Abstract
Objective
Although chronic exposure to air pollution is associated with an increased risk of dementia in normal elderlies, the effect of chronic exposure to air pollution on the rates of cognitive decline in Alzheimer's disease (AD) has not been elucidated.
Methods
In this longitudinal study, a total of 269 patients with mild cognitive impairment or early dementia due to AD with the evidence of brain β‐amyloid deposition were followed‐up for a mean period of 4 years. Five‐year normalized hourly cumulative exposure value of each air pollutant, such as carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2), and particulate matter (PM2.5 and PM10), was computed based on nationwide air pollution database. The effects of chronic exposure to air pollution on longitudinal cognitive decline rate were evaluated using linear mixed models.
Results
Higher chronic exposure to SO2 was associated with a faster decline in memory score, whereas chronic exposure to CO, NO2, and PM10 were not associated with the rate of cognitive decline. Higher chronic exposure to PM2.5 was associated with a faster decline in visuospatial score in apolipoprotein E ε4 carriers. These effects remained significant even after adjusting for potential confounders.
Interpretation
Our findings suggest that chronic exposure to SO2 and PM2.5 is associated with faster clinical progression in AD.
Introduction
Several epidemiological studies have revealed an association between chronic exposure to air pollution and the risk of developing dementia. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 In specific, chronic exposure to fine (<2.5 μm) and coarse (<10 μm) particulate matter (PM2.5 and PM10) and nitrogen dioxide (NO2) is associated with faster cognitive decline in normal elderly population. 10 , 11 , 12 , 13 Multiple air pollutants, including PM, NO2, sulfur dioxide (SO2), and carbon monoxide (CO), are neurotoxic, 14 by increasing oxidative stress, disrupting integrity of blood–brain–barrier (BBB). Based on this evidence, the Lancet Commission added air pollution as a potential modifiable risk factor for dementia in 2020, 15 and emphasize that the management of air pollution could reduce the risk of developing dementia.
The longitudinal effects of air pollution on cognitive decline in patients with AD remain unclear. One study reported that neuropathologic burden of AD are increased in those with chronic exposure to air pollution. 16 A study based on two cities in Taiwan showed that CO, NO2, SO2, and PM10 exposure was associated with faster clinical deterioration in AD. 17 However, another study based in the United States showed no effect of PM2.5 on longitudinal decline in cognitively impaired patients. 18 As previous studies lacked detailed neuropsychological evaluation and did not provide evidence related to biomarkers of Aβ deposition, it is not clear specifically which air pollutants are associated with cognitive decline in AD. Furthermore, comprehensive analysis of multiple air pollutants was not conducted.
In the present study, we aimed to investigate the impact of air pollutants on cognitive deterioration based on longitudinal neuropsychological test in 269 patients with clinical AD with brain Aβ deposition. We hypothesized that chronic exposure to air pollutants would be associated with faster cognitive decline in patients with AD. By using 5‐year normalized hourly cumulative exposure to air pollutants for each participant, we focused on the chronic effects of air pollutants to suggest a target for both clinical and policy‐making perspectives.
Methods
Study participants
This study included 342 consecutive patients of AD who first visited the Dementia Outpatient Clinic at Severance Hospital, Yonsei University Health System, between January, 2014 and February, 2020. The patients were clinically diagnosed as AD with brain Aβ deposition confirmed by 18F‐florbetaben (FBB) positron emission tomography (PET). Clinical stage of the patients were mild cognitive impairment (MCI) or early dementia with Clinical Dementia Rating® (CDR) either 0.5 or 1, respectively. Subjects were followed‐up for more than 2 years and underwent detailed neuropsychological testing at least twice. Seventy‐three patients were excluded, as their addresses had changed within the 5 years preceding baseline or the air pollutant monitoring station was farther than 15 km from their address. Therefore, 269 patients were included in the final analysis (Fig. 1). Information regarding previous occupational history, smoking and alcohol history, body mass index (BMI), comorbidities—including hypertension, type 2 diabetes, dyslipidemia, and stroke—and apolipoprotein E ε4 (APOE4) carrier status was obtained based on a review of patient medical records.
Figure 1.
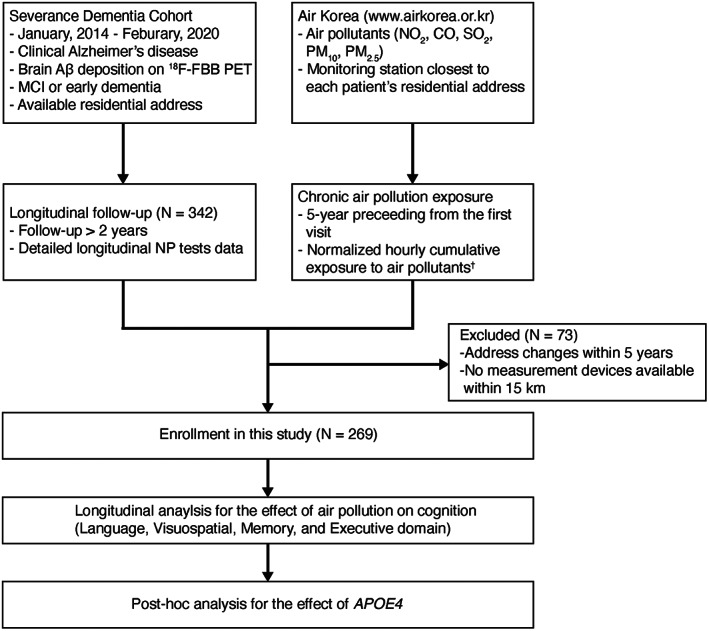
Participant selection flowchart. Aβ, β‐amyloid; APOE4, apolipoprotein E ε4; CO, carbon monoxide; 18F‐FBB, 18F‐florbetaben; NO2, nitrogen dioxide; MCI, mild cognitive impairment; NP, neuropsychological; PET, positron emission tomography, PM, particulate matter; SO2, sulfur dioxide. †Normalized hourly cumulative value = .
Standard protocol approval, registration, and patient consent
This study was approved by the Institutional Review Board of Severance Hospital (IRB 4‐2022‐0221); the requirement for informed consent was waived because this study was based on a retrospective chart review.
Clinical assessment and neuropsychological evaluation
All participants underwent testing using the standardized Seoul Neuropsychological Screening Battery 2nd Edition (SNSB‐II), 19 , 20 at their first visit and during the follow‐up period. This battery includes the following scorable tests: the digit span test (forward and backward), Korean version of the Boston Naming Test (K‐BNT), Seoul Verbal Learning Test (SVLT; includes immediate recall, 20‐min delayed recall, and recognition tests), Rey‐Osterrieth Complex Figure Test (RCFT; copying, immediate recall, 20‐min delayed recall, and recognition tests), Clock‐Drawing Test (CDT), set‐shifting ability (go‐no‐go), phonemic and semantic Controlled Oral Word Association Test (COWAT), Stroop Word Reading (Stroop‐WR), Stroop Color Reading (Stroop‐CR), Digit Symbol Coding (DSC), and Korean‐Trail Making Test (K‐TMT). 21 Age‐ and education‐specific norms were based on 1,067 overall and cognitively healthy community‐dwelling individuals. General cognitive status was assessed using the Korean version of the Mini‐Mental Status Examination (K‐MMSE) and the CDR.
Normally distributed test scores, such as the K‐BNT, SVLT, RCFT, COWAT, Stroop‐CR, DSC, and K‐TMT scores, were z‐transformed using the mean and standard deviation values of the 1,067 norms. Scores that did not follow a normal distribution, such as the CDT and go‐no‐go results, were standardized using percentile scores. The composite scores of the language, visuospatial, memory, and executive domains were calculated using the average of the standardized scores of the corresponding test. Specifically, the language domain composite score was based on the K‐BNT; the visuospatial domain score on the copying items of the RCFT and CDT; the memory domain score on the immediate recall, delayed recall, and recognition tests of the SVLT and RCFT; and the executive domain score on the go‐no‐go, phonemic COWAT, Stroop‐CR, DSC, and K‐TMT part B.
Patient exposure to air pollutants
We calculated patient exposure to air pollutants using publicly available Air Korea (www.airkorea.or.kr, Korea Environment Corporation) data. Monitoring station‐wise data sets were converted to patient‐wise data sets using a previously reported algorithm. 22 Briefly, we extracted the monitoring station closest to each patient's residential address from the Air Korea dataset. PM2.5 measurements were not available for 18 patients, who were therefore excluded from all statistical analyses for PM2.5. We calculated prehospital visit pollutant exposure of 5 years preceding from the date of the first visit to the outpatient clinic of our hospital between January, 2014 and February, 2020. We included exposure data if the measurement value was more than zero (negative or zero values were excluded during the preprocessing step, and these exposure data were not included during the averaging algorithm). All 5‐year cumulative values of hourly exposure were divided by the hour duration of valid pollutant measurements to obtain the normalized average pollutant exposure for a patient (Fig. S1). We excluded cases in which the patient reported house movement during the continuous exposure time‐window of the prehospital visit.
Global Aβ burden measurement using 18 F‐florbetaben PET imaging
FBB PET was performed using a Discovery 600 platform (General Electric Healthcare, Milwaukee, MI, USA). The FBB PET images were acquired 90 min after the administration of 300 MBq (8 mCi) FBB for 20 min. Images were acquired with a 256 × 256 matrix and reconstructed with an ordered‐subsets expectation–maximization algorithm in iso‐0.98‐mm voxel size. We measured the global FBB PET standardized uptake value ratio (SUVR) using a MATLAB‐based MRI‐free pipeline as previously described. 23 Briefly, individual scans were registered nonlinearly to an FBB PET template generated by the Alzheimer's Disease Neuroimaging Initiative. We then estimated the full width at half maximum (FWHM) of an individual scan to apply a differential smoothing kernel with a target resolution of 10 mm3 using the AFNI 3dFWHMx function. 24 Finally, using the standard Global Alzheimer's Association Interactive Network (http://www.gaain.org/centiloid‐project) volumes‐of‐interest (VOIs), we quantified individual global SUVRs using cortical VOIs as a target and cerebellar gray matter VOIs as a reference region. We (Y. Lee, S. Jeon, and B.S. Ye) visually inspected the automated pipeline outcomes for quality control and corrected minor registration defects manually.
Statistical analyses
Statistical analyses of the demographic and clinical data were performed using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). General linear models were used to evaluate the effects of chronic exposure to air pollutants on baseline cognitive scores. Five‐year normalized hourly cumulative value for each air pollutant was used as a predictor. Age, sex, education, APOE4 carrier status, and baseline CDR were included as covariates in Model 1 and occupational history, insurance type, smoking and alcohol history, and BMI were further included as covariates in Model 2. Thereafter, comorbidities, including hypertension, type 2 diabetes, dyslipidemia, and stroke, were further added as covariates in Model 3.
To evaluate the effect of chronic exposure to air pollutants on the rate of cognitive declines, we used linear mixed models (LMMs) for domain‐specific cognitive scores using 5‐year normalized hourly cumulative value for each air pollutant (exposure), follow‐up years from baseline (time), and interaction between the exposure and time terms (exposure × time) were used as predictors. Random intercepts and random slopes were included in the models to allow the subject specific cognitive changes. Each exposure × time interaction term was used to measure the effect of 5‐year normalized cumulative exposure to air pollutants on the rate of change of cognitive scores. We first analyzed the effect of each pollutant separately in a single‐pollutant model. The same covariates of general linear models were used in Models 1–3. Then multiple pollutants were simultaneously included in a multi‐pollutant model with the same covariates. To avoid multicollinearity, if a pair of cumulative exposure of air pollutants were highly correlated (r > 0.7) (Table S1), one of the variables were excluded in the multi‐pollutant models. In the sensitivity analysis, we treated 5‐year normalized hourly cumulative values of chronic exposure to air pollutants as categorical variables based on quartile distribution. In each analysis, the false discovery rate method was used to correct for multiple comparisons across the five air pollutants. To evaluate the independent effect of quantified global amyloid deposition on the longitudinal cognitive decalin, we further conducted sensitivity analysis after further consideration of global SUVR in LMMs for domain‐specific cognitive scores.
To investigate the interacting effects of APOE4 on the relationship between chronic e xposure to air pollutants and longitudinal cognitive decline rate, we further conducted LMMs including three‐way interaction term of APOE4 × exposure × time as predictors. Then, LMMs for domain‐specific cognitive scores were conducted in the subgroups stratified by APOE4 carrier status.
Results
Participants characteristics
The demographic and clinical characteristics of the study subjects (n = 269) are summarized in Table 1. At baseline, the mean age of participants was 74.2 years (SD, 7.0 years), and 63% were female. The mean K‐MMSE total score was 23.5 (SD, 3.2). CDR scores were 0.5 (84%) or 1.0 (16%). During a mean 4.0 years of follow‐up, the participants underwent a median of 2 (range, 2–5) detailed neuropsychological testing with a mean interval of 2.7 years (SD, 1.2 years) between the tests. Five‐year normalized hourly cumulative exposures were as follows: CO, 0.57 parts per million (ppm) (SD, 0.11 ppm); NO2, 0.03 ppm (SD, 0.01 ppm); SO2, 0.01 ppm (SD, 0.001 ppm); PM10, 0.05 ng/m3 (SD, 0.01 ng/m3) and PM2.5, 0.03 ng/m3 (SD, 0.003 ng/m3). The geographic distributions of each participant and 5‐year normalized hourly cumulative exposure to each air pollutant are depicted in Figure S2.
Table 1.
Baseline characteristics of the study subjects.
| Characteristic | N = 269 |
|---|---|
| Age, years | 74.2 (±7.0) |
| Female, no. (%) | 170 (63.2%) |
| Education, years | 10.2 (±4.8) |
| Medical aid type insurance, no. (%) | 4 (1.5%) |
| Follow‐up duration, years | 4.0 (±1.3) |
| Number of cognitive tests, no (range) | 2 (2–5) 2 |
| Interval between cognitive tests, years | 2.7 (±1.2) |
| APOE4 carriers, no. (%) | 144 (53.5%) |
| K‐MMSE total score | 23.5 (±3.2) |
| CDR, no. (%) | |
| 0.5 | 227 (84.4%) |
| 1.0 | 42 (15.6%) |
| Hypertension, no. (%) | 152 (56.5%) |
| Type 2 diabetes, no. (%) | 55 (20.4%) |
| Dyslipidemia, no. (%) | 68 (25.3%) |
| Stroke, no. (%) | 19 (7.1%) |
| BMI (kg/m2) | 23.6 (±3.7) |
| CO (ppm) 1 | 0.57 (±0.11) |
| NO2 (ppm) 1 | 0.03 (±0.01) |
| SO2 (ppm) 1 | 0.01 (±0.001) |
| PM10 (ng/m3) 1 | 0.05 (±0.01) |
| PM2.5 (ng/m3) 1 , 3 | 0.03 (±0.003) |
Plus‐minus values are mean ± SD.
APOE4, apolipoprotein E ε4; BMI, body mass index; CDR, Clinical Dementia Rating®; CO, carbon monoxide; K‐MMSE, Korean version of the Mini‐Mental Status Exam; NO2, nitrogen dioxide; PM, particulate matter; SO2, sulfur dioxide.
Normalized hourly cumulative value.
Median with range.
N = 251.
Effects of chronic exposure to air pollution on baseline cognitive scores
The 5‐year normalized hourly cumulative exposures to air pollutants were not associated with baseline cognitive scores after adjusting for possible cofounders (Table S2).
Effects of chronic exposure to air pollution on cognitive decline rates
In single‐pollutant LMMs for longitudinal cognitive scores (Table 2 and Tables S3–S7), we examined the relation of 5‐year normalized hourly cumulative exposure to each air pollutant with the rate of cognitive decline. Higher exposure to SO2 was associated with a faster rate of decline in memory domain (β = −66.18, 95% confidence interval [CI] = −113.18 to −19.54, p = 0.006) after adjusting for age, sex, education, APOE4 carrier status, and baseline CDR (Model 1). The interacting effect of SO2 and time remained significant after adjustment for demographic cofounders (Model 2) and comorbidities (Model 3). Chronic exposure to other air pollutants (CO, NO2, PM10, and PM2.5) were not associated with cognitive decline rates. When amyloid deposition was further considered as covariates, the effect of SO2 remained significant (Table S8).
Table 2.
Effects of chronic exposure to air pollutant on the rate of cognitive declines.
| Domain | Predictor | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | ||
| Language | CO × year | −0.28 (−0.67–0.10) | 0.151 | −0.28 (−0.67–0.10) | 0.149 | −0.29 (−0.67–0.10) | 0.145 |
| NO2 × year | −3.58 (−8.53–1.37) | 0.157 | −3.65 (−8.57–1.30) | 0.148 | −3.65 (−8.57–1.30) | 0.147 | |
| SO2 × year | −10.18 (−62.92–42.42) | 0.705 | −8.56 (−61.13–43.97) | 0.750 | −8.33 (−60.84–44.22) | 0.756 | |
| PM10 × year | −5.54 (−13.83–2.74) | 0.191 | −5.55 (−13.82–2.72) | 0.189 | −5.54 (−13.80–2.73) | 0.190 | |
| PM2.5 × year | −12.63 (−28.48–3.39) | 0.119 | −12.67 (−28.48–3.30) | 0.117 | −12.73 (−28.54–3.23) | 0.115 | |
| Visuospatial | CO × year | −0.23 (−1.02–0.56) | 0.575 | −0.21 (−1.01–0.57) | 0.597 | −0.21 (−1.00–0.57) | 0.596 |
| NO2 × year | 5.28 (−4.78–15.43) | 0.306 | 5.15 (−4.84–15.33) | 0.316 | 5.15 (−4.80–15.34) | 0.315 | |
| SO2 × year | 14.53 (−91.92–122.50) | 0.789 | 20.00 (−85.76–128.53) | 0.711 | 19.69 (−85.70–127.97) | 0.715 | |
| PM10 × year | −8.18 (−25.07–8.62) | 0.342 | −8.18 (−25.10–8.49) | 0.339 | −8.25 (−25.17–8.39) | 0.335 | |
| PM2.5 × year | −32.42 (−64.61–0.67) | 0.048 | −32.06 (−64.40–0.79) | 0.048 | −32.24 (−64.72–1.06) | 0.047 | |
| Memory | CO × year | −0.28 (−0.64–0.07) | 0.116 | −0.29 (−0.64–0.07) | 0.114 | −0.28 (−0.64–0.07) | 0.116 |
| NO2 × year | −4.08 (−8.58–0.43) | 0.078 | −4.05 (−8.56–0.47) | 0.080 | −4.04 (−8.54–0.48) | 0.081 | |
| SO2 × year | −66.18 (−113.18–19.54) | 0.006 1 | −66.22 (−113.36–19.67) | 0.006 1 | −66.48 (−113.64–20.05) | 0.006 1 | |
| PM10 × year | −3.96 (−11.50–3.62) | 0.305 | −3.94 (−11.49–3.64) | 0.307 | −3.97 (−11.52–3.61) | 0.304 | |
| PM2.5 × year | 6.65 (−7.41–20.84) | 0.356 | 6.52 (−7.53–20.72) | 0.365 | 6.46 (−7.59–20.66) | 0.369 | |
| Executive | CO × year | 0.01 (−0.53–0.55) | 0.974 | 0.01 (−0.53–0.56) | 0.969 | 0.01 (−0.53–0.56) | 0.971 |
| NO2 × year | 1.40 (−5.55–8.34) | 0.693 | 1.38 (−5.57–8.32) | 0.698 | 1.38 (−5.57–8.32) | 0.697 | |
| SO2 × year | −25.94 (−97.26–45.63) | 0.477 | −24.47 (−95.91–47.40) | 0.503 | −24.40 (−95.79–47.46) | 0.504 | |
| PM10 × year | 0.19 (−11.39–11.81) | 0.975 | 0.21 (−11.36–11.85) | 0.971 | 0.22 (−11.35–11.85) | 0.971 | |
| PM2.5 × year | −2.92 (−24.71–19.15) | 0.790 | −3.15 (−25.05–18.93) | 0.773 | −3.16 (−25.07–18.91) | 0.773 | |
Data represent the results of linear mixed model analysis for longitudinal cognitive scores using air pollutants, year, and the interaction term between air pollutants and year (air pollutants × year) as predictors. Model 1 was adjusted for age, sex, education, APOE4 carrier status, and baseline CDR. Model 2: model 1 + further adjusted for the occupational history, insurance type, smoking and alcohol history, and BMI. Model 3: model 2 + further adjusted for comorbidities including hypertension, dyslipidemia, type 2 diabetes, and stroke.
APOE4, apolipoprotein E ε4; BMI, body mass index; CDR, Clinical Dementia Rating®; CO, carbon monoxide; NO2, nitrogen dioxide; PM, particulate matter; SO2, sulfur dioxide.
Significant after multiple comparison corrections across five air pollutants using the false discovery rate method.
Next, we examined the relation of average 5‐year normalized hourly cumulative exposure to multiple air pollutants simultaneously (Table S9). Higher exposure to SO2 was associated with a faster rate of decline in memory domain, while other pollutants were not associated with the cognitive decline rate (Table S9).
In sensitivity analyses, we used quartiles of 5‐year normalized hourly cumulative exposure to each air pollutant (Table 3). Compared to the participants with the lowest SO2 exposure quartile group (2.40–4.80 ppb), participants with the highest SO2 exposure quartile group (5.70–9.43 ppb) had more rapid decline in memory domain score (β = −0.16, 95% CI = −0.27 to −0.04, p = 0.008; Fig. S3).
Table 3.
Effects quartiles of chronic exposure to air pollutants on the rate of cognitive declines.
| Predictor | Category | Language domain | Visuospatial domain | Memory domain | Executive domain | ||||
|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | ||
| CO | 1st quartile [267.54–493.84 ppb] | Reference | Reference | Reference | Reference | ||||
| 2nd quartile [493.91–536.96 ppb] | −0.10 (−0.22–0.03) | 0.122 | 0.04 (−0.21–0.30) | 0.737 | −0.01 (−0.12–0.11) | 0.882 | −0.06 (−0.24–0.12) | 0.499 | |
| 3rd quartile [537.26–626.75 ppb] | −0.09 (−0.22–0.03) | 0.133 | −0.20 (−0.45–0.05) | 0.112 | 0.00 (−0.12–0.11) | 0.955 | −0.06 (−0.23–0.12) | 0.512 | |
| 4th quartile [628.0–932.24 ppb] | −0.15 (−0.27–0.02) | 0.020 | −0.11 (−0.36–0.14) | 0.387 | −0.08 (−0.20–0.03) | 0.145 | −0.09 (−0.26–0.08) | 0.302 | |
| Interquartile increase 1 | −0.04 (−0.08–0.00) | 0.029 | −0.06 (−0.14–0.02) | 0.159 | −0.03 (−0.06–0.01) | 0.167 | −0.03 (−0.08–0.03) | 0.325 | |
| NO2 | 1st quartile [9.06–26.17 ppb] | Reference | Reference | Reference | Reference | ||||
| 2nd quartile [26.60–31.36 ppb] | −0.05 (−0.17–0.08) | 0.471 | 0.05 (−0.21–0.30) | 0.716 | −0.04 (−0.16–0.07) | 0.472 | −0.10 (−0.27–0.08) | 0.280 | |
| 3rd quartile [31.44–34.57 ppb] | −0.02 (−0.14–0.10) | 0.762 | −0.03 (−0.27–0.22) | 0.819 | −0.09 (−0.20–0.02) | 0.113 | −0.12 (−0.29–0.05) | 0.170 | |
| 4th quartile [34.58–58.94 ppb] | −0.12 (−0.24–0.01) | 0.074 | 0.13 (−0.12–0.39) | 0.299 | −0.09 (−0.20–0.03) | 0.133 | 0.00 (−0.17–0.18) | 0.977 | |
| Interquartile increase 1 | −0.03 (−0.07–0.01) | 0.118 | 0.03 (−0.05–0.11) | 0.442 | −0.03 (−0.07–0.00) | 0.087 | 0.00 (−0.06–0.05) | 0.944 | |
| SO2 | 1st quartile [2.40–4.80 ppb] | Reference | Reference | Reference | Reference | ||||
| 2nd quartile [4.81–5.17 ppb] | 0.00 (−0.13–0.13) | 0.974 | 0.10 (−0.15–0.36) | 0.454 | −0.08 (−0.20–0.03) | 0.165 | −0.04 (−0.22–0.14) | 0.649 | |
| 3rd quartile [5.18–5.70 ppb] | −0.02 (−0.14–0.11) | 0.802 | 0.00 (−0.26–0.26) | 0.985 | −0.08 (−0.20–0.03) | 0.153 | −0.05 (−0.23–0.13) | 0.607 | |
| 4th quartile [5.70–9.43 ppb] | −0.01 (−0.14–0.12) | 0.886 | 0.15 (−0.10–0.41) | 0.255 | −0.16 (−0.27–0.04) | 0.008 2 | −0.01 (−0.19–0.16) | 0.870 | |
| Interquartile increase 1 | 0.00 (−0.04–0.04) | 0.846 | 0.04 (−0.04–0.12) | 0.380 | −0.05 (−0.08–0.01) | 0.012 | 0.00 (−0.06–0.05) | 0.892 | |
| PM10 | 1st quartile [37.42–44.49 μg/m3] | Reference | Reference | Reference | Reference | ||||
| 2nd quartile [44.50–47.43 μg/m3] | 0.11 (−0.02–0.24) | 0.088 | 0.11 (−0.14–0.36) | 0.373 | ‐0.01 (−0.13–0.10) | 0.836 | 0.19 (0.02–0.36) | 0.033 | |
| 3rd quartile [47.45–52.54 μg/m3] | −0.02 (−0.14–0.10) | 0.739 | −0.28 (−0.53–0.04) | 0.023 | −0.07 (−0.18–0.04) | 0.213 | −0.01 (−0.18–0.16) | 0.891 | |
| 4th quartile [52.56–64.01 μg/m3] | −0.03 (−0.16–0.10) | 0.627 | 0.01 (−0.24–0.26) | 0.920 | −0.07 (−0.19–0.04) | 0.217 | 0.10 (−0.08–0.27) | 0.287 | |
| Interquartile increase 1 | −0.02 (−0.06–0.02) | 0.246 | −0.04 (−0.12–0.04) | 0.320 | −0.03 (−0.07–0.01) | 0.127 | 0.01 (−0.05–0.06) | 0.822 | |
| PM2.5 | 1st quartile [20.65–24.03 μg/m3] | Reference | Reference | Reference | Reference | ||||
| 2nd quartile [24.03–25.61 μg/m3] | 0.02 (−0.11–0.16) | 0.723 | 0.01 (−0.26–0.28) | 0.961 | −0.01 (−0.13–0.11) | 0.875 | −0.18 (−0.36–0.01) | 0.058 | |
| 3rd quartile [25.62–27.98 μg/m3] | −0.04 (−0.17–0.09) | 0.543 | −0.12 (−0.37–0.14) | 0.378 | 0.06 (−0.05–0.18) | 0.282 | −0.10 (−0.28–0.07) | 0.257 | |
| 4th quartile [28.02–35.82 μg/m3] | −0.02 (−0.15–0.11) | 0.739 | −0.23 (−0.49–0.03) | 0.086 | 0.03 (−0.08–0.15) | 0.554 | −0.03 (−0.21–0.14) | 0.720 | |
| Interquartile increase 1 | −0.01 (−0.05–0.03) | 0.529 | −0.08 (−0.16–0.00) | 0.057 | 0.02 (−0.02–0.05) | 0.350 | 0.00 (−0.06–0.05) | 0.940 | |
Data represent the results of linear mixed model analysis for longitudinal cognitive scores using air pollutants, year, and the interaction term between air pollutants and year (air pollutants × year) as predictors. Covariates included age, sex, education, APOE4 carrier status, and baseline CDR. Air pollutants were treated as categorical variables based on the quartile distribution, and the lowest quartile group was used as a reference.
APOE4, apolipoprotein E ε4; BMI, body mass index; CDR, Clinical Dementia Rating; CO, carbon monoxide; NO2, nitrogen dioxide; PM, particulate matter; SO2, sulfur dioxide.
Air pollutant quartiles were treated as ordinal variables.
Significant after multiple comparison corrections across five air pollutants using the false discovery rate method.
Effects of chronic exposure to air pollution and APOE4 on cognitive decline rates
To understand whether the effect of air pollution on cognitive decline rate was modified by APOE4 carrier, we first conducted LMMs for longitudinal cognitive scores using three‐way interaction term between exposure, time, and APOE4 (Table 4). Higher exposure to PM2.5 and APOE4 carrier status was associated with a faster rate of decline in visuospatial domain (β = −94.21, 95% CI = −155.98 to −32.44, p = 0.003).
Table 4.
Effects of chronic exposure to air pollutants and APOE4 carrier status on the rate of cognitive declines.
| Predictor | Language domain | Visuospatial domain | Memory domain | Executive domain | ||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| CO × APOE4 × year | 0.49 (−0.29–1.26) | 0.224 | 0.48 (−1.11–2.08) | 0.558 | −0.20 (−0.90–0.51) | 0.587 | 0.62 (−0.47–1.71) | 0.266 |
| NO2 × APOE4 × year | 6.54 (−3.59–16.67) | 0.208 | 4.07 (−16.72–24.87) | 0.702 | −5.24 (−14.49–4.01) | 0.269 | 6.88 (−7.40–21.15) | 0.348 |
| SO2 × APOE4 × year | 17.57 (−88.32–123.69) | 0.746 | 29.83 (−182.92–242.75) | 0.785 | −23.60 (−117.72–70.33) | 0.622 | 43.62 (−99.97–187.16) | 0.552 |
| PM10 × APOE4 × year | −7.27 (−23.84–9.28) | 0.393 | −16.11 (−49.85–17.47) | 0.351 | −5.14 (−20.11–9.85) | 0.504 | 1.02 (−22.18–24.24) | 0.932 |
| PM2.5 × APOE4 × year | −13.85 (−45.40–17.71) | 0.394 | −94.21 (−155.98–32.44) | 0.003 1 | −14.59 (−42.21–13.05) | 0.306 | −30.59 (−73.44–12.28) | 0.166 |
Data represent the results of linear mixed model analysis for longitudinal cognitive scores using a three‐way interaction term between air pollutants, APOE4 carrier status, and year (air pollutants × APOE4 × year) as a predictor. The covariates included age, sex, education, and baseline CDR.
APOE4, apolipoprotein E ε4; BMI, body mass index; CDR, Clinical Dementia Rating; CO, carbon monoxide; NO2, nitrogen dioxide; PM, particulate matter; SO2, sulfur dioxide.
Significant after multiple comparison corrections across five air pollutants using the false discovery rate method.
We further investigate the effect of air pollution on cognitive decline rate in APOE4 carrier and noncarrier subgroups (Table S10). Higher exposure to PM2.5 was associated with a faster rate of decline in visuospatial domain only in APOE4 carrier group (β = −94.21, 95% CI = −155.98 to −32.44, p = 0.003).
Discussion
In this study, we assessed the effects of chronic exposure to air pollutants on the cognitive decline rate in AD patients with brain Aβ deposition and whose detailed demographic and socioeconomic information, residential history, comorbidities, and longitudinal neuropsychological evaluations were available. The major findings of this study are as follows: first, we found that higher 5‐year normalized hourly cumulative exposure to SO2 was associated with a faster rate of decline in the memory domain. Second, higher 5‐year normalized hourly cumulative exposure to PM2.5 was associated with a faster rate of decline in the visuospatial domain only in APOE4 carriers. These associations remained statistically robust after adjusting for possible confounders, including age, sex, education, APOE4 carrier status, baseline CDR, occupational history, smoking and alcohol history, BMI, and comorbidities, including hypertension, dyslipidemia, type 2 diabetes, and stroke. These findings suggest that chronic exposure to air pollutants could have deleterious effects on the clinical deterioration in the patients with AD.
Our first major finding was that higher 5‐year normalized hourly cumulative exposure to SO2 was associated with a faster rate of decline in the memory domain. This suggest that among the air pollutants, SO2 is important target for the management of AD. The detrimental effects of SO2 could be explained by neuroinflammation, 25 synaptic dysfunction, 26 and tau phosphorylation. 27 Our finding is consistent with a previous study that showed the strongest effects of exposure to SO2 on CDR deterioration in patients with AD compared to exposure to CO, NO2, PM10, SO2, and ozone. 17 Another study also showed detrimental effects of SO2 on the total and memory subdomain score of the MMSE in normal elderly population. 28 However, most of previous studies evaluating the effect of air pollution on the risk of dementia or cognitive decline have been performed in normal elderly individuals without dementia from community‐based cohorts and most of these studies did not include SO2 as a predictor, 1 , 4 , 5 , 6 , 7 , 11 , 12 , 29 , 30 , 31 , 32 , 33 it may be possible that the effects of SO2 in AD were unrevealed in previous studies. Further studies are warranted to evaluate whether SO2 is associated with rapid clinical progression after AD occurrence rather than the occurrence of AD.
Our findings raise concerns about the current guidelines on the exposure limit of SO2 and suggest that dementia patients may need disease‐specific guidelines. As the highest SO2 exposure quartile (5.70–9.43 ppb/h) group showed faster decline of memory function than the lowest quartile group (2.40–4.80 ppb/h), current guidelines for general population may not be safe for dementia patients. The US Environmental Protection Agency National Ambient Air Quality Standards value for SO2 is 75 ppb (1‐h concentration), 34 that according to a general guideline of the World Health Organization is 125 μg/m3 (24‐h mean concentration), 35 and that as per the Korean Ministry of Environment Framework Act On Environmental Policy is 150 ppb (1‐h concentration), 36 all of which are 7–15 times higher than the level of SO2 in the highest exposure group.
Our second major finding was that APOE4 status and chronic exposure to PM2.5 were interactively associated with a faster decline in the visuospatial domain score. This result is consistent with previous longitudinal studies showing a significant interaction between PM2.5 and APOE4 on cognitive deterioration. 32 , 37 Given that PM2.5 penetrates the BBB by disrupting tight junctions 38 and that APOE4 accelerates the breakdown of the BBB, 39 aggravated disruption of BBB integrity could explain the interaction effect between PM2.5 and APOE4 on the longitudinal decline in visuospatial domain score. However, the previous studies included community‐dwelling elderly individuals without dementia 32 , 37 ; moreover, the interaction effect was significant for all cognitive domains, including memory, language, and executive function, 32 and all air pollutants, including not only PM2.5 32 , 37 but also NO2 and PM10. 32 Considering that PM is also associated with an increased risk of Aβ accumulation in elderly individuals without dementia 16 and animal models, 37 the limited variance of Aβ due to the inclusion of cognitively impaired patients with confirmed Aβ deposition in our study could explain the discrepancy.
This study has several limitations. First, it included subjects from a tertiary referral center in Seoul, a metropolitan city in Korea, and 63.2% of the subjects had lived in Seoul for at least 5 years prior to baseline. Thus, various demographic features related to the residential environment may have been major confounders. Although the sensitivity analyses with further adjustment for living in Seoul showed similar results (data not shown), careful interpretation of our results in this context is required. Second, we included patients only if their residential addresses were located within 15 km of air monitoring stations. While such an inclusion criterion has been found appropriate for other diseases, 22 dementia may need further examination to identify disease‐related optimal distance. Further evaluation using satellite‐based air pollutant measurement data would be required. Despite these limitations, our study shows a strong association between chronic exposure to air pollution and faster cognitive decline in patients with AD. Our findings emphasize that prompt consideration of air pollution is needed in the management of patients with AD from the policy‐making perspective as well as in clinical settings.
Author Contributions
Young‐gun Lee, Seon‐Jin Yoon, and Byoung Seok Ye contributed to the conception and design of the study; Young‐gun Lee and Seon‐Jin Yoon contributed to the acquisition and analysis of data; Young‐gun Lee, Seon‐Jin Yoon, So Hoon Yoon, Sung Woo Kang, Seun Jeon, Minseok Kim, Dong Ah Shin, Chung Mo Nam, and Byoung Seok Ye contributed to drafting the text or preparing the figures.
Conflict of Interest
The authors report no competing interests.
Supporting information
Appendix S1.
Acknowledgments
The authors are grateful to all the participants who have taken part in this study. This research was supported by grants of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant numbers: HI22C0977, HU22C0138, and HR22C1411).
Funding Statement
This work was funded by Korea Health Industry Development Institute grants HI22C0977, HR22C1411, and HU22C0138.
Data Availability Statement
Data of air pollution level excluding address information and de‐identified clinical information supporting the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Jung C‐R, Lin Y‐T, Hwang B‐F. Ozone, particulate matter, and newly diagnosed Alzheimer's disease: a population‐based cohort study in Taiwan. J Alzheimers Dis. 2015;44(2):573‐584. [DOI] [PubMed] [Google Scholar]
- 2. Oudin A, Forsberg B, Adolfsson AN, et al. Traffic‐related air pollution and dementia incidence in northern sweden: a longitudinal study. Environ Health Perspect. 2016;124(3):306‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen H, Kwong JC, Copes R, et al. Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis: a population‐based cohort study. Lancet. 2017;389(10070):718‐726. [DOI] [PubMed] [Google Scholar]
- 4. Carey IM, Anderson HR, Atkinson RW, et al. Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open. 2018;8(9):e022404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oudin A, Segersson D, Adolfsson R, Forsberg B. Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in Northern Sweden. PLoS ONE. 2018;13(6):e0198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air pollution and dementia: a systematic review. J Alzheimers Dis. 2019;70:S145‐S163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu P, Yung KKL. Air pollution and Alzheimer's disease: a systematic review and meta‐analysis. J Alzheimers Dis. 2020;77(2):701‐714. [DOI] [PubMed] [Google Scholar]
- 8. He F, Tang J, Zhang T, et al. Impact of air pollution exposure on the risk of Alzheimer's disease in China: a community‐based cohort study. Environ Res. 2022;205:112318. [DOI] [PubMed] [Google Scholar]
- 9. Semmens EO, Leary CS, Fitzpatrick AL, et al. Air pollution and dementia in older adults in the Ginkgo Evaluation of Memory Study. Alzheimers Dement. 2023;19:549‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F. Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med. 2012;172(3):219‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tonne C, Elbaz A, Beevers S, Singh‐Manoux A. Traffic‐related air pollution in relation to cognitive function in older adults. Epidemiology (Cambridge, Mass.). 2014;25(5):674‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kulick ER, Wellenius GA, Boehme AK, et al. Long‐term exposure to air pollution and trajectories of cognitive decline among older adults. Neurology. 2020;94(17):e1782‐e1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petkus AJ, Younan D, Widaman K, et al. Exposure to fine particulate matter and temporal dynamics of episodic memory and depressive symptoms in older women. Environ Int. 2020;135:105196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Block ML, Calderón‐Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32(9):506‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JH, Byun MS, Yi D, et al. Long‐term exposure to PM10 and in vivo Alzheimer's disease pathologies. J Alzheimers Dis. 2020;78(2):745‐756. [DOI] [PubMed] [Google Scholar]
- 17. Lin FC, Chen CY, Lin CW, Wu MT, Chen HY, Huang P. Air pollution is associated with cognitive deterioration of Alzheimer's disease. Gerontology. 2021;68:53‐61. [DOI] [PubMed] [Google Scholar]
- 18. Cleary EG, Cifuentes M, Grinstein G, Brugge D, Shea TB. Association of low‐level ozone with cognitive decline in older adults. J Alzheimers Dis. 2017;61:67‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang Y, Jahng S, Na DL. Seoul Neuropsychological Screening Battery 2nd Edition (SNSB‐II). Human Brain Research & Consulting; 2012. [Google Scholar]
- 20. Kim H, Yu KH, Lee BC, Kim BC, Kang Y. Validity of the Montreal Cognitive Assessment (MoCA) Index Scores: a comparison with the cognitive domain scores of the Seoul Neuropsychological Screening Battery (SNSB). Dement Neurocogn Disord. 2021;20(3):28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahn H‐J, Chin J, Park A, et al. Seoul Neuropsychological Screening Battery‐Dementia Version (SNSB‐D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010;25(7):1071‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoon SJ, Noh J, Son HY, et al. Ambient carbon monoxide exposure and elevated risk of mortality in the glioblastoma patients: a double‐cohort retrospective observational study. Cancer Med. 2020;9:9018‐9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iaccarino L, La Joie R, Koeppe R, et al. rPOP: robust PET‐only processing of community acquired heterogeneous amyloid‐PET data. Neuroimage. 2022;246:118775. [DOI] [PubMed] [Google Scholar]
- 24. Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10(4–5):171‐178. [DOI] [PubMed] [Google Scholar]
- 25. Yao G, Yue H, Yun Y, Sang N. Chronic SO2 inhalation above environmental standard impairs neuronal behavior and represses glutamate receptor gene expression and memory‐related kinase activation via neuroinflammation in rats. Environ Res. 2015;137:85‐93. [DOI] [PubMed] [Google Scholar]
- 26. Yun Y, Yao G, Yue H, et al. SO2 inhalation causes synaptic injury in rat hippocampus via its derivatives in vivo. Chemosphere. 2013;93(10):2426‐2432. [DOI] [PubMed] [Google Scholar]
- 27. Ku T, Chen M, Li B, Yun Y, Li G, Sang N. Synergistic effects of particulate matter (PM2.5) and sulfur dioxide (SO2) on neurodegeneration via the microRNA‐mediated regulation of tau phosphorylation. Toxicol Res (Camb). 2017;6(1):7‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen M‐C, Wang C‐F, Lai B‐C, et al. Air pollution is associated with poor cognitive function in Taiwanese adults. Int J Environ Res Public Health. 2021;18(1):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schikowski T, Vossoughi M, Vierkötter A, et al. Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ Res. 2015;142:10‐16. [DOI] [PubMed] [Google Scholar]
- 30. Gatto NM, Henderson VW, Hodis HN, et al. Components of air pollution and cognitive function in middle‐aged and older adults in Los Angeles. Neurotoxicology. 2014;40:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duchesne J, Gutierrez L‐A, Carrière I, et al. Exposure to ambient air pollution and cognitive decline: results of the prospective Three‐City cohort study. Environ Int. 2022;161:107118. [DOI] [PubMed] [Google Scholar]
- 32. Kulick ER, Elkind MSV, Boehme AK, et al. Long‐term exposure to ambient air pollution, APOE‐ε4 status, and cognitive decline in a cohort of older adults in northern Manhattan. Environ Int. 2020;136:105440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sakhvidi MJZ, Yang J, Lequy E, et al. Outdoor air pollution exposure and cognitive performance: findings from the enrolment phase of the CONSTANCES cohort. The Lancet Planetary Health. 2022;6(3):e219‐e229. [DOI] [PubMed] [Google Scholar]
- 34. EPA . Review of the Primary National Ambient Air Quality Standards for Sulfur Oxides. Environmental Protection Agency; 2019. [Google Scholar]
- 35. WHO . Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulfur Dioxide: Global Update 2005: Summary of Risk Assessment. Environmental Health Team; World Health Organization; 2006. [Google Scholar]
- 36. Framework Act On Environmental Policy . Formulation of Environmental Standards. Ministry of Environment; 2020. [Google Scholar]
- 37. Cacciottolo M, Wang X, Driscoll I, et al. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry. 2017;7(1):e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kang YJ, Tan HY, Lee CY, Cho H. An air particulate pollutant induces neuroinflammation and neurodegeneration in human brain models. Adv Sci (Weinh). 2021;8(21):e2101251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Montagne A, Nation DA, Sagare AP, et al. APOE4 leads to blood‐brain barrier dysfunction predicting cognitive decline. Nature. 2020;581(7806):71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
Data of air pollution level excluding address information and de‐identified clinical information supporting the findings of this study are available from the corresponding author upon reasonable request.


