Abstract
Background
The comparative efficacy and tolerability of methylphenidate (MPH) and neurofeedback (NF) in individuals with attention-deficit/hyperactivity disorder (ADHD) remains uncertain. This study aimed to fill this gap by means of a systematic review/meta-analysis.
Methods
PubMed, OVID, ERIC, Web of Science, ClinialTrials.gov and a set of Chinese databases were searched until 22 August 2018. Standardised mean differences (SMD) were pooled using comprehensive meta-analysis software.
Results
18 randomised controlled trials (RCTs) were included (778 individuals with ADHD in the NF arm and 757 in the MPH group, respectively; 13 studies in Chinese, five in English). At the study first endpoint, MPH was significantly more efficacious than NF on ADHD core symptoms (ADHD symptoms combined: SMD=−0.578, 95% CI (−1.063 to –0.092)) and on two neuropsychological parameters (inattention:−0.959 (-1.711 to –0.208); inhibition:−0.469 (-0.872 to –0.066)). Dropouts were significantly lower in NF versus MPH (OR=0.412, 0.186 to 0.913). Results were robust to sensitivity analyses, with two important exceptions: removing Chinese studies and non-funded studies, no differences emerged between MPH and NF, although the number of studies was small. At the study follow-up, MPH was superior to NF in some outcomes, but results were inconsistent across raters.
Conclusions
Due to the risk of bias of included studies, the results of the sensitivity analysis excluding Chinese and non-funded studies, and the mixed findings on at the follow-up endpoint, further high quality studies are needed to assess the comparative efficacy and acceptability of NF and MPH in individuals with ADHD.
Trial registration number
CRD42018090256.
Keywords: impulse control disorders
Background
With a worldwide estimated prevalence around 5% in school-age children and 2.5% in adults,1 2 attention-deficit/hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders. In the USA, annual incremental costs have been found to range from $143 to $266 billion,3 and social costs are substantial in other countries as well.4 Currently, proposed treatment for ADHD include pharmacological and non-pharmacological options, such as, among others, behavioural interventions/parenting skills and cognitive training.5–7 Several guidelines recommend psychostimulants, including methylphenidate (MPH), as a first-line pharmacological option.8–10 MPH inhibits the reuptake of dopamine and norepinephrine, increasing dopaminergic and noradrenergic activity in the prefrontal cortex, which may contribute to its efficacy and effectiveness in ADHD. Although MPH is highly efficacious on ADHD symptoms of ADHD in the short-term,11 there are concerns over its tolerability and long-term effects.12 For instance, a recent study found a rate of psychotic events of 0.4% and 0.2% during treatment with amphetamines and methylphenidate, respectively,13 although this study could not prove causality.14
Therefore, alternative non-pharmacological options directly targeting the pathophysiology of ADHD are currently being actively investigated. Among these, neurofeedback (NF) has been proposed by a number of research groups as an effective and safe option for ADHD.15 16NF is a process of operant conditioning which aims at improving self-regulation of brain activity through the correction of electroencephalography (EEG) abnormalities.17 18 When applied to ADHD, NF is meant to address the alterations in EEG that have been reported in this disorder (at least in a subsample of patients), in particular the increase in slow-wave activity in frontal regions.19 Meta-analytic evidence on the efficacy of NF for ADHD is currently mixed. An early meta-analysis pooled 15 studies and concluded that standard protocols such as theta/beta ratio (TBR), sensorimotor rhythm (SMR) and slow cortical potentials (SCP) NF are well investigated and have demonstrated specificity.18 The meta-analysis concluded that NF treatment for ADHD can be considered ‘efficacious and specific’, with a large effect size (ES) for inattention and impulsivity (0.8097 and 0.6862, respectively) and a medium ES (0.3962) for hyperactivity. By contrast, a more recent meta-analysis with different inclusion criteria,20 after pooling 13 randomised controlled trials (RCTs) (including 520 participants with ADHD) found that, while ratings from unblinded assessors show significant effects of NF in reducing ADHD core symptoms, ratings from probably blinded assessors fail to support NF as an effective treatment for ADHD core symptoms. Evidence on the comparative efficacy/effectiveness and tolerability of stimulants (including MPH and amphetamines) and NF needs further investigation. In a recent network meta-analysis,21 stimulants emerged as significantly more efficacious than NF on ADHD symptoms and global functioning. However, this network meta-analysis did not focus on the effects of stimulants (or, more specifically, MPH) and NF on subdomains of ADHD separately (ie, inattention and hyperactivity/impulsivity). This is of relevance given that previous studies have shown that inattention and hyperactivity/impulsivity symptoms may have different degrees of sensitivity to different treatments.22 Additionally, the network meta-analysis21 chose to use a dichotomous outcome (ie, proportion of patients who displayed improvements in the symptoms of ADHD or global functioning on standardised rating scales), which may be less informative compared with continuous outcomes.23
Furthermore, when considering the comparison between MPH and NF, and the treatment of ADHD more in general, a key aspect, highly relevant from a clinical standpoint, relates to sustained effects. Another meta-analysis24 focused on sustained effects (defined by these authors as those at follow-up at 2 to 12 months) of NF in ADHD. It found that, compared with non-active control treatments, NF had significantly more durable treatment effects for at least 6 months following treatment, although the authors concluded that additional studies are needed for a properly powered comparison of follow-up effects between NF and active treatments.24 Indeed, this meta-analysis could not inform on the sustained effect of NF and MPH directly because it considered MPH combined with other active treatments including attention training, cognitive training, physical activity training and self-management.
Finally, another aspect that deserves further investigation relates to the comparative efficacy of MPH and NF on neuropsychological measures, such as working memory or sustained attention. This is of relevance because executive dysfunctions, although far from being universal in ADHD, affect a sizeable portion of individuals with ADHD and impact on their academical and global functioning.25 Therefore, a number of questions still need to be answered in relation to the comparative efficacy and tolerability of MPH and NF.
Objectives
Our study aimed to fill these gaps by means of a systematic review and meta-analysis of head-to-head RCTs comparing the effects (at trial end point and, if available, at follow-up) of MPH and NF in terms of efficacy on ADHD core symptoms (combined, inattention and hyperactivity/impulsivity), using continuous measures as outcome. We also assessed the comparative tolerability of MPH and NF and the comparative effects on neuropsychological variables.
Methods
For this systematic review/meta-analysis we followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).26 The protocol of this systematic review/meta-analysis was registered in PROSPERO (CRD42018090676) and published as peer-reviewed article.27
Eligibility criteria
Population
We included studies recruiting participants (children/adolescents (<18 years) and/or adults (≥18 years)) with a categorical diagnosis of ADHD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) (III, III-R, IV, IV-TR or 5), hyperkinetic disorder as per the International Classification of Diseases, 10th revision (ICD-10) or previous ICD versions or with ADHD defined based on scores above cut-off point on any validated ADHD measure, as in previous meta-analyses.28 29
Intervention(s)
We included trials comparing head-to-head NF and MPH. Both fixed-dose and flexible-dose designs (in relation to the MPH regime) were allowed. Studies assessing the efficacy of multimodal treatments including the combination of NF plus other treatments were excluded, to avoid confounders.
Comparator(s)/control
Studies including a non-active comparator were retained if they included at least two other active arms, that is, MPH and NF.
Types of outcomes
The primary outcome was the efficacy (as a continuous outcome) on the severity of ADHD core symptoms at the end of the study (first available time point) and, if available, at follow-up. We performed an analysis focusing on the total (combined) ADHD score, that is inattentive plus hyperactive/impulsive symptoms, and another set of analyses focusing on ADHD subdomains, that is analysing separately inattention and hyperactivity/impulsivity. Validated ADHD rating scales that we considered eligible for the measurement of the outcomes are reported in table 1. As in previous studies,30 we planned to conduct separate analyses for measures rated by (1) clinicians, (2) parents, (3) teachers and (4) patients (self), if available. Secondary outcomes were the number of dropouts for any reasons at the end of the intervention (and, if available, at follow-up) and neuropsychological laboratory-based measures of working memory (eg, visual-spatial working memory task),31 attention (eg, test of variables of attention,32 33 attention endurance test34 and inhibition (eg, integrated visual and auditory continuous performance test).35
Table 1.
Descriptive table of the studies included in the meta-analysis
| First author (year) | NF(standard) | MPH | Treatment duration | Follow-up | Assessment instrument | Outcome | Diagnosis of ADHD | Country | Rater | |||||||
| Age | N | Male | Drop out | Age | N | Male | Drop out | Treatment | ||||||||
| Chen (2007) | 6–13 | 43 | NR | 4 | 6–13 | 43 | NR | 11 | 5 mg/day | 3 months | 2 months | Conner-parent | HI IA |
DSM-IV | China | P |
| Chen (2009) | 9.16± 2.09 |
25 | 80 | 0 | 9.38± 2.16 |
30 | 79.31 | 1 | 18–54 mg/day | 6 weeks | 2 months | IVA-CPT IOWA Conners |
FRCQ FAQ |
DSM-III-R | China | P |
| Chen (2011) | 7.6± 1.5 |
45 | 77.8 | NR | 7.5± 1.7 |
36 | 80 | NR | 10 mg/day, Monday to Friday | 6 months | 3 months | Conner-parent IVA-CPT |
HI IA FRCQ FAQ |
DSM-IV | China | P |
| Du (2014) | 6–14 | 60 | NR | 6 | 6–14 | 60 | NR | 0 | 5 mg/day | 3–4 months | 6 months |
SNAP-IV | TS | DSM-IV | China | P |
| Duric (2017) | 11.4± 3.1 |
42 | 73 | 12 | 10.9± 2.4 |
44 | 87 | 13 | 1 mg/kg/day; range: 20–60 mg | 3 months | 6 months | Barkley (teacher) |
TS HI IA |
ICD-10 | Norway | T/P |
| Fan (2012) | 6–13 | 89 | NR | NR | 6–13 | 80 | NR | NR | 5 mg/day - 20 mg/day | 3 months | 3 months | Conner-parent | HI IA |
DSM-IV | China | P |
| Gelade (2018) | 9.8± 1.9 |
39 | 72.7 | 1 | 9.0± 1.2 |
36 | 75.0 | 5 | 5–20 mg/day | 12 weeks | 6 months | SWAN | HI IA |
DSM-IV | Netherlands | T/P |
| Ji (2009) | 8.75± 1.66 |
69 | 76.8 | NR | 9.19± 1.72 |
63 | 76.2 | NR | >5 mg/day | 3–4 months | NR | IVA-CPT | FRCQ FAQ |
DSM-IV | China | NA |
| Kong (2007) | 8.6±1.2 | 90 | 75.6 | 0 | 8.4±1.4 | 90 | 74.4 | 10 | 5 mg/day and constantly adjusted | 3 months | 6 months | Conner-parent TOVA1 |
HI IA IQ omissions RT variation |
DSM-IV | China | P |
| Li (2001) | 8–13 | 28 | NR | 2 | 8–13 | 29 | NR | 8 | Initial dose 5 mg/day and constantly adjusted | 3–4 months | 1–3 months | Conner-parent | HI IA |
DSM-III | China | P |
| Meisel (2013) | 9.5±1.8 | 14 | 50 | 2 | 8.9±1.5 | 13 | 54.55 | 2 | 1 mg/kg/day | 2 months | 2 months | ADHD RS-IV | TS HI IA |
DSM-IV | Spain | T/P |
| Moreno (2015) | 9.21±1.9 | 19 | 79 | NR | 9.21±2.2 | 19 | 79 | NR | Immediate, intermediate release or osmotic controlled release oral system | 20 weeks | NR | IVA-CPT | IVA/CPT | ADHDRS-IV | Spain | NA |
| Sudnawa (2018) | 8.4±1.6 | 20 | 90 | 1 | 9.0±1.5 | 20 | 90 | 0 | 5–20 mg/day | 12 weeks | NR | VADTRS | HI IA TS |
NR | Thailand | T/P |
| Tang (2017) | 8.64±1.54 | 43 | 55.8 | NR | 8.75±1.51 | 43 | 53.4 | NR | 5 mg/day | 3 months | NR | Conner-parent | HI IA |
ICD-10 | China | P |
| Yang (2016) | Child | 63 | NR | NR | Child | 63 | NR | NR | 5 mg/day | 6 months | NR | SNAP-IV | Total score | DSM-IV | China | P |
| Zhang (2006) | 6.5–11.9 | 21 | 79.5 | 1 | 6.5–11.9 | 22 | 79.5 | 6 | 5 mg/day | 3–4 months | 3 months | Conner-parent | HI IA |
DSM-IV | China | P |
| Zhou (2012) | 8.4±1.7 | 38 | 78.9 | 0 | 9.1±1.5 | 36 | 77.8 | 4 | 18–36 mg/day | 6 months | NR | IVA-CPT | FRCQ FAQ |
DSM-IV | China | NA |
| Zuo (2009) | 8.4±2.3 | 30 | NR | NR | 8.4±2.3 | 30 | NR | NR | 0.1–0.61 mg/kg/day | 2 months | 3 months | IVA-CPT | FRCQ FAQ |
DSM-III-R | China | NA |
ADHD, attention-deficit/hyperactivity disorder; IVA/CPT, integrated visual and auditory continuous performance test; MPH, methylphenidate; NF, neurofeedback; NR, not recorded; ADHD RS-IV, ADHD Rating Scales IV; FAQ, full attention quotient; FRCQ, Full scale of Response Control Quotient; HI, Hyperactivity/Impulsivity; IA, Inattention; IQ, Intelligence Quotient; RT, Response Time; SNAP-IV, Swanson, Nolan and Pelham Scale-Version IV; SWAN, Strengths and Weaknesses of ADHD Symptoms and Normal Behavior Rating Scale; TS, Total score; VADTRS, Vanderbilt ADHD Diagnostic Teacher Rating Scale.
With regards to timing, the primary analysis focused on endpoint minus baseline changes, while secondary analyses focused on data, when available, at a follow-up (time point closest to 12 months after treatment, following the same approach as in Van Doren et al 2019 and other studies,24 36 or longer follow-up, if available).
Types of study
RCTs were included regardless the level of blinding. We planned to include parallel-group RCTs as well as crossover trials, but no cross-over studies were found for the present meta-analysis.
Search strategy
We included published and unpublished studies pertinent to our criteria. An electronic literature search was conducted independently by two authors. The following electronic databases were searched with no language, date or type of document restrictions: PubMed, OVID, ERIC and Web of Science. Chinese databases, including China National Knowledge Infrastructure, CQVIP and WanFang data, were also searched. We also checked ClinicalTrials.gov, clinicaltrialsregister.eu and osf.io for additional reports not published in peer-reviewed journals. Details about the search strategy/syntax are reported in the online supplemental material 1. The references of all selected studies were hand-searched to detect any additional pertinent reference not found with the electronic search.
ebmental-2019-300088supp001.pdf (712.6KB, pdf)
Data extraction
Studies identified through electronic and manual searches were listed with citation, titles and abstracts in EndNote. First, two authors independently screened title and abstracts of all non-duplicated papers and excluded those not pertinent to the criterion. Second, full-text version of the articles was downloaded and assessed for eligibility by two authors, independently. Data from multiple reports of the same study were linked together. The following data were extracted from each included study:
Study details: First author/study ID, year(s) of study or publication, location (country or continent), setting, diagnostic criteria, funding/sponsor (industry or academic);
Participants details, including number, gender distribution, mean and range of age, presence, and type of co-morbid (neuro) psychiatric conditions, mean (and SD) IQ, sample size in each group and number of dropouts for side effects in both groups;
Interventions details, including mean and maximum doses of MPH, type of NF, the duration of interventions and whether forced dose or optimised treatment with MPH; time of outcome measurement;
Outcomes: mean, SD or percentage in both groups at pretest, post-test and follow-up (any time point reported);
Information as to whether participants in the NF studies learnt to regulate the feedback.
Risk of bias (quality) assessment
Risk of bias was assessed for each included study using the Cochrane Collaboration risk of bias tool, as a reference.37 Two independent investigators assessed the risk of bias in selected studies. Any disagreement was resolved through discussion and in consultation with the principal investigators. Where necessary (ie, unclear information for the published report), the corresponding authors of the studies were contacted for further information. As in a previous meta-analysis,38 the overall rating of risk of bias for each study was the lowest rating for any of the criteria (eg, if any domain is scored high risk of bias, the study was considered high risk of bias).
Data synthesis
Meta-analyses of standardised mean differences (SMD) were performed by means of comprehensive meta-analysis (CMA) software. Additionally, we used the appropriate function in CMA, to combine outcomes within study from the same subjects. Heterogeneity was assessed and measured with Cochran’s Q and I2 statistics, which estimates the percentage of variation among effect sizes that can be attributed to heterogeneity.39 Clinically significant values were indicated by SMD >0.4.40
Sensitivity analyses
We performed the following sensitivity analyses: (1) excluding studies not using the Conners’ scale to measure the outcome, (2) excluding studies with small sample size trials (less than 30 children per arm), (3) excluding studies where the diagnosis was not made according to standardised DSM/ICD criteria and (4) removing studies on non-standard NF (ie, TBR, SMR and SCP) as per the criteria set in a previous meta-analysis.18
Publication bias
Publication bias was assessed via funnel plots and Eggers’ test.41
Results
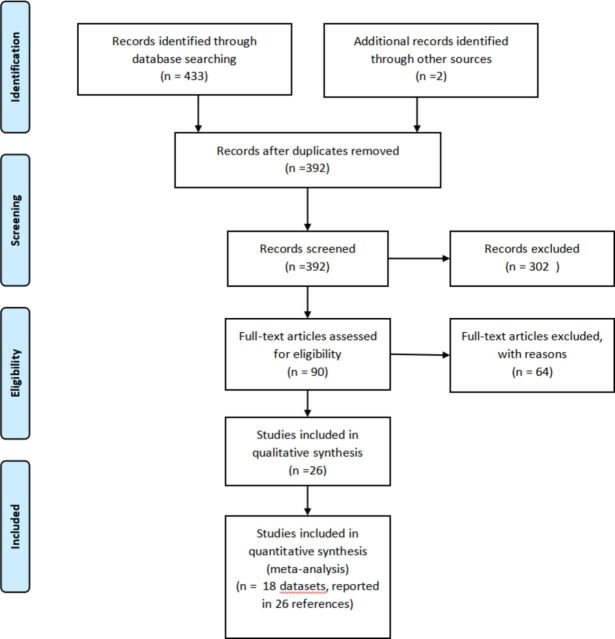
A total of 18 studies meeting study criteria were included (see table 1), encompassing a total of 778 participants in NF group and 757 in MPH group. A detailed description of selection process is shown in figure 1, showing the PRISMA flow diagram. Reasons for studies exclusion after a deep examination in full-text length are listed in online supplemental material 2. A list of included studies is provided in online supplemental material 3. Overall, 13 studies recruited participants from China, two from Spain, one from Norway, one from Thailand and one from Netherlands. Thirteen studies were published in Chinese, while five in English. ADHD core symptoms were rated by parents in Chinese studies (n=10 studies) and by teachers as well as parents in English studies (four studies). In all the retained studies, NF procedures were standard ones, according to the criteria by Arns et al.18 No studies directly assessed whether learning occurred after NF training. In all studies, measures were collected at baseline and first endpoint. Additionally, 12 studies provided also measures at follow-up (in the absence of treatment after the first endpoint). Neuropsychological measures were reported in six studies at endpoint and three studies at follow-up. Rating of risk of bias for each study is listed in the online supplemental material 4. Of note, allocation concealment and blinding of participants/personnel were considered at high risk in all studies of bias and only two studies used blinded assessors.42 43
Figure 1.
PRISMA flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Comparison of NF versus MPH at post treatment (first study endpoint)
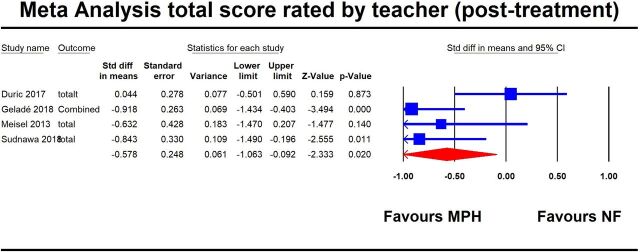
The results based on teachers’ evaluation on ADHD core symptoms are shown in table 2 and figure 2. At the study endpoint, MPH was significantly more efficacious than NF in decreasing the severity of ADHD core symptoms, both when considering the combined symptoms and the individual domains (ADHD symptoms combined: SMD=−0.58, 95% CI −1.06 to −0.09, I2=59.1%; hyperactivity/impulsivity: SMD=−0.47, 95% CI −0.86 to −0.09, I2=37.8%; inattention: SMD = - 0.68, 95% CI −1.25 to −0.11, I2=69.9%).
Table 2.
Summary of the results rated by teachers
| Timepoint | Outcome | Type of analysis | N studies |
N subjects |
SMD | Lower limit | Upper limit | P | Heterogeneity | Egger’s Test publication bias | ||||
| Q | df | p | I2 | t | p | |||||||||
| Post treatment | Total score | 4 | 228 | −0.578 | −1.063 | −0.092 | 0.020 | 7.14 | 3 | 0.062 | 59.126 | 0.155 | 0.890 | |
| Hyperactivity/impulsivity | 4 | 228 | −0.474 | −0.860 | −0.088 | 0.016 | 4.825 | 3 | 0.156 | 37.818 | 0.311 | 0.784 | ||
| Inattention | 4 | 228 | −0.677 | −1.245 | −0.109 | 0.020 | 9.951 | 3 | 0.019 | 69.852 | 0.103 | 0.927 | ||
| Follow-up | Total score | 3 | 198 | −0.192 | −0.531 | 0.148 | 0.268 | 0.045 | 2 | 0.978 | 0.000 | 0.134 | 0.914 | |
| Hyperactivity/impulsivity | 3 | 188 | 0.105 | −0.263 | 0.473 | 0.576 | 2.287 | 2 | 0.319 | 12.565 | 0.078 | 0.475 | ||
| Inattention | 3 | 188 | −0.489 | −0.833 | −0.144 | 0.005 | 1.475 | 2 | 0.478 | 0.000 | 0.149 | 0.905 | ||
SMD, standardised mean differences.
Figure 2.
First plot for the primary outcome (ADHD core symptoms, combined) rated by teacher(post-treatment). ADHD, attention-deficit/hyperactivity disorder.
The results based on parents’ evaluation on ADHD core symptoms are shown in online supplemental table 3 and online supplemental figure 3. At the study endpoint, MPH was significantly more efficacious than NF in decreasing the severity of ADHD core symptoms (ADHD symptoms combined: SMD=−0.50, 95% CI −0.81 to −0.19, I2=81.2%; hyperactivity/impulsivity: SMD=−0.51, 95% CI −0.89 to −0.13, I2=85.7%; inattention SMD=−0.41, 95% CI −0.73 to −0.09, I2=77.2%). Results were substantially confirmed in the sensitivity analyses, as shown in online supplemental table 3.
NF was associated with significantly lower dropout ratio than MPH: 29 dropouts in 420 ADHD participants in NF group and 60 dropouts in 423 ADHD participants in MPH group, (OR=0.41, 95% CI 0.19 to 0.91, I2=40.5%) (see online supplemental table 5 and supplemental figure 5).
With regards to neuropsychological measures, MPH was significantly more efficacious than NF for inattention (SMD=−0.96, 95% CI −1.71 to −0.21, I2=92.4%) and inhibition (SMD=−0.47, 95% CI −0.87 to −0.07, I2=76.5%). For more information, see online supplemental table 4 and online supplemental figure 4. Results were substantially confirmed in the sensitivity analyses, as shown in online supplemental table 4.
Comparison of NF versus MPH at follow-up
As per teachers’ evaluation, at 6 month follow-up, MPH was significantly more efficacious than NF in decreasing the severity of inattention (SMD=−0.49, 95% CI −0.83 to −0.14, I2=0.0%). There was no difference between MPH and NF on total score (SMD=−0.19, 95% CI −0.53 to 0.15, I2=0.0%) and hyperactivity/impulsivity (SMD=0.11, 95% CI −0.26 to 0.47, I2=12.6%). See table 2 and online supplemental figure 6 for additional information.
Based on the parents’ evaluation of ADHD core symptoms (online supplemental table 3 and online supplemental figure 7), at the study endpoint, NF was significantly more efficacious than MPH in decreasing the severity of ADHD core symptoms (ADHD symptoms combined: SMD=0.83, 95% CI 0.42 to 1.25, I2=85.6%; hyperactivity/impulsivity: SMD=0.69, 95% CI 0.40 to 0.97, I2=69.5%; inattention: SMD=0.45, 95% CI 0.04 to 0.86, I2=85.1%). Results were in general robust to the sensitivity analyses, as shown in online supplemental table 3. However, after removing non-funded trials or Chinese studies, no significant differences emerged between NF and MPH.
For neuropsychological measure outcomes, there was no clinically significant difference between two treatments considering inhibition (SMD=−0.21, 95% CI −2.61 to 2.19, I2=98.3%) and inattention (SMD=0.38, 95% CI −0.79 to 1.56, I2=94.4%) as outcomes (see online supplemental table 4 and online supplemental figure 8). Results were robust to the sensitivity analyses, as shown in online supplemental table 4.
Discussion
To our knowledge, this is the first meta-analysis comparing the effects of MPH versus NF for individuals with ADHD on ADHD core symptoms (combined, inattention and hyperactive/impulsive) and neuropsychological measures (inattention and inhibition), as well as their comparative acceptability in terms of participants dropouts.
The findings of our main analysis are consistent with and extend those by Catalá-López et al,21 who, by means of a network meta-analysis, provided comparative evidence on the effects of stimulants (rather than, specifically, MPH) and NF on ADHD combined symptoms relying on a dichotomous outcome (ie, proportion of patients who displayed improvements in the symptoms of ADHD or global functioning on standardised rating scales). They found that stimulants (including MPH and amphetamines) were superior to NF on ADHD combined symptom. We found that, at the first study endpoint, MPH was significantly better than NF both based on teachers’ and parents’ reports not only on combined ADHD symptoms, but also on individual dimensions of ADHD symptoms (ie, inattention and hyperactivity/impulsivity) measured on ADHD rating scales commonly used in clinical practice. Therefore, we believe our results add important clinical information for practitioners. In previous meta-analyses on non-pharmacological treatment for ADHD,20 30 44 45 the European ADHD Guidelines Group used two types of outcomes: those rated by individuals most proximal to the treatment setting (typically unblinded) and those by ‘probably blinded’ raters. As it may be challenging to assess to which extent a rater was ‘probably’ blinded, we analyses separately teachers and parents ratings. The concordance of findings across raters makes our results stronger.
While Catalá-López et al (2017) found no difference, in terms of acceptability between stimulants and NF, we did find NF significantly more acceptable. It should be pointed out that another recent network meta-analysis11 found MPH better tolerated than amphetamines. This may explain differences in terms of findings on acceptability between our meta-analysis and the one by Catalá-López et al (2017). Furthermore, it should be pointed out that we included additional studies, especially the Chinese ones, not included by Catalá-López et al (2017).
We also provided new meta-analytic evidence showing that MPH was superior to NF in terms of its effects on inattention and inhibition. It is important to highlight that this does not provide evidence that MPH improves ADHD symptoms via improvement in neuropsychological functions. Indeed, it has been argued that the two represent two distinct aspects in ADHD.20
Of note, results were robust to a series of sensitivity analyses, but there were two important exceptions. Removing non-funded trials or Chinese studies, no significant differences emerged between NF and MPH. The number of studies in these sensitivity analyses was small (n=3) so these conclusions need to be taken with cautions. It has been noted that the quality of some Chinese trials may be questionable and indeed we rated all the included trials at high risk of bias due to concerns of allocation concealment.46 This may have contributed to placebo effects, which may affect the results based on the studies conducted in China.
Of note, both MPH and NF were beneficial to the improvement of ADHD symptoms at study endpoint, but it is likely that the neural mechanism underlying their clinical action were different. MPH binds with high affinity to the dopamine transporter, and with lower affinity to the norepinephrine transporter and serotonin transporter and inhibits the transport of synaptic monoamines back into the neuron.47 Functional MRI was used to investigate the effects of a single dose of MPH on brain activation during interference inhibition in medication-naive ADHD boys and it was found that MPH significantly normalised the front-striatal under functioning in ADHD patients relative to controls during interference inhibition, but did not affect medial frontal or temporal dysfunction.48
The mechanism underlying NF effects is different. Compared with normal children, a subsample of children with ADHD usually have increased fronto-central theta band activity and increased theta to beta power ratio during rest, though increased theta is not specific to ADHD and is not present in all subjects with ADHD. Attempts to correct these EEG abnormalities provided the rationale for NF in ADHD.49 NF is performed by using an electrode placed on the head and using interactive computer software. In an operant learning procedure, children are taught how to control their brain waves, and NF has the potential to enhance the patient’s capacity to form and execute plans of action. Once learnt and trained, the brain waves can be improved to the normal range of health. However, since brain waves cannot be easily remodelled after simple training several times, there is a process for learning, so the training course is longer, and it usually takes about 40 sessions. NF also has the disadvantage that children should reach the age when they can play video games to reach their goals and are willing to train. As to safety and side effects, NF was categorised as minimal risk and non-severe adverse events in NF training are documented.
However, at the study follow-up, the findings were mixed. Neuropsychological measure outcomes showed that there was no significant difference between MPH and NF. Teachers’ evaluation found that the effect of MPH was better than NF at total score and HI (Hyperactivity/Impulsivity), but parents’ evaluation was on the contrary, showing that the effect of NF was better than that of MPH.
Our results should be considered in the light of some limitations.50 We found significant heterogeneity in many comparisons. There are also some differences in the dose of drugs, the number of feedbacks, which may introduce some bias in the statistical analyses. Another possible limitation is the inclusion of different rating scales to assess the core symptoms of ADHD. However, we selected only validated scales that measure exclusively the same triad of symptoms, that is, inattention, hyperactivity and impulsivity. In future trials, risk of bias should be reduced, in particular, blinding of outcome assessment should be implemented. Finally, our results, as they are based on aggregate data, are true at the group level, but they are not informative at the individual patient level. Indeed, MPH may be the best choice for some patients, while others may benefit more from NF. Individual patient network meta-analyses will be needed to address these issues.
Conclusions
In conclusion, due to the risk of bias of included studies, the discrepancy between the main analysis and the sensitivity analyses excluding Chinese and non-funded studies, and the mixed findings on at the follow-up endpoint, we cannot draw clinically meaningful interpretation of results from this study. Further high quality and larger studies are needed to more properly assess the comparative efficacy and acceptability of NF and MPH in individuals with ADHD.
Acknowledgments
We are very grateful to Dr. Vilawan Chirdkiatgumchai and Dr. Khemika Khemakanok Sudnawa from Mahidol University, Thailand, for providing additional information.
Footnotes
LY and SW contributed equally.
Contributors: JZ conceived the study and supervised screening, data extraction and analyses and wrote the first draft of the manuscript. LY, SW and YY did the screening, data extraction and analyses and revised the manuscript.
Funding: This study was supported by the projects of Postdoctoral Fund of Jiangsu (1401038c) and Jiangsu Overseas Research & Training Programme for University Prominent Young & Middle-Aged Teachers and Presidents ((2017)3523).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not required.
References
- 1. Polanczyk GV, Salum GA, Sugaya LS, et al. Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry 2015;56:345–65. 10.1111/jcpp.12381 [DOI] [PubMed] [Google Scholar]
- 2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry 2009;194:204–11. 10.1192/bjp.bp.107.048827 [DOI] [PubMed] [Google Scholar]
- 3. Doshi JA, Hodgkins P, Kahle J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry 2012;51:990–1002. 10.1016/j.jaac.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 4. Hakkaart-van Roijen L, Zwirs BW, Bouwmans C, et al. Societal costs and quality of life of children suffering from attention deficient hyperactivity disorder (ADHD). Eur Child Adolesc Psychiatry 2007;16:316–26. 10.1007/s00787-007-0603-6 [DOI] [PubMed] [Google Scholar]
- 5. Sonuga-Barke EJS, Brandeis D, Cortese S, et al. Nonpharmacological Interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry 2013;170:275–89. 10.1176/appi.ajp.2012.12070991 [DOI] [PubMed] [Google Scholar]
- 6. Cortese S, Rosello-Miranda R. [Treatments for children and adolescents with attention deficit hyperactivity disorder: what is the evidence base to date?]. Rev Neurol 2017;64:S3–S7. [PubMed] [Google Scholar]
- 7. Richardson M, Moore DA, Gwernan-Jones R, et al. Non-pharmacological interventions for attention-deficit/hyperactivity disorder (ADHD) delivered in school settings: systematic reviews of quantitative and qualitative research. Health Technol Assess 2015;19:1–470. 10.3310/hta19450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bolea-Alamañac B, Nutt DJ, Adamou M, et al. Evidence-based guidelines for the pharmacological management of attention deficit hyperactivity disorder: update on recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2014;28:179–203. 10.1177/0269881113519509 [DOI] [PubMed] [Google Scholar]
- 9. Kooij SJ, Bejerot S, Blackwell A, et al. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry 2010;10:67. 10.1186/1471-244X-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pliszka S. AACAP Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2007;46:894–921. 10.1097/chi.0b013e318054e724 [DOI] [PubMed] [Google Scholar]
- 11. Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry 2018;5:727–38. 10.1016/S2215-0366(18)30269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banaschewski T, Buitelaar J, Chui CS, et al. Methylphenidate for ADHD in children and adolescents: throwing the baby out with the bathwater. Evid Based Ment Health 2016;19:97–9. 10.1136/eb-2016-102461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moran LV, Ongur D, Hsu J, et al. Psychosis with Methylphenidate or Amphetamine in Patients with ADHD. N Engl J Med 2019;380:1128–38. 10.1056/NEJMoa1813751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cortese S. Psychosis during Attention Deficit-Hyperactivity Disorder Treatment with Stimulants. N Engl J Med 2019;380:1178–80. 10.1056/NEJMe1900502 [DOI] [PubMed] [Google Scholar]
- 15. Duric NS, Aßmus J, Elgen IB. Self-reported efficacy of neurofeedback treatment in a clinical randomized controlled study of ADHD children and adolescents. Neuropsychiatr Dis Treat 2014;10:1645–54. 10.2147/NDT.S66466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flisiak-Antonijczuk H, Adamowska S, Chladzinska-Kiejna S, et al. Treatment of ADHD: comparison of EEG-biofeedback and methylphenidate. Archives of Psychiatry and Psychotherapy 2015;17:31–8. [Google Scholar]
- 17. Strehl U. What learning theories can teach us in designing neurofeedback treatments. Front Hum Neurosci 2014;8:894. 10.3389/fnhum.2014.00894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arns M, Heinrich H, Strehl U. Evaluation of neurofeedback in ADHD: the long and winding road. Biol Psychol 2014;95:108–15. 10.1016/j.biopsycho.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 19. Holtmann M, Sonuga-Barke E, Cortese S, et al. Neurofeedback for ADHD: a review of current evidence. Child Adolesc Psychiatr Clin N Am 2014;23:789–806. 10.1016/j.chc.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 20. Cortese S, Ferrin M, Brandeis D, et al. Neurofeedback for Attention-Deficit/Hyperactivity Disorder: meta-analysis of Clinical and Neuropsychological Outcomes From Randomized Controlled Trials. J Am Acad Child Adolesc Psychiatry 2016;55:444–55. 10.1016/j.jaac.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 21. Catalá-López F, Hutton B, Núñez-Beltrán A, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: A systematic review with network meta-analyses of randomised trials. PLoS One 2017;12:e0180355. 10.1371/journal.pone.0180355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arns M, de Ridder S, Strehl U, et al. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin EEG Neurosci 2009;40:180–9. 10.1177/155005940904000311 [DOI] [PubMed] [Google Scholar]
- 23. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006;332:1080.1. 10.1136/bmj.332.7549.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Doren J, Arns M, Heinrich H, et al. Sustained effects of neurofeedback in ADHD: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry 2019;28. 10.1007/s00787-018-1121-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Willcutt EG, Doyle AE, Nigg JT, et al. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry 2005;57:1336–46. 10.1016/j.biopsych.2005.02.006 [DOI] [PubMed] [Google Scholar]
- 26. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yan L, Zhang J, Yuan Y, et al. Effects of neurofeedback versus methylphenidate for the treatment of attention-deficit/hyperactivity disorder protocol for a systematic review and meta-analysis of head-to-head trials. Medicine 2018;97:e12623–e23. 10.1097/MD.0000000000012623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cortese S. Are concerns about DSM-5 ADHD criteria supported by empirical evidence? BMJ 2013;347:f7072. 10.1136/bmj.f7072 [DOI] [PubMed] [Google Scholar]
- 29. De Crescenzo F, Cortese S, Adamo N, et al. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health 2017;20:4–11. 10.1136/eb-2016-102415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sonuga-Barke EJ, Brandeis D, Cortese S, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry 2013;170:275–89. 10.1176/appi.ajp.2012.12070991 [DOI] [PubMed] [Google Scholar]
- 31. Westerberg H, Hirvikoski T, Forssberg H, et al. Visuo-spatial working memory span: a sensitive measure of cognitive deficits in children with ADHD. Child Neuropsychol 2004;10:155–61. 10.1080/09297040409609806 [DOI] [PubMed] [Google Scholar]
- 32. Greenberg LM. An objective measure of methylphenidate response: clinical use of the MCA. Psychopharmacol Bull 1987;23:279–82. [PubMed] [Google Scholar]
- 33. Fuchs T, Birbaumer N, Lutzenberger W, et al. Neurofeedback treatment for attention-deficit/hyperactivity disorder in children: a comparison with methylphenidate. Appl Psychophysiol Biofeedback 2003;28:1–12. 10.1023/A:1022353731579 [DOI] [PubMed] [Google Scholar]
- 34. Brickenkamp R. Test d2, Aufmerksamkeits-Belastungs-Test G¨ottingen: Hogrefe. 1994.
- 35. Moreno-García I, Meneres-Sancho S, Camacho-Vara de Rey C, et al. A Randomized Controlled Trial to Examine the Posttreatment Efficacy of Neurofeedback, Behavior Therapy, and Pharmacology on ADHD Measures. J Atten Disord 2019;23:1087054717693371–71. 10.1177/1087054717693371 [DOI] [PubMed] [Google Scholar]
- 36. Steiner NJ, Frenette EC, Rene KM, et al. In-school neurofeedback training for ADHD: sustained improvements from a randomized control trial. Pediatrics 2014;133:483–92. 10.1542/peds.2013-2059 [DOI] [PubMed] [Google Scholar]
- 37. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley and Sons, 2011. [Google Scholar]
- 38. Cortese S, Adamo N, Mohr-Jensen C, et al. Comparative efficacy and tolerability of pharmacological interventions for attention-deficit/hyperactivity disorder in children, adolescents and adults: protocol for a systematic review and network meta-analysis. BMJ Open 2017;7:e013967. 10.1136/bmjopen-2016-013967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis New Jersey: John Wiley & Sons, Ltd, 2009:24. [Google Scholar]
- 40. Citrome L. Quantifying clinical relevance. Innov Clin Neurosci 2014;11(5-6):26–30. [PMC free article] [PubMed] [Google Scholar]
- 41. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geladé K, Janssen TWP, Bink M, et al. A 6-month follow-up of an RCT on behavioral and neurocognitive effects of neurofeedback in children with ADHD. Eur Child Adolesc Psychiatry 2018;27:581–93. 10.1007/s00787-017-1072-1 [DOI] [PubMed] [Google Scholar]
- 43. Moreno-García I, Delgado-Pardo G, Camacho-Vara de Rey C, et al. Neurofeedback, pharmacological treatment and behavioral therapy in hyperactivity: Multilevel analysis of treatment effects on electroencephalography. Int J Clin Health Psychol 2015;15:217–25. 10.1016/j.ijchp.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cortese S, Ferrin M, Brandeis D, et al. Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry 2015;54:164–74. 10.1016/j.jaac.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Daley D, van der Oord S, Ferrin M, et al. Behavioral interventions in attention-deficit/hyperactivity disorder: a meta-analysis of randomized controlled trials across multiple outcome domains. J Am Acad Child Adolesc Psychiatry 2014;53:835–47. 10.1016/j.jaac.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 46. Purgato M, Cipriani A, Barbui C. Randomized trials published in Chinese or Western journals: comparative empirical analysis. J Clin Psychopharmacol 2012;32:354–61. 10.1097/JCP.0b013e3182546ef6 [DOI] [PubMed] [Google Scholar]
- 47. Han DD, Gu HH, Hh G. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol 2006;6:6:6. 10.1186/1471-2210-6-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rubia K, Halari R, Cubillo A, et al. Methylphenidate normalizes fronto-striatal underactivation during interference inhibition in medication-naïve boys with attention-deficit hyperactivity disorder. Neuropsychopharmacology 2011;36:1575–86. 10.1038/npp.2011.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Loo SK, Makeig S. Clinical utility of EEG in attention-deficit/hyperactivity disorder: a research update. Neurotherapeutics 2012;9:569–87. 10.1007/s13311-012-0131-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cortese S, Coghill D. Twenty years of research on attention-deficit/hyperactivity disorder (ADHD): looking back, looking forward. Evid Based Ment Health 2018;21:173–6. 10.1136/ebmental-2018-300050 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ebmental-2019-300088supp001.pdf (712.6KB, pdf)