Abstract
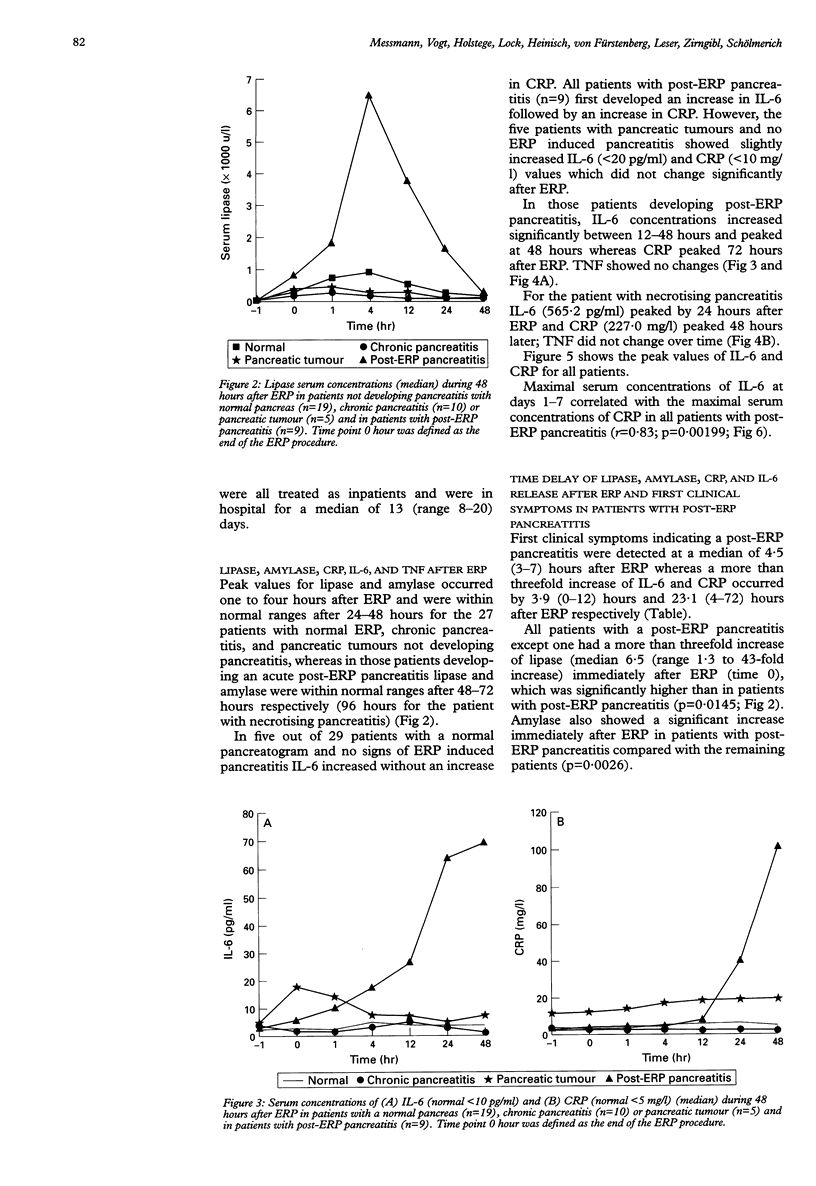
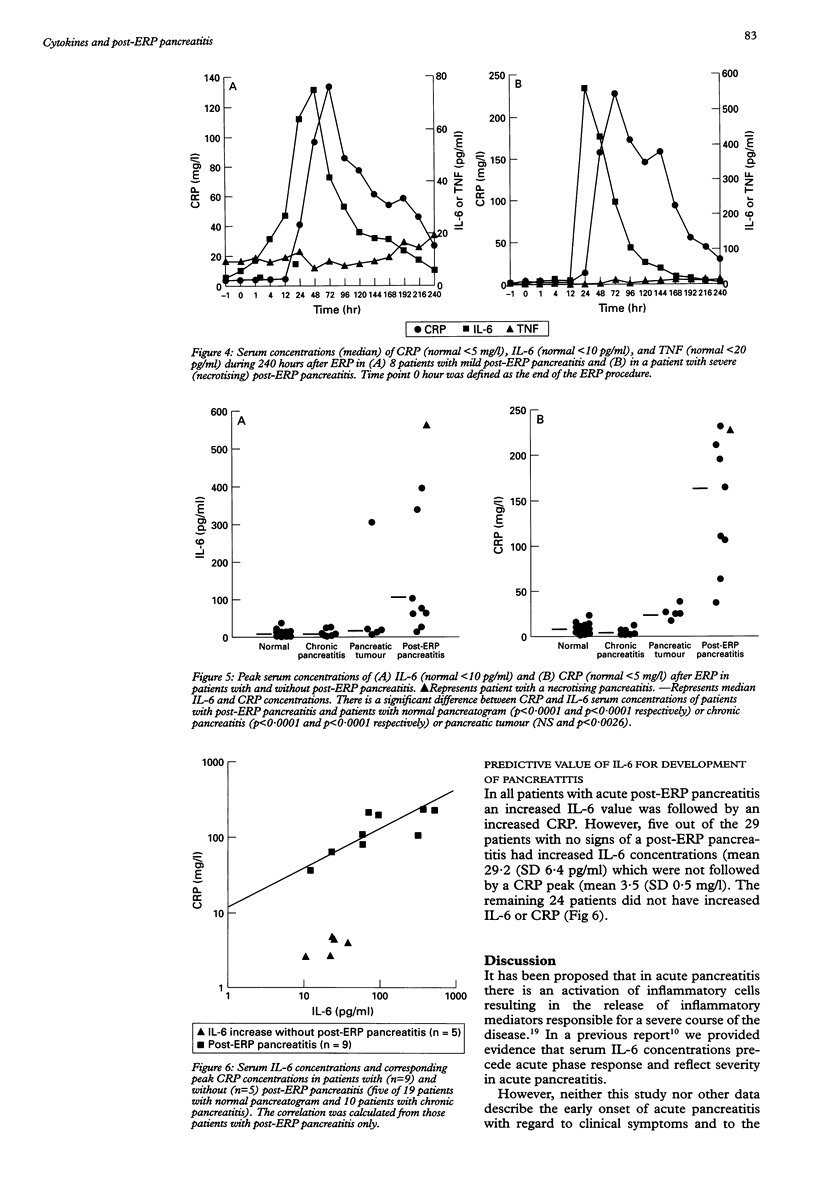
BACKGROUND/AIMS: By contrast with animal models, in most cases it is not possible to examine the systemic response in patients in the first hours after onset of acute pancreatitis. The aim was to determine whether endoscopic retrograde cholangiopancreaticography (ERP)-induced pancreatitis can be used as a human model for the study of cytokine release and acute phase response in the first hours of the disease. PATIENTS AND METHODS: Seventy consecutive patients undergoing ERP for different reasons were prospectively evaluated by sampling blood before and 0, 1, 4, 12, 24, and 48 hours after ERP and, in patients who developed an acute post-ERP pancreatitis, daily until C reactive protein (CRP) was within normal range. A post-ERP pancreatitis was defined as a three-fold increase of amylase or lipase and at least two of the clinical symptoms: abdominal pain, nausea, vomiting, and peritonism during 24 hours after ERP. RESULTS: Nine out of 70 patients developed an acute pancreatitis. Cytokines and other biochemical variables were measured in those nine and in 34 patients out of the 61 not developing pancreatitis. In the nine patients amylase and lipase increased within the first hour after ERP with maximum values between four and 12 hours. Interleukin-6 increased to maximal concentrations after 24-48 hours and the highest CRP concentrations were found 72 hours after ERP. Tumour necrosis factor did not change. CONCLUSION: Post-ERP pancreatitis is an ideal model in which to examine the initial cytokine and acute phase response in the first hours after the initiation of the disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cotton P. B. ERCP. Gut. 1977 Apr;18(4):316–341. doi: 10.1136/gut.18.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruickshank A. M., Fraser W. D., Burns H. J., Van Damme J., Shenkin A. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond) 1990 Aug;79(2):161–165. doi: 10.1042/cs0790161. [DOI] [PubMed] [Google Scholar]
- Exley A. R., Leese T., Holliday M. P., Swann R. A., Cohen J. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut. 1992 Aug;33(8):1126–1128. doi: 10.1136/gut.33.8.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjøsne U., Waldum H. L., Romslo I., Kleveland P. M., Johnsen H., Engebretsen L. F. Amylase, pancreatic isoamylase and lipase in serum before and after endoscopic pancreatography. Acta Med Scand. 1986;219(3):301–304. doi: 10.1111/j.0954-6820.1986.tb03315.x. [DOI] [PubMed] [Google Scholar]
- Gross V., Schölmerich J., Leser H. G., Salm R., Lausen M., Rückauer K., Schöffel U., Lay L., Heinisch A., Farthmann E. H. Granulocyte elastase in assessment of severity of acute pancreatitis. Comparison with acute-phase proteins C-reactive protein, alpha 1-antitrypsin, and protease inhibitor alpha 2-macroglobulin. Dig Dis Sci. 1990 Jan;35(1):97–105. doi: 10.1007/BF01537230. [DOI] [PubMed] [Google Scholar]
- Gross V., Schölmerich J., Leser H. G., Salm R., Lausen M., Rückauer K., Schöffel U., Lay L., Heinisch A., Farthmann E. H. Granulocyte elastase in assessment of severity of acute pancreatitis. Comparison with acute-phase proteins C-reactive protein, alpha 1-antitrypsin, and protease inhibitor alpha 2-macroglobulin. Dig Dis Sci. 1990 Jan;35(1):97–105. doi: 10.1007/BF01537230. [DOI] [PubMed] [Google Scholar]
- Heath D. I., Cruickshank A., Gudgeon M., Jehanli A., Shenkin A., Imrie C. W. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993 Jan;34(1):41–45. doi: 10.1136/gut.34.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. B., Gaber L. W., Kotb M., Mohey el-Din A. B., Pabst M., Gaber A. O. Induction of acute pancreatitis in germ-free rats: evidence of a primary role for tumor necrosis factor-alpha. Surgery. 1995 Feb;117(2):201–205. doi: 10.1016/s0039-6060(05)80086-8. [DOI] [PubMed] [Google Scholar]
- Hughes C. B., Gaber L. W., Kotb M., Mohey el-Din A. B., Pabst M., Gaber A. O. Induction of acute pancreatitis in germ-free rats: evidence of a primary role for tumor necrosis factor-alpha. Surgery. 1995 Feb;117(2):201–205. doi: 10.1016/s0039-6060(05)80086-8. [DOI] [PubMed] [Google Scholar]
- LaFerla G., Gordon S., Archibald M., Murray W. R. Hyperamylasaemia and acute pancreatitis following endoscopic retrograde cholangiopancreatography. Pancreas. 1986;1(2):160–163. doi: 10.1097/00006676-198603000-00009. [DOI] [PubMed] [Google Scholar]
- Leser H. G., Gross V., Scheibenbogen C., Heinisch A., Salm R., Lausen M., Rückauer K., Andreesen R., Farthmann E. H., Schölmerich J. Elevation of serum interleukin-6 concentration precedes acute-phase response and reflects severity in acute pancreatitis. Gastroenterology. 1991 Sep;101(3):782–785. doi: 10.1016/0016-5085(91)90539-w. [DOI] [PubMed] [Google Scholar]
- Mayer A. D., McMahon M. J., Bowen M., Cooper E. H. C reactive protein: an aid to assessment and monitoring of acute pancreatitis. J Clin Pathol. 1984 Feb;37(2):207–211. doi: 10.1136/jcp.37.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon M. J., Bowen M., Mayer A. D., Cooper E. H. Relation of alpha 2-macroglobulin and other antiproteases to the clinical features of acute pancreatitis. Am J Surg. 1984 Jan;147(1):164–170. doi: 10.1016/0002-9610(84)90052-7. [DOI] [PubMed] [Google Scholar]
- Nishimoto N., Yoshizaki K., Tagoh H., Monden M., Kishimoto S., Hirano T., Kishimoto T. Elevation of serum interleukin 6 prior to acute phase proteins on the inflammation by surgical operation. Clin Immunol Immunopathol. 1989 Mar;50(3):399–401. doi: 10.1016/0090-1229(89)90147-5. [DOI] [PubMed] [Google Scholar]
- Norman J. G., Fink G. W., Franz M. G. Acute pancreatitis induces intrapancreatic tumor necrosis factor gene expression. Arch Surg. 1995 Sep;130(9):966–970. doi: 10.1001/archsurg.1995.01430090052018. [DOI] [PubMed] [Google Scholar]
- Paajanen H., Laato M., Jaakkola M., Pulkki K., Niinikoski J., Nordback I. Serum tumour necrosis factor compared with C-reactive protein in the early assessment of severity of acute pancreatitis. Br J Surg. 1995 Feb;82(2):271–273. doi: 10.1002/bjs.1800820244. [DOI] [PubMed] [Google Scholar]
- Panteghini M., Pagani F., Alebardi O., Lancini G., Cestari R. Time course of changes in pancreatic enzymes, isoenzymes and, isoforms in serum after endoscopic retrograde cholangiopancreatography. Clin Chem. 1991 Sep;37(9):1602–1605. [PubMed] [Google Scholar]
- Pitchumoni C. S., Agarwal N., Jain N. K. Systemic complications of acute pancreatitis. Am J Gastroenterol. 1988 Jun;83(6):597–606. [PubMed] [Google Scholar]
- Puolakkainen P., Valtonen V., Paananen A., Schröder T. C-reactive protein (CRP) and serum phospholipase A2 in the assessment of the severity of acute pancreatitis. Gut. 1987 Jun;28(6):764–771. doi: 10.1136/gut.28.6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson J. H., Rifkind K. M., Roses D. F., Fink S. D., Eng K., Spencer F. C. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet. 1974 Jul;139(1):69–81. [PubMed] [Google Scholar]
- Rinderknecht H. Fatal pancreatitis, a consequence of excessive leukocyte stimulation? Int J Pancreatol. 1988 Mar;3(2-3):105–112. doi: 10.1007/BF02798921. [DOI] [PubMed] [Google Scholar]
- Schölmerich J., Heinisch A., Leser H. G. Diagnostic approach to acute pancreatitis: diagnosis, assessment of etiology and prognosis. Hepatogastroenterology. 1993 Dec;40(6):531–537. [PubMed] [Google Scholar]
- Sherman S., Lehman G. A. ERCP- and endoscopic sphincterotomy-induced pancreatitis. Pancreas. 1991 May;6(3):350–367. doi: 10.1097/00006676-199105000-00013. [DOI] [PubMed] [Google Scholar]
- Uhl W., Büchler M., Malfertheiner P., Martini M., Beger H. G. PMN-elastase in comparison with CRP, antiproteases, and LDH as indicators of necrosis in human acute pancreatitis. Pancreas. 1991 May;6(3):253–259. doi: 10.1097/00006676-199105000-00001. [DOI] [PubMed] [Google Scholar]
- Viedma J. A., Pérez-Mateo M., Domínguez J. E., Carballo F. Role of interleukin-6 in acute pancreatitis. Comparison with C-reactive protein and phospholipase A. Gut. 1992 Sep;33(9):1264–1267. doi: 10.1136/gut.33.9.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner G. R., Geenen J. E., Hogan W. J., Catalano M. F. Use of corticosteroids in the prevention of post-ERCP pancreatitis. Gastrointest Endosc. 1995 Dec;42(6):579–583. doi: 10.1016/s0016-5107(95)70014-5. [DOI] [PubMed] [Google Scholar]
- Wilson C., Heads A., Shenkin A., Imrie C. W. C-reactive protein, antiproteases and complement factors as objective markers of severity in acute pancreatitis. Br J Surg. 1989 Feb;76(2):177–181. doi: 10.1002/bjs.1800760224. [DOI] [PubMed] [Google Scholar]



