Abstract
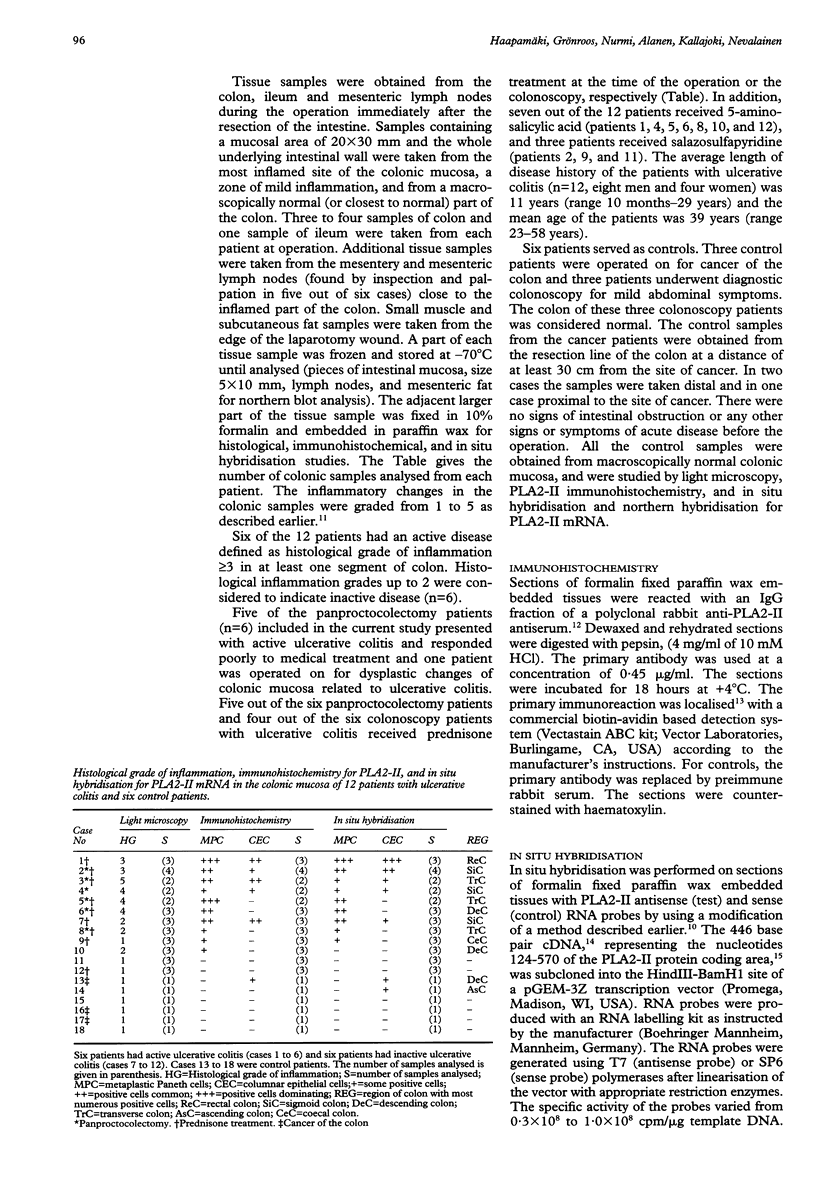
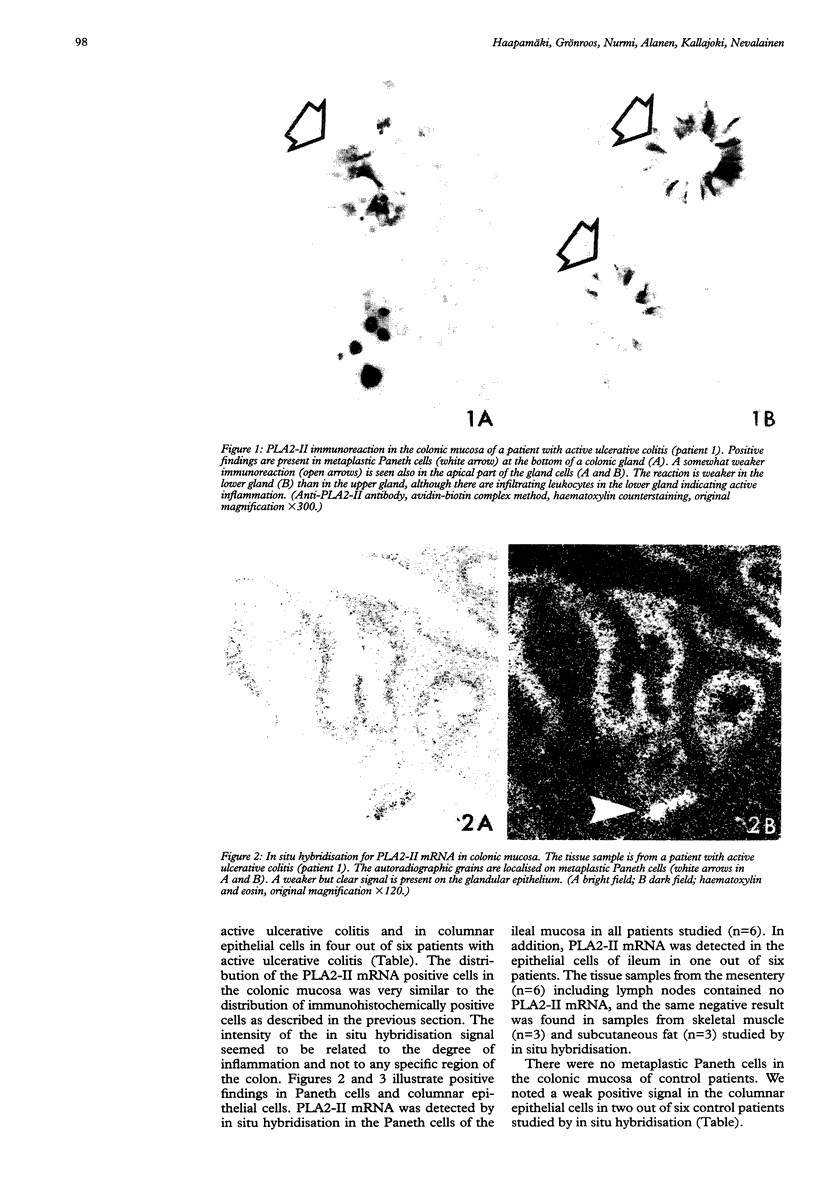
BACKGROUND: It has been suggested that phospholipase A2 (PLA2) has an essential role in the pathogenesis of inflammatory bowel diseases. AIMS: This study aimed at identifying cells in intestinal and mesenteric tissue samples that might express group II phospholipase A2 (PLA2-II) at the mRNA and enzyme protein levels in patients with ulcerative colitis. PATIENTS AND TISSUE SAMPLES: Tissue samples were obtained from the intestine, mesentery, skeletal muscle, and subcutaneous fat of six patients who underwent panproctocolectomy for severe ulcerative colitis. Mucosal biopsy specimens were obtained from the colon of another group of six patients with ulcerative colitis during routine diagnostic colonoscopies. Tissues from six patients without intestinal inflammatory diseases served as controls. METHODS: Tissue samples were studied by light microscopy, immunohistochemistry for PLA2-II enzyme protein, and in situ hybridisation and northern hybridisation for PLA2-II mRNA. RESULTS: PLA2-II mRNA and PLA2-II protein were detected in metaplastic Paneth cells in six patients and in the columnar epithelial cells of colonic mucosa in four out of six patients with active ulcerative colitis. Positive findings were less numerous in patients with mild ulcerative colitis. Only two out of six control patients had a weak positive signal for PLA2-II mRNA and one of these two patients had a weak positive immunoreaction for PLA2-II in columnar epithelial cells in the colonic mucosa. None of the control patients had metaplastic Paneth cells. CONCLUSIONS: Metaplastic Paneth cells and colonic epithelial cells synthesise PLA2-II in ulcerative colitis. The activity of the PLA2-II synthesis seems to be related to the degree of inflammation in the diseased bowel.
Full text
PDF

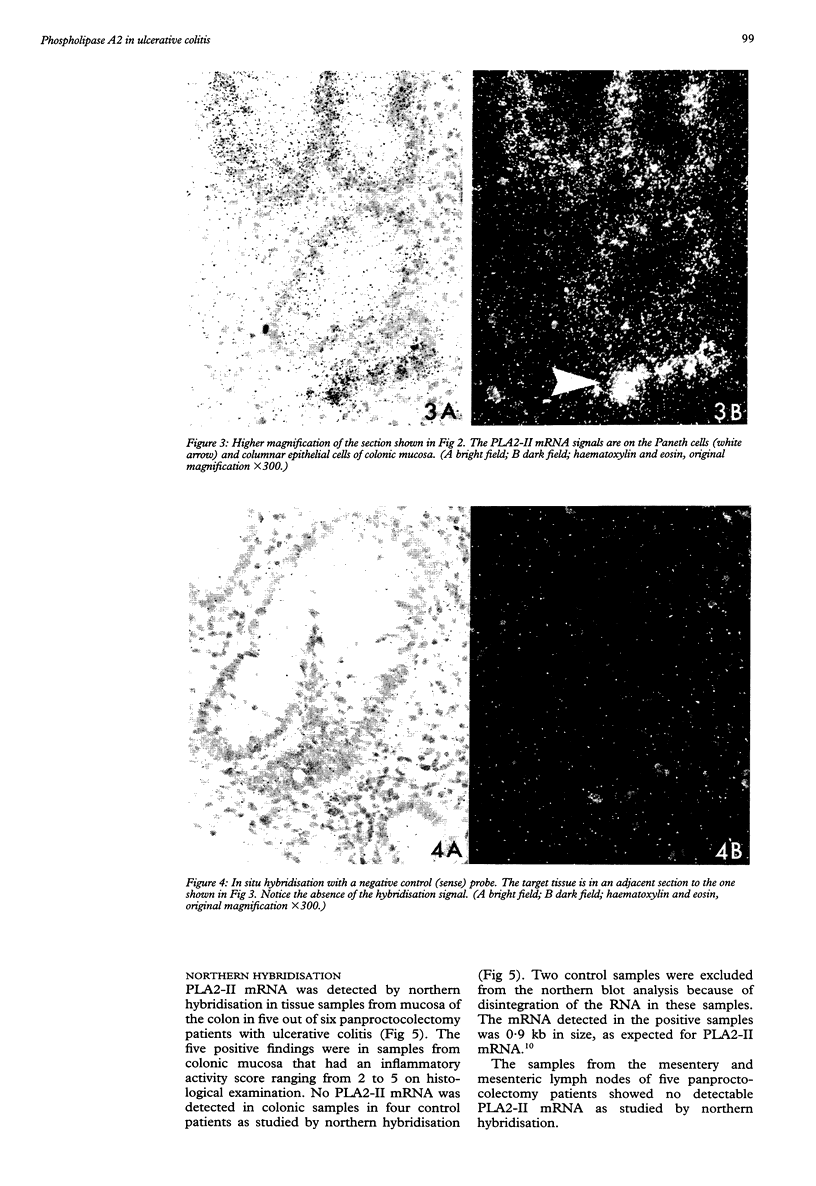


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste J., Chignard M., Le Couedic J. P., Vargaftig B. B. Biosynthesis of platelet-activating factor (PAF-ACETHER). II. Involvement of phospholipase A2 in the formation of PAF-ACETHER and lyso-PAF-ACETHER from rabbit platelets. Thromb Res. 1982 Mar 1;25(5):375–385. doi: 10.1016/0049-3848(82)90128-1. [DOI] [PubMed] [Google Scholar]
- Bomalaski J. S., Baker D. G., Brophy L., Resurreccion N. V., Spilberg I., Muniain M., Clark M. A. A phospholipase A2-activating protein (PLAP) stimulates human neutrophil aggregation and release of lysosomal enzymes, superoxide, and eicosanoids. J Immunol. 1989 Jun 1;142(11):3957–3962. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Di Marco S., Märki F., Hofstetter H., Schmitz A., van Oostrum J., Grütter M. G. Purification, analysis, and enzymatic activity of recombinant human synovial fluid phospholipase A2 and N-terminal variants. J Biochem. 1992 Sep;112(3):350–354. doi: 10.1093/oxfordjournals.jbchem.a123904. [DOI] [PubMed] [Google Scholar]
- Di Marco S., Märki F., Hofstetter H., Schmitz A., van Oostrum J., Grütter M. G. Purification, analysis, and enzymatic activity of recombinant human synovial fluid phospholipase A2 and N-terminal variants. J Biochem. 1992 Sep;112(3):350–354. doi: 10.1093/oxfordjournals.jbchem.a123904. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Grönroos J. M., Kuttila K., Nevalainen T. J. Group II phospholipase A2 in serum in critically ill surgical patients. Crit Care Med. 1994 Jun;22(6):956–959. doi: 10.1097/00003246-199406000-00013. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kiyohara H., Egami H., Shibata Y., Murata K., Ohshima S., Ogawa M. Light microscopic immunohistochemical analysis of the distribution of group II phospholipase A2 in human digestive organs. J Histochem Cytochem. 1992 Nov;40(11):1659–1664. doi: 10.1177/40.11.1431054. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Hession C., Johansen B., Hayes G., McGray P., Chow E. P., Tizard R., Pepinsky R. B. Structure and properties of a human non-pancreatic phospholipase A2. J Biol Chem. 1989 Apr 5;264(10):5768–5775. [PubMed] [Google Scholar]
- Lilja I., Smedh K., Olaison G., Sjödahl R., Tagesson C., Gustafson-Svärd C. Phospholipase A2 gene expression and activity in histologically normal ileal mucosa and in Crohn's ileitis. Gut. 1995 Sep;37(3):380–385. doi: 10.1136/gut.37.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja I., Smedh K., Olaison G., Sjödahl R., Tagesson C., Gustafson-Svärd C. Phospholipase A2 gene expression and activity in histologically normal ileal mucosa and in Crohn's ileitis. Gut. 1995 Sep;37(3):380–385. doi: 10.1136/gut.37.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTS S. G. The value of rectal biopsy in the diagnosis of ulcerative colitis. Q J Med. 1961 Oct;30:393–407. [PubMed] [Google Scholar]
- Minami T., Tojo H., Shinomura Y., Matsuzawa Y., Okamoto M. Increased group II phospholipase A2 in colonic mucosa of patients with Crohn's disease and ulcerative colitis. Gut. 1994 Nov;35(11):1593–1598. doi: 10.1136/gut.35.11.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T., Tojo H., Shinomura Y., Tarui S., Okamoto M. Raised serum activity of phospholipase A2 immunochemically related to group II enzyme in inflammatory bowel disease: its correlation with disease activity of Crohn's disease and ulcerative colitis. Gut. 1992 Jul;33(7):914–921. doi: 10.1136/gut.33.7.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen T. J., Grönroos J. M., Kallajoki M. Expression of group II phospholipase A2 in the human gastrointestinal tract. Lab Invest. 1995 Feb;72(2):201–208. [PubMed] [Google Scholar]
- Nevalainen T. J., Grönroos J. M., Kortesuo P. T. Pancreatic and synovial type phospholipases A2 in serum samples from patients with severe acute pancreatitis. Gut. 1993 Aug;34(8):1133–1136. doi: 10.1136/gut.34.8.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen T. J., Haapanen T. J. Distribution of pancreatic (group I) and synovial-type (group II) phospholipases A2 in human tissues. Inflammation. 1993 Aug;17(4):453–464. doi: 10.1007/BF00916585. [DOI] [PubMed] [Google Scholar]
- Nevalainen T. J., Märki F., Kortesuo P. T., Grütter M. G., Di Marco S., Schmitz A. Synovial type (group II) phospholipase A2 in cartilage. J Rheumatol. 1993 Feb;20(2):325–330. [PubMed] [Google Scholar]
- Nevalainen T. J. Serum phospholipases A2 in inflammatory diseases. Clin Chem. 1993 Dec;39(12):2453–2459. [PubMed] [Google Scholar]
- Pruzanski W., Saito S., Stefanski E., Vadas P. Comparison of group I and II soluble phospholipases A2 activities on phagocytic functions of human polymorphonuclear and mononuclear phagocytes. Inflammation. 1991 Apr;15(2):127–135. doi: 10.1007/BF00917507. [DOI] [PubMed] [Google Scholar]
- Seilhamer J. J., Pruzanski W., Vadas P., Plant S., Miller J. A., Kloss J., Johnson L. K. Cloning and recombinant expression of phospholipase A2 present in rheumatoid arthritic synovial fluid. J Biol Chem. 1989 Apr 5;264(10):5335–5338. [PubMed] [Google Scholar]
- Vadas P., Browning J., Edelson J., Pruzanski W. Extracellular phospholipase A2 expression and inflammation: the relationship with associated disease states. J Lipid Mediat. 1993 Aug;8(1):1–30. [PubMed] [Google Scholar]
- Weinrauch Y., Elsbach P., Madsen L. M., Foreman A., Weiss J. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J Clin Invest. 1996 Jan 1;97(1):250–257. doi: 10.1172/JCI118399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Inada M., Elsbach P., Crowl R. M. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J Biol Chem. 1994 Oct 21;269(42):26331–26337. [PubMed] [Google Scholar]
- Wright G. C., Weiss J., Kim K. S., Verheij H., Elsbach P. Bacterial phospholipid hydrolysis enhances the destruction of Escherichia coli ingested by rabbit neutrophils. Role of cellular and extracellular phospholipases. J Clin Invest. 1990 Jun;85(6):1925–1935. doi: 10.1172/JCI114655. [DOI] [PMC free article] [PubMed] [Google Scholar]