testing. Instruments that failed verification were sent to the manufacturer for service.
User training and competency.
Testers were trained to use the Pima CD4 assay, including software, QC testing, specimen collection and handling, testing, documentation, and data transfer. The trainings included instructor-led lectures, demonstrations, small group discussions, practical hands-on training, and instructor feedback from direct observation. The final competency of each trainee was assessed via direct observation, a valid QC run, and a comparison of tester results to specimens of known CD4 values.
QA and data analysis.
QC materials, both low and normal, that were provided by the manufacturer were tested daily for each analyzer. A valid QC run was mandatory, prior to the testing of participant specimens. QC results outside acceptable ranges were repeated once. Repeated failures were reported to the laboratory manager-in-charge for additional follow-up. Instruments with repeated QC failures were not used for the survey. The data were transferred daily via modem into a Sympheos System (formerly Data Point) cloud database and were reviewed by the laboratory and data teams.
The Pima CD4 analyzer has internal checks to validate the mechanics, sample movement, optics, and reagent. The analyzer automatically flags any testing errors if a system check fails, resulting in an error code associated with the failure. The test is considered invalid with any error, and there is no CD4 test result. Any testing event that resulted in an error was repeated to get a valid CD4 result. If errors were not resolved by the HH tester, participants were referred to the nearest health care facility, and the instrument was removed from the field for maintenance. The frequency of error rates was tracked for each analyzer and operator. Results of low and normal QC and coefficient of variation (CV) values were monitored for the precision of each Pima instrument.
All of the CD4 data analyses are limited only to the descriptive techniques that were conducted on the PHIA data without using weights. The median CD4 (interquartile range [IQR]) measurements were analyzed for HIV-positive and HIV negative participants by country and were further disaggregated by age, sex, and the presence or absence of ARVs. The Wilcoxon test was conducted to compare the median CD4 values among the HIV-positive individuals, both with and without detectable ARVs. Additional analyses were conducted among the HIV-positive cases to assess the proportion of individuals with CD4 values of <200 cells/mm3, both overall and in each country. The CD4 data among HIV-positive individuals focuses on those who had ARV measurements. The analyses were performed using SAS software version 9.4 (TS1M6) (SAS Institute Inc., Cary, NC, USA) and R version 4.1.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Ethical considerations.
The PHIA surveys were approved by Institutional Review Boards at the U.S Centers for Disease Control and Prevention, Columbia University, Westat, as well as by the local ethics boards in the respective countries. Written consent was obtained from eligible survey participants. HIV-positive participants were referred to HIV care and treatment services. Further, HIV-positive participants were asked to consent to be contacted by qualified health care professionals, regardless of their CD4 counts, to facilitate active linkage to HIV care and treatment services.
Data Availability.
Data described are publicly available at the following URL: PHIA Project - Guiding the Global HIV Response (columbia.edu).
ACKNOWLEDGMENTS
We thank the many people who have worked toward the success of the PHIA surveys, including, foremost, the participants, as well as the interviewers, laboratory technicians, support staff, analysts, Ministries of Health in each country, and our implementing partner. Additionally, the authors acknowledge the following individuals for their contribution to the implementation of the survey and data collection: Trudy Dobbs, Mervi Detorio, Carole Moore, Katrina Sleeman, Christiane Adje-Toure, Judith Shang, Danielle Payne, Nellie W. Wadonda-Kabondo, Yared Tedla, Yohannes M. Eshete, Norah Vere, Souleymane Sawadogo, Anita Beukes, Ndumbu Pentikainen, Gerhard van Rooyen, Salomo Natanael, Christina Mwangi, Mary Naluguza, Trong Ao, Michael Mwasekaga, Heather Alexander, Larry E. Westerman, Gregory Khoury, Julius Manjengwa, Yvonne Mavengere, Tafadzwa Dzinamarira, Francis Ogollah, Belete Tegbaru Erkyhun, Sakhile Sithole, Peter Nkurunziza, Silas Nunu, and Wessen Nega.
The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), through the CDC under the terms of cooperative agreements number U2GGH001271 and number U2GGH001226, financially supported this work.
The findings and conclusions of this document are those of the authors and do not necessarily represent the official positions of the funding agencies.
Footnotes
[This article was published on 18 April 2023 with errors in Fig. 1 and 2, the Table 2 footnotes, Acknowledgments, and the text. These errors were corrected on 9 May 2023, and further corrections to Fig. 2 and the text were made in the current version, posted on 15 May 2023.]
Contributor Information
Hetal K. Patel, Email: byg7@cdc.gov.
Eleanor A. Powell, University of Cincinnati
REFERENCES
- 1.World Health Organization. 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection (second edition). Accessed October 08, 2020. Geneva. https://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf;jsessionid=5616E5674843EE885681FB0D86B3EA92?sequence=1. [PubMed] [Google Scholar]
- 2.Ford N, et al. 2017. The evolving role of CD4 cell counts in HIV care. Curr Opin HIV AIDS 12:123–128. doi: 10.1097/COH.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. 2018. Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children, Accessed 08 October 2020. Geneva. [PubMed] [Google Scholar]
- 4.Awotiwon AA, et al. 2018. Primary antifungal prophylaxis for cryptococcal disease in HIV-positive people. Cochrane Database Syst Rev 8:CD004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molloy SF, et al. 2018. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med 378:1004–1017. doi: 10.1056/NEJMoa1710922. [DOI] [PubMed] [Google Scholar]
- 6.Rajasingham R, et al. 2017. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 17:873–881. doi: 10.1016/S1473-3099(17)30243-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habiyambere V, et al. 2018. Forecasting the global demand for HIV monitoring and diagnostic tests: a 2016–2021 analysis. PLoS One 13:e0201341. doi: 10.1371/journal.pone.0201341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balachandra S, et al. 2020. Concurrent advanced HIV disease and viral load suppression in a high-burden setting: findings from the 2015–6 ZIMPHIA survey. PLoS One 15:e0230205. doi: 10.1371/journal.pone.0230205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenkranz PD, et al. 2019. The missed potential of CD4 and viral load testing to improve clinical outcomes for people living with HIV in lower-resource settings. PLoS Med 16:e1002820. doi: 10.1371/journal.pmed.1002820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, Lehe JD, Peter TF. 2011. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet 378:1572–1579. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 11.Malagun M, et al. 2014. Multisite evaluation of point of care CD4 testing in Papua New Guinea. PLoS One 9:e112173. doi: 10.1371/journal.pone.0112173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang J, et al. 2015. Evaluation of PIMA point-of-care CD4 analyzer in Yunnan, China. Chin Med J 128:890–895. doi: 10.4103/0366-6999.154283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott LE, et al. 2015. A meta-analysis of the performance of the Pima CD4 for point of care testing. BMC Med 13:168. doi: 10.1186/s12916-015-0396-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leeme TB, et al. 2021. Utility of CD4 count measurement in the era of universal antiretroviral therapy: an analysis of routine laboratory data in Botswana. HIV Med 22:1–10. doi: 10.1111/hiv.12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNITAID. 2015. HIV/AIDS diagnostic technology landscape, 5th edition. Cited 2022 4/1/2022. Available from: http://www.unitaid.org/assets/UNITAID_HIV_Nov_2015_Dx_Landscape-1.pdf.
- 16.Waruiru W, et al. 2014. The Kenya AIDS Indicator Survey 2012: rationale, methods, description of participants, and response rates. J Acquir Immune Defic Syndr 66 Suppl 1:S3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Odhiambo JO, et al. 2014. Antiretroviral treatment scale-up among persons living with HIV in Kenya: results from a nationally representative survey. J Acquir Immune Defic Syndr 66 Suppl 1:S116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel HK, et al. 2021. A comprehensive approach to assuring quality of laboratory testing in HIV surveys: lessons learned from the population-based HIV Impact Assessment Project. J Acquir Immune Defic Syndr 87(Suppl 1):S17–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malaza A, et al. 2013. Population-based CD4 counts in a rural area in South Africa with high HIV prevalence and high antiretroviral treatment coverage. PLoS One 8:e70126. doi: 10.1371/journal.pone.0070126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaubius R, et al. 2021. Disease progression and mortality with untreated HIV infection: evidence synthesis of HIV seroconverter cohorts, antiretroviral treatment clinical cohorts and population-based survey data. J Int AIDS Soc 24 Suppl 5:e25784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sachathep K, et al. 2021. Population-based HIV impact assessments survey methods, response, and quality in Zimbabwe, Malawi, and Zambia. J Acquir Immune Defic Syndr 87(Suppl 1):S6–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koal T, et al. 2005. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 19:2995–3001. doi: 10.1002/rcm.2158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described are publicly available at the following URL: PHIA Project - Guiding the Global HIV Response (columbia.edu).