Abstract
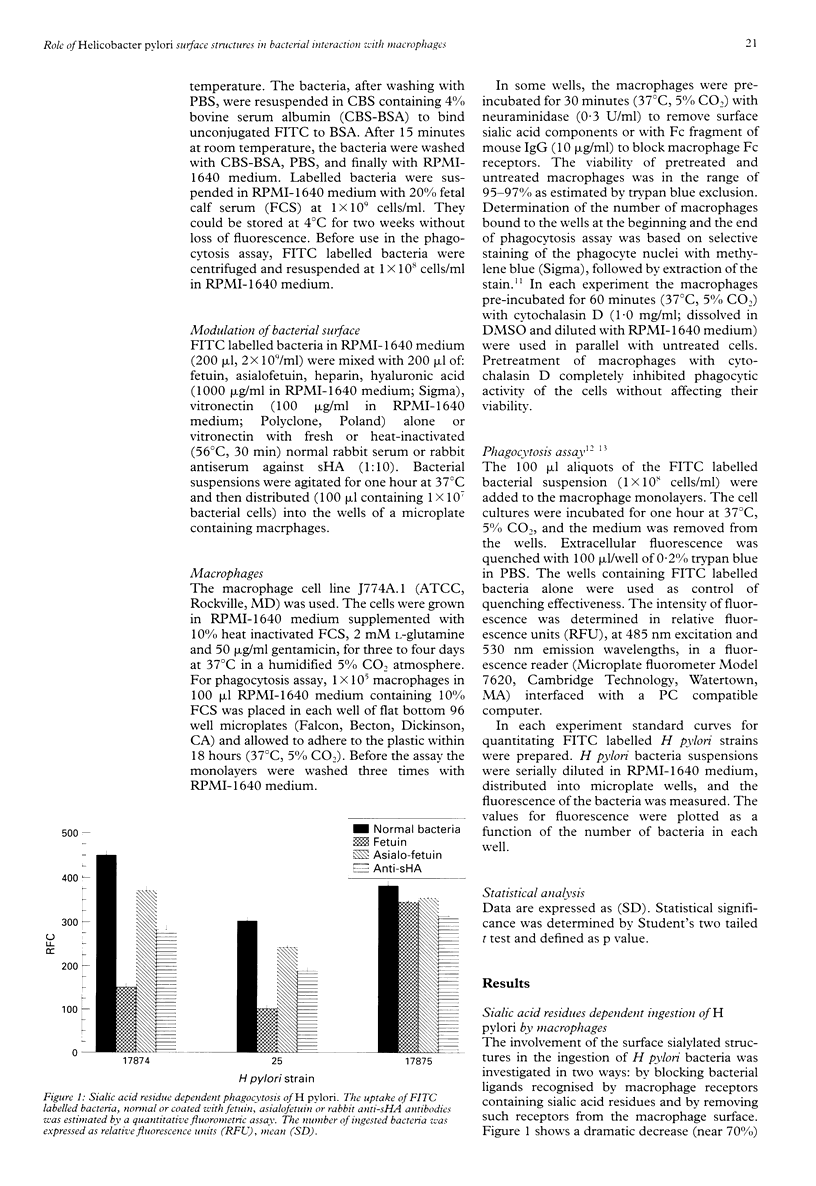
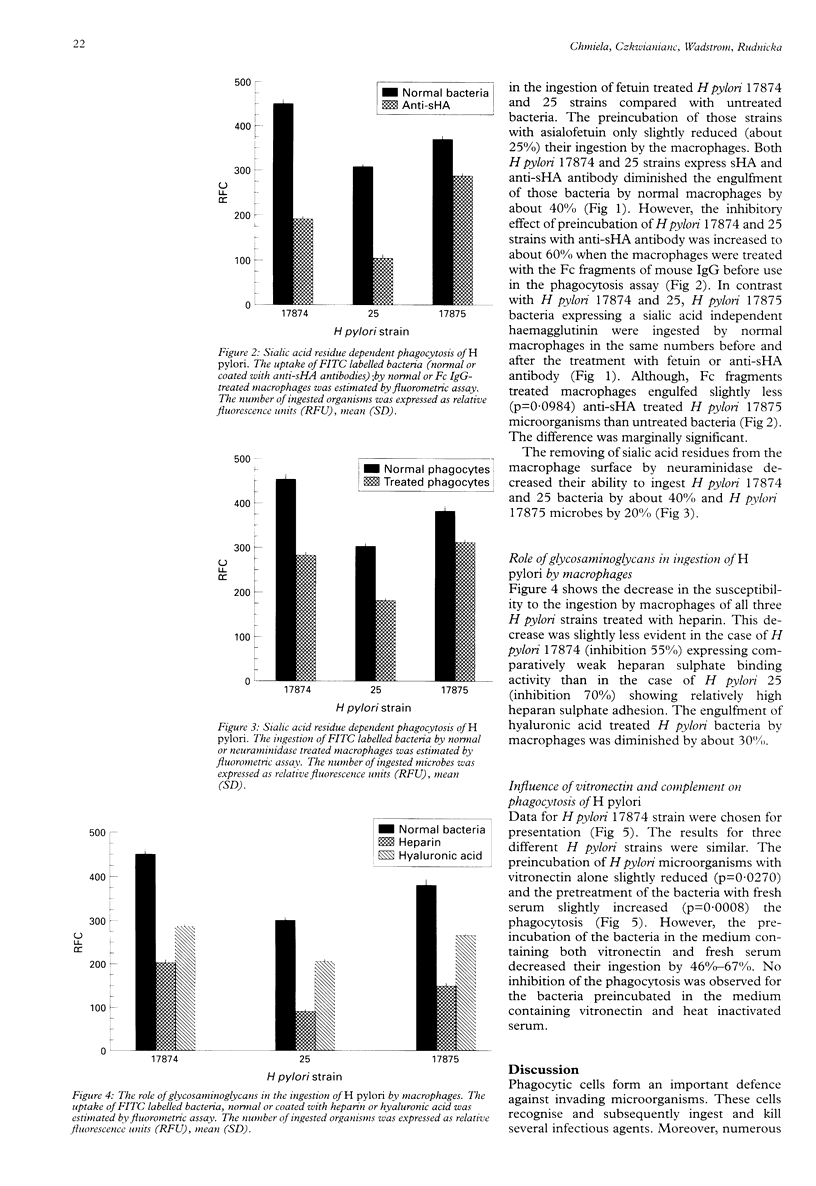
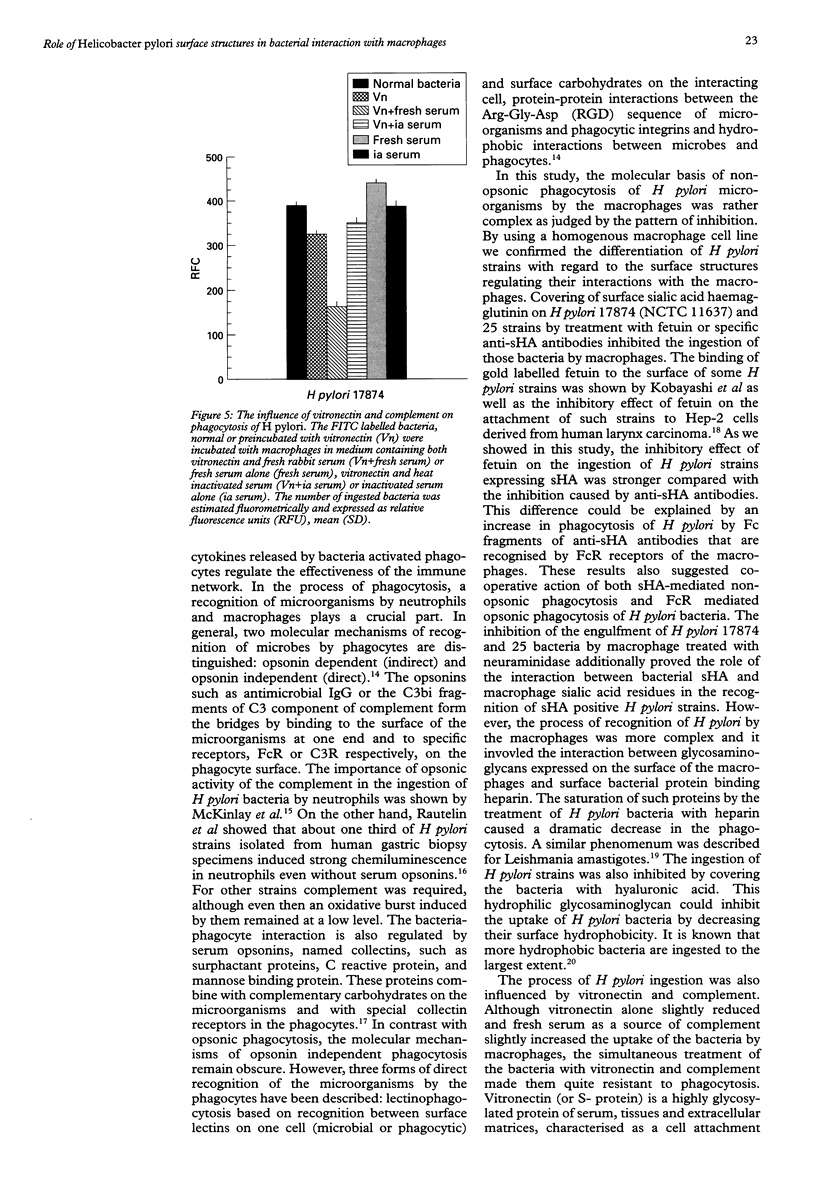
BACKGROUND: Helicobacter pylori infection is associated with a marked infiltration of the gastric epithelium by neutrophils, macrophages, lymphocytes, and plasma cells. Despite the presence of phagocytes in close vicinty to H pylori microbes a great number of people are unable to eradicate bacteria. AIMS: To investigate the involvement of multiple bacterial 'adhesins' and some phagocytic receptors in the process of the ingestion of H pylori by macrophages. BACTERIA: H pylori strains differing in the expression of sialic acid dependent (sHA) or sialic acid independent (HA) haemagglutinin and heparan sulphate binding were selected for the study. METHODS: The uptake of fluorescein labelled H pylori bacteria by a homogenous macrophage cell line J 774A.1 was estimated in a quantitative fluorometric assay. RESULTS: The ingestion of H pylori 17874 and 25 strains expressing sHA was inhibited by the pretreatment of the bacteria with anti-sHA antibodies or fetuin as well as by treatment of the macrophages with neuraminidase. In contrast the uptake of H pylori 17875 strain expressing HA remained unchanged. The phagocytosis of all investigated bacteria was inhibited after the treatment with heparin, hyaluronic acid or vitronectin with fresh but not heat inactivated serum. CONCLUSIONS: The results suggest that H pylori surface compounds binding host proteins such as fetuin, heparin/haparan sulphate, hyaluronic acid, and vitronectin in the presence of complement, could allow the bacteria to avoid phagocytosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absolom D. R. The role of bacterial hydrophobicity in infection: bacterial adhesion and phagocytic ingestion. Can J Microbiol. 1988 Mar;34(3):287–298. doi: 10.1139/m88-054. [DOI] [PubMed] [Google Scholar]
- Ascencio F., Fransson L. A., Wadström T. Affinity of the gastric pathogen Helicobacter pylori for the N-sulphated glycosaminoglycan heparan sulphate. J Med Microbiol. 1993 Apr;38(4):240–244. doi: 10.1099/00222615-38-4-240. [DOI] [PubMed] [Google Scholar]
- Bracha R., Mirelman D. Virulence of Entamoeba histolytica trophozoites. Effects of bacteria, microaerobic conditions, and metronidazole. J Exp Med. 1984 Aug 1;160(2):353–368. doi: 10.1084/jem.160.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmiela M., Lelwala-Guruge J., Wadström T. Interaction of cells of Helicobacter pylori with human polymorphonuclear leucocytes: possible role of haemagglutinins. FEMS Immunol Med Microbiol. 1994 Jun;9(1):41–48. doi: 10.1111/j.1574-695X.1994.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Chmiela M., Paziak-Domanska B., Rudnicka W., Wadström T. The role of heparan sulphate-binding activity of Helicobacter pylori bacteria in their adhesion to murine macrophages. APMIS. 1995 Jun;103(6):469–474. doi: 10.1111/j.1699-0463.1995.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Chmiela M., Paziak-Domanska B., Wadström T. Attachment, ingestion and intracellular killing of Helicobacter pylori by human peripheral blood mononuclear leukocytes and mouse peritoneal inflammatory macrophages. FEMS Immunol Med Microbiol. 1995 Feb;10(3-4):307–316. doi: 10.1111/j.1574-695X.1995.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Dahlbäck B., Podack E. R. Characterization of human S protein, an inhibitor of the membrane attack complex of complement. Demonstration of a free reactive thiol group. Biochemistry. 1985 Apr 23;24(9):2368–2374. doi: 10.1021/bi00330a036. [DOI] [PubMed] [Google Scholar]
- Hirmo S., Utt M., Ringner M., Wadström T. Inhibition of heparan sulphate and other glycosaminoglycans binding to Helicobacter pylori by various polysulphated carbohydrates. FEMS Immunol Med Microbiol. 1995 Feb;10(3-4):301–306. doi: 10.1111/j.1574-695X.1995.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Holmskov U., Malhotra R., Sim R. B., Jensenius J. C. Collectins: collagenous C-type lectins of the innate immune defense system. Immunol Today. 1994 Feb;15(2):67–74. doi: 10.1016/0167-5699(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Okazaki K., Murakami K. Adhesion of Helicobacter pylori to gastric epithelial cells in primary cultures obtained from stomachs of various animals. Infect Immun. 1993 Oct;61(10):4058–4063. doi: 10.1128/iai.61.10.4058-4063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelwala-Guruge J., Ljungh A., Wadström T. Haemagglutination patterns of Helicobacter pylori. Frequency of sialic acid-specific and non-sialic acid-specific haemagglutinins. APMIS. 1992 Oct;100(10):908–913. [PubMed] [Google Scholar]
- Love D. C., Esko J. D., Mosser D. M. A heparin-binding activity on Leishmania amastigotes which mediates adhesion to cellular proteoglycans. J Cell Biol. 1993 Nov;123(3):759–766. doi: 10.1083/jcb.123.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay A. W., Young A., Russell R. I., Gemmell C. G. Opsonic requirements of Helicobacter pylori. J Med Microbiol. 1993 Mar;38(3):209–215. doi: 10.1099/00222615-38-3-209. [DOI] [PubMed] [Google Scholar]
- Mooney C., Keenan J., Munster D., Wilson I., Allardyce R., Bagshaw P., Chapman B., Chadwick V. Neutrophil activation by Helicobacter pylori. Gut. 1991 Aug;32(8):853–857. doi: 10.1136/gut.32.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oben J. A., Foreman J. C. A simple quantitative fluorimetric assay of in vitro phagocytosis in human neutrophils. J Immunol Methods. 1988 Aug 9;112(1):99–103. doi: 10.1016/0022-1759(88)90039-7. [DOI] [PubMed] [Google Scholar]
- Ofek I., Goldhar J., Keisari Y., Sharon N. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- Rautelin H., Blomberg B., Fredlund H., Järnerot G., Danielsson D. Incidence of Helicobacter pylori strains activating neutrophils in patients with peptic ulcer disease. Gut. 1993 May;34(5):599–603. doi: 10.1136/gut.34.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A., Pangburn M. K. Identification of multiple sites of interaction between heparin and the complement system. Mol Immunol. 1993 May;30(7):679–684. doi: 10.1016/0161-5890(93)90079-q. [DOI] [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990 Jan 11;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Sobala G. M., Crabtree J. E., Dixon M. F., Schorah C. J., Taylor J. D., Rathbone B. J., Heatley R. V., Axon A. T. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991 Nov;32(11):1415–1418. doi: 10.1136/gut.32.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Hasubski A. T., Fox J. G., Lee A. Haemagglutination profiles of Helicobacter species that cause gastritis in man and animals. J Med Microbiol. 1992 Nov;37(5):299–303. doi: 10.1099/00222615-37-5-299. [DOI] [PubMed] [Google Scholar]
- Tomasini B. R., Mosher D. F. Vitronectin. Prog Hemost Thromb. 1991;10:269–305. [PubMed] [Google Scholar]
- Wan C. P., Park C. S., Lau B. H. A rapid and simple microfluorometric phagocytosis assay. J Immunol Methods. 1993 Jun 4;162(1):1–7. doi: 10.1016/0022-1759(93)90400-2. [DOI] [PubMed] [Google Scholar]



