Abstract
Children with rare cholestatic liver diseases, such as Alagille syndrome, progressive familial intrahepatic cholestasis, and biliary atresia typically require liver transplantation (LT). The objective of this analysis was to assess the economic burden of LT on these patients. Health care resource utilization and costs associated with pediatric LT were retrospectively assessed using insurance claims data from the US IBM MarketScan Commercial and Medicaid databases collected between October 2015 and December 2019. Inclusion criteria were as follows: ≥1 procedure code for LT, <18 years old at transplant, and ≥6 months of insurance eligibility at baseline. A cholestatic liver disease population who received LT was selected in the absence of specific diagnosis codes by excluding other severe liver conditions (ie, acute liver failure, malignancy) and by excluding severely decompensated individuals requiring ICU admission before LT. Annualized rates were reported. Over a mean study duration of 1.8 years, 53 commercially insured and 100 Medicaid-insured children received LT, with mean (SD) ages at baseline of 6.9 (6.0) and 5.7 (5.4) years, respectively. During this period, commercially insured and Medicaid-insured patients had annualized means of 65.3 and 52.8 medical visits, respectively. Most were outpatient visits, although the burden of inpatient visits was also high, with mean inpatient stays (inclusive of LT stay) of 37.2 and 31.6 days per year, respectively. Commercially insured and Medicaid-insured patients averaged US$512,124 and $211,863 in medical costs and $26,998 and $15,704 in pharmacy costs, respectively. These costs remained substantial throughout the first year after transplant. Overall, pediatric LT resulted in substantial health care resource utilization and cost burden in both commercially- and Medicaid-insured patients. Novel targeted medications that negate the need for pediatric LT could decrease the associated morbidity and costs.
INTRODUCTION
Liver transplantation (LT) is an effective therapy for a range of chronic liver diseases in children; however, it can result in several potentially fatal or long-term complications, such as graft rejection and failure, hypertension, and chronic renal impairment, with a 5-year mortality rate of 11.6%.1,2 Moreover, 5-year graft survival rates were reported to be 86.5% in pediatric patients who underwent LT.3 Graft rejection, which is more common in children, is associated with the need for additional medical procedures such as liver biopsies and hospital admissions.4,5 Furthermore, an estimated 8% to 29% of patients who receive a liver transplant will require retransplantation, which has higher associated morbidity and mortality rates than the initial transplant.5–7 In addition, posttransplant immunosuppression increases the risk of infections and malignant complications,8–10 and can result in a significant burden for patients and caregivers.
Between 2011 and 2021, 5,741 liver transplants were performed on pediatric patients in the US.11 A significant proportion of these transplants (46.0%) were performed due to cholestatic disease, including biliary atresia, but also rarer cholestatic diseases, such as Alagille syndrome (ALGS) and progressive familial intrahepatic cholestasis (PFIC).2
Pediatric LT is associated with significant health care resource utilization (HRU), including inpatient and outpatient medical visits and cost burden. However, there is a paucity of data focused on the cost of LT in pediatric patients with cholestatic liver disease. Therefore, this analysis estimated the economic burden associated with LT in pediatric patients with cholestatic liver disease in the US following their surgery.
METHODS
Data sources
Health insurance claims data from October 1, 2015, through December 31, 2019, from the IBM Watson Health MarketScan Commercial Claims and Encounters and Multi-State Medicaid databases were analyzed retrospectively. The MarketScan Commercial Claims and Encounters database contains data from ~100 different insurance plans representing around 93 million covered individuals. This database provides information on private sector health care and the health care claims of employees and dependents covered by health benefit programs of large employers. The Medicaid database provides information on over 44 million Medicaid enrollees across multiple states in the US. These databases provide information on enrollment history and claims for medical (eg, professional and institutional services) and pharmacy services. Inpatient services are also recorded at the claim and summarized stay levels. The children in this analysis are drawn from a subset of the US population with available claims in the database. This research was exempt from Institutional Review Board approval as the data were completely de-identified from a health care claims database.
Study design and cohort selection
Pediatric liver transplant cohorts were identified based on the following criteria within each population: ≥1 procedure code (Supporting Table 1, http://links.lww.com/LVT/A329) for a liver transplant on a claim between October 1, 2015, and December 31, 2019; age <18 years as of the liver transplant date, defined as the earliest occurrence of liver transplant in the data cut; and ≥6 months of continuous eligibility (ie, continuous enrollment in commercial or Medicaid insurance plans and continuous prescription drug coverage) before the liver transplant date.
Rare cholestatic liver diseases, like ALGS and PFIC, do not have their own International Classification of Diseases codes; thus, an algorithm for cholestatic liver disease was needed to better approximate the burden of LT among pediatric patients with cholestatic liver disease. This algorithm was developed with input from clinical experts and cross-referenced with the United Network for Organ Sharing (UNOS) transplant database to assess the accuracy of our selected patient population (Figure 1; Supporting Fig. 1, http://links.lww.com/LVT/A329).12 To better characterize the costs after a liver transplant for individuals with cholestatic liver disease apart from costs associated with severe decompensation and acute-on-chronic liver failure in the absence of being able to directly identify status 1A and 1B patients, the following exclusion criteria were used: stay in an ICU beginning >24 hours before and including the liver transplant date; a diagnosis of any malignancy (hepatic or extrahepatic) on or before the liver transplant date; and a solid organ transplant in the year before liver or after liver transplant.
FIGURE 1.

Identification of children who underwent LT due to conditions similar in severity to cholestatic liver disease. *The liver transplant date was defined as the earliest occurrence of a procedure code for LT. †Continuous eligibility was defined as continuous enrollment in commercial or Medicaid insurance plans and continuous prescription drug coverage. ‡This step was taken to exclude status 1A patients; however, all pediatric ICU stays >24 hours were excluded. §Patients with the following solid organ transplants were excluded: heart, lung, kidney, pancreas, or intestine. Abbreviations: ICU, intensive care unit; LT, liver transplantation.
A subset of pediatric patients with ≥12 months of follow-up after the liver transplant date was selected for additional analyses. The study period started 90 days before the liver transplant date, and patients were followed for 12 months after transplant. To ensure the analysis accounted for differences in insurance type, HRU and the total cost of pediatric LT were evaluated separately in commercially insured and Medicaid-insured populations using insurance claims data.
Study outcomes
Baseline characteristics were assessed during the baseline period (defined as the 6 mo before liver transplant) for each population and included clinical characteristics, extrahepatic manifestations and procedures relevant to cholestatic liver disease, and treatment with off-label medications. All-cause HRU was assessed throughout the study period within each population. The HRU categories assessed included all visits and the following subcategories: outpatient visits (overall and by provider categories); home care visits; inpatient visits; emergency department visits, and other visits. Home care visits were defined as any visit with a home health care provider. “Other” visits were defined as visits associated with durable medical equipment or dental and vision care. Outpatient provider categories of interest were defined using revenue codes and MarketScan provider variables and included primary care, cardiology, gastrointestinal, lab/imaging, and nephrology.
All-cause costs were defined as the total costs reimbursed by insurers and were assessed throughout the study period within each population. Medical costs, other costs, and pharmacy costs were assessed. Pharmacy costs were derived from outpatient pharmacy claims specifically.
All-cause HRU and costs were also described over 3-month periods for the subset of pediatric patients who received a liver transplant with ≥12 months of follow-up after the liver transplant date. HRU and costs were assessed across the following mutually exclusive periods: 3 months before surgery; liver transplant date to 3 months after liver transplant; 3–6 months after surgery; 6–9 months after surgery, and 9–12 months after surgery.
Statistical analyses
All statistical analyses were implemented using the Statistical Analysis Software (SAS) Enterprise Guide 7.1. Means, medians, SDs, and ranges were reported for continuous variables; frequencies and percentages were reported for categorical variables. Costs were reported in 2019 US dollars. HRU and costs were reported as annualized rates, which were calculated by dividing each patient’s total number of visits or inpatient days (HRU) or costs by their study period duration. After calculating each patient’s annualized outcome, the average of the patients’ annualized HRU and cost values were reported. HRU and costs for the exploratory subgroup with ≥12 months of follow-up were reported as the average total number of visits and average total costs per patient over every 3 months.
RESULTS
Patient characteristics
This analysis included 53 commercially insured and 100 Medicaid-insured pediatric patients who underwent liver transplants. In the commercially insured population, the mean (SD) age was 6.9 (6.0) years, and 43.4% of patients were male. Among the Medicaid-insured population, the mean (SD) age was 5.7 (5.4) years, and 51.0% of patients were male (Table 1). The most common clinical characteristics of patients at baseline were cirrhosis (commercial: 75.5%; Medicaid: 64.0%), portal hypertension (commercial: 60.4%; Medicaid: 50.0%), and liver failure (commercial: 54.7%; Medicaid: 31.0%) (Supporting Table 2, http://links.lww.com/LVT/A329). Rates of nutritional deficiencies or malnutrition and congenital heart disease were similar between commercially insured and Medicaid-insured patients, but the incidence of rickets was numerically higher in the Medicaid-insured population (commercial: 26.4%; Medicaid: 42.0%). At baseline, many patients were taking off-label medications in both the commercially insured and Medicaid-insured populations (see Supporting Table 3, http://links.lww.com/LVT/A329).
TABLE 1.
Demographic characteristics of the pediatric liver transplant patient cohorts at liver transplant date
| Patient characteristics | Commercially insured population (n=53), n (%) | Medicaid-insured population (n=100), n (%) |
|---|---|---|
| Age, mean (SD) | 6.9 (6.0) | 5.7 (5.4) |
| Age categories | ||
| >0-<2 y | 20 (37.7) | 42 (42.0) |
| ≥2-<6 y | 6 (11.3) | 20 (20.0) |
| ≥6-<10 y | 7 (13.2) | 15 (15.0) |
| ≥10 y | 20 (37.7) | 23 (23.0) |
| Male | 23 (43.4) | 51 (51.0) |
Note: All values are n (%) unless otherwise specified.
All-cause HRU
The mean study period duration was 1.8 years (median 1.5; range 0.3–3.9) for the commercially insured population and 1.8 years (median 1.6; range 0.3–4.0) for the Medicaid-insured population. During this period, commercially insured patients had an annualized mean of 65.3 medical visits (median 56.1; range 9.5–256.1; Figure 2), and Medicaid-insured patients had a mean of 52.8 medical visits (median 44.2; range 5.5–240.7; Figure 3). Of note, multiple visits could occur on the same day. Medical visits were defined as laboratory tests, outpatient procedures, and clinic visits. Outpatient visits accounted for most of the total number of medical visits. Commercially insured and Medicaid-insured patients had mean numbers of 41.0 (median 37.1; range 8.9–108.8) and 33.6 (median 30.9; range 4.5–100.9) outpatient visits, respectively. The most common types of outpatient visits in both populations were lab/imaging and primary care specialty.
FIGURE 2.

Annualized, all-cause HRU among pediatric patients receiving an LT in the commercially-insured population (n = 53). *Mean values. Box-and-whisker plots represent the medians, interquartile ranges, and ranges of the overall annualized cost data; asterisks denote mean values. Max. values denote the upper limits of the ranges, which are off-scale. Abbreviations: HRU, health care resource utilization; LT, liver transplantation.
FIGURE 3.
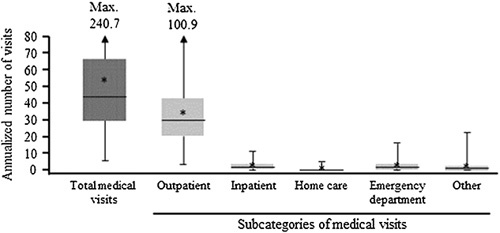
Annualized, all-cause HRU among pediatric patients receiving an LT in the Medicaid-insured population (n = 100). *Mean values. Box-and-whisker plots represent the medians, interquartile ranges, and ranges of the overall annualized cost data; asterisks denote meal values. Max. values denote the upper limits of the ranges, which are off-scale. Abbreviations: HRU, health care resource utilization; LT, liver transplantation.
Commercially insured patients had a mean of 3.5 (median 2.3; range 0.0–13.3) hospitalizations, with a mean length of stay of 37.2 (median 30.6; range 0.0–139.5) days per year and 2.3 (median 1.6; range 0.0–13.7) emergency department visits per year. Medicaid-insured patients had a mean of 2.6 (median 1.7; range 0.0–11.1) hospitalizations, with a mean length of stay of 31.6 (median 16.7; range 0.0–206.4) days per year and 2.7 (median 1.9; range 0.0–16.9) emergency department visits per year.
All-cause costs
Mean annualized medical costs among commercially insured patients were $512,124 (median $438,818; range $1,066–$1,638,008) per year (Figure 4), and among Medicaid-insured patients were $211,863 (median $161,373; range $176–$1,020,575) per year (Figure 5). The majority of these costs were incurred during inpatient visits (mean $452,744 [median $371,544; range $0–$1,557,333] per year for commercially insured patients and mean $194,483 [median $130,086; range $0–$1,007,760] per year for Medicaid-insured patients). Lab/imaging visits were the largest contributors to outpatient costs in both populations. Annualized mean pharmacy costs were $26,998 (median $13,277; range $0–$433,346) among commercially insured patients and $15,704 (median $7,211; range $0–$154,528) among Medicaid-insured patients.
FIGURE 4.

Annualized costs in the commercially-insured population (n = 53). Mean all-cause total medical costs are reported in 2019 US dollars. *Mean values. †Categories of total medical costs. Box-and-whisker plots represent the medians, interquartile ranges, and ranges of the overall annualized cost data; asterisks denote mean values. Max. values denote the upper limits of the ranges, which are off-scale.
FIGURE 5.

Annualized costs in the Medicaid-insured population (n = 100). Mean all-cause total medical costs are reported in 2019 US dollars. *Mean values. †Categories of total medical costs. Box-and-whisker plots represent the medians, interquartile ranges, and ranges of the overall annualized cost data; asterisks denote mean values. Max. values denote the upper limits of the ranges, which are off-scale.
HRU across 3-month periods
A total of 32 commercially insured patients and 61 Medicaid-insured patients who had ≥12 months of continuous eligibility after the liver transplant date were included in an analysis of HRU and costs across 3-month periods. This analysis aimed to examine the impact of follow-up on HRU and costs. The mean (SD) age at liver transplant was 8.6 (5.7) years among commercially insured patients and 5.6 (5.6) years among Medicaid-insured patients. Other baseline characteristics among these subgroups were comparable with those of the overall pediatric liver transplant population described in Table 1 and Supporting Table 2, http://links.lww.com/LVT/A329.
Among commercially insured patients, the greatest number of medical visits occurred during the 3- to 6-month period after surgery (mean 19.7 visits), although a substantial number of follow-up visits also occurred during each period that was assessed, continuing through the 12 months following transplant (full results can be found in Supporting Table 4, http://links.lww.com/LVT/A329). The largest driver of total medical visits during each period was outpatient visits, which was highest during the 3- to 6-month period after surgery (mean 13.3). In the 3 months before surgery, commercially insured patients had a mean of 1.0 inpatient visits, with a mean duration of 7.3 days. In the 3 months after surgery (inclusive of transplant day), this rose to a mean of 1.4 inpatient visits, with a mean duration of 39.3 days. Since all patients were required to have an LT, it was expected that all patients would have an inpatient stay; however, 4 patients (1 Commercial, 3 Medicaid) had LTs that were flagged as an outpatient stay, likely due to miscoding in the insurance data.
When the initial stay relating to the liver transplant was excluded from the analysis, the frequency and duration of additional inpatient visits during the 0–3 months after surgery remained notable, with a mean of 0.4 visits lasting a mean of 3.5 days. A similar frequency and duration of visits were maintained over each subsequent period up to 12 months after surgery. The relationship between the total costs for each patient at 0–3 months and 9–12 months after surgery is shown in Supporting Fig. 2, http://links.lww.com/LVT/A329. High initial costs 0–3 months after surgery did not appear to predict sustained higher costs at 9–12 months after surgery.
Among Medicaid-insured patients, the greatest number of medical visits occurred during the first 3 months immediately after surgery (mean 16.4), which was largely driven by outpatient visits (Supporting Table 4, http://links.lww.com/LVT/A329). This population also made a substantial number of health care visits through the 12 months following the transplant. The number of outpatient visits peaked during the 3 months after surgery (mean 11.4). In the 3 months before surgery, Medicaid-insured patients had a mean of 0.7 inpatient visits, with a mean duration of 5.5 days. In the 3 months after surgery, this rose to a mean of 1.4 inpatient visits, with a mean duration of 25.9 days. When the initial stay relating to the liver transplant was excluded from the analysis, the frequency and duration of additional inpatient visits during the 0–3 months after surgery remained notable, with a mean of 0.5 (range 0.0–4.0) visits lasting a mean of 6.0 (range 0.0–134.0) days, though the frequency and duration of visits did then decrease over each subsequent period up to 12 months after surgery. Supporting Fig. 3, http://links.lww.com/LVT/A329, shows the relationship between the total costs for each patient at 0–3 months and 9–12 months after surgery. High initial costs 0–3 months after surgery did not appear to predict sustained higher costs at 9–12 months after surgery.
Costs across 3-month periods
Total medical costs were highest in the 0–3-month period after surgery (which included the liver transplant) for both the commercially insured and Medicaid-insured populations, with mean costs of $609,100 (Fig. 6) and $221,428 (Fig. 7), respectively. Costs throughout the first year after surgery in the commercially insured and Medicaid-insured populations can be found in Supporting Fig. 4, http://links.lww.com/LVT/A329.
FIGURE 6.

All-cause total medical costs by 3-month periods among commercially-insured patients who had ≥12 months of continuous eligibility post–liver transplant date (n = 32). Mean all-cause total medical costs are reported in 2019 US dollars. Ranges for costs were: 3 months before surgery ($0-$283,191); 0–3 months after surgery ($1208-$2,052,575); 3–6 months after surgery ($0-$791,275); 6–9 months after surgery ($112–$276,029); and 9–12 months after surgery ($0-$256,287).
FIGURE 7.

All-cause total medical costs by 3-month periods among Medicaid-insured patients who had ≥12 months of continuous eligibility post–liver transplant date (n = 61). Mean all-cause total medical costs are reported in 2019 US dollars. Ranges for costs were: 3 months before surgery ($0-$99,753); 0–3 months after surgery ($0-$768,590); 3–6 months after surgery ($0-$171,914); 6–9 months after surgery ($0-$52,802); and 9–12 months after surgery ($0-$67,002).
In the commercially insured population, inpatient visits were the highest cost across each period, with a peak (mean $598,217) during the first 3 months after surgery (Supporting Fig. 4, http://links.lww.com/LVT/A329). This was largely due to the cost of the initial stay relating to the liver transplant (mean $559,864), with a lower mean cost of $38,344 for additional inpatient visits 0–3 months after surgery. Outpatient costs peaked during the 3–6-month period after surgery (mean $13,781). Pharmacy costs were relatively consistent across all periods and peaked during the first 3-month period after surgery (mean $8,052). Among pharmacy costs, those associated with cytomegalovirus infection agents steadily declined over the 12 months after surgery, whereas costs associated with immunosuppressants remained relatively stable throughout this period.
Overall trends were similar in the Medicaid-insured population. Inpatient visits were the costliest component during each period, peaking in the first 3 months after surgery (mean $215,157; Supporting Fig. 5, http://links.lww.com/LVT/A329). This was again largely due to the cost of the initial stay relating to the liver transplant (mean $209,798), with a lower mean cost of $5,077 for additional inpatient visits 0–3 months after surgery. Among Medicaid-insured patients, the peak in outpatient costs occurred during the first 3 months after surgery (mean $5,145). Peak pharmacy costs occurred during the 3 months before transplant (mean $7,723) and remained relatively consistent over 12 months of observation. The pattern of costs associated with cytomegalovirus infection agents and immunosuppressants mirrored those incurred by the commercial population.
DISCUSSION
Cholestatic liver disease is the leading indication for a pediatric liver transplant.13 Therefore, in this analysis, we aimed to exclude status 1A patients who may have had an acute liver failure or other conditions more severe than cholestatic liver disease, as well as severely decompensated chronic liver disease requiring ICU admission. This analysis used an enriched population for rarer cholestatic liver diseases like biliary atresia, ALGS, and PFIC. The results demonstrated that pediatric LT imposes a significant economic burden on this specific population of patients, which suggests that improved medical management of some liver diseases could help reduce this burden. For example, ALGS is a cholestatic liver disease with an estimated incidence of 1 in 30,000–50,000 live births,14 of which as few as 24% to 41% of patients reach 18.0–18.5 years without requiring a liver transplant.15,16 In this case, LT may be performed for reasons other than end-stage liver disease, including intractable pruritus, xanthomas, and failure to thrive, all of which result in poor health-related quality of life.17,18 Improved management of these clinical manifestations of ALGS, and other rare cholestatic liver diseases like PFIC, could therefore potentially delay or negate the need for liver transplants in these patients. Reducing the need for LT in patients with cholestatic liver diseases would increase the availability of donor organs for other patients and have cost and HRU benefits across the health care system.
This real-world study assessed HRU and total costs of care during the first year after surgery among pediatric patients with cholestatic liver disease who received a liver transplant in the US. The results show that LT imposed a significant economic burden on both commercially insured and Medicaid-insured populations, with the costs primarily driven by lengthy inpatient stays.
The number of health care visits made by patients included in this analysis appeared higher than that made by US-based children with no medical complexities between 2007 and 2009, who were estimated to make a mean of 0.03 inpatient visits, 2.64 outpatient visits, and 0.21 emergency department visits per year.19 Patients included in this analysis also experienced substantial medical costs, especially when considering the average annual health care costs of a child without medical complexities, which are estimated to be $804.19 A previous study examining the costs of pediatric LT among patients with biliary atresia was estimated to be $458,059 per year in 2018 US dollars, similar to what was observed in the current analysis.20 A separate analysis, focused only on total cost during the transplant surgery admission, found median costs of $162,902.4 for children with ALGS and $150,186.7 for children with BA (in 2018 US dollars), with no significant difference between the 2 groups.21As would be expected, these single-admission costs were lower than the all-cause total medical costs described here. The cost estimates described here for pediatric transplant recipients are also higher than those reported for children with other chronic conditions. For example, the annual cost of treating a child with asthma in the US has been estimated to range from $3,076–$13,612 per child.22–24 It is also useful to compare our results with other severe, complex medical diseases. For example, the results of a French analysis suggest that the mean annual cost of treating a pediatric patient with cystic fibrosis is lower than that described here (€29,746 per patient, which is equivalent to $32,851 per patient, per 2022 exchange rates).25
This analysis demonstrated that pediatric LT was associated with frequent health care visits and significant medical costs in the first year after surgery. Inpatient visits were the costliest type of visit for patients, regardless of insurance type. Most of these costs were due to the initial stay after the transplant, with lower inpatient costs after surgery. The frequency and duration of inpatient visits and associated costs remained fairly steady during each 3-month time period after the initial transplant visit through to 12 months, although the length of inpatient stays declined with time in the Medicaid-insured population. In addition, higher initial total costs at 0–3 months after surgery did not appear to predict sustained costs later in the treatment course at 9–12 months. There was wide variation in the medical costs incurred by individual patients within each cohort, and the mean values were often higher than the corresponding medians, demonstrating that some individuals experienced significantly higher costs compared with the rest of their cohort. This highlights that the care required for patients who undergo LT is complex and can vary according to individual needs.
While there are advantages to using claims data to analyze the economic burden of pediatric LT, there are certain general limitations associated with these types of observational studies. For example, the nature of claims data does not allow an understanding of the burden of specific cholestatic liver diseases such as ALGS and PFIC. The elimination of costs associated with ICU stays more than 24 hours before transplant also served to focus on the LT rather than the costs that occur in severe decompensation or acute-on-chronic liver failure, but this process would not eliminate individuals with other non-cholestatic diseases. In addition, while we eliminated from the analysis individuals with tumors, a condition with clear diagnostic codes, we did not exclude individuals with rare metabolic diseases; these conditions represent a small fraction (ie, ~one-fifth) the number of cases of children transplanted for cholestatic disease.2 These diseases represent rare indications for liver transplants.26 Moreover, individual claims may have been miscoded or contained inaccuracies. In addition, many claims were missing information required to attribute HRU and cost data accurately to specific physician specialties. Among the commercially insured and Medicaid-insured samples, 22.6% and 47.0% of patients were missing a physician specialty code on at least 1 claim in the study period, respectively. For this reason, the breakdown of claims by specialty is likely to underrepresent costs and HRU. As a result, this study adopted a broad approach when assessing the costs and HRU associated with outpatient visits. The reliance on diagnostic and treatment codes, which were designed for administrative purposes, may have led to the misclassification of pediatric patients with LT.
The HRU and cost burdens presented in the analysis do not necessarily reflect all costs incurred but only costs reimbursed by insurers. Several factors such as out-of-pocket expenses (eg, transportation costs), the lifelong need for immunosuppression or retransplantation, additional pharmacologic treatments, loss of education and wages, and the societal impact of these diseases were unaccounted for. Furthermore, these results are limited geographically to patients with commercial or Medicaid insurance in the US, and temporally to October 1, 2015, through December 31, 2019, and thus may not apply to other patient populations. These data cannot be used to estimate the long-term economic burden of pediatric LT; this analysis provides an estimation based on costs reported during the study period. Immunosuppression to ensure that the graft is not rejected by the host is required for some patients throughout their lives, and this analysis does not reflect the costs associated with prolonged immunosuppressant therapy.27 The routine follow-up care required for the recipients of liver transplants, including monitoring of height and weight, supporting adherence to immunosuppressive medication, and screening for potential late surgical complications, was not accounted for in this analysis.8
In addition to this, the small sample sizes in these analyses limit the power of the conclusions. The annualized estimates of HRU and medical costs include what are arguably unrealistic assumptions about the stability of a patient’s health over time, and therefore may result in overestimates of the true average HRU and costs and must be interpreted with care beyond the first-year after surgery. Finally, the exploratory subgroup analysis was restricted to patients who had ≥12 months of posttransplant follow-up, which may have resulted in the selection of a subgroup with less severe disease, as this inherently excludes patients who died or were lost to follow-up within a year of transplantation. However, the baseline characteristics for the subgroup and the overall pediatric population did not appear qualitatively different.
This analysis demonstrates that pediatric LT, in a population of patients selected to exclude those with severe liver disease and failure, resulted in substantial HRU and cost burdens in both the commercially-insured and Medicaid-insured US populations, which were mainly driven by lengthy inpatient stays. The optimization of medical management to slow the progression of liver disease and fibrosis in cholestatic liver disorders is needed. Novel targeted medications may delay or negate the need for LT and could decrease the associated morbidity and costs.
Supplementary Material
ACKNOWLEDGMENTS
This manuscript was written in memory of our co-author Tamir Miloh, who was an important part of this study and passed away during the development of this publication. Medical writing support for the development of this manuscript was provided by Charlotte Rowan, PhD, formerly of Health Interactions, and funded by Mirum Pharmaceuticals, Inc. The authors also thank Allison Briggs, formerly of Analysis Group, Inc., for their data analytic expertise and thoughtful input on the study design. This research was previously presented at the American Association for the Study of Liver Diseases (AASLD) The Liver Meeting® Digital Experience (TLMdX); November 12 -15, 2021. Tamir Miloh, Andrea Goldstein, Robin Howard, Jessica R Marden, Katherine Gaburo, Philip Rosenthal. Cost of pediatric liver transplant among commercial and Medicaid insured patients. Poster number: 1946.
FUNDING INFORMATION
This analysis was funded by Mirum Pharmaceuticals, Inc.
CONFLICTS OF INTEREST
Tamir Miloh consulted for Mirum. Andrea Goldstein owns stock in and is employed by Mirum. Robin Howard and Douglas B. Mogul own stock in and are employed by Mirum. Jessica R. Marden, Annika Anderson, Noam Kirson, and Katherine Gaburo consult for Mirum and are employed by Analysis Group, Inc. Phillip Rosenthal consults for and received grants from Gilead, Abbvie, Travere, MedinCell, and Albiero. He consults for, is on the speakers’ bureau for, and received grants from Mirum. He received grants from Merck and Arrowhead. He consults for Takeda, Audentes, BioMarin, Dicerna, Encoded, and Vertex.
Footnotes
Abbreviations: ALGS, Alagille syndrome; HRU, health care resource utilization; ICD-10, International Classification of Diseases; LT, liver transplantation; PFIC, progressive familial intrahepatic cholestasis; Tenth Revision; ICU, intensive care unit
Data for these analyses were made available to the authors through a third-party license from IBM, a commercial data provider in the US. As such, the authors cannot make these data publicly available due to a data use agreement. Other researchers can access these data by purchasing a license through IBM. Inclusion criteria specified in the Methods section will allow other researchers to identify the same cohort of patients that we used for these analyses. Interested individuals may refer to https://www.ibm.com/products/marketscan-research-databases for more information on accessing the IBM MarketScan data.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.ltxjournal.com.
Contributor Information
Tamir Miloh, Email: jolan.terner-rosenthal@mirumpharma.com.
Andrea Goldstein, Email: agoldstein@mirumpharma.com.
Robin Howard, Email: rhoward@mirumpharma.com.
Douglas B. Mogul, Email: douglas.mogul@mirumpharma.com.
Jessica R. Marden, Email: jessica.marden@analysisgroup.com.
Annika Anderson, Email: annika.anderson@analysisgroup.com.
Katherine Gaburo, Email: katherine.gaburo@analysisgroup.com.
Noam Kirson, Email: noam.kirson@analysisgroup.com.
Philip Rosenthal, Email: prosenth@ucsf.edu.
REFERENCES
- 1.Muiesan P, Vergani D, Mieli-Vergani G. Liver transplantation in children. J Hepatol. 2007;46:340–348. [DOI] [PubMed] [Google Scholar]
- 2.Kwong AJ, Ebel NH, Kim WR, Lake JR, Smith JM, Schladt DP, et al. OPTN/SRTR 2020 annual data report: liver. Am J Transpl. 2022;22:204–309. [DOI] [PubMed] [Google Scholar]
- 3.Leiskau C, Junge N, Pfister ED, Goldschmidt I, Mutschler F, Laue T, et al. Recipient-specific risk factors impairing patient and graft outcome after pediatric liver transplantation-analysis of 858 transplantations in 38 years. Children (Basel). 2021;8:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannuri AC, Lima F, Mello ES, Tanigawa RY, Tannuri U. Prognostic factors for the evolution and reversibility of chronic rejection in pediatric liver transplantation. Clinics (Sao Paulo). 2016;71:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spada M, Riva S, Maggiore G, Cintorino D, Gridelli B. Pediatric liver transplantation. World J Gastroenterol. 2009;15:648–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cañon Reyes I, Halac E, Aredes D, Lauferman L, Cervio G, Dip M, et al. Prognostic factors in pediatric early liver retransplantation. Liver Transpl. 2020;26:528–536. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande RR, Rela M, Girlanda R, Bowles MJ, Muiesan P, Dhawan A, et al. Long-term outcome of liver retransplantation in children. Transplantation. 2002;74:1124–1130. [DOI] [PubMed] [Google Scholar]
- 8.Kelly DA, Bucuvalas JC, Alonso EM, Karpen SJ, Allen U, Green M, et al. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation.. Liver Transpl. 2013;19:798–825. [DOI] [PubMed] [Google Scholar]
- 9.Soltys KA, Mazariegos GV, Squires RH, Sindhi RK, Anand R. SPLIT Research Group. Late graft loss or death in pediatric liver transplantation: An analysis of the SPLIT database. Am J Transpl. 2007;7:2165–2171. [DOI] [PubMed] [Google Scholar]
- 10.Wallot MA, Mathot M, Janssen M, Hölter T, Paul K, Buts JP, et al. Long-term survival and late graft loss in pediatric liver transplant recipients--a 15-year single-center experience. Liver Transpl. 2002;8:615–622. [DOI] [PubMed] [Google Scholar]
- 11.Organ Procurement and Transplantation Network. Transplants in the U.S. by Recipient Age. Accessed March 29, 2022. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#
- 12.Miloh T, Gonzalez-Peralta RP, Mogul D, Baek M, Baker SM, Terner-Rosenthal J, et al. Outcomes of liver transplantation in children with Alagille syndrome compared to a biliary atresia population: Analysis of 2015-2019 data from the UNOS transplant registry [oral presentation]. Oral presentation at the Society of Pediatric Liver Transplantation (SPLIT) Annual Meeting. Pittsburgh, PA, USA. 30 September–1 October 2021.
- 13.Venick RS, Farmer DG, Soto JR, Vargas J, Yersiz H, Kaldas FM, et al. One thousand pediatric liver transplants during thirty years: lessons learned. J Am Coll Surg. 2018;226:355–366. [DOI] [PubMed] [Google Scholar]
- 14.Leonard LD, Chao G, Baker A, Loomes K, Spinner NB. Clinical utility gene card for: alagille syndrome (ALGS). Eur J Hum Genet. 2014;22. doi: 10.1038/ejhg.2013.140. Epub 2013 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamath BM, Stein P, Houwen RHJ, Verkade HJ. Potential of ileal bile acid transporter inhibition as a therapeutic target in Alagille syndrome and progressive familial intrahepatic cholestasis. Liver Int. 2020;40:1812–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandriel S, Li L, She H, Wang J, Czubkowski P, Jankowska I, et al. Clinical features and natural history of 1154 Alagille syndrome patients: results from the international multicenter GALA study group. J Hepatol. 2020;73:S554–S555; (Abstract FRI312). [Google Scholar]
- 17.Kamath BM, Baker A, Houwen R, Todorova L, Kerkar N. Systematic review: the epidemiology, natural history, and burden of Alagille syndrome. J Pediatr Gastroenterol Nutr. 2018;67:148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lykavieris P, Hadchouel M, Chardot C, Bernard O. Outcome of liver disease in children with Alagille syndrome: a study of 163 patients. Gut. 2001;49:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuo DZ, Melguizo-Castro M, Goudie A, Nick TG, Robbins JM, Casey PH. Variation in child health care utilization by medical complexity. Matern Child Health J. 2015;19:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raghu VK, Squires JE, Mogul DB, Squires RH, McKiernan PJ, Mazariegos GV, et al. Cost-effectiveness of primary liver transplantation versus hepatoportoenterostomy in the management of biliary atresia in the United States. Liver Transpl. 2021;27:711–718. [DOI] [PubMed] [Google Scholar]
- 21.Black K, Ziogas IA, Thurm C, Hall M, Hafberg E, Alexopoulos SP, et al. Pediatric liver transplant survival in alagille syndrome is comparable to biliary atresia-A linked database analysis. J Pediatr Gastroenterol Nutr. 2022;75:257–263. [DOI] [PubMed] [Google Scholar]
- 22.Perry R, Braileanu G, Palmer T, Stevens P. The economic burden of pediatric asthma in the United States: literature review of current evidence. Pharmacoeconomics. 2019;37:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan PW, Ghushchyan V, Navaratnam P, Friedman HS, Kavati A, Ortiz B, et al. The national cost of asthma among school-aged children in the United States. Ann Allergy, Asthma Immunol. 2017;119:246–252.e241. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Zhao Y, Keshishian A, Xie L, Yuce H, Baser O. Evaluating asthma-related expenses and health care resource utilization among children in the United States medicaid population [abstract]. Value Health. 2016;19:A114. [Google Scholar]
- 25.Chevreul K, Berg Brigham K, Michel M, Rault G. BURQOL-RD Research Network. Costs and health-related quality of life of patients with cystic fibrosis and their carers in France. J Cyst Fibros. 2015;14:384–391. [DOI] [PubMed] [Google Scholar]
- 26.Sanada Y, Sakuma Y, Onishi Y, Okada N, Yamada N, Hirata Y, et al. Outcomes after living donor liver transplantation in pediatric patients with inherited metabolic diseases. Ann Transplant. 2021;26:e932994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng S. Long-term management of immunosuppression after pediatric liver transplantation: Is minimization or withdrawal desirable or possible or both? Curr Opin Organ Transplant. 2008;13:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]


