Abstract
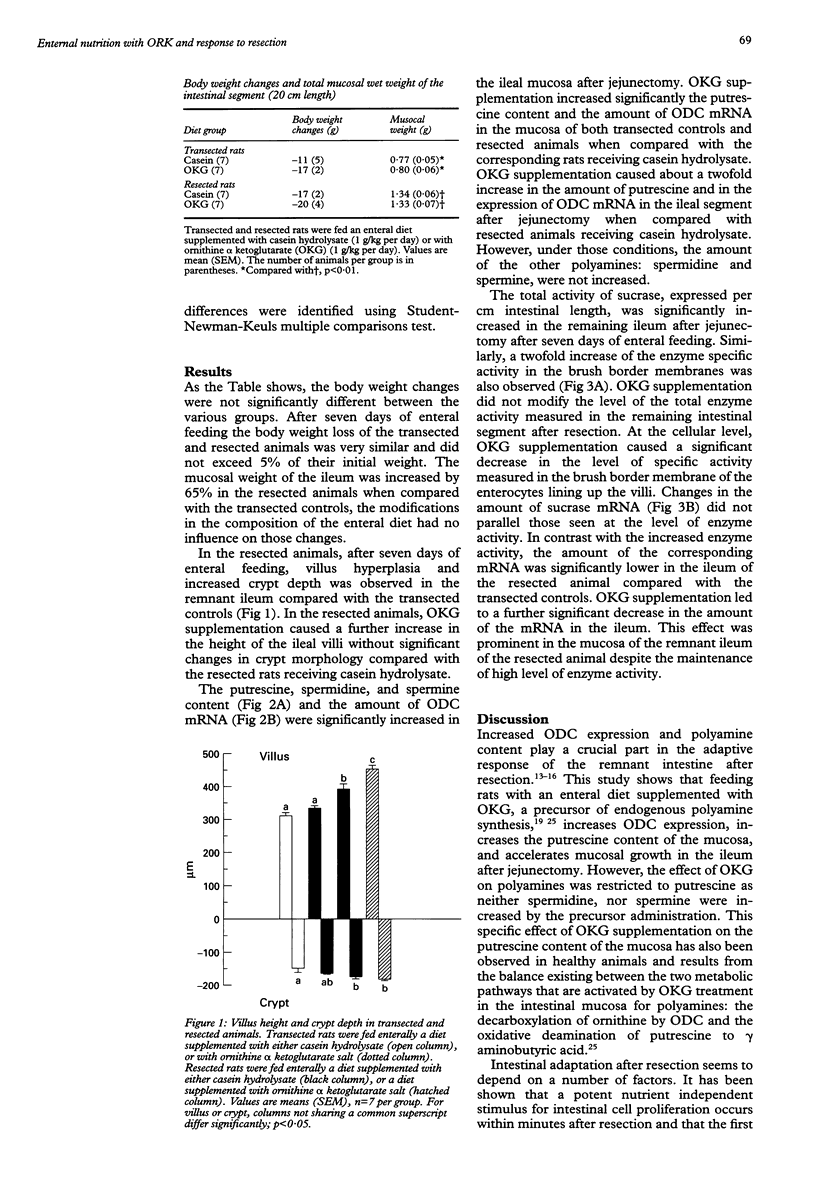
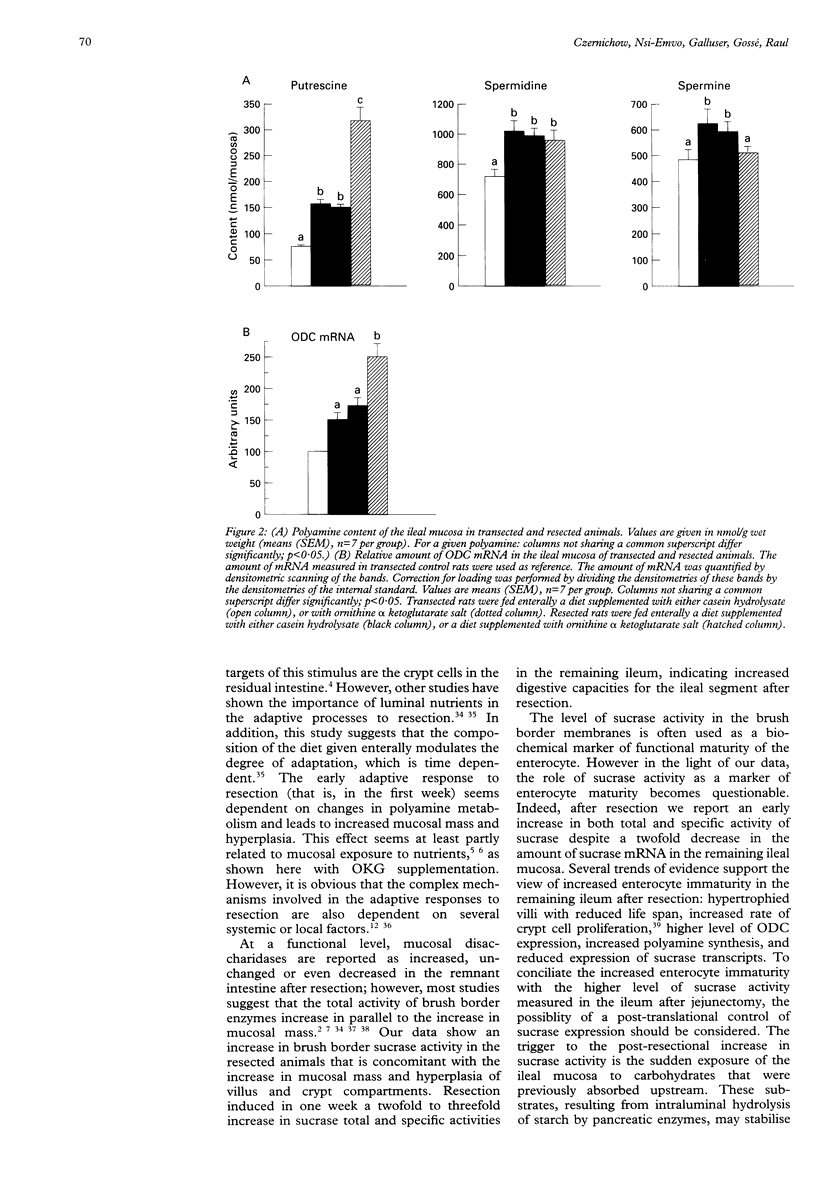
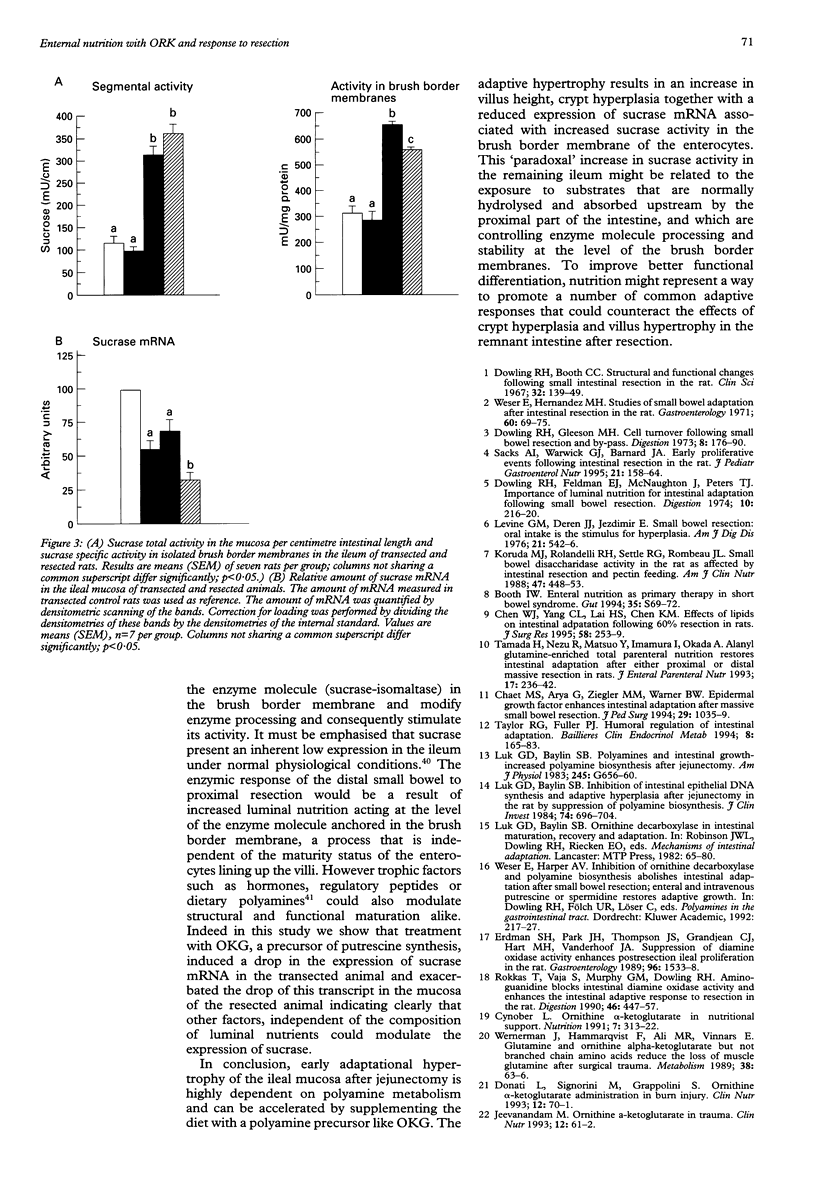
BACKGROUND: Polyamine synthesis or uptake, or both, might be an important event that initiates the adaptive hyperplasia seen in the intestinal remnant after partial small bowel resection. AIM: The ability of an enteral diet supplemented with the ornithine salt: ornithine alpha ketoglutarate (OKG), a precursor for polyamine synthesis, to modulate the adaptive response of the remnant ileum after jejunectomy was evaluated. METHODS: Adult Wistar rats underwent a resection of the proximal 50% of the small intestine. Controls underwent a single transection. The rats were fed intragastrically with a nutritive mixture supplemented either with casein hydrolysate or with OKG (1 g/kg). The isoenergetic and isonitrogeneous diets was given continuously for seven days. RESULTS: Villus and crypt hyperplasia was observed in the remnant ileum compared with transfected controls. OKG supplementation started after resection a further increase in villus height. After resection, OKG supplementation increased significantly the putrescine content and the amount of ornithine decarboxylase mRNA. A twofold to threefold increase of sucrase activity was measured in the resected animals compared with the transected rats. In contrast, the amount of sucrase mRNA was significantly lower in the ileum of the resected rats and OKG supplementation initiated a further drop in the amount of sucrase mRNA without pronounced changes in enzyme activity. CONCLUSIONS: The adaptive hypertrophy seen after resection can be accelerated by supplementing the diet with ornithine (OKG) a precursor of polyamine synthesis. In the remnant ileum, the reduced amount of sucrase mRNA, despite the increased level of sucrase activity, suggests a post-translational control of sucrase expression.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth I. W. Enteral nutrition as primary therapy in short bowel syndrome. Gut. 1994 Jan;35(1 Suppl):S69–S72. doi: 10.1136/gut.35.1_suppl.s69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaet M. S., Arya G., Ziegler M. M., Warner B. W. Epidermal growth factor enhances intestinal adaptation after massive small bowel resection. J Pediatr Surg. 1994 Aug;29(8):1035–1039. doi: 10.1016/0022-3468(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Chaves M., Smith M. W., Williamson R. C. Increased activity of digestive enzymes in ileal enterocytes adapting to proximal small bowel resection. Gut. 1987 Aug;28(8):981–987. doi: 10.1136/gut.28.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. J., Yang C. L., Lai H. S., Chen K. M. Effects of lipids on intestinal adaptation following 60% resection in rats. J Surg Res. 1995 Mar;58(3):253–259. doi: 10.1006/jsre.1995.1040. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Yang C. L., Lai H. S., Chen K. M. Effects of lipids on intestinal adaptation following 60% resection in rats. J Surg Res. 1995 Mar;58(3):253–259. doi: 10.1006/jsre.1995.1040. [DOI] [PubMed] [Google Scholar]
- Cheng H., McCulloch C., Bjerknes M. Effects of 30% intestinal resection on whole population cell kinetics of mouse intestinal epithelium. Anat Rec. 1986 May;215(1):35–41. doi: 10.1002/ar.1092150106. [DOI] [PubMed] [Google Scholar]
- Cynober L. Ornithine alpha-ketoglutarate in nutritional support. Nutrition. 1991 Sep-Oct;7(5):313–322. [PubMed] [Google Scholar]
- Cynober L., Vaubourdolle M., Dore A., Giboudeau J. Kinetics and metabolic effects of orally administered ornithine alpha-ketoglutarate in healthy subjects fed with a standardized regimen. Am J Clin Nutr. 1984 Apr;39(4):514–519. doi: 10.1093/ajcn/39.4.514. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Dowling R. H., Gleeson M. H. Cell turnover following small bowel resection and by-pass. Digestion. 1973;8(2):176–190. doi: 10.1159/000197312. [DOI] [PubMed] [Google Scholar]
- Duluc I., Freund J. N., Mantei N., Raul F. L'ADNc de la lactase détecte un ARNm qui s'exprime différemment dans le jéjunum et le côlon au cours du développement du rat. Gastroenterol Clin Biol. 1990;14(8-9):635–640. [PubMed] [Google Scholar]
- Duluc I., Galluser M., Raul F., Freund J. N. Dietary control of the lactase mRNA distribution along the rat small intestine. Am J Physiol. 1992 Jun;262(6 Pt 1):G954–G961. doi: 10.1152/ajpgi.1992.262.6.G954. [DOI] [PubMed] [Google Scholar]
- Erdman S. H., Park J. H., Thompson J. S., Grandjean C. J., Hart M. H., Vanderhoof J. A. Suppression of diamine oxidase activity enhances postresection ileal proliferation in the rat. Gastroenterology. 1989 Jun;96(6):1533–1538. doi: 10.1016/0016-5085(89)90523-4. [DOI] [PubMed] [Google Scholar]
- Erdman S. H., Park J. H., Thompson J. S., Grandjean C. J., Hart M. H., Vanderhoof J. A. Suppression of diamine oxidase activity enhances postresection ileal proliferation in the rat. Gastroenterology. 1989 Jun;96(6):1533–1538. doi: 10.1016/0016-5085(89)90523-4. [DOI] [PubMed] [Google Scholar]
- Hanson W. R., Osborne J. W., Sharp J. G. Compensation by the residual intestine after intestinal resection in the rat. II. Influence of postoperative time interval. Gastroenterology. 1977 Apr;72(4 Pt 1):701–705. [PubMed] [Google Scholar]
- Jeevanandam M. Ornithine alpha-ketoglutarate in trauma. Clin Nutr. 1993 Feb;12(1):61–62. doi: 10.1016/0261-5614(93)90155-w. [DOI] [PubMed] [Google Scholar]
- Kahana C., Nathans D. Isolation of cloned cDNA encoding mammalian ornithine decarboxylase. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3645–3649. doi: 10.1073/pnas.81.12.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koruda M. J., Rolandelli R. H., Settle R. G., Rombeau J. L. Small bowel disaccharidase activity in the rat as affected by intestinal resection and pectin feeding. Am J Clin Nutr. 1988 Mar;47(3):448–453. doi: 10.1093/ajcn/47.3.448. [DOI] [PubMed] [Google Scholar]
- Levine G. M., Deren J. J., Yezdimir E. Small-bowel resection. Oral intake is the stimulus for hyperplasia. Am J Dig Dis. 1976 Jul;21(7):542–546. doi: 10.1007/BF01464760. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Inhibition of intestinal epithelial DNA synthesis and adaptive hyperplasia after jejunectomy in the rat by suppression of polyamine biosynthesis. J Clin Invest. 1984 Sep;74(3):698–704. doi: 10.1172/JCI111485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Polyamines and intestinal growth--increased polyamine biosynthesis after jejunectomy. Am J Physiol. 1983 Nov;245(5 Pt 1):G656–G660. doi: 10.1152/ajpgi.1983.245.5.G656. [DOI] [PubMed] [Google Scholar]
- Nsi-Emvo E., Foltzer-Jourdainne C., Raul F., Gosse F., Duluc I., Koch B., Freund J. N. Precocious and reversible expression of sucrase-isomaltase unrelated to intestinal cell turnover. Am J Physiol. 1994 Apr;266(4 Pt 1):G568–G575. doi: 10.1152/ajpgi.1994.266.4.G568. [DOI] [PubMed] [Google Scholar]
- Raul F., Gosse F., Galluser M., Hasselmann M., Seiler N. Functional and metabolic changes in intestinal mucosa of rats after enteral administration of ornithine alpha-ketoglutarate salt. JPEN J Parenter Enteral Nutr. 1995 Mar-Apr;19(2):145–150. doi: 10.1177/0148607195019002145. [DOI] [PubMed] [Google Scholar]
- Rokkas T., Vaja S., Murphy G. M., Dowling R. H. Aminoguanidine blocks intestinal diamine oxidase (DAO) activity and enhances the intestinal adaptive response to resection in the rat. Digestion. 1990;46 (Suppl 2):447–457. doi: 10.1159/000200420. [DOI] [PubMed] [Google Scholar]
- Sacks A. I., Warwick G. J., Barnard J. A. Early proliferative events following intestinal resection in the rat. J Pediatr Gastroenterol Nutr. 1995 Aug;21(2):158–164. doi: 10.1097/00005176-199508000-00007. [DOI] [PubMed] [Google Scholar]
- Seiler N., Sarhan S., Grauffel C., Jones R., Knödgen B., Moulinoux J. P. Endogenous and exogenous polyamines in support of tumor growth. Cancer Res. 1990 Aug 15;50(16):5077–5083. [PubMed] [Google Scholar]
- Tamada H., Nezu R., Matsuo Y., Imamura I., Takagi Y., Okada A. Alanyl glutamine-enriched total parenteral nutrition restores intestinal adaptation after either proximal or distal massive resection in rats. JPEN J Parenter Enteral Nutr. 1993 May-Jun;17(3):236–242. doi: 10.1177/0148607193017003236. [DOI] [PubMed] [Google Scholar]
- Vaubourdolle M., Jardel A., Coudray-Lucas C., Ekindjian O. G., Agneray J., Cynober L. Fate of enterally administered ornithine in healthy animals: interactions with alpha-ketoglutarate. Nutrition. 1989 May-Jun;5(3):183–187. [PubMed] [Google Scholar]
- Weser E., Hernandez M. H. Studies of small bowel adaptation after intestinal resection in the rat. Gastroenterology. 1971 Jan;60(1):69–75. [PubMed] [Google Scholar]
- Weser E., Tawil T. Epithelial cell loss in remaining intestine after small bowel resection in the rat. Gastroenterology. 1976 Sep;71(3):412–415. [PubMed] [Google Scholar]
- Williamson R. C. Intestinal adaptation (second of two parts). Mechanisms of control. N Engl J Med. 1978 Jun 29;298(26):1444–1450. doi: 10.1056/NEJM197806292982604. [DOI] [PubMed] [Google Scholar]
- Wilson H. D., Miller T., Ogesen B., Schedl H. P., Failla M. L., Loven D. P. Adaptation of the duodenum and ileum of the rat to mid-gut resection: enzyme activity and trace metal status. Am J Clin Nutr. 1986 Feb;43(2):185–193. doi: 10.1093/ajcn/43.2.185. [DOI] [PubMed] [Google Scholar]



