ABSTRACT
Selenium (Se) is a micronutrient in most eukaryotes, and Se-enriched yeast is the most common selenium supplement. However, selenium metabolism and transport in yeast have remained unclear, greatly hindering the application of this element. To explore the latent selenium transport and metabolism mechanisms, we performed adaptive laboratory evolution under the selective pressure of sodium selenite and successfully obtained selenium-tolerant yeast strains. Mutations in the sulfite transporter gene ssu1 and its transcription factor gene fzf1 were found to be responsible for the tolerance generated in the evolved strains, and the selenium efflux process mediated by ssu1 was identified in this study. Moreover, we found that selenite is a competitive substrate for sulfite during the efflux process mediated by ssu1, and the expression of ssu1 is induced by selenite rather than sulfite. Based on the deletion of ssu1, we increased the intracellular selenomethionine content in Se-enriched yeast. This work confirms the existence of the selenium efflux process, and our findings may benefit the optimization of Se-enriched yeast production in the future.
IMPORTANCE Selenium is an essential micronutrient for mammals, and its deficiency severely threatens human health. Yeast is the model organism for studying the biological role of selenium, and Se-enriched yeast is the most popular selenium supplement to solve Se deficiency. The cognition of selenium accumulation in yeast always focuses on the reduction process. Little is known about selenium transport, especially selenium efflux, which may play a crucial part in selenium metabolism. The significance of our research is in determining the selenium efflux process in Saccharomyces cerevisiae, which will greatly enhance our knowledge of selenium tolerance and transport, facilitating the production of Se-enriched yeast. Moreover, our research further advances the understanding of the relationship between selenium and sulfur in transport.
KEYWORDS: adaptive laboratory evolution, selenium tolerance, selenium efflux, Se-enriched yeast, selenium, yeasts
INTRODUCTION
Selenium (Se) is an essential trace element in human nutrition that widely participates in the formation of selenoproteins and enzymes required for antioxidation, inflammation reduction, thyroid hormone production, DNA synthesis, fertility, and reproduction (1, 2). Due to rapid proliferation and efficient assimilation of many minerals from the environment, yeast cells are commonly used for the production of food and fodder for humans and livestock in the biotechnology industry, especially for the production of selenium supplements. Se-enriched Saccharomyces cerevisiae is one of the most popular sources of selenium supplements (3). During the process of enrichment, yeasts are cultured with inorganic Se sources, selenite (SeO32−) or selenate (SeO42−), to create low-molecular-weight and less toxic organic Se compounds, while selenomethionine (SeMet) is the dominant and bioavailable form in humans and animals (4). Considering the extensive application of Se-enriched yeasts, focusing on the mechanism of selenium accumulation and its metabolism in yeast cells is worthwhile.
Generally, selenium metabolism in yeast principally refers to other elements adjacent to the periodic table, especially sulfur (S). Selenium has high chemical/physical similarity to S; for this reason, Se compounds follow the same metabolic routes as those of S compounds (5). Selenium reduction depends primarily on glutathione (GSH), and selenium assimilation is conducted by sulfur metabolism pathways (4, 6). A few previous studies have noted that the process of intracellular selenium accumulation occurs mainly through the active transport of other ions inside yeast cells. The known selenium transport systems include the sulfate permeases SUL1 and SUL2 for selenate (7) and monocarboxylic conveyer JEN1 and low-affinity (PHO87, PHO90, and PHO91)/high-affinity (PHO84 and PHO89) phosphate transporters for selenite (8, 9). However, until now, there have been no reports related to the selenium efflux process, which may play a crucial part in selenium metabolism and the production of Se-enriched yeast. Consequently, it is still necessary to explore the unknown selenium transport and metabolism mechanisms.
Adaptive laboratory evolution (ALE) is a powerful method to improve strain tolerance against artificial stress and expand the current understanding of molecular mechanisms that confer stress tolerance (10). Under the influence of the proper selective pressure, a microbial strain is propagated continually for hundreds of generations until the desired phenotypes emerge because of the accumulated mutations in the evolution process (11). Combined with whole-genome resequencing, ALE helps investigate the links between the genotype and the phenotype of evolved strains. The genetic basis or potential metabolic mechanism related to tolerance against selective pressure can be easily determined (12). Previously, ALE was frequently used to explore the mechanisms of tolerance to diverse substances in S. cerevisiae, such as glycerol (13), urea (14), weak acids (15), and salinity (16). In most cases, the accumulated mutations during ALE can lead to an elevated metabolism capacity in the cell against selective pressure (17, 18). In other cases, enhanced transporters, or efflux pumps, that are responsible for the generated tolerance can be identified by researchers (19, 20). Finally, based on the findings of ALE, the yield of the corresponding target products can be improved (19).
Inspired by the above-mentioned ALE cases, we employed this method to increase the selenium tolerance of S. cerevisiae to explore the unknown selenium transport and metabolism mechanisms. The workflow used here is summarized in Fig. 1, from the initial determination of the optimal concentration of selenite (prescreening) to the validation of selected mutations (reverse engineering). We found that mutations in the sulfite efflux pump gene ssu1 and its transcription factor gene fzf1 are responsible for increased selenium tolerance. Finally, we confirmed the selenium efflux process mediated by ssu1, which leads to decreased intracellular selenium accumulation and consequently confers hyper-selenium tolerance to the evolved strains. Overall, our work extends the understanding of selenium metabolism and transport and provides a foundation for the optimization of relevant industrial production.
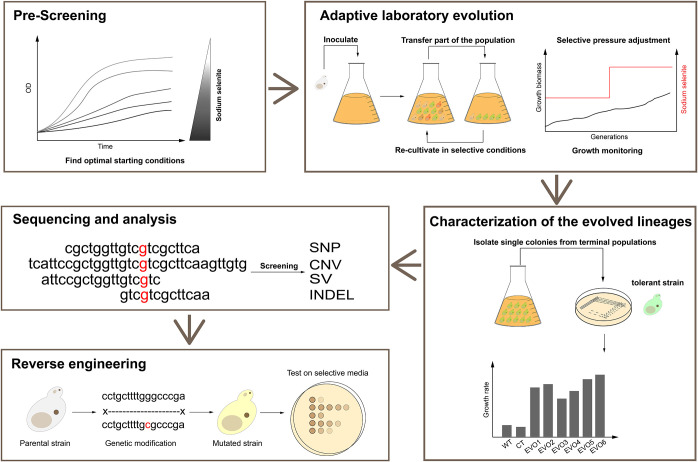
FIG 1.
Scheme of the experimental procedures to investigate selenium tolerance in S. cerevisiae. For prescreening, the growth inhibition of parental strains was measured in the presence of different concentrations of sodium selenite, and the optimal starting conditions were found and applied for subsequent serial cultivations. For adaptive laboratory evolution, strains were serially transferred into fresh YPD medium with the addition of increasing concentrations of sodium selenite for 100 days. For the characterization of the evolved yeast lineages, six clones were isolated from Ev1 to Ev6, and their selenium tolerance was characterized and evaluated. For sequencing and analysis, whole-genome sequencing of six single clones from E1 to E6 and one clone from the starting group was performed, and potentially functional mutations generated in the evolved strains were screened and identified. For reverse engineering, the determined mutations were introduced into the wild-type strain, and their contribution to the tolerance phenotype was tested.
RESULTS AND DISCUSSION
ALE conferred selenium adaptation and resistance to evolved strains.
To determine the suitable selenite concentration before the initialization of the evolution experiment, the growth of S. cerevisiae BY4742 during serial passage with different concentrations of sodium selenite was measured. We found that 50 μM sodium selenite is exactly the starting point to allow the stable reproduction of yeast (data not shown). Three control groups and six evolved groups were set to perform serial transfer for 100 days, and their growth was persistently monitored (see Fig. S1 in the supplemental material).
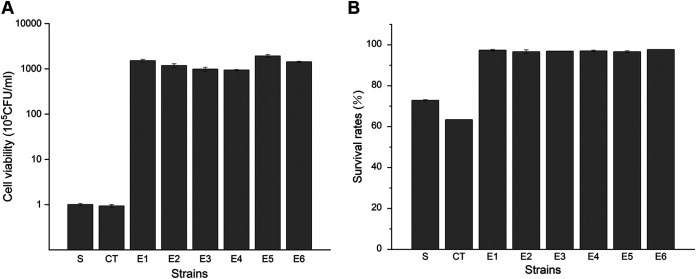
Six strains from the final evolved groups were isolated (denoted E1, E2, E3, E4, E5, and E6), and their selenium adaptation was evaluated by the cell viability and survival rate after selenium treatment. All six evolved strains maintained considerable cell proliferation abilities after incubation with sodium selenite, indicating that they still had high cell viabilities, while the unevolved starting strain (S) and the control strain (CT) showed only very limited cell viability (Fig. 2A). Moreover, after evolving under selenium-selective pressure for 100 days, E1 to E6 could effectively resist continuous selenite stress, and more than 95% of the cells survived, while many cells died in the unevolved S and CT strains (Fig. 2B). Hence, the evolved strains greatly improved their selenium adaptation after long-term evolution from the aspects of cell viability and survival rates.
FIG 2.
Selenium adaptation of the unevolved and evolved strains. The starting strain is denoted S, the control strain (after serial transfer without sodium selenite) is denoted CT, and the six evolved strains are denoted E1 to E6. (A) Viability of various strains after selenium treatment. Cell samples from all tested strains were harvested after culture in YPD medium with 100 μM Na2SeO3 for 16 h, diluted, and plated. After incubation at 30°C for 3 days, the number of colonies on the plates was counted. (B) Survival rates of various strains after selenium treatment. Yeast cells were sampled during serial transfer in selenium-containing YPD medium, stained with propidium iodide, and analyzed by flow cytometry. The proportion of unstained cells was regarded as alive. The error bars represent the means and ranges from three independent experiments.
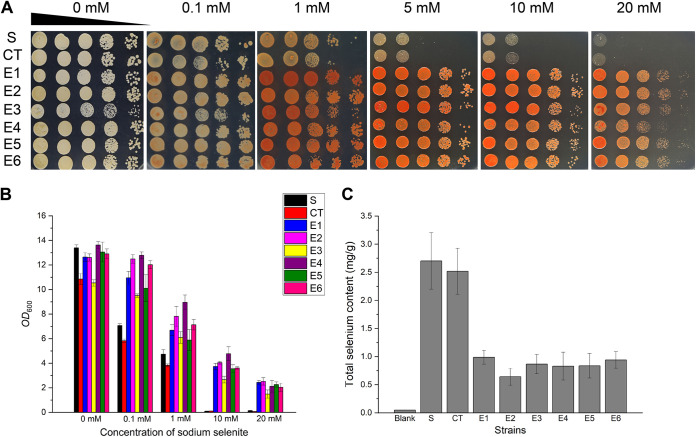
Next, we evaluated the selenium tolerance of evolved and unevolved strains in liquid medium and agar plates. With the same trend as the one described above, the evolved strains showed vigorous growth, even when exposed to 20 mM sodium selenite pressure, whether in liquid medium or on agar plates, while the unevolved S and CT strains could not tolerate 10 mM sodium selenite pressure (Fig. 3A and B). The inconspicuous growth defect of CT may come from degeneration during long-term passage in nutritious yeast extract-peptone-dextrose (YPD) medium without selective pressure. Therefore, all six evolved strains successfully generated strong adaptation and tolerance to sodium selenite after laboratory evolution.
FIG 3.
Selenium tolerance and intracellular selenium content in evolved strains. The starting strain is denoted S, the control strain (after serial transfer without sodium selenite) is denoted CT, and the six evolved strains are denoted E1 to E6. (A) Growth assays on agar plates. After incubation in liquid YPD medium overnight, cells were sampled and spotted onto 1.5% YPD agar plates supplemented with increasing concentrations of Na2SeO3. Pictures were taken 2 days later. (B) Growth assays in liquid medium. All strains were grown at 30°C overnight and then subcultured into fresh YPD liquid medium with increasing concentrations of sodium selenite for 16 h. The final OD600 of each strain was measured and recorded. (C) Intracellular selenium content of the evolved strains. After growth in YPD medium with 100 μM Na2SeO3, yeast cells were sampled and digested, and the selenium content was then analyzed using ICP-MS. The error bars represent the means and ranges from three independent experiments.
To further investigate the possible mechanisms of selenium tolerance in the evolved strains, we measured the intracellular selenium contents of all tested strains. Compared to the unevolved and intolerant S and CT strains, selenium-tolerant strains E1 to E6 had distinctly lower levels of intracellular selenium (Fig. 3C). The lower intracellular selenium content means less selenium-induced toxicity in yeast cells, so the decrease in the intracellular selenium content in E1 to E6 may be the biochemical basis of their tolerant phenotypes. Given that some previous ALE studies of tolerance involved functions of efflux pumps or transporters (20–22), we reasonably speculated that the reduction in the intracellular selenium content in E1 to E6 may be related to an improved selenium efflux capacity. Thus, we performed whole-genome sequencing of S and E1 to E6 to identify potential mutations and verify this hypothesis.
Mutated ssu1 and fzf1 were screened by sequencing analysis.
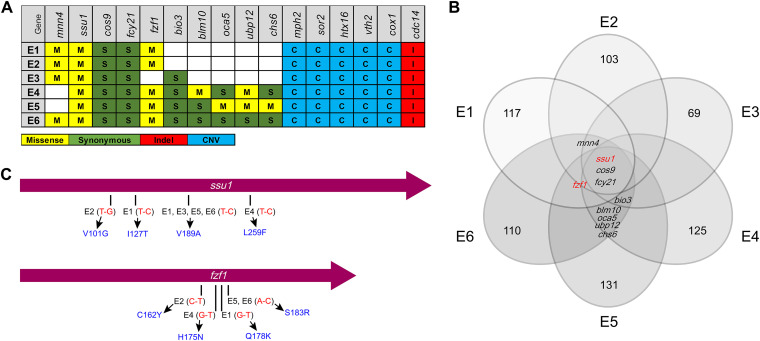
Six evolved strains (E1, E2, E3, E4, E5, and E6) and the S strain were analyzed by whole-genome sequencing, and potential mutations related to the selenium-tolerant phenotype were identified. An average genome coverage of 130× was utilized to screen mutations, and the mutations possibly related to the evolution of E1 to E6 are shown in Fig. 4A and B. To identify potential functional mutations and genes, we determined the most frequently mutated genes (ssu1, cos9, fcy21, fzf1, mnn4, and bio3) that simultaneously occurred in more than 4 parallel groups for further functional verification. Notably, ssu1 is the only gene that occurred with nonsynonymous mutations in all six parallel evolved groups in concert, and missense mutations in fzf1 existed in five evolved groups (Fig. 4C). Thus, the unusual patterns of mutations of ssu1 and fzf1 indicated their paramount effect on selenium tolerance during ALE.
FIG 4.
Summary of the mutated genes identified in evolution experiments. (A) Table of gene mutations present in more than three clones from each independently evolved population. (B) Venn diagram of genes with mutations that occurred and accumulated within E1 to E6. Each circle indicates the number of mutated genes found in the clone. ssu1, cos9, and fcy21 were found in all six evolved groups simultaneously, and fzf1 was found in five evolved groups simultaneously except for E3. mnn4 and bio3 were found in four evolved groups, while blm10, oca5, ubp12, and chs6 were found only in E4, E5, and E6. (C) Mutated positions of E1 to E6 in sequence diagrams of ssu1 and fzf1. The length of ssu1 is 1,377 bp, encoding a 458-aa membrane protein, and the length of fzf1 is 900 bp, encoding a 299-aa transcription factor. Red represents the base substitution, and blue represents the corresponding amino acid substitution.
ssu1 encodes a plasma membrane protein conferring sulfite tolerance to S. cerevisiae (23). Ssu1p is required for efficient sulfite efflux and can directly decrease intracellular sulfite accumulation (24). fzf1 encodes a transcription factor that is involved in sulfite metabolism (25). Fzf1p is composed of five zinc fingers (ZFs) and plays a positive role in the transcription of ssu1 (26). Given the tight regulatory relationship between ssu1 and fzf1 and that they were both mutated in most evolved groups within several positions coincidentally, these results suggested that ssu1 and fzf1 are likely to perform homogeneous functions in selenium metabolism combined with the chemical similarity between selenium and sulfur (27–29). Therefore, we investigated the latent functions of ssu1 and fzf1 in the selenium tolerance of yeasts and then confirmed that both ssu1 and fzf1 indeed play vital roles, while the other four most frequently mutated genes (fcy21, cos9, mnn4, and bio3) screened by sequencing analysis were found to be irrelevant for selenium tolerance (Fig. S2).
ssu1 and fzf1 are involved in selenium tolerance.
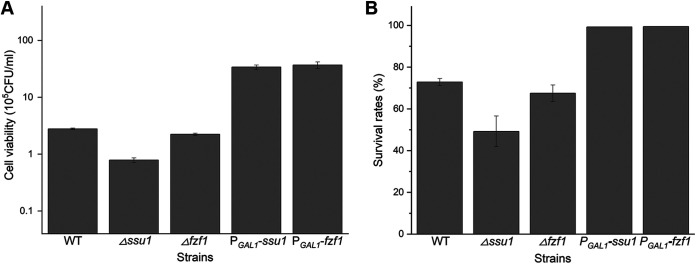
To verify their contributions to selenium adaptation and tolerance, reverse-engineered deletion and overexpression strains of ssu1 and fzf1 were used here. For the deleted strains, after selenium treatment, the Δssu1 strain displayed low cell viabilities (approximately 30% of that of the wild type [WT]) and decreased survival rates (the survival rate of the Δssu1 strain is 45%, while that of the WT is almost 70% in yeast extract-peptone-galactose [YPG] medium), but the Δfzf1 strain did not demonstrate a distinct recession in selenium adaptation compared to the WT, as seen for the Δssu1 strain (Fig. 5). However, the overexpression of ssu1 or fzf1 (PGAL1-ssu1 and PGAL1-fzf1) led to increases in the two indices (their cell viabilities were 12-fold higher than that of the WT, and their survival rates were approximately 100%). Therefore, the expression of ssu1 and fzf1 affected the selenium adaptation of S. cerevisiae.
FIG 5.
ssu1 and fzf1 contribute to selenium adaptation in S. cerevisiae. (A) Viability of deletion and overexpression strains after selenium treatment. Cell samples from all tested strains were harvested after culture in YPG medium with 100 μM Na2SeO3 for 16 h, diluted, and plated. After incubation at 30°C for 3 days, the number of colonies on the plates was counted. (B) Survival rates of deletion and overexpression strains after selenium treatment. Yeast cells were sampled during serial transfer in selenium-containing YPG medium, stained with propidium iodide, and analyzed by flow cytometry. The proportion of unstained cells was regarded as alive. The error bars represent the means and ranges from three independent experiments.
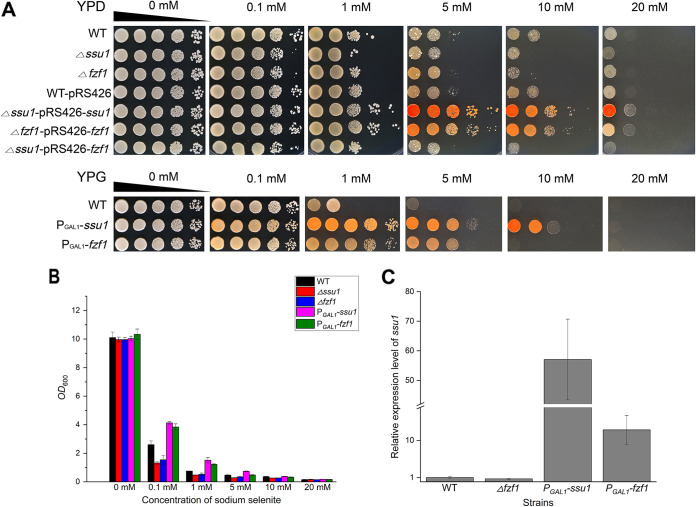
Next, the effect of ssu1 and fzf1 on selenium tolerance was further evaluated. The deletion of ssu1 or fzf1 weakened the growth on selenium-containing plates, and the complemented strains (Δssu1-pRS426-ssu1 and Δfzf1-pRS426-fzf1) efficiently restored the selenium-tolerant phenotype, whereas the overexpression of ssu1 or fzf1 enhanced the growth on selenium-containing plates (Fig. 6A); the same trend was found in assays in liquid medium (Fig. 6B). However, the above-described inferior phenotypes of fzf1 deletion and overexpression compared to ssu1 may be because fzf1 functions by altering the expression level of ssu1 (Fig. 6C). The Δfzf1 strain caused the insufficient activation of ssu1 and caused ssu1 to be expressed at a lower level (approximately 0.7-fold lower than that of the WT), so its tolerance to selenium was lower than that of the WT but higher than that of the Δssu1 strain. Similarly, although PGAL1-fzf1 caused the excessive activation of ssu1 (approximately 12.5-fold higher than that of the WT) by producing many transcription factors to bind the promoter of ssu1, the GAL1 promoter in the PGAL1-ssu1 strain is more powerful and directly upregulates the transcription of ssu1 (approximately 57-fold higher than that of the WT). Therefore, the PGAL1-ssu1 strain is more tolerant to selenium than the PGAL1-fzf1 strain because of the higher expression level of ssu1. The above-mentioned results combined with the abortive tolerance recovery by introducing fzf1 into the Δssu1 strain (Δssu1-pRS426-fzf1) (Fig. 6A) comprehensively indicated that ssu1 is the direct executant in selenium tolerance.
FIG 6.
Selenium tolerance and relative expression levels of ssu1 in the WT, Δfzf1, PGAL1-ssu1, and PGAL1-fzf1 strains. (A) Growth assays on agar plates. After incubation in YPD/YPG medium overnight, cells were sampled and spotted onto 1.5% YPD/YPG agar plates supplemented with increasing concentrations of Na2SeO3. Pictures were taken 2 days later. (B) Growth assays in liquid medium. All strains were grown at 30°C overnight and then subcultured into fresh YPG liquid medium with increasing concentrations of sodium selenite for 16 h. The final OD600 of each strain was measured and recorded. (C) The expression levels of ssu1 in the WT, Δfzf1, PGAL1-ssu1, and PGAL1-fzf1 strains were determined by qRT-PCR. The error bars represent the means and ranges from three independent experiments.
Moreover, the selenium-tolerant phenotype of the overexpression strains (PGAL1-ssu1 and PGAL1-fzf1) is closely associated with E1 to E6, implying that the excellent selenium tolerance of the evolved strains is likely attributed to the overexpression of ssu1 or the increased protein activity of Ssu1p. Hence, we speculated that the mutations of ssu1 and fzf1 screened by sequencing may be the key factors in the selenium tolerance of the evolved strains, and we demonstrated this assumption in the work described below by measuring their contributions to selenium tolerance.
ssu1 decreases intracellular selenium accumulation and is involved in selenium efflux.
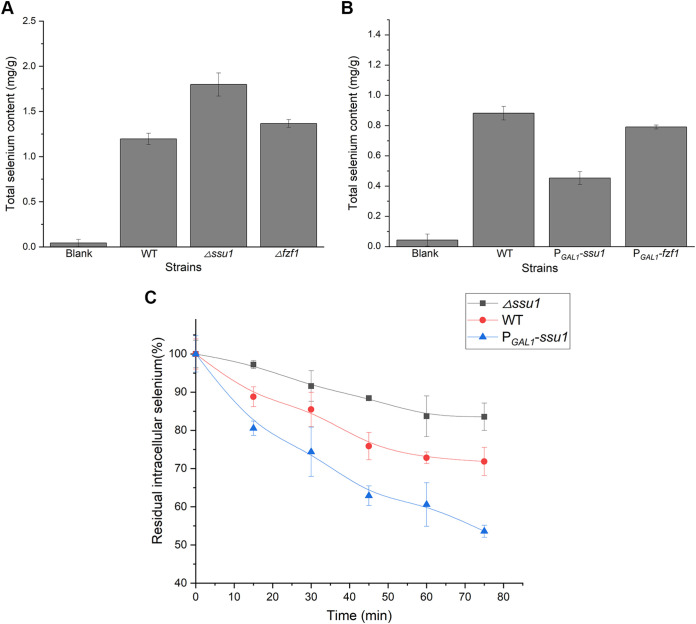
To verify the specific transport function of ssu1 in selenium tolerance, we measured the intracellular selenium contents of the Δssu1, Δfzf1, PGAL1-ssu1, and PGAL1-fzf1 strains. The intracellular selenium content of the Δssu1 strain was 1.5-fold that of WT, while for PGAL1-ssu1, the selenium content was almost half that of the WT (Fig. 7A and B). Meanwhile, the effect of fzf1 on the intracellular selenium content was the same as that of ssu1 but was less obvious. The efflux kinetics of the WT, Δssu1, and PGAL1-ssu1 strains were separately measured, and we found that ssu1 affected intracellular selenium accumulation by altering the selenium efflux capabilities of yeasts. At the end of the selenium efflux measurements, the WT retained approximately 75% residual intracellular selenium, and the Δssu1 strain showed a defective efflux pattern, with approximately 85% residual intracellular selenium remaining, while the PGAL1-ssu1 strain displayed an extremely strong efflux capacity, with only 55% residual intracellular selenium remaining (Fig. 7C).
FIG 7.
ssu1 and fzf1 affect intracellular selenium accumulation, and ssu1 is involved in selenium efflux. After growth in YPD/YPG medium with 100 μM Na2SeO3, yeast cells were sampled and digested, and the selenium content was then analyzed using ICP-MS. (A) The absence of ssu1 and fzf1 contributes to selenium accumulation in yeast cells. (B) The overexpression of ssu1 and fzf1 leads to a decrease in intracellular selenium. (C) Efflux of selenium from the WT, Δssu1, and PGAL1-ssu1 strains. Cells were loaded into YPG medium supplemented with 1 mM Na2SeO3 for 1 h to yield a final total intracellular concentration of 2 to 3 mg/g (dry weight). Selenium efflux was determined in Se-enriched cells suspended in fresh YPG medium by measuring the percent reduction of intracellular selenium. The error bars represent the means and ranges from three independent experiments.
The content of intracellular selenium directly determines the toxicity induced by selenium (30); therefore, the changes in the selenium sensitivity of the Δssu1 and PGAL1-ssu1 strains may originate from the altered ability for intracellular selenium accumulation. When ssu1 was absent (Δssu1) or downregulated (Δfzf1), cells were unable to efficiently pump out toxic selenite to avoid induced damage or stress. When ssu1 was overexpressed (PGAL1-ssu1 and PGAL1-fzf1), the intracellular selenium content was maintained at a minimum level so that cells could tolerate extremely high concentrations of selenium. Our results show that the expression of ssu1 intensely shifted the selenium efflux pattern in yeast and controlled the residual intracellular selenium content, similar to its function in sulfite metabolism (31). Consequently, we concluded that the sulfite efflux pump Ssu1p is also involved in selenium efflux and exerts a similar function.
Mutations in Ssu1p and Fzf1p contribute to selenium tolerance in yeast.
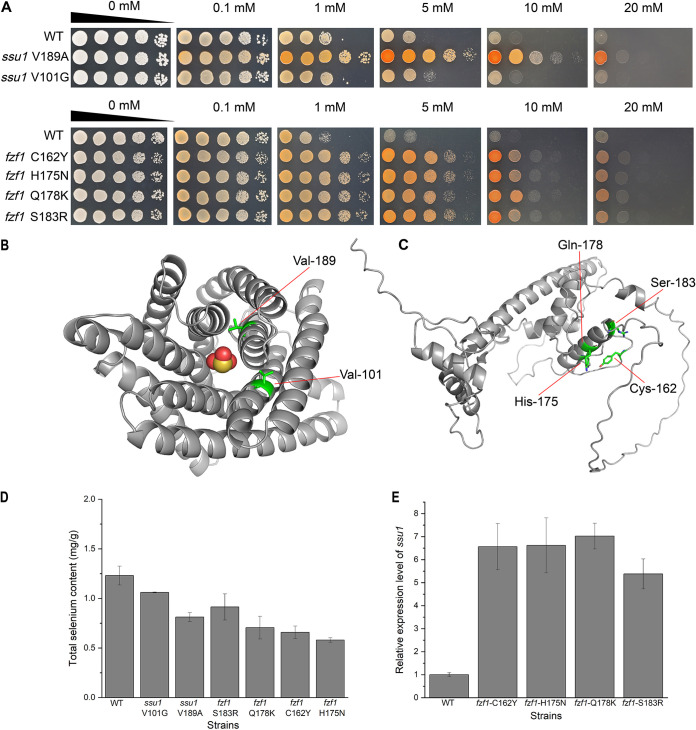
After identifying the essential efflux function of ssu1 for selenite, we began to investigate the point mutations screened from the evolved groups and tried to quantify their contributions to the hyper-selenium tolerance of yeast. Four missense mutations of ssu1 and fzf1 existed in E1 to E6 (Fig. 4C). We separately introduced these mutations into the WT and performed a selenium tolerance assay. Next, two mutations of ssu1 (V101G and V189A) and all four mutations of fzf1 (C162Y, H175N, Q178K, and S183R) were found to confer a considerable enhancement in the selenium tolerance of yeast (Fig. 8A). These mutations not only enabled yeast cells to grow well on YPD agar plates supplemented with up to 10 mM Na2SeO3 but also allowed cells to accumulate less intracellular selenium than in WT cells (Fig. 8D). The growth of the ssu1 V189A mutant was more vigorous against selenite, with a low selenium content compared to that of ssu1 V101G, which may indicate their different contributions to the enhancement of selenium tolerance. All point mutations of fzf1 share similar phenotypes against selenite on plates and decreased intracellular selenium contents, and they all contribute to the expression of ssu1 (Fig. 8E). Undoubtedly, these mutations are functional and significant for the selenium tolerance of E1 to E6.
FIG 8.
Selenium tolerance of point mutation strains and structures of Ssu1p and Fzf1p. (A) Growth assays on agar plates. After incubation in YPD medium overnight, cells were sampled and spotted onto 1.5% YPD agar plates supplemented with increasing concentrations of Na2SeO3. Pictures were taken 2 days later. (B) AlphaFold-predicted structure and functional mutated sites of Ssu1p (https://alphafold.ebi.ac.uk/entry/P41930). The central molecule represents SeO32−. (C) AlphaFold-predicted structure and functional mutated sites of Fzf1p (https://alphafold.ebi.ac.uk/entry/P32805). (D) Measurement of the intracellular selenium contents of mutated strains. (E) Expression levels of ssu1 in mutated fzf1 strains.
The structures predicted by the AlphaFold Protein Structure Database are shown in Fig. 8B. Ssu1p is a transmembrane protein composed of 10 surrounding α-helices and a central transport channel, and the two mutations (V101G and V189A) were located exactly in the channel center or active site of anion transport. Considering that the two substitutions induced by mutations were all from complex and large amino acids (valine) to simple and small amino acids (glycine and alanine), we reasonably suspected that the two mutations may expand the size of the channel center by relieving the steric hindrance produced by the original valine. As shown in Table S4, after evaluating the binding energy of selenite and the channel center of Ssu1p by using AutoDock, we found that the two mutations (V101G and V189A) in Ssu1p both resulted in higher binding energies than that of the WT, indicating that they may lead to an increased affinity for selenite in mutated Ssu1p. Therefore, the efflux ability of point-mutated Ssu1p was likely enhanced so that the mutated strains could tolerate higher selenium stress.
Fzf1p is a transcription factor that contains five ZFs of the Cys2His2 type with striking similarity to the TFIIIA-like zinc finger motif (32). As described in a previous study (33), the fourth ZF of glioblastoma protein (Glip), which is also a five-ZF protein, was involved in DNA recognition. Based on the similarity to Glip, a point mutation of the fourth ZF of Fzf1p was found to detrimentally affect the DNA binding activity (34). Hence, it is likely that the fourth ZF of Fzf1p, ranging from 99 to 182 amino acids (aa) (26), may have an important function in the DNA binding process to impact the expression of ssu1. The four mutations of Fzf1p found in this study (C162Y, H175N, Q178K, and S183R) are all located in the fourth ZF region (Fig. 8C). Moreover, the mutations C162Y and H175N are adjacent in space and are exactly part of the fourth ZF motif (shown in Fig. S3), while the other two mutations nearby (Q178K and S183R) are involved in amino acid charge transformation, so they all may lead to the collapse of the fourth ZF motif and consequently improve the expression of ssu1.
Thus, we have verified that the mutations found in Ssu1p (V101G and V189A) may result in efflux activity promotion, and the mutations found in Fzf1p (C162Y, H175N, Q178K, and S183R) could presumably strengthen the expression of ssu1 by increasing the DNA binding activity. Therefore, the hyper-selenium tolerance of E1 to E6 may come from a combined effect of mutated ssu1 and fzf1.
Selenite is a competitive substrate for sulfite in the efflux process mediated by ssu1.
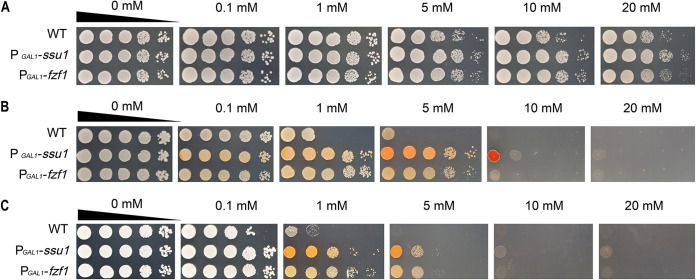
Next, the relationship between the two substrates during their efflux process was explored as our above-described results indicated that the sulfite efflux pump Ssu1p can also function in selenite transport. The growth inhibitions caused by sulfite, selenite, and both were simultaneously measured (Fig. 9). Sulfite shows a very limited inhibition effect on yeast growth; all tested strains can grow well on agar plates with 20 mM sulfite (Fig. 9A), while with the addition of selenite, the growth of the WT was severely suppressed and could be partially restored by the overexpression of ssu1 or fzf1 (Fig. 9B). When yeast cells were enriched with enough sulfite before being spotted onto selenium-containing YPG agar plates, all strains (including PGAL1-ssu1 and PGAL1-fzf1) showed a distinct growth decline even with 1 mM selenite (Fig. 9C).
FIG 9.
The addition of sulfite impairs the selenite tolerance of yeast on YPG agar plates. (A) The growth inhibition of all tested strains was very limited on YPG agar plates with the addition of Na2SO3. (B) On YPG agar plates with the addition of Na2SeO3, the growth of the WT was severely inhibited, while the PGAL1-ssu1 strain could still tolerate up to 10 mM sodium selenite. (C) After incubation with 1 mM Na2SO3 for 6 h, yeast cells were washed, diluted, and spotted onto YPG agar plates with the addition of Na2SeO3, and the growth of all tested strains was inhibited further. The WT can only be sustained with 1 mM, and the PGAL1-ssu1 and PGAL1-fzf1 strains can only be maintained with 5 mM.
Typically, selenium metabolism always refers to the pathways of sulfur (4), and selenite reduction possibly related to upregulated sulfite metabolism pathways has also been reported (35). The sulfite level in the medium had an impact on selenite toxicity (36) and even directly affected selenite uptake or transport (37–39). In our experiment, the presence of intracellular sulfite greatly weakened the selenite tolerance of yeast. When ssu1 was overexpressed (PGAL1-ssu1 and PGAL1-fzf1), cells acquired a more powerful efflux capacity so that selenite and sulfite could be effulged promptly, and the impairment of selenite tolerance induced by sulfite could consequently be relieved. Therefore, we suggest that selenite may be a competitive substrate for sulfite during the efflux process mediated by ssu1, and the existence of sulfite would greatly depress the efflux efficiency of selenite and increase the selenite sensitivity of yeast.
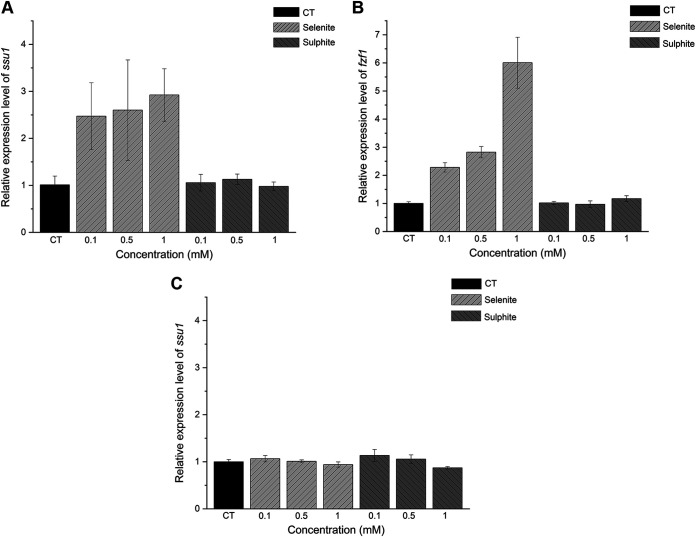
The expression of ssu1 is induced in response to selenite rather than sulfite.
Although selenite and sulfite share the same efflux pathway mediated by ssu1, the expression model of ssu1 for different substrates remains to be discovered. After induction by selenite in the medium for 2 h, the expression level of ssu1 was upregulated, and the inductive effect was gradually improved with increasing selenite concentrations (Fig. 10A). Finally, ssu1 could be upregulated up to 2.9-fold compared to the control with 1 mM sodium selenite. In contrast, after treatment with different concentrations of sulfite, cells did not show a distinct transcription discrepancy, so sulfite seems to have little influence on the expression of ssu1, consistent with the results of a previous report (40). With the same pattern as that of ssu1, the expression level of fzf1 was gradually upregulated by increasing concentrations of selenite (6-fold higher than that of the control in 1 mM sodium selenite) (Fig. 10B). However, when fzf1 is absent, the expression level of ssu1 cannot be altered, implying that the response of ssu1 to sodium selenite is mediated by its transcription factor fzf1 (Fig. 10C).
FIG 10.
The expression level of ssu1 responds to selenite rather than sulfite in S. cerevisiae. (A) Expression levels of ssu1 in S. cerevisiae with different concentrations of sodium selenite or sodium sulfite. (B) Expression levels of fzf1 in S. cerevisiae with different concentrations of sodium selenite or sodium sulfite. (C) Expression levels of ssu1 in the Δfzf1 strain with different concentrations of sodium selenite or sodium sulfite. Yeast cells were incubated in YPD medium for 16 h at 30°C, different concentrations of selenite or sulfite were then added, and the cells were incubated for 2 h at 30°C. The same volume of water was added for the control groups. Finally, cells were harvested, and total RNA was extracted for qRT-PCR analysis.
Although the expression of ssu1 can lead to an increase in the sulfite tolerance of yeast (41), sulfite itself is not a powerful inducer of ssu1 transcription (42). Notably, the expression of ssu1 is induced in response to selenite rather than sulfite, and this response is likely regulated by its sole transcription factor, fzf1. The different responses of cells to selenite and sulfite may come from their divergence of toxicity as the stress imposed by selenite is more urgent and lethal than that imposed by sulfite.
Blocking the selenium efflux process mediated by ssu1 is a potential way to increase intracellular SeMet in Se-enriched yeast.
The efficiency of selenium enrichment is one of the most important qualities of Se-enriched yeast (43). The selenium efflux process mediated by Ssu1p would have negative effects on the production of Se-enriched yeast, which will result in the severely low utilization of raw Se sources. In our work, intracellular selenium accumulation was found to be closely related to the expression of ssu1, and the deletion of ssu1 led to an increased content of intracellular selenium, implying a feasible way to advance Se-enriched yeast production by blocking selenium efflux. Hence, based on the deletion of ssu1, we optimized yeast strains to gain more advantageous Se-enriched characteristics, such as the most stable and valuable intracellular organic form of selenium—SeMet.
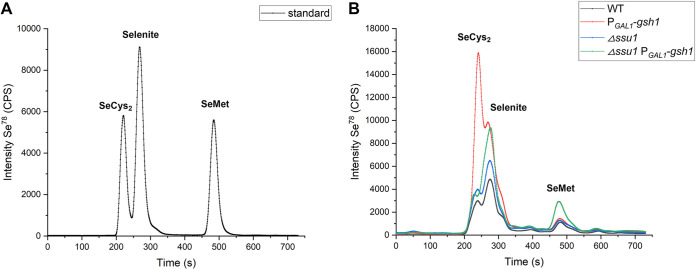
To ensure abundant reducing power in yeast cells to convert inorganic selenium into organic selenium, we overexpressed gsh1 (encoding γ-glutamylcysteine synthetase, which catalyzes the first step in glutathione biosynthesis) in the Δssu1 strain. Compared to the WT, both the Δssu1 and PGAL1-gsh1 strains had higher intracellular levels of SeCys2 and more unconverted inorganic selenium, but they seemed not to increase the SeMet content (Fig. 11B). However, when they were combined, our modified Δssu1-PGAL1-gsh1 strain successfully achieved increased SeMet yields, approximately twice those of the other three strains, meaning that intracellular trapped inorganic selenium can be efficiently transformed into SeMet by enough reduced glutathione. Therefore, our strain construction strategy works well and demonstrates the considerable potential for the industrial production of Se-enriched yeast, especially for intracellular SeMet.
FIG 11.
Characterization of selenium species of Se-enriched yeasts by reversed-phase HPLC–ICP-MS. (A) Chromatograms of selenium species standards (150 ppb) of SeMet (selenomethionine), SeCys2 (selenocystine), and selenite. (B) Chromatograms of the tested yeast strains. All tested strains were inoculated into YPG medium containing 0.1 mM sodium selenite and cultivated for 16 h before being sampled and analyzed.
Conclusion.
In this study, through the adaptive laboratory evolution of sodium selenite, we obtained selenium-tolerant strains and demonstrated that the combined effect generated by mutated Ssu1p and Fzf1p was sufficient to confer selenium tolerance to S. cerevisiae. For the first time, the selenium efflux process mediated by ssu1 has been described. Ssu1p helps yeast cells protect against selenium by decreasing intracellular selenium accumulation, while its transcription factor Fzf1p exerts the same function by adjusting the expression level of ssu1. We found that selenite is a competitive substrate for sulfite during the efflux process, while the expression of ssu1 responds to selenite rather than sulfite. Moreover, based on the deletion of ssu1 and additional intracellular reducing power, we achieved higher intracellular SeMet concentrations in yeast, which may contribute to the industrial production of Se-enriched yeast.
In conclusion, our results suggest a latent selenium transport pathway through the efflux process, which would be a significant complement to the understanding of selenium metabolism and application.
MATERIALS AND METHODS
Yeast strains and media.
The S. cerevisiae strains used in this study are derived from BY4742 (MATa his3Δ1 leu2Δ0 ura3Δ0), obtained from Euroscarf. All yeast strains used in this work are listed in Table 1. YPD medium contained 1% yeast extract (BD, USA), 2% peptone (BD, USA), and 2% dextrose (Amresco, USA). YPG medium contained 1% yeast extract (BD, USA), 2% peptone (BD, USA), and 2% galactose (Amresco, USA). All cultivations were carried out at 30°C.
TABLE 1.
List of S. cerevisiae strains used in this work
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| WT | BY4742 (MATa his3Δ1 leu2Δ0 ura3Δ0) | Euroscarf |
| Δssu1 | BY4742 ssu1Δ::KANMX6 | This work |
| Δfzf1 | BY4742 fzf1Δ::KANMX6 | This work |
| WT-pRS426 | BY4742/pRS426 | This work |
| Δssu1-pRS426-ssu1 | BY4742 ssu1Δ::KANMX6/pRS426-ssu1 | This work |
| Δfzf1-pRS426-fzf1 | BY4742 fzf1Δ::KANMX6/pRS426-fzf1 | This work |
| Δssu1-pRS426-fzf1 | BY4742 ssu1Δ::KANMX6/pRS426-fzf1 | This work |
| Δssu1-PGAL1-gsh1 | BY4742 ssu1Δ::KANMX6 PGAL1-gsh1::URA3 | This work |
| PGAL1-ssu1 | BY4742 PGAL1-ssu1::KANMX6 | This work |
| PGAL1-fzf1 | BY4742 PGAL1-fzf1::KANMX6 | This work |
| PGAL1-gsh1 | BY4742 PGAL1-gsh1::URA3 | This work |
| ssu1 V189A | BY4742 ssu1-V189A::URA3 | This work |
| ssu1 V101G | BY4742 ssu1-V101G::URA3 | This work |
| ssu1 I127T | BY4742 ssu1-I127T::URA3 | This work |
| ssu1 L259F | BY4742 ssu1-L259F::URA3 | This work |
| fzf1 C162Y | BY4742 fzf1-C162Y::URA3 | This work |
| fzf1 H175N | BY4742 fzf1-H175N::URA3 | This work |
| fzf1 Q178K | BY4742 fzf1-Q178K::URA3 | This work |
| fzf1 S183R | BY4742 fzf1-S183R::URA3 | This work |
Adaptive laboratory evolution.
Selenium-tolerant strains were generated from strain BY4742 (MATa his3Δ1 leu2Δ0 ura3Δ0) by serial cultivations in YPD medium containing increasing amounts of sodium selenite for 100 days (Fig. 1). The design of laboratory evolution refers to methods in a previous study (10). Specifically, the parental strain was chosen from a single colony of BY4742 and inoculated into YPD medium with agitation at 200 rpm overnight at 30°C. The culture grown overnight was denoted S (starting strain), and the cells were then reinoculated into nine 250-mL flasks containing 50 mL fresh YPD with agitation at 200 rpm overnight at 30°C. The nine cultures were serially propagated in YPD medium after 24 h independently (adjusting the initial optical density at 600 nm [OD600] to 0.2). Six cultures were supplemented with 50 μM sodium selenite and denoted Ev1 to Ev6 (evolved group) to endow yeast with selenite resistance, while the other three cultures were not and were denoted CT1 to CT3 (control group) to eliminate interference from other factors. When adaptive evolution was conducted for 50 days, another 50-day serial passage was performed to further enhance the selenium resistance phenotype of the evolved groups, and the selenium concentration was increased to 100 μM. In addition, to prevent occasional contamination, the cultures were periodically sampled for microscopic observation and stored in 25% glycerol at −80°C. Finally, the evolution of Ev1 to Ev6 was conducted under continuous sodium selenium for 100 days, and six strains from the final evolved groups were isolated for the following research and denoted E1, E2, E3, E4, E5, and E6.
Strain construction.
The primers and plasmids used in this study are listed in Tables S1 and S3 in the supplemental material, respectively. The deletion strains were constructed by replacing the entire reading frame with a PCR-generated G418 resistance marker cassette (KANMX), and the complemented strains were constructed by introducing yeast expression plasmid pRS426 containing the corresponding gene into the deletion strains. The yeast shuttling expression plasmid pRS426 has an AmpR marker for selection in Escherichia coli and a URA3 marker for selection in yeast, and we placed a TEF promoter and a CYC1 terminator at the multiple-cloning site to control the expression of the inserted gene. The overexpression strains were constructed by integrating a URA3-targeted GAL1 promoter in front of the gene. For the point mutant strains, site-directed PCR mutagenesis was performed to replace the position of interest coupled with a URA3 marker. The manipulation of plasmid DNA or genomic DNA and the transformation of yeast cells were performed according to standard procedures (44).
Selenite tolerance assay.
For the growth assays on agar plates, the tested strains were inoculated into YPD liquid medium and cultivated for 16 h (during the exponential growth phase). Next, all cultures were adjusted to an OD600 of 6.0 and washed twice with sterile water. After serial dilution with sterile water, 3 μL of each dilution was spotted onto 1.5% YPD or YPG agar plates supplemented with various concentrations of sodium selenite. Pictures were taken after 2 days of incubation at 30°C.
For the growth assays in liquid medium, the tested strains were grown at 30°C overnight and then subcultured into fresh YPD or YPG liquid medium with various concentrations of sodium selenite. After 16 h of incubation, the final OD600 of each strain was measured and recorded.
Cell viability and survival tests.
To estimate the cell viability of each strain after selenite treatment, cultures of growing yeast cells were sampled after 16 h of incubation in selenium-containing liquid medium, adjusted to an OD600 of 6.0, and washed twice with sterile water. After proper dilution, cultures of each strain were plated onto 1.5% YPD agar plates. The plates were incubated at 30°C for 3 days, and the number of colonies was counted.
The cell survival rates of each strain after selenite treatment were measured by flow cytometry. After 16 h of incubation in selenium-containing liquid medium, 6 × 107 cells were harvested by centrifugation, washed three times with deionized water, resuspended in 1 mL of 0.85% (wt/vol) NaCl, and stained with 20 μL of the fluorescent probe propidium iodide (PI) (100 μg/mL) for 15 min. Stained cells were analyzed by using a flow cytometer (Beckman Cytoflex), and the fluorescence emission was measured in the logarithmic mode for the PI signal. Unstained and ethanol-treated cells were used as controls. The flow rate during analysis never exceeded 1,000 cells s−1. A total of 2 × 104 cells were measured for each sample. The proportion of PI-stained cells was regarded as dead cells, while the other proportion was counted as alive.
Whole-genome sequencing and analysis.
Genomic DNA samples of six single colonies from the evolved strains (E1, E2, E3, E4, E5, and E6) and one clone from the starting strain (S) were isolated using the Tianamp yeast DNA kit (Tiangen, China) according to the manufacturer’s recommendations.
The DNA samples were subjected to whole-genome resequencing using the Illumina NovaSeq platform with paired-end 2× 150-bp reads targeting a genome coverage of 130× per sample. The processed reads were mapped to the S. cerevisiae BY4742 reference genome (GenBank accession number JRIR00000000.1) with the aligner BWA (version 0.7.12). To improve the accuracy of the prediction of single nucleotide polymorphisms (SNPs), PICARD software (version 1.107) and the GATK package were applied to remove potential errors in sequencing and mapping. SNPs and indels (insertions-deletions) were detected by using GATK and annotated by using ANNOVAR, while the CNV (copy number variation) and SV (structural variation) were investigated using CNVnator (version 0.2.7) and Break Dancer (version 1.1), respectively. Finally, the total variations between S and E1 to E6 were counted and analyzed, and the potential mutated site was determined for further functional verification.
Selenium efflux and intracellular selenium content quantification.
After selenium treatment, 600 μL of yeast cells was rapidly harvested by centrifugation and washed with ice-cold MilliQ water at least three times. Next, the samples were dried in a metal bath at 80°C to obtain the dry weight. To determine selenium efflux, cells during the exponential growth phase were incubated in YPG medium supplemented with 1 mM sodium selenium at 30°C for 1 h, and the cells were then rapidly filtered and washed with ice-cold 75 mM tartaric acid (pH 3.5). Selenium efflux was initiated by resuspending the cell pellet in fresh YPG medium with agitation at 30°C. At appropriate time intervals, 1-mL aliquots were taken and rapidly collected for further measurements.
The samples were digested with 1 mL 65% nitric acid at 120°C for 2 h, diluted properly, and measured by inductively coupled plasma mass spectrometry (ICP-MS). The results are the means of data obtained for each isotope and are expressed in milligrams per gram (selenium content/dry weight).
qRT-PCR analysis.
RNA extraction was conducted according to a method described in a previous study (45). The CFX384 real-time PCR detection system (Bio-Rad Laboratories, CA) was used to perform quantitative reverse transcription–real-time PCR (qRT-PCR) experiments with SYBR quantitative PCR (qPCR) master mix (Vazyme, China). The thermocycler program used was 95°C for 3 min followed by 39 cycles of 95°C for 30 s and 60°C for 1 min. ACT1 was chosen as the reference gene, and the primers used in the qRT-PCR experiments are listed in Table S2. Three parallel experiments were conducted for each gene, and the 2−ΔΔCT method was employed to analyze the data.
Measurement of selenium species.
To determine the intracellular organic selenium contents, especially SeCys2 and SeMet, 6 mL of yeast cells was harvested by centrifugation, washed, and resuspended in 1× phosphate-buffered saline (PBS) (pH 7.5). Cells were disrupted using a Mini-Bead beater-16 instrument for 30 s and cooled on ice for another 30 s, four times. After centrifugation, the supernatant of the lysates was collected, trypsin (1-mg/mL final concentration) and TCS buffer (10×) (0.1 mM Tris-HCl, 10 mM CaCl2, 0.5% SDS) were added, and the samples were incubated at 37°C for 20 h. Next, the samples were further treated with pronase E (1-mg/mL final concentration) for 20 h and finally filtered with 0.22-μm filters to remove undigested proteins. Reversed-phase high-performance liquid chromatography (HPLC)–ICP-MS measurements were performed using an ICP-MS system (Thermo Scientific, USA) for online element-specific detection, coupled with an HPLC system (Dionex, USA) equipped with a Hypersil BDS C18 column (3 μM, 150 mm by 2.1 mm; Thermo Scientific, USA).
Data availability.
All of the data and materials generated in this study can be provided upon a request submitted to the corresponding author for at least 5 years following the publication date.
ACKNOWLEDGMENTS
This work is supported by the National Natural Science Foundation of China (no. 31570090), the National Basic Research Program of China (no. 2013CB933904), and the National Infrastructure of Natural Resources for Science and Technology Program of China (no. NIMR-2022-8).
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplemental material is available online only.
Contributor Information
Zhixiong Xie, Email: zxxie@whu.edu.cn.
Kate S. Howell, University of Melbourne
REFERENCES
- 1.Hariharan S, Dharmaraj S. 2020. Selenium and selenoproteins: it’s [sic] role in regulation of inflammation. Inflammopharmacology 28:667–695. doi: 10.1007/s10787-020-00690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rayman MP. 2000. The importance of selenium to human health. Lancet 356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 3.Yoshinaga M, How S, Blanco D, Murdoch IS, Grudny M, Powers SL, Molina N, Rosen BP, Welch AZ. 2018. Directed evolution of Saccharomyces cerevisiae for increased selenium accumulation. Microorganisms 6:81. doi: 10.3390/microorganisms6030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kieliszek M, Błażejak S, Gientka I, Bzducha-Wróbel A. 2015. Accumulation and metabolism of selenium by yeast cells. Appl Microbiol Biotechnol 99:5373–5382. doi: 10.1007/s00253-015-6650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birringer M, Pilawa S, Flohé L. 2002. Trends in selenium biochemistry. Nat Prod Rep 19:693–718. doi: 10.1039/b205802m. [DOI] [PubMed] [Google Scholar]
- 6.Tarze A, Dauplais M, Grigoras I, Lazard M, Ha-Duong N-T, Barbier F, Blanquet S, Plateau P. 2007. Extracellular production of hydrogen selenide accounts for thiol-assisted toxicity of selenite against Saccharomyces cerevisiae. J Biol Chem 282:8759–8767. doi: 10.1074/jbc.M610078200. [DOI] [PubMed] [Google Scholar]
- 7.Mapelli V, Hillestrøm PR, Patil K, Larsen EH, Olsson L. 2012. The interplay between sulphur and selenium metabolism influences the intracellular redox balance in Saccharomyces cerevisiae. FEMS Yeast Res 12:20–32. doi: 10.1111/j.1567-1364.2011.00757.x. [DOI] [PubMed] [Google Scholar]
- 8.McDermott JR, Rosen BP, Liu Z. 2010. Jen1p: a high affinity selenite transporter in yeast. Mol Biol Cell 21:3934–3941. doi: 10.1091/mbc.E10-06-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazard M, Blanquet S, Fisicaro P, Labarraque G, Plateau P. 2010. Uptake of selenite by Saccharomyces cerevisiae involves the high and low affinity orthophosphate transporters. J Biol Chem 285:32029–32037. doi: 10.1074/jbc.M110.139865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkler J, Reyes LH, Kao KC. 2013. Adaptive laboratory evolution for strain engineering. Methods Mol Biol 985:211–222. doi: 10.1007/978-1-62703-299-5_11. [DOI] [PubMed] [Google Scholar]
- 11.Conrad TM, Lewis NE, Palsson BØ. 2011. Microbial laboratory evolution in the era of genome-scale science. Mol Syst Biol 7:509. doi: 10.1038/msb.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portnoy VA, Bezdan D, Zengler K. 2011. Adaptive laboratory evolution—harnessing the power of biology for metabolic engineering. Curr Opin Biotechnol 22:590–594. doi: 10.1016/j.copbio.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Kutyna DR, Varela C, Stanley GA, Borneman AR, Henschke PA, Chambers PJ. 2012. Adaptive evolution of Saccharomyces cerevisiae to generate strains with enhanced glycerol production. Appl Microbiol Biotechnol 93:1175–1184. doi: 10.1007/s00253-011-3622-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Cheng Y, Li Y, Du G, Xie G, Zou H, Zhou J, Chen J. 2018. Adaptive evolution relieves nitrogen catabolite repression and decreases urea accumulation in cultures of the Chinese rice wine yeast strain Saccharomyces cerevisiae XZ-11. J Agric Food Chem 66:9061–9069. doi: 10.1021/acs.jafc.8b01313. [DOI] [PubMed] [Google Scholar]
- 15.Mira NP, Teixeira MC, Sá-Correia I. 2010. Adaptive response and tolerance to weak acids in Saccharomyces cerevisiae: a genome-wide view. OMICS 14:525–540. doi: 10.1089/omi.2010.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dhar R, Sägesser R, Weikert C, Yuan J, Wagner A. 2011. Adaptation of Saccharomyces cerevisiae to saline stress through laboratory evolution. J Evol Biol 24:1135–1153. doi: 10.1111/j.1420-9101.2011.02249.x. [DOI] [PubMed] [Google Scholar]
- 17.Strucko T, Zirngibl K, Pereira F, Kafkia E, Mohamed ET, Rettel M, Stein F, Feist AM, Jouhten P, Patil KR, Forster J. 2018. Laboratory evolution reveals regulatory and metabolic trade-offs of glycerol utilization in Saccharomyces cerevisiae. Metab Eng 47:73–82. doi: 10.1016/j.ymben.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Matson MM, Cepeda MM, Zhang A, Case AE, Kavvas ES, Wang X, Carroll AL, Tagkopoulos I, Atsumi S. 2022. Adaptive laboratory evolution for improved tolerance of isobutyl acetate in Escherichia coli. Metab Eng 69:50–58. doi: 10.1016/j.ymben.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Pereira R, Wei Y, Mohamed E, Radi M, Malina C, Herrgård MJ, Feist AM, Nielsen J, Chen Y. 2019. Adaptive laboratory evolution of tolerance to dicarboxylic acids in Saccharomyces cerevisiae. Metab Eng 56:130–141. doi: 10.1016/j.ymben.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, Sun T, Li S, Chen L, Zhang W. 2018. Adaptive laboratory evolution of cadmium tolerance in Synechocystis sp. PCC 6803. Biotechnol Biofuels 11:205. doi: 10.1186/s13068-018-1205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kusumawardhani H, Furtwängler B, Blommestijn M, Kaltenytė A, van der Poel J, Kolk J, Hosseini R, de Winde JH. 2021. Adaptive laboratory evolution restores solvent tolerance in plasmid-cured Pseudomonas putida S12: a molecular analysis. Appl Environ Microbiol 87:e00041-21. doi: 10.1128/AEM.00041-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Q, Yang Y, Tang Y, Wang X, Chen Y, Shen W, Zhan Y, Gao J, Wu B, He M, Chen S, Yang S. 2020. Development and characterization of acidic-pH-tolerant mutants of Zymomonas mobilis through adaptation and next-generation sequencing-based genome resequencing and RNA-Seq. Biotechnol Biofuels 13:144. doi: 10.1186/s13068-020-01781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avram D, Bakalinsky AT. 1997. SSU1 encodes a plasma membrane protein with a central role in a network of proteins conferring sulfite tolerance in Saccharomyces cerevisiae. J Bacteriol 179:5971–5974. doi: 10.1128/jb.179.18.5971-5974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H, Bakalinsky AT. 2000. SSU1 mediates sulphite efflux in Saccharomyces cerevisiae. Yeast 16:881–888. doi:. [DOI] [PubMed] [Google Scholar]
- 25.Avram D, Bakalinsky AT. 1996. Multicopy FZF1 (SUL1) suppresses the sulfite sensitivity but not the glucose derepression or aberrant cell morphology of a grr1 mutant of Saccharomyces cerevisiae. Genetics 144:511–521. doi: 10.1093/genetics/144.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avram D, Leid M, Bakalinsky AT. 1999. Fzf1p of Saccharomyces cerevisiae is a positive regulator of SSU1 transcription and its first zinc finger region is required for DNA binding. Yeast 15:473–480. doi:. [DOI] [PubMed] [Google Scholar]
- 27.Chawla M, Poater A, Besalú-Sala P, Kalra K, Oliva R, Cavallo L. 2018. Theoretical characterization of sulfur-to-selenium substitution in an emissive RNA alphabet: impact on H-bonding potential and photophysical properties. Phys Chem Chem Phys 20:7676–7685. doi: 10.1039/c7cp07656h. [DOI] [PubMed] [Google Scholar]
- 28.Đorđević IS, Popadić M, Sarvan M, Petković-Benazzouz M, Janjić GV. 2020. Supramolecular insight into the substitution of sulfur by selenium, based on crystal structures, quantum-chemical calculations and biosystem recognition. Acta Crystallogr B Struct Sci Cryst Eng Mater 76:122–136. doi: 10.1107/S2052520619016287. [DOI] [PubMed] [Google Scholar]
- 29.Melnikov P, Nascimento VA, Silva AF, Consolo LZ. 2014. Structural modeling of djenkolic acid with sulfur replaced by selenium and tellurium. Molecules 19:4847–4856. doi: 10.3390/molecules19044847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spallholz JE. 1994. On the nature of selenium toxicity and carcinostatic activity. Free Radic Biol Med 17:45–64. doi: 10.1016/0891-5849(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 31.Casalone E, Colella CM, Daly S, Gallori E, Moriani L, Polsinelli M. 1992. Mechanism of resistance to sulphite in Saccharomyces cerevisiae. Curr Genet 22:435–440. doi: 10.1007/BF00326407. [DOI] [PubMed] [Google Scholar]
- 32.Breitwieser W, Price C, Schuster T. 1993. Identification of a gene encoding a novel zinc finger protein in Saccharomyces cerevisiae. Yeast 9:551–556. doi: 10.1002/yea.320090512. [DOI] [PubMed] [Google Scholar]
- 33.Pavletich NP, Pabo CO. 1993. Crystal structure of a five-finger GLI-DNA complex: new perspectives on zinc fingers. Science 261:1701–1707. doi: 10.1126/science.8378770. [DOI] [PubMed] [Google Scholar]
- 34.Casalone E, Colella CM, Daly S, Fontana S, Torricelli I, Polsinelli M. 1994. Cloning and characterization of a sulphite-resistance gene of Saccharomyces cerevisiae. Yeast 10:1101–1110. doi: 10.1002/yea.320100812. [DOI] [PubMed] [Google Scholar]
- 35.Jia H, Huang S, Cheng S, Zhang X, Chen X, Zhang Y, Wang J, Wu L. 2022. Novel mechanisms of selenite reduction in Bacillus subtilis 168: confirmation of multiple-pathway mediated remediation based on transcriptome analysis. J Hazard Mater 433:128834. doi: 10.1016/j.jhazmat.2022.128834. [DOI] [PubMed] [Google Scholar]
- 36.Li Z-Y, Guo S-Y, Li L. 2003. Bioeffects of selenite on the growth of Spirulina platensis and its biotransformation. Bioresour Technol 89:171–176. doi: 10.1016/s0960-8524(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 37.Hudman JF, Glenn AR. 1984. Selenite uptake and incorporation by Selenomonas ruminantium. Arch Microbiol 140:252–256. doi: 10.1007/BF00454937. [DOI] [PubMed] [Google Scholar]
- 38.Gharieb MM, Gadd GM. 2004. The kinetics of 75[Se]-selenite uptake by Saccharomyces cerevisiae and the vacuolization response to high concentrations. Mycol Res 108:1415–1422. doi: 10.1017/s0953756204001418. [DOI] [PubMed] [Google Scholar]
- 39.Park YC, Whanger PD. 1995. Toxicity, metabolism and absorption of selenite by isolated rat hepatocytes. Toxicology 100:151–162. doi: 10.1016/0300-483x(95)03080-y. [DOI] [PubMed] [Google Scholar]
- 40.Park H, Hwang Y-S. 2008. Genome-wide transcriptional responses to sulfite in Saccharomyces cerevisiae. J Microbiol 46:542–548. doi: 10.1007/s12275-008-0053-y. [DOI] [PubMed] [Google Scholar]
- 41.Liu XZ, Sang M, Zhang XA, Zhang TK, Zhang HY, He X, Li SX, Sun XD, Zhang ZM. 2017. Enhancing expression of SSU1 genes in Saccharomyces uvarum leads to an increase in sulfite tolerance and a transcriptome profile change. FEMS Yeast Res 17:fox023. doi: 10.1093/femsyr/fox023. [DOI] [PubMed] [Google Scholar]
- 42.Aranda A, Jiménez-Martí E, Orozco H, Matallana E, Del Olmo M. 2006. Sulfur and adenine metabolisms are linked, and both modulate sulfite resistance in wine yeast. J Agric Food Chem 54:5839–5846. doi: 10.1021/jf060851b. [DOI] [PubMed] [Google Scholar]
- 43.Demirci A, Pometto AL, III, Cox DJ. 1999. Enhanced organically bound selenium yeast production by fed-batch fermentation. J Agric Food Chem 47:2496–2500. doi: 10.1021/jf9811976. [DOI] [PubMed] [Google Scholar]
- 44.Gietz RD, Woods RA. 2006. Yeast transformation by the LiAc/SS carrier DNA/PEG method. Methods Mol Biol 313:107–120. doi: 10.1385/1-59259-958-3:107. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Liu J, Wang X, Zhao L, Chen Q, Zhao W. 2009. A waterbath method for preparation of RNA from Saccharomyces cerevisiae. Anal Biochem 384:189–190. doi: 10.1016/j.ab.2008.09.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download spectrum.01326-23-s0001.docx, DOCX file, 6.8 MB (6.8MB, docx)
Data Availability Statement
All of the data and materials generated in this study can be provided upon a request submitted to the corresponding author for at least 5 years following the publication date.