Abstract
Background
The cortisol awakening response (CAR) is characterised by an increase in cortisol in the 30 to 60 min after waking. Research has found significant associations between an atypical CAR and symptoms of stress and anxiety in typically developing (TD) children and adolescents. A number of studies have explored the CAR in autism spectrum disorder (ASD), but no evidence synthesis is available to date.
Objective and methods
Based on a preregistered protocol (PROSPERO: CRD42017051187), we carried out a systematic review (SR) and meta-analysis (MA) of CAR studies to explore potential significant differences between children and adolescents with ASD and TD controls. Web of Science, PubMed and PsychInfo were searched until January 2019. A random-effects model was used to pool studies and we used the Newcastle-Ottawa scale (NOS) to assess study quality and risk of bias.
Findings
The SR retrieved a total of nine studies, with mixed findings on the comparison of the CAR between children and adolescents with ASD and TD controls. The MA, based on four studies (ASD; n=117 and TD n=118), suggested no differences between the CAR in ASD and TD populations (SMD: −0.21, 95% CI −0.49 to 0.08). In terms of NOS items, no study specified Representativeness of the cases and Non-response rate.
Discussion and clinical implications
Given the relatively few studies and lack of appropriately matched TD controls, additional research is needed to further understand and recommend the utility of the CAR as a reliable marker to differentiate ASD and TD.
Keywords: anxiety disorders
Summary box.
What is already known about this subject?
A high proportion of children and adolescents diagnosed with autism spectrum disorder (ASD) experience elevated anxiety
The cortisol awakening response (CAR) represents an objective index of anxiety and stress
The CAR is sensitive to an individual’s environment and environmental change
What are the new findings?
The systematic exploration and meta-analysis of the CAR highlighted a limited evidence base to understand its potential as a biomarker to differentiate children with ASD and TD
It indicated that future research should ensure comparison of matched groups of individuals with ASD and typically developing (TD) populations
It also suggested that additional research should more carefully explore gender differences in the CAR
How might it impact on clinical practice in the foreseeable future?
The study makes clear recommendations for further research that has the potential to inform future clinical practice
Background
Recent surveys in the USA and UK estimate that 1.5% of children and 0.8% of adolescents have a diagnosis of autism spectrum disorder (ASD), with prevalence rates in males and females of 1.5% and 0.2%, respectively.1 2 Up to half of individuals with ASD meet the diagnostic criteria for an anxiety disorder,3 4 and symptom severity is increased in females5 and positively associated with autism severity.6 Elevated anxiety in children with ASD can decrease adaptive functioning and reduce social communication over time,7 highlighting a need to understand its prevalence and course in development.
Measurement of anxiety in youth can be difficult. For example, studies have found poor consistency between reports of anxiety from parents and teachers with children or adolescents themselves.8 Self-report of anxiety may be additionally challenging for children and adolescents with ASD, who can show a ‘more fragmented understanding of emotions and their own emotion experience’ (p.667).9 Anxiety can become heightened in anticipation of or when faced with threat, and this response is associated with behavioural avoidance of situations perceived to be threatening.10 Researchers have suggested that anxiety can reflect feelings of stress that are associated with the extent to which individuals feel they can manage and adapt to anticipated or actual threat.11 The cortisol awakening response (CAR) can provide an objective index of an individual’s anxiety or fear and reactivity to stress.12 13
The cortisol awakening response
The hypothalamic-pituitary-adrenal (HPA) axis is the main hormonal system involved in the stress response. It follows a daily diurnal cycle characterised by peak cortisol levels in the morning, that fall throughout the day and with a nadir around midnight.14 15 Approximately 75% of healthy individuals show CAR – an initial peak in cortisol (ie, a rise of 2.49 nmol/L or more in adults or any increase in children) in the 30 to 60 min after waking.16 17 The CAR is a distinct component of the cortisol diurnal rhythm, in part caused by the process of awakening.18 Its measurement involves the collection of one salivary sample directly after waking and at least one additional sample 30 min later.16
Several studies have found significant associations between the CAR and anxiety disorders in adulthood,19 and chronic symptoms of anxiety in adolescence,20 as well as adult reports of being unable to manage daily stress or worry and work overload.21–23 Overstimulation of this system has been linked to ‘hyposecretion’ (p.239).24 In this case, the CAR becomes smaller and less reactive in the context of prolonged and severe stress. For example, a diminished CAR has been associated with posttraumatic stress disorder,25 first episode psychosis,26 severe depression,27 fatigue, exhaustion and burnout,23 as well as the exposure to significant early childhood adversity, such as being reared in institutions.28 Additional studies have found that a reduced CAR is linked to lower scores on cognitive ability measures in adults (eg, slower processing speed on cognitive tasks and lower performance on verbal and non-verbal working memory tasks).26 29
Objective
In this study, for the first time, we meta-analytically explored possible differences in the CAR between children and adolescents with ASD and typically developing (TD) controls.
Search strategy
Our protocol, including the search strategy, for the systematic review (SR) and meta-analysis (MA) was pre-registered on the International Prospective Register of Systematic Reviews PROSPERO (http://www.crd.york.ac.uk/ PROSPERO, protocol number: CRD42017051187). Two researchers blindly searched Web of Science, PubMed and PsychInfo (via EBSCO) to January 2019 (with no date/language/type of document restriction), and completed initial searches and data extraction, and quality assessment. The following search terms were used: Autis*, ASD, Asperger* or ‘Pervasive Developmental Disorder’, and ‘Cortisol Awakening’ or ‘Awakening Cortisol’ and syntax was adapted for each database, and included papers with terms in the keywords, title or abstract. References of relevant review papers were hand-searched to identify any possible study missed during the electronic search.
Participants and studies
A study was included if it (1) recruited children and adolescents ≤18 years of age with a diagnosis of ASD according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth or Fifth Edition (DSM-IV or DSM-5) or the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) criteria, and (2) used a quantitative methodology that involved an analysis of cortisol levels at waking and at least one additional sample 30 min later. For the SR, we retained any type of observational study in which the CAR was measured in participants with ASD, regardless of the presence of a control group. For the MA, we included only case-control studies contrasting the CAR in ASD and TD participants. Studies were excluded if they only considered family members (ie, parent, sibling) of a child with ASD. Dissertations, conference presentations and unpublished works were excluded if further data necessary for the MA could not be obtained by the study authors.
Data extraction and quality/risk of bias assessment
Based on expert consensus guidelines on the assessment of the cortisol awakening response,30 we extracted data on (1) the presence of a control sample; the inclusion of a basic description of participants including (2) age, (3) gender, (4) diagnosis; recognition of factors that impact the CAR, linked to (5) medication and (6) IQ; detail reflecting best practice for the CAR measurement including (7) timing, (8) instructions and (9) compliance. We used the Newcastle Ottawa Scale (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) to assess the risk of bias of the studies included in the SR/MA.
Statistical analysis
To be pooled in the MA, studies had to include a case control design that compared the CAR between groups.31 Means and SD for the CAR value in participants with ASD and TD controls were extracted by one reviewer and double checked by a second one, and entered into Review Manager V.5.3 (http://handbook-5-1.cochrane.org/). Standardised mean differences (SMD) were calculated and combined for each study, using the inverse variance method. A random effects model was used, given the inherent heterogeneity of the studies, an I2 statistic was calculated to allow an estimate of between study heterogeneity in SMD. An I2 value of more than 50% would indicate substantial heterogeneity between the studies used in the meta-analysis.32
Results
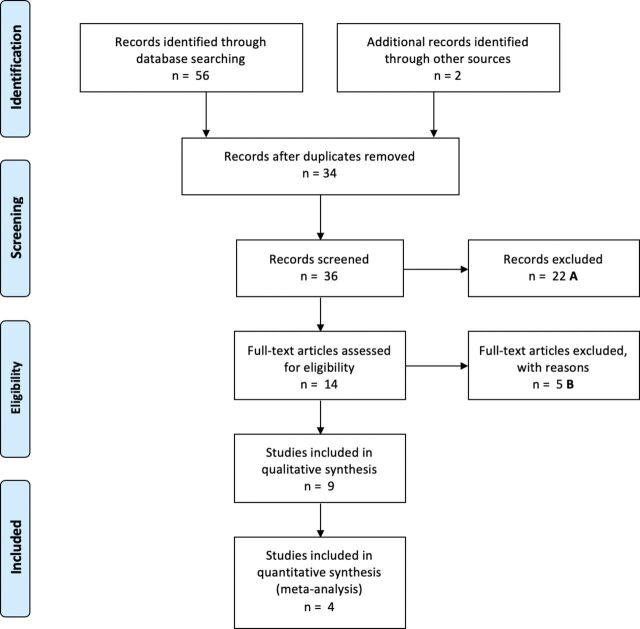
Fifty six papers were identified by both researchers, and after removal of duplications 34 were assessed for eligibility for the review. Two further articles were identified using reference lists of identified papers, making a total of 36 papers. Twenty two papers were excluded from screening titles and abstracts. The full text of 14 papers were explored and of these, nine were included in the SR, and four in the MA. Reasons for exclusion of the remaining six papers are reported in the online supplementary table a. Figure 1 shows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram detailing the selection process. Tables 1 and 2 summarise the core characteristics of the studies included in the SR and MA.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow-chart. (A) No ASD population (n=2), conference presentation (n=1), review paper (n=2), focus on other family member (n=17). (B) No ASD population (n=1), conference presentation (n=1), review paper (n=3). ASD, autism spectrum disorder.
Table 1.
Key characteristics of the studies included in the systematic review and the cortisol awakening response results
| Study information | ASD | Results | |||||
| Author/year | Country | Cortisol measure Other measures |
Diagnosis | Exclusion | N (M/F) |
Mean age (SD) range | CAR |
| Gabriels et al, 201333 | USA | Cortisol: Immediate wake,+30 mins; before lunch; 4 pm; 3 days. Other: repetitive behaviour; IQ, sleep patterns. |
Met or exceeded cut-off on Social Communication Questionnaire (SCQ)and ADOS; diagnosed using clinical records, SCQ and ADOS. | IQ<40; female; pubertal. | 21 male | 3–9 years. | Compared CAR between groups of children who showed low and high RB; wake+30 mins CAR NS. Both groups the showed expected circadian rhythm; the high RB group cortisol levels <low RB group at all time points. |
| Marinović- Curin et al, 200834 | Croatia | Cortisol: Immediate wake,+30 mins; before lunch; after lunch; 6 pm; just before sleep (~10 pm). 1 day. Other: adrenocorticotropic hormone (ACTH) stimulation test. | DSM-IV and ICD-10 criteria; ADI and childhood autism rating scale. | Psychiatric or endocrinology disorders; female. | 9 male | 11.9 years; (±2.97). | This paper included an age matched TD control group (7 males, mean age=11.6 (±4.27). The paper reported no difference in the CAR between groups and a typical circadian rhythm, but did not tabulate cortisol levels at awakening/30 mins later. |
| Sharpley et al, 201935 | Australia | Cortisol: Immediate wake +30 mins; 1 day. Other: Self-reported anxiety (GAD, SP) and depression symptoms; IQ. | ADOS; ADOS-2 cut-off (DSM-5). | Female; IQ<70; genetic or neurological conditions, or previous or comorbid psychiatric disorder. | 32 male | 14.3 years (2.7). | Inter-individual variability in the CAR with around half of the adolescents showing no or a reverse CAR. No association between emotional symptoms and the CAR. Age and IQ, and ADOS score was not associated with the CAR. |
| Sharpley et al, 201638 | Australia | Cortisol: Immediate wake,+30 mins; between 2-4 pm; 1 day. Other: Self-reported anxiety (GAD) and depression symptoms; IQ. | DSM-IV criteria, and ADOS-2. | Male; genetic or neurological conditions, or previous or comorbid psychiatric disorder; IQ<70. | 39 female | 10.1 years (SD 2.7). | Inter-individual variability in the CAR with around half (59%) of the adolescents showing no or a reverse CAR. Circadian rhythm present for~86%. Self-reported symptoms of major depresive disorder (MDD) was associated with a flattened CAR. Age, IQ and ADOS score, RB, was not associated with the CAR. |
| Viau et al, 201039 | Canada | Cortisol: Immediate wake,+30 min; bedtime; 1 day; a week; averages computed for each time point (2 weeks pre-dog; 4 weeks during dog, and 2 weeks post dog). Other: Family income. |
Diagnostic evaluation by professionals. | Allergic to dogs or taking oral steroids. | 42 (37 male) | 7.1 years (±3.1). | Normal diurnal rhythms in salivary cortisol. Reduction in CAR when the service dog was introduced. Age and family income were not associated with the CAR. |
Five studies were included in the SR, and these were not included in the MA due to the absence of a TD control group (n=4), or *where the data presented did not allow a group comparison, and where no further information could be obtained following unsuccessful efforts to make contact with the authors (n=1).
ADI; Autism Diagnostic Interview; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; CAR, cortisol awakening response; DSM, Diagnostic and Statistical Manual of Mental Disorders; F, female; GAD, generalised anxiety disorders; ICD, International Statistical Classification of Diseases and Related Health Problems; M, male; MA, meta-analysis; NS, not specified; RB, repetitive behaviours; SP, social phobia; SR, systematic review; TD, typically developing.
Table 2.
Key characteristics of the studies included in the systematic review and meta-analysis and a comparison of the cortisol awakening response results between TD and individuals with ASD
| Study information | ASD group | TD group | Results | ||||||||
| Author/year | Origin | Cortisol measures | Diagnosis | Exclusion | N (F/M) | Age (Mean; SD; range) | CAR (Mean, SD) | N (F/M) |
Mean age (SD/range) | CAR (Mean; SD) | CAR difference |
| Brosnan et al, 200936 | UK | Cortisol: Immediate wake +30 mins; evening; 2 days. Other measure: daily hassles. | DSM-IV formal diagnosis. | Learning or physical disability; medication; female; comorbid conditions. |
20 male | 12.8 (1.91) 11–16 |
0.29 (1.21) | 28 male | 13.3 (1.91) 11–16 |
1.63 (2.58) | Flattened CAR in ASD group, and typical circadian rhythm in both groups (decrease from AM to PM). Age and daily hassles not linked to the CAR. |
| Corbett & Schupp, 201437 | USA | Cortisol: Immediate wake +30 mins; afternoon; evening; 3 days. Other measure: IQ; social communication. |
DSM-IV and ADOS formal diagnosis. | IQ<70; pubertal; female; medications control: neurodevelopmental conditions. |
46 male | 10.3 8–12.1 |
0.21 (0.50) | 48 male | 9.9 8–12.1 |
0.23 (0.28) | Group difference NS. IQ and social communication was not associated with the CAR. Circadian rhythm data not reported. |
| Tomarken, et al, 201540 | USA | Cortisol: Immediate wake +30 mins; afternoon; evening; 3 days. Other: IQ, stress, sensory sensitivity, child behaviour, self-reported state and trait anxiety. | DSM-IV and ADOS formal diagnosis. | IQ<70; medication. | 36 (30 male) | 10.2 (1.96) | 0.64 (2.00) | 27 (23 male) | 9.71 (1.54) | 0.68 (1.44) | Group difference CAR NS. (ASD had increased cortisol in the evening; morning/afternoon to evening was flatter and more variable). IQ was not associated with cortisol. Parent and self-report anxiety was associated with lower awakening cortisol. |
| Zinke et al, 201041 | Germany | Cortisol: Immediate wake +30 mins; 2 days. Other: IQ; sleep; mediation; morning stress; comorbidity. | ICD-10; ADI; ADOS. | Insufficient verbal ability; IQ<78; genetic, infectious or metabolic disorders. | 15 (13 male) | 9.1 6–12 |
5.16 (5.17) | 25 (21 male) | 9.0 6–12 |
7.59 (5.89) | Group difference CAR NS. |
ADI; Autism Diagnostic Interview; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; CAR, cortisol awakening response; DSM, Diagnostic and Statistical Manual of Mental Disorders; F, female; ICD, International Statistical Classification of Diseases and Related Health Problems; M, male; NS, not specified; TD, typically developing.
ebmental-2019-300098supp001.pdf (109.6KB, pdf)
Study characteristics
Tables 1 and 2 show that the age range of participants in the nine papers was 3 to 18 years across 135 TD and 276 children and adolescents with ASD. All studies included a sample with a diagnosis of autism, Asperger’s or pervasive developmental disorder - not otherwise specified. The majority of participants were male (ASD: 77%; TD: 94%), with five studies including all male participants,33–37 one with all female participants38 and three with mixed gender.39–41 Most studies (eight out of nine) confirmed diagnoses of ASD with validated and widely accepted diagnostic measures, including the Autism Diagnostic Interview-Revised42 or the Autism Diagnostic Observation Schedule.43 The remaining study relied on prior diagnostic evaluation by ‘independent professionals’ and no further information was provided.39
Systematic review
Following the inclusion criteria, all studies used the preferred sampling method for the CAR data, taking samples at awakening and 30 min after. Studies focused on investigating the CAR and its correlates in children and adolescents with ASD and/or comparing the CAR with TD controls. Five studies included an age matched control comparison group, and the remaining four studies had no control sample (table 2). Of those with a control group, four studies provided evidence to suggest that the CAR (any rise in cortisol 30 min post awakening) in children and adolescents with ASD is similar to that seen in the general population,34 37 40 41 and one study found a blunted CAR in ASD (vs a TD group).36 Considering the four studies that did not have a TD comparison, one study showed increases in cortisol from immediate awakening to 30 min in children with ASD (changes were illustrated only in figures).39 A further study showed a similar pattern, but the difference did not reach statistical significance.33 Two further studies reported that a CAR was present in around half of adolescents with ASD (with others showing a flattened or reverse CAR, ie, a decrease from awakening to 30 min later).35 38 Other researchers also found that some children and adolescents with ASD did not show an increase in cortisol from awakening to 30 min later, though noted that the number of young people who did not show a CAR was similar between ASD and TD groups.37 41
The only intervention study included in the present review found an expected CAR that decreased during the intervention stage (introduction of a therapy dog) for children and adolescents with ASD, and increased once the intervention was removed.39 Reductions in the CAR following the intervention were reflected in parent reports of fewer behavioural difficulties and the authors suggested that the change in the CAR reflected increased positivity in the young person.
Two studies focused only on the CAR (sampling at two time points),35 41 while six measured the diurnal rhythm more broadly, sampling cortisol across the day (mean number of samples across all studies=3.11 (SD=0.73, range=2 to 4). Four studies reported a typical cortisol circadian rhythm33 34 36 40 (though one study noted a shallower decline in the ASD group).40 One study did not report this data37 and a further study reported a flattened slope from morning to evening cortisol for a subset of adolescents with ASD (vs a TD group).38
Several studies measured additional variables to ensure that key factors (eg, IQ, bedtime behaviour, medication, social communication, puberty level) were either matched between comparison groups33 37 41 or to control for these variables in analyses.35 Other researchers explored whether these additional variables were associated with the CAR or the diurnal rhythm more broadly. For example, one paper reported a flattened (or reverse) CAR for some girls diagnosed with ASD and where this was most evident for those young people who self-reported elevated symptoms of depression.38 Another study found a negative association between increased parent report symptoms of anxiety/depression with waking cortisol.40 In addition, one paper showed that children with ASD with more repetitive behaviour had lower cortisol levels at every measurement point across the day (ie, with samples taken at awakening +30 min, lunch and dinner).30
Seven studies reported adequate cortisol analysis techniques, with sufficient detail of extraction and assay techniques.33 35–38 40 41 Across studies, there was some variation in instructions given to participants regarding protocols and kits used for collecting saliva samples. For example, four (out of nine) studies provided inadequate information regarding methods for measuring participant compliance with cortisol sampling procedures.33 34 38 40Compliance is important for measuring the CAR and differences between actual and recorded time of sampling can skew the reported pattern of cortisol levels. Consensus guidelines likens the sampling of the CAR following awakening to a quadratic function, indicating that short and long delays increase or decrease the CAR respectively (and where delays >40 min can lead to a negative CAR).30 Further studies reported methods to increase compliance.
Two studies employed a TrackCap system; an electronic monitoring system that tracks when test tubes are opened and swabs removed and returned to the collection kits.37 41 Other studies increased compliance with researchers taking the cortisol samples themselves36 or visiting the home before and after samples were collected.35 38 Other methods involved the use of established standardised procedures and practices,40 or researchers asking parents to use a diary to record sampling times.36 For one study, different methods led to a lack of equivalence between participant groups, where the researcher collected samples from the ASD group and parents collected them in the control group.39 The presence of the researcher can introduce an artificial (and likely stressful) component to a young person’s normal waking routine, that may have influenced cortisol levels.
Tables 1 and 2 show that five studies excluded children and adolescents with IQs below 70 or 80, and one study33 excluded those with an IQ below 40 to control for the effect of cognitive functioning on cortisol level. The broader measurement, reporting and analysis of IQ across studies is mixed. For example, six studies measured IQ.33 35 37 38 40 41 Of the five studies that included a TD comparison group, three reported statistically significant differences in IQ between groups (with increased IQ in the TD group).37 40 41 Across studies there was no association between IQ and cortisol level,41 with the degree of change from cortisol at awakening to 30 min later37 or with the presence or absence of a CAR.35 In one paper it was unclear that group differences in IQ had been explored in the analysis43 or the authors reported that IQ was not explored due to a small sample sizes.33
Tables 1 and 2 show that to further reduce the influence of confounding variables, three studies excluded participants taking any medication36 37 40 and six excluded all participants with current or historical comorbid psychiatric disorders or a neurodevelopmental disorder (eg, attention deficit hyperactivity disorder). Exclusion based on the presence of a psychiatric disorder could serve to reduce the external validity of studies and their applicability to the wider ASD population, because psychiatric disorders, especially anxiety disorders are highly prevalent in individuals with ASD.3
Studies that included additional analyses comparing medicated and non-medicated participants reported no significant group differences on cortisol levels41 or noted that the medication did not affect cortisol secretion (as reported by the manufacturer) and so these participants were not excluded from their analyses.38
Meta-analysis
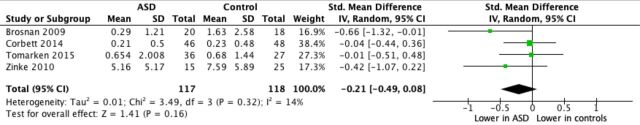
Five studies identified from the SR met the eligibility criteria for inclusion in the MA (see table 2 and figure 2). One paper was excluded from the meta-analysis due to unsuccessful contact with authors (ie, no further information could be obtained regarding specific cortisol values that were not reported in the paper; see online supplementary table a). Therefore, in total, four studies were included in the meta-analysis.
Figure 2.
Forest plot for meta-analysis of differences in CAR between children and adolescents diagnosed with ASD versus TD control groups. Note: Mean and SD values represent raw scores for all studies except Corbett & Schupp (2014) which show log CAR values. ASD, autism spectrum disorder; CAR, cortisol awakening response; TD, typically developing.
While the overall CAR was smaller in the ASD versus the TD group, collectively the results indicated no significant difference between ASD and TD groups in the mean CAR (SMD: 0.21, 95% CI −0.49 to 0.08), suggesting that both groups showed a similar pattern of a rise in cortisol in the 30 min after wakening (figure 2). The I2between study test for heterogeneity indicated that only 14% of the variability in effect estimate was likely to be due to the heterogeneity of the studies. While there is no established minimum of studies that can be included in a MA,44 this analysis was limited by a small sample of studies. However, the I2 of our pooled estimated was low (14%) indicating consistency across the pooled studies, suggesting that our results are likely to be generalisable and representative of the true effect size.
Assessment of study quality/risk of bias
Online supplementary table b indicates that all studies were considered at low risk in terms of definition of cases and ascertainment of exposure, however, no study provided information representativeness of cases and non-response rate. Definition of controls, when applicable, was in general appropriate. In relation to the item comparability of cases and controls, most studies matched cases and controls on one, rather than two or more, factors.
Discussion
This study explored, using a SR and MA, possible differences in the CAR between children and adolescents with ASD and TD. The SR highlighted that few studies (n=9) have explored the CAR in children and adolescent with ASD and fewer studies (n=5) have compared the results to an age-matched control group. When pooling studies in the MA, even though participants with ASD showed an overall lower CAR in ASD compared with TD, the difference was not significant.
This null result could be accounted for by the limited power due to the small number of included studies, but also to heterogeneity across studies with respect to a number of characteristics, as well as methodological issues of the individual studies included in the MA. For example, of the five studies with a control group only three measured IQ. No study included groups matched on both age and IQ. The MA did however indicate that children and adolescents with ASD showed a comparable CAR to TD children and these studies were of increased quality relative to those across the SR more broadly. One further study indicated that CAR could be effectively utilised as an index of positive change following an intervention.39
Some studies included in the MA did highlight cortisol differences for children and adolescents diagnosed with ASD (vs TD) and this included a blunted CAR.36 A further study also noted a blunted CAR in children with ASD (no TD comparison).33 This profile of findings fits with previous research which suggests that chronic stress or prolonged exposure to stressors is associated with a smaller CAR.23 27 A blunted CAR was found in adolescent males with ASD who lived in residential care compared with TD adolescents who lived at home.36 A later study proposed that the group difference might potentially reflect daily adaptation challenges in this alternative care (vs care at home) setting,41 that negatively impacted HPA axis functioning28. Further studies also raised the possibility that findings highlighting a flattened or reversed CAR,35 or a smaller decrease in cortisol from the morning to the evening,40 may also reflect chronic stress and anxiety in individuals with ASD.
Some studies noted that while there is some evidence for the absence of a CAR in children and adolescents with ASD, the percentage who show this profile is similar to age-matched TD control groups.37 41 This finding emphasises the need to more carefully explore underpinning reasons for different CAR profiles in ASD and TD populations. While differences might reflect challenges in daily life,36 results in the SR showed a negative association between the CAR with symptoms of depression in girls with ASD.38 Moreover, moving beyond the CAR, increased repetitive behaviour in male children with ASD was also associated with lowered cortisol across the day. The authors argued that this behaviour might serve an adaptive function to moderate daily stressors.33 The investigation of individual differences and associations with the cortisol diurnal cycle fits with findings that have shown a positive association between self-reported and parent-reported symptoms of generalised anxiety with increased (afternoon) cortisol in children with ASD.45
Explaining individual differences in the CAR has extended to consideration of cognitive functioning, with several studies in the broader literature reporting positive associations with cognitive ability.26 29 46 These link to research that has identified a positive association between IQ with symptoms of anxiety in adults47 and children and adolescents diagnosed with ASD.48 Further studies have found that indices of lower cognitive functioning (eg, the absence (vs presence) of spoken language) were associated with a reduced fall in cortisol across the day in children and adolescents with ASD.49
Summary and clinical implications
This MA represents the first quantitative synthesis of studies on the CAR in ASD, which complement the larger qualitative synthesis provided by the SR. The MA suggests no differences between the CAR in TD and ASD populations, though across the SR results are more mixed. The findings should be considered in the light of possible limitations of our MA. Even though we endeavoured to conduct a comprehensive search, we cannot exclude the possibility that we missed relevant studies. Additionally, we could not gather information/data from all the contacted authors. Finally, due to the limited number of studies, we could not test publication bias, subgroup or meta-regression analyses. Given the small evidence base, the review highlights a need for further well-designed and controlled studies to develop a clearer understanding of the CAR as a reliable and valid measure of stress and anxiety in children and young people with ASD. To control for developmental and gender differences, future research should aim to include comparison groups that are matched for chronological age and cognitive ability, and that allow some comparison between gender.
Acknowledgments
This study formed part a Doctorate in Clinical Psychology research thesis. We are grateful to Leanne Fahey for help with data extraction.
Footnotes
Contributors: All authors contributed to the conception and design of the study. EL organised the database and the project administration. EL and SC performed the statistical analysis. EL and JH wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Brugha T, Cooper S, Gullon-Scott FJ, et al. Autism spectrum disorder. Adult psychiatric morbidity survey. NHS Digital 2014. [Google Scholar]
- 2. Doherty J, Cooper M, Thapar A. Advances in our understanding of the genetics of childhood neurodevelopmental disorders. Evid Based Ment Health 2018;21:171–2. 10.1136/ebmental-2018-300067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Bruin EI, Ferdinand RF, Meester S, et al. High rates of psychiatric co-morbidity in PDD-NOS. J Autism Dev Disord 2007;37:877–86. 10.1007/s10803-006-0215-x [DOI] [PubMed] [Google Scholar]
- 4. van Steensel FJ, Bögels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clin Child Fam Psychol Rev 2011;14:302–17. 10.1007/s10567-011-0097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murray C, Kovshoff H, Brown A, et al. Exploring the anxiety and depression profile in individuals diagnosed with an autism spectrum disorder in adulthood. Res Autism Spectr Disord 2019;58:1–8. 10.1016/j.rasd.2018.11.002 [DOI] [Google Scholar]
- 6. Lever AG, Geurts HM. Psychiatric co-occurring symptoms and disorders in young, middle-aged, and older adults with autism spectrum disorder. J Autism Dev Disord 2016;46:1916–30. 10.1007/s10803-016-2722-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duvekot J, van der Ende J, Verhulst FC, et al. Examining bidirectional effects between the autism spectrum disorder (ASD) core symptom domains and anxiety in children with ASD. J Child Psychol Psychiatry 2018;59:277–84. 10.1111/jcpp.12829 [DOI] [PubMed] [Google Scholar]
- 8. De Los Reyes A, Augenstein TM, Wang M, et al. The validity of the multi-informant approach to assessing child and adolescent mental health. Psychol Bull 2015;141:858–900. 10.1037/a0038498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rieffe C, Oosterveld P, Terwogt MM, et al. Emotion regulation and internalizing symptoms in children with autism spectrum disorders. Autism 2011;15:655–70. 10.1177/1362361310366571 [DOI] [PubMed] [Google Scholar]
- 10. Raymond JG, Steele JD, Seriès P. Modeling trait anxiety: from computational processes to personality. Front Psychiatry 2017;8:1. 10.3389/fpsyt.2017.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Putwain D. Researching academic stress and anxiety in students: some methodological considerations. Br Educ Res J 2007;33:207–19. 10.1080/01411920701208258 [DOI] [Google Scholar]
- 12. Rickard NS, Chin T-C, Vella-Brodrick DA. Cortisol awakening response as an index of mental health and well-being in adolescents. J Happiness Stud 2016;17:2555–68. 10.1007/s10902-015-9706-9 [DOI] [Google Scholar]
- 13. Taylor JL, Corbett BA. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology 2014;49:207–28. 10.1016/j.psyneuen.2014.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gröschl M, Rauh M, Dörr HG. Circadian rhythm of salivary cortisol, 17alpha-hydroxyprogesterone, and progesterone in healthy children. Clin Chem 2003;49:1688–91. 10.1373/49.10.1688 [DOI] [PubMed] [Google Scholar]
- 15. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 2002;53:865–71. 10.1016/S0022-3999(02)00429-4 [DOI] [PubMed] [Google Scholar]
- 16. Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 2009;72:67–73. 10.1016/j.ijpsycho.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 17. Wüst S, Wolf J, Hellhammer DH, et al. The cortisol awakening response - normal values and confounds. Noise Health 2000;2:79–88. [PubMed] [Google Scholar]
- 18. Wilhelm I, Born J, Kudielka BM, et al. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology 2007;32:358–66. 10.1016/j.psyneuen.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 19. Vreeburg SA, Zitman FG, van Pelt J, et al. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom Med 2010;72:340–7. 10.1097/PSY.0b013e3181d2f0c8 [DOI] [PubMed] [Google Scholar]
- 20. Greaves-Lord K, Ferdinand RF, Oldehinkel AJ, et al. Higher cortisol awakening response in young adolescents with persistent anxiety problems. Acta Psychiatr Scand 2007;116:137–44. 10.1111/j.1600-0447.2007.01001.x [DOI] [PubMed] [Google Scholar]
- 21. Gartland N, O’Connor DB, Lawton R, et al. Exploring day-to-day dynamics of daily stressor appraisals, physical symptoms and the cortisol awakening response. Psychoneuroendocrinology 2014;50:130–8. 10.1016/j.psyneuen.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 22. Schlotz W, Hellhammer J, Schulz P, et al. Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom Med 2004;66:207–14. 10.1097/01.psy.0000116715.78238.56 [DOI] [PubMed] [Google Scholar]
- 23. Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol 2009;80:265–78. 10.1016/j.biopsycho.2008.10.004 [DOI] [PubMed] [Google Scholar]
- 24. Mondelli V, Dazzan P, Hepgul N, et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res 2010;116:234–42. 10.1016/j.schres.2009.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wessa M, Rohleder N, Kirschbaum C, et al. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology 2006;31:209–15. 10.1016/j.psyneuen.2005.06.010 [DOI] [PubMed] [Google Scholar]
- 26. Aas M, Dazzan P, Mondelli V, et al. Abnormal cortisol awakening response predicts worse cognitive function in patients with first-episode psychosis. Psychol Med 2011;41:463–76. 10.1017/S0033291710001170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dedovic K, Ngiam J. The cortisol awakening response and major depression: examining the evidence. Neuropsychiatr Dis Treat 2015;11:1181. 10.2147/NDT.S62289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumsta R, Schlotz W, Golm D, et al. HPA axis dysregulation in adult adoptees twenty years after severe institutional deprivation in childhood. Psychoneuroendocrinology 2017;86:196–202. 10.1016/j.psyneuen.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 29. Almela M, van der Meij L, Hidalgo V, et al. The cortisol awakening response and memory performance in older men and women. Psychoneuroendocrinology 2012;37:1929–40. 10.1016/j.psyneuen.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 30. Stalder T, Kirschbaum C, Kudielka BM, et al. Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology 2016;63:414–32. 10.1016/j.psyneuen.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 31. Efthimiou O. Practical guide to the meta-analysis of rare events. Evid Based Ment Health 2018;21:72–6. 10.1136/eb-2018-102911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Furukawa TA, Salanti G, Atkinson LZ, et al. Comparative efficacy and acceptability of first-generation and second-generation antidepressants in the acute treatment of major depression: protocol for a network meta-analysis. BMJ Open 2016;6:e010919. 10.1136/bmjopen-2015-010919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gabriels RL, Agnew JA, Pan Z, et al. Elevated repetitive behaviors are associated with lower diurnal salivary cortisol levels in autism spectrum disorder. Biol Psychol 2013;93:262–8. 10.1016/j.biopsycho.2013.02.017 [DOI] [PubMed] [Google Scholar]
- 34. Marinović-Curin J, Marinović-Terzić I, Bujas-Petković Z, et al. Slower cortisol response during ACTH stimulation test in autistic children. Eur Child Adolesc Psychiatry 2008;17:39–43. 10.1007/s00787-007-0632-1 [DOI] [PubMed] [Google Scholar]
- 35. Sharpley CF, Bitsika V, McMillan ME, et al. Incidence, profiles and correlates of the Cortisol Awakening Response in high-functioning young males with ASD. Res Autism Spectr Disord 2019;57:145–53. 10.1016/j.rasd.2018.11.001 [DOI] [Google Scholar]
- 36. Brosnan M, Turner-Cobb J, Munro-Naan Z, et al. Absence of a normal cortisol awakening response (CAR) in adolescent males with Asperger syndrome (AS). Psychoneuroendocrinology 2009;34:1095–100. 10.1016/j.psyneuen.2009.02.011 [DOI] [PubMed] [Google Scholar]
- 37. Corbett BA, Schupp CW. The cortisol awakening response (CAR) in male children with autism spectrum disorder. Horm Behav 2014;65:345–50. 10.1016/j.yhbeh.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharpley CF, Bitsika V, Andronicos NM, et al. Further evidence of HPA-axis dysregulation and its correlation with depression in Autism Spectrum Disorders: Data from girls. Physiol Behav 2016;167:110–7. 10.1016/j.physbeh.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 39. Viau R, Arsenault-Lapierre G, Fecteau S, et al. Effect of service dogs on salivary cortisol secretion in autistic children. Psychoneuroendocrinology 2010;35:1187–93. 10.1016/j.psyneuen.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 40. Tomarken AJ, Han GT, Corbett BA. Temporal patterns, heterogeneity, and stability of diurnal cortisol rhythms in children with autism spectrum disorder. Psychoneuroendocrinology 2015;62:217–26. 10.1016/j.psyneuen.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zinke K, Fries E, Kliegel M, et al. Children with high-functioning autism show a normal cortisol awakening response (CAR). Psychoneuroendocrinology 2010;35:1578–82. 10.1016/j.psyneuen.2010.03.009 [DOI] [PubMed] [Google Scholar]
- 42. Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 1994;24:659–85. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- 43. Lord C, Rutter M, DiLavore PC, et al. Autism diagnostic observation schedule: manual. Los Angeles, CA: Western Psychological Services, 1994. [Google Scholar]
- 44. Mavridis D, Chaimani A, Efthimiou O, et al. Missing outcome data in meta-analysis. Evid Based Ment Health 2018;21:123. 10.1136/eb-2014-101899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bitsika V, Sharpley CF, Andronicos NM, et al. Hypothalamus-pituitary-adrenal axis daily fluctuation, anxiety and age interact to predict cortisol concentrations in boys with an autism spectrum disorder. Physiol Behav 2015;138:200–7. 10.1016/j.physbeh.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 46. Evans PD, Fredhoi C, Loveday C, et al. The diurnal cortisol cycle and cognitive performance in the healthy old. Int J Psychophysiol 2011;79:371–7. 10.1016/j.ijpsycho.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 47. Mayes SD, Calhoun SL, Murray MJ, et al. Variables associated with anxiety and depression in children with autism. J Dev Phys Disabil 2011;23:325–37. 10.1007/s10882-011-9231-7 [DOI] [Google Scholar]
- 48. van Steensel FJA, Heeman EJ. Anxiety levels in children with autism spectrum disorder: a meta-analysis. J Child Fam Stud 2017;26:1753–67. 10.1007/s10826-017-0687-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tordjman S, Anderson GM, Kermarrec S, et al. Altered circadian patterns of salivary cortisol in low-functioning children and adolescents with autism. Psychoneuroendocrinology 2014;50:227–45. 10.1016/j.psyneuen.2014.08.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ebmental-2019-300098supp001.pdf (109.6KB, pdf)