ABSTRACT
Skin microbiome dysbiosis with a Staphylococcus overabundance is a feature of actinic keratosis (AK) and squamous skin carcinoma (SCC) patients. The impact of lesion-directed treatments for AK lesions such as diclofenac (DIC) and cold atmospheric plasma (CAP) on the lesional microbiome is not established. We studied 321 skin microbiome samples of 59 AK patients treated with DIC 3% gel versus CAP. Microbial DNA from skin swabs taken before start of treatment (week 0), at the end of the treatment period (week 24), and 3 months after end of treatment (week 36) was analyzed after sequencing the V3/V4 region of the 16S rRNA gene. The relative abundance of S. aureus was scrutinized by a tuf gene specific TaqMan PCR assay. The total bacterial load and both, relative and absolute abundance of Staphylococcus genus was reduced upon both therapies at week 24 and 36 compared to week 0. Notably, the lesional microbiome of patients responding to CAP therapy at week 24 was characterized by an increased relative abundance of Corynebacterium genus compared to nonresponders. A higher relative abundance of Staphylococcus aureus at week 36 was a feature of patients classified as nonresponders for both treatments 12 weeks after therapy completion. The reduction of the Staphylococcus abundance after treatment of AK lesions and alterations linked to treatment response encourage further studies for investigation of the role of the skin microbiome for both, the carcinogenesis of epithelial skin cancer and its function as predictive therapeutic biomarker in AK.
IMPORTANCE The relevance of the skin microbiome for development of actinic keratosis (AK), its progression into squamous skin cancer, and for field-directed treatment response is unknown. An overabundance of staphylococci characterizes the skin microbiome of AK lesions. In this study, analyses of the lesional microbiome from 321 samples of 59 AK patients treated with diclophenac gel versus cold atmospheric plasma (CAP) revealed a reduced total bacterial load and reduced relative and absolute Staphylococcus genus abundance upon both treatments. A higher relative Corynebacterium abundance was a feature of patients classified as responders at the end of CAP-treatment period (week 24) compared with nonresponders and the Staphylococcus aureus abundance of patients classified as responders 3 months after treatment completion was significantly lower than in nonresponders. The alterations of the skin microbiome upon AK treatment encourage further investigations for establishing its role for carcinogenesis and its function as predictive biomarker in AK.
KEYWORDS: skin microbiome, CAP, cold atmospheric plasma, diclofenac, actinic keratosis, treatment
INTRODUCTION
Actinic keratoses (AK) are premalignant keratotic lesions of the skin that may progress to squamous cell carcinoma (SCC), the second most common skin malignancy worldwide after basal cell carcinomas (1). Chronic UV-light exposure is a major risk factor for development of AK. Immunosuppressive medication, high age, light skin color, and chronic inflammation additionally predispose for AK development (2, 3). Microorganisms are supposed to contribute to chronic inflammatory skin diseases such as atopic dermatitis (4), but the relevance of the skin microbiome for AK development, for its progression into SCC, and for AK treatment response is not clarified yet. However, an increased prevalence of β-human papillomavirus and Staphylococcus aureus characterizes the skin microbiome of AK patients (2). β-human papillomavirus has been suggested to promote the survival of UV-light exposed DNA-damaged cells and thereby promote the evolvement of cancer cells (5). Staphylococcus aureus may cause or maintain skin inflammation and induces beta-defensin production of keratinocyte cell lines increasing keratinocyte proliferation (6). AK treatment includes field-directed anti-inflammatory therapies, e.g., diclofenac (DIC) 3% gel that inhibits prostaglandin synthesis (7). We have shown that cold atmospheric argon plasma (CAP), a partly ionized gas that contains reactive oxygen and nitrogen species, is a new option for AK treatment with comparable efficiency to conventional treatments and less side effects compared with DIC (8). In the present study, we analyzed the skin microbiome longitudinally in patients with AK treated for a period of 24 weeks with CAP versus DIC 3% gel of two comparable AK regions. To our knowledge, changes of the skin microbiome of AK patients in response to field-directed treatments have not been investigated longitudinally yet. We observed a reduction of the absolute and relative abundance of Staphylococcus after treatment, mainly attributed to coagulase-negative staphylococci (CoNS). Treatment response to either DIC or CAP therapy at week 36 was linked to a lower relative S. aureus abundance in responders than in nonresponding patients. The AK microbiome of patients responding to CAP therapy at the time point of therapy completion (week 24) was characterized by a higher relative abundance of the Corynebacterium genus compared to the AK microbiome of patients not responding to CAP treatment.
RESULTS
Regional differences in the skin microbiome of AK lesions.
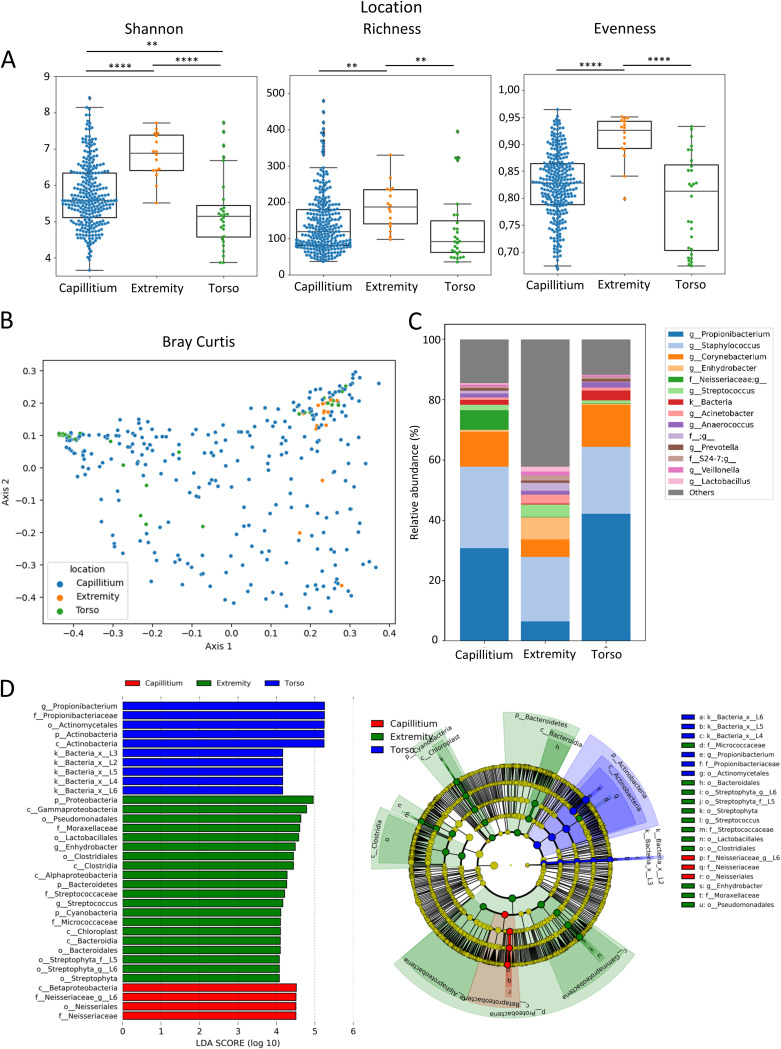
The skin microbiome of two comparable AK areas was investigated in 59 participants. In each patient, one area was treated with CAP and the other with DIC. Skin microbiome swabs were taken at three time points: before start of treatment (week 0), at the end of treatment (week 24), and at the end of the study at week 36. The mean age of the individuals was 74 years and 56 of 59 patients (95%) were male. The skin microbiome from AK lesions was investigated from capillitium in 51, torso in 5, and extremities in 3 patients. As the skin microbiome of healthy individuals varies significantly depending on the body region, we first studied the extent of the differences of the skin microbiome linked to the AK localization. Alpha diversity metrics, including Shannon diversity, richness, and evenness were significantly higher in skin swabs from extremity AK lesions compared to those from capillitium and torso (Fig. 1A). In addition, Shannon diversity was higher from AK areas of capillitium compared to the torso region. Significant differences in the skin microbiome beta diversity were determined by Principal Coordinates analysis (PCoA) of Bray Curtis distance matrix with P < 0.001 (Fig. 1B). While the genus Cutibacterium (previous nomenclature Propionibacterium) and phylum Actinobacteria were enriched in the torso region and were biomarkers of the skin microbiome for this location as identified by LEfSe (Linear discriminant analysis Effect Size) analysis, the family Neisseriaceae was a biomarker for the skin microbiome of patients with AK of the capillitium (Fig. 1 C and D). Several taxa, including the genera Streptococcus, Enhydrobacter, and the phylum Proteobacteria were enriched and biomarkers for AK of extremities.
FIG 1.
(A) Shannon diversity, bacterial richness, determined by observed features (amplicon sequence variants [ASV]), and evenness in skin microbiome samples from capillitium (blue), extremity (orange) and torso (green). Kruskal-Wallis test was used to test for significant differences among groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. (B) Principal coordinates analysis (PCoA) of skin microbiome samples from capillitium (blue), extremity (orange) and torso (green) of Bray-Curtis distance matrix. PERMANOVA multivariate analysis was used to test for significant differences. (C) Mean relative abundance of the 15 most abundant genera in skin microbiome samples from capillitium, extremity, and torso. (D) Linear discriminant effect size (LEfSe) analysis of bacterial taxa enriched in Capillitium (red), extremity (green) and torso (blue). The cladogram reports the taxa showing different abundance values (LDA score >4) according to LEfSe. AK locations are presented in the color of the most abundant group.
Relative Staphylococcus abundance is reduced in the AK skin microbiome of treated patients.
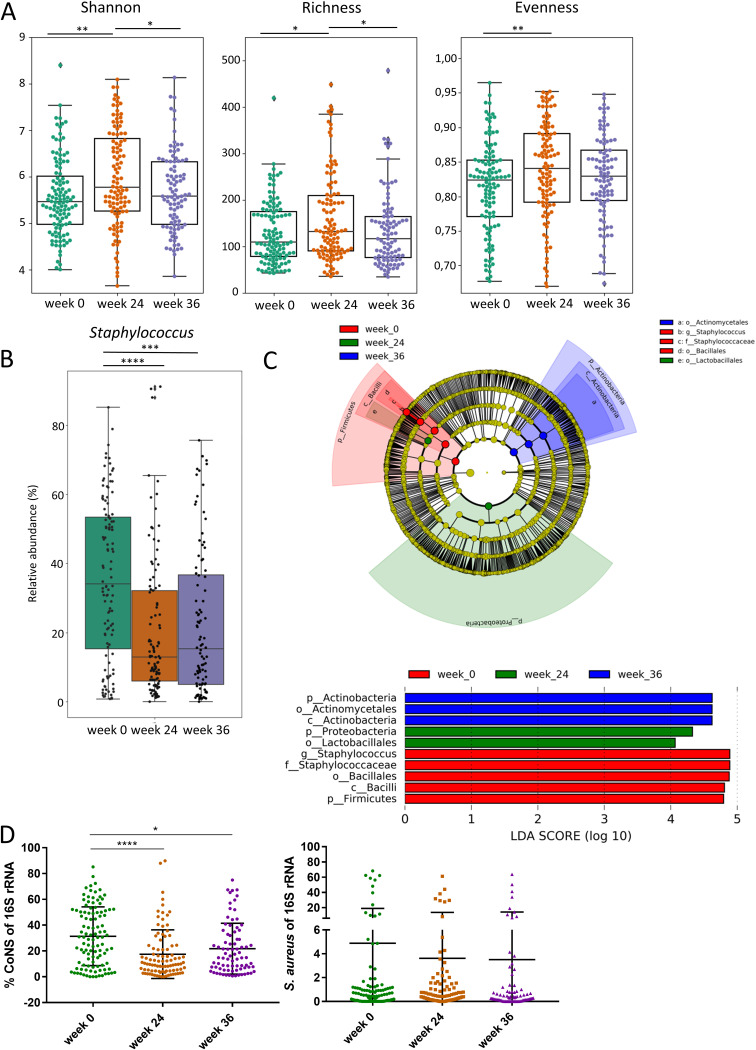
We determined the differences in the skin microbiome of AK patients before start of therapy (week 0), at the time point of treatment completion (week 24), and 3 months after completion of treatment (week 36). Shannon diversity (P = 0.0022), richness (P = 0.0213) and evenness (P = 0.0095) were higher at treatment completion (week 24) compared to week 0 before start of therapy (Fig. 2A). Shannon diversity (P = 0.0457) and richness (P = 0.0465) were significantly lower at week 36 compared with week 24 and were comparable with week 0. No significant differences in Shannon diversity, richness, and evenness were existent between week 0 and 36. Interestingly, the relative abundance of the genus Staphylococcus of the skin microbiome of AK lesions before start of therapy (week 0) was significantly higher, with a mean relative abundance of 35.2%, compared to a mean relative abundance of 19.8% at week 24 (P = 0.0001) and a mean relative abundance of 22.8% at week 36 (P = 0.0001) (Fig. 2B). Staphylococcus was the only genus that was identified as biomarker with a linear discriminant analysis (LDA) score >4 and was enriched at week 0 (Fig. 2C). The reduction of the relative Staphylococcus abundance at week 24 and 36 was mainly attributed to a reduction of CoNS, while the mean relative S. aureus abundance was lower but not significantly different between the different time points (Fig. 2D).
FIG 2.
(A) Shannon diversity, bacterial richness determined by observed features (amplicon sequence variants [ASV]) and evenness in skin microbiome samples at week 0 (green), week 24 (brown) and week 36 (purple). Kruskal-Wallis test was used to test for significant differences among groups. *, P < 0.05; **, P < 0.01. (B) Relative abundance of Staphylococcus genus in skin microbiome samples from week 0, week 24 and week 36. ***, P < 0.001; ****, P < 0.0001. (C) Linear discriminant effect size (LEfSe) analysis of bacterial taxa enriched in week 0 (red), week 24 (green), and week 36 (blue). The cladogram reports the taxa showing different abundance values (LDA score >4) according to LEfSe. Colors are presented in the color of the most abundant group. (D) Relative abundance of coagulase negative staphylococci (CoNS) and S. aureus of 16S rRNA sequences as determined by quantitative real-time PCR at week 0 (green), week 24 (brown) and week 36 (purple). *, P < 0.05; ****, P < 0.0001.
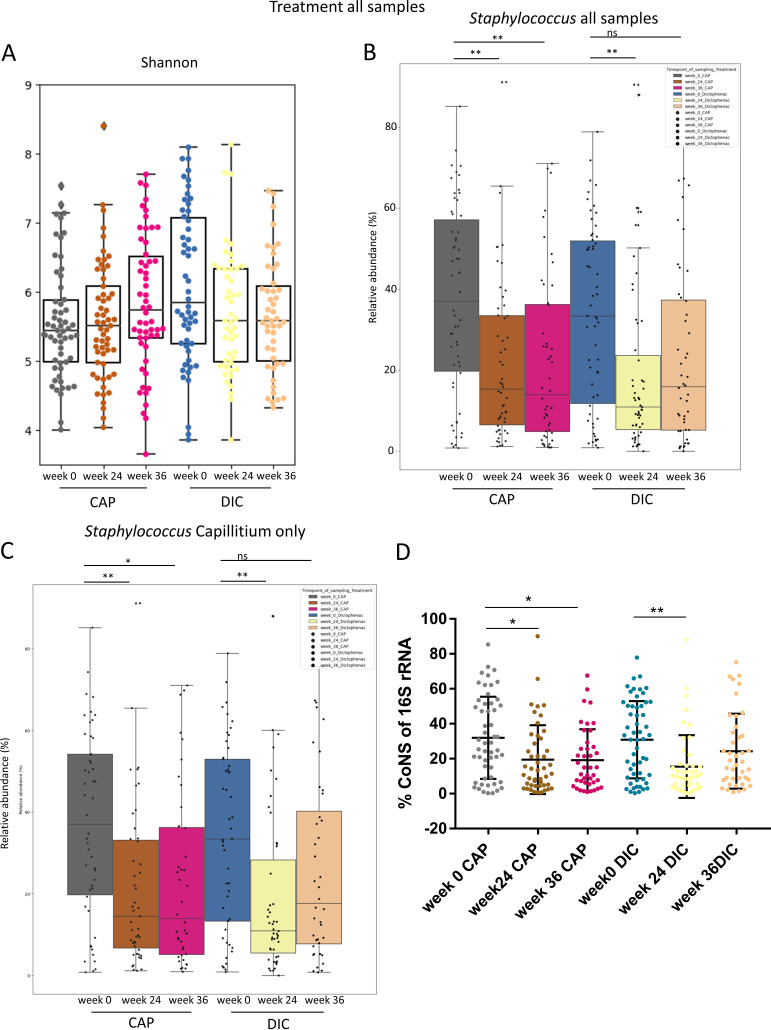
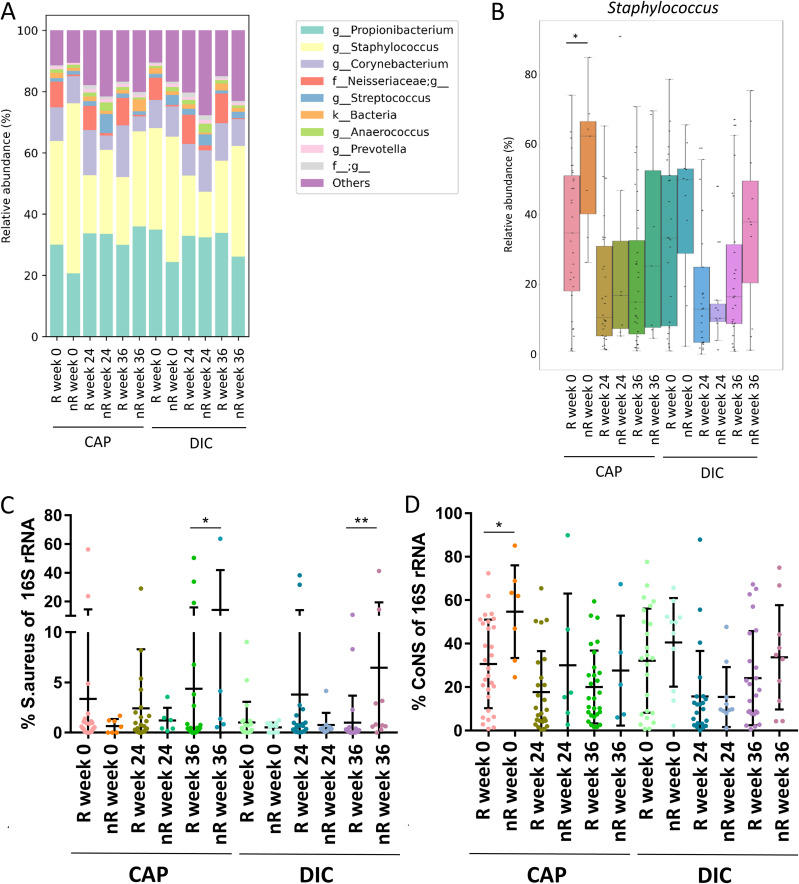
The relative abundance of CoNS is reduced after CAP and DIC-treatment at week 24 and remains significantly reduced in CAP-treated patients at week 36.
To study whether CAP and DIC treatment may differently affect the skin microbiome of AK patients, we compared the skin microbiome of both treatments for all samples at all time points and in addition also at individual time points. The alpha diversity metrics Shannon index, richness, and evenness did not differ significantly (Fig. 3A), and LEfSe did not identify biomarkers with an LDA score ≥ 3 associated with the treatment at time point of treatment completion (week 24) or 12 weeks after treatment completion (week 36), indicating that the differences are minor (Fig. S1). Notably, the reduction of relative Staphylococcus genus abundance was observed for both CAP and DIC treatment and for capillitium samples only (Fig. 3 B and C), which represented the majority of the samples of the study. The reduction of the relative abundance of Staphylococcus of 16S rRNA sequences was due largely to CoNS-reduction and was observed in both CAP- and DIC-treated AK regions (Fig. 3D).
FIG 3.
(A) Shannon diversity in skin microbiome samples receiving CAP versus DIC treatment at week 0, week 24, and week 36. Relative abundance of Staphylococcus in all samples (B) and Capillitium samples only (C) receiving CAP versus DIC treatment at week 0, week 24, and week 36. (D) Relative abundance of coagulase-negative staphylococci (CoNS) of 16S rRNA sequences for week 0, week 24 and week 36 for CAP versus DIC treatment. Kruskal-Wallis test was used to test for significant differences among groups. *, P < 0.05; **, P < 0.01.
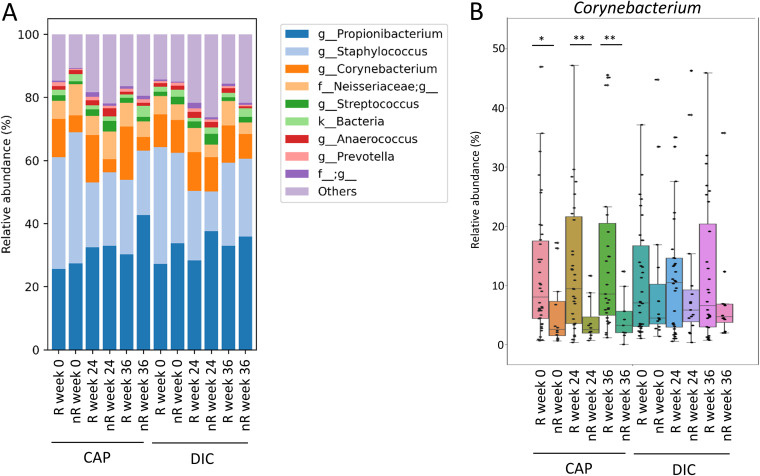
The skin microbiome of AK at the capillitium responding to CAP at the end of treatment (week 24) is associated with an increased relative abundance of Corynebacterium.
Response to therapy at the capillitium (largest subcohort of ACTICAP) was determined directly after end of treatment (week 24), and additionally at the end of the study at week 36 to assess long-term response. Response was assessed for both treatments in each patient separately. A response to treatment (responder, R) was defined by the reduction of the AK area by at least 25% compared to baseline. At time point of treatment completion (week 24), a total of 38 patients were classified as R to CAP and 35 patients were classified as R to DIC treatment. The skin microbiome of AK patients responding to therapy at week 24 was characterized by a significantly higher mean relative abundance of Corynebacterium genus of 14.9% at this time point (P = 0.0038) compared to those patients who did not respond to CAP treatment (mean 3.98%) (Fig. 4A and B). Corynebacterium was the only genus that was identified as significantly different by ANCOM (9) to discriminate R from nonresponder (nR) at week 24 and the only genus identified by LEfSe as biomarker with an LDA score >4 for treatment response at week 24. Interestingly, the relative Corynebacterium abundance was increased in patients classified as responders to CAP treatment at week 24 already before the start of the therapy (week 0). They exhibited a mean relative Corynebacterium abundance of 12.1% compared to 5.3% in nR at that time point (P = 0.0151). In addition, the relative Corynebacterium abundance of patients classified as responders at week 24 was also higher 12 weeks after completion of therapy (week 36) with a relative abundance of 16.80% in R and 4.5% in nR (P = 0.0078). Corynebacterium kroppenstedtii, Corynebacterium bovis, and two species of the Corynebacterium genus that were not identified by the Greengenes database were the four most abundant species accounting for about 98.9% of the capillitium reads that were assigned to bacteria of the Corynebacterium genus. Furthermore, C. durum, C. lubricantis, C. mastitidis, C. simulans, C. stationis, and C. variabile were identified by the Greengenes database from 16S rRNA gene sequences of the samples of our study.
FIG 4.
(A) Mean relative abundance of the 10 most abundant genera in skin microbiome samples from capillitium of individuals classified as responders (R) and nonresponders (nR) for CAP or DIC treatment at week 24 for all time points (week 0, week 24, and week 36). (B) Mean relative Corynebacterium genus abundance of patients classified as R and nR for CAP or DIC treatment individually at week 24. Relative Corynebacterium abundance is shown for all time points of R and nR.
The skin microbiome of AK at the capillitium of CAP nonresponders at follow up (week 36) is characterized by a higher relative S. aureus abundance compared to responders.
In addition, we compared the skin microbiome of patients that were classified as R 12 weeks after completion of therapy (week 36) with those that were classified as nR at this time point. nR were characterized by a higher relative abundance of Staphylococcus genus at week 0 before start of therapy than R (Fig. 5A and B). However, the relative Staphylococcus abundance was reduced at week 24 in both, R and nR, and did not significantly differ between both groups at that time point. Interestingly, nR exhibited a significantly higher relative S. aureus abundance compared to R at week 36 for both treatments, while the S. aureus relative abundance in these patients did not differ significantly at week 0 and 24 (Fig. 5C) The relative abundance of CoNS was significantly higher in CAP-treated nR at week 0 compared to R at that time point but was not significantly different at the other time points between both groups (Fig. 5D).
FIG 5.
(A) Mean relative abundance of the 10 most abundant genera in skin microbiome samples from capillitium of individuals classified as responders (R) and nonresponders (nR) for CAP or DIC treatment at week 36 for all time points (week 0, week 24, and week 36). (B) Mean relative Staphylococcus genus abundance of patients with capillitium AK classified as responders (R) and nonresponders (nR) for CAP or DIC treatment individually at week 36. Relative Staphylococcus abundance is shown for all time points of R and nR. (C) Relative abundance of Staphylococcus aureus (S. aureus) in capillitium samples of patients classified as R and nR for CAP or DIC treatment at week 36. The relative S. aureus abundance in these patients is shown for all time points. (D) Relative abundance of coagulase-negative staphylococci (CoNS) in capillitium samples of patients classified as R and nR for CAP or DIC treatment at week 36. The relative CoNS bundance in these patients is shown for all time points.
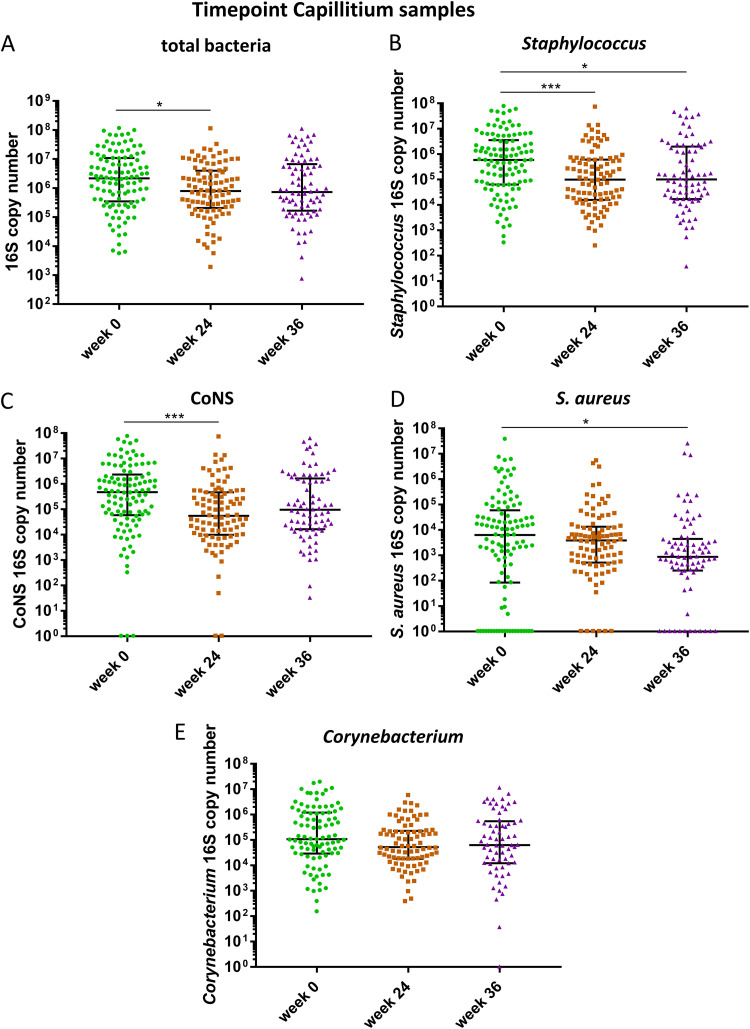
The lesional AK microbiome is characterized by a reduction of the total bacterial and Staphylococcus loads after treatment and an increased absolute S. aureus abundance is a biomarker for CAP long-term nonresponders 12 weeks after the end of treatment.
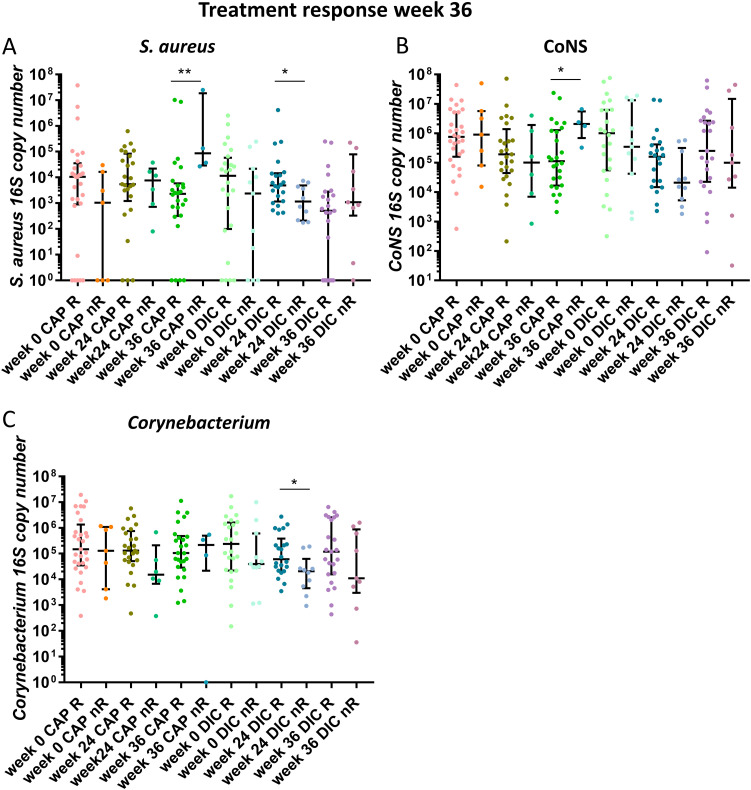
To determine effects of CAP and DIC treatments on the absolute bacterial abundance, we performed a qPCR assay allowing for the quantification of the 16S rRNA gene copies. The bacterial load was lower at week 24 (P = 0.0286) with a median/mean absolute abundance of 793,252/11,246,154 copies compared with week 0 with a median/mean abundance of 2,186,940/4,594566 (Fig. 6A). Also, the absolute Staphylococcus abundance decreased from a median/mean of 586,168/5,303,343 16S rRNA copies at week 0 to a median/mean abundance of 99,358/1,774,733 at week 24 (P = 0.0004) and the median/mean abundance was 100,290/4,006,945 copies at week 36, which was also significantly lower than the abundance at week 0 (P = 0.0147) (Fig. 6B). The decrease of Staphylococcus abundance at week 24 was mainly due to a decrease in the absolute CoNS abundance from 471,979 at week 0 to 55,135 at week 24 (P = 0.0003) (Fig. 6C), while the absolute S. aureus abundance was not significantly different between week 0 and week 24 (Fig. 6D). Interestingly, the absolute S. aureus abundance decreased after treatment completion and was lowest at week 36 with a median of 872.3 16S rRNA gene copies. The 16S rRNA gene copy number was significantly lower at week 36 (872.3) compared with week 0 (P = 0.0217) with a median 16S rRNA gene copy number of 6312, suggesting ongoing skin microbiota modulation beyond the treatment period. The median CoNS abundance however increased again between week 24 and week 36. The absolute Corynebacterium abundance did not change significantly between the three time points (Fig. 6E), suggesting unequal treatment effects on the absolute abundance for individual species. Although both treatments differ in their mechanism of action, the trends on the reduction of the absolute abundance were similar for both treatments. However, the absolute Staphylococcus and CoNS abundances were significantly reduced at week 24 after DIC treatment compared with week 0, while there was a tendency for CAP, which did not reach significance (Fig. S2). In addition, the reduction of the absolute S. aureus abundance was significant at week 36 for DIC only (Fig. S2). A tendency for higher absolute abundances of the Corynebacterium genus was present for patients classified as R for week 24 (Fig. S3) and week 36 (Fig. 7) compared with nR for the majority of time points. However, a significant higher Corynebacterium abundance was observed only for DIC treated patients classified as R at week 36 compared with nR for the time point week 24 (Fig. 7). Interestingly, the absolute S. aureus abundance was significantly higher for CAP treated patients classified as nR at follow-up (week 36) compared to R at that time point (Fig. 7), indicating that proliferation of S. aureus 12 weeks after CAP therapy completion is a biomarker for long-term treatment failure.
FIG 6.
Absolute abundance of bacteria in individual samples assessed by qPCR quantifying the 16S rRNA gene copy number in skin swab samples from capillitium at week 0 (green), week 24 (brown) and week 36 (purple). (A) total bacterial load, (B) Staphylococcus genus load, (C) coagulase negative staphylococci, (CoNS) (D) S. aureus, (E) Corynebacterium genus. Bars indicate median ± interquartile range. Kruskal-Wallis test was used to test for significant differences among groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
FIG 7.
Absolute 16S rRNA gene copy number from capillitium skin swab samples linked to treatment response at week 36. The relative abundance of the respective bacteria for patients classified as R versus nR at week 36 is shown for all time points (week 0, 24 and 36) (A) S. aureus, (B) coagulase negative staphylococci (CoNS), (C) Corynebacterium genus. Bars indicate median ± interquartile range. Mann-Whitney U test was performed to test for significant differences between R and nR for the respective time point and treatment.
DISCUSSION
In our longitudinal prospective study, the skin microbiome of AK patients differed significantly between AK lesions of the capillitium, torso, and extremities. The highest diversity was present in AK lesions of the extremities. We showed that the relative abundance of the Staphylococcus genus was significantly higher before start of therapy compared with the time points of end of therapy at week 24 and at week 36 for both, CAP and DIC therapy. The reduction of Staphylococcus relative abundance was due to a reduction of the relative CoNS abundance and observed in both, CAP and DIC treated patients. The skin microbiome of patients classified as R at time point of CAP therapy completion was characterized by a higher relative abundance of Corynebacterium genus at all investigated time points, also before start of therapy. The skin microbiome of patients classified as nR at week 36 exhibited a higher relative abundance of S. aureus than R at week 36. In addition, the total bacterial and Staphylococcus loads were higher before therapy initiation than at time point of therapy completion; and absolute S. aureus abundance 12 weeks after therapy completion was higher in CAP treated patients classified as nR than in R at that time point.
The differences associated with the location of the AK lesions mimic those described for these skin microbiome regions in healthy individuals, indicating that the diseased skin microbiome is not basically altered and the regional microbiota characteristics are maintained in AK areas. The back of the torso is a sebaceous area characterized by a low diversity with Cutibacterium (Propionibacterium) as the dominant genus in healthy individuals (4). In AK patients of our study, the torso was the site with the lowest alpha diversity metrics and the highest relative abundance of Cutibacterium—the dominating genus of the skin microbiome of this site. The skin microbiome of AK lesions from extremities in our study showed the highest diversity and richness of the three body regions and was enriched in Proteobacteria, Bacteroidia, and Clostridiales among other taxa. This is in line with previous studies that observed that dry skin areas of healthy individuals, that include large parts of the extremities, are known to harbor the greatest diversity of the skin microbiome (4, 10). This location is also colonized by Gram-negative bacteria, including Proteobacteria, and has a phylogenetic diversity that may even exceed the diversity of the oral cavity and gut of these individuals (4).
In our study, the genus Staphylococcus was the second most abundant genus of the skin microbiome of AK patients after Cutibacterium (Propionibacterium) and the genus with the most pronounced alterations of the relative abundance over the different time points of treatment. The relative abundance of the genus Staphylococcus has repeatedly been found to be increased in AK and SCC patients (6, 11, 12). It has been suggested that Staphylococcus, particularly S. aureus, is involved in AK pathogenesis and may play a protumorigenic role, e.g., by stimulating the production of beta-defensins of keratinocytes that have antibacterial properties, but may increase proliferation of keratinocytes in an autocrine manner (6). Besides, S. aureus can promote skin inflammation by producing toxins that may also mediate protumorigenic inflammatory responses (13). The relative Staphylococcus abundance was significantly reduced at the end of both treatments, CAP and DIC, and was significantly lower in R compared to nR 12 weeks after treatment completion, indicating that both treatments affect the skin microbiome composition similarly. The reduction of the relative Staphylococcus abundance was largely due to the reduction of the relative abundance of CoNS. Although S. aureus is the pathogen most commonly reported to promote tumor progression in skin malignancies, CoNS has been found to be overrepresented in transplant patients with SCC and was linked to tumor progression (14). However, the long-term response toward treatment at week 36 in our study was associated with a lower relative and absolute S. aureus abundance in R than in nR, supporting a relevance of S. aureus for this disease and suggesting that an increase of S. aureus after completion of treatment is a biomarker for long-term treatment failure. The formation of microbial communities, their function and stability depend on host factors and the interaction between these microbes and effects may be context specific. Interactions between microbes can be competitively or synergistically. While the growth of S. aureus is prohibited, e.g., by the production of lugdunin by Staphylococcus lugdunensis (15), some Cutibacterium species induce the aggregation of S. aureus and its biofilm formation (16). In our study, the relative abundance of Corynebacterium genus was higher in R than in nR at the end of CAP treatment, and the skin microbiome of R was also characterized by a higher relative Corynebacterium abundance compared to that of nR before start of CAP treatment. Corynebacterium genus has been shown to shift S. aureus toward commensalism by reducing its virulence factor expression (17), which might be exploited to modulate S. aureus behavior in future therapies. Shifting S. aureus toward commensalism was observed for several different commensal Corynebacterium species by others (17), indicating a genus specific rather than species specific potential. In our study, sequencing reads were assigned to several species; however, four Corynebacterium species, two that could not be identified to the species level using 16S rRNA gene sequencing and the two species C. kroppenstedtii and C. bovis, were responsible for more than 98% of the sequences of the Corynebacterium genus.
The total bacterial loads were significantly lower at the end of treatment (week 24) compared to week 0, suggesting that antibacterial effects of CAP and DIC treatments reduce bacterial load of AK skin lesions. The total abundance was not reduced equally for all bacterial species, indicating differential susceptibility toward treatments. Several mechanisms have been reported for antibacterial CAP activity. Reactive oxygen and nitrogen species produced by CAP can trigger programmed cell death with hallmarks of apoptosis by short-term treatment, but long-term CAP treatment can physically destruct intracellular components (18). In addition, CAP may promote the potency of macrophages to kill S. aureus by improving the formation of degradative phagosomes (19). Besides the anti-inflammatory effects by inhibiting the prostaglandin synthesis, DIC was suggested to affect in vitro bacterial growth by modulating DNA synthesis (20). Despite the different nature of both treatments, the differences on the skin microbiome composition linked to the treatments in our study were minor. However, absolute abundance of Staphylococcus and CoNS were significantly lower at week 24 than at week 0 for DIC but not CAP. Also, the reduction of absolute S. aureus abundance at week 36 was significant for DIC but not CAP. Patients classified as nR 12 weeks after end of CAP therapy were characterized by significantly higher S. aureus relative and absolute abundance than R, indicating that a high S. aureus abundance may be a biomarker for CAP treatment failure. Although most studies have focused on S. aureus as most important species of the Staphylococcus genus, a recent report links S. epidermidis, a common species of CoNS, to SCC progression in transplant patients (14). Pathologies might be context dependent. Pathogens like S. aureus may asymptomatically colonize the skin, whereas mutualists like S. epidermidis or corynebacteria sometimes then promote disease (17, 21, 22).
This study is limited by its monocentric design. Although the longitudinal design of our study investigating the skin microbiome in patients over a period of about 9 months, we cannot exclude that other unconsidered factors contribute to the differences in the skin microbiome observed in our study.
Conclusions.
Our study reveals a reduction of the absolute bacterial abundance of AK lesions and alterations of the AK lesional microbiome composition after field-directed therapies. The microbiome alterations affect bacteria that are considered to be involved in pathogenesis of skin malignancies. The reduction of the overabundance of Staphylococcus of the skin microbiome after treatment of AK lesions, the persistent lower relative Staphylococcus genus und relative and absolute S. aureus abundance in long-term responders, and the link of Corynebacterium genus with response to CAP treatment encourage further studies to investigate the role of the skin microbiome for the carcinogenesis of epithelial skin cancer as well as its function as predictive therapeutic biomarker in AK.
MATERIALS AND METHODS
Study population.
We included 59 AK patients of the ACTICAP study, a prospective randomized study that was performed between 2017 and 2019 (8). The Ethics Committee of the University Hospital Essen reviewed and approved the study (17-7546-BO). The study was performed in accordance with the latest version of the Declaration of Helsinki. All patients signed written informed consent.
Sampling and sample processing.
Two anatomically comparable areas of approximately 19.6 cm2 exhibiting similar numbers of AK at baseline (at least n = 2) were chosen for sampling in each participant. The ACTICAP study design included a washout phase of potentially prohibited medication, including photosensitizing agents, immunomodulators, antineoplastic agents and systemic retinoids for 4 to 12 weeks before treatment with CAP or DIC was started, depending on drug type. Patients were included after a minimum of 6 months after physical procedures (e.g., surgery, laser, PDT). The identified treatment areas were computationally randomized to either CAP that was applied for 180 s twice a week or topical DIC (diclofenac 3%) twice a day (8). Each area was sampled at three time points: first, before start of treatment (week 0); second, at the end of the treatment period (week 24); third, 12 weeks after completion of the treatment period at follow-up (week 36). Of 59 patients, 51 patients had AK of the capillitium, 5 of the torso and 3 of extremities (two patients with AK of the hands, one patients with AK of the lower leg). The clinical diagnosis had been confirmed by routine histology previously. Skin swabs were taken using Puritan sterile standard foam swabs (Puritan Medical Products, Guilford, ME, US) moistened with sterile SCF-1 solution (5 mm Tris buffer, 1 mM EDTA and 0.5% Tween 20) by rubbing the swabs about 50 times back and forth at the site of AK for 30 s. The swabs were placed in PowerBead Tubes of the DNeasy PowerLyzer PowerSoil kit (Qiagen, Hilden, Germany) filled with 250 μL SCF1 solution. Samples were frozen at −20°C until further processing. In addition, swabs not used for sampling were shaken in SCF1 solution as negative-control samples and were prepared in parallel with the patient samples. Sixteen patients were lost for the follow-up examination, unrelated to treatment but mainly due to incompliance. Extraction of microbial DNA was performed using the DNeasy PowerLyzer PowerSoil kit (Qiagen) according to the manufacturer´s guidelines. DNA was eluted in 30 μL TE buffer. Amplicon PCR was performed as described previously (23) amplifying the V3/V4 16S rRNA gene region with 25 cycles. We used the Nextera XT Index kit V2 primers for index PCR. Sequencing was performed using the Illumina MiSeq with the 600 cycle reagent kit. Swabs shaked in SCF1 solution and a PCR water sample were used as negative controls.
For absolute quantification of microbes in individual samples, we used the Femto Bacterial DNA Quantification kit (Zymo Research, Freiburg, Germany) according to the manufacturer´s guidelines. This qPCR assay includes bacterial standards, allowing for determination of 16S rRNA gene copy numbers in individual samples. As the DNA of all swab samples had been eluted in a volume of 30 μL and 2 μL were used for individual PCRs, the resulting 16S rRNA gene copies of individual samples calculated from the standard curves were multiplied with 15 to obtain the copy number/sample.
Microbiome data analysis.
The QIIME2 (Quantitative Insights Into Microbial Ecology2) pipeline (24) was used for analysis of fastq files with the DADA2-package integrated in QIIME2 to correct sequences and to filter chimeric sequences using the consensus-method. A total of 4,076,151 quality filtered reads representing 20,314 amplicon sequence variants (ASV) were obtained from 321 patient samples and eight negative-control samples. Of 321 patient samples, a total of 4,053,981 quality filtered reads represented 20,222 ASV. For the identification of contaminating ASVs, we used the Decontam software package in R (25) using the “prevalence” method, that identified 124 contaminating ASVs. Contaminating ASVs were removed from all samples, resulting in a total of 3,969,719 quality filtered reads from 321 samples, including 20,098 ASVs. Four patient samples with a frequency ≤ 2,000 quality filtered reads were removed from data analysis. The remaining 317 samples represented 20,083 ASVs and 3,967,062 quality filtered sequences with a mean and median frequency of 12,514 and 11,944 reads and a minimum of 2,065 reads.
The q2 diversity plugin was used for computing alpha and beta diversity metrics. We used the q2-feature-classifier plugin for taxonomy assignment with a Naïve Bayes classifier, trained on the Greengenes 13_8_99% OTUs 16S rRNA gene full-length sequences. Comparisons between categorical metadata columns and alpha diversity metrics were computed using the qiime diversity alpha-group-significance command with Kruskal-Wallis test. To test for significant differences in beta diversity among groups, we used the qiime diversity beta-group significance command with permutational multivariate analysis of variance (PERMANOVA) of distance matrices with 999 permutations. ANCOM (9) and Kruskal–Wallis test was used for analysis of differences in the relative taxa abundance. Biomarkers were assessed using LEfSe (26) with a P < 0.05 for bacterial class comparison using Kruskal-Wallis test and a linear discriminant analysis (LDA) score >4.
Patient characteristics were tested using t test for metric variables and Fisher exact test for categorical variables with a significance level <0.05. Alpha diversity metrics, barplots, boxplots and principle coordinates analysis (PCoA) were visualized using Dokdo, Version 1.7 (https://github.com/sbslee/dokdo). Dotplots for assessing absolute bacterial abundances and S. aureus and CoNS relative abundance were visualized using GraphPad Prism Ver. 7 software (San Diego, CA, US).
TaqMan PCR for quantification of the relative S. aureus and CoNS abundance.
S. aureus and other members of the Staphylococcus genus are inadequately classified to the species level using 16S rRNA gene sequencing (27). The TaqMan assay described here allows for the quantification of the relative abundance of S. aureus from staphylococci present in microbial communities using two MGB-probes in a single PCR tube for relative quantification, as described elsewhere (28, 29). We used TaqMan probes, that had been validated previously to specifically amplify and bind the tuf gene (30) of either S. aureus (FAM labeled) or Staphylococcus genus (HEX labeled). The relative abundance of S. aureus to Staphylococcus genus was determined using a calibration curve of several mixtures of S. aureus (DSMZ 799) and S. epidermidis (DSMZ 1498). For the generation of the mixtures, S. aureus and S. epidermidis, single colonies grown on Columbia blood agar were subcultured for 6 h in brain heart infusion (Oxoid, Wesel, Germany). Dilutions in PBS from 10−1 to 10−7 were plated on Columbia blood agar and incubated at 37°C with 5% CO2 atmosphere. After 24 h, CFU (CFU) were counted to determine the concentration of S. aureus (Deutsche Sammlung von Mikroorganismen und Zellkulturen [DSMZ] 799) and S. epidermidis (DSMZ 1498) in the pure cultures to generate various mixtures. Besides the single strain cultures, various S. aureus/S. epidermidis mixtures of the reference isolates were generated for the calibration curve with a relative S. aureus abundance ranging between 1% and 99.9%. DNA of the calibration standards was extracted using the ZymoBIOMICS DNA Miniprep kit (Zymo Research, Freiburg, Germany) according to the manufacturer´s recommendations. Mixtures were subjected to NGS deep amplicon sequencing to determine the precise composition of the relative S. aureus abundance of the mixtures. The TaqMan PCR was run on a Roche LightCycler 480 (Roche, Basel, Swiss) with the LightCycler 480 Probes MasterMix in a reaction volume of 20 μL in a two-step PCR with 40 cycles at 60°C for 60 s. Calibration standards were used in duplicate and included in each LightCycler run. All samples were analyzed in duplicates. Data analysis was performed using the LightCycler 480 (Roche) software using the “fixed-points” method. Of 33 samples, the relative abundance of S. aureus and CoNS could not be calculated due to low total amount of bacterial DNA of the samples. Those were excluded from analyses. The relative CoNS abundance was calculated as follows: relative CoNS abundance = relative Staphylococcus abundance – relative S. aureus abundance.
Data availability.
Sequencing data are available in the National Center for Biotechnology Information Sequence Read Archive under BioProject accession ID PRJNA894468.
Supplementary Material
ACKNOWLEDGMENTS
This study was technically advised by Mary McGovern and Jeiram Jeyaratnam and financially supported in parts by Adtec Europe Limited.
We have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
Contributor Information
Jan Kehrmann, Email: Jan.Kehrmann@uk-essen.de.
Victor Gonzalez, CCG-UNAM.
Gregor Gorkiewicz, Medical University of Graz.
REFERENCES
- 1.Urban K, Mehrmal S, Uppal P, Giesey RL, Delost GR. 2021. The global burden of skin cancer: a longitudinal analysis from the Global Burden of Disease Study, 1990–2017. JAAD Int 2:98–108. doi: 10.1016/j.jdin.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Que SKT, Zwald FO, Schmults CD. 2018. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol 78:237–247. doi: 10.1016/j.jaad.2017.08.059. [DOI] [PubMed] [Google Scholar]
- 3.Tang L, Wang K. 2016. Chronic inflammation in skin malignancies. J Mol Signal 11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grice EA, Segre JA. 2011. The skin microbiome. Nat Rev Microbiol 9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson S, Harwood C, Thomas M, Banks L, Storey A. 2000. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev 14:3065–3073. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madhusudhan N, Pausan MR, Halwachs B, Durdevic M, Windisch M, Kehrmann J, Patra V, Wolf P, Boukamp P, Moissl-Eichinger C, Cerroni L, Becker JC, Gorkiewicz G. 2020. Molecular profiling of keratinocyte skin tumors links Staphylococcus aureus overabundance and increased human beta-defensin-2 expression to growth promotion of squamous cell carcinoma. Cancers (Basel) 12:541. doi: 10.3390/cancers12030541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dianzani C, Conforti C, Giuffrida R, Corneli P, di Meo N, Farinazzo E, Moret A, Magaton RG, Zalaudek I. 2020. Current therapies for actinic keratosis. Int J Dermatol 59:677–684. doi: 10.1111/ijd.14767. [DOI] [PubMed] [Google Scholar]
- 8.Koch F, Salva KA, Wirtz M, Hadaschik E, Varaljai R, Schadendorf D, Roesch A. 2020. Efficacy of cold atmospheric plasma vs. diclofenac 3% gel in patients with actinic keratoses: a prospective, randomized and rater-blinded study (ACTICAP). J Eur Acad Dermatol Venereol 34:e844–e846. doi: 10.1111/jdv.16735. [DOI] [PubMed] [Google Scholar]
- 9.Lin H, Peddada SD. 2020. Analysis of compositions of microbiomes with bias correction. Nat Commun 11:3514. doi: 10.1038/s41467-020-17041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Program NCS, Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML, Segre JA. 2009. Topographical and temporal diversity of the human skin microbiome. Science 324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kullander J, Forslund O, Dillner J. 2009. Staphylococcus aureus and squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev 18:472–478. doi: 10.1158/1055-9965.EPI-08-0905. [DOI] [PubMed] [Google Scholar]
- 12.Wood DLA, Lachner N, Tan JM, Tang S, Angel N, Laino A, Linedale R, Le Cao KA, Morrison M, Frazer IH, Soyer HP, Hugenholtz P. 2018. A natural history of actinic keratosis and cutaneous squamous cell carcinoma microbiomes. mBio 9:e01432-18. doi: 10.1128/mBio.01432-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krueger A, Zaugg J, Chisholm S, Linedale R, Lachner N, Teoh SM, Tuong ZK, Lukowski SW, Morrison M, Soyer HP, Hugenholtz P, Hill MM, Frazer IH. 2021. Secreted toxins from Staphylococcus aureus strains isolated from keratinocyte skin cancers mediate pro-tumorigenic inflammatory responses in the skin. Front Microbiol 12:789042. doi: 10.3389/fmicb.2021.789042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krueger A, Zaugg J, Lachner N, Bialasiewicz S, Lin LL, Gabizon S, Sobarun P, Morrison M, Soyer HP, Hugenholtz P, Frazer IH. 2022. Changes in the skin microbiome associated with squamous cell carcinoma in transplant recipients. ISME Communications 2:13. doi: 10.1038/s43705-022-00095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, Burian M, Schilling NA, Slavetinsky C, Marschal M, Willmann M, Kalbacher H, Schittek B, Brotz-Oesterhelt H, Grond S, Peschel A, Krismer B. 2016. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535:511–516. doi: 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]
- 16.Wollenberg MS, Claesen J, Escapa IF, Aldridge KL, Fischbach MA, Lemon KP. 2014. Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation. mBio 5:e01286-14–e01214. doi: 10.1128/mBio.01286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsey MM, Freire MO, Gabrilska RA, Rumbaugh KP, Lemon KP. 2016. Staphylococcus aureus shifts toward commensalism in response to corynebacterium species. Front Microbiol 7:1230. doi: 10.3389/fmicb.2016.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan D, Malyavko A, Wang Q, Ostrikov KK, Sherman JH, Keidar M. 2021. Multi-modal biological destruction by cold atmospheric plasma: capability and mechanism. Biomedicines 9:1259. doi: 10.3390/biomedicines9091259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duchesne C, Frescaline N, Blaise O, Lataillade JJ, Banzet S, Dussurget O, Rousseau A. 2021. Cold atmospheric plasma promotes killing of Staphylococcus aureus by macrophages. mSphere 6:e0021721. doi: 10.1128/mSphere.00217-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazumdar K, Dastidar SG, Park JH, Dutta NK. 2009. The anti-inflammatory non-antibiotic helper compound diclofenac: an antibacterial drug target. Eur J Clin Microbiol Infect Dis 28:881–891. doi: 10.1007/s10096-009-0739-z. [DOI] [PubMed] [Google Scholar]
- 21.Chen YE, Fischbach MA, Belkaid Y. 2018. Skin microbiota-host interactions. Nature 553:427–436. doi: 10.1038/nature25177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otto M. 2009. Staphylococcus epidermidis—the “accidental” pathogen. Nat Rev Microbiol 7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kehrmann J, Menzel J, Saeedghalati M, Obeid R, Schulze C, Holzendorf V, Farahpour F, Reinsch N, Klein-Hitpass L, Streeck H, Hoffmann D, Buer J, Esser S, Group H-HS. 2019. Gut microbiota in human immunodeficiency virus-infected individuals linked to coronary heart disease. J Infect Dis 219:497–508. doi: 10.1093/infdis/jiy524. [DOI] [PubMed] [Google Scholar]
- 24.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodriguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis NM, Proctor DM, Holmes SP, Relman DA, Callahan BJ. 2018. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6:226. doi: 10.1186/s40168-018-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. 2011. Metagenomic biomarker discovery and explanation. Genome Biol 12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrd AL, Belkaid Y, Segre JA. 2018. The human skin microbiome. Nat Rev Microbiol 16:143–155. doi: 10.1038/nrmicro.2017.157. [DOI] [PubMed] [Google Scholar]
- 28.Tatura R, Zeschnigk M, Adamzik M, Probst-Kepper M, Buer J, Kehrmann J. 2012. Quantification of regulatory T cells in septic patients by real-time PCR-based methylation assay and flow cytometry. PLoS One 7:e49962. doi: 10.1371/journal.pone.0049962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeschnigk M, Bohringer S, Price EA, Onadim Z, Masshofer L, Lohmann DR. 2004. A novel real-time PCR assay for quantitative analysis of methylated alleles (QAMA): analysis of the retinoblastoma locus. Nucleic Acids Res 32:e125. doi: 10.1093/nar/gnh122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martineau F, Picard FJ, Ke D, Paradis S, Roy PH, Ouellette M, Bergeron MG. 2001. Development of a PCR assay for identification of staphylococci at genus and species levels. J Clin Microbiol 39:2541–2547. doi: 10.1128/JCM.39.7.2541-2547.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S3. Download spectrum.04401-22-s0001.pdf, PDF file, 0.8 MB (816.6KB, pdf)
Data Availability Statement
Sequencing data are available in the National Center for Biotechnology Information Sequence Read Archive under BioProject accession ID PRJNA894468.